刺葡萄(Vitis davidii Foёx.)属于葡萄科葡萄属东亚种群的一个种,被认定为中国南方地区重要的野生葡萄种质资源[1],该物种的地理分布主要集中在湖南[2]、福建[3]等地。湘刺1 号作为湖南农业大学葡萄团队选育的刺葡萄新品种,已在湖南省怀化市中方县等地区成功种植,其果皮呈紫黑色,富含原花青素、花色苷及黄酮醇等多种生物活性成分[4]。这些成分为刺葡萄的鲜食和酿酒提供优质原料[5]。
可溶性糖含量不仅是衡量果实品质的重要指标,也是影响水果经济效益和市场竞争力的关键指标。葡萄作为典型的己糖积累型水果作物[3],其成熟期糖分主要以葡萄糖和果糖的形式积累于液泡内,此过程主要受到单糖转运蛋白的调控[6]。单糖转运蛋白主要包括液泡膜糖转运蛋白(TST)、早期响应干旱类似蛋白(ERD6L)、液泡葡萄糖转运蛋白(VGT)、己糖转运蛋白(STP)、质体葡萄糖转运蛋白(PGlCT)、肌醇转运蛋白(INT)及糖醇转运蛋白(PMT)。ERD6L(early response to dehydration six like)是定位在液泡膜上的糖转运蛋白[7],Kiyosue等[8]首次在拟南芥中发现第一个ERD6基因。迄今,已在拟南芥中鉴定出多个ERD6L蛋白家族成员,其中Poschet 等[9]通过研究发现AtERD6L6 基因突变体中液泡葡萄糖含量显著增加,推断AtERD6L6 可能参与从液泡到细胞质的葡萄糖转运。有趣的是,在苹果中过表达MdERD6L-1 导致大量葡萄糖流入胞质中,使得MdTST1 和MdTST2 的表达量上调,从而增加液泡中葡萄糖、果糖、蔗糖的含量[10-11]。在葡萄中,Breia等[12]发现VvERD6L13编码的蛋白质定位于细胞的质膜上,能将质外体中的糖类物质转运至葡萄果实细胞中。此外,ERD6L 直系同源基因在柑橘[13]、梨[14]等水果作物中的表达与果实糖分积累之间的密切相关性也逐步被研究证实。上述表明水果作物中的ERD6L与果实糖的积累密切相关,研究其调控果实发育过程中可溶性糖的分子机制,对理解水果作物的糖分积累调控机制及开展品质改良具有重要的理论意义和应用价值。
已有研究发现,葡萄中存在18个ERD6L家族成员,而目前仅对VvERD6L13完成了功能验证[12],其他ERD6L功能未知。本研究中,基于前期研究基础,发现VdERD6L15 在果实早期表达且与刺葡萄果实糖的积累呈负相关,探究其是否参与刺葡萄果实细胞中糖的转运以及其在果实发育早期发挥何种生物学功能对刺葡萄果实品质改良具有重要意义。
1 材料和方法
1.1 试验材料
试验材料为刺葡萄湘刺1 号品种,保存于湖南农业大学长安教学基地。从花后13 d 开始每隔7 d采集1次,共采集18个时期的果实,将采集后的果实用液氮速冻研磨成粉末,保存于-80 ℃冰箱中备用。本氏烟草种植于25 ℃培养室中。
1.2 湘刺1号果实总RNA提取、cDNA合成
使用南京诺唯赞公司植物快速RNA 提取试剂盒(FastPure Universal Plant Total RNA Isolation Kit)提取湘刺1 号果实的总RNA,通过微量分光光度计(Nano-530)测定RNA 浓度,使用TaKaRa 公司反转录试剂盒(PrimeScript™RT reagent Kit with gDNA Eraser)进行反转录得到cDNA,存放于-20 ℃冰箱中备用。
1.3 刺葡萄不同发育时期中VdERD6L15基因的表达分析
根据课题组前期研究基础,提取刺葡萄VdERD6LI5 基因在果实发育时期的FPKM 及糖(葡萄糖、果糖、蔗糖)含量数据,绘制折线图(https://www.chiplot.online/),使用R 包GGally 和ggplot2 计算VdERD6LI5基因的表达数据和糖(葡萄糖、果糖、蔗糖)含量皮尔逊相关系数及FDR 值,并进行可视化处理。
1.4 刺葡萄不同发育时期可溶性糖含量测定方法
将采集的刺葡萄果实用液氮进行快速冷冻研磨处理,称取3 g处理后的样品并向其中加入80%乙醇提取液,35 ℃提取20 min,并每隔5 min混匀1次,将混合物在6500 r·min-1的条件下离心15 min,收集上清液。重复提取3次后,将提取得到的上清液合并,并使用超纯水定容至20.0 mL。上清液经0.45 μm水相滤膜过滤后置于1.5 mL进样瓶中,用于高效液相色谱(HPLC)测定葡萄糖、果糖、蔗糖的含量。
1.5 VdERD6L15基因及启动子引物设计
在葡萄基因组数据库(http://grapegenomics.com)下载VdERD6L15基因序列(Vitvi04g01302),利用Oligo7 软件设计VdERD6L15 基因编码区(CDS)特异性引物VdERD6L15- F1、VdERD6L15- R1、VdERD6L15-F2、VdERD6L15-R2,采用Primer primer5.0 软件从翻译起始位点ATG 上游3000、2200、1500、1000、500 bp设计特异性引物(表1)。
表1 引物序列
Table 1 Primer sequences
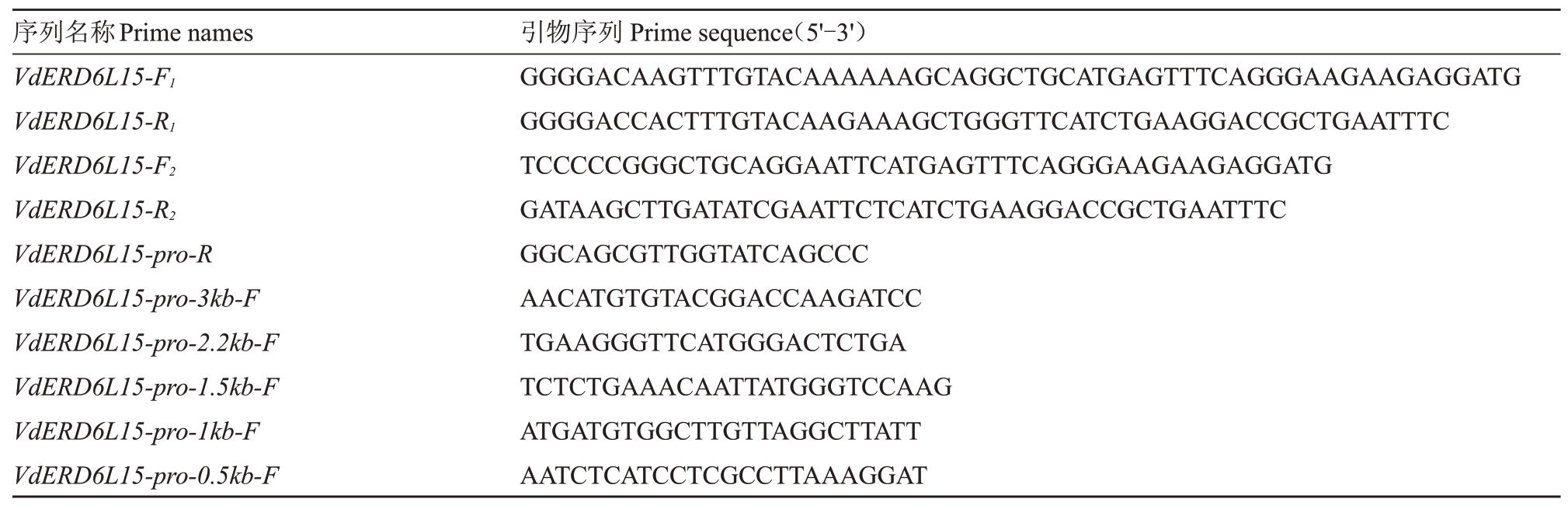
序列名称Prime names VdERD6L15-F1 VdERD6L15-R1 VdERD6L15-F2 VdERD6L15-R2 VdERD6L15-pro-R VdERD6L15-pro-3kb-F VdERD6L15-pro-2.2kb-F VdERD6L15-pro-1.5kb-F VdERD6L15-pro-1kb-F VdERD6L15-pro-0.5kb-F引物序列Prime sequence(5'-3')GGGGACAAGTTTGTACAAAAAAGCAGGCTGCATGAGTTTCAGGGAAGAAGAGGATG GGGGACCACTTTGTACAAGAAAGCTGGGTTCATCTGAAGGACCGCTGAATTTC TCCCCCGGGCTGCAGGAATTCATGAGTTTCAGGGAAGAAGAGGATG GATAAGCTTGATATCGAATTCTCATCTGAAGGACCGCTGAATTTC GGCAGCGTTGGTATCAGCCC AACATGTGTACGGACCAAGATCC TGAAGGGTTCATGGGACTCTGA TCTCTGAAACAATTATGGGTCCAAG ATGATGTGGCTTGTTAGGCTTATT AATCTCATCCTCGCCTTAAAGGAT
1.6 VdERD6L15基因克隆及表达载体构建
将提取的刺葡萄根系组织中的RNA 逆转录为cDNA 并作为模板,用Oligo 7 软件设计特异性引物扩增得到VdERD6L15基因CDS序列(表1),PCR产物经1%的琼脂糖凝胶电泳后用胶回收试剂盒(TSINGKE TSP602-200 Trelief® DNA Gel Extraction Kit)纯化回收,利用Gateway 系统(Thermo Fisher Scientific Gateway™LR Clonase™Ⅱ)构建35S:GFPVdERD6L15载体,并用热激法转入大肠杆菌DH5α,将阳性单菌落送至生工生物工程(长沙)有限公司进行测序。糖转运蛋白在酵母中异源功能验证的载体为酵母表达载体PDR196,带有-Ura筛选标记。酵母表达载体pDR196 通过限制性内切酶Eco RⅠ(New England Biolabs B6004s)进行酶切并进行胶回收处理,使用Tsingke 公司的In-Fusion® HD Cloning Kit进行无缝克隆,并用热激法转入DH5α大肠杆菌,涂板过夜,挑取阳性克隆测序比对完全正确后进行质粒提取。
1.7 VdERD6L15生物信息学分析
通过NCBI 数据库(https://www.ncbi.nlm.nih.gov/)查询黑比诺VvERD6L15、苹果MdERDL6及拟南芥AtERDL6 的氨基酸序列,并采用TBtools 软件进行多重序列比对;使用ExPasY 网站(https://web.expasy.org/protparam/)预测VdERD6L15蛋白的理化性质;使用TMHMM2.0 网站(https://services.healthtech.dtu.dk/services/TMHMM-2.0)对VdERD6L15 蛋白跨膜螺旋结构进行预测;采用SWISS-MODEL在线网站(https://swissmodel.expasy.org/)预测VdERD6L15蛋白的二、三级结构。
1.8 VdERD6L15蛋白亚细胞定位
亚细胞定位试验参照Xie 等[15]的方法并稍加改进。将测序正确的质粒通过电转法转入农杆菌EHA105,以定位于细胞膜的载体RFP-SYP122 作为对照[16],将GFP-VdERD6L15 与RFP-SYP122 菌液重悬并混合均匀,使用1 mL一次性注射器吸取重悬液注射于生长健康的本氏烟草叶片背面。光照培养72 h后,使用激光共聚焦显微镜(Zeiss LSM710)观察烟草叶片中GFP融合蛋白的分布,并保存图片,GFP激发光波长为488 nm,RFP激发光波长为562 nm。
1.9 VdERD6L15基因酵母中异源表达
酵母异源表达试验参照毛常清[17]的方法操作并稍加改进。将测序比对完全正确的质粒转入到只能在麦芽糖培养基上正常生长的己糖缺陷型酵母菌株EBY.VW4000 中,以空载体pDR196 作为阴性对照。培养3 d 后挑取单克隆进行PCR 鉴定,选取阳性单克隆菌液,按每次1∶10 体积比稀释菌液,使菌液终体积比分别为:原液、1∶10、1∶100 和1∶1000,将原液和3 个比例的稀释菌液各取5 μL 分别在SC/-Ura/Mal、SC/-Ura/Glc、SC/-Ura/Fru、SC/-Ura/Suc 固体培养基上进行点样,放入30 ℃培养箱,倒置培养3 d,通过观察不同糖源的培养基上己糖缺陷型酵母菌株EBY.VW4000 的生长情况,判断VdERD6L15的功能。
1.10 VdERD6L15启动子克隆及表达载体构建
从葡萄基因组数据库下载VdERD6L15 基因组序列,分析获得其上游启动子序列,将提取刺葡萄组培苗叶片组织的gDNA 作为模板,用Primer primer5.0 软件设计特异性引物并进行扩增(表1),扩增长度分别为3000、2200、1500、1000、500 bp,PCR 产物经1%的琼脂糖凝胶电泳后进行胶回收(TSINGKE TSP602-200 Trelief® DNA Gel Extraction Kit)。利用Gateway 系统(Thermo Fisher Scientific Gateway™LR Clonase™Ⅱ)将扩增片段构建到以GUS基因为报告基因的植物表达载体pKGWS7,0rfa中并转化到大肠杆菌DH5α 中,挑选单菌落进行阳性鉴定,将阳性单菌落送至生工生物工程(长沙)有限公司进行测序。使用PlantCARE(https://bioinformatics.psb.ugent.be/webtools/plantcare/html/)预 测VdERD6L15启动子顺式作用元件的功能和位置。
1.11 GUS染色
GUS染色试验参照杨静静[18]的方法操作并稍加改进。GUS染色液的配置(200 mL):NaH2PO4 1.56 g+Na2HPO4 3.58 g+K4Fe(CN)6 0.169 g+K3Fe(CN)6 0.132 g+EDTA 0.372 g+TritonX-100 200 μL+XGluc 0.2 g,4 ℃避光保存。将测序正确的质粒通过电转法转入农杆菌EHA105 后,挑取含有重组质粒proVdERD6L15:GFP-GUS 的农杆菌于LB 液体培养基中,摇菌至OD600=0.6~0.8,弃上清液加入侵染液,用注射器抽取1 mL 菌液注射于长势较好且一致的本氏烟草叶片中。注射菌液3 d后,将烟草叶片进行1 cm 打孔并置于烧杯中,加入染色液,37 ℃培养24 h。先后分别用50%、70%的乙醇漂洗样品,每次漂洗10 min,随后加入100%的乙醇溶液浸泡,每6 h换1次溶液,观察染色情况并拍照。
2 结果与分析
2.1 刺葡萄果实发育时期VdERD6L15基因表达及糖含量的相关性分析
为检测整个刺葡萄果实发育过程中VdERD6LI5基因的表达情况,从花后第7天开始每隔7 d采集一次直至成熟(共19个采样节点)。通过分析VdERD6LI5 基因在刺葡萄果实发育期的FPKM,发现VdERD6LI5在果实发育早期表达量高,在后期表达量低(图1-A)。对刺葡萄果实采样并进行转录组测序和可溶性糖含量的测定(图1-B),基于VdERD6L15 的基因表达和糖含量的皮尔逊相关性分析,发现其与刺葡萄果实中的可溶性糖(葡萄糖、果糖和蔗糖)积累呈显著负相关(-0.652、-0.642和-0.712)。
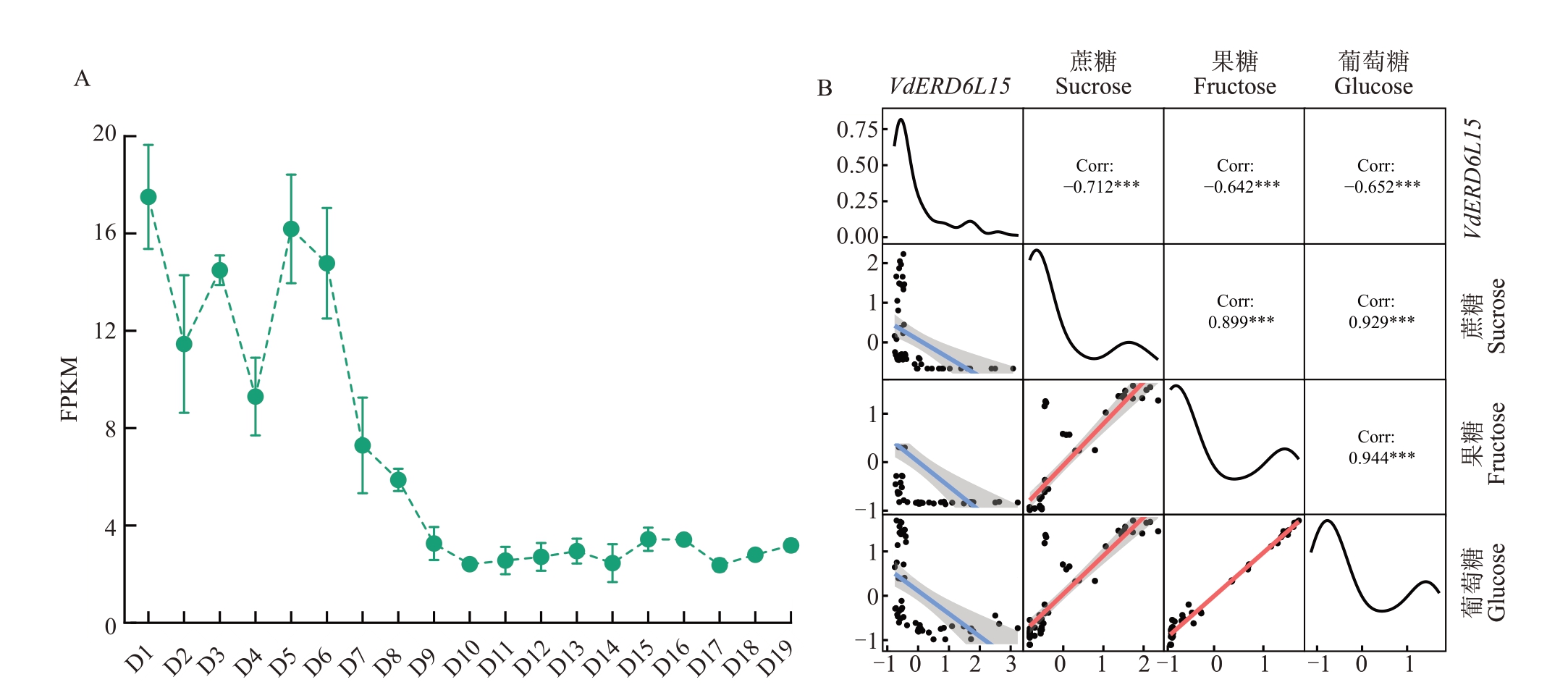
图1 刺葡萄VdERD6L15 基因表达量及糖含量的相关性分析
Fig.1 Correlation analysis of VdERD6L15 gene expression and sugar content in V.davidii
A.VdERD6L15 在刺葡萄果实发育过程中表达量分析;B.刺葡萄果实发育过程中VdERD6L15 的表达量和糖含量的相关性分布矩阵。D1表示花后第7 天,D19 表示花后130 d,每隔7 d 采一次样;显著性p-value 小于0.001、0.01、0.05 分别使用***、**、*表示。
A.Analysis of VdERD6L15 expression during the fruit development in V. davidii; B. Correlation distribution matrix between the expression and sugar content of VdERD6L15 during fruit development in V.davidii.D1 indicates the 7th day after flowering,and D19 indicates 130 days after flowering,and samples are taken every seven days;Significant p-values less than 0.001,0.01,and 0.05 were indicated by ***,**,and *,respectively.
2.2 VdERD6L15基因克隆
为探究VdERD6L15 基因在刺葡萄果实发育中的作用,以刺葡萄果实组织中的cDNA作为模板,用特异引物VdERD6L15-F1和VdERD6L15-R1进行PCR扩增,扩增得到片段与预期大小一致(图2-A),经测序发现VdERD6L15 的编码序列区域长为1461 bp,编码486 个氨基酸。如图2-B 所示,通过对VdERD6L15与黑比诺VvERD6L15、苹果MdERDL6及拟南芥AtERDL6进行氨基酸多重序列比对,发现VdERD6L15与黑比诺VvERD6L15、苹果MdERDL6及拟南芥AtERDL6 的同源率分别为100%、82%、78%,均属于单糖转运蛋白家族(monosaccharide transporters,MSTs)蛋白。
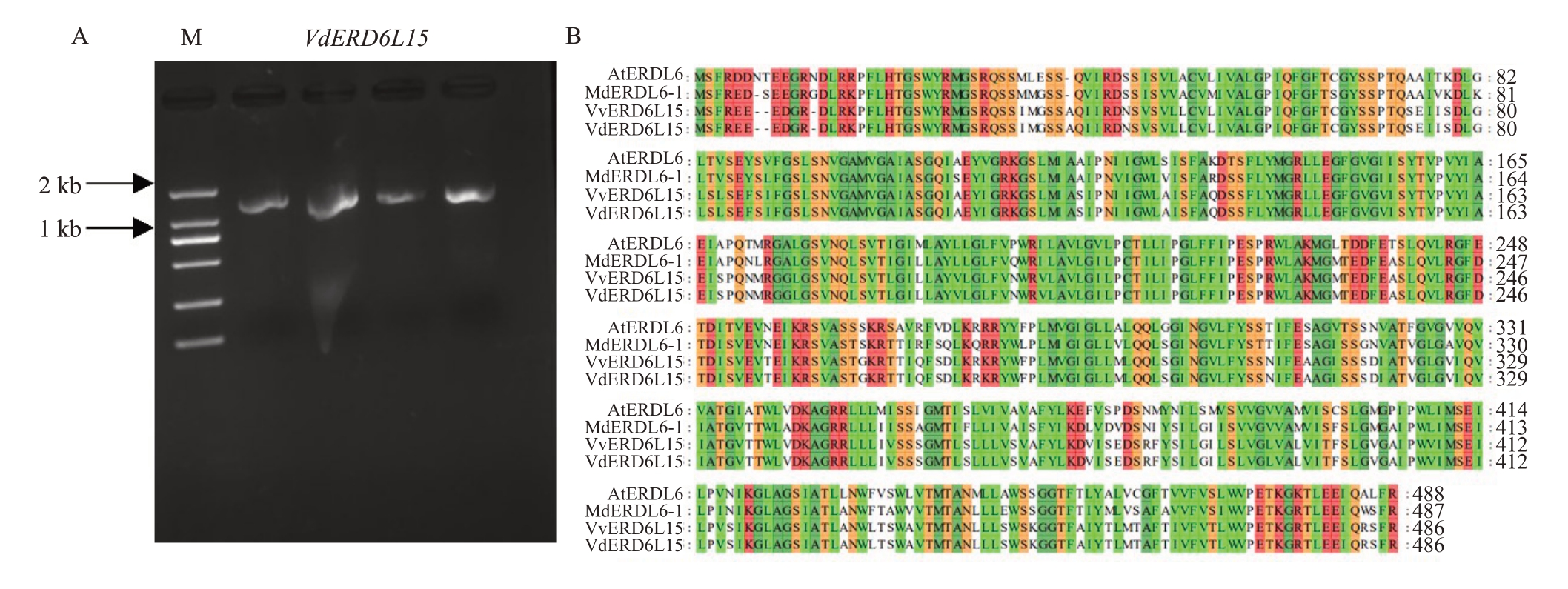
图2 VdERD6L15 基因克隆
Fig.2 The cloning of VdERD6L15 gene
A.VdERD6L15 基因RT-PCR 扩增胶图;B.VdERD6L15 与黑比诺、苹果及拟南芥同源基因氨基酸多重序列比对。M.DL2000 DNA marker。
A. RT-PCR amplification gel map of VdERD6L15; B. Multiple sequence alignment of amino acids sequence of VdERD6L15 with homologues from Pinot Noir;Malus domestica and Arabidopsis.M.DL2000 DNA marker.
2.3 VdERD6L15蛋白生物信息学分析
通过ExPASy 在线网站分析,发现VdERD6L15编码的蛋白含有468 个氨基酸,分子式为C2413H3839N601O673S18,相对分子质量为52 614.73,理论等电点(pI)为8.34,脂肪系数为118.91,亲水性平均系数为0.628,是一个不稳定类蛋白(不稳定性指数为42.08)。使用TMHMM 2.0 网站对VdERD6L15蛋白跨膜结构域进行预测,发现其可能包含12个跨膜结构域,推测VdERD6L15 是一个膜蛋白。通过分析VdERD6L15蛋白二级结构发现其由49.18%的α-螺旋、3.10%的β-折叠、18.11%的延伸链和26.54%的随机卷曲构成(图3-A)。利用SWISS-MODEL软件对VdERD6L15 蛋白三级结构进行同源建模,如图3-B所示。
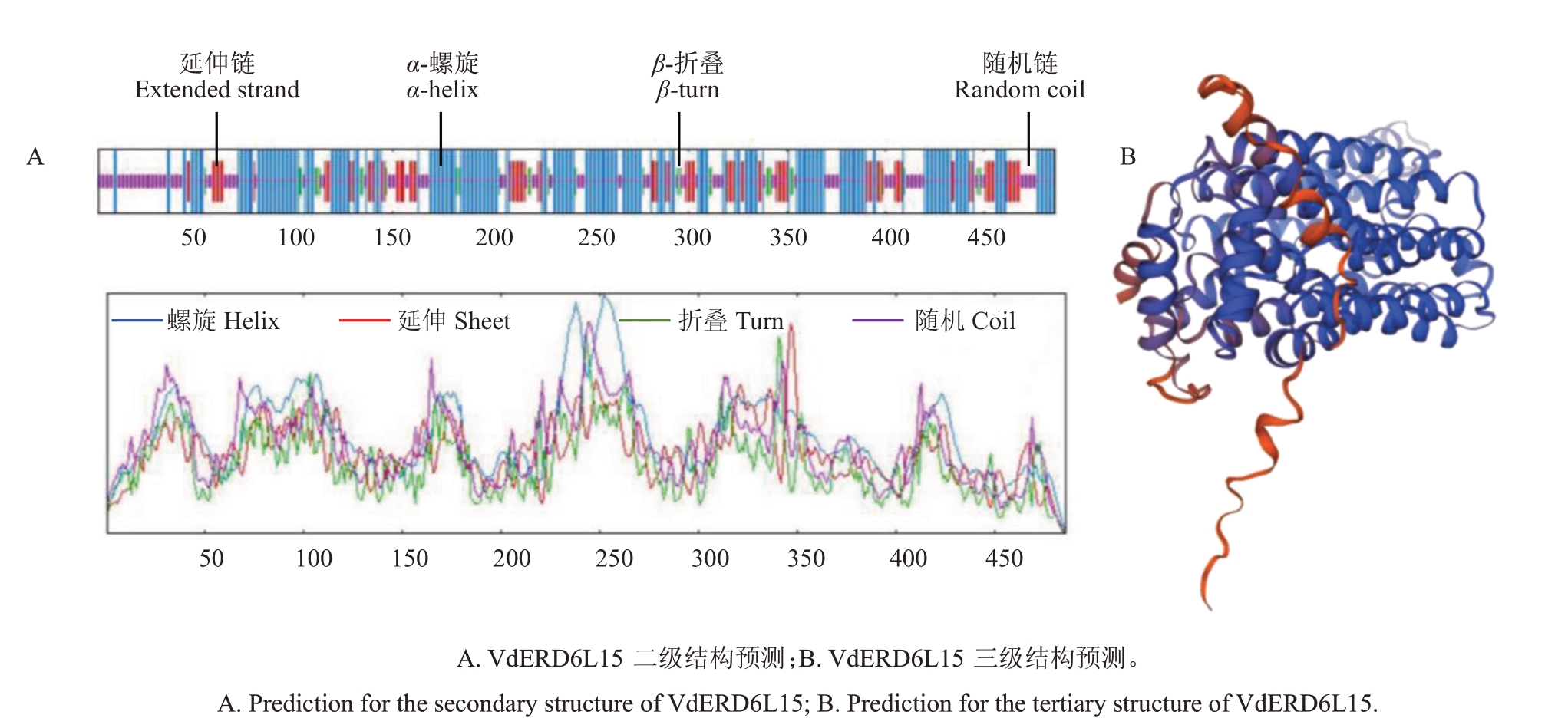
图3 VdERD6L15 蛋白结构预测
Fig.3 Structural analysis of VdERD6L15
2.4 VdERD6L15蛋白的亚细胞定位分析
为探究VdERD6L15 的细胞定位,构建GFPVdERD6L15 载体并转化到EHA105 农杆菌中,将活化后含有GFP-VdERD6L15 的农杆菌菌液与含有细胞膜定位的载体RFP-SYP122 农杆菌菌液混合均匀注射到本氏烟草叶片中,培养3 d 后,在激光共聚焦显微镜下观察荧光分布情况。如图4 所示,通过激光共聚焦显微镜观察发现GFP-VdERD6L15蛋白为膜蛋白,其定位于膜且具有典型的液泡膜内陷结构(如图中白色箭头所示),而RFP-SYP122 标记的细胞膜不具有内陷,表明GFP-VdERD6L15 主要定位于液泡膜上。
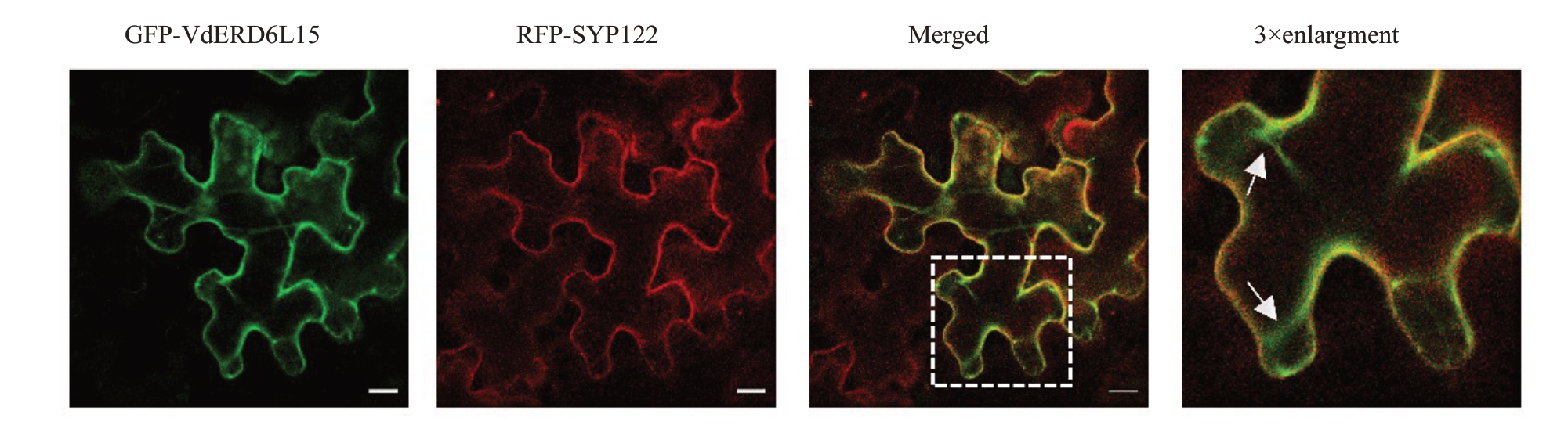
图4 VdERD6L15 亚细胞定位分析
Fig.4 Subcellular localization of VdERD6L15
激光通道从左自右分别为GFP 荧光、细胞膜标记RFP 荧光、合并图和局部图;标尺=20 μm。
From left to right:GFP,RFP,merged channel and local channel;Scale=20 μm.
2.5 VdERD6L15酵母异源表达
为探究VdERD6L15 是否具有糖转运活性,对在己糖转运蛋白缺陷型酵母菌株EBY.VW4000 中异源表达的VdERD6L15进行研究。将含有pDR196-VdERD6L15 质粒的己糖缺陷型酵母菌株点种于含有不同糖源的培养基上,以转化pDR196 空载的己糖缺陷型酵母菌株作为阴性对照,观察酵母生长情况。如图5所示,培养3 d后,转化pDR196空载的阴性对照己糖缺陷型酵母菌株在麦芽糖培养基上可以正常生长,在葡萄糖、果糖及蔗糖培养基上都不能够正常生长;pDR196-VdERD6L15 的己糖缺陷型酵母菌株在含有葡萄糖的培养基上生长良好,而在果糖、蔗糖培养基上生长明显受到抑制,表明VdERD6L15在酵母中具有一定的葡萄糖转运活性。
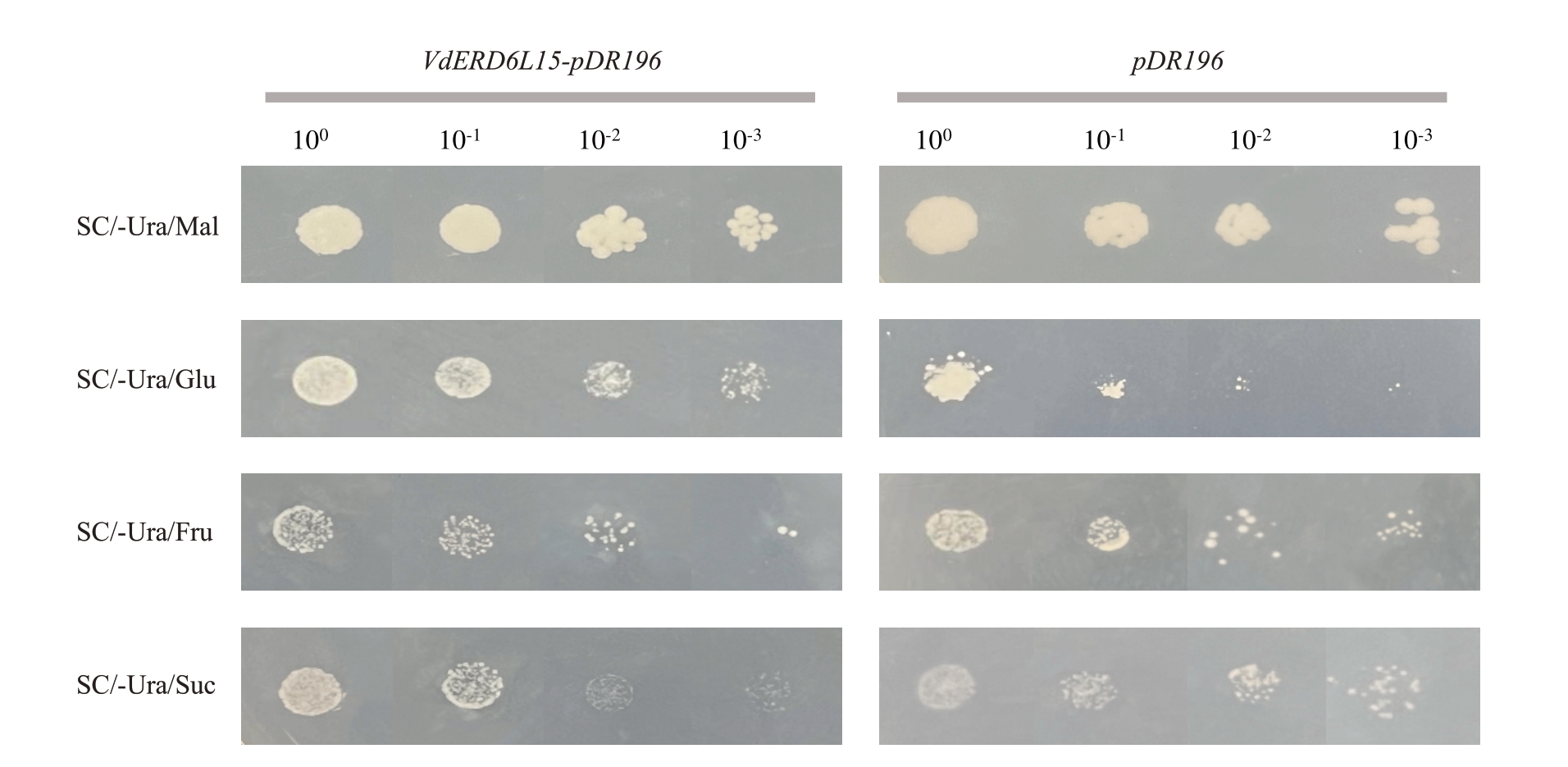
图5 VdERD6L15 基因在己糖缺陷型酵母菌株EBY.VW40000 中的功能验证
Fig.5 Functional verification of VdERD6L15 gene in yeast mutant strain EBY.VW4000
Mal.麦芽糖;Glu.葡萄糖;Fru.果糖;Suc.蔗糖。
Mal.Maltose;Glu.Glucose;Fru.Fructose;Suc.Sucrose.
2.6 VdERD6L15启动子顺式作用元件预测及活性分析
使用PlantCARE 网站对VdERD6L15 启动子进行预测分析,发现VdERD6L15启动子区域包含了大量顺式作用元件(图6),如光响应元件L-box、Box4,MeJA 反应的顺式作用元件CGTCA-motif,参与干旱诱导元件MBS 等,推测VdERD6L15 基因表达可能受光调控。通过GUS 染色试验分析,发现对照组的烟草叶片无染色,而试验组P1(-3000 bp)、P2(-2200 bp)、P3(-1500 bp)及P4(-1000 bp)烟草叶片均可正常染色,且颜色随片段长度的截断而变浅(图6),表明4 个启动子缺失体都能正常表达,而P5(-500 bp)烟草叶片不可正常染色,由此进一步明确VdERD6L15 启动子核心区域位于-500 bp到-1000 bp之间。
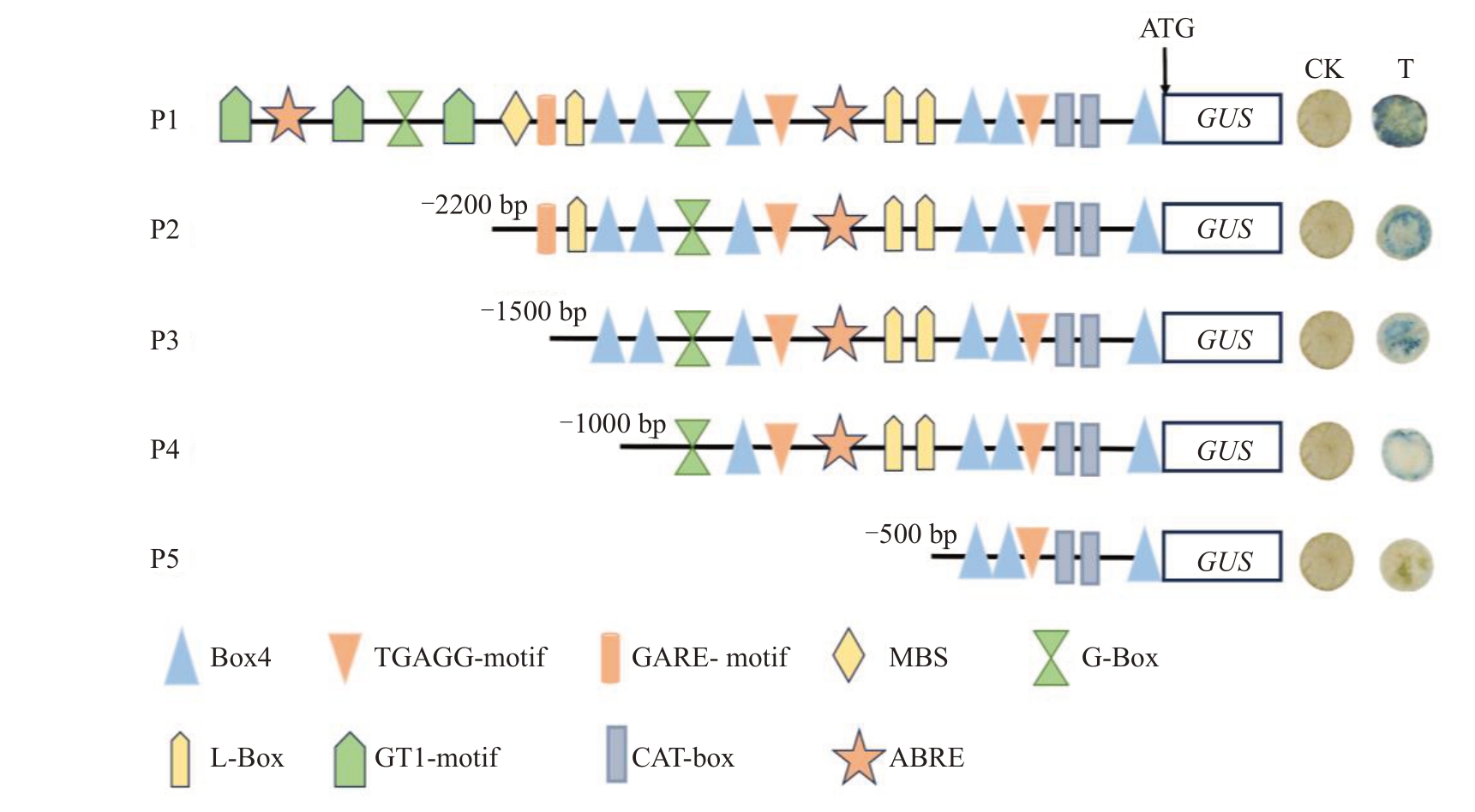
图6 VdERD6L15 启动子顺式作用元件预测及截短活性分析
Fig.6 Cis-acting element prediction and promoter deletion analysis of VdERD6L15
P1~P5.5 段启动子缺失片段;CK.对照;T. P1~P5VdERD6L15:GUS-GFP。
P1-P5.5-segment promoter deletion fragment;CK.control;T. P1~P5VdERD6L15:GUS-GFP.
3 讨论
在果实发育过程中,糖分的积累是决定果实品质和市场价值的关键因素之一。迄今为止,在葡萄[19-21]、苹果[22-23]、梨[24]、菠萝[25]等水果作物中先后揭示糖转运蛋白与果实中糖分积累之间存在着紧密的相关性。其中,属于单糖转运蛋白家族的ERD6L在果实糖的积累中也发挥着重要功能。笔者在本研究中通过分析刺葡萄果实发育时期VdERDL6L15的表达量,并关联分析果实发育时期可溶性糖的积累规律,发现VdERDL6L15 在果实早期表达且与刺葡萄果实糖的积累呈负相关。为探究VdERDL6L15在葡萄果实发育中的作用,笔者在本研究中首次成功克隆VdERDL6L15 的CDS 序列,并对其编码的氨基酸序列进行了初步的生物信息学分析。通过氨基酸多重序列比对,发现VdERD6L15 与黑比诺VvERD6L15、苹果MdERDL6及拟南芥AtERDL6具有高度同源。已有研究发现,MdERDL6 及AtER-DL6 编码蛋白定位于液泡膜[9,26-27],在果实糖的积累中扮演着重要角色,由此推测与它们同源的VdERD6L15可能具有相似生物学功能。本研究中,通过亚细胞定位研究发现VdERD6L15 也定位于液泡膜中,在己糖转运蛋白缺陷型酵母菌株EBY.VW4000 中对VdERD6L15 异源表达,初步明确了VdERD6L15 具有葡萄糖转运活性。这一发现与之前对苹果MdERDL6-1的试验结果一致[11]。综上,笔者在本研究中初步确定VdERD6L15 定位于液泡膜中,具有转运葡萄糖的功能。
目前,已有研究发现MdERDL6-1在苹果果实发育后期表达量与果实中糖的积累呈显著正相关,其可将果实细胞液泡中的葡萄糖转运到细胞质中,激活液泡膜上糖转运蛋白编码基因MdTST1 的表达,进而促使果实细胞中糖的积累[26]。然而,笔者在本研究中发现VdERD6L15在坐果期早期表达量高,与葡萄果实中糖的积累呈负相关,其在果实发育早期将液泡中的葡萄糖转移到细胞质中以发挥何种生物学功能尚不明确。在果实发育早期,果实快速膨大,果实中大部分可溶性糖通过转运代谢后用于果实细胞生长发育[28-29]。基于此,推测果实发育早期VdERD6L15转运的葡萄糖,可在细胞质中分解代谢成ATP 和碳源,用于葡萄果实生长发育,但关于VdERD6L15 是如何调控刺葡萄果实发育早期细胞内可溶性糖的动态平衡进而影响果实发育的仍有待进一步探究。
启动子作为基因表达调控的关键序列,包含了大量不同功能的顺式调控元件[31],这些元件在基因转录调控中扮演着至关重要的角色,因此对启动子中的顺式作用元件进行研究对揭示基因功能具有重要意义[31-32]。笔者在本研究中借助PlantCARE 数据库对VdERD6L15 基因ATG 上游3000 bp 启动子序列进行了分析,发现其启动子区域包含多种响应元件,如ABA 响应元件、MeJA 反应的顺式作用元件、MYB结合位点参与干旱诱导响应元件、光响应元件等。通过对VdERD6L15启动子进行5′端缺失克隆,并以5 段不同长度缺失体为目的片段构建表达载体,进行烟草瞬时转化。通过GUS染色试验发现仅启动子缺失体P5 建立的瞬时表达体系的烟草叶片没有染上蓝色,表明P5 片段没有启动子活性,从而初步判断VdERD6L15 基因启动子的核心元件的位置在P4 和P5 之间(-500 bp~-1000 bp)。进一步分析P4 和P5 序列的顺式作用元件,发现序列中包含L-box、G-box 等光响应元件,推测VdERD6L15 通过响应光而参与刺葡萄果实细胞中糖的转运。目前在苹果[33]、香蕉[34]、番茄[35]等水果作物中也先后发现光照会影响果实中糖的积累。但由于启动子与顺式作用元件之间调控机制复杂且多样[36],VdERD6L15 表达是否受光调控进而参与刺葡萄果实细胞中糖的转运和果实的发育仍需进一步研究。
4 结论
笔者在本研究中首次克隆了刺葡萄VdERD6L15并对其功能进行了分析,结合前人研究基础,初步发现VdERD6L15 是一个定位在液泡膜上的糖转运蛋白,具有转运葡萄糖的功能;通过对VdERD6L15 启动子不同长度的5′端缺失克隆及活性分析,发现其核心元件位置位于ATG 上游500 bp 到1000 bp 之间,以上研究可为解析VdERD6L15在刺葡萄果实生长发育过程中的功能提供一定的理论基础。
[1] 黎炎夏,罗飞雄,许延帅,李双江,陈文婷,谭君,王美军,徐丰,杨国顺,白描.刺葡萄新品种湘刺3 号的选育[J].果树学报,2024,41(4):781-785.LI Yanxia,LUO Feixiong,XU Yanshuai,LI Shuangjiang,CHEN Wenting,TAN Jun,WANG Meijun,XU Feng,YANG Guoshun,BAI Miao.Breeding report of a new spine grape cultivar Xiangci No.3[J].Journal of Fruit Science,2024,41(4):781-785.
[2] 石雪晖,杨国顺,熊兴耀,刘昆玉,钟晓红,王先荣,倪建军,郭光银.湖南省刺葡萄种质资源的研究与利用[J].湖南农业科学,2010(19):1-4.SHI Xuehui,YANG Guoshun,XIONG Xingyao,LIU Kunyu,ZHONG Xiaohong,WANG Xianrong,NI Jianjun,GUO Guangyin. Research and utilization status quo of germplasm resources of Vitis davidii Foёx.in Hunan[J].Hunan Agricultural Sciences,2010(19):1-4.
[3] 李佳秀,张春岭,刘慧,陈大磊,刘杰超,焦中高.葡萄汁中糖酸组成分析及在掺假鉴别中的应用[J].果树学报,2019,36(11):1566-1577.LI Jiaxiu,ZHANG Chunling,LIU Hui,CHEN Dalei,LIU Jiechao,JIAO Zhonggao. Profiles of soluble sugars and organic acids in grape juice and their application for authentication[J].Journal of Fruit Science,2019,36(11):1566-1577.
[4] 杨梅,潘永杰,杨国顺,石雪晖,刘昆玉,白描,罗飞雄. 刺葡萄新品种湘刺1 号的选育[J]. 果树学报,2023,40(9):2001-2005.YANG Mei,PAN Yongjie,YANG Guoshun,SHI Xuehui,LIU Kunyu,BAI Miao,LUO Feixiong. A new spine grape cultivar Xiangci No. 1 (Vitis davidii Foёx.)[J]. Journal of Fruit Science,2023,40(9):2001-2005.
[5] 王静,周广胜.中国毛葡萄和刺葡萄分布的气候适宜性[J].应用生态学报,2020,31(1):97-103.WANG Jing,ZHOU Guangsheng. Climatic suitability for the distribution of Vitis heyneana and V.davidii in China[J].Chinese Journal of Applied Ecology,2020,31(1):97-103.
[6] SHIRAISHI M,FUJISHIMA H,CHIJIWA H. Evaluation of table grape genetic resources for sugar,organic acid,and amino acid composition of berries[J].Euphytica,2010,174(1):1-13.
[7] 祝令成.苹果液泡葡萄糖外排蛋白MdERDL6 调控糖积累的机制研究[D].杨凌:西北农林科技大学,2022.ZHU Lingcheng. Mechanism study on an apple tonoplast glucose exporter MdERDL6 in regulating sugar accumulation[D].Yangling:Northwest A&F University,2022.
[8] KIYOSUE T,ABE H,YAMAGUCHI-SHINOZAKI K,SHINOZAKI K.ERD6,a cDNA clone for an early dehydration-induced gene of Arabidopsis,encodes a putative sugar transporter[J].Biochimica et Biophysica Acta,1998,1370(2):187-191.
[9] POSCHET G,HANNICH B,RAAB S,JUNGKUNZ I,KLEMENS P A W,KRUEGER S,WIC S,NEUHAUS H E,BÜTTNER M.A novel Arabidopsis vacuolar glucose exporter is involved in cellular sugar homeostasis and affects the composition of seed storage compounds[J]. Plant Physiology,2011,157(4):1664-1676.
[10] WEI X Y,LIU F L,CHEN C,MA F W,LI M J.The Malus domestica sugar transporter gene family:Identifications based on genome and expression profiling related to the accumulation of fruit sugars[J].Frontiers in Plant Science,2014,5:569.
[11] ZHU L C,LI B Y,WU L M,LI H X,WANG Z Y,WEI X Y,MA B Q,ZHANG Y F,MA F W,RUAN Y L,LI M J.MdERDL6-mediated glucose efflux to the cytosol promotes sugar accumulation in the vacuole through up-regulating TSTs in apple and tomato[J]. Proceedings of the National Academy of Sciences of the United States of America,2021,118(1):e2022788118.
[12] BREIA R,CONDE A,CONDE C,FORTES A M,GRANELL A,GERÓS H. VvERD6l13 is a grapevine sucrose transporter highly up-regulated in response to infection by Botrytis cinerea and Erysiphe necator[J]. Plant Physiology and Biochemistry,2020,154:508-516.
[13] ZHENG Q M,TANG Z,XU Q,DENG X X.Isolation,phylogenetic relationship and expression profiling of sugar transporter genes in sweet orange (Citrus sinensis)[J]. Plant Cell,Tissue and Organ Culture,2014,119(3):609-624.
[14] LI J M,ZHENG D M,LI L T,QIAO X,WEI S W,BAI B,ZHANG S L,WU J. Genome-wide function,evolutionary characterization and expression analysis of sugar transporter family genes in pear (Pyrus bretschneideri Rehd.)[J]. Plant & Cell Physiology,2015,56(9):1721-1737.
[15] XIE X B,LI S,ZHANG R F,ZHAO J,CHEN Y C,ZHAO Q,YAO Y X,YOU C X,ZHANG X S,HAO Y J.The bHLH transcription factor MdbHLH3 promotes anthocyanin accumulation and fruit colouration in response to low temperature in apples[J].Plant,Cell&Environment,2012,35(11):1884-1897.
[16] VERWEIJ W,SPELT C E,BLIEK M,DE VRIES M,WIT N,FARACO M,KOES R,QUATTROCCHIO F M. Functionally similar WRKY proteins regulate vacuolar acidification in Petunia and hair development in Arabidopsis[J]. The Plant Cell,2016,28(3):786-803.
[17] 毛常清. 玉米糖转运蛋白基因的鉴定、系统发育和表达分析[D]. 雅安:四川农业大学,2019.MAO Changqing. Identification,Phylogenetic and expression analysis of sugar transporter gene in maize[D]. Ya’an:Sichuan Agricultural University,2019.
[18] 杨静静.苹果果糖激酶基因MdFRK2 在调控糖代谢中的功能研究[D].杨凌:西北农林科技大学,2019.YANG Jingjing. Function study of apple fructokinase gene Md-FRK2 in regulating sugar metabolism[D]. Yangling:Northwest A&F University,2019.
[19] BAI Q,CHEN X X,ZHENG Z Z,FENG J J,ZHANG Y J,SHEN Y Y,HUANG Y. Vacuolar Phosphate Transporter1(VPT1) may transport sugar in response to soluble sugar status of grape fruits[J].Horticulture Research,2022,10(2):uhac260.
[20] CHONG J L,PIRON M C,MEYER S,MERDINOGLU D,BERTSCH C,MESTRE P. The SWEET family of sugar transporters in grapevine:VvSWEET4 is involved in the interaction with Botrytis cinerea[J]. Journal of Experimental Botany,2014,65(22):6589-6601.
[21] CAI Y M,TU W R,ZU Y Y,JING Y,XU Z M,LU J,ZHANG Y L.Overexpression of a grapevine sucrose transporter(VvSUC27)in tobacco improves plant growth rate in the presence of sucrose in vitro[J].Frontiers in Plant Science,2017,8:1069.
[22] FAN R C,PENG C C,XU Y H,WANG X F,LI Y,SHANG Y,DU S Y,ZHAO R,ZHANG X Y,ZHANG L Y,ZHANG D P.Apple sucrose transporter SUT1 and sorbitol transporter SOT6 interact with cytochrome b5 to regulate their affinity for substrate sugars[J].Plant Physiology,2009,150(4):1880-1901.
[23] PENG Q,CAI Y M,LAI E H,NAKAMURA M,LIAO L,ZHENG B B,OGUTU C,CHERONO S,HAN Y P.The sucrose transporter MdSUT4.1 participates in the regulation of fruit sugar accumulation in apple[J]. BMC Plant Biology,2020,20(1):191.
[24] CHENG R,CHENG Y S,LÜ J H,CHEN J Q,WANG Y Z,ZHANG S L,ZHANG H P. The gene PbTMT4 from pear (Pyrus bretschneideri)mediates vacuolar sugar transport and strongly affects sugar accumulation in fruit[J].Physiologia Plantarum,2018,164(3):307-319.
[25] FAKHER B,ASHRAF M A,WANG L L,WANG X M,ZHENG P,ASLAM M,QIN Y. Pineapple SWEET10 is a glucose transporter[J].Horticulture Research,2023,10(10):uhad175.
[26] ZHU L C,LI Y Z,WANG C C,WANG Z Q,CAO W J,SU J,PENG Y J,LI B Y,MA B Q,MA F W,RUAN Y L,LI M J.The SnRK2.3-AREB1-TST1/2 cascade activated by cytosolic glucose regulates sugar accumulation across tonoplasts in apple and tomato[J].Nature Plants,2023,9(6):951-964.
[27] KLEMENS P A W,PATZKE K,TRENTMANN O,POSCHET G,BÜTTNER M,SCHULZ A,MARTEN I,HEDRICH R,NEUHAUS H E. Overexpression of a proton-coupled vacuolar glucose exporter impairs freezing tolerance and seed germination[J].New Phytologist,2014,202(1):188-197.
[28] 苏静,祝令成,刘茜,彭云静,马百全,马锋旺,李明军.果实糖代谢与含量调控的研究进展[J]. 果树学报,2022,39(2):266-279.SU Jing,ZHU Lingcheng,LIU Xi,PENG Yunjing,MA Baiquan,MA Fengwang,LI Mingjun. Research progress on sugar metabolism and concentration regulation in fruit[J]. Journal of Fruit Science,2022,39(2):266-279.
[29] 田晓成,祝令成,邹晖,李白云,马锋旺,李明军.果实可溶性糖的积累模式及其调控研究进展[J].园艺学报,2023,50(4):885-895.TIAN Xiaocheng,ZHU Lingcheng,ZOU Hui,LI Baiyun,MA Fengwang,LI Mingjun. Research progress on accumulation pattern and regulation of soluble sugar in fruit[J]. Acta Horticulturae Sinica,2023,50(4):885-895.
[30] 张春晓,王文棋,蒋湘宁,陈雪梅. 植物基因启动子研究进展[J].遗传学报,2004,31(12):1455-1464.ZHANG Chunxiao,WANG Wenqi,JIANG Xiangning,CHEN Xuemei.Review on plant gene promoters[J].Acta Genetica Sinica,2004,31(12):1455-1464.
[31] POTENZA C,ALEMAN L,SENGUPTA-GOPALAN C.Targeting transgene expression in research,agricultural,and environmental applications:Promoters used in plant transformation[J].In Vitro Cellular&Developmental Biology-Plant,2004,40(1):1-22.
[32] 杨晓娜,赵昶灵,李云,李会容,苏丽,周燕琼.启动子序列克隆和功能分析方法的研究进展[J].云南农业大学学报(自然科学版),2010,25(2):283-290.YANG Xiaona,ZHAO Changling,LI Yun,LI Huirong,SU Li,ZHOU Yanqiong. Research advances in the methods of cloning and function-analyzing of promoters[J].Journal of Yunnan Agricultural University(Natural Science),2010,25(2):283-290.
[33] MEI Z X,LI Z Q,LU X,ZHANG S H,LIU W J,ZOU Q,YU L,FANG H C,ZHANG Z Y,MAO Z Q,CHEN X S,WANG N.Supplementation of natural light duration promotes accumulation of sugar and anthocyanins in apple (Malus domestica Borkh.)fruit[J].Environmental and Experimental Botany,2023,205:105133.
[34] HUANG J Y,XU F Y,ZHOU W B. Effect of LED irradiation on the ripening and nutritional quality of postharvest banana fruit[J].Journal of the Science of Food and Agriculture,2018,98(14):5486-5493.
[35] THWE A A,KASEMSAP P,VERCAMBRE G,GAY F,PHATTARALERPHONG J,GAUTIER H.Impact of red and blue nets on physiological and morphological traits,fruit yield and quality of tomato (Solanum lycopersicum Mill.)[J]. Scientia Horticulturae,2020,264:109185.
[36] VAN DER DOES D,LEON-REYES A,KOORNNEEF A,VAN VERK M C,RODENBURG N,PAUWELS L,GOOSSENS A,KÖRBES A P,MEMELINK J,RITSEMA T,VAN WEES S C M,PIETERSE C M J. Salicylic acid suppresses jasmonic acid signaling downstream of SCFCOI1-JAZ by targeting GCC promoter motifs via transcription factor ORA59[J].The Plant Cell,2013,25(2):744-761.