化州柚(Citrus grandis‘Tomentosa’)是柚[C.grandis(L.)Osbeck]的一个变种,起源于广东化州,是中药化橘红(Exocarpium Citri Grandis)的源植物。化州柚主要有正毛、副毛、光青、黄龙和假西洋等地方品系[1],其中正毛、副毛和光青的主要区别在于果实表面的茸毛形态不同[2]。正毛果实表面茸毛长而密,副毛果实表面茸毛稀且短,光青果实表面光滑无毛(图1)[3]。

图1 正毛、副毛和光青3 个品系化州柚花和果实形态
Fig.1 Flower and fruit morphology of three varieties of C.grandis‘Tomentosa’
化州柚花期在3月,开花量大,为保证果实产量和品质,果农一般会进行疏花,疏下的花在化州当地被制成花茶。化州柚花与果实中的有效成分在种类和含量上都较为接近,化州柚花茶同化橘红一样具有理气化痰的功效[4]。柚皮苷被认为是化橘红的主要功效成分[5],其通过对气道炎症以及气道上皮、气道平滑肌的调控发挥消炎、止咳化痰的作用[6-7]。Duan等[8]研究发现幼果期化州柚果实中柚皮苷含量(w,后同)可达果实干质量的28%,而化州柚花中柚皮苷含量也达16%,高于叶片及近成熟果实和成熟果实。化州柚花中挥发油含量约为0.7%,含量较高的成分有D-柠檬烯、γ-萜品烯、β-月桂烯、芳樟醇、反-橙花叔醇等[4]。韩寒冰等[9]从化州柚花和果实的挥发油中分别鉴定出30 种和36 种成分,其中28 种成分相同。
不同品系的化州柚果实在有效成分含量和功效上存在显著差异。Fan等[10]对化州柚中16种有效成分进行定量分析,发现正毛品系含有更多的类黄酮,其野漆树苷的含量明显高于光青。李宇邦等[11]的研究发现毛橘红中柚皮苷、野漆树苷、柚皮素的含量高于光橘红。研究还发现[12-13],由正毛化州柚未成熟果实和近成熟果实外果皮炮制而成的“毛橘红”抗炎和化痰作用最突出,其效果优于由光青化州柚制成的“光橘红”。然而,目前对不同品系的化州柚花之间的成分差异缺乏深入研究。因此,笔者在本研究中选择同一生长期的正毛、副毛和光青3 个品系的化州柚花作为研究对象,运用代谢组学的方法比较它们之间的成分差异,通过多元统计分析筛选出差异代谢物。旨在明确不同品系化州柚花的化学成分差异,为化州柚花的综合利用提供理论依据。
1 材料和方法
1.1 材料与试剂
正毛、副毛和光青3个品系化州柚花,采集自广东省化州市化橘红药材开发有限公司GMP 标准苗圃。内标核糖醇和2-氯苯丙氨酸购自德国Sigma-Aldrich 公司;甲氧胺盐购自上海梯希爱公司;N,O-双(三甲基硅)三氟乙酰胺(BSTFA)(含1%三甲基氯硅烷TMCS)购自美国REGIS Technologies 公司;饱和脂肪酸甲酯(FAMEs)购自德国Dr.Ehrenstorfer公司;氯仿和吡啶(色谱纯)及甲醇、乙腈和甲酸(质谱纯)购自美国Thermo Fisher Scientific公司。
1.2 仪器与设备
ExionLC AD-trap 6500+UPLC-MS/MS 液质联用仪,美国Sciex 公司生产;7890B GC 气相色谱仪,美国安捷伦公司生产;PEGASUS HT飞行时间质谱仪,美国LECO公司生产;Millipore D23 UV纯水仪,美国Merck 公司生产;Heraeus Fresco17 高速离心机,美国Thermo Fisher Scientific 公司生产;LNGT98真空干燥仪,太仓市华美生化仪器厂生产。
1.3 试验方法
1.3.1 UPLC-MS/MS样本前处理 称取50 mg冷冻干燥研磨后的样本加入700 μL 含0.1%2-氯苯丙氨酸内标的25%甲醇水溶液,然后冰水浴超声提取5 min,重复提取3 次后在混匀仪上4 ℃过夜,然后将样本在4 ℃下13 800g 离心15 min,取上清液经0.22 μm 微孔滤膜过滤,用提取液稀释上清液20倍,涡旋30 s,每个样本各取50 μL 混合成质控(QC)样本,-80 ℃储存直到上机检测。
1.3.2 GC-QTOF/MS样本前处理 称取50 mg冷冻干燥研磨后的样本加入500 μL 含核糖醇内标的25%甲醇水溶液,然后冰水浴超声提取5 min,重复提取3次后在4 ℃下13 800g 离心15 min,每个样本各取100 μL上清液混合成QC样本。将试验样本及QC样本在真空浓缩器中干燥,然后加入50 μL溶解于吡啶的20 mg·mL-1甲氧胺盐试剂,混匀后在烘箱中80 ℃孵育30 min,再加入70 μL BSTFA(含1%TMCS)70 ℃孵育1.5 h,冷却至室温后向混合的样本中加入5 μL FAMEs(溶于氯仿),随机顺序上机检测。
1.3.3 UPLC-MS/MS数据采集 液相色谱采用Waters Acquity UPLC HSS T3(2.1 mm×100 mm,1.8 μm)色谱柱,流动相A 相为0.1%甲酸水,B 相为乙腈,梯度洗脱:0 min,98% A;0.5 min,98% A;10 min,50%A;11 min,5%A;13 min,5%A;13.1 min,98%A;15 min,98%A。流速为0.4 mL·min-1,进样体积为2 μL,柱温为40 ℃。质谱采用ESI 离子源,以多反应监测模式(MRM)进行质谱分析。离子源参数如下:离子化电压+5500/-4500 V,气帘气35 psi,温度400 ℃,喷雾气60 psi,辅助加热气60 psi。样品随机进样,每个样本正离子模式和负离子模式各进1针,在每进3针样本后,进1针QC样本和1针空白样本(25%甲醇),进行质量控制和矫正峰的漂移。所有质谱数据采集及目标化合物定量分析工作均通过SCIEX Analyst Work Station Software (Version 1.6.3)来完成。使用MSconventer 软件将质谱原始转成TXT 格式再使用自撰写R 程序包结合自建数据库完成提峰、注释等工作。
1.3.4 GC-QTOF/MS数据采集 气相色谱采用Agilent DB-5MS 毛细管柱(30 m×250 μm×0.25 μm,J W Scientific,Folsom,CA,USA),以不分流的方式进1 μL 样品。以氦气为载气,隔垫吹扫流速为3 mL·min-1,柱流速为1 mL·min-1。程序升温条件为:初始温度50 ℃,保持1 min;然后以10 ℃·min-1的速率升至310 ℃,然后在310 ℃下保持8 min。前进样口温度为280 ℃,传输线温度为280 ℃,离子源温度为250 ℃。电离电压为70 eV。在溶剂延迟6.25 min 后,以12.5 scan·s-1的速率,在m/z 范围为50~500 的全扫描模式下获得质谱数据。使用ChromaTOF 软件(V 4.3x,LECO)对质谱数据进行峰提取、基线矫正、解卷积、峰积分、峰对齐等处理。使用LECO-Fiehn Rtx5 数据库通过质谱匹配及保留时间指数匹配进行化合物的注解。最后,将QC 样本中检出率50%以下或RSD>30%的峰去除。
W Scientific,Folsom,CA,USA),以不分流的方式进1 μL 样品。以氦气为载气,隔垫吹扫流速为3 mL·min-1,柱流速为1 mL·min-1。程序升温条件为:初始温度50 ℃,保持1 min;然后以10 ℃·min-1的速率升至310 ℃,然后在310 ℃下保持8 min。前进样口温度为280 ℃,传输线温度为280 ℃,离子源温度为250 ℃。电离电压为70 eV。在溶剂延迟6.25 min 后,以12.5 scan·s-1的速率,在m/z 范围为50~500 的全扫描模式下获得质谱数据。使用ChromaTOF 软件(V 4.3x,LECO)对质谱数据进行峰提取、基线矫正、解卷积、峰积分、峰对齐等处理。使用LECO-Fiehn Rtx5 数据库通过质谱匹配及保留时间指数匹配进行化合物的注解。最后,将QC 样本中检出率50%以下或RSD>30%的峰去除。
1.3.5 代谢组学数据分析 将采集到的UPLC-MS/MS 和GC-QTOF/MS 数据采用SIMCA(V 14.1,Umetrics)进行多元统计分析。数据在对数(Log)转换和Mean-centered 尺度化后,进行3 个品系样本的PCA 分析和品系间两两对比的OPLS-DA 分析。以OPLS-DA 模型中的变量投影重要性分析值(VIP)、差异倍数(Fold change,FC)以及单因素方差分析(student’s t-test)的p值综合筛选UPLC-MS/MS检测到的差异代谢物。以OPLS-DA分析得到的S-plot图筛选GC-TOF/MS 检测到的差异代谢物。用R 软件和Origin(V 9.1,OriginLab)软件进行数据可视化。
2 结果与分析
2.1 UPLC-MS/MS广泛靶向数据主成分分析
通过与自建数据库比对,从正毛、副毛和光青3组共9 个样本中检测到978 种代谢物,其中黄酮类154种,占比15.75%;酚类129种,占比13.19%;生物碱108 种,占比11.04%;萜类90 种,占比9.20%。此外,还检测到香豆素、木质素、脂质、植物激素、芳香族化合物、糖和糖醇、有机酸及其衍生物、氨基酸及其衍生物、甾体及其衍生物以及核苷酸及其衍生物等,检测到的各类代谢物数量如表1。为描绘正毛、副毛和光青3 个品系间代谢物的整体差异,对以上检测到的代谢物进行主成分分析(PCA)。得分图(图2)显示主成分1 和主成分2 的贡献率分别为44.4%和8.5%,且正毛、副毛和光青3组样本点完全分离,说明3个品系成分差异显著,并且检测到的代谢物能够代表三者之间的差异。
表1 UPLC-MS/MS广泛靶向代谢组学检测的代谢物种类和数量
Table 1 The classification and quantity of metabolites detected by widely-targeted metabolomics using UPLCMS/MS
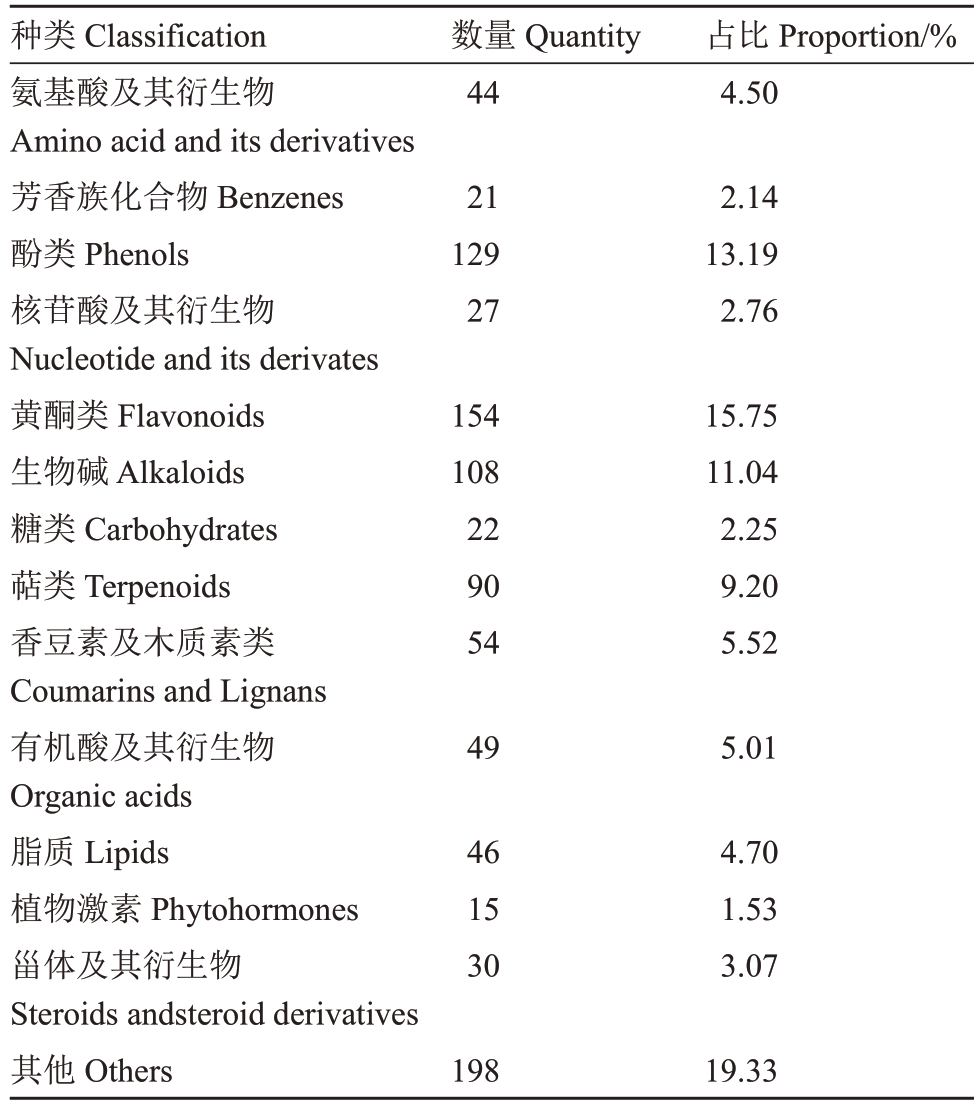
种类Classification氨基酸及其衍生物Amino acid and its derivatives芳香族化合物Benzenes酚类Phenols核苷酸及其衍生物Nucleotide and its derivates黄酮类Flavonoids生物碱Alkaloids糖类Carbohydrates萜类Terpenoids香豆素及木质素类Coumarins and Lignans有机酸及其衍生物Organic acids脂质Lipids植物激素Phytohormones甾体及其衍生物Steroids andsteroid derivatives其他Others数量Quantity 44 21 129 27 154 108 22 90 54 49 46 15 30 198占比Proportion/%4.50 2.14 13.19 2.76 15.75 11.04 2.25 9.20 5.52 5.01 4.70 1.53 3.07 19.33
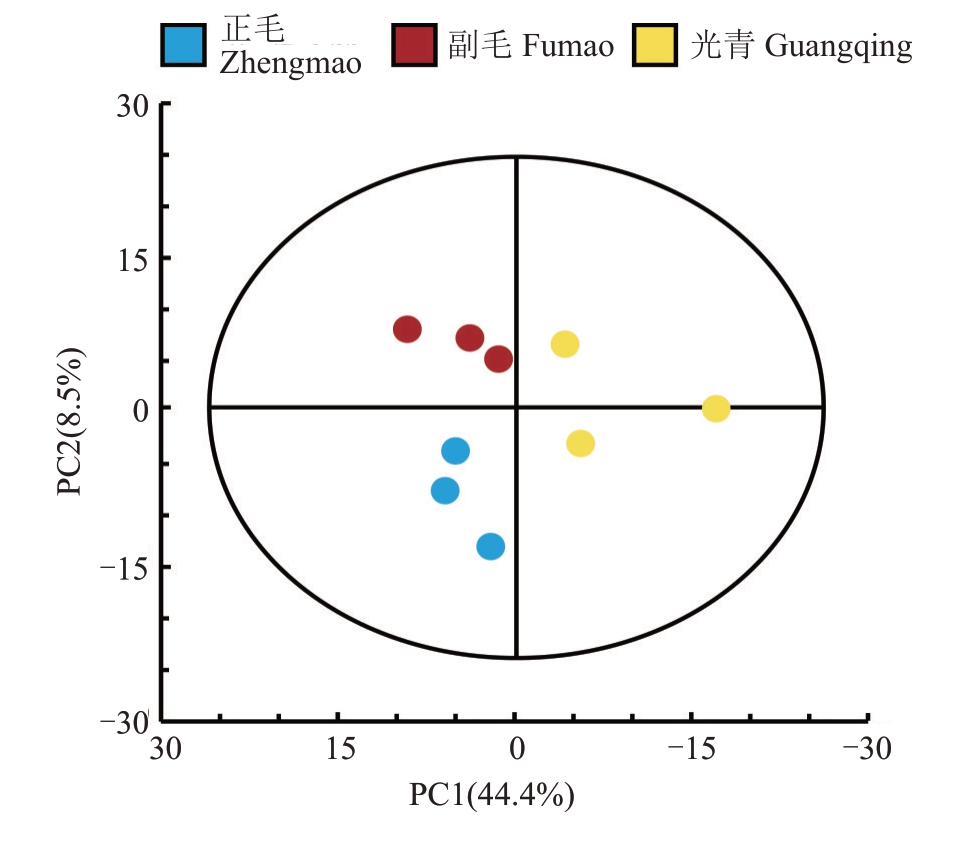
图2 UPLC-MS/MS 检测的代谢物主成分分析
Fig.2 Principal component analysis(PCA)of metabolites detected by UPLC-MS/MS
2.2 UPLC-MS/MS广泛靶向代谢组差异代谢物筛选
采用OPLS-DA模型将正毛、副毛和光青3组样本进行两两对比。以单因素方差分析(student’s ttest)p 值小于0.05、差异倍数(Fold change,FC)大于2或者小于0.5,以及OPLS-DA模型中变量投影重要性分析值(VIP)大于1 这3 个条件筛选差异代谢物。从ZM vs FM 中筛选出34 种代谢物,其中25 种上调,9种下调;从ZM vs GQ中筛选出48种代谢物,其中37种上调,11种下调;从FM vs GQ中筛选出58种代谢物,其中34 种代谢物上调,24 种代谢物下调。以差异倍数(FC)和p值分别为横纵坐标作火山图对数据进行可视化(图3)。共有芸香柚皮苷、野漆树苷、橙皮内酯、芦丁、木犀草素-7-O-芸香糖苷、邻苯三酚、驴食草酚、9-甲氧基-α-拉帕醌、无梗五加苷B、荭草苷、8-甲基壬烯酸酯、甜茶苷、5-尿嘧啶核苷酸、7-甲基鸟嘌呤和Nemorensine 15 种代谢物在两组比较中同时具有差异。将各组对比中上调及下调前15 位的代谢物以火柴杆图的形式展示,如图4。将以上火柴杆图中所示代谢物汇总,共有63 种代谢物,以热图的形式展示其在3 个品系间的相对丰度,并将代谢物和样品都进行基于欧式距离(Euclidean distance)的聚类分析,结果如图5。地奥司明、山柰酚-3-O-芸香糖苷、芦丁、牡荆素-2''-O-葡萄糖苷、木犀草素-7-O-芸香糖苷、维采宁-2、芸香柚皮苷、香草酸、佛手苷内酯和橙皮内酯这10 种化州柚中活性物质在正毛、副毛中的含量高于光青,说明副毛和正毛化州柚花可能具有更高的保健和药用价值。
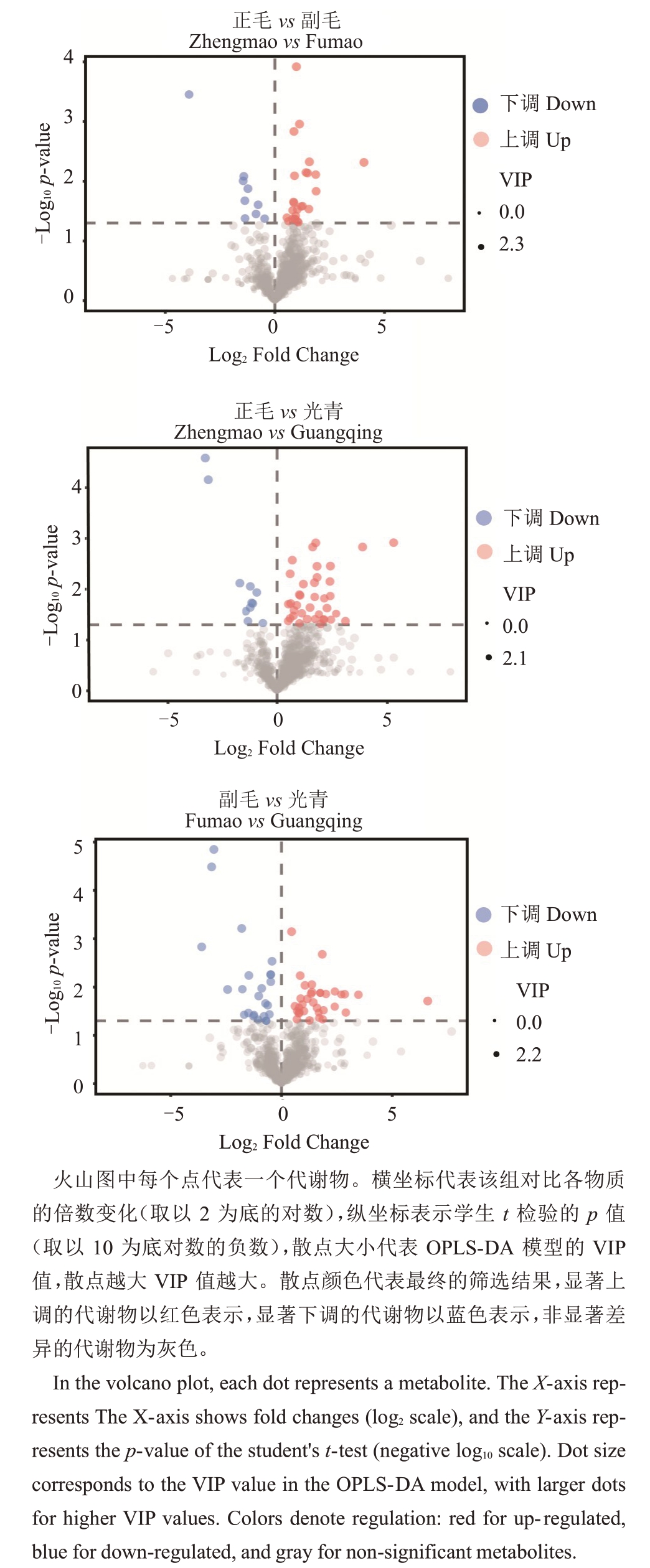
图3 UPLC-MS/MS 检测的代谢物火山图
Fig.3 Volcano plot of metabolites detected by UPLC-MS/MS
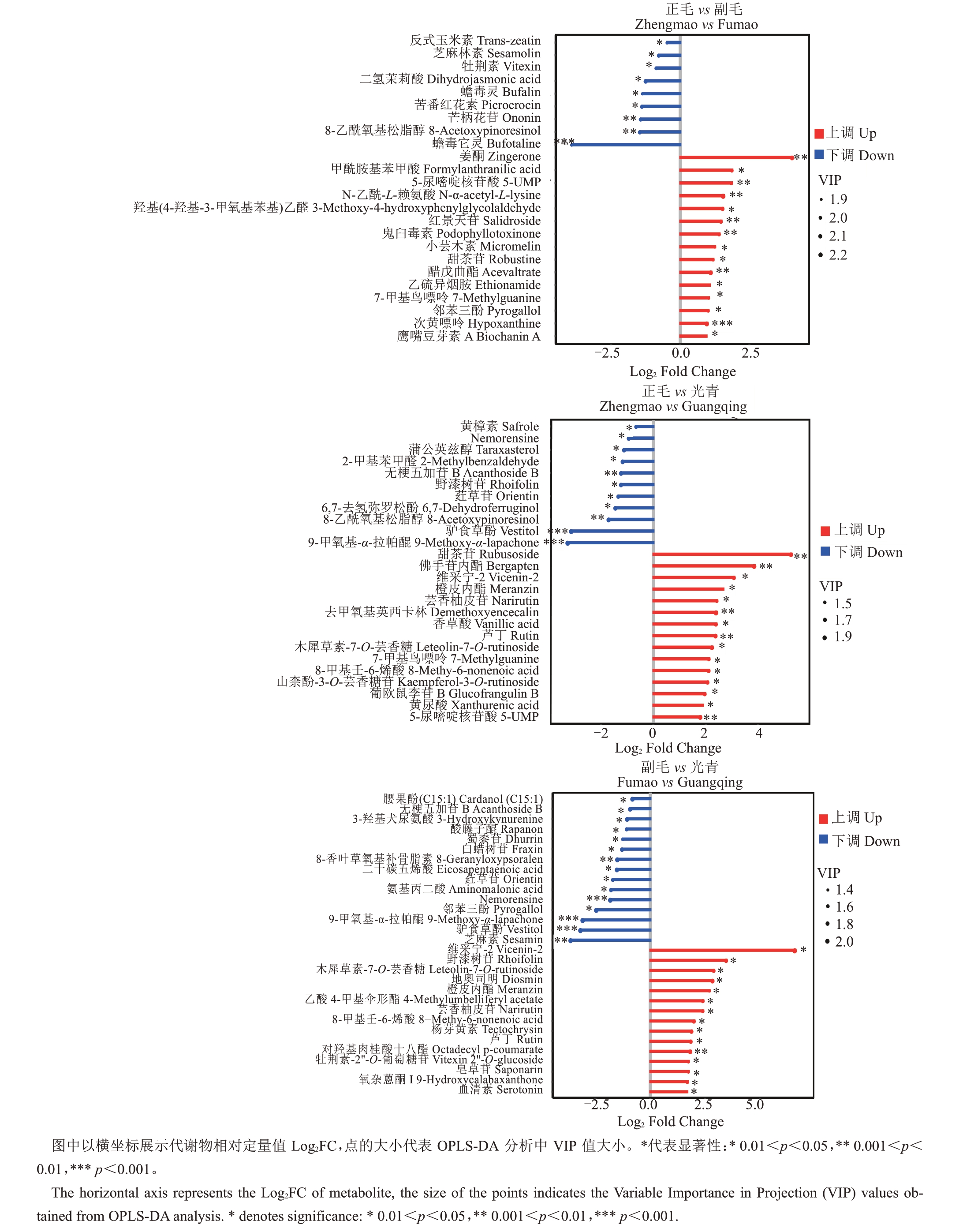
图4 UPLC-MS/MS 差异代谢物差异倍数火柴杆图
Fig.4 Matchstick Analysis of differential metabolites detected by UPLC-MS/MS

图5 UPLC-MS/MS 差异代谢物热图
Fig.5 UPLC-MS/MS differential metabolites heatmap
2.3 GC-TOF/MS非靶向数据主成分分析
从正毛、副毛和光青3 个品系样本的GC-TOF/MS原始数据中共提取到594个质谱峰,与标准数据库比对后共检测到236 个代谢物,其中有机酸及其衍生物66 种,占比27.97%;有机氧化合物60 种,占比25.42%;脂质23种,占比9.75%;苯及其衍生物17种,占比7.20%,其他类代谢物的占比如图6。以上述代谢物归一化后的相对丰度为变量进行PCA 分析,得分图如图7。主成分1和主成分2的贡献度分别为39.8%和17.1%,并且ZM、FM和GQ3组样本点的分离度良好,说明以上代谢物可以代表正毛、副毛和光青3个品系之间的差异。
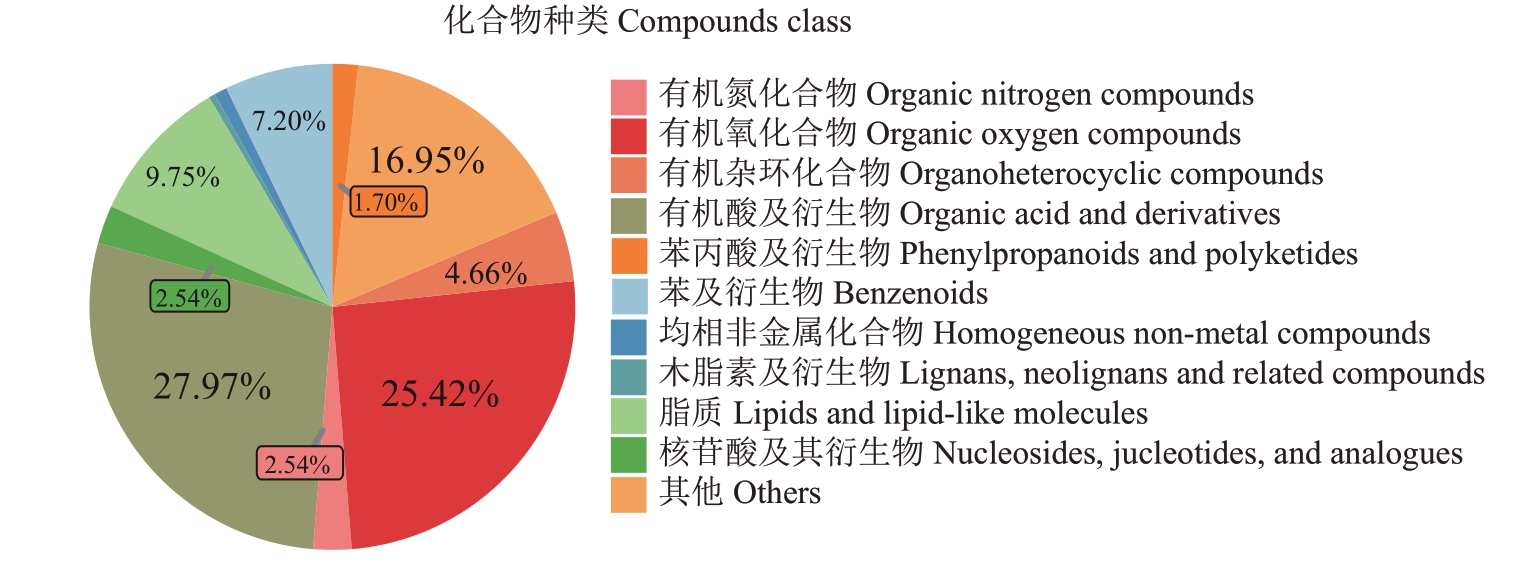
图6 GC-TOF/MS 非靶向代谢组学检测的代谢物种类和数量
Fig.6 The classification and quantity of metabolites detected by untargeted metabolomics using GC-TOF/MS
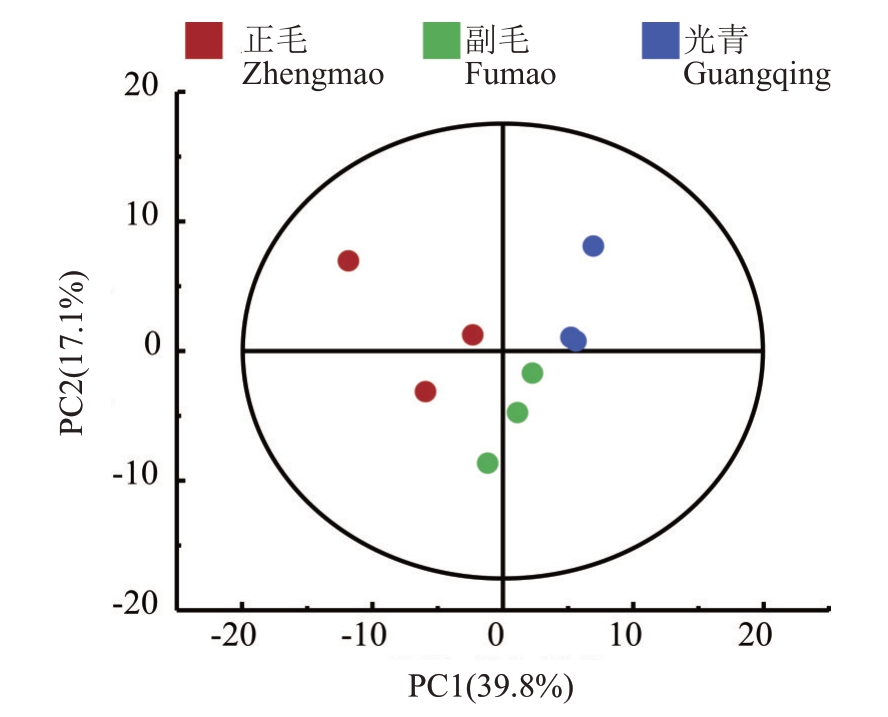
图7 GC-TOF/MS 检测的代谢物主成分分析
Fig.7 Principal component analysis(PCA)of metabolites detected by GC-TOF/MS
2.4 GC-TOF/MS非靶向代谢组差异代谢物筛选
以检测到的236种代谢物的相对丰度为变量,采用OPLS-DA模型对正毛、副毛和光青3个品系进行两两对比,筛选差异代谢物。将OPLS-DA分析的结果以S-plots 的形式进行展示,如图8,横坐标p[1]为负载向量在主成分1上的协方差,纵坐标p(corr)[1]为负载向量与主成分1的相关性,以|p(corr)[1]|>0.5,|p[1]|>0.15为条件筛选差异代谢物。从FM vs ZM中筛选出6种差异代谢物,柚皮素-7-O-葡萄糖苷和柠檬酸上调,咖啡酸、反式-4-羟基肉桂酸、阿魏酸和丙酮酸下调。从GQ vs ZM中筛选出8种差异代谢物,柚皮素-7-O-葡萄糖苷、莽草酸、柠檬酸和景天酮庚糖上调,D-纤维二糖、咖啡酸、α-槐糖和D-(-)-果糖下调。从GQ vs FM中筛选出7种差异代谢物,柚皮素-7-O-葡萄糖苷、阿魏酸、丙酮酸、景天酮庚糖和反式-4-羟基肉桂酸上调,D-纤维二糖和D-(-)-果糖下调。上述差异代谢物汇总后共有11种,其中D-(-)-果糖、α-槐糖、丙酮酸、咖啡酸、阿魏酸、柚皮素-7-O-葡萄糖苷在3组对比中都有差异,D-纤维二糖、景天酮庚糖、柠檬酸、莽草酸、反式-4-羟基肉桂酸在两组对比中有差异。上述差异代谢物在正毛、副毛和光青3组中的相对含量如图9所示。
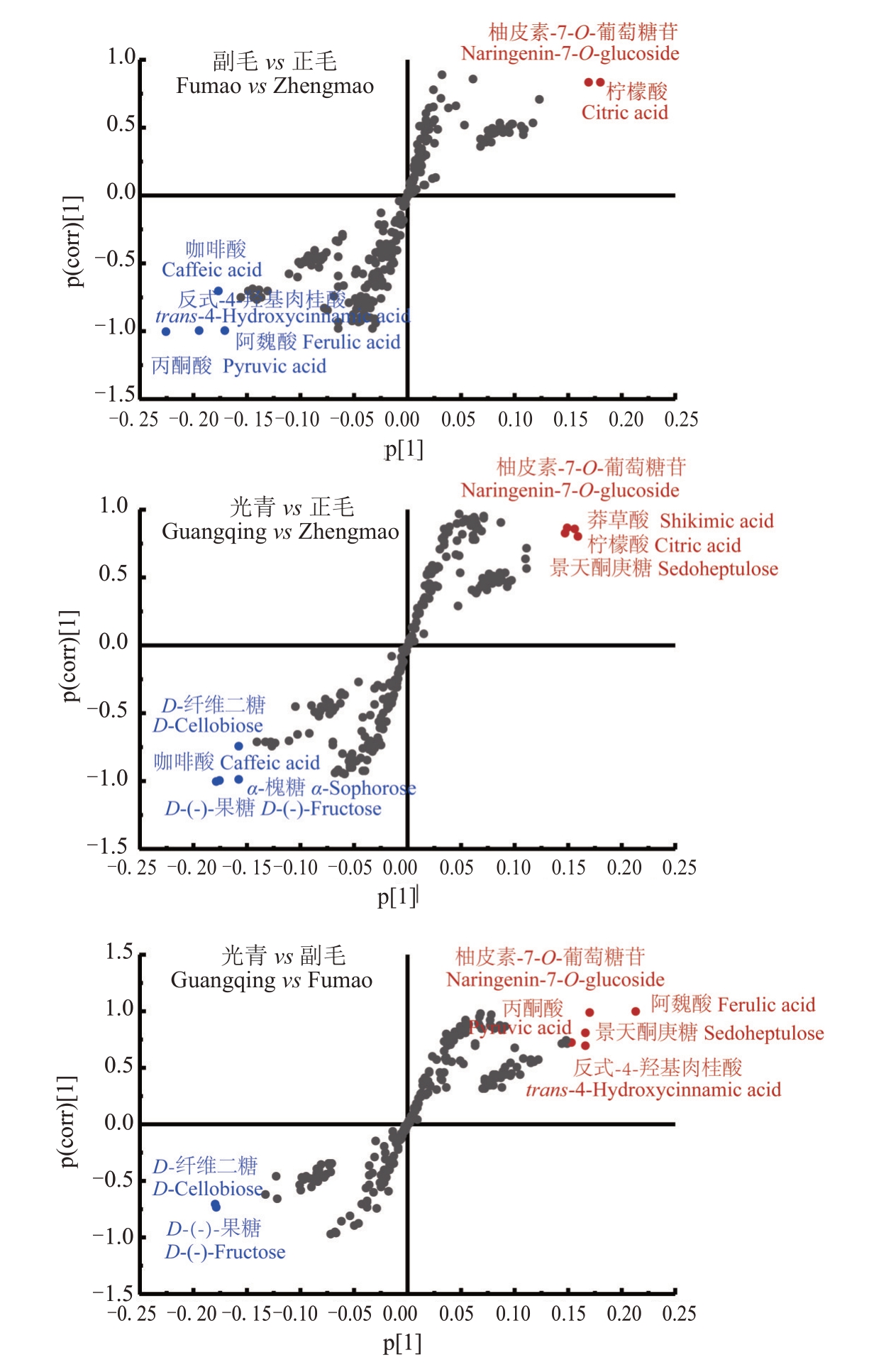
图8 代谢物的OPLS-DA 分析S-plot 图
Fig.8 S-plot of metabolites in the in OPLS-DA model
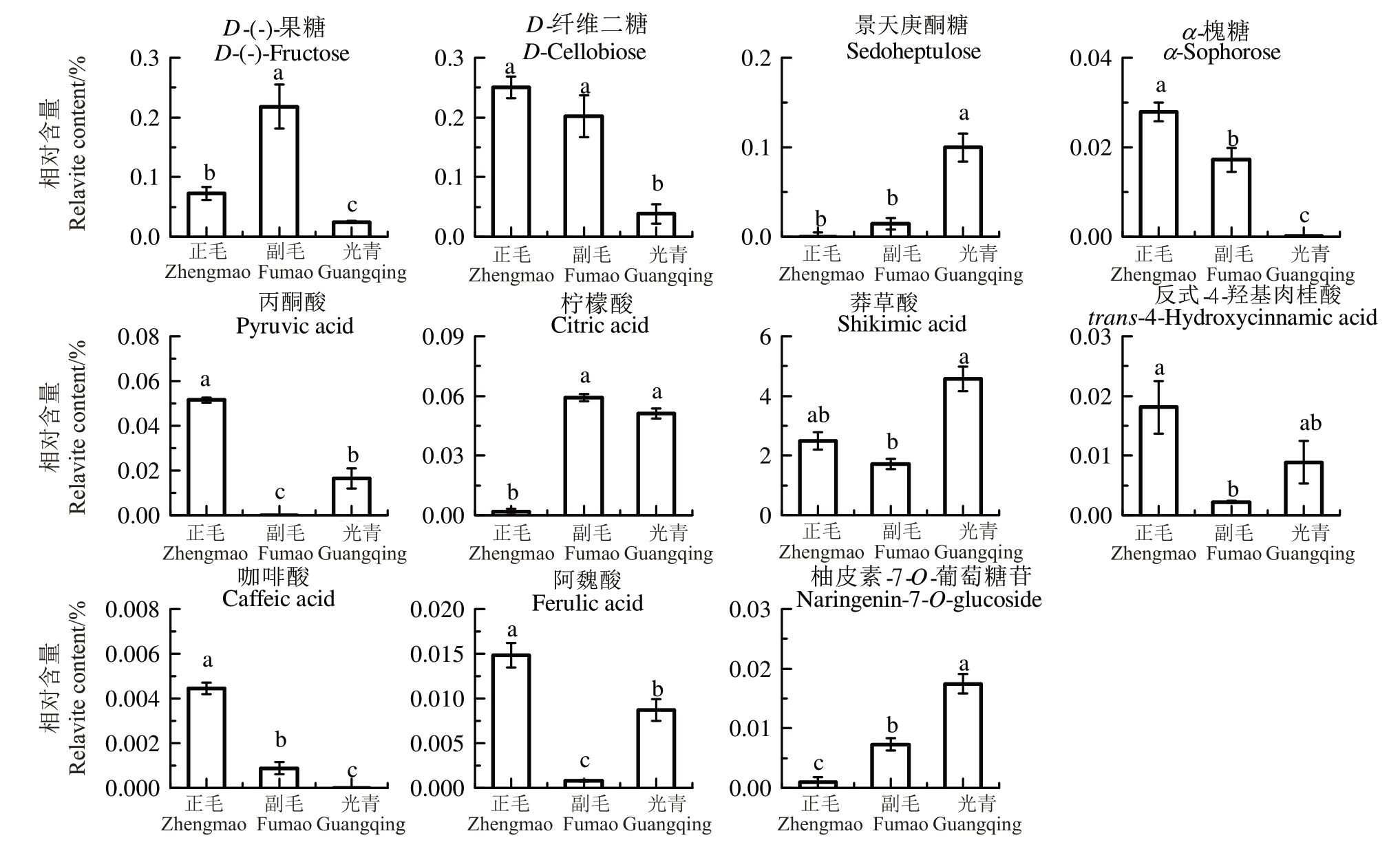
图9 GC-TOF/MS 差异代谢物在不同品系间的相对含量
Fig.9 The relative abundance of differential metabolites detected by GC-TOF/MS among different cultivars
3 讨 论
评价食用和药用植物的品质往往需要对其中的氨基酸、糖、有机酸、维生素等初级代谢物和酚酸、黄酮、生物碱、萜类等次级代谢产物的组成和含量进行综合评估,采用传统的检测和定量方法存在成本高、耗时长、过程复杂等问题,越来越多的研究开始采用代谢组学完成食品和药用植物中代谢物的高通量整体分析[14]。通过液质联用(LC-MS)、气质联用(GCMS)和核磁共振(nuclear magnetic resonance,NMR)平台,采集植物样品中成百上千种代谢物的信息,再通过多元统计分析从中筛选出不同品种、品系,不同生长时期以及不同处理方式的植物样本中差异代谢物或者标志物,是代谢组学在植物学领域的经典研究方法,广泛应用于遗传育种、资源鉴定和保护以及天然产物开发与利用等研究领域[15]。
笔者在本研究中利用超高效液相色谱-三重四极杆质谱(UPLC-MS/MS)和气相色谱-四级杆飞行时间质谱(GC-TOF/MS)分别进行广泛靶向代谢组学和非靶向代谢组学分析,从化州柚正毛、副毛和光青3 个品系的花中分别筛选出63 种和11 种差异代谢物。野漆树苷、维采宁-2、芸香柚皮苷、地奥司明和木犀草素-7-O-芸香糖苷、山柰酚-3-O-芸香糖苷、芦丁等是已经报道的化州柚中主要的类黄酮成分[16]。笔者在本研究中发现地奥司明、山柰酚-3-O-芸香糖苷、芦丁、牡荆素-2''-O-葡萄糖苷、木犀草素-7-O-芸香糖苷、维采宁-2 和芸香柚皮苷这6 种类黄酮在正毛和副毛化州柚花中的含量高于光青。这与在化州柚果实中的研究结果相一致[17]。采用正毛和副毛化州柚制成的化橘红被认为具有更高的药用价值,也可能与其更高的类黄酮含量有关[18]。地奥司明、维采宁-2、野漆树苷、芦丁等黄酮类化合物具有抗炎、抗感染、抗肿瘤、预防心脑血管疾病等功效[19-21]。橙皮内酯、佛手柑内酯、小芸木素、白蜡树苷等属于香豆素。橙皮内酯和佛手柑内酯是化州柚中典型的香豆素成分[22]。而白蜡树苷也有研究从酸橙和甜橙花中鉴定到[23]。本研究中橙皮内酯和佛手柑内酯在正毛和副毛花中的含量也显著高于光青。大量研究显示柑橘香豆素具有抗炎、抗癌、抗病毒以及保护心脑血管、神经和消化系统等多种生物学活性[24-26]。
筛选出的差异代谢物中还包含香草酸、咖啡酸、阿魏酸等反式-4-羟基肉桂酸等酚酸化合物,以及柠檬酸、莽草酸、果糖、7-甲基鸟嘌呤、5-尿嘧啶核苷酸、二十碳五烯酸等初级代谢产物。这些差异代谢物涉及三羧酸循环、多酚生物合成、嘌呤代谢、果糖和甘露糖代谢等代谢途径。以多酚生物合成为例,酚酸类化合物在植物体内主要由糖酵解及磷酸戊糖途径生成的中间体经莽草酸途径和苯丙烷类代谢途径合成[27],而苯丙烷类代谢途径最终产物对香豆酰辅酶A是类黄酮类化合物合成的重要前体[28]。
柑橘属植物花量过大会导致坐果率低,对花朵进行稀疏处理是必不可少的管理措施。柑橘花香味馥郁清爽,将疏下的花制作成精油和花茶可以提高柑橘的经济价值。沙田柚[29]、温州蜜柑[30]、甜橙和酸橙花[23]都已被开发为精油或花茶产品。化州柚花在化州当地也被用来制作花茶,但目前对化州柚花的成分分析、加工工艺和功能活性的研究较少,化州柚花茶并没有得到广泛应用和推广。本研究对3个不同品系化州柚花的成分进行了鉴定,并筛选出不同品系间的差异代谢物,为未来的品种改良、药物开发和保健产品设计提供研究基础。
4 结 论
通过代谢组学比较化州柚正毛、副毛和光青3个品系花中的代谢物差异。利用UPLC-MS/MS 进行广泛靶向代谢组学分析,筛选出63 种差异代谢物。其中,地奥司明、山柰酚-3-O-芸香糖苷、芦丁、牡荆素-2''-O-葡萄糖苷、木犀草素-7-O-芸香糖苷、维采宁-2、芸香柚皮苷、香草酸、佛手苷内酯和橙皮内酯这10 种化州柚中活性物质含量在正毛和副毛花中高于光青中。此外,基于GC-TOF/MS 进行非靶向代谢组学分析,共筛选出11 种差异代谢物,分别为D-(-)果糖、D-纤维二糖、景天酮庚糖、α-槐糖、丙酮酸、柠檬酸、莽草酸、反式-4-羟基肉桂酸、咖啡酸、阿魏酸和柚皮素-7-O-葡萄糖苷。笔者在本研究中探讨不同品系化州柚花的成分差异,发现正毛和副毛化州柚花的有效成分含量显著高于光青花,说明正毛和副毛化州柚花可能具有更高的药用和保健价值,为化州柚花精油或花茶产品的开发提供了理论依据,有望促进化橘红的综合利用与产业发展。
[1] 李润唐,李映志,汪永保,余庆,李映晖.中药“化橘红”原料植物化州柚种质资源初步研究[J].中国南方果树,2012,41(4):53-55.LI Runtang,LI Yingzhi,WANG Yongbao,YU Qing,LI Yinghui.Preliminary study on germplasm resources of Huazhou Pomelo,the raw material plant of“Hua Ju Hong”[J].South China Fruits,2012,41(4):53-55.
[2] 田静,庞一波,陈嘉景,袁子彧,曾继吾,徐娟.化州柚种质资源的SSR 分析及其果实不同发育期柚皮苷含量变化[J].华中农业大学学报,2019,38(5):64-70.TIAN Jing,PANG Yibo,CHEN Jiajing,YUAN Ziyu,ZENG Jiwu,XU Juan. SSR analyses of germplasm resources and changes of naringin content at different developmental stages of Citrus grandis‘Tomentosa’fruit[J]. Journal of Huazhong Agricultural University,2019,38(5):64-70.
[3] 庄洁旋,陈浪,张丹华,龚丽珍,詹若挺,陈立凯.不同化州柚品系新橘皮糖苷合成关键酶基因的差异表达分析[J].中药材,2023,46(8):1850-1857.ZHUANG Jiexuan,CHEN Lang,ZHANG Danhua,GONG Lizhen,ZHAN Ruoting,CHEN Likai.Differential expression analysis of neohesperidin synthesis key enzyme genes of Citrus grandis Tomentosa’from different strains[J].Journal of Chinese Medicinal Materials,2023,46(8):1850-1857.
[4] 李武国,苏乔,魏洁书,朱烨妍,刘翠婷,李雯雯,赵广银,黄浩机,詹若挺.化州柚花挥发油的GC-MS 分析及其对肺癌细胞A549 增殖、迁移的影响研究[J].中国药房,2018,29(22):3093-3097.LI Wuguo,SU Qiao,WEI Jieshu,ZHU Yeyan,LIU Cuiting,LI Wenwen,ZHAO Guangyin,HUANG Haoji,ZHAN Ruoting.GC-MS analysis of volatile oil from the flowers of Citrus grandis and study on effects of it on the proliferation and migration of lung cancer A549 cells[J]. China Pharmacy,2018,29(22):3093-3097.
[5] CHEN Z,CHEN P,WU H,SHI R,SU W W,WANG Y G,LI P B. Evaluation of naringenin as a promising treatment option for COPD based on literature review and network pharmacology[J].Biomolecules,2020,10(12):1644.
[6] ZHOU C Y,LAI Y L,HUANG P,XIE L P,LIN H Y,ZHOU Z T,MO C,DENG G H,YAN W X,GAO Z W,HUANG S H,CHEN Y Y,SUN X G,LV Z P,GAO L. Naringin attenuates alcoholic liver injury by reducing lipid accumulation and oxidative stress[J].Life Sciences,2019,216:305-312.
[7] ZENG X,SU W W,LIU B M,CHAI L,SHI R,YAO H L.A review on the pharmacokinetic properties of naringin and its therapeutic efficacies in respiratory diseases[J]. Mini Reviews in Medicinal Chemistry,2020,20(4):286-293.
[8] DUAN L,GUO L,DOU L L,YU K Y,LIU E H,LI P.Comparison of chemical profiling and antioxidant activities of fruits,leaves,branches,and flowers of Citrus grandis‘Tomentosa’[J].Journal of Agricultural and Food Chemistry,2014,62(46):11122-11129.
[9] 韩寒冰,张啟,魏国程,刘杰凤,马超.南药化橘红花果叶中挥发油成分比较分析[J].中医药导报,2018,24(7):33-36.HAN Hanbing,ZHANG Qi,WEI Guocheng,LIU Jiefeng,MA Chao. Composition analysis of volatile components extracted from flowers,fruitlets and leaves of exocarpium Citrus grandis[J]. Guiding Journal of Traditional Chinese Medicine and Pharmacy,2018,24(7):33-36.
[10] FAN R Y,ZHU C Y,QIU D Y,MAO G L,MUELLER-ROEBER B,ZENG J W. Integrated transcriptomic and metabolomic analyses reveal key genes controlling flavonoid biosynthesis in Citrus grandis‘Tomentosa’fruits[J].Plant Physiology and Biochemistry,2023,196:210-221.
[11] 李宇邦,肖凤霞,宋小欣,陈周华,林励,李海波,罗立莹.一测多评法比较毛橘红与光橘红5 种黄酮类成分含量[J].中草药,2018,49(2):444-449.LI Yubang,XIAO Fengxia,SONG Xiaoxin,CHEN Zhouhua,LIN Li,LI Haibo,LUO Liying. Comparison of content of five flavones in Citrus grandis“Tomentosa”and Citrus grandis with quantitative analysis of muli-components by single marker[J].Chinese Traditional and Herbal Drugs,2018,49(2):444-449.
[12] 张秀明,陈志霞,林励.毛橘红与光橘红的化痰及抗炎作用比较研究[J].中药材,2004,27(2):122-123.ZHANG Xiuming,CHEN Zhixia,LIN Li. Comparison on dissolving sputum and anti-inflammation of“Mao ju hong”and“Guang ju Hong”[J]. Journal of Chinese Medicinal Materials,2004,27(2):122-123.
[13] 陈岩,胡燕琴. 橘红与化橘红临床药用不同[J]. 北京中医,2001,20(2):43.CHEN Yan,HU Yanqin. Clinical medicinal differences between Citrus grandis‘Tomentosa’and Citrus grandis[J].Beijing Journal of Traditional Chinese Medicine,2001,20(2):43.
[14] 曹小汉,毛惠敏,任莉萍.代谢组学技术在植物次生代谢产物研究中的应用[J].农业与技术,2022,42(11):1-3.CAO Xiaohan,MAO Huimin,REN Liping.Application of metabolomics in the study of plant secondary metabolites[J].Agriculture and Technology,2022,42(11):1-3.
[15] 淡墨,高先富,谢国祥,刘忠,赵爱华,贾伟.代谢组学在植物代谢研究中的应用[J]. 中国中药杂志,2007,32(22):2337-2341.DAN Mo,GAO Xianfu,XIE Guoxiang,LIU Zhong,ZHAO Aihua,JIA Wei.Application of metabolomics in research of plant metabolites[J].China Journal of Chinese Materia Medica,2007,32(22):2337-2341.
[16] XIAN L,SAHU S K,HUANG L Y,FAN Y N,LIN J H,SU J M,BAI M,CHEN Y W,WANG S J,YE P,WANG F,LUO Q,BAI H Y,LIN X J,YUAN C H,GENG X D,LIU H,WU H.The draft genome and multi-omics analyses reveal new insights into geo-herbalism properties of Citrus grandis‘Tomentosa’[J].Plant Science,2022,325:111489.
[17] FAN R Y,ZHU C Y,QIU D Y,ZENG J W. Comparison of the bioactive chemical components and antioxidant activities in three tissues of six varieties of Citrus grandis‘Tomentosa’fruits[J]. International Journal of Food Properties,2019,22(1):1848-1862.
[18] 廖弈秋,李泮霖,廖文波,苏薇薇.南药化橘红基原考证[J].中药材,2015,38(2):401-404.LIAO Yiqiu,LI Panlin,LIAO Wenbo,SU Weiwei.Investigation on the origin of Citrus grandis‘Tomentosa’[J]. Journal of Chinese Medicinal Materials,2015,38(2):401-404.
[19] ANMOL R J,MARIUM S,HIEW F T,HAN W C,KWAN L K,WONG A K Y,KHAN F,SARKER M M R,CHAN S Y,KIFLI N,MING L C. Phytochemical and therapeutic potential of Citrus grandis (L.) Osbeck:A review[J]. Journal of Evidence-Based Integrative Medicine,2021,26:2515690X211043741.
[20] ZHANG Y F,SUN J,DONG Y,SHEN X R,ZHANG Z Y. Vicenin-2 inhibits the Helicobacterium pylori infection associated gastric carcinogenic events through modulation of PI3K/AKT and Nrf2 signaling in GES-1 cells[J]. Journal of Biochemical and Molecular Toxicology,2021,35(3):e22680.
[21] ADEM Ş,EYUPOGLU V,IBRAHIM I M,SARFRAZ I,RASUL A,ALI M,ELFIKY A A. Multidimensional in silico strategy for identification of natural polyphenols-based SARS-CoV-2 main protease (Mpro) inhibitors to unveil a hope against COVID- 19[J]. Computers in Biology and Medicine,2022,145:105452.
[22] 牛艳,王磊,黄晓君,张晓琦,殷志琦,叶文才.化橘红香豆素类的化学成分[J].暨南大学学报(自然科学与医学版),2012,33(5):501-505.NIU Yan,WANG Lei,HUANG Xiaojun,ZHANG Xiaoqi,YIN Zhiqi,YE Wencai.Chemical constituents of Citrus grandis‘Tomentosa’[J]. Journal of Jinan University (Natural Science  Medicine Edition),2012,33(5):501-505.
Medicine Edition),2012,33(5):501-505.
[23] 张忠立,李淑琪,左月明. 酸橙花和甜橙花的UPLC-Q-TOFMS/MS 定性分析及HPLC 含量测定[J]. 中南药学,2022,20(11):2480-2489.ZHANG Zhongli,LI Shuqi,ZUO Yueming. Qualitative and quantitative analysis of flower of Citrus aurantium L.and Citrus sinensis (L.) Osbeck by UPLC-Q-TOF-MS/MS and content determination by HPLC[J].Central South Pharmacy,2022,20(11):2480-2489.
[24] KAUR M,KOHLI S,SANDHU S,BANSAL Y,BANSAL G.Coumarin:A promising scaffold for anticancer agents[J]. Anti-Cancer Agents in Medicinal Chemistry,2015,15(8):1032-1048.
[25] MEDINA F G,MARRERO J G,MACÍAS- ALONSO M,GONZÁLEZ M C,CÓRDOVA- GUERRERO I,TEISSIER GARCÍA A G,OSEGUEDA-ROBLES S. Coumarin heterocyclic derivatives:chemical synthesis and biological activity[J].Natural Product Reports,2015,32(10):1472-1507.
[26] PATIL S B. Medicinal significance of novel coumarin analogs:Recent studies[J].Results in Chemistry,2022,4:100313.
[27] KOŁTONA,DŁUGOSZ-GROCHOWSKAO,WOJCIECHOWSKA R,CZAJA M. Biosynthesis regulation of folates and phenols in plants[J].Scientia Horticulturae,2022,291:110561.
[28] ZHENG W K,ZHANG W,LIU D H,YIN M Q,WANG X,WANG S C,SHEN S Q,LIU S J,HUANG Y,LI X X,ZHAO Q,YAN L,XU Y T,YU S Q,HU B,YUAN T,MEI Z N,GUO L P,LUO J,DENG X X,XU Q,HUANG L Q,MA Z C.Evolution-guided multiomics provide insights into the strengthening of bioactive flavone biosynthesis in medicinal pummelo[J].Plant Biotechnology Journal,2023,21(8):1577-1589.
[29] 廖远东,夏红玲,刘容飞,李志威,王洁,黄海英,周梦珍,曾浩.不同茶坯窨制柚花茶中化学成分变化及感官品质差异[J].广东茶业,2023(6):9-12.LIAO Yuandong,XIA Hongling,LIU Rongfei,LI Zhiwei,WANG Jie,HUANG Haiying,ZHOU Mengzhen,ZENG Hao.Chemical composition changes and sensory quality differences in jasmine-scented grapefruit flower tea made from different tea bases[J].Guangdong Tea Industry,2023(6):9-12.
[30] 杨慧,李玉壬,吴神群,陈春凤,杨晓萍.不同杀青方式对柑橘花茶品质的影响[J].食品科学,2023,44(3):105-111.YANG Hui,LI Yuren,WU Shenqun,CHEN Chunfeng,YANG Xiaoping. Effect of different fixation methods on the quality of Citrus flower tea[J].Food Science,2023,44(3):105-111.