中国是全球桃树栽培面积和产量最大的国家,其中以河北、山东等北方桃主产区为代表,面积约占全国的22%,产量超过30%,总产值达到400 多亿元[1]。然而,在生产过程中,害虫的危害严重影响了桃树的产量和果实品质。蚜虫以刺吸危害枝叶、嫩梢为主,影响果树生长发育,间接影响果实品质。在桃树上常见的蚜虫主要包括桃蚜[Myzus persicae(Sulzer)]、桃粉蚜[Hyalopterus amygdali(Blanchard)]和桃瘤蚜[Tuberocephalus momonis(Matsumura)][2]。桃蚜又名烟蚜,是一种分布广泛且危害严重的害虫,能够寄生于50多个科400多种植物上。除了对桃的危害外,还会危害十字花科蔬菜、烟草和马铃薯等茄科作物。桃蚜除通过刺吸植物汁液吸收营养外,还可以传播植物病毒,对植物造成更大的间接伤害,从而对农业生产造成巨大的经济损失[3]。桃蚜除直接取食植物造成危害外,其分泌的蜜露也会引起植物的煤污病,进一步影响果树的光合作用[4]。
对桃蚜的防治历来以化学防治为主。但由于桃蚜具有种群数量大、生命周期短、繁殖率高和迁飞能力强等特点,导致他们对许多常用杀虫剂产生了不同程度的抗药性[3-7]。目前,我国对桃蚜的防控策略以“化学防治为主、生物防治为辅”。因此,监测桃蚜对常用杀虫剂的敏感度变化显得尤为重要。这不仅有助于评估药剂的实际效果,还可以根据抗药性的发展程度,及时调整防治策略,延缓或避免抗药性的出现,实施更精准的害虫管理措施,从而实现农业生产的可持续性。
吡虫啉(Imidacloprid)是一种高效、广谱的新烟碱类杀虫剂,对蚜虫类、飞虱类、蓟马和果蝇等害虫具有良好的防控效果[8]。其杀虫机制是选择性地作用于昆虫神经系统后突触的烟碱型乙酰胆碱受体,通过干扰害虫中枢神经系统使化学信号传递受阻,造成昆虫出现麻痹而死亡[9]。1992年以来,吡虫啉在中国已广泛用于防治桃蚜等刺吸式口器害虫[10]。尽管吡虫啉对桃蚜具有良好的防治效果,但长期单一使用可能导致桃蚜对其敏感度降低甚至产生抗药性。前期研究表明,我国多地区的桃蚜对吡虫啉产生了不同程度的抗药性,且已明确靶标突变位点。桃蚜抗吡虫啉种群烟碱型乙酰胆碱受体(nAChR)β1亚基81位的精氨酸(R)突变为苏氨酸(T)导致受体对吡虫啉的敏感性降低[11]。此外,桃蚜细胞色素P450 酶系基因CYP6CY3 的过量表达也是其对吡虫啉产生抗药性的一个原因[12]。
双丙环虫酯(Afidopyropen)是一种源自丝状真菌粪生青霉(Penicillium coprobium)的新型丙烯类杀虫剂[13],其防治对象为刺吸式口器和吮吸式口器的害虫,如蚜虫、粉虱、木虱、介壳虫、粉蚧和叶蝉等[14-15]。双丙环虫酯作用于香草酸型瞬时感受器电位通道,通过阻碍运动协调和汁液型害虫的摄食能力而导致其死亡[16-17]。研究表明,双丙环虫酯对非靶标昆虫的毒性较低或无毒[14]。由于其独特的作用模式,与拟除虫菊酯和吡虫啉无交互抗性,可高效防治对有机磷和新烟碱类杀虫剂产生抗药性的害虫[18]。因此,双丙环虫酯为杀虫剂抗性管理提供了另一种改变杀虫剂种类的选择。2019年,该药剂在中国登记使用,用于防治桃蚜等刺吸式口器害虫。
为了提高桃园蚜虫的精准防控水平,合理使用化学药剂,提升桃果实品质,同时减少对环境的负面影响,笔者连续两年监测了国内主要桃生产区桃园中主要蚜虫桃蚜和桃粉蚜对传统杀虫剂吡虫啉和新进杀虫剂双丙环虫酯的药剂敏感度,以期为桃园蚜虫化学防治的药剂选择和轮换使用提供重要的数据支撑。
1 材料和方法
1.1 试验昆虫
2022年和2023年,在我国14 个省24 个市区的桃产业试验站采集桃蚜和桃粉蚜样本,具体样本采集点如表1所示。样本采集后放置于北京市农林科学院植物保护研究所室内养虫室饲养3 d后,选取体型大小一致的成蚜进行毒力测定。
表1 桃蚜和桃粉蚜样本采集信息
Table 1 Sources of green peach aphid(M.persicae)and peach mealy aphid(H.amygdali)
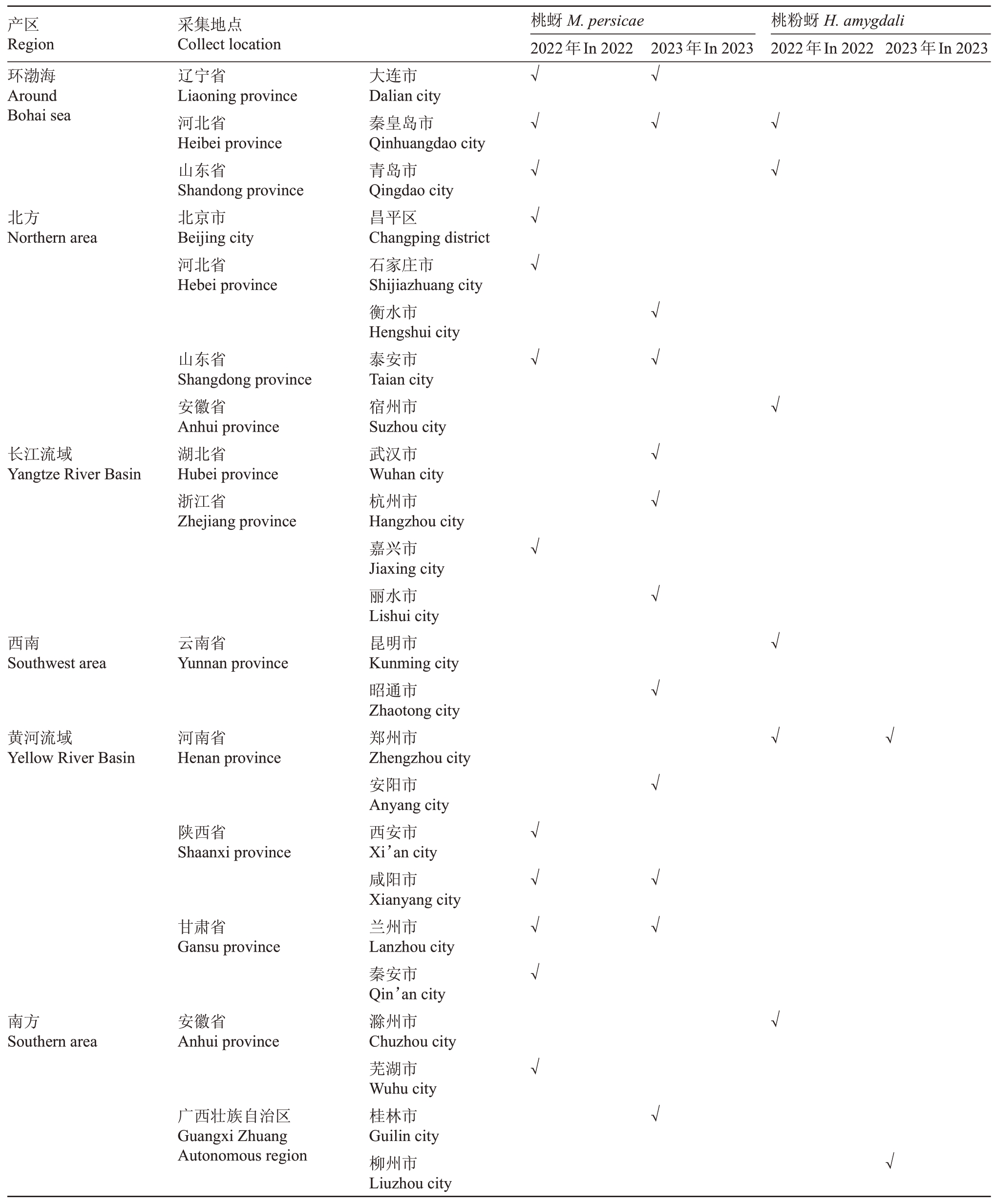
产区Region环渤海Around Bohai sea桃蚜M.persicae 2022年In 2022采集地点Collect location辽宁省Liaoning province河北省Heibei province山东省Shandong province北京市Beijing city河北省Hebei province 2023年In 2023桃粉蚜H.amygdali 2022年In 2022 2023年In 2023√√√√北方Northern area√√√√√√√长江流域Yangtze River Basin山东省Shangdong province安徽省Anhui province湖北省Hubei province浙江省Zhejiang province√西南Southwest area云南省Yunnan province√黄河流域Yellow River Basin河南省Henan province√√陕西省Shaanxi province甘肃省Gansu province√√√√√√√√√南方Southern area安徽省Anhui province√√√√√√广西壮族自治区Guangxi Zhuang Autonomous region大连市Dalian city秦皇岛市Qinhuangdao city青岛市Qingdao city昌平区Changping district石家庄市Shijiazhuang city衡水市Hengshui city泰安市Taian city宿州市Suzhou city武汉市Wuhan city杭州市Hangzhou city嘉兴市Jiaxing city丽水市Lishui city昆明市Kunming city昭通市Zhaotong city郑州市Zhengzhou city安阳市Anyang city西安市Xi’an city咸阳市Xianyang city兰州市Lanzhou city秦安市Qin’an city滁州市Chuzhou city芜湖市Wuhu city桂林市Guilin city柳州市Liuzhou city√√
1.2 供试药剂
吡虫啉原药(96%)采购于河北野田农用化学有限公司,双丙环虫酯原药(97.3%)采购于巴斯夫公司(中国)。
1.3 试验方法
药剂配制:用丙酮溶剂将吡虫啉和双丙环虫酯配置成1000 mg·L-1的母液储存备用。称取104.17 mg吡虫啉原药加入100 mL丙酮配置成1000 mg·L-1的母液。称取102.78 mg双丙环虫酯原药加入100 mL丙酮配置成1000 mg·L-1的母液。
采用玻璃管药膜法进行桃蚜和桃粉蚜对药剂敏感度的检测。将供试药剂吡虫啉和双丙环虫酯用丙酮按照等比序列配置成7个浓度,每个浓度设置3个技术重复。用移液器吸取250 μL 药液转入玻璃管中(长:7.3 cm;底面直径:1.2 cm;内表面积:27.51 cm2),利用微型旋转器旋转玻璃管直至药膜形成。以丙酮处理为对照。每个药膜管中转入20头桃蚜或桃粉蚜。然后将玻璃管放置于养虫室中,温度(25±1)℃,湿度70%,光/暗周期16 h/8 h。24 h后检查死亡率,以毛笔轻触虫体,不能活动者视为死亡。
1.4 数据分析
使用PoloPlus 2.0 软件进行数据分析,得出吡虫啉和双丙环虫酯对桃蚜和桃粉蚜种群的LC50值,并计算95%置信区间。使用卡方值(χ2)进行统计分析,以评估观察值与期望值之间的偏差程度,从而确定药剂对害虫种群的毒力效果。
2 结果与分析
2.1 桃蚜对吡虫啉的敏感度
桃园桃蚜种群对杀虫剂吡虫啉的敏感度如表2所示,2022年度在所有监测的地区中,浙江省嘉兴市的桃蚜种群表现最敏感,其LC50为0.222 mg·L-1,可作为敏感基线,用于分析其他检测地区桃蚜对吡虫啉的敏感度水平。其次为陕西省咸阳市的种群,其LC50为0.388 mg·L-1。相对而言,山东省泰安市和青岛市、河北省石家庄市、北京市昌平区以及甘肃省秦安市的桃蚜种群对吡虫啉处于相对敏感水平,而河北省秦皇岛市、陕西省西安市和甘肃省兰州市的桃蚜种群对吡虫啉的敏感度显著降低。
表2 桃园桃蚜种群对吡虫啉的敏感度
Table 2 The sensitivity of green peach aphid(M.persicae)population to imidacloprid in peach orchard
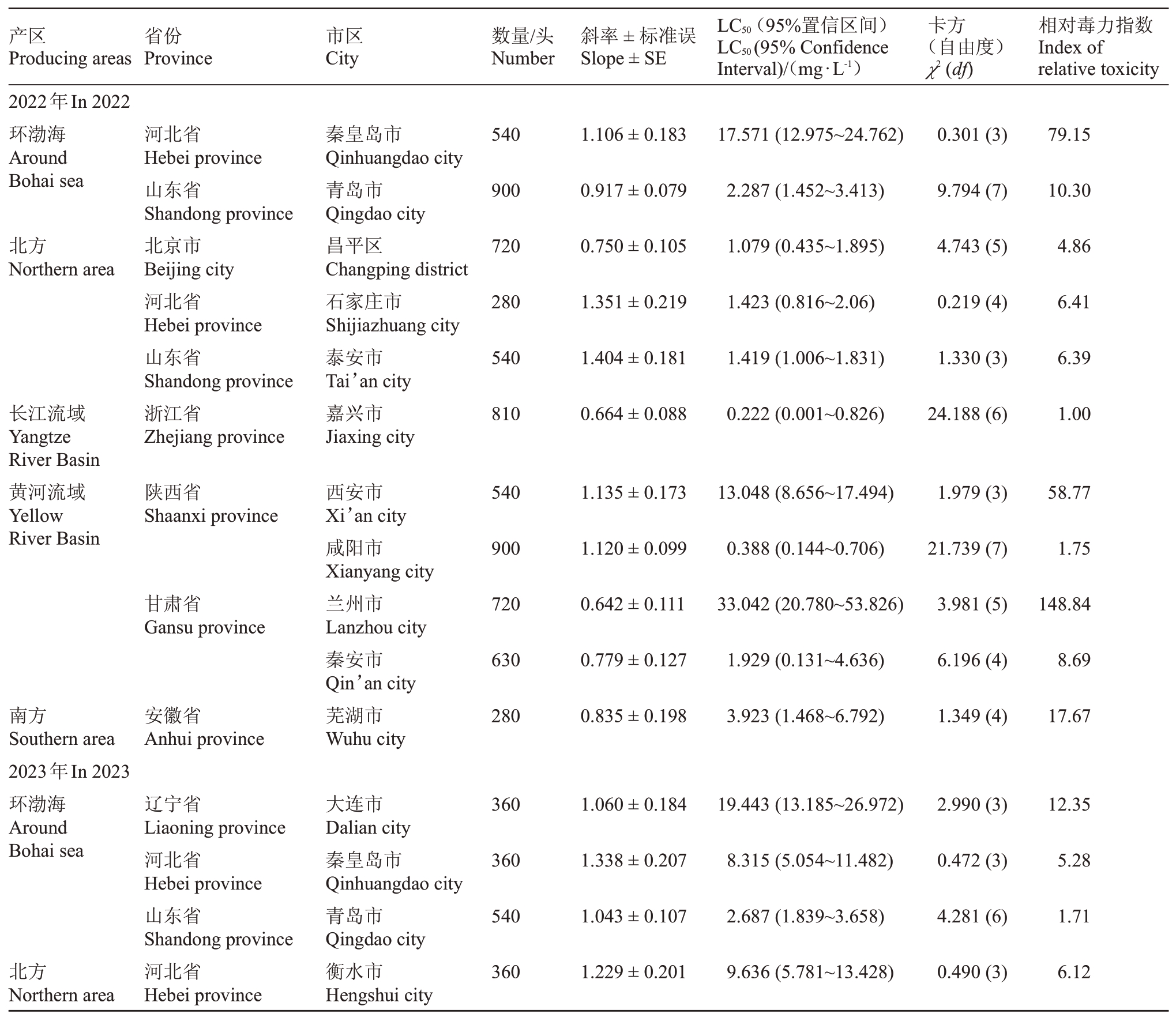
产区Producing areas省份Province市区City数量/头Number斜率±标准误Slope±SE LC50(95%置信区间)LC50(95%Confidence Interval)/(mg·L-1)卡方(自由度)χ2(df)相对毒力指数Index of relative toxicity 2022年In 2022环渤海Around Bohai sea 540 1.106±0.183河北省Hebei province山东省Shandong province北京市Beijing city河北省Hebei province山东省Shandong province浙江省Zhejiang province秦皇岛市Qinhuangdao city青岛市Qingdao city昌平区Changping district石家庄市Shijiazhuang city泰安市Tai’an city嘉兴市Jiaxing city 17.571(12.975~24.762)0.301(3)79.15 900 0.917±0.079 2.287(1.452~3.413)9.794(7)10.30北方Northern area 720 0.750±0.105 1.079(0.435~1.895)4.743(5)4.86 280 1.351±0.219 1.423(0.816~2.06)0.219(4)6.41 540 1.404±0.181 1.419(1.006~1.831)1.330(3)6.39长江流域Yangtze River Basin黄河流域Yellow River Basin 810 0.664±0.088 0.222(0.001~0.826)24.188(6)1.00陕西省Shaanxi province 540 1.135±0.173西安市Xi’an city咸阳市Xianyang city兰州市Lanzhou city秦安市Qin’an city芜湖市Wuhu city 13.048(8.656~17.494)1.979(3)58.77 900 1.120±0.099 0.388(0.144~0.706)21.739(7)1.75甘肃省Gansu province 720 0.642±0.111 33.042(20.780~53.826)3.981(5)148.84 630 0.779±0.127 1.929(0.131~4.636)6.196(4)8.69南方Southern area 2023年In 2023环渤海Around Bohai sea安徽省Anhui province 280 0.835±0.198 3.923(1.468~6.792)1.349(4)17.67大连市Dalian city秦皇岛市Qinhuangdao city青岛市Qingdao city衡水市Hengshui city辽宁省Liaoning province河北省Hebei province山东省Shandong province河北省Hebei province 360 1.060±0.184 19.443(13.185~26.972)2.990(3)12.35 360 1.338±0.207 8.315(5.054~11.482)0.472(3)5.28 540 1.043±0.107 2.687(1.839~3.658)4.281(6)1.71北方Northern area 360 1.229±0.201 9.636(5.781~13.428)0.490(3)6.12
表2 (续) Table 2 (Continued)
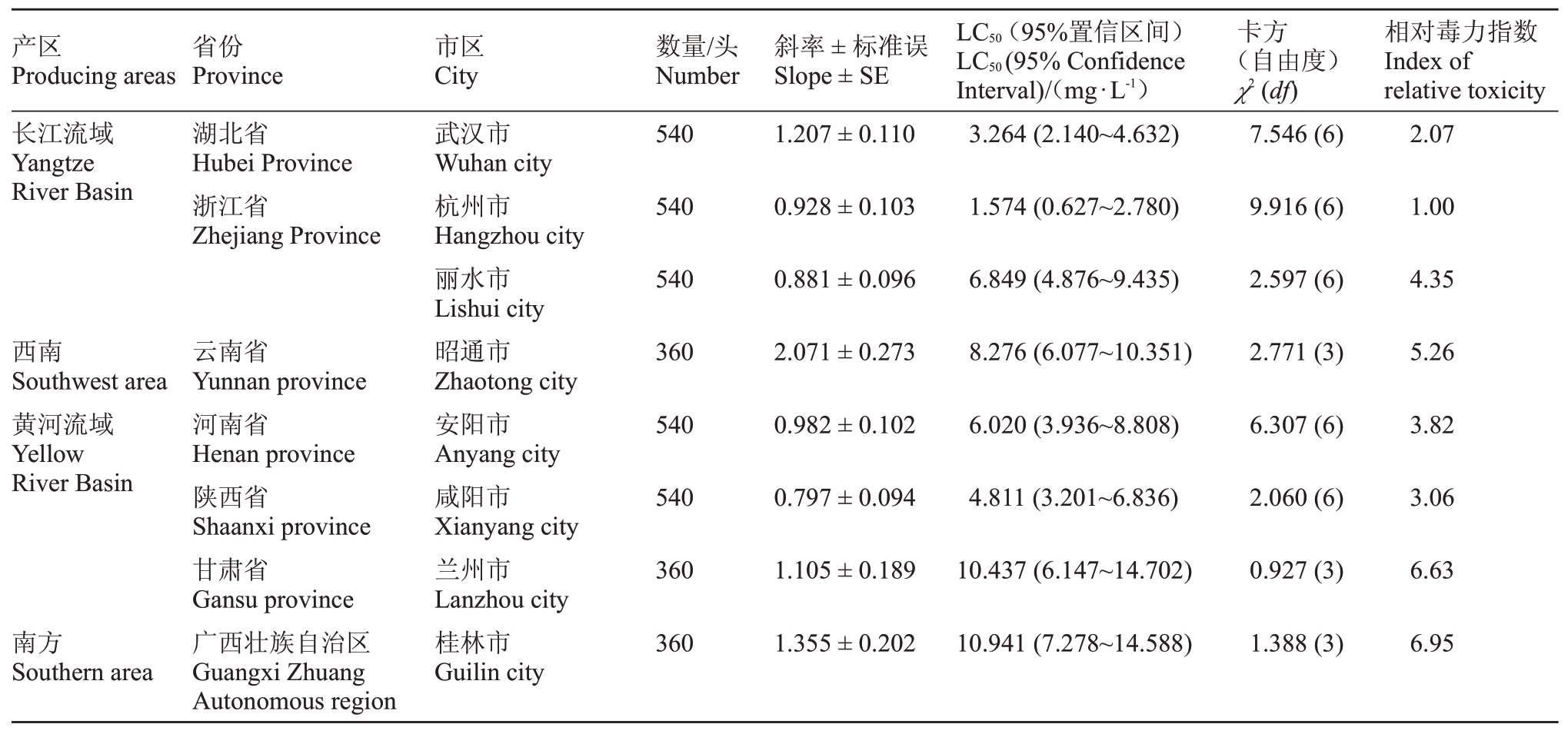
产区Producing areas省份Province市区City数量/头Number斜率±标准误Slope±SE LC50(95%置信区间)LC50(95%Confidence Interval)/(mg·L-1)卡方(自由度)χ2(df)相对毒力指数Index of relative toxicity长江流域Yangtze River Basin湖北省Hubei Province浙江省Zhejiang Province 540 1.207±0.110武汉市Wuhan city杭州市Hangzhou city丽水市Lishui city昭通市Zhaotong city安阳市Anyang city咸阳市Xianyang city兰州市Lanzhou city桂林市Guilin city 3.264(2.140~4.632)7.546(6)2.07 540 0.928±0.103 1.574(0.627~2.780)9.916(6)1.00 540 0.881±0.096 6.849(4.876~9.435)2.597(6)4.35西南Southwest area黄河流域Yellow River Basin 360 2.071±0.273云南省Yunnan province河南省Henan province陕西省Shaanxi province甘肃省Gansu province广西壮族自治区Guangxi Zhuang Autonomous region 8.276(6.077~10.351)2.771(3)5.26 540 0.982±0.102 6.020(3.936~8.808)6.307(6)3.82 540 0.797±0.094 4.811(3.201~6.836)2.060(6)3.06 360 1.105±0.189 10.437(6.147~14.702)0.927(3)6.63南方Southern area 360 1.355±0.202 10.941(7.278~14.588)1.388(3)6.95
2023年度在所有监测地区中,浙江省杭州市的桃蚜种群表现为对吡虫啉最敏感,其LC50 为1.574 mg·L-1,同样可作为敏感基线用于分析其他监测地区的敏感度水平。其次为山东省青岛市的种群,其LC50为2.687 mg·L-1。以浙江省杭州市种群的LC50值为基础,分析判断除辽宁省大连市的桃蚜种群外,其他地区的桃蚜种群对吡虫啉处于相对敏感水平。
通过对同一地区两年的数据分析,笔者发现山东省青岛市桃蚜种群对吡虫啉的敏感度变化较小。相反,河北省秦皇岛市和甘肃省兰州市的桃蚜种群对吡虫啉的敏感度呈现增高的趋势,而陕西省咸阳市桃蚜种群则显示出对吡虫啉的敏感度降低的趋势。
2.2 桃蚜对双丙环虫酯的敏感度
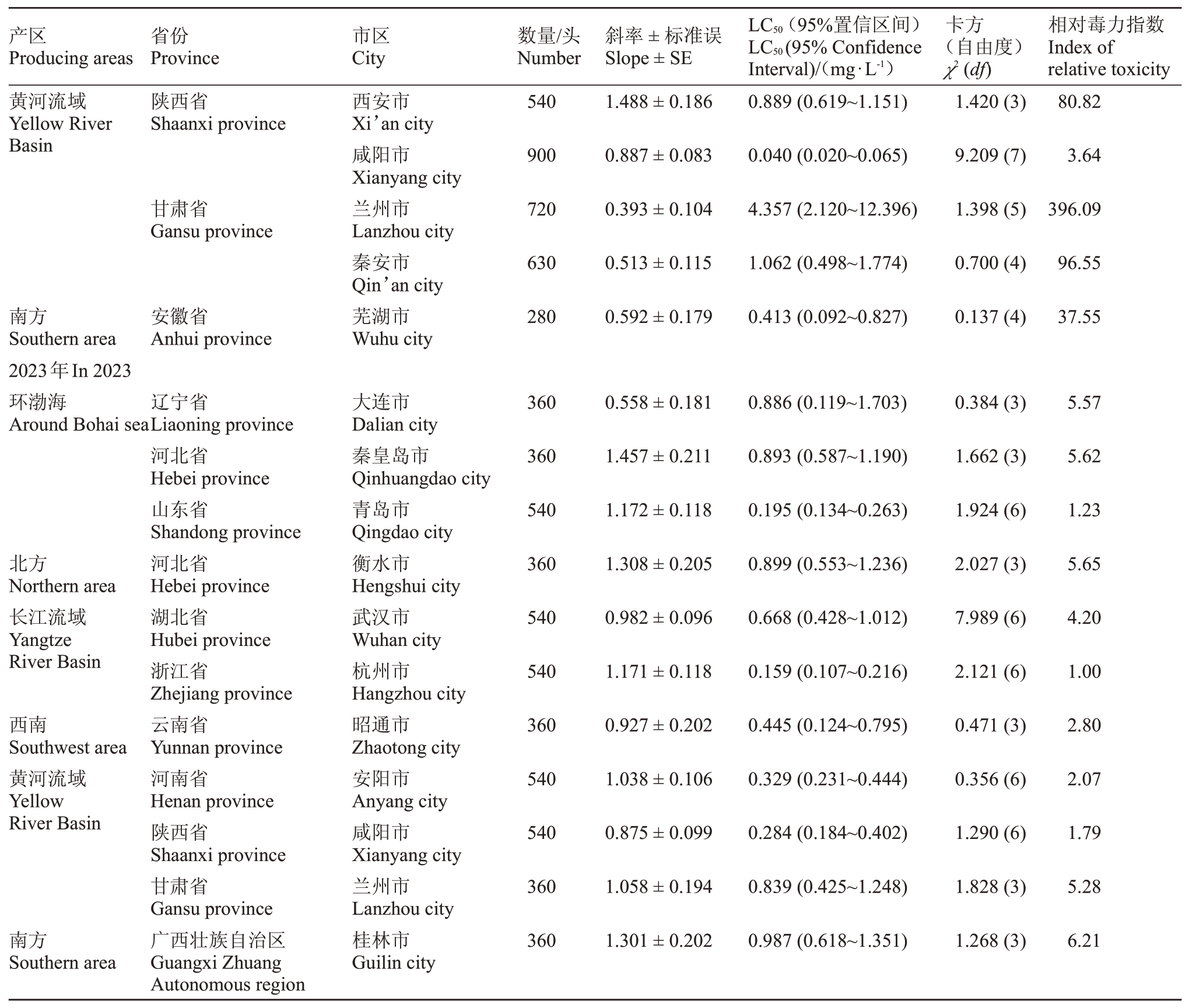
桃园桃蚜种群对杀虫剂双丙环虫酯的敏感度如表3所示,2022年度在所有监测的地区中,河北省秦皇岛市的桃蚜种群对双丙环虫酯最敏感,其LC50为0.011 mg·L-1,可作为敏感基线用于分析其他监测地区桃蚜种群对双丙环虫酯的敏感度水平。其次为陕西省咸阳市和浙江省嘉兴市的桃蚜种群,其LC50分别为0.040 和0.050 mg·L-1。相对而言,甘肃省的桃蚜种群对双丙环虫酯的敏感度不高,其LC50分别为4.357 和1.062 mg·L-1,北京市昌平区的桃蚜种群对双丙环虫酯的敏感度最低,其LC50达到7.819 mg·L-1。
表3 桃园桃蚜种群对双丙环虫酯的敏感度
Table 3 The sensitivity of green peach aphid(M.persicae)population to afidopyropen in peach orchard
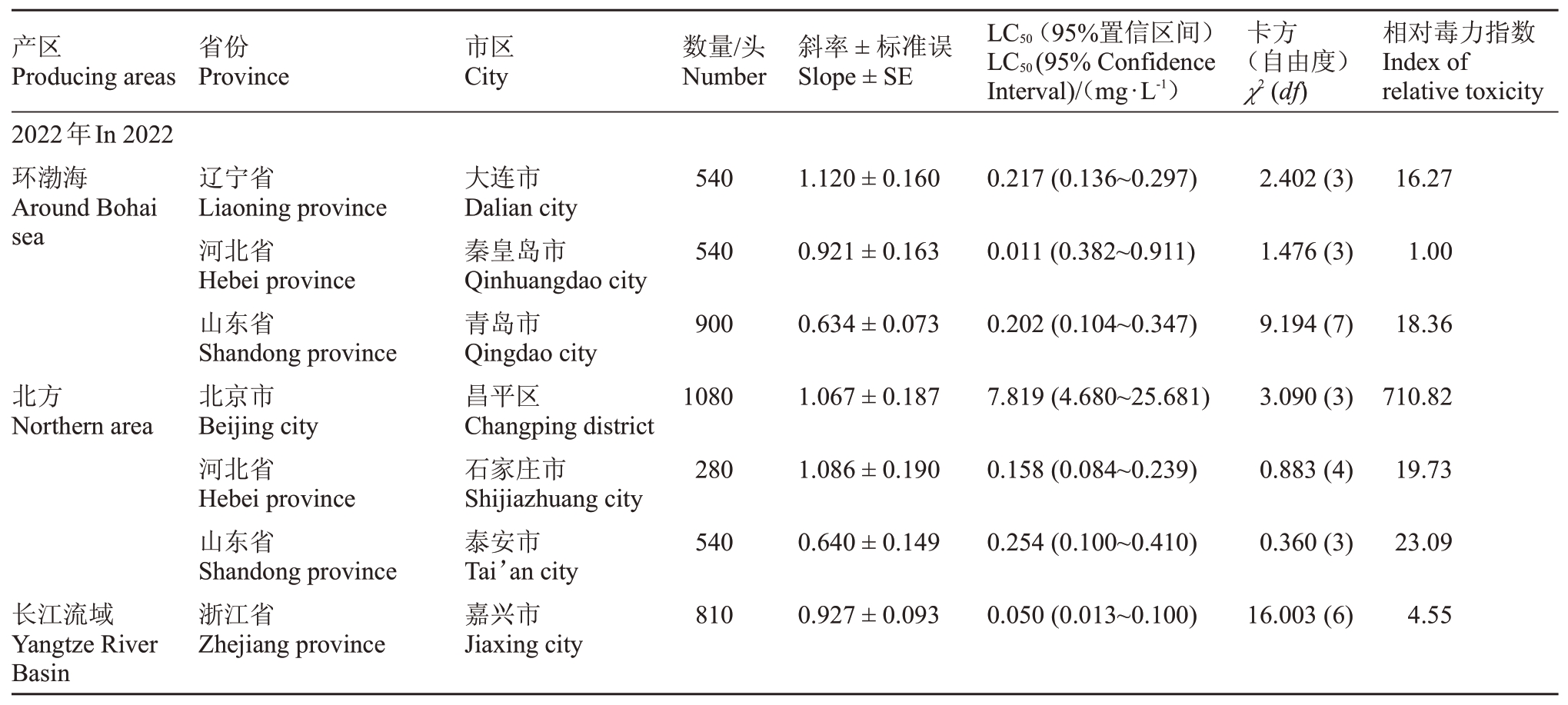
产区Producing areas省份Province市区City数量/头Number斜率±标准误Slope±SE LC50(95%置信区间)LC50(95%Confidence Interval)/(mg·L-1)卡方(自由度)χ2(df)相对毒力指数Index of relative toxicity 2022年In 2022环渤海Around Bohai sea 540 1.120±0.160辽宁省Liaoning province河北省Hebei province山东省Shandong province北京市Beijing city河北省Hebei province山东省Shandong province浙江省Zhejiang province大连市Dalian city秦皇岛市Qinhuangdao city青岛市Qingdao city昌平区Changping district石家庄市Shijiazhuang city泰安市Tai’an city嘉兴市Jiaxing city 0.217(0.136~0.297)2.402(3)16.27 540 0.921±0.163 0.011(0.382~0.911)1.476(3)1.00 900 0.634±0.073 0.202(0.104~0.347)9.194(7)18.36北方Northern area 1080 1.067±0.187 7.819(4.680~25.681)3.090(3)710.82 280 1.086±0.190 0.158(0.084~0.239)0.883(4)19.73 540 0.640±0.149 0.254(0.100~0.410)0.360(3)23.09长江流域Yangtze River Basin 810 0.927±0.093 0.050(0.013~0.100)16.003(6)4.55
表3 (续) Table 3 (Continued)
产区Producing areas省份Province市区City数量/头Number斜率±标准误Slope±SE LC50(95%置信区间)LC50(95%Confidence Interval)/(mg·L-1)卡方(自由度)χ2(df)相对毒力指数Index of relative toxicity黄河流域Yellow River Basin陕西省Shaanxi province 540 1.488±0.186西安市Xi’an city咸阳市Xianyang city兰州市Lanzhou city秦安市Qin’an city芜湖市Wuhu city 0.889(0.619~1.151)1.420(3)80.82 900 0.887±0.083 0.040(0.020~0.065)9.209(7)3.64甘肃省Gansu province 720 0.393±0.104 4.357(2.120~12.396)1.398(5)396.09 630 0.513±0.115 1.062(0.498~1.774)0.700(4)96.55南方Southern area 2023年In 2023环渤海Around Bohai sea安徽省Anhui province 280 0.592±0.179 0.413(0.092~0.827)0.137(4)37.55大连市Dalian city秦皇岛市Qinhuangdao city青岛市Qingdao city衡水市Hengshui city武汉市Wuhan city杭州市Hangzhou city昭通市Zhaotong city安阳市Anyang city咸阳市Xianyang city兰州市Lanzhou city桂林市Guilin city 0.558±0.181 360 0.886(0.119~1.703)0.384(3)5.57 1.662(3)1.924(6)360 540 1.457±0.211 1.172±0.118 0.893(0.587~1.190)0.195(0.134~0.263)5.62 1.23北方Northern area长江流域Yangtze River Basin 360 0.899(0.553~1.236)2.027(3)1.308±0.205 5.65 0.982±0.096 540 0.668(0.428~1.012)辽宁省Liaoning province河北省Hebei province山东省Shandong province河北省Hebei province湖北省Hubei province浙江省Zhejiang province云南省Yunnan province河南省Henan province陕西省Shaanxi province甘肃省Gansu province广西壮族自治区Guangxi Zhuang Autonomous region 7.989(6)4.20 540 1.171±0.118 0.159(0.107~0.216)2.121(6)1.00西南Southwest area黄河流域Yellow River Basin 360 0.927±0.202 0.445(0.124~0.795)0.471(3)2.80 540 1.038±0.106 0.329(0.231~0.444)0.356(6)2.07 540 0.875±0.099 0.284(0.184~0.402)1.290(6)1.79 360 1.058±0.194 0.839(0.425~1.248)1.828(3)5.28南方Southern area 360 1.301±0.202 0.987(0.618~1.351)1.268(3)6.21
2023年度在所有监测地区中,浙江省杭州市的桃蚜种群表现为对双丙环虫酯最为敏感,其LC50为0.159 mg·L-1,同样可作为敏感基线用于分析其他监测地区的敏感度水平。其他监测地区的桃蚜种群对双丙环虫酯也均处于相对敏感水平,LC50在0.195~0.987 mg·L-1之间。
通过对同一地区两年数据的分析,笔者发现山东省青岛市桃蚜种群对双丙环虫酯的敏感度基本无变化,河北省秦皇岛市和陕西省咸阳市的桃蚜种群对双丙环虫酯敏感度有所降低。相反,甘肃省兰州市的桃蚜种群对双丙环虫酯的敏感度呈增高的趋势。
2.3 桃粉蚜对吡虫啉的敏感度
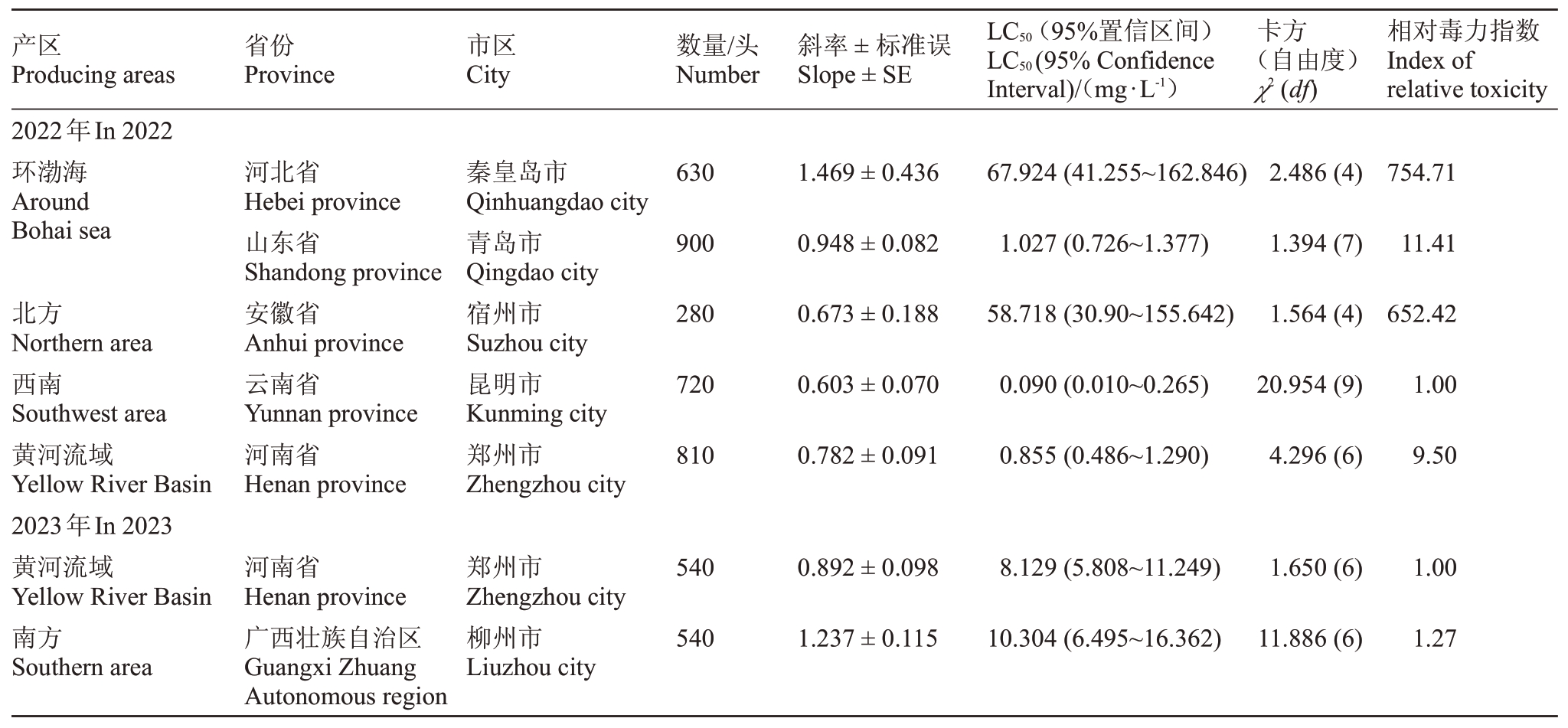
桃粉蚜田间种群对杀虫剂吡虫啉的敏感度如表4 所示,2022年度在所有监测的地区中,云南省昆明市的桃粉蚜种群对吡虫啉最敏感,其LC50为0.090 mg·L-1,以其为敏感基线分析其他监测地区对吡虫啉的敏感度水平。山东省青岛市和河南省郑州市的桃粉蚜种群对吡虫啉仍处于相对敏感水平。河北省秦皇岛市和安徽省宿州市的桃粉蚜种群对吡虫啉的敏感度显著降低,推测其可能产生抗药性。2023年所监测的两个地区的桃粉蚜对吡虫啉也处于相对敏感水平。河南省郑州市和广西壮族自治区柳州市的桃粉蚜种群的LC50值分别为8.129和10.304 mg·L-1。通过对同一地区两年数据的分析,笔者发现河南省郑州市的桃粉蚜种群对吡虫啉的敏感度降低。
表4 桃园桃粉蚜种群对吡虫啉的敏感度
Table 4 The sensitivity of peach mealy aphid(H.amygdali)population to imidacloprid in peach orchard
产区Producing areas 2022年In 2022环渤海Around Bohai sea省份Province市区City数量/头Number斜率±标准误Slope±SE LC50(95%置信区间)LC50(95%Confidence Interval)/(mg·L-1)卡方(自由度)χ2(df)相对毒力指数Index of relative toxicity 1.469±0.436河北省Hebei province山东省Shandong province安徽省Anhui province云南省Yunnan province河南省Henan province秦皇岛市Qinhuangdao city青岛市Qingdao city宿州市Suzhou city昆明市Kunming city郑州市Zhengzhou city 630 67.924(41.255~162.846)2.486(4)754.71 900 0.948±0.082 1.027(0.726~1.377)1.394(7)11.41北方Northern area西南Southwest area黄河流域Yellow River Basin 2023年In 2023黄河流域Yellow River Basin南方Southern area 280 0.673±0.188 58.718(30.90~155.642)1.564(4)652.42 720 0.603±0.070 0.090(0.010~0.265)20.954(9)1.00 810 0.782±0.091 0.855(0.486~1.290)4.296(6)9.50河南省Henan province广西壮族自治区Guangxi Zhuang Autonomous region郑州市Zhengzhou city柳州市Liuzhou city 540 0.892±0.098 8.129(5.808~11.249)1.650(6)1.00 540 1.237±0.115 10.304(6.495~16.362)11.886(6)1.27
2.4 桃粉蚜对双丙环虫酯的敏感度
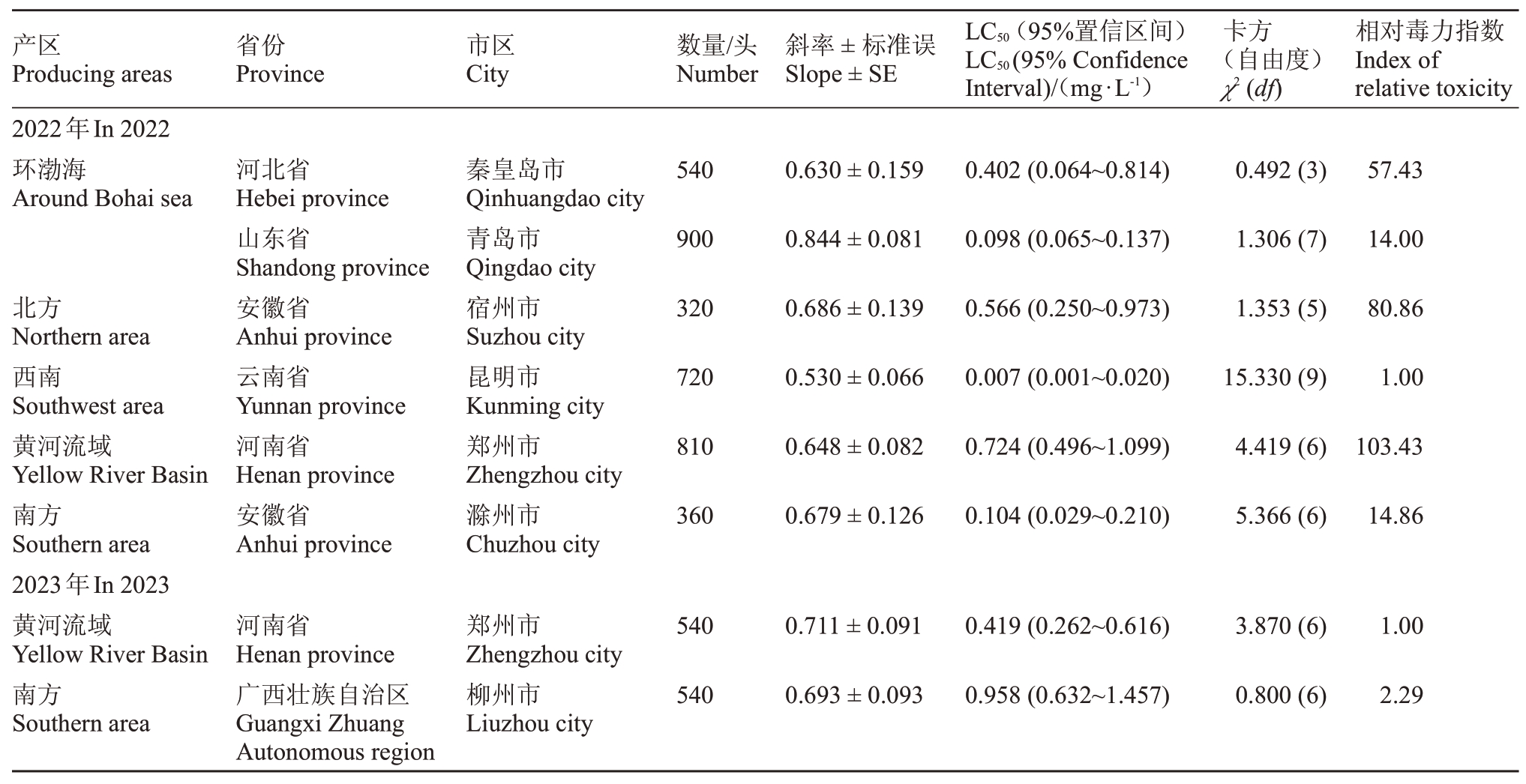
桃粉蚜田间种群对杀虫剂双丙环虫酯的敏感度如表5所示,2022年度在所有监测的地区中,云南省昆明市的桃粉蚜种群对双丙环虫酯最敏感,LC50为0.007 mg·L-1,以其为敏感基线分析其他监测地区对双丙环虫酯的敏感度水平。其次为山东省青岛市的种群,LC50为0.098 mg·L-1。其他监测地区的桃粉蚜种群对双丙环虫酯也均处于相对敏感水平,LC50在0.104~0.724 mg·L-1之间。2023年度在两个监测地区中,河南省郑州市和广西壮族自治区柳州市的桃粉蚜种群对双丙环虫酯均处于敏感水平,其LC50值分别为0.419和0.958 mg·L-1。河南省郑州市连续两年进行采样监测,监测数据表明,该地区桃粉蚜对双丙环虫酯敏感度变化不大。
表5 桃园桃粉蚜种群对双丙环虫酯的敏感度
Table 5 The sensitivity of peach mealy aphid(H.amygdali)population to afidopyropen in peach orchard
产区Producing areas 2022年In 2022环渤海Around Bohai sea省份Province市区City数量/头Number斜率±标准误Slope±SE LC50(95%置信区间)LC50(95%Confidence Interval)/(mg·L-1)卡方(自由度)χ2(df)相对毒力指数Index of relative toxicity 0.630±0.159河北省Hebei province山东省Shandong province安徽省Anhui province云南省Yunnan province河南省Henan province安徽省Anhui province秦皇岛市Qinhuangdao city青岛市Qingdao city宿州市Suzhou city昆明市Kunming city郑州市Zhengzhou city滁州市Chuzhou city 540 0.402(0.064~0.814)0.492(3)57.43 900 0.844±0.081 0.098(0.065~0.137)1.306(7)14.00北方Northern area西南Southwest area黄河流域Yellow River Basin南方Southern area 2023年In 2023黄河流域Yellow River Basin南方Southern area 320 0.686±0.139 0.566(0.250~0.973)1.353(5)80.86 720 0.530±0.066 0.007(0.001~0.020)15.330(9)1.00 810 0.648±0.082 0.724(0.496~1.099)4.419(6)103.43 360 0.679±0.126 0.104(0.029~0.210)5.366(6)14.86河南省Henan province广西壮族自治区Guangxi Zhuang Autonomous region郑州市Zhengzhou city柳州市Liuzhou city 540 0.711±0.091 0.419(0.262~0.616)3.870(6)1.00 540 0.693±0.093 0.958(0.632~1.457)0.800(6)2.29
3 讨论
监测害虫对常用药剂的敏感度不仅可以为害虫的高效防控提供关键信息,还有助于合理选择农药并进行科学管理[19]。这一过程对理解害虫对药剂抗性的演变尤为重要。通过对敏感度变化趋势的详细分析,能够及早地发现抗性问题,并据此为制定长期、可持续的防治策略提供坚实的科学依据。作为一种广泛危害十字花科蔬菜和桃树的害虫,桃蚜的繁殖周期短且快速,这加速了其对药剂的抗药性发展[20-21]。因此,在药剂的选择和使用方法上,不仅要求对靶标害虫的快速有效作用,还需要考虑药剂的时效性和使用的便捷性。由此可见,对桃蚜药剂敏感度的监测需要快速有效的检测方法。
笔者连续两年对我国主要桃产区的桃蚜和桃粉蚜种群对常用药剂吡虫啉和双丙环虫酯的敏感度进行监测。结果表明,桃蚜种群对吡虫啉的敏感度在不同地区之间存在显著差异。2022年浙江省嘉兴市的桃蚜种群对吡虫啉的敏感度最高。相比之下,河北省秦皇岛市、陕西省西安市和甘肃省兰州市的桃蚜种群则表现出较低的敏感性。此外,桃粉蚜的监测结果表明,河北省秦皇岛市和安徽省宿州市的种群对吡虫啉的敏感度显著降低。刘俊丽[21]对我国9 个地区桃蚜田间种群对吡虫啉的抗药性进行监测,结果表明,桃蚜种群的整体抗性水平存在地区差异。不同地区桃蚜种群对药剂敏感度差异可能受到多种因素的影响,包括地理、环境和人为因素等。首先,地理和环境因素可能对桃蚜种群的生态学特征产生影响,从而影响其对药剂的敏感度。其次,不同地区的用药历史和农业实践也可能导致种群对药剂的敏感度差异。吡虫啉作为一种兼具高效性和广谱性的新烟碱类杀虫剂,在我国已有近20年的田间推广使用历史,其过量和频繁使用可能导致了多种靶标害虫对其产生不同程度的抗药性。宫亚军等[4]在2011年对北京地区不同桃蚜种群的抗药性进行了研究,发现不同地区的桃蚜对吡虫啉均产生了高倍抗性。这表明长期的杀虫剂使用可能导致抗性基因在某些地区的桃蚜种群中积累,从而降低了对特定杀虫剂的敏感性。此外,交互抗性也可能导致害虫对其他类似结构或相似作用机制的杀虫剂的敏感度降低或抗药性的产生。因此,针对本研究中桃蚜对吡虫啉敏感度较低的地区,应明确是否存在靶标基因突变导致敏感度显著降低的情况。此外,为降低桃蚜或桃粉蚜对吡虫啉产生抗性的风险,建议轮换使用不同作用机制的杀虫剂,这不仅有助于持续有效的害虫管理,也有助于减少对环境的负面影响。
双丙环虫酯自2019年在中国登记后被广泛应用于对蚜虫和粉虱等刺吸式口器害虫的防治中。为了提高药剂的防控效果,延缓抗药性的发生,科研人员已开始监测蚜虫和粉虱田间种群对双丙环虫酯的敏感度。研究结果表明,我国棉蚜田间种群对双丙环虫酯处于敏感水平,但敏感度呈现逐年下降趋势[22-23]。相对而言,烟粉虱的监测结果则显示北京市海淀区的种群已对双丙环虫酯产生约40 倍的抗性[24]。在此基础上进行抗药性机制的分析研究,初步推断细胞色素P450 酶可能参与抗性的形成[25]。本研究结果表明,除北京市昌平区和甘肃省兰州市的桃蚜种群外,其余地区的桃蚜和桃粉蚜种群对双丙环虫酯的LC50 值均小于1.0 mg·L-1。北京市昌平区的桃蚜种群对双丙环虫酯的LC50 值高达7.819 mg·L-1,明显高于其他监测地区。结合烟粉虱的监测结果分析,笔者推断北京地区双丙环虫酯的药剂使用频次较高,这可能导致该地区的靶标害虫蚜虫和粉虱对其敏感度下降,且产生一定的抗药性。后续应加强对重点区域桃蚜种群的监测,以便及时发现抗药性的发展趋势。这些研究结果对制定有效的抗药性管理策略至关重要,通过持续的监测和分析,可以更好地了解抗药性发展的模式,从而有助于减缓抗药性的发展速度。
本研究结果揭示了桃蚜和桃粉蚜种群对常用杀虫剂的敏感性存在显著的地域和年份差异。这些差异可能源于多种因素,包括各地区的杀虫剂使用历史、不同的环境因素以及种群间的遗传多样性。鉴于这些影响因素的复杂性,制订害虫防控策略时,应综合考虑这些变量,以便采取更为有效和针对性的控制措施,不仅有助于提高防治效果,还有助于减缓抗性的发展速度。
4 结论
本研究结果表明,陕西省咸阳市和河南省郑州市的桃蚜或桃粉蚜种群对吡虫啉的敏感度呈现逐渐降低的趋势。除北京市昌平区和甘肃省兰州市的桃蚜种群外,其余地区的桃蚜或桃粉蚜对双丙环虫酯均处于敏感水平。对于敏感度降低的地区,建议加强对重点区域桃蚜种群的监测,同时轮换使用不同作用机制的杀虫剂。
致谢:感谢国家桃产业技术体系的大力支持!文中测试虫源由国家桃产业技术体系相关综合试验站协助提供。
[1] 王力荣.我国桃产业现状与发展建议[J].中国果树,2021(10):1-5.WANG Lirong. Current situation and development suggestions of peach industry in China[J].China Fruits,2021(10):1-5.
[2] 宫庆涛,李桂祥,李素红,武海斌,姜莉莉,孙瑞红,张安宁.桃树蚜虫调查及药效与安全性评价[J].农学学报,2022,12(3):23-29.GONG Qingtao,LI Guixiang,LI Suhong,WU Haibin,JIANG Lili,SUN Ruihong,ZHANG Anning. Investigation on peach aphids and study on insecticides' control efficacy and safety for natural enemies[J].Journal of Agriculture,2022,12(3):23-29.
[3] 闫乐乐,卜璐璐,牛良,曾文芳,鲁振华,崔国朝,苗玉乐,潘磊,王志强.广泛靶向代谢组学解析桃蚜危害对桃树次生代谢产物的影响[J].中国农业科学,2022,55(6):1149-1158.YAN Lele,BU Lulu,NIU Liang,ZENG Wenfang,LU Zhenhua,CUI Guochao,MIAO Yule,PAN Lei,WANG Zhiqiang. Widely targeted metabolomics analysis of the effects of Myzus persicae feeding on Prunus persica secondary metabolites[J]. Scientia Agricultura Sinica,2022,55(6):1149-1158.
[4] 宫亚军,王泽华,石宝才,康总江,朱亮,郭晓军,刘建华,魏书军.北京地区不同桃蚜种群的抗药性研究[J].中国农业科学,2011,44(21):4385-4394.GONG Yajun,WANG Zehua,SHI Baocai,KANG Zongjiang,ZHU Liang,GUO Xiaojun,LIU Jianhua,WEI Shujun. Resistance status of Myzus persicae (Sulzer) (Hemiptera:Aphididae)populations to pesticide in Beijing[J]. Scientia Agricultura Sinica,2011,44(21):4385-4394.
[5] 高希武,郑炳宗,曹本钧.北京地区桃蚜抗药性研究初报[J].农药,1991,30(2):37-39.GAO Xiwu,ZHENG Bingzong,CAO Benjun.Preliminary report on the study of peach aphid resistance in the Beijing Area[J].Pesticides,1991,30(2):37-39.
[6] 顾春波,王开运,辛海军,郭庆龙,王刚. 山东省主要烟区烟(桃)蚜[Myzus persicae (Sulzer)]抗药性研究[J]. 中国烟草学报,2005,11(4):21-23.GU Chunbo,WANG Kaiyun,XIN Haijun,GUO Qinglong,WANG Gang. Study on the resistance of Myzus persicae (Sulzer)in major tobacco planting areas in Shandong province[J].Acta Tabacaria Sinica,2005,11(4):21-23.
[7] 宋春满,吴兴富,邓建华,雷朝亮.云南主要烟区烟蚜抗药性的监测[J].昆虫知识,2006,43(4):500-503.SONG Chunman,WU Xingfu,DENG Jianhua,LEI Chaoliang.Monitoring insecticide resistance of Myzus persicae in tobacco fields in Yunnan[J]. Chinese Bulletin of Entomology,2006,43(4):500-503.
[8] 郭媛,郭一凡,申红英,张云毅.苜蓿田喷施高剂量吡虫啉对蜜蜂采集行为的影响[J].中国植保导刊,2023,43(11):15-19.GUO Yuan,GUO Yifan,SHEN Hongying,ZHANG Yunyi. Effects of high doses of imidacloprid sprayed in alfalfa fields on honey bees foraging[J]. China Plant Protection,2023,43(11):15-19.
[9] QU Y Y,XIAO D,LI J Y,CHEN Z,BIONDI A,DESNEUX N,GAO X W,SONG D L.Sublethal and hormesis effects of imidacloprid on the soybean aphid Aphis glycines[J]. Ecotoxicology,2015,24(3):479-487.
[10] 吴世雄,王晓军.吡虫啉在我国的生产与应用[J].农药科学与管理,1999,20(1):37-38.WU Shixiong,WANG Xiaojun. Production and application of imidacloprid in China[J]. Pesticide Science and Administration,1999,20(1):37-38.
[11] BASS C,PUINEAN A M,ANDREWS M,CUTLER P,DANIELS M,ELIAS J,PAUL V L,CROSSTHWAITE A J,DENHOLM I,FIELD L M,FOSTER S P,LIND R,WILLIAMSON M S,SLATER R. Mutation of a nicotinic acetylcholine receptor β subunit is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae[J].BMC Neuroscience,2011,12:51.
[12] PUINEAN A M,FOSTER S P,OLIPHANT L,DENHOLM I,FIELD L M,MILLAR N S,WILLIAMSON M S,BASS C.Amplification of a cytochrome P450 gene is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae[J].PLoS Genetics,2010,6(6):e1000999.
[13] HORIKOSHI R,GOTO K,MITOMI M,OYAMA K,HIROSE T,SUNAZUKA T,ŌMURA S. Afidopyropen,a novel insecticide originating from microbial secondary extracts[J]. Scientific Reports,2022,12(1):2827.
[14] KOCH R L,DA SILVA Q O,AITA R C,HODGSON E W,POTTER B D,NYOIKE T,ELLERS-KIRK C D. Efficacy of afidopyropen against soybean aphid (Hemiptera:Aphididae) and toxicity to natural enemies[J]. Pest Management Science,2020,76(1):375-383.
[15] KUMAR V,MCKENZIE C L,OSBORNE L S. Effect of foliar application of afidopyropen on Bemisia tabaci and Amblyseius swirskii,2018[J]. Arthropod Management Tests,2018,43(1):tsy071.
[16] KANDASAMY R,LONDON D,STAM L,VON DEYN W,ZHAO X L,SALGADO V L,NESTEROV A. Afidopyropen:New and potent modulator of insect transient receptor potential channels[J]. Insect Biochemistry and Molecular Biology,2017,84:32-39.
[17] LEICHTER C A,THOMPSON N,JOHNSON B R,SCOTT J G. The high potency of ME-5343 to aphids is due to a unique mechanism of action[J].Pesticide Biochemistry and Physiology,2013,107(2):169-176.
[18] ZHANG Z,SHI H J,XU W,LIU J T,GENG Z Q,CHU D,GUO L. Pymetrozine-resistant whitefly Bemisia tabaci (Gennadius)populations in China remain susceptible to afidopyropen[J].Crop Protection,2021,149:105757.
[19] 陈黔,杨朗,黄立飞,曹雪梅,张建民,姜建军.瓜实蝇成虫对不同杀虫剂相对敏感基线的建立[J].中国瓜菜,2023,36(4):106-112.CHEN Qian,YANG Lang,HUANG Lifei,CAO Xuemei,ZHANG Jianmin,JIANG Jianjun. The relative sensitivity baseline of Zeugodacus cucurbitae (Diptera:Tephritidae) adults to different insecticides[J]. China Cucurbits and Vegetables,2023,36(4):106-112.
[20] 汤秋玲.我国桃蚜田间种群抗药性和遗传变异研究[D].北京:中国农业大学,2015.TANG Qiuling. Insecticide resistance and genetic variation of field population of Myzus persicae (Sulzer),in China[D]. Beijing:China Agricultural University,2015.
[21] 刘俊丽.我国桃蚜抗药性机制及检测[D].北京:中国农业大学,2010.LIU Junli. Insecticide resistance mechanisms and detection of the peach-potato aphid,Myzus persicae,in China[D]. Beijing:China Agricultural University,2010.
[22] LI R,CHENG S H,LIANG P Z,CHEN Z B,ZHANG Y J,LIANG P,ZHANG L,GAO X W.Status of the resistance of Aphis gossypii Glover,1877 (Hemiptera:Aphididae) to afidopyropen originating from microbial secondary metabolites in China[J].Toxins,2022,14(11):750.
[23] SHI D D,LIANG P Z,ZHANG L,LV H X,GAO X W,YOU H,LI J H,MA K S. Susceptibility baseline of Aphis gossypii Glover (Hemiptera:Aphididae) to the novel insecticide afidopyropen in China[J].Crop Protection,2022,151:105834.
[24] WANG R,GAO B L,CHE W N,QU C,ZHOU X,LUO C.First report of field resistance to afidopyropen,the novel pyropene insecticide,on Bemisia tabaci Mediterranean (Q Biotype) from China[J].Agronomy,2022,12(3):724.
[25] WANG R,ZHANG Q H,ZHOU X,ZHANG M,YANG Q Y,SU Q,LUO C.Characterization of field-evolved resistance to afidopyropen,a novel insecticidal toxin developed from microbial secondary metabolites,in Bemisia tabaci[J].Toxins,2022,14(7):453.