花青苷是植物重要的次生代谢产物,主要存在于高等植物的花、果实、种皮等器官中,使植物呈现不同的色彩,在避免植物受到紫外线伤害、吸引昆虫传粉和抵御低温胁迫等方面起着重要的作用[1]。花青苷的合成由一系列的酶促反应构成,主要包括苯丙氨酸解氨酶(phenylalanine ammonialyase,PAL)、查尔酮合成酶(chalcone synthase,CHS)、查尔酮异构酶(chalcone isomerase,CHI)、黄烷酮-3-羟化酶(flavanone 3-hydroxylase,F3H)、二氢黄酮醇-4-还原酶(dihydroflavonol 4-reductase,DFR)、花青素合成酶(anthocyanidin synthase,ANS)、类黄酮-3-O-糖基转移酶(UDP-glucose:flavonoid-3-O-glucosyltransferase,UFGT)等[2]。
查尔酮合成酶(chalcone synthase,CHS)是类黄酮生物合成途径中的第一个关键酶,催化3 分子的丙二酰-CoA 和1 分子的4-香豆酰-CoA 结合形成查尔酮,是类黄酮途径中的第一个呈色物质[3]。大量研究表明,CHS能够影响花青苷的累积水平。在智利草莓[Fragaria chiloensis(L.)Mill.]果实发育过程中,ABA 通过激活FcPAL、FcCHS、FcANS 等花青素途径的关键基因加速果实颜色的积累[4];套袋处理降低了杏果实中包括PaCHS 在内的花青苷合成基因的表达量,从而导致花青苷含量的下降[5]。苹果[6]、梨[7]和柑橘[8]中,CHS基因的表达量随花青苷积累量的增加而升高;实验室前期在红瓤核桃自然杂交后代中鉴定了4 个与花青苷含量呈正相关的JrCHSs基因,但其功能还没有相关研究[9]。
bHLH(basic Helix-Loop-Helix,碱性螺旋-环-螺旋)转录因子是植物第二大转录因子家族,其蛋白结构包含两个功能不同的区域,即位于N 端的碱性区域(DNA 识别区)和C 端的HLH 区域(可形成同源或异源二聚体),在植物的生长发育、抵抗胁迫和转导信号等方面发挥着重要作用,是植物花青苷合成的关键调控因子[3]。研究发现,茄子SmbHLH13 可以正向调控茄子F3H和CHS基因的表达,促进茄子花青素的合成[10]。笔者课题组前期根据生物信息学与表达分析,筛选出了4 个与红瓤核桃花青苷合成相关的bHLH转录因子基因JrbHLHA1、JrbHLHA2、JrEGL1a、JrEGL1b[11],但对其调控红瓤核桃花青苷生物合成的分子机制比如与JrCHSs 基因的调控关系没有相关研究。
核桃(Juglans regia L.)是世界四大坚果之首,含有丰富的营养成分,被广泛种植和加工利用[12]。据联合国粮食及农业组织(FAO)(http://faostat.fao.org)最新数据统计,2022 年中国核桃收获面积占世界核桃收获面积的28.58%,产量占世界核桃产量的36.14%,均稳居世界首位。中国核桃栽培历史已有3000 多年,具有极丰富的种质资源,目前广泛栽培的核桃品种种皮均为黄白色或浅黄色[13],可选择的外观性状较少。笔者课题组前期在太行山区域发现了珍稀的红瓤核桃种质资源(J.regia L.RW-1),其叶片、果皮和种皮均因富含花青苷而呈红色,但其呈色机制目前尚不清楚,限制了核桃的色泽品质改良。因此,笔者在前期研究的基础上,筛选红瓤核桃种皮花青苷合成关键CHS 基因,探究其与上游Jrb-HLHs 的调控关系,并验证其在花青苷生物合成与积累中发挥的功能与作用,以期解析红瓤核桃种皮花青苷合成与积累的分子机制,为促进红瓤核桃色泽品质的改良及育种奠定基础。
1 材料和方法
1.1 试验材料
供试材料为野生资源红瓤核桃(J.regia L.RW-1,资源编号JUREG4108210002)和普通核桃中林1号(J.regia L.‘Zhonglin 1’),均种植于河南农业大学科教园区毛庄果树资源圃,南北向定植,株行距2 m×3 m,常规肥水管理。选择生长状况良好、长势一致的植株,于花后60、90、120 d采集红瓤核桃RW-1(RW)和普通核桃中林1号(GW)的种皮,采集样品于液氮速冻后置于-80 ℃超低温冰箱中保存备用。
所有用于注射的烟草(Nicotiana tabacum L.)均在温度22 ℃、湿度60%、光照16 h/黑暗8 h 的培养箱中进行培养。
1.2 总花青素含量测定
将样品于液氮中速冻并研磨至粉末状,悬浮于预冷的1%盐酸甲醇溶液中,充分混匀后于4 ℃黑暗浸提24 h,4 ℃条件下12 000g离心10 min收集上清液,检测上清液在波长为530、620、650 nm处的吸光值,代入公式计算:总花青素含量(w,后同)/(mg·g-1)=[(A530-A620)-0.25(A650-A620)]/0.1,进行3次生物学重复[14]。
1.3 DNA提取及启动子克隆
使用EZ-10 Spin Column Plant Genomic DNA Purification Kit(生工生物工程股份有限公司,上海)对样品进行DNA 提取[15]。JrCHS4 的启动子序列通过核桃基因组[16]预测获得,关键顺式作用元件的分析通过PLACE(https://www.dna.affrc.go.jp/PLACE/?action=newplace)获得。
1.4 RNA 提取与cDNA 合成、实时荧光定量PCR(qRT-PCR)
使用快速通用植物RNA提取试剂盒(北京华越洋生物科技有限公司,北京)对样品进行RNA提取,利用HiScript® ⅢRT SuperMix for qPCR(+gDNA wiper)反转录试剂盒(南京诺唯赞生物科技股份有限公司,南京)对质量合格的RNA进行cDNA合成。
于ABI 7500 实时PCR 系统(Applied Biosystems,Foster City,CA,United States)使用ChamQ Universal SYBR qPCR Master Mix(南京诺唯赞生物科技股份有限公司,南京)进行qRT-PCR 试验。以Jr18S(XM_019004991.1)作为内参基因,基因相对表达水平使用2-△△Ct法计算[17],引物序列见表1。
表1 qRT-PCR、基因克隆和载体构建引物序列
Table 1 Primer sequences for qRT-PCR,gene cloning and vector construction

qRT-JrCHS1-F qRT-JrCHS1-R qRT-JrCHS2-F qRT-JrCHS2-R qRT-JrCHS3-F qRT-JrCHS3-R qRT-JrCHS4-F qRT-JrCHS4-R qRT-18S-F qRT-18S-R qRT-NtActin-F qRT-NtActin-R JrCHS4pro-F JrCHS4pro-R JrCHS4-F JrCHS4-R JrbHLHA1-F JrbHLHA1-R JrbHLHA2-F JrbHLHA2-R JrEGL1a-F JrEGL1a-R JrEGL1b-F JrEGL1b-R CATTCCGAGGGCCTAGTGAC GATGGCCCCATCACTATCGG CATACCCTGACTACTACTTCCG GTGATTTCCGAGCAGACG GCGAAGTAGGCTTGACAT AATATGGCGACTTGCTCTAA CACTCCCTCAAACTGCGTCT CTTGATTGCCTTCGATGCCG ATTGGTTGCGGATCAGGACT GCTCCAATGCAACATCAAGC AATGATCGGAATGGAAGCTG TGGTACCACCACTGAGGACA CTTTGCAATTATGGAGTCCTTTTG GCCTCTTGCTCGGTCCTAGTT ATGGCGTCCATGGAGGA TTAGATATTGACACTGTGCAGCA ATGGCTGCACCGCCGAG TTAAGAGTCTGTGTGGGGGATG ATGGCTGCACCGCCAA CTAAGAGTCATTGTGGGGTATG ATGGCTAATGGCTGTCAAAC TCAACACTTACAAGCAATTTTCC ATGGAGGGGAGAATGCTAGAAAAC TCAACACTTCCTAGTTGATCTCTGG引物名称Primer name引物序列(5'-3')Primer sequence(5'-3')
表1 (续) Table 1 (Continued)
JrCHS4pro-1381-F JrCHS4pro-1381-R JrCHS4pro-pAbAi-F JrCHS4pro-pAbAi-R JrCHS4pro-LUC-F JrCHS4pro-LUC-R JrbHLHA1-AD-F JrbHLHA1-AD-R JrbHLHA2-AD-F JrbHLHA2-AD-R JrEGL1a-AD-F JrEGL1a-AD-R JrEGL1b-AD-F JrEGL1b-AD-R JrCHS4-2300-F JrCHS4-2300-R JrbHLHA2-2300-F JrbHLHA2-2300-R TGGGCCCGGCGCGCCGAATTCCTTTGCAATTATGGAGTCCTTTTG CCTCTTAAAGCTTGGCTGCAGGCCTCTTGCTCGGTCCTAGTT AAATGATGAATTGAAAAGCTTCTTTGCAATTATGGAGTCCTTTTG ATACAGAGCACATGCCTCGAGGCCTCTTGCTCGGTCCTAGTT ACTATAGGGCGAATTGGGTACCCTTTGCAATTATGGAGTCCTTTTG ATCGATACCGTCGACCTCGAGGCCTCTTGCTCGGTCCTAGTT TACGACGTACCAGATTACGCTCATATGATGGCTGCACCGCCGAG TCTACGATTCATCTGCAGCTCGAGTTAAGAGTCTGTGTGGGGGATG TACGACGTACCAGATTACGCTCATATGATGGCTGCACCGCCAA TCTACGATTCATCTGCAGCTCGAGCTAAGAGTCATTGTGGGGTATG TACGACGTACCAGATTACGCTCATATGATGGCTAATGGCTGTCAAAC TCTACGATTCATCTGCAGCTCGAGTCAACACTTACAAGCAATTTTCC TACGACGTACCAGATTACGCTCATATGATGGAGGGGAGAATGCTAGAAAAC TCTACGATTCATCTGCAGCTCGAGTCAACACTTCCTAGTTGATCTCTGG ACGGGGGACGAGCTCGGTACCATGGCGTCCATGGAGGA GGTGTCGACTCTAGAGGATCCGATATTGACACTGTGCAGCA ACGGGGGACGAGCTCGGTACCATGGCTGCACCGCCAA GGTGTCGACTCTAGAGGATCCAGAGTCATTGTGGGGTATG引物名称Primer name引物序列(5'-3')Primer sequence(5'-3')
1.5 GUS染色与GUS蛋白定量检测
从2 种核桃DNA 中分别克隆JrCHS4 启动子片段插入至植物表达载体pCAMBIA1381-GUS,分别转入农杆菌GV3101-pSoup 感受态细胞(北京庄盟国际生物基因科技有限公司,北京),瞬时转化本氏烟草叶片,进行GUS 染色和GUS 蛋白定量分析[18]。
1.6 酵母单杂交(Y1H)
将红瓤核桃JrCHS4 启动子片段插入至pAbAi载体,从红瓤核桃cDNA 中克隆JrbHLHs 转录因子编码序列插入至pGADT7 载体。使用经典酵母转化试剂盒(北京酷来搏科技有限公司,北京)进行酵母感受态的制备与转化,以pGADT7 为阴性对照,将含有JrbHLHs-AD 重组质粒的Y1HGold(含RWJrCHS4pro-pAbAi重组质粒)菌株点至AbA浓度梯度的固体SD/-Leu 培养基平板上于29 ℃培养箱培养2~4 d后观察互作情况。
1.7 双荧光素酶报告基因检测(LUC)
将分别带有JrCHS4pro-LUC、JrbHLHA2-2300 重组质粒的农杆菌菌液按1∶9 的体积比混合,注射本氏烟草叶片,使用Dual-Luciferase® Reporter Assay System试剂盒(普洛麦格生物技术有限公司,北京)测定萤火虫荧光素酶LUC 和海肾萤光素酶REN 酶活性,计算LUC/REN比值[19]。
1.8 烟草叶片瞬时表达分析
将带有JrCHS4-2300重组质粒的农杆菌菌液注射至大叶烟草叶片,其间仔细观察叶片的颜色变化情况,后采集经注射的烟草叶片于液氮速冻研磨后进行总花青素含量的测定与分析[20]。
1.9 数据分析
采用Microsoft Excel 2019软件进行试验数据整理;采用SPSS 21.0 软件进行试验数据统计分析;采用Adobe Photoshop 2021、GraphPad Prism 8 软件绘图。
2 结果与分析
2.1 红瓤核桃不同发育时期种皮CHSs的表达分析
课题组前期根据核桃基因组数据筛选CHS 家族,通过功能注释分析筛选到了4 个可能与花青苷合成相关的CHSs[9]。利用qRT-PCR 检测CHSs 基因在2 种核桃不同发育时期种皮中的表达模式,结果表明花后60、120 d 时4 个CHSs 基因在红瓤核桃种皮中的表达量均显著高于在普通核桃种皮中的表达量,其中JrCHS4(gene35863,XM_018966498.2)在2种核桃种皮中的表达量差异最大,分别约为66.04、11 970.93倍;花后90 d时除JrCHS4在2种核桃种皮中的表达量基本相同外,其他3个JrCHSs在红瓤核桃种皮中的表达量均显著低于在普通核桃种皮中的表达量(图1)。因此,推测JrCHS4可能是红瓤核桃种皮花青苷合成的关键基因。
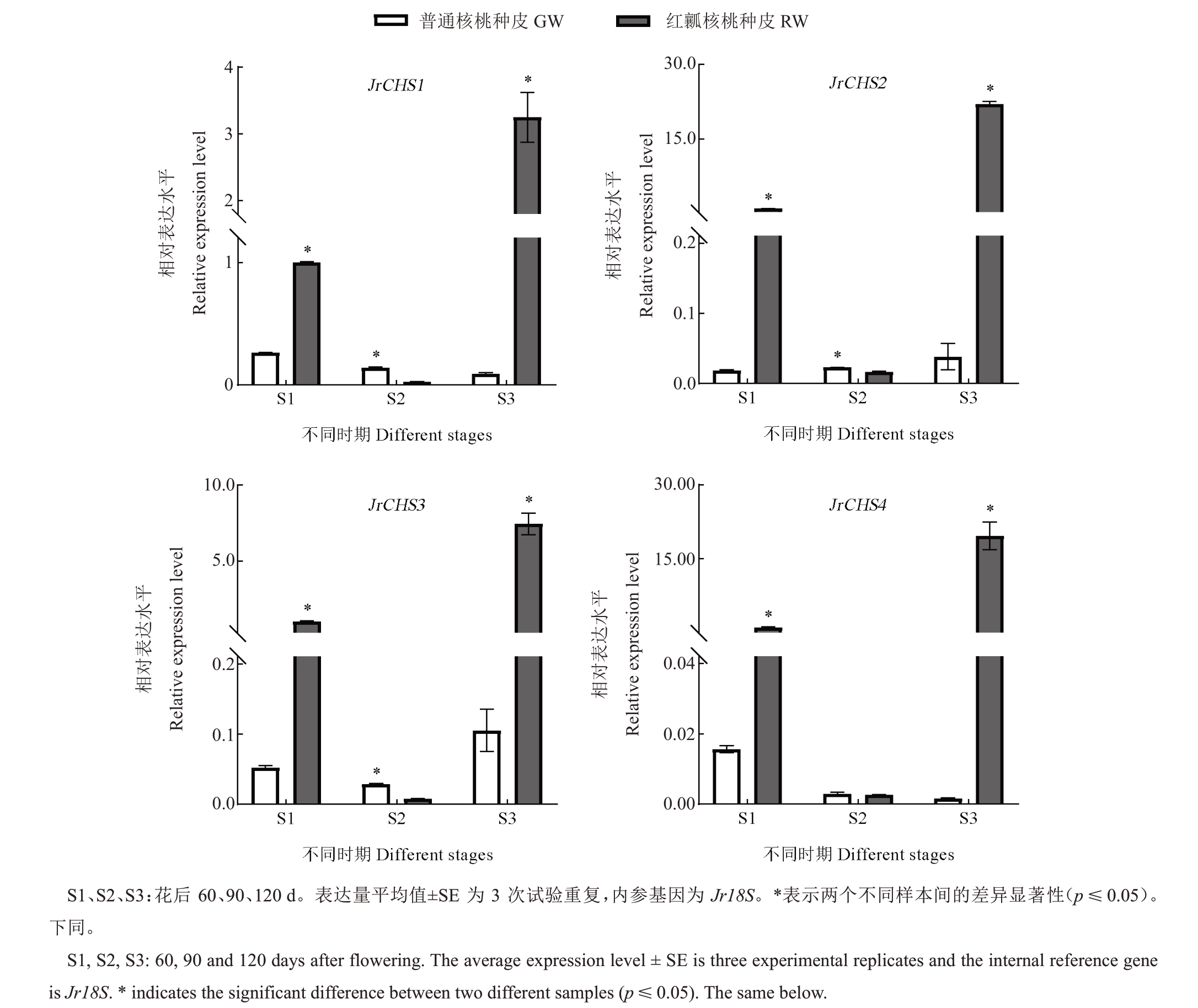
图1 JrCHSs 基因在不同颜色核桃种皮各发育时期的qRT-PCR 表达分析
Fig.1 qRT-PCR expression analysis of JrCHSs gene in different developmental stages of walnut seed coat with different colors
2.2 红瓤核桃JrCHS4启动子的克隆及启动子活性分析
为了研究CHS4基因在红瓤核桃和普通核桃种皮发育中表达趋势的不同是否与其启动子有关,对2 种核桃的CHS4 启动子序列进行了克隆。通过序列比对,GW-JrCHS4启动子与RW-JrCHS4启动子具有98.50%的同源性(图2)。红瓤核桃JrCHS4 启动子含有许多响应激素如脱落酸、乙烯、赤霉素以及与逆境胁迫相关的顺式作用元件,如ABRE、MYC、ERE、GARE、MYB1AT等,与普通核桃JrCHS4启动子相比,缺失了1 个MYB 结合位点MYB1AT,插入了1 个bHLH 结合位点MYCCONSENSUSAT(表2)。

图2 红瓤核桃和普通核桃种皮中JrCHS4 启动子序列比对
Fig.2 Comparison of JrCHS4 promoter sequences between red walnut and normal walnut seed coats
表2 JrCHS4 基因启动子关键顺式作用元件分析
Table 2 Pivotal cis-acting elements analysis of JrCHS4 gene promoter

顺式作用元件Cis-acting element ABRE ERE GARE MBS MYC功能Function脱落酸响应元件Abscisic acid responsive element乙烯响应元件Ethylene-responsive element赤霉素响应元件Gibberellin-responsive element MYB转录因子结合位点;逆境胁迫响应元件MYB transcription factor binding site;Adversity stress responsive element bHLH转录因子结合位点bHLH transcription factor binding site顺式作用元件数量Number of cis-acting elements GW 32588 RW 32579
GUS 染色结果表明,RW-JrCHS4启动子诱导产生的蓝色深于GW-JrCHS4 启动子诱导产生的蓝色(图3-A)。GUS 蛋白定量结果显示,RW-JrCHS4 启动子活性显著高于GW-JrCHS4 启动子活性,约是GW-JrCHS4启动子活性的1.17倍,与上述GUS染色结果相一致(图3-B)。
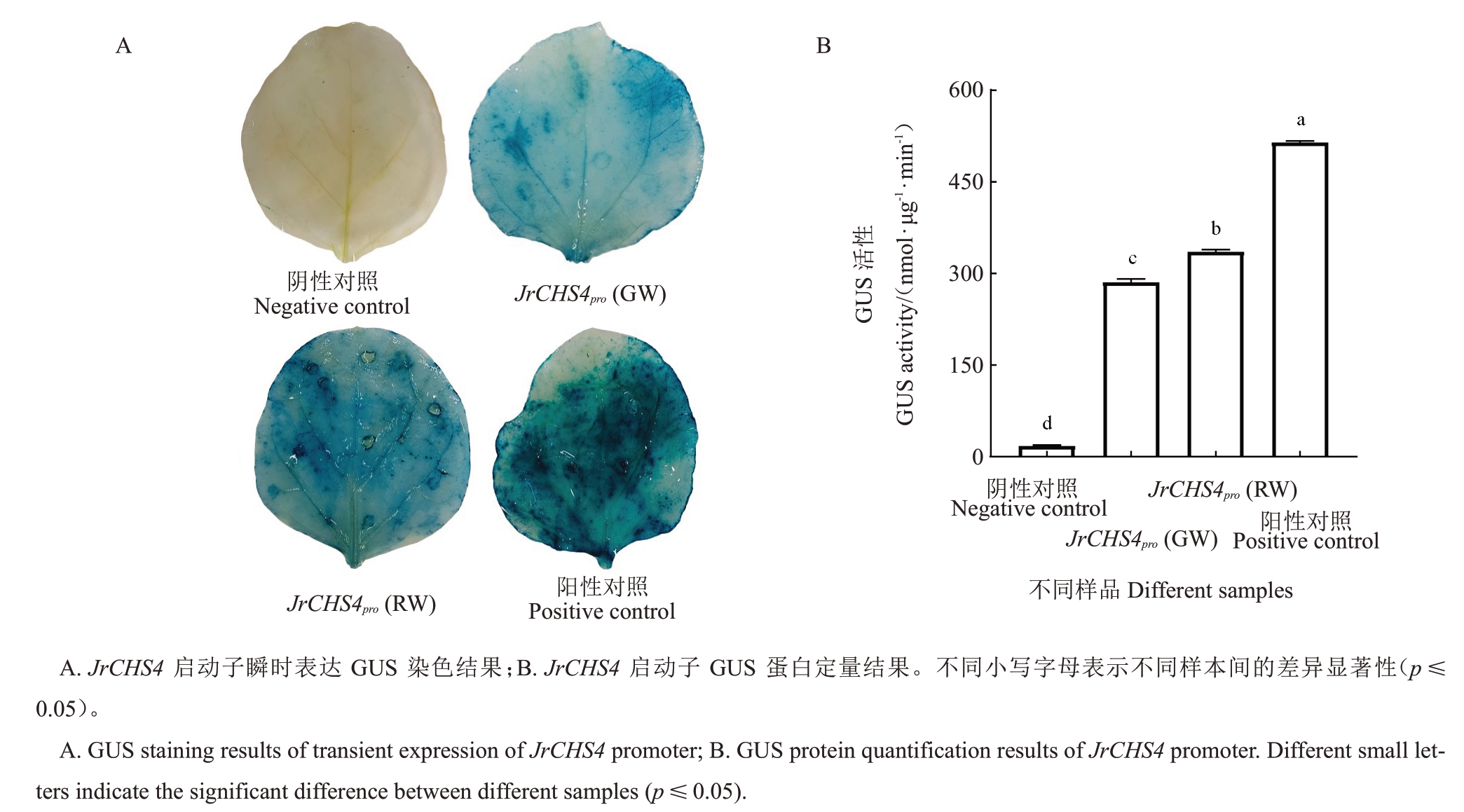
图3 红瓤核桃和普通核桃种皮中JrCHS4 启动子GUS 活性分析
Fig.3 GUS activity analysis of JrCHS4 promoter in red walnut and normal walnut seed coats
2.3 红瓤核桃JrCHS4启动子上游bHLH转录因子的筛选及验证
为了探究JrCHS4 启动子与4 个花青苷合成相关的bHLH 转录因子JrbHLHA1、JrbHLHA2、JrEGL1a、JrEGL1b 的调控关系,进行了酵母单杂交试验。结果显示,抑制JrCHS4启动子自身表达的最佳AbA 质量浓度为150 ng·mL-1,且仅JrbHLHA2-AD+JrCHS4pro在此AbA质量浓度的SD/-Leu筛选培养基上能够正常生长,其他组合均无法生长(图4-A)。LUC 试验进一步验证了JrbHLHA2 能够显著激活JrCHS4 基因启动子的活性,其LUC/REN 比值约是对照LUC/REN 比值的2.45 倍(图4-B)。以上结果表明,JrbHLHA2 转录因子可以与JrCHS4 的启动子特异性结合并激活其表达。

图4 JrbHLHA2 对JrCHS4 启动子的调控作用分析
Fig.4 Analysis of the regulatory effect of JrbHLHA2 on JrCHS4 promoter
2.4 烟草叶片中过表达红瓤核桃JrCHS4促进花青苷积累
为了验证JrCHS4 在花青苷生物合成与积累中发挥的功能与作用,将JrCHS4构建植物表达载体并瞬时转化至大叶烟草叶片,转化后7 d 左右观察发现,瞬时转化JrCHS4 的烟草叶片与对照相比,绿色变浅,呈现轻微的红色(图5-A)。其中,JrCHS4 在瞬时转化JrCHS4 烟草叶片中的表达量约是在对照烟草叶片中的30.07 倍(图5-B)。对瞬转烟草叶片测定总花青素含量的结果显示,瞬时转化JrCHS4烟草叶片的总花青素含量显著高于对照烟草叶片,约是对照烟草叶片的1.09 倍(图5-C)。以上结果表明,JrCHS4能够促进花青苷的生物合成与积累。

图5 JrCHS4 瞬时转化烟草叶片表型与总花青素含量分析
Fig.5 Analysis of phenotypes and total anthocyanins content in JrCHS4 transiently transformed tobacco leaves
3 讨 论
花青苷是重要的天然抗氧化剂,在清除人体自由基、改善血糖平衡、预防心脑血管疾病等方面有着积极的作用[21];花青苷在红瓤核桃种皮中积累不仅提高了核桃的营养价值,也丰富了种仁的外观品质,市场前景广阔,但其呈色机制目前尚不清楚,限制了核桃的色泽品质改良。因此,探究红瓤核桃种皮着色机制、挖掘关键调控基因,对培育优质红瓤核桃新品种具有重要的理论意义和应用价值。
查尔酮合成酶是花青苷合成通路的第一个限速酶,决定着花青苷合成的种类及含量[22]。笔者课题组前期基于转录组数据,首先进行基因功能注释筛选出了17个注释为“Chalcone synthetase”的基因,后又通过构建核桃CHSs基因表达图谱筛选获得了4个具有显著差异表达的JrCHSs基因JrCHS1~JrCHS4,且表达量与花青苷含量呈正相关[9]。笔者以前期获得的4 个与花青苷合成相关的JrCHSs 基因为研究对象,通过qRT-PCR 发现,花后60、120 d 时JrCHS4 在红瓤核桃种皮中的表达量显著高于普通核桃种皮且表达量差异最大,分别约为66.04、11 970.93倍,该结果与MaCHS2基因在红皮香蕉各组织中的表达量高于天宝香蕉各组织[23]、PeCHS基因在紫色西番莲果皮中的表达量明显高于黄色西番莲果皮[24]和IbCHS1基因在紫肉甘薯中的表达量高于黄肉、白肉甘薯[25]等研究结果一致,表明JrCHS4可能是红瓤核桃种皮花青苷合成的关键基因。
本研究结果表明,红瓤核桃不同时期种皮JrCHSs 的表达量受到了果实发育的影响,在花后60 d和120 d时表达量较高,而在花后90 d时表达量显著降低。在红瓤核桃种皮颜色形成过程中,花后60 d是花青苷积累的关键时期,花青苷大量合成,因此4 个JrCHSs 基因在花后60 d 红瓤核桃种皮中的表达量较高;在花后90 d时,红瓤核桃种皮花青苷合成速度减慢,此时4个JrCHSs基因在红瓤核桃种皮中便保持了较低的表达水平;花后120 d 时,核桃果实在发育成熟时期通常伴随有含水量降低现象,推测可能诱导了红瓤核桃种皮中的花青苷再次大量合成,因此4个JrCHSs基因在红瓤核桃种皮中的表达量又再一次升高。
根据PLACE 数据库,2 种核桃JrCHS4 启动子中均含有ABA相关的ABRE元件、乙烯相关的ERE元件、赤霉素相关的GARE 元件[26-28],以及MYB、bHLH 转录因子的结合位点[29]。根据顺式作用元件分析结果推测,JrCHS4基因可能参与激素信号转导以及逆境胁迫响应等生物学过程,并受到MYB 和bHLH 转录因子的调控。根据前人研究,MYB 和bHLH 是影响花青苷生物合成的关键转录因子,如彭亚丽等[30]阐述了MYB 转录因子在蔬菜花青苷合成中的激活作用与抑制作用;荔枝中与LcMYB1起协同作用的LcbHLH1、LcbHLH3能够调控荔枝花青素生物合成的晚期结构基因,进而调控荔枝中花青素的合成与积累[31];过表达MdMYC2 的转基因苹果愈伤组织中能够积累更多的花青素且显著提升MdCHS、MdDFR 等花青素生物合成相关基因的表达水平[32]。而笔者在本研究中发现与GW-JrCHS4启动子相比,RW-JrCHS4启动子缺失了1个MYB结合位点MYB1AT,插入了1个bHLH结合位点MYCCONSENSUSAT,推测bHLH 结合位点MYCCONSENSUSAT 的插入可能会导致bHLH 转录因子对JrCHS4启动子结合作用的差异,进而影响bHLH转录因子对JrCHS4的调控,从而影响红瓤核桃种皮花青苷的积累,同样MYB 结合位点MYB1AT 的缺失也将会影响MYB 转录因子对JrCHS4 的调控,具体影响将会在之后的研究中继续进行深入探索。
前人研究表明,bHLH 是花青苷合成通路结构基因的主要调控因子之一[33],探究JrCHS4 与上游JrbHLHs的调控关系能够为解析红瓤核桃种皮花青苷生物合成分子机制提供数据支撑。通过酵母单杂交试验表明JrbHLHA2 能够特异地结合到JrCHS4的启动子上,通过LUC 试验证明JrbHLHA2 能够提高JrCHS4启动子的启动活性。在蓝莓中,酵母单杂交试验表明,3 个花青素生物合成VcbHLHs(VcAN1、VcbHLH1-1和VcbHLH1-2)可特异性结合VcCHS21启动子的G-box序列(CACGTG)进而调控VcCHS21 的表达[34],说明bHLH 转录因子对CHS 在花青苷合成中的调节作用具有普遍性。
瞬时转化烟草叶片是验证果树花青苷合成相关基因功能的常用方法,在苹果[35]、梨[36]等物种中应用广泛。为了进一步研究JrCHS4 在花青苷合成中的作用,将JrCHS4 的过表达载体瞬时转化烟草叶片,结果表明过表达JrCHS4 显著提高了烟草叶片花青苷含量,与马铃薯StCHS4、StCHS5[37]瞬时转化烟草叶片能够提高花青苷含量的结果一致,表明JrCHS4能够促进花青苷的生物合成与积累。
4 结 论
探究了JrbHLHA2 靶向JrCHS4 调控花青苷合成的分子机制。红瓤核桃JrCHS4 在种皮发育过程中持续高表达,且启动子活性高于GW-JrCHS4启动子。JrbHLHA2能够直接结合RW-JrCHS4启动子并促进其上调表达,JrCHS4过表达烟草叶片能够促进花青苷的积累。推测JrbHLHA2 靶向JrCHS4 启动子促进了红瓤核桃花青苷的积累,这对红瓤核桃的改良育种提供了一定的理论依据。
[1] 王欣,张天柱.园艺作物花青素合成调控研究进展[J].生物技术进展,2022,12(1):10-16.WANG Xin,ZHANG Tianzhu.Research progress on the regulation of anthocyanin synthesis in horticultural crops[J]. Current Biotechnology,2022,12(1):10-16.
[2] TANAKA Y,SASAKI N,OHMIYAA.Biosynthesis of plant pigments:Anthocyanins,betalains and carotenoids[J]. Plant Journal,2008,54(4):733-749.
[3] 刘恺媛,王茂良,辛海波,张华,丛日晨,黄大庄.植物花青素合成与调控研究进展[J].中国农学通报,2021,37(14):41-51.LIU Kaiyuan,WANG Maoliang,XIN Haibo,ZHANG Hua,CONG Richen,HUANG Dazhuang. Anthocyanin biosynthesis and regulate mechanisms in plants:A review[J]. Chinese Agricultural Science Bulletin,2021,37(14):41-51.
[4] MATTUS-ARAYA E,GUAJARDO J,HERRERA R,MOYALEÓN M A.ABA speeds up the progress of color in developing F. chiloensis fruit through the activation of PAL,CHS and ANS,key genes of the Phenylpropanoid/Flavonoid and anthocyanin pathways[J]. International Journal of Molecular Sciences,2022,23(7):3854.
[5] XI W P,FENG J,LIU Y,ZHANG S K,ZHAO G H.The R2R3-MYB transcription factor PaMYB10 is involved in anthocyanin biosynthesis in apricots and determines red blushed skin[J].BMC Plant Biology,2019,19(1):287.
[6] XU Y T,FENG S Q,JIAO Q Q,LIU C C,ZHANG W W,CHEN W Y,CHEN X S. Comparison of MdMYB1 sequences and expression of anthocyanin biosynthetic and regulatory genes between Malus domestica Borkh. cultivar‘Ralls’and its blushed sport[J].Euphytica,2012,185(2):157-170.
[7] ZHANG X D,C ALLAN A,YI Q,CHEN L M,LI K Z,SHU Q,SU J. Differential gene expression analysis of Yunnan red pear,Pyrus pyrifolia,during fruit skin coloration[J]. Plant Molecular Biology Reporter,2011,29(2):305-314.
[8] BERNARDI J,LICCIARDELLO C,PATRIZIA RUSSO M,LUISA CHIUSANO M,CARLETTI G,REFORGIATO RECUPERO G,MAROCCO A. Use of a custom array to study differentially expressed genes during blood orange (Citrus sinensis L.Osbeck) ripening[J]. Journal of Plant Physiology,2010,167(4):301-310.
[9] 赵伟,李琳,刘永辉,章露露,杨莹,孟海军,王磊,吴国良.红仁核桃自然杂交后代不同表型叶片差异表达CHS 基因的鉴定及生物信息学分析[J].果树学报,2021,38(2):179-191.ZHAO Wei,LI Lin,LIU Yonghui,ZHANG Lulu,YANG Ying,MENG Haijun,WANG Lei,WU Guoliang. Identification and bioinformatics analysis of CHS genes in different phenotypic leaves of natural hybrid progenies of red-kernel walnut[J]. Journal of Fruit Science,2021,38(2):179-191.
[10] XI H C,HE Y J,CHEN H Y. Functional characterization of SmbHLH13 in anthocyanin biosynthesis and flowering in eggplant[J].Horticultural Plant Journal,2021,7(1):73-80.
[11] ZHAO W,LIU Y H,LI L,MENG H J,YANG Y,DONG Z B,WANG L,WU G L. Genome-wide identification and characterization of bHLH transcription factors related to anthocyanin biosynthesis in red walnut(Juglans regia L.)[J].Frontiers in Genetics,2021,12:632509.
[12] 张翰生,昌秦湘,康建忠,梁宗锁.核桃的营养价值及其开发利用研究进展[J].浙江农业学报,2024,36(4):905-919.ZHANG Hansheng,CHANG Qinxiang,KANG Jianzhong,LIANG Zongsuo. Research progress on nutritional value and utilization of walnut[J]. Acta Agriculturae Zhejiangensis,2024,36(4):905-919.
[13] 裴东,鲁新政. 中国核桃种质资源[M]. 北京:中国林业出版社,2011.PEIDong,LUXinzheng.WalnutgermplasmresourcesinChina[M].Beijing:China Forestry Publishing House,2011.
[14] ZHAO W,FAN L,WU W J,LI Y Q,MENG H J,WANG G X,DONG Z B,WANG L,WU G L. Re-sequencing and transcriptomic analysis reveal differential expression patterns and sequence variation in glucosyltransferase gene related to anthocyanin biosynthesis in walnut(Juglans regia L.)[J].Scientia Horticulturae,2023,317:112077.
[15] 李恺睿,史庆瑶,樊铭玺,谭浩然,陈晓峰.应用STR 荧光标记分析烟台地区草莓种质资源遗传多样性[J].山东农业科学,2024,56(1):43-49.LI Kairui,SHI Qingyao,FAN Mingxi,TAN Haoran,CHEN Xiaofeng. Genetic diversity analysis of strawberry germplasm resources in Yantai Region using STR fluorescent markers[J].Shandong Agricultural Sciences,2024,56(1):43-49.
[16] MARRANO A,BRITTON M,ZAINI P A,ZIMIN A V,WORKMAN R E,PUIU D,BIANCO L,PIERRO E A D,ALLEN B J,CHAKRABORTY S,TROGGIO M,LESLIE C A,TIMP W,DANDEKAR A,SALZBERG S L,NEALE D B. High-quality chromosome-scale assembly of the walnut(Juglans regia L.)reference genome[J].GigaScience,2020,9(5):giaa050.
[17] LIVAK K J,SCHMITTGEN T D.Analysis of relative gene expression data using real-time quantitative PCR and the 2- ΔΔCt method[J].Methods,2001,25(4):402-408.
[18] WANG C H,GAO G,CAO S X,XIE Q J,QI H Y.Isolation and functional validation of the CmLOX08 promoter associated with signalling molecule and abiotic stress responses in oriental melon,Cucumis melo var.makuwa Makino[J].BMC Plant Biology,2019,19(1):75.
[19] ALABD A,AHMAD M,ZHANG X,GAO Y H,PENG L,ZHANG L,NI J B,BAI S L,TENG Y W.Light-responsive transcription factor PpWRKY44 induces anthocyanin accumulation by regulating PpMYB10 expression in pear[J]. Horticulture Research,2022,9:uhac199.
[20] KIM D H,LEE J,RHEE J,LEE J Y,LIM S H. Loss of the R2R3 MYB transcription factor RsMYB1 shapes anthocyanin biosynthesis and accumulation in Raphanus sativus[J]. International Journal of Molecular Sciences,2021,22(20):10927.
[21] KHOO H E,AZLAN A,TANG S T,LIM S M.Anthocyanidins and anthocyanins:colored pigments as food,pharmaceutical ingredients,and the potential health benefits[J]. Food  Nutrition Research,2017,61(1):1361779.
Nutrition Research,2017,61(1):1361779.
[22] 万东璞,于卓,吴燕民,丁梦琦,李金博,周美亮.花青素代谢调控植物彩叶研究进展[J].中国农业科技导报,2020,22(2):30-38.WAN Dongpu,YU Zhuo,WU Yanmin,DING Mengqi,LI Jinbo,ZHOU Meiliang. Regulation of anthocyanin metabolism on colored leaves of plants[J]. Journal of Agricultural Science and Technology,2020,22(2):30-38.
[23] 李文飞,寇萍,陈春玲,解鸿磊,黄玉吉.红皮香蕉CHS 基因的克隆和表达模式分析[J].分子植物育种,2023,21(3):697-707.LI Wenfei,KOU Ping,CHEN Chunling,XIE Honglei,HUANG Yuji. Cloning and expression pattern analysis of CHS gene in Musa acuminata‘Red Green’(AAA)[J].Molecular Plant Breeding,2023,21(3):697-707.
[24] 何锐杰,方庭,余伟军,张梦媛,饶娅,梁钒,魏秀清,曾黎辉.西番莲查尔酮合成酶(CHS)基因家族全基因组鉴定及表达模式[J].应用与环境生物学报,2022,28(4):1066-1075.HE Ruijie,FANG Ting,YU Weijun,ZHANG Mengyuan,RAO Ya,LIANG Fan,WEI Xiuqing,ZENG Lihui. Genome-wide identification and expression analysis of the CHS gene family in passion fruit[J]. Chinese Journal of Applied and Environmental Biology,2022,28(4):1066-1075.
[25] 徐靖,朱家红,王效宁,韩义胜,唐力琼,朱红林.甘薯查尔酮合成酶基因IbCHS1 的克隆和表达分析[J]. 分子植物育种,2018,16(6):1752-1757.XU Jing,ZHU Jiahong,WANG Xiaoning,HAN Yisheng,TANG Liqiong,ZHU Honglin. Cloning and expression analysis of Chalcone synthase gene IbCHS1 in Ipomoea batatas[J]. Molecular Plant Breeding,2018,16(6):1752-1757.
[26] 徐献斌,李慧,耿晓月,郑焕,陶建敏.ABA 信号通路对葡萄果皮花青苷生物合成的调控机制研究[J].西北植物学报,2021,41(3):406-415.XU Xianbin,LI Hui,GENG Xiaoyue,ZHENG Huan,TAO Jianmin.Regulation mechanism of ABA pathway genes on anthocyanin biosynthesis in grape skins[J].Acta Botanica Boreali-Occidentalia Sinica,2021,41(3):406-415.
[27] 孙玉帅,王菲,管雪强,郗慧茹,姚玉新.ABA 和乙烯互作调控葡萄VlMybA1 和VlMybA2 表达并促进果皮着色[J].园艺学报,2023,50(11):2323-2336.SUN Yushuai,WANG Fei,GUAN Xueqiang,CHI Huiru,YAO Yuxin.ABA and ethylene enhance the expression VlMybA1 and VlMybA2 and promote pigmentation in the berry skin via their interaction[J]. Acta Horticulturae Sinica,2023,50(11):2323-2336.
[28] NARDI C F,VILLARREAL N M,OPAZO M C,MARTÍNEZ G A,MOYA- LEÓN M A,CIVELLO P M. Expression of FaXTH1 and FaXTH2 genes in strawberry fruit.Cloning of promoter regions and effect of plant growth regulators[J]. Scientia Horticulturae,2014,165:111-122.
[29] 郭晋艳,郑晓瑜,邹翠霞,李秋莉.植物非生物胁迫诱导启动子顺式元件及转录因子研究进展[J].生物技术通报,2011,27(4):16-20.GUO Jinyan,ZHENG Xiaoyu,ZOU Cuixia,LI Qiuli. Research progress of cis-elements of abiotic stress inducible promoters and associated transcription factors[J]. Biotechnology Bulletin,2011,27(4):16-20.
[30] 彭亚丽,高倩,董文,熊安平,秦玉芝,林原,熊兴耀,胡新喜.MYB 转录因子调控蔬菜花青素生物合成的研究进展[J].中国瓜菜,2020,33(12):1-7.PENG Yali,GAO Qian,DONG Wen,XIONG Anping,QIN Yuzhi,LIN Yuan,XIONG Xingyao,HU Xinxi.Advances of MYB transcription factors regulating vegetable anthocyanins biosynthesis[J].China Cucurbits and Vegetables,2020,33(12):1-7.
[31] LAI B,DU L N,LIU R,HU B,SU W B,QIN Y H,ZHAO J T,WANG H C,HU G B. Two LcbHLH transcription factors interacting with LcMYB1 in regulating late structural genes of anthocyanin biosynthesis in Nicotiana and Litchi chinensis during anthocyanin accumulation[J]. Frontiers in Plant Science,2016,7:166.
[32] AN J P,LI H H,SONG L Q,SU L,LIU X,YOU C X,WANG X F,HAO Y J. The molecular cloning and functional characterization of MdMYC2,a bHLH transcription factor in apple[J].Plant Physiology and Biochemistry,2016,108:24-31.
[33] 王华,李茂福,杨媛,金万梅.果实花青素生物合成分子机制研究进展[J].植物生理学报,2015,51(1):29-43.WANG Hua,LI Maofu,YANG Yuan,JIN Wanmei. Recent advances on the molecular mechanisms of anthocyanin synthesis in fruits[J].Plant Physiology Journal,2015,51(1):29-43.
[34] ZHANG Z N,QU P Y,HAO S Y,LI R D,ZHANG Y Y,ZHAO Q,WEN P F,CHENG C Z.Characterization and functional analysis of Chalcone synthase genes in highbush blueberry(Vaccinium corymbosum)[J]. International Journal of Molecular Sciences,2023,24(18):13882.
[35] CHAGNÉ D,KUI L W,ESPLEY R V,VOLZ R K,HOW N M,ROUSE S,BRENDOLISE C,CARLISLE C M,KUMAR S,DE SILVA N,MICHELETTI D,MCGHIE T,CROWHURST R N,STOREY R D,VELASCO R,HELLENS R P,GARDINER S E,ALLAN A C.An ancient duplication of apple MYB transcription factors is responsible for novel red fruit-flesh phenotypes[J].Plant Physiology,2013,161(1):225-239.
[36] YAO G F,MING M L,ALLAN A C,GU C,LI L T,WU X,WANG R Z,CHANG Y J,QI K J,ZHANG S L,WU J. Mapbased cloning of the pear gene MYB114 identifies an interaction with other transcription factors to coordinately regulate fruit anthocyanin biosynthesis[J].Plant Journal,2017,92(3):437-451.
[37] 王文静.马铃薯CHS 基因家族鉴定及功能解析[D].合肥:安徽农业大学,2023.WANG Wenjing. Genome- wide identification and functional analysis of CHS gene family in Solanum tuberosum[D]. Hefei:Anhui Agricultural University,2023.