菌根是自然界中普遍存在的一种共生现象,是由土壤中的菌根真菌与高等植物根系形成的共生关系[1]。研究表明,菌根真菌所获取的氮和磷可占植物根系吸收的80%[2]。外生菌根是菌根中的一种主要类型,主要在木本植物中形成,如板栗、杨树和松树等。与丛枝菌根真菌入侵植物根系皮层细胞形成细胞内丛枝结构不同,外生菌根具有典型的结构特征,真菌菌丝在根尖外层形成菌套,在根表皮细胞之间形成哈蒂氏网以及根外形成外延菌丝[3-4]。外生菌根真菌的外延菌丝从土壤中吸收水分及矿质营养元素通过远距离运输给菌根,然后通过相关转运蛋白转运至共生界面提供给宿主植物。
氮素是植物生长发育过程中必不可少的营养元素[5]。在自然界中,植物根系吸收氮的主要形式是硝酸盐(NO3-)和铵盐(NH4+)[6-8]。在好氧土壤条件下,NO3-是大多数植物吸收氮的主要形式[9]。为了适应土壤中硝酸盐的浓度变化,高等植物发展出了两种不同的硝酸盐吸收系统,分别为高亲和力运输系统(HATs)及低亲和力运输系统(LATs)[10]。
在植物根系中,NO3-的吸收及运输主要由硝酸盐转运蛋白家族(nitrate transporter,NRT)负责。NRTs 包含了3 个基因家族:NRT1 家族[又称PTR(peptide transporter)基因家族或NPF(NRT1 PTR FAMILY)基因家族][11]、NRT2 家族及NRT3 家族(又称NAR 家族)[9]。NRT1 基因家族十分庞大,除转运NO3-外,还能转运NO2-、多肽和氨基酸等物质,大多数NRT1 家族成员主要在LATs 中起作用[12-14]。NRT2家族主要在HATs系统中起作用,他们往往需要NRT3家族作为伴侣蛋白共同促进植物在低氮水平下对硝酸盐的吸收[15]。在番茄[16]、苜蓿[17]和水稻[18]中均发现了受丛枝菌根诱导表达的硝酸盐转运蛋白基因,其中水稻OsNPF4.5在丛枝菌根中的转录水平比对照组升高了500倍以上;当以硝酸盐作为唯一氮源时,该基因在氮吸收中的贡献可占菌根氮吸收贡献的45%[18]。目前部分丛枝菌根植物共生途径的硝酸盐转运已得到证实,但外生菌根诱导的硝酸盐转运的研究相对较少,其转运机制尚未明确。有研究表明,外生菌根真菌与杨树共生后,杨树PcNRT1.1和PcNRT2.1基因转录水平明显高于对照组[19]。
板栗(Castanea mollissima)为壳斗科栗属植物,属于坚果类乔木经济植物的一种,具有极高的营养价值,有着“木本粮食”、“干果之王”的美称[20],大多生长在土壤贫瘠、干旱的地区[21]。板栗抗旱,耐瘠薄,与其能够形成外生菌根的能力密切相关。板栗可与多种外生菌根真菌共生[22],外生菌根形成后,显著促进板栗对土壤水分、养分的吸收,增强植物对干旱、盐碱、重金属胁迫的耐受性,以及对病虫害的抗性,满足了板栗植株正常生长的需求[23]。
笔者通过同源序列比对,在板栗基因组中鉴定出60 个CmNRT 基因,进化分析表明,他们分布于3个进化支,其中CmNRT3 为NRT3 进化支中仅有的成员,前期研究发现该基因受外生菌根响应后上调表达[24]。笔者在本研究中进一步对板栗CmNRT3基因开展了时空表达定位及相关功能等方面的研究,以期为板栗外生菌根促氮吸收提供科学依据。
1 材料和方法
1.1 试验材料
试验于2021—2022 年在北京农学院农业应用新技术北京市重点实验室进行。本研究用到的植物材料包括板栗(C.mollissima)京暑红、蒺藜苜蓿A17(Medicago truncatula)及本氏烟草(Nicotiana benthamiana)。板栗种子果实饱满、无病虫害,蒺藜苜蓿A17 种子、本氏烟草种子均为实验室扩繁所得。外生菌根真菌橙黄硬皮马勃菌(Scleroderma citrinum,Sc)为笔者实验室分离培养,丛枝菌根真菌异形根孢囊霉(Rhizophagus irregularis,Ri)由北京农林科学院丛枝菌根真菌种质资源库提供。
1.2 外生菌根真菌培养
配制P20 固体培养基(0.5 g·L-1 Di-NH4-tartrat、1 g·L-1磷酸二氢钾、0.5 g·L-1七水合硫酸镁、1 g·L-1葡萄糖、Kanieltra 1000×母液1 mL、100 mg·L-1 Thiamine 母液1 mL、18 g·L-1琼脂、氢氧化钾调节pH 至5.5),用于培养外生菌根真菌橙黄硬皮马勃菌。将继代培养的马勃菌丝切割成5 mm×5 mm 的方块,倒置于P20 固体培养基上,随后将培养基正置于25 ℃培养箱中,黑暗培养15 ~20 d即可获得菌丝活力较强的真菌。
1.3 板栗外生菌根土盆共生
在干净的生长钵中放入2/3 无菌蛭石,将板栗幼苗放置于生长钵中央,自来水浇透。利用固态橙黄硬皮马勃菌接种板栗根系,每株板栗苗接种3 个3 cm×3 cm 的固态橙黄硬皮马勃菌菌块。同时,用保鲜膜封住生长钵,一周后,逐步揭开保鲜膜。隔周分别浇灌水和营养液,2 个月左右即可观察到外生菌根的形成。
1.4 CmNRT3序列克隆及载体构建
利用Omega 植物RNA 提取试剂盒对板栗菌根及未接种根系进行RNA 提取,反转录获得cDNA。根据CmNRT3序列设计引物CmNRT3-ORF-F(5'-ATGGCAGCACGTGGAATTCTCT-3')/CmNRT3-ORFR(5'-TCACTTCTTCTGAGACTGTTTTGCCCTTC-3')和CmNRT3- PRO- F(5'- TCGGGCAGAGTGGAATCTGAATAC- 3')/CmNRT3- PRO- R(5'- TTGCTGCTCTGAGTTGTTGCCA- 3'),分别扩增CmNRT3 ORF序列及起始密码子上游1.8 kb启动子序列。将扩增产物连接到TOPO 载体上,测序正确后,分别构建CmNRT3pro::GUS、35S::CmNRT3::GFP及CmNRT3pro::CmNRT3::GFP表达载体。
1.5 CmNRT3序列比对及进化分析
通过已知的拟南芥、水稻硝酸盐转运蛋白与板栗基因组进行BLASTp 比对分析,并下载相应的板栗硝酸盐转运蛋白氨基酸序列;在NCBI 数据库(https://www.ncbi.nlm.nih.gov/)中下载拟南芥(Arabidopsis thaliana)、水稻(Oryza sativa)、蒺藜苜蓿(M.truncatula)及毛果杨(Populus trichocarpa)等草本及木本植物NRT3 家族蛋白序列。使用MEGA7.0.14 软件中的ClustalW 功能进行序列比对,导入GeneDoc 中标记跨膜结构域与保守结构域;使用MEGA7.0.14 软件中的NJ 邻接法构建进化树,参数Bootstrap值设为1000。
1.6 CmNRT3的表达分析
分别取板栗未接种根系及外生菌根的样品提取RNA,反转录获得cDNA。所用引物通过Primer 3(v.0.4.0)进行设计(http://bioinfo.ut.ee/primer3-0.4.0/),并通过BioEdit 进行特异性分析(引物序列为CmNRT3-F:5'-GTCTAGCTGTAACTTGTTATGGA-3';CmNRT3-R:5'-CTGGCAAACTCTGGTTTAGA-3')。利用CFX96 Touch荧光定量PCR检测系统(BIO-RAD,美国)进行qRT-PCR反应,数据用2-△△CT算法进行处理,并通过SPSS软件进行差异显著性分析。所有试验均设置3 次重复,CmACTIN 作为内参基因(引物序列为CmACTIN-F:5'-GTGGCGGTTCAACCATGTTC-3';CmACTIN-R:5'-GGATGGACCACTCTCATCGT-3')。
1.7 CmNRT3启动子GUS分析
将CmNRT3pro::GUS表达载体转化至发根农杆菌MSU440中,利用毛根转化方法[24-25]获得转基因根系。板栗和苜蓿毛根转化的外植体均是新长出的2~3 d的胚根。将含有转基因根系的板栗/苜蓿植株分别与外生菌根真菌橙黄硬皮马勃菌/丛枝菌根真菌异形根孢囊霉进行共生,获得转基因菌根。取板栗/苜蓿转基因的菌根与未接种根系放入含有GUS染液的离心管中,避光抽真空1~2 h 后放入37 ℃培养箱,避光反应40 h左右,将根段进行树脂包埋和切片处理。切片完成后,采用0.1%钌红染色15 min,即可使用显微镜观察切片。
1.8 CmNRT3蛋白亚细胞定位
将35S::CmNRT3::GFP及Marker质膜标记蛋白pm-rb CD3-1008 表达载体转入根癌农杆菌GV3101中,并制备侵染液。选择状态较好的烟草,吸取侵染液注射烟草叶片下表皮。过夜暗培养后,光照培养2~3 d。将侵染后的烟草叶片剪成小块放置于载玻片上(下表皮朝上),盖上盖玻片,利用激光共聚焦显微镜(Leica,STELLARIS 5)观察拍照。
利用发根农杆菌介导的毛根转化方法,将含有CmNRT3pro::CmNRT3::GFP 表达载体的发根农杆菌MSU440侵染苜蓿,获得转基因根系,进而与异形根孢囊霉共生获得转基因丛枝菌根。将菌根放置于体视显微镜下用双面刀片从中间切成两部分,使用激光共聚焦显微镜观察。由于转基因材料带有GFP融合蛋白及DsRed 红色荧光蛋白,因此可通过GFP(激发光为489 nm,接收光为495~560 nm)和DsRed(激发光为561 nm,接收光为565~680 nm)双激发光,于40倍水镜下观察拍照。
1.9 酵母功能互补
利用Invitrogen 公司Gateway® LR ClonaseTM II重组酶及其试剂盒,将已构建好的pENTR-TOPOCmNRT3载体(含终止密码子TGA)与酵母表达载体pDR-F1-GW 进行LR 重组反应,获得CmNRT3-pDR-F1-GW 表达载体。将试验组CmNRT3-pDR-F1-GW表达载体及对照组pDR-F1-GW载体转入硝酸盐缺陷型的多形汉逊酵母菌株(△ynt-Leu-)中[26-27]。配制不同硝酸盐浓度的选择培养基,以培养基中加入亮氨酸作为阳性对照。选择PCR鉴定为阳性的重组酵母单菌落进行活化(PCR鉴定引物为pDR-F:5'-ATTATGACCGGTGACGAAACGTG-3'和CmNRT3-ORFR:5'-TCACTTCTTCTGAGACTGTTTTGCCCTTC-3'),3000 r·min-1离心5 min,无菌水清洗2 次后再用无菌水重悬,调整酵母OD600为1.0,最后用无菌水稀释10 倍。在不同硝酸盐浓度的培养基上吸取2 μL菌液点接。37 ℃倒置培养,2 d后观察。
2 结果与分析
2.1 板栗NRT基因家族成员CmNRT3的鉴定
为鉴定板栗基因组中的硝酸盐转运蛋白成员,利用拟南芥、水稻硝酸盐转运蛋白对板栗基因组进行BLASTp 筛选,共鉴定出60 个NRT 家族成员。进化树分析表明,NRT1 家族成员56 个,NRT2 家族成员3 个,NRT3 家族成员1 个(图1)。在NRT3 基因家族中,只有1 个板栗基因(Cm06G00423),命名为CmNRT3。对板栗、拟南芥、水稻、苜蓿和杨树NRT3 家族成员的进化树分析表明,这些物种分别存在1、2、2、2和3个NRT3基因,因此利用板栗研究NRT3 基因的功能可以避免同源基因功能的冗余性。

图1 板栗NRT 基因家族系统进化树
Fig.1 Phylogenetic tree of NRT gene family in Chinese chestnut
蛋白序列比对结果(图2)表明,前述5个物种的NRT3 中均含有NAR2(high-affinity nitrate transporter)保守结构域及两个跨膜结构域。在水稻中NAR2结构域参与了硝酸盐的信号转导[28];而跨膜结构域则参与硝酸盐的吸收及运输。这些结果预示了CmNRT3 可能参与板栗根系氮素利用的生物学功能。

图2 CmNRT3 与其他物种NRT3 蛋白的多序列比对
Fig.2 Multiple sequence alignments of CmNRT3 protein with NRT3 proteins in other species
2.2 CmNRT3在外生菌根中诱导上调表达
对板栗60 个NRT 家族成员进行转录组数据分析[29],发现与对照组相比,共有14 个基因在外生菌根中极显著上调表达,且上调倍数大于2 倍(图3)。其中Cm06G00423(CmNRT3)在外生菌根中高度表达,与对照组相比上调了8 倍。为研究CmNRT3 是否在外生菌根中具有作用,qRT-PCR 结果表明,与未接种的对照根相比,该基因在外生菌根中的表达量上调了3.13 倍,因此该基因为外生菌根诱导上调表达的基因(图4),可能在外生菌根共生中发挥作用。
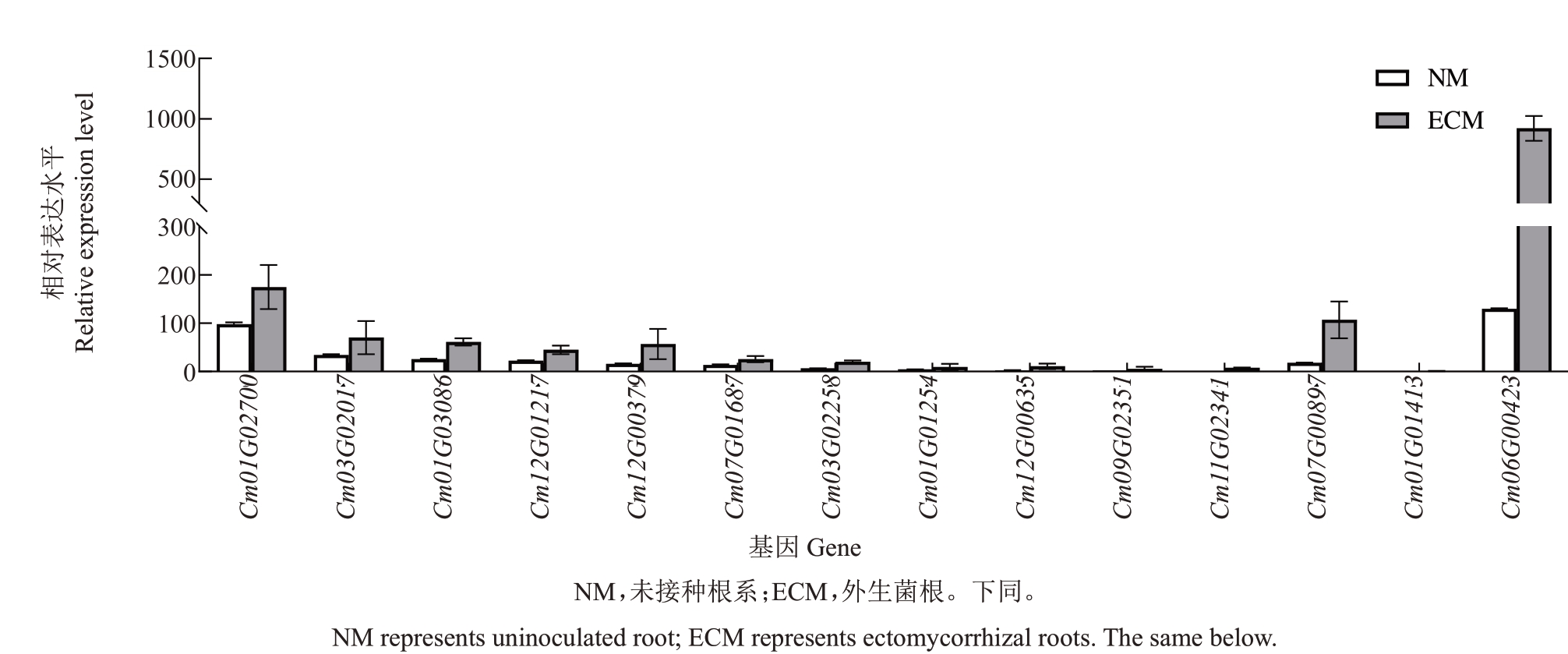
图3 板栗菌根中上调极显著的NRT 基因家族成员转录组数据分析
Fig.3 Transcriptome data analysis of NRT gene family members in chestnut mycorrhizal group that were significantly upregulated
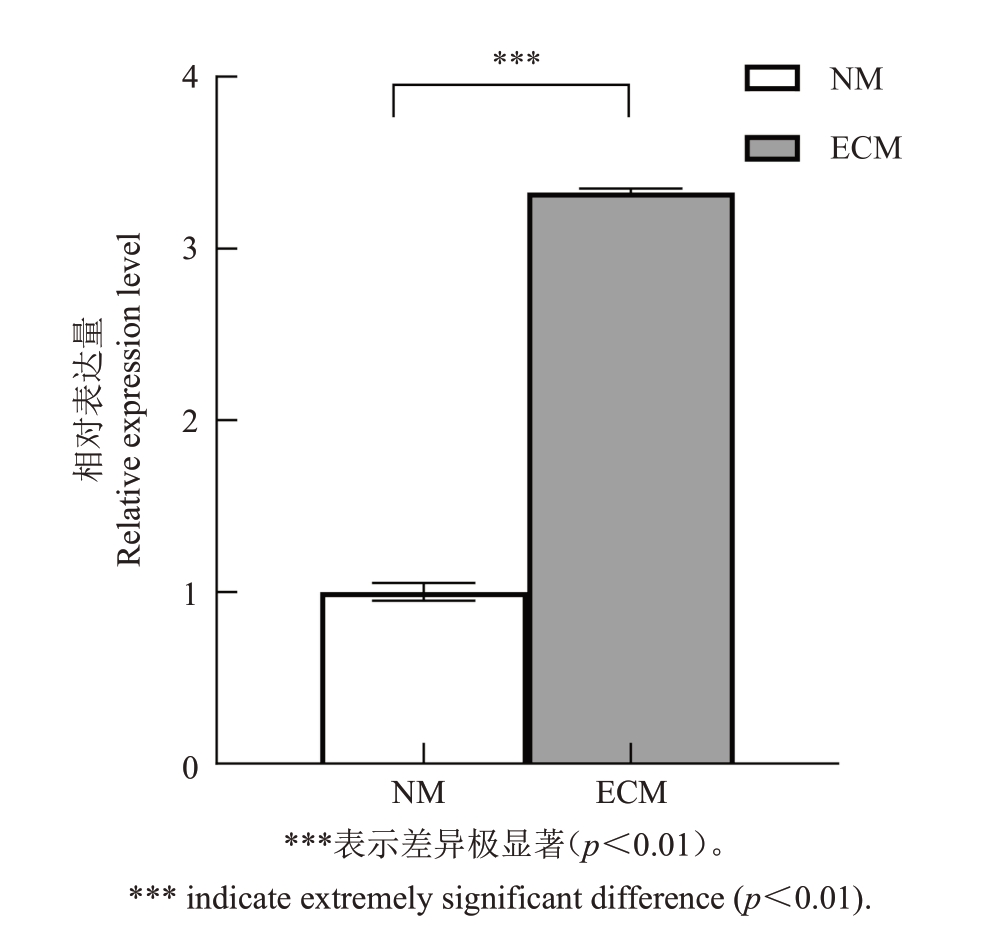
图4 CmNRT3 在板栗未接种根系与外生菌根中的相对表达量
Fig.4 Relative expression of CmNRT3 in non-inoculated roots and ectomycorrhiza of chestnut
2.3 CmNRT3基因启动子在板栗根中的表达定位
为明确CmNRT3 基因的表达定位,构建了由CmNRT3起始密码子上游1.8 kb启动子序列驱动的GUS 表达载体CmNRT3pro::GUS,将该表达载体瞬时转化至板栗根系中。对含有转基因根的板栗苗接种橙黄硬皮马勃菌或不接菌处理(对照组)。但与真菌互作一个月后,所有的转基因根均未能形成外生菌根(n=6)。将对照组及真菌互作组的转基因根进行GUS 染色,并利用半薄塑料切片观察。如图5 所示,对照组的板栗转基因根中(n=6),GUS信号在其表皮细胞中强烈表达,在皮层细胞中也有部分表达。真菌互作组中未形成外生菌根的转基因根也有GUS信号,其表达模式与对照组一致。
图5 CmNRT3 在未接种板栗根系中的启动子活性
Fig.5 Promoter activity of CmNRT3 in uninoculated chestnut roots
2.4 CmNRT3基因启动子在苜蓿根中的表达定位
在未接种丛枝菌根真菌的苜蓿转基因对照根中,GUS活性在苜蓿转基因根系各个组织中均有表达,其中表皮细胞中的表达量最高(图6-A),这与板栗中的表达模式一致,推测不同物种中CmNRT3 表达模式较为保守。转基因苜蓿根系接种丛枝菌根真菌后,CmNRT3 基因在表皮细胞和未形成丛枝的细胞中表达微弱,其主要表达于丛枝细胞。同时,在成熟丛枝的细胞中,GUS 信号最为强烈(图6-B,红箭头指示的细胞类型),而在衰退丛枝的细胞中GUS信号减弱(图6-B,黑箭头指示的细胞类型),表明CmNRT3 的表达与丛枝菌根的发育阶段具有相关性。CmNRT3基因在成熟丛枝细胞中表达量高预示其参与了丛枝的功能,推测该基因介导了丛枝细胞中氮信号转导或硝酸盐的运输。

图6 在接种与不接种丛枝菌根真菌的苜蓿根中CmNRT3 启动子的活性
Fig.6 CmNRT3 promoter activity in M.truncatula roots during mycorrhizal symbiosis and non-inoculated with AM fungus
2.5 CmNRT3蛋白亚细胞定位
为准确定位CmNRT3所编码的蛋白发挥功能的位置,构建了35S::CmNRT3::GFP 表达载体并进行烟草叶片亚细胞定位分析。观察发现,CmNRT3-GFP 融合蛋白与Marker 质膜标记蛋白pm-rb CD3-1008 共定位(图7),说明CmNRT3 蛋白定位在细胞膜上,是膜转运蛋白,与其具有两个跨膜结构域一致(图2)。
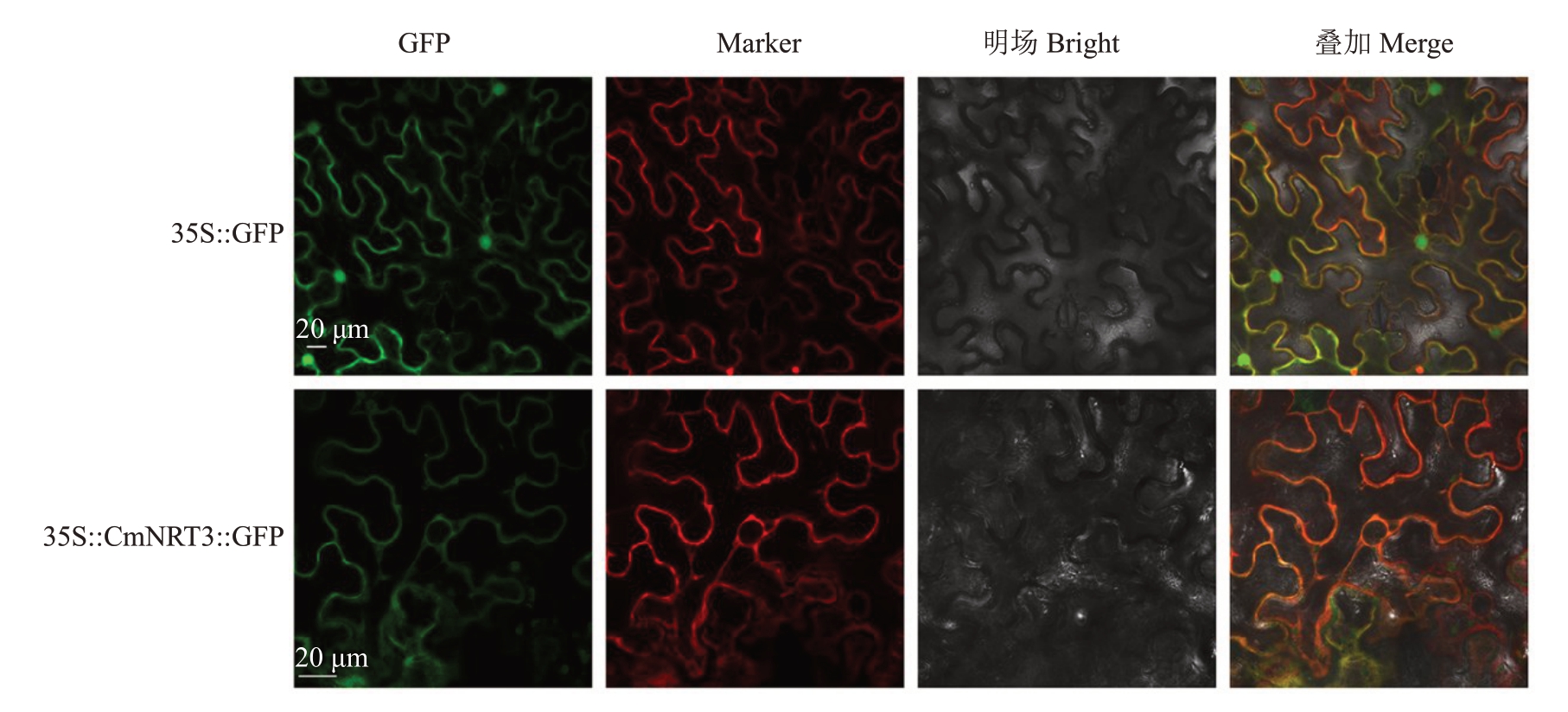
图7 CmNRT3 在烟草叶片中的亚细胞定位
Fig.7 Subcellular localization of CmNRT3 in tobacco leaves
为进一步探究CmNRT3 在菌根共生中的功能,利用苜蓿丛枝菌根体系研究CmNRT3 在苜蓿丛枝中的亚细胞定位。首先利用CmNRT3起始密码子上游1.8 kb 的启动子构建了CmNRT3pro::CmNRT3::GFP 融合表达载体,瞬时转化获得苜蓿转基因根后,接种丛枝菌根真菌异形根孢囊霉或不进行接种(对照组),4 周后利用激光扫描共聚焦显微镜对GFP 信号进行定位观察。在对照根中,CmNRT3 蛋白定位于细胞膜上(图8),与烟草中的观察结果一致;在苜蓿的丛枝菌根中观察到菌丝分支及丛枝主干被GFP 包围,该部位类似于丛枝围膜,推测CmNRT3蛋白定位于丛枝围膜上(图8)。

图8 CmNRT3 在苜蓿接种与不接种丛枝菌根真菌根系中的亚细胞定位
Fig.8 Subcellular localization of CmNRT3 in the M.truncatula roots inoculated and non-inoculated arbuscular mycorrhizal fungi
2.6 酵母功能互补
为了研究CmNRT3 是否具有硝酸盐转运功能,构建了CmNRT3-pDR-F1-GW 酵母表达载体以开展同源互补试验,分别将上述载体与空载分别转化酵母突变体。在Leu 缺陷培养基中,CmNRT3 基因及空载转化的缺陷型酵母突变体均不能正常生长(图9-A)。向酵母培养基中添加Leu 后,恢复了缺陷型酵母体内的氨基酸合成途径,缺陷型酵母及转基因酵母生长状况良好(图9-B)。由此可以推断,CmNRT3基因编码的蛋白不具有硝酸盐吸收或转运的能力。
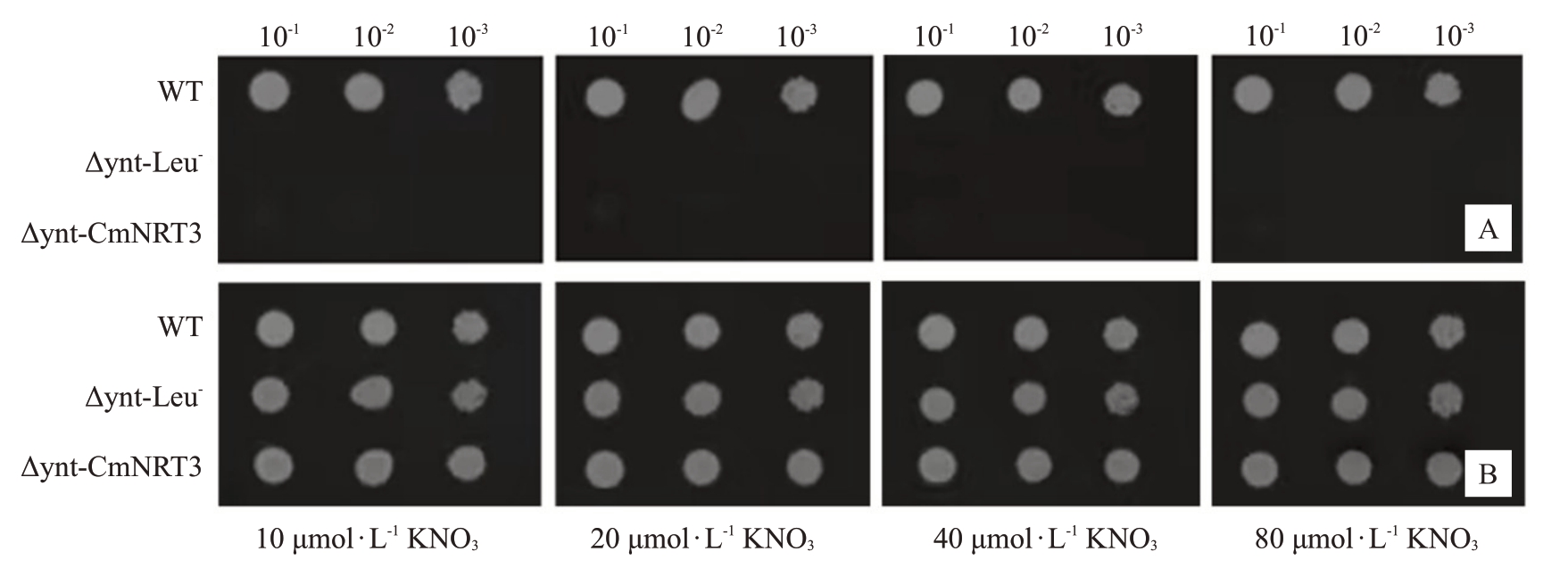
图9 CmNRT3 在酵母突变体△ynt-Leu-中的功能验证
Fig.9 Functional verification of CmNRT3 in yeast mutant △ynt-Leu-
3 讨 论
目前由于板栗转基因效率低及转基因根系共生难等原因,难以获得板栗转基因外生菌根。与板栗不同,其亲缘支中豆目豆科的苜蓿转基因效率高,常用来作为研究丛枝菌根共生机制的模式材料,且有研究表明,外生菌根与丛枝菌根的进化共生机制具有一定的相似性和保守性[30-31]。因此笔者利用菌根模式材料苜蓿来研究CmNRT3 基因的表达模式,从而推测其相关功能。
CmNRT3 受外生菌根信号响应上调表达,推测可能介导了板栗外生菌根的硝酸盐途径。试验结果表明,CmNRT3pro::GUS 表达载体分别转化苜蓿和板栗后,在未接种转基因根系中,两者均表现出在根表皮组织中表达信号强烈,而在皮层组织中表达相对较弱的现象,推测NRT3 在板栗与苜蓿的空间表达模式上具有高度的进化保守性。当苜蓿与丛枝菌根真菌共生后,发现GUS信号在含有成熟丛枝的皮层细胞中表达强烈,而在含有衰退丛枝的细胞中表达较弱,这与水稻OsNPF4.5 的表达模式基本一致[18]。此外,前人报道大豆GmAMT4.1[6]、高粱SbAMT3.1[32]均在丛枝中特异表达,且定位于丛枝周膜中,负责将NH4+从丛枝交换界面运输到宿主植物中,证明了这些基因在丛枝菌根促氮吸收中起关键作用。笔者通过构建CmNRT3pro::CmNRT3::GFP表达载体并转化苜蓿,发现CmNRT3蛋白定位于苜蓿丛枝菌根的丛枝上。基于CmNRT3在外生菌根中显著上调表达,且丛枝菌根与外生菌根具有一定的进化保守性,猜测CmNRT3 基因在外生菌根共生途径中具有重要功能。
CmNRT3可能作为氮信号分子参与外生菌根途径中的硝酸盐运输。在拟南芥中,酵母双杂和拟南芥原生质体试验表明,所有NRT2(除AtNRT2.7 外)基因均与AtNAR2.1(AtNRT3.1)基因相互作用[33]。同样地,水稻NRT2 家族成员中的OsNRT2.1、2.2 和2.3a 也需要在OsNAR2.1(OsNRT3.1)的协助下吸收硝酸盐[34]。此外,许多硝酸盐转运蛋白除运输硝酸盐外,还常常充当硝态氮的信号分子感受器,介导相关信号的传递工作[35]。AtNPF6.3/AtNRT1.1 突变后不再具有转运硝酸盐的能力,但仍具有传递硝酸盐信号的作用,表明其同时具有信号分子的功能[36]。酵母功能互补结果表明,CmNRT3 不能直接参与吸收或转运硝酸盐,推测其可能以氮信号分子感受器的形式参与外生菌根硝酸盐吸收。在未接种的对照根中表皮细胞的根毛往往是根感受氮信号以及硝酸盐吸收的主要部位,qRT-PCR 结果以及启动子分析分别证明了CmNRT3在未接种的板栗对照根中有表达,且在表皮细胞表达量最高。在形成菌根后,植物氮吸收途径不再局限于根毛,CmNRT3 在苜蓿未接种根系及丛枝菌根中的表达模式也发生了相应的改变。在苜蓿丛枝菌根研究中,感受磷信号的SPX1和SPX3在未接种的对照根和丛枝菌根中的表达模式也发生了变化,在未接种的对照根中均匀表达,但在形成丛枝菌根后在丛枝细胞中特异表达[37],这与本研究的结果一致。
4 结 论
笔者通过转录组数据分析及实时荧光定量PCR 验证筛选出了在外生菌根中高度表达的硝酸盐转运蛋白基因CmNRT3。通过组织学定位发现,板栗、苜蓿非菌根的表皮细胞中检测到CmNRT3 启动子的GUS 信号;在苜蓿菌根中,CmNRT3 仅在含丛枝的细胞中特异性表达。亚细胞定位结果表明,CmNRT3 在烟草叶片的细胞膜上具有活性,是膜转运蛋白;在苜蓿中主要在含丛枝的细胞中表达并定位于丛枝围膜上。酵母功能互补验证表明,转化CmNRT3基因的缺陷型多形汉逊酵母突变体仍不能生长,说明该基因不具备运输NO3-的能力。
[1] SHI J C,WANG X L,WANG E T. Mycorrhizal symbiosis in plant growth and stress adaptation:From genes to ecosystems[J].Annual Review of Plant Biology,2023,74:569-607.
[2] VAN DER HEIJDEN M G A,MARTIN F M,SELOSSE M A,SANDERS I R. Mycorrhizal ecology and evolution:The past,the present,and the future[J]. New Phytologist,2015,205(4):1406-1423.
[3] BONFANTE P,GENRE A. Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis[J]. Nature Communications,2010,1:48.
[4] TEDERSOO L,BAHRAM M. Mycorrhizal types differ in ecophysiology and alter plant nutrition and soil processes[J]. Biological Reviews of the Cambridge Philosophical Society,2019,94(5):1857-1880.
[5] KUMAR S,KUMAR S,MOHAPATRA T. Interaction between macro- and micro-nutrients in plants[J]. Frontiers in Plant Science,2021,12:665583.
[6] KOEGEL S,AIT LAHMIDI N,ARNOULD C,CHATAGNIER O,WALDER F,INEICHEN K,BOLLER T,WIPF D,WIEMKEN A,COURTY P E. The family of ammonium transporters(AMT) in Sorghum bicolor:Two AMT members are induced locally,but not systemically in roots colonized by arbuscular mycorrhizal fungi[J].New Phytologist,2013,198(3):853-865.
[7] CHEN A Q,GU M,WANG S S,CHEN J D,XU G H.Transport properties and regulatory roles of nitrogen in arbuscular mycorrhizal symbiosis[J].Seminars in Cell Developmental Biology,2018,74:80-88.
Developmental Biology,2018,74:80-88.
[8] HESTRIN R,HAMMER E C,MUELLER C W,LEHMANN J.Synergies between mycorrhizal fungi and soil microbial communities increase plant nitrogen acquisition[J].Communications Biology,2019,2:233.
[9] WANG Y Y,CHENG Y H,CHEN K E,TSAY Y F.Nitrate transport,signaling,and use efficiency[J].Annual Review of Plant Biology,2018,69:85-122.
[10] FAN X R,NAZ M,FAN X R,XUAN W,MILLER A J,XU G H.Plant nitrate transporters:From gene function to application[J].Journal of Experimental Botany,2017,68(10):2463-2475.
[11] LÉRAN S,VARALA K,BOYER J C,CHIURAZZI M,CRAWFORD N,DANIEL-VEDELE F,DAVID L,DICKSTEIN R,FERNANDEZ E,FORDE B,GASSMANN W,GEIGER D,GOJON A,GONG J M,HALKIER B A,HARRIS J M,HEDRICH R,LIMAMI A M,RENTSCH D,SEO M,TSAY Y F,ZHANG M Y,CORUZZI G,LACOMBE B. A unified nomenclature of nitrate transporter 1/peptide transporter family members in plants[J].Trends in Plant Science,2014,19(1):5-9.
[12] SUGIURA M,GEORGESCU M N,TAKAHASHI M. A nitrite transporter associated with nitrite uptake by higher plant chloroplasts[J].Plant Cell Physiology,2007,48(7):1022-1035.
Cell Physiology,2007,48(7):1022-1035.
[13] KOMAROVA N Y,THOR K,GUBLER A,MEIER S,DIETRICH D,WEICHERT A,SUTER GROTEMEYER M,TEGEDER M,RENTSCH D. AtPTR1 and AtPTR5 transport dipeptides in planta[J].Plant Physiology,2008,148(2):856-869.
[14] VON WITTGENSTEIN N J J B,LE C H,HAWKINS B J,EHLTING J. Evolutionary classification of ammonium,nitrate,and peptide transporters in land plants[J].BMC Evolutionary Biology,2014,14:11.
[15] 李赢.大麦NRT2/3 基因家族分析及其功能验证[D].扬州:扬州大学,2019.LI Ying. Genome-wide analysis and functional identification of NRT2/3 gene family in barley[D].Yangzhou:Yangzhou University,2019.
[16] HILDEBRANDT U,SCHMELZER E,BOTHE H. Expression of nitrate transporter genes in tomato colonized by an arbuscular mycorrhizal fungus[J]. Physiologia Plantarum,2002,115(1):125-136.
[17] HOHNJEC N,VIEWEG M F,PÜHLER A,BECKER A,KÜSTER H. Overlaps in the transcriptional profiles of Medicago truncatula roots inoculated with two different Glomus fungi provide insights into the genetic program activated during arbuscular mycorrhiza[J]. Plant Physiology,2005,137(4):1283-1301.
[18] WANG S S,CHEN A Q,XIE K,YANG X F,LUO Z Z,CHEN J D,ZENG D C,REN Y H,YANG C F,WANG L X,FENG H M,LÓPEZ-ARREDONDO D L,HERRERA-ESTRELLA L R,XU G H. Functional analysis of the OsNPF4.5 nitrate transporter reveals a conserved mycorrhizal pathway of nitrogen acquisition in plants[J]. Proceedings of the National Academy of Sciences of the United States of America,2020,117(28):16649-16659.
[19] SA G,YAO J,DENG C,LIU J,ZHANG Y N,ZHU Z M,ZHANG Y H,MA X J,ZHAO R,LIN S Z,LU C F,POLLE A,CHEN S L. Amelioration of nitrate uptake under salt stress by ectomycorrhiza with and without a Hartig net[J]. New Phytologist,2019,222(4):1951-1964.
[20] 刘帅,陈良珂,房克凤,杨瑞,邢宇,曹庆芹,秦岭.板栗种子淀粉体发育的扫描电镜观察[J].电子显微学报,2015,34(4):346-350.LIU Shuai,CHEN Liangke,FANG Kefeng,YANG Rui,XING Yu,CAO Qingqin,QIN Ling. Observation of amyloplast development in chestnut seed by scanning electron microscope[J].Journal of Chinese Electron Microscopy Society,2015,34(4):346-350.
[21] 赵彦华.板栗良种资源[J].果树资源学报,2020,1(6):91-94.ZHAO Yanhua. Chestnut seed resources[J]. Journal of Fruit Resources,2020,1(6):91-94.
[22] 秦岭,徐践,马萱,苑虎,郑来友,王有智.板栗共生菌根真菌种类及其发生规律的研究[J].北京农学院学报,1995,10(1):71-76.QIN Ling,XU Jian,MA Xuan,YUAN Hu,ZHENG Laiyou,WANG Youzhi. Research on symbiotical fungi species and ectomycorrhizae occurrence of chestnut (Castanea mollissima BL.)[J]. Journal of Beijing University of Agriculture,1995,10(1):71-76.
[23] 王腾.板栗两种菌根形态的鉴定及Pht1 家族基因的挖掘与表达分析[D].北京:北京农学院,2017.WANG Teng.Identification of two types of mycorrhizas and expression profiles of Pht1 gene family in mycorrhizal Castanea mollissima Blume.[D]. Beijing:Beijing University of Agriculture,2017.
[24] 李光栋.板栗田间外生菌根转录组分析及共生相关转运蛋白基因挖掘[D].北京:北京农学院,2020.LI Guangdong. Transcriptome analysis on ectomycorrhiza of Castanea mollissima revealed symbiotic related nutrient transporters[D].Beijing:Beijing University of Agriculture,2020.
[25] 安剑勇.柑橘菌根比较转录组学分析及菌根共生寄主糖输出转运蛋白SWEET1b 的功能鉴定[D]. 武汉:华中农业大学,2018.AN Jianyong. Comparative transcriptome analysis of citrus AM symbiosis and functional characterization of AM-host plant sugar efflux transporter SWEET1b[D]. Wuhan:Huazhong Agricultural University,2018.
[26] MACHÍN F,MEDINA B,NAVARRO F J,PÉREZ M D,VEENHUIS M,TEJERA P,LORENZO H,LANCHA A N,SIVERIO J M.The role of Ynt1 in nitrate and nitrite transport in the yeast Hansenula polymorpha[J].Yeast,2004,21(3):265-276.
[27] SIVERIO J M. Assimilation of nitrate by yeasts[J]. FEMS Microbiology Reviews,2002,26(3):277-284.
[28] 张辰明.水稻OsNAR2.1 参与硝酸盐调控根系生长的机制[D].南京:南京农业大学,2011.ZHANG Chenming.The rice OsNAR2.1 participates the regulating root growth by nitrate[D]. Nanjing:Nanjing Agricultural University,2011.
[29] LI H C,GE Y Y,ZHANG Z Y,ZHANG H L,WANG Y Y,WANG M D,ZHAO X,YAN J D,LI Q,QIN L,CAO Q Q,BISSELING T.Arbuscular mycorrhizal conserved genes are recruited for ectomycorrhizal symbiosis[J].New Phytologist,2024,242(5):1860-1864.
[30] LOTH- PEREDA V,ORSINI E,COURTY P E,LOTA F,KOHLER A,DISS L,BLAUDEZ D,CHALOT M,NEHLS U,BUCHER M,MARTIN F. Structure and expression profile of the phosphate Pht1 transporter gene family in mycorrhizal Populus trichocarpa[J].Plant Physiology,2011,156(4):2141-2154.
[31] HARRISON M J,DEWBRE G R,LIU J Y. A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi[J]. The Plant Cell,2002,14(10):2413-2429.
[32] KOBAE Y,TAMURA Y,TAKAI S,BANBA M R,HATA S.Localized expression of arbuscular mycorrhiza-inducible ammonium transporters in soybean[J].Plant Cell Physiology,2010,51(9):1411-1415.
Cell Physiology,2010,51(9):1411-1415.
[33] KOTUR Z,MACKENZIE N,RAMESH S,TYERMAN S D,KAISER B N,GLASS A D M. Nitrate transport capacity of the Arabidopsis thaliana NRT2 family members and their interactions with AtNAR2.1[J]. New Phytologist,2012,194(3):724-731.
[34] YAN M,FAN X R,FENG H M,MILLER A J,SHEN Q R,XU G H. Rice OsNAR2.1 interacts with OsNRT2.1,OsNRT2.2 and OsNRT2.3a nitrate transporters to provide uptake over high and low concentration ranges[J]. Plant,Cell  Environment,2011,34(8):1360-1372.
Environment,2011,34(8):1360-1372.
[35] XU G H,FAN X R,MILLER A J. Plant nitrogen assimilation and use efficiency[J]. Annual Review of Plant Biology,2012,63:153-182.
[36] HO C H,LIN S H,HU H C,TSAY Y F.CHL1 functions as a nitrate sensor in plants[J].Cell,2009,138(6):1184-1194.
[37] WANG P,SNIJDERS R,KOHLEN W,LIU J Y,BISSELING T,LIMPENS E. Medicago SPX1 and SPX3 regulate phosphate homeostasis,mycorrhizal colonization,and arbuscule degradation[J].The Plant Cell,2021,33(11):3470-3486.