树体矮化容易实现果园的机械化、集约化管理,可以增加种植密度,提高单位面积果实产量和土地利用率,对果树生产具有重要意义。在果树栽培中,控制树体大小最常用的就是使用矮化砧木。通过应用矮化砧木实现矮化集约栽培已成为世界苹果栽培的主要趋势,而优良矮化砧木是实现这一模式的基础[1]。
世界苹果生产国都非常重视矮化砧木的选育,如英国东茂林试验站选育出的M 系和MM 系矮化和半矮化砧木[2-3],美国康奈尔大学几内瓦试验站选育的G系矮化砧木[4],前苏联米丘林大学选育的B系抗寒半矮化和矮化砧木[2],波兰选育的P 系矮化砧木[4],日本选育的JM 系矮化砧木[5],中国选育的GM系抗寒矮化砧木、SH 系半矮化砧木、冀砧系列矮化砧木等[6-8]。尽管国内外选育了多种矮化砧木,但受知识产权保护、适应性、繁育技术等因素影响,长期以来中国苹果产业应用矮化砧木品种仍以M 系砧木为主,相对单一,在应对多样、不利的条件中,难以满足生产需求,亟须选育一批抗逆性、适应性强,生产性能好且具有自主知识产权的苹果优良砧木品种,丰富中国砧木品种结构,促进矮砧集约栽培模式的应用,保证苹果产业健康发展。
苹果受基因型和遗传背景差异影响,不同品种的叶片再生和不定梢生根难易不同[9-10]。碳源是对叶片再生率影响较大的因素之一,研究表明,M9-T337[11]、山丁子砧木[12]及富士品种[13]等对碳源要求严格,三者仅在碳源为D-山梨醇时有较高的不定芽再生率,砧木54-118[14]和品种鲁丽[15]在蔗糖和D-山梨醇上都可以获得较高不定梢再生率,但以加D-山梨醇的培养基再生率最高,D-山梨醇更有利于提高砧木BP-176的叶片不定梢再生率[16],蔗糖能够诱导多数砧木品种,如M7A、M26、平邑甜茶和G.41的不定梢再生[17-19]。生长素(主要是吲哚丁酸IBA、1-萘乙酸NAA、吲哚-3-乙酸IAA)在苹果根诱导中是必需的激素成分[10]。在苹果砧木的离体生根诱导中以IBA 最常用[20-22],也有IBA 和NAA 共同使用的报道[3]。
鲁砧1号是山东省果树研究所以M9×60-160杂交育成的苹果半矮化砧木新品种,该砧木具有生长势中庸、半矮化、易生根、抗寒性强、与栽培品种嫁接亲和性好的特点,获农业农村部植物新品种权。笔者在本研究中拟建立鲁砧1号砧木新品种离体叶片高效不定梢再生技术体系,为苹果矮化栽培优良苗木的快速繁殖奠定技术基础,并为苹果矮化砧遗传改良提供技术参考。
1 材料和方法
1.1 材料
山东省果树研究所选育的半矮化砧木新品种鲁砧1号,压条繁殖自根树,5年生,定植于山东省泰安市岱岳区天平湖苹果试验基地(117.03°E,36.23°N),常规管理。
1.2 方法
1.2.1 无菌苗的培养 4 月中下旬,剪取正在生长的鲁砧1 号半木质化健康新梢(图1-A),去掉叶片,把枝条剪成一芽一段的枝段,按孙清荣等[16]杀菌流程对枝段进行表面杀菌,杀菌后的芽段种植于装有腋芽启动培养基MS + 1 mg·L-1 6-苄基氨基嘌呤(BA)+0.2 mg·L-1 IBA 的试管内,每管1 个芽段,放培养室培养,促进腋芽萌发。培养室昼夜温度为(25±2)℃,光照周期为14 h·d-1。除特别说明外,所有处理的培养条件都相同。

图1 苹果半矮化砧木品种鲁砧1 号离体无菌苗培养及增殖
Fig.1 Establishment of micropropagation of aseptic plantlets of semi-dwarfing apple rootstock Luzhen 1
A.剪取的半木质化枝条;B.腋芽萌发;C.无菌苗增殖。
A.Semi-lignified shoots;B.Outgrowth of the axillary buds;C.In vitro shoots proliferation.
1.2.2 无菌苗的增殖 芽段在启动培养基上培养获得的腋芽萌发生长的绿苗,即是离体无菌苗,无菌苗用作进一步增殖培养的材料。无菌苗转移到增殖培养基MS+0.5 mg·L-1 BA+0.05 mg·L-1 IBA上进行继代增殖培养,4~5 周继代1 次,继代3 次后获得叶片培养所用足够试材。
1.2.3 离体叶片不定芽的诱导 根据叶片大小,切3~5刀。切伤的叶片接种于叶片不定芽诱导培养基上,先置连续黑暗条件下培养4周,然后转光周期为14 h·d-1的光照条件下继续培养2 周,共诱导培养6周后,观察计数产生不定芽(梢)叶片数及每叶不定芽(梢)数,计算不定芽再生率和平均每叶不定芽(梢)数。不定芽诱导培养基设两种碳源D-山梨醇和蔗糖,每一碳源分别添加细胞分裂素(TDZ)(0.6和1 mg·L-1)和BA(2 和4 mg·L-1),共构成8 个处理(表1),所有处理都添加0.3 mg·L-1 IBA。
表1 不同不定芽诱导培养基对苹果半矮化砧木新品种鲁砧1 号离体叶片不定芽再生的影响
Table 1 The effect of different bud induction media on bud regeneration from leaf explants
of apple semi-dwarfing rootstock new cultivar Luzhen 1
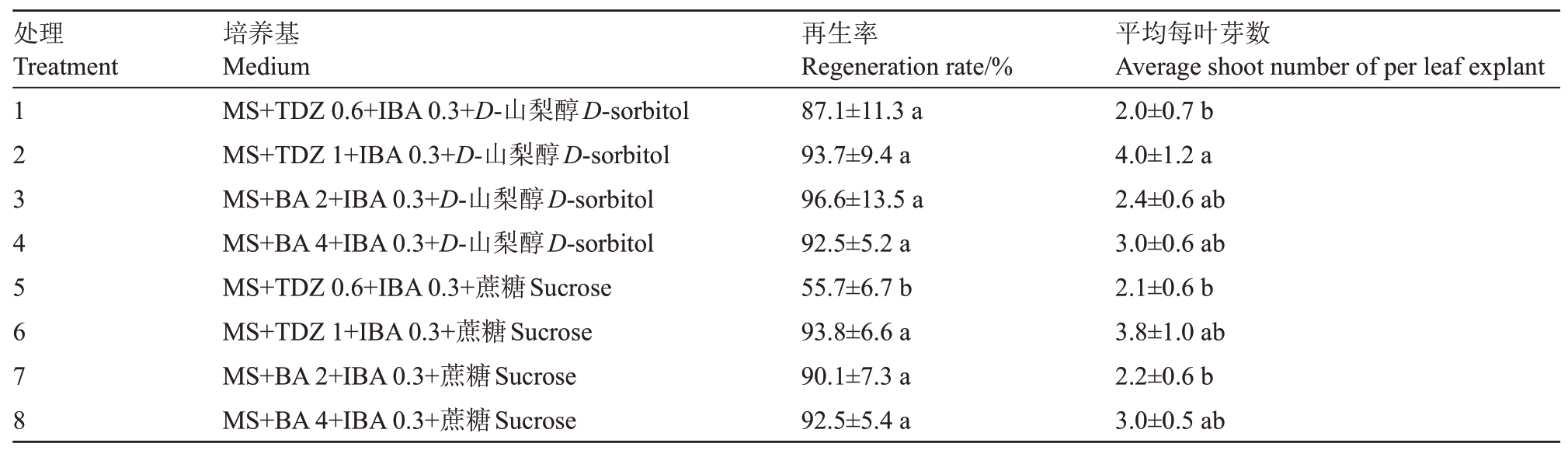
注:培养基使用激素单位均为mg·L-1;D-山梨醇和蔗糖质量浓度均为30 g·L-1;再生率和平均每叶芽均为均值±标准差。
Note:The unit of hormones used in medium was mg·L-1; the concentrations of sorbitol and sucrose used in medium were 30 g·L-1; the regeneration rates and average shoot number per leaf explant were showed with mean±standard deviation.
平均每叶芽数Average shoot number of per leaf explant 2.0±0.7 b 4.0±1.2 a 2.4±0.6 ab 3.0±0.6 ab 2.1±0.6 b 3.8±1.0 ab 2.2±0.6 b 3.0±0.5 ab处理Treatment 12345678培养基Medium MS+TDZ 0.6+IBA 0.3+D-山梨醇D-sorbitol MS+TDZ 1+IBA 0.3+D-山梨醇D-sorbitol MS+BA 2+IBA 0.3+D-山梨醇D-sorbitol MS+BA 4+IBA 0.3+D-山梨醇D-sorbitol MS+TDZ 0.6+IBA 0.3+蔗糖Sucrose MS+TDZ 1+IBA 0.3+蔗糖Sucrose MS+BA 2+IBA 0.3+蔗糖Sucrose MS+BA 4+IBA 0.3+蔗糖Sucrose再生率Regeneration rate/%87.1±11.3 a 93.7±9.4 a 96.6±13.5 a 92.5±5.2 a 55.7±6.7 b 93.8±6.6 a 90.1±7.3 a 92.5±5.4 a
1.2.4 不定芽增殖伸长培养和不定梢生根诱导 将叶片产生的不定芽(梢)转移到继代增殖培养基上进行增殖和伸长生长培养,获得足够用以生根培养的健壮生长的不定梢绿苗。选取高度在1.5 cm以上的不定梢转移到生根培养基上,置连续黑暗条件下诱导培养5 d,然后转光下培养,生根培养20 d,计数统计不定梢生根率及单株生根条数。生根培养基设基本培养基1/4MS 和1/2MS,蔗糖设两个质量浓度20 g·L-1和30 g·L-1,IBA设两个质量浓度0.3 mg·L-1和0.5 mg·L-1,共构成8种处理。
1.3 统计分析
试验结果采用DPS v3.01 软件进行统计分析,不同处理间的平均值用最小显著性差异LSD 法进行差异性分析比较。
2 结果与分析
2.1 无菌苗的培养和增殖
将半木质化新梢芽段在腋芽启动培养基上培养3 周,腋芽萌发并伸长生长形成无菌绿苗(图1-B)。无菌绿苗在继代增殖培养基上生长良好,既表现出良好的增殖生长,又有良好的伸长生长,月增殖倍数超过5.0(图1-C),表明鲁砧1号容易增殖扩繁。
2.2 离体叶片不定芽诱导
2.2.1 碳源对叶片不定芽再生的影响 供试的两种碳源D-山梨醇和蔗糖对诱导不定芽再生都有效,除了TDZ 在较低质量浓度0.6 mg·L-1时,D-山梨醇上的不定芽再生率显著高于蔗糖外,其他处理之间的不定芽再生率两种碳源之间差异不显著,都超过90%(表1)。平均每叶不定芽数,在相同细胞分裂素种类和浓度条件下,两种碳源之间差异不显著。这些结果表明新优系砧木鲁砧1号离体叶片不定芽再生对碳源要求不严格。
2.2.2 细胞分裂素TDZ和BA及其质量浓度对叶片不定芽再生的影响 在同一碳源条件下,TDZ 在较低质量浓度0.6 mg·L-1时,获得的不定芽再生率和平均每叶芽数最低,两种碳源上结果一致(表1)。
当培养基中的碳源为D-山梨醇时,TDZ 和BA之间及其不同质量浓度之间,不定芽再生率都没有达到显著差异水平(处理1~4),但当TDZ 质量浓度为1 mg·L-1时,平均每叶不定芽数显著高于质量浓度0.6 mg·L-1 TDZ时,而在BA的两个质量浓度之间差异不显著。
当培养基中碳源为蔗糖时,TDZ 在较低质量浓度0.6 mg·L-1时的再生率显著低于其他处理的再生率(处理5~8),但平均每叶不定芽数不同处理间差异不显著。
2.2.3 TDZ和BA对不定芽伸长生长的影响 在不定芽诱导培养基上,细胞分裂素BA(图2-A~D)比TDZ(图2-E~H)更容易直接诱导形成伸长生长的不定梢,而TDZ诱导产生的不定芽在诱导培养基上不易获得此类不定梢,需转移到不加TDZ的增殖培养基上进行诱导。

图2 苹果半矮化砧木新品种鲁砧1 号离体叶片在不同不定芽诱导培养上的不定芽再生
Fig.2 Adventitious bud regeneration from in vitro leaf explants of the new semi-dwarfing apple rootstock Luzhen 1 on different adventitious bud induction media
A.MS+2 mg·L-1 BA+30 g·L-1 蔗糖;B.MS+4 mg·L-1 BA+30 g·L-1 蔗糖;C.MS+2 mg·L-1 BA+30 g·L-1 D-山梨醇;D.MS+4 mg·L-1 BA+30 g·L-1 D-山梨醇;E.MS+0.6 mg·L-1 TDZ+30 g·L-1蔗糖;F.MS+1 mg·L-1 TDZ+30 g·L-1蔗糖;G.MS+0.6 mg·L-1 TDZ+30 g·L-1 D-山梨醇;H.MS+1 mg·L-1 TDZ+30 g·L-1 D-山梨醇。
A.MS+2 mg·L-1 BA+30 g·L-1 sucrose;B.MS+4 mg·L-1 BA+30 g·L-1 sucrose;C.MS+2 mg·L-1 BA+30 g·L-1 D-sorbitol;D.MS+4 mg·L-1 BA+30 g·L-1 D-sorbitol;E.MS+0.6 mg·L-1 TDZ+30 g·L-1 sucrose;F.MS+1 mg·L-1 TDZ+30 g·L-1 sucrose;G.MS+0.6 mg·L-1 TDZ+30 g·L-1 D-sorbitol;H.MS+1 mg·L-1 TDZ+30 g·L-1 D-sorbitol.
综上,鲁砧1 号是一个离体叶片比较容易诱导再生不定芽的优良砧木无性系品种,对碳源无论是D-山梨醇还是蔗糖要求不严格,但蔗糖比D-山梨醇价格便宜,所以推荐诱导叶片不定芽再生的最佳培养基组合是添加1 mg·L-1 TDZ、0.3 mg·L-1 IBA 和30 g·L-1蔗糖的MS培养基。
2.3 不定梢生根诱导
2.3.1 不定根产生进程 生根诱导培养12 d 时,裸眼可观察到早期不定根突起的产生,培养至18 d,可观察到明显的短根形成(图3-A~D),并且此时可达最终生根率的88%(详细资料没有列出),培养至22 d时,基本上不再生新的不定根,生根率达到最高值,之后随着培养时间的延长,主要是根的伸长生长(图3-E~H),个别也有单株根数的增加,但根数增加并不明显。

图3 苹果半矮化砧木新品种鲁砧1 号在不同生根培养和不同培养时间的生根表现
Fig.3 In vitro rooting behavior of the semi-dwarfing apple rootstock cultivar Luzhen 1 on different rooting media and under different culturing time
A. 1/4MS+0.3 mg·L-1 IBA+20 g·L-1 蔗糖; B. 1/4MS+0.3 mg·L-1 IBA+30 g·L-1 蔗糖; C. 1/4MS+0.5 mg·L-1 IBA+20 g·L-1 蔗糖; D. 1/4MS+0.5 mg·L-1 IBA+30 g·L-1 蔗糖;E.1/4MS+0.3 mg·L-1 IBA+20 g·L-1 蔗糖;F.1/4MS+0.3 mg·L-1 IBA+30 g·L-1 蔗糖;G.1/4MS+0.5 mg·L-1 IBA+20 g·L-1 蔗糖;H.1/4MS+0.5 mg·L-1 IBA+30 g·L-1 蔗糖.A~D.离体生根第18 天;E~H.离体生根第30 天。
A.1/4MS+0.3 mg·L-1 IBA+20 g·L-1 sucrose;B.1/4MS+0.3 mg·L-1 IBA+30 g·L-1 sucrose;C.1/4MS+0.5 mg·L-1 IBA+20 g·L-1 sucrose;D.1/4MS+0.5 mg·L-1 IBA+30 g·L-1 sucrose;E.1/4MS+0.3 mg·L-1 IBA+20 g·L-1 sucrose;F.1/4MS+0.3 mg·L-1 IBA+30 g·L-1 sucrose;G.1/4MS+0.5 mg·L-1 IBA+20 g·L-1 sucrose;H.1/4MS+0.5 mg·L-1 IBA+30 g·L-1 sucrose.A-D.In vitro rooting in the 18th day;E-H.In vitro rooting in the 30th day.
2.3.2 基本培养基和蔗糖质量浓度对生根的影响供试的8种生根培养基处理都能成功诱导不定梢生根(表2),但不同处理间生根率差异较大,不同处理间平均每株生根条数差异不显著。
表2 不同生根培养基对苹果半矮化砧木新品种鲁砧1 号不定梢生根的影响
Table 2 Effect of different rooting media on in vitro rooting of the new semi-dwarfing apple rootstock cultivar Luzhen 1
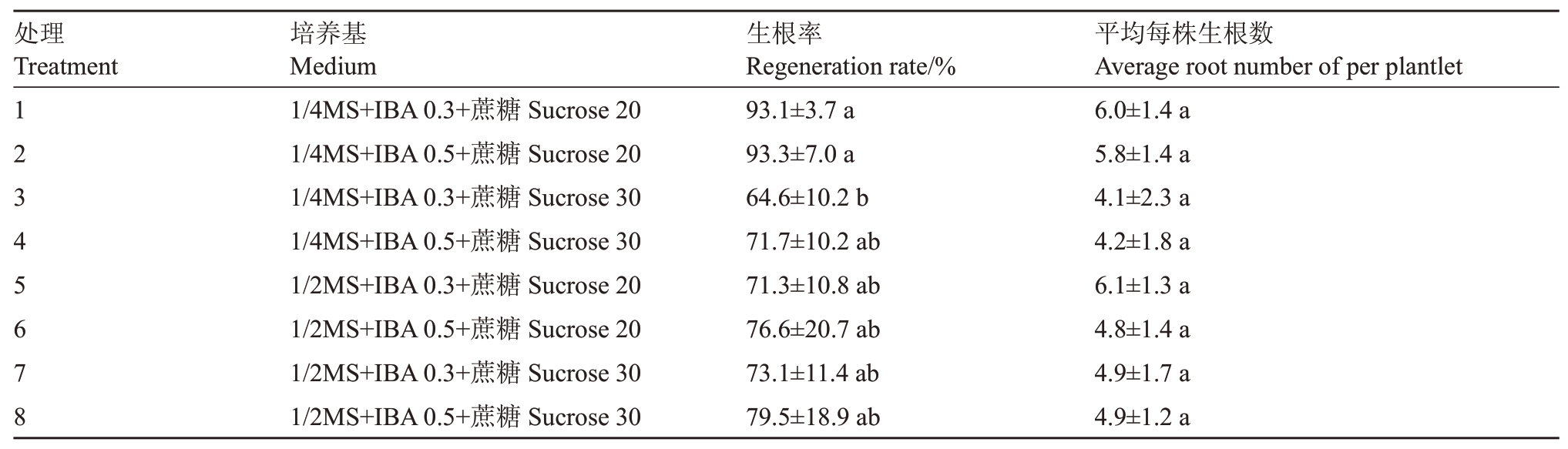
注:IBA 后的数字单位为mg·L-1;蔗糖后的数字单位为g·L-1。
Note:The units of IBA and sucrose are mg·L-1 and g·L-1,respectively.
处理Treatment 12345678平均每株生根数Average root number of per plantlet 6.0±1.4 a 5.8±1.4 a 4.1±2.3 a 4.2±1.8 a 6.1±1.3 a 4.8±1.4 a 4.9±1.7 a 4.9±1.2 a培养基Medium 1/4MS+IBA 0.3+蔗糖Sucrose 20 1/4MS+IBA 0.5+蔗糖Sucrose 20 1/4MS+IBA 0.3+蔗糖Sucrose 30 1/4MS+IBA 0.5+蔗糖Sucrose 30 1/2MS+IBA 0.3+蔗糖Sucrose 20 1/2MS+IBA 0.5+蔗糖Sucrose 20 1/2MS+IBA 0.3+蔗糖Sucrose 30 1/2MS+IBA 0.5+蔗糖Sucrose 30生根率Regeneration rate/%93.1±3.7 a 93.3±7.0 a 64.6±10.2 b 71.7±10.2 ab 71.3±10.8 ab 76.6±20.7 ab 73.1±11.4 ab 79.5±18.9 ab
在基本培养基1/4MS 上,不依赖于生长素IBA质量浓度,都表现蔗糖在较低质量浓度20 g·L-1(处理1和2)比高质量浓度30 g·L-1(处理3和4)更有利于提高生根率和平均单株生根条数。在添加0.3 mg·L-1 IBA处理上,20 g·L-1蔗糖上的生根率显著高于30 g·L-1蔗糖(处理1 和3),这一结果表明,在基本培养基1/4MS上,蔗糖质量浓度20 g·L-1比30 g·L-1更有效。
在基本培养基1/2MS 上,不管是2 个蔗糖质量浓度之间还是2 个IBA 质量浓度之间,其生根率和单株生根条数都无显著差异,4 个处理上的生根率都可达70%以上,表明在基本培养基1/2MS上,鲁砧1号不定梢的生根对蔗糖质量浓度和IBA质量浓度的要求不严格,供试的2个蔗糖质量浓度和2个IBA质量浓度对鲁砧1 号不定梢的生根具有相似的效果。
综合以上分析,表明鲁砧1号组织培养获得的不定梢生根较易,最佳生根培养基为1/4MS 添加0.3~0.5 mg·L-1 IBA和20 g·L-1蔗糖,生根率超过93%,平均单株生根条数为5.9(处理1和2)。
3 讨论
对于果树作物,其砧木育种比接穗品种的育种需时更长,难度更大,一般情况下,杂交后获得的种子实生苗经过生长期初步评价,或有利性状分子标记筛选后,再进行砧木选育的其他评价阶段[23]。单株实生苗嫁接后,就不能继续繁殖,所以必须把单株实生苗繁殖足够数量才能用以嫁接品种和其他测试,以满足重复试验的要求,才能最终选出优良砧木,因此选育出一个可在生产上推广应用的优良砧木相当不易。所以世界不同育种机构对自己选育出的砧木都注册专利品种保护。笔者在本研究中使用的砧木新品种鲁砧1号是山东省果树研究所选育的苹果优良半矮化砧木,已申请并获农业农村部植物新品种权授权(CNA20191005762)。为尽早在生产上推广应用这一优良砧木,同时为遗传改良奠定基础,笔者进行了鲁砧1 号组织培养的离体叶片不定梢诱导及不定梢生根研究,发现该砧木叶片不定梢再生及不定梢生根都相对较易,符合作为砧木较易繁殖和生根的要求,易于用组培快繁工厂化育苗技术进行苗木繁育。
由于苹果砧木对苹果优质丰产和省力化栽培的重要性,世界各国不断选育砧木新品种。不同砧木的组织培养及离体叶片不定梢的研究,以及叶片不定芽再生成功的品种也都在日益增多[7,12,14,16,20],但由于基因型和遗传背景的差异,不同品种的再生难易不同[9],至今研究者们还不能实现对所有品种的叶片培养再生。在本研究中,苹果半矮化砧木鲁砧1 号离体叶片的不定梢再生,不同于对碳源要求严格的砧木M9-T337[11]、山丁子[12]及品种富士[13]等,M9-T337、山丁子及富士仅在碳源为D-山梨醇上有较高的不定芽再生率;也不同于砧木54-118[13]和栽培品种鲁丽[15],虽然在蔗糖和D-山梨醇上都可以获得较高不定梢再生率,但最高再生率以加D-山梨醇的培养基最高;与蔗糖相比D-山梨醇更有利于提高砧木BP-176 的不定梢再生率[9];与蔗糖能够诱导多数砧木品种如G.41[14]、M7A 和M26[9,17-18]获得90%的不定梢再生率相似。
无菌苗离体生根是组培快繁成功的关键一步。苹果砧木离体生根研究已有较多报道[24],业内专业领域普遍接受的是生长素(主要是IBA、NAA、IAA)是根诱导所必需的[10],但在苹果砧木的离体生根诱导中以IBA 最常用[20-22],也有IBA 和NAA 共同使用的[3]。不同基因型的生根难易不同,存在难生根品种和易生根品种,Da Silva 等[10]研究报道生根率在18%~100%之间。在本研究中,半矮化砧木品种鲁砧1 号的试管苗的离体生根结果表明,蔗糖质量浓度和基本培养基组成对生根率的高低也有明显影响,在蔗糖质量浓度为20 g·L-1条件下,基本培养基1/4MS 比1/2MS 显著提高生根率,但在蔗糖质量浓度为30 g·L-1时,基本培养基1/4MS与1/2MS上的生根率无显著差异。在基本培养基1/4MS 上,蔗糖质量浓度20 g·L-1比30 g·L-1显著提高生根率,而在1/2MS 上,蔗糖质量浓度在20 g·L-1与30 g·L-1之间无显著差异,表明了蔗糖和基本培养基对鲁砧1 号的离体生根具有协同效应。
4 结论
鲁砧1 号离体叶片容易诱导再生不定芽,对碳源要求不严格,推荐诱导叶片不定芽再生的最佳培养基是添加1 mg·L-1 TDZ、0.3 mg·L-1 IBA和30 g·L-1蔗糖的MS 培养基。该砧木也容易实现不定梢生根,最佳生根培养基为1/4MS 添加0.3~0.5 mg·L-1 IBA和20 g·L-1蔗糖,生根率达93%,平均单株生根5.9条。
[1] 韩明玉.苹果矮砧集约栽培技术模式刍议[J].中国果树,2015(3):76-79.HAN Mingyu.Discussion on intensive apple orchard systems[J].China Fruits,2015(3):76-79.
[2] AUTIO W,ROBINSON T,BLACK B,CRASSWELLER R,FALLAHI E,HOYING S,PARKER M,QUEZADA R P,REIG G,WOLFE D. Budagovsky,geneva,pillnitz,and malling apple rootstocks affect‘Fuji’performance over eight years in the 2010 NC-140‘Fuji’apple rootstock trial[J]. Journal of the American Pomological Society,2020,74(4):196-209.
[3] MODGIL M,THAKUR M. In vitro culture of clonal rootstocks of apple for their commercial exploitation[J]. Acta Horticulturae,2017,1155:331-336.
[4] CZYNCZYK A,BIELICKI P. Eleven year evaluation of American (Geneva®) and Polish rootstocks with‘Golden Delicious Reinders’apple in Poland[J]. Journal of Fruit and Ornamental Plant Research,2012,20(2):11-21.
[5] 副島淳一,吉田義雄,羽生田忠敬,別所英男,土屋七郎,増田哲男,小森貞男,真田哲朗,伊藤祐司,定盛昌助,樫村芳記.リンゴわい性台木の新品種‘JM 1’,‘JM 7’および‘JM 8’[J].果樹研究所研究報告,2010(11):1-16.SOEJIMA J,YOSHIDA Y,HANIUD T,BESSHO H,TSUCHIYA S,MASUDA T,KOMORI S,SANADA T,ITO Y,SADAMORI S,KASHIMURA Y. New dwarfing apple rootstocks‘JM 1’,‘JM 7’and‘JM 8’[J].Bulletin of the National Institute of Fruit Tree Science,2010(11):1-16.
[6] 张冰冰,李粤渤,宋洪伟,赵晨辉.苹果抗寒矮化砧木新品种GM310 的选育[J].中国果树,2011(6):4-5.ZHANG Bingbing,LI Yuebo,SONG Hongwei,ZHAO Chenhui. The selection of apple cold-resistance dwarfing rootstock GM310[J].China Fruits,2011(6):4-5.
[7] 郑亚杰,姚环宇.苹果矮化砧GM256 组织培养与快繁技术研究[J].吉林农业科学,2008,33(1):26-27.ZHENG Yajie,YAO Huanyu.Studies on tissue culture technology of GM256,a dwarf rootstock of apple[J].Journal of Jilin Agricultural Sciences,2008,33(1):26-27.
[8] 张学英,李中勇,邵建柱,陈海江,徐继忠.苹果矮化砧木新品种‘冀砧1 号’[J].园艺学报,2020,47(1):195-196.ZHANG Xueying,LI Zhongyong,SHAO Jianzhu,CHEN Haijiang,XU Jizhong.A new apple dwarfing rootstock cultivar‘Jizhen 1’[J].Acta Horticulturae Sinica,2020,47(1):195-196.
[9] SUN Q R,SUN M J,SUN H Y,BELL R L,LI L G,ZHANG W,TAO J H. Comparative organogenic response of six clonal apple rootstock cultivars[J].HortScience,2016,51(3):272-278.
[10] DA SILVA J A T,GULYÁS A,MAGYAR-TÁBORI K,WANG M R,WANG Q C,DOBRÁNSZKI J. In vitro tissue culture of apple and other Malus species:Recent advances and applications[J].Planta,2019,249(4):975-1006.
[11] HÖHNLE M K,WEBER G. Efficient adventitious shoot formation of leaf segments of in vitro propagated shoots of the apple rootstock M.9/T337[J]. European Journal of Horticultural Science,2010,75(3):128-131.
[12] SUN C Y,WANG Y,XU X F,SUN Y,ZHU L H,HAN Z H.Regeneration from leaf segments of in vitro-grown shoots of Malus baccata[J].New Zealand Journal of Crop and Horticultural Science,2008,36(4):233-238.
[13] LEE Y K,KWON Y,HYUNG N I. Optimal medium compositions for plant regeneration via adventitious shoot formation using‘Fuji’apple leaf explants[J]. Journal of Plant Biotechnology,2019,46(4):310-317.
[14] 孙清荣,关秋竹,孙洪雁,李林光,陶吉寒,王海波,何平.苹果抗寒半矮化砧木‘54-118’的组织培养及其离体叶片不定梢再生[J].植物生理学报,2017,53(11):2007-2012.SUN Qingrong,GUAN Qiuzhu,SUN Hongyan,LI Linguang,TAO Jihan,WANG Haibo,HE Ping.Tissue culture and shoot regeneration from leaf explants of cold-hardy and semi-dwarf apple rootstock‘54-118’[J]. Plant Physiology Journal,2017,53(11):2007-2012.
[15] 孙清荣,关秋竹,李林光,何平,王海波,孙洪雁.苹果新品种‘鲁丽’离体叶片高效不定芽再生体系的建立[J].北方园艺,2021(19):42-47.SUN Qingrong,GUAN Qiuzhu,LI Linguang,HE Ping,WANG Haibo,SUN Hongyan.Establishment of high-efficiency adventitious bud regeneration from leaf explants of Malus domestica‘Luli’[J].Northern Horticulture,2021(19):42-47.
[16] 孙清荣,关秋竹,王海波,李林光,陶吉寒,孙洪雁.苹果抗寒矮化砧木‘BP-176’的组织培养及其叶片不定梢诱导[J].果树学报,2019,36(6):812-818.SUN Qingrong,GUAN Qiuzhu,WANG Haibo,LI Linguang,TAO Jihan,SUN Hongyan. Tissue culture and induction of adventitious shoot regeneration from leaf explants of cold-hardy dwarfing apple rootstock‘BP-176’[J].Journal of Fruit Science,2019,36(6):812-818.
[17] YEPES L M,ALDWINEKLE H S.Factors that effect leaf regeneration efficiency in apple,and effect of antibiotics in morphogenesis[J]. Plant Cell,Tissue and Organ Culture,1994,37(3):257-269.
[18] JIN W M,WANG Y H,WANG H.Adventitious shoot regeneration from leaves of apple rootstock‘Pingyitiancha’(Malus hupehensis var. pinyiensis) and genetic fidelity of regenerated plantlets using SSR markers[J]. Canadian Journal of Plant Science,2014,94(8):1345-1354.
[19] ZHANG X,QIN Y,LIANG D,ZOU Y J,MA F W. Enhancement of in vitro shoot regeneration from leaf explants of apple rootstock G. 41[J]. In Vitro Cellular & Developmental Biology-Plant,2014,50(2):263-270.
[20] YASSEN M,AHMAD T,ABBASI N A,HAFIZ I A. Assessment of apple rootstock M9 and M26 for in vitro rooting potential using different carbon sources[J]. Pakistan Journal of Botany,2009,41(2):769-781.
[21] SUN Q R,SUN H Y,BELL R L,LI L G,XIN L,TAO J H,LI Q. Optimisation of the media for in vitro shoot proliferation and root induction in three new cold-hardy and dwarfing or semidwarfing clonal apple rootstocks[J].The Journal of Horticultural Science and Biotechnology,2014,89(4):381-388.
[22] BAHMANI R,GHOLAMI M,MOZAFARI A A,ALIVAISIS R.Effects of salinity on in vitro shoot proliferation and rooting of apple rootstock MM. 106[J]. World Applied Sciences Journal,2012,17(3):292-295.
[23] COUSINS P.Rootstock breeding:An analysis of intractability[J].HortScience,2005,40(7):1945-1946.
[24] SHARMA T,MODGIL M,THAKUR M.Factors affecting induction and development of in vitro rooting in apple rootstocks[J].Indian Journal of Experimental Biology,2007,45(9):824-829.