腐烂病由黑腐皮壳属(Valsa)致病腐生真菌引起,是苹果和梨产业的重大真菌病害[1-2]。其病原菌主要侵染主干韧皮组织,也可对新梢和果实致病[2]。目前,主要通过刮除病斑并涂抹药剂对其进行防控,但该措施用工量大且复发率较高。此外,由于病原菌菌丝可深入到木质部深处,增加了有效防控的难度[3]。抗病育种是持久、有效且环保的措施,但育种进程缓慢,育成的兼具果实优质和抗病性强的品种极为罕见。当下,鉴定关键抗病基因并系统研究其抗病机制,是加快抗病育种进程的重要基础。
在与病原菌长期共进化的过程中,植物形成了复杂且有序的免疫系统,主要包括水平抗性和垂直抗性[4]。植物的水平抗性常由位于细胞膜的模式识别受体(pattern recognition receptor,PRR)激发,在植物对腐生病害的抗性中起至关重要的作用[5]。作为重要的PRRs,类受体激酶(receptor-like kinases,RLKs)在识别病原信号和激发免疫反应中起重要的调控作用[6]。CRINKLY4(CR4)是独立的RLKs亚家族,N末端存在至少1个染色体聚集调控因子1(regulator of chromosome condensation 1,RCC1)结构域,在拟南芥和苹果中分别发现5个和8个成员[7-8]。功能分析发现,该基因家族成员在植物生长发育和病原菌抗性中都具有重要作用。对苹果CR4 基因家族进行鉴定和分析,发现部分成员在苹果响应腐烂病菌信号过程中显著上调表达[8]。
山荆子(Malus baccata)是中国北方苹果产区的重要砧木,对多种生物逆境和非生物逆境都具有较强的适应能力。腐烂病抗性评价表明,东北山荆子为抗腐烂病材料,是进行抗病基因挖掘的优良材料[9]。笔者在本研究中从东北山荆子中鉴定出一个正调控腐烂病抗性的CR4 家族成员,命名为MbCCR4,并发现水杨酸和茉莉酸信号参与了该基因的功能。
1 材料和方法
1.1 植物材料
东北山荆子(M.baccata)一年生枝条由国家果树种质兴城梨、苹果圃友情提供。烟富3 号苹果和黄冠梨果实分别采自甘肃省静宁县果树研究所和景泰县条山农场。东北山荆子和杜梨-G03(Duli-G03,Pyrus betulifolia)悬浮细胞由笔者课题组诱导获得。
1.2 病原菌和病原菌代谢物获得
苹果腐烂病菌(Valsa mali,Vm)菌株Vm-A-003和梨腐烂病菌(Valsa pyri,Vp)菌株Vp-P-007由笔者课题组保存。将菌株在马铃薯葡萄糖琼脂培养基(potato dextrose agar,PDA)中培养3 d 后用于后续试验。Vp 代谢物(Vp metabolism,VpM)或Vm 代谢物(Vm metabolism,VmM)为10 块直径为5 mm 的Vp-P-007或Vm-A-003菌饼在100 mL马铃薯葡萄糖液体培养基(Potato Dextrose Broth,PDB)中培养3 d后过滤获得滤液,经去离子水稀释后获得的不同浓度的代谢物[10]。
1.3 载体构建
设计上游引物F1(CGC GGA TCC ATG GCA ATC AGC AGA AGG,含AscⅠ酶切位点)和下游引物R1(TGC TCT AGA CGG ACA TAC CGT TGG GTT G,含AvrⅡ酶切位点),克隆MbCCR4 全长序列,并经双酶切和连接导入表达载体pFGC5941,将重组质粒转入大肠杆菌DH5α。提取重组质粒和pFGC5941 空载体质粒,并利用冻融法转入农杆菌GV3101,经PCR 验证获得携带目标基因和空载体的农杆菌转化子。
1.4 果实瞬时表达
将携带目标基因和空载体(对照)的农杆菌活化,并大量扩繁至OD600为0.6~1.0时,离心并重悬于MES-KOH 溶液,4 ℃静置4 h 后,吸取0.2 mL 重悬液注射至果实,25 ℃培养3 d 后用于病原菌接种和注射部位目标基因的表达量分析。将Vm和Vp菌饼分别接种至苹果和梨果实注射部位,并在发病后36、48、60、72和84 h测量各处理的病斑大小。以上试验设5次生物学重复。
1.5 杜梨-G03悬浮细胞遗传转化和抗病性分析
用细胞过滤器(孔径40 目)过滤并收集杜梨-G03 悬浮细胞小细胞团,黑暗、110 r·min-1振荡培养3 d备用。携带目标基因的农杆菌的活化、扩繁和重悬方法同上。将农杆菌悬浮液和杜梨悬浮细胞以体积比1∶10混合,静置5 min后除去多余的农杆菌,黑暗静置培养48 h后用头孢菌素杀灭农杆菌,并转移至含有抗生素的MS 培养基中培养约20 d,挑取新长出的细胞团进行继代扩繁并利用PCR 和实时荧光定量PCR(quantitative real time polymerase chain reaction,qRT-PCR)筛选转化细胞系[11]。
选取过表达效果理想的3 个细胞系,对其进行Vp 和VpM 的抗性分析。悬浮细胞对Vp 的抗性分析:取野生型和过表达细胞系的细胞团1 mL(密实体积)平铺至MS平板,并在中心位置接种Vp菌饼,分别于接种后36、48、60 和72 h 测量病斑大小。在接种72 h时,利用MTT染料对接种的细胞进行染色并观察细胞活性[12]。悬浮细胞对VpM的抗性分析:用上述方法过滤收集各细胞系小细胞团并将浓度(φ)调整至20µL(密实体积)·mL-1,接种不同浓度的VpM,并于处理后1、3和6 h用于免疫反应相关基因的表达分析和细胞活性测定。各细胞系的细胞活性采用MTT染色法测定。
1.6 基因表达分析
苹果、梨果实和杜梨-G03 悬浮细胞总RNA 提取、cDNA 反转录和qRT-PCR 检测参考田丹等[13]的方法。根据孙娥等[14]的结果,选取模式触发免疫(PTI)、活性氧(ROS)、茉莉酸(JA)和水杨酸(SA)等免疫反应信号相关的基因共7 个,基因名称和引物序列等信息详见表1。内参基因Actin序列和引物选取根据Sun 等[12]的方法。基因的相对表达量采用2-ΔΔCT法计算[15]。
表1 qRT-PCR 分析相关基因及引物信息
Table 1 Information of gene and primers using for qRT-PCR assay
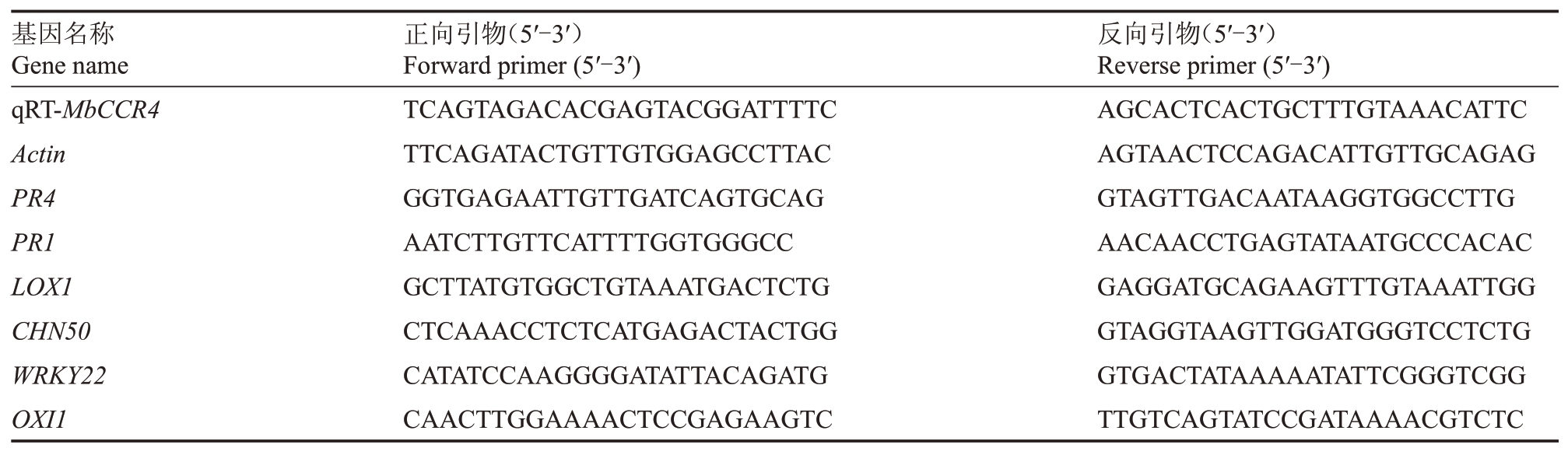
基因名称Gene name qRT-MbCCR4 Actin PR4 PR1 LOX1 CHN50 WRKY22 OXI1正向引物(5′-3′)Forward primer(5′-3′)TCAGTAGACACGAGTACGGATTTTC TTCAGATACTGTTGTGGAGCCTTAC GGTGAGAATTGTTGATCAGTGCAG AATCTTGTTCATTTTGGTGGGCC GCTTATGTGGCTGTAAATGACTCTG CTCAAACCTCTCATGAGACTACTGG CATATCCAAGGGGATATTACAGATG CAACTTGGAAAACTCCGAGAAGTC反向引物(5′-3′)Reverse primer(5′-3′)AGCACTCACTGCTTTGTAAACATTC AGTAACTCCAGACATTGTTGCAGAG GTAGTTGACAATAAGGTGGCCTTG AACAACCTGAGTATAATGCCCACAC GAGGATGCAGAAGTTTGTAAATTGG GTAGGTAAGTTGGATGGGTCCTCTG GTGACTATAAAAATATTCGGGTCGG TTGTCAGTATCCGATAAAACGTCTC
1.7 生物信息学分析
从美国国家生物技术信息中心(National Center for Biotechnology Information,NCBI;https://www.ncbi.nlm.nih.gov/)、拟南芥基因组数据库(Arabidopsis information resource,TAIR;http://www.arabidopsis.org)和蔷薇科数据库(Genome Database for Rosaceae,GDR;https://www.rosaceae.org/)下载所需基因组信息,使用Mafft v7.505[16]对17个物种的部分CR4L家族成员氨基酸全长序列进行多序列比对。采用Fast Tree[17]最大似然法构建系统发育树,利用JTT模型(1000次bootstrap重复)估算遗传距离。系统发育树的显示、操作和注释使用Interactive Tree of Life(iTOL,http://itol.embl.de/)。利用SMART(http://smart.embl.de/)进行结构域预测[18]。使用TBtools提取MbCCR4 基因上游2000 bp 序列,使用在线工具PlantCARE(http://bioinformatics.psb.ugent.be/webtools/plantcare/html/)预测其启动子区域的顺式作用元件[19]。
1.8 统计分析
采用Microsoft Excel(2016)软件进行数据的初步整理,采用t-test进行差异显著性检测(*p<0.05;**p<0.01)。
2 结果与分析
2.1 MbCCR4是CR4L亚家族成员
将MbCCR4的蛋白序列提交至拟南芥基因组网站,发现其与CCR4(AT5G47850.1)同源,故将其命名为MbCCR4。进一步通过序列比对获得该基因在17 个物种中的30 个同源基因,并构建了系统发育树,发现其与苹果中MD08G1217500 的同源性最高(图1-A)。结构域分析表明,MbCCR4 蛋白在N 端含有1个信号肽,1个RCC1_2结构域,1个跨膜区和1 个C 端的激酶结构域,为典型的CR4L 家族成员(图1-B)。顺式作用元件预测分析发现,MbCCR4启动子区域含有与MeJA、ABA以及信号传导、胁迫响应相关的元件(图1-C)。如上所述,MbCCR4 是典型的CR4L 的家族成员,其表达可能响应多种激素和逆境信号。
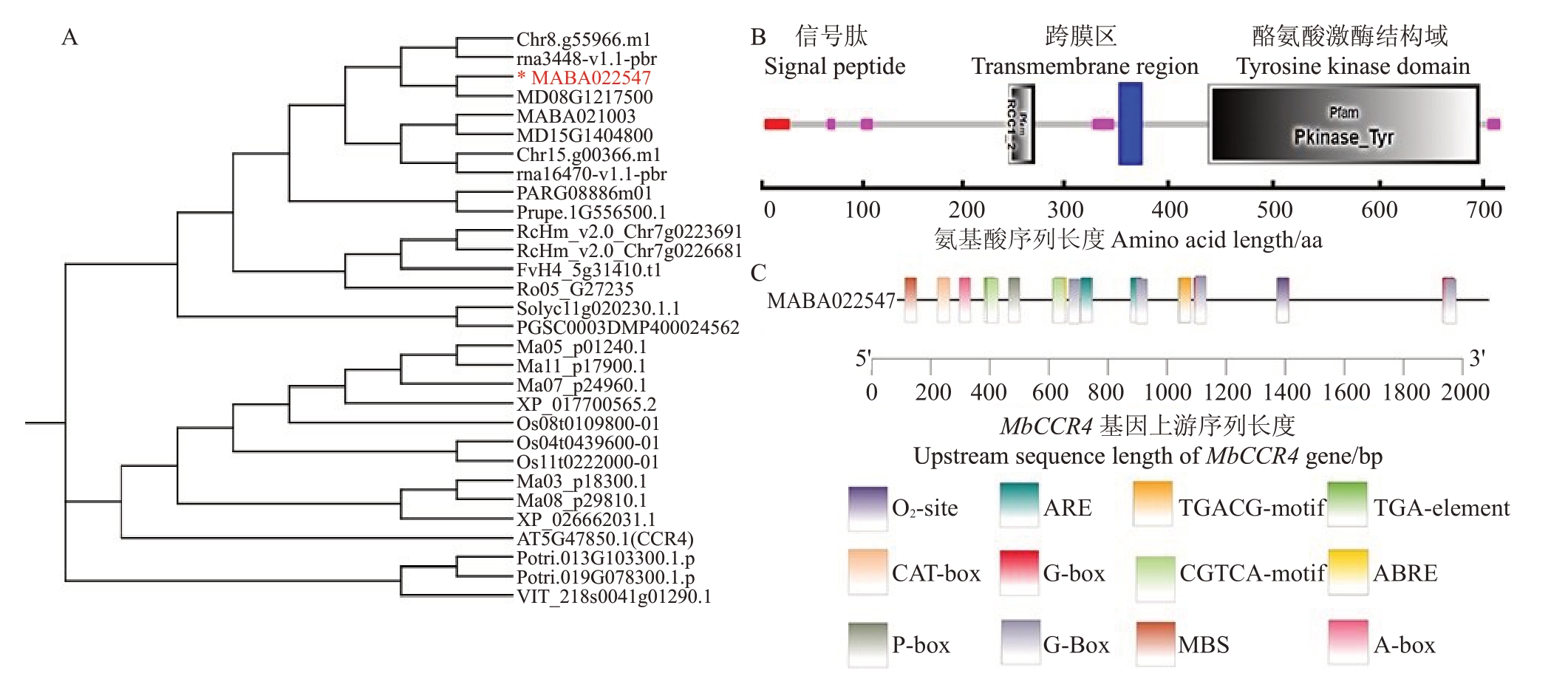
图1 MbCCR4 基本参数和序列系统发育树
Fig.1 Basic parameters and sequence phylogenetic tree of MbCCR4
A.进化分析;B.保守结构域分析;C.顺式作用元件预测。
A.Evolutionary analysis;B.Conserved domain analysis;C.Cis-acting element prediction.
2.2 MbCCR4响应腐烂病信号
根据20%VmM 处理山荆子悬浮细胞后的转录组数据,筛选出MbCCR4基因的表达量(图2)[20]。与对照相比,20%VmM 处理1 h 后,MbCCR4 的FPKM值由对照的47.37 上升至237.08。利用qRT-PCR 分析了20%VmM 处理后1 h、3 h 和6 h 山荆子野生型细胞中MbCCR4的表达模式。结果显示,VmM处理后MbCCR4 的表达被显著激活,处理1、3 和6 h 后MbCCR4 的表达上调至对照的456、202 和155 倍。以上结果表明,在山荆子响应腐烂病信号过程中,MbCCR4的表达被显著诱导。
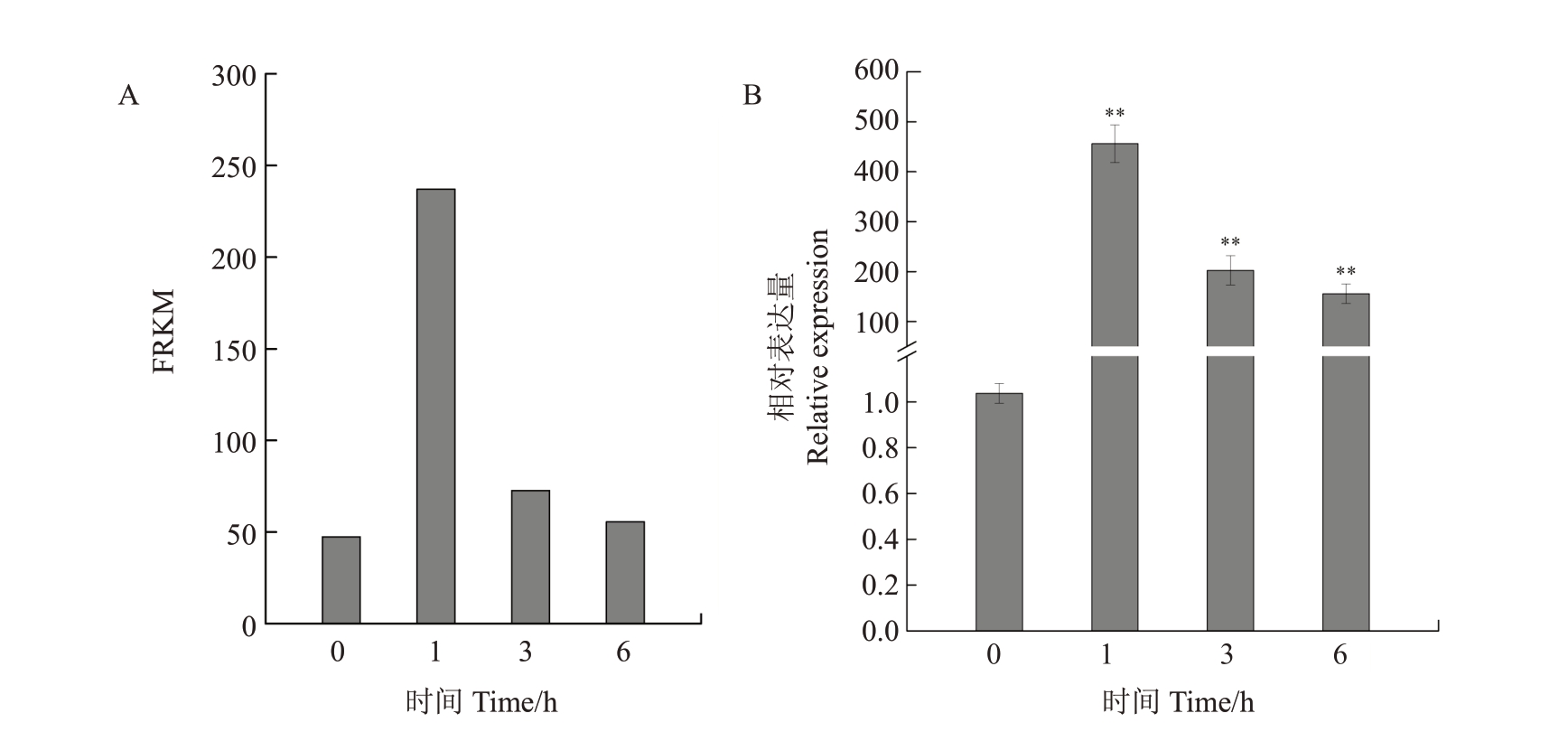
图2 MbCCR4 基因响应腐烂病信号的表达分析
Fig.2 The expression analysis of MbCCR4 respond to Valsa canker signals
用20%(φ)Valsa mali 代谢物(VmM)处理Malus baccata 悬浮细胞后通过RNA-seq(A)和qRT-PCR(B)检测MbCCR4 的表达。FPKM.每千个碱基的转录每百万映射读取的fragments。
Expression of MbCCR4 as determined by RNA-seq and qRT-PCR(B)in Malus baccata suspension cells inoculated with 20%(φ)solution of Valsa mali metabolites(VmM).FPKM.Fragments per kilobase of exon model per million mapped fragments.
2.3 MbCCR4正调控苹果和梨果实的腐烂病抗性
利用农杆菌介导的瞬时表达检测山荆子基因MbCCR4 对苹果和梨腐烂病的抗性(图3)。结果表明,接种病原菌36 h时,烟富3号苹果和黄冠梨果实接种部位逐渐发病,60、72和84 h时,过表达苹果和梨果实(A-pFGC5941-MbCCR4 和P-pFGC5941-MbCCR4)的病斑直径都显著小于空载体(A-pFGC5941 和P-pFGC5941)(图3-C)。qRT-PCR 检测证实,与空载体相比,MbCCR4在过表达后其表达量显著上调(图3-B)。因此,MbCCR4 基因的过表达显著增强了苹果和梨果实的腐烂病抗性。
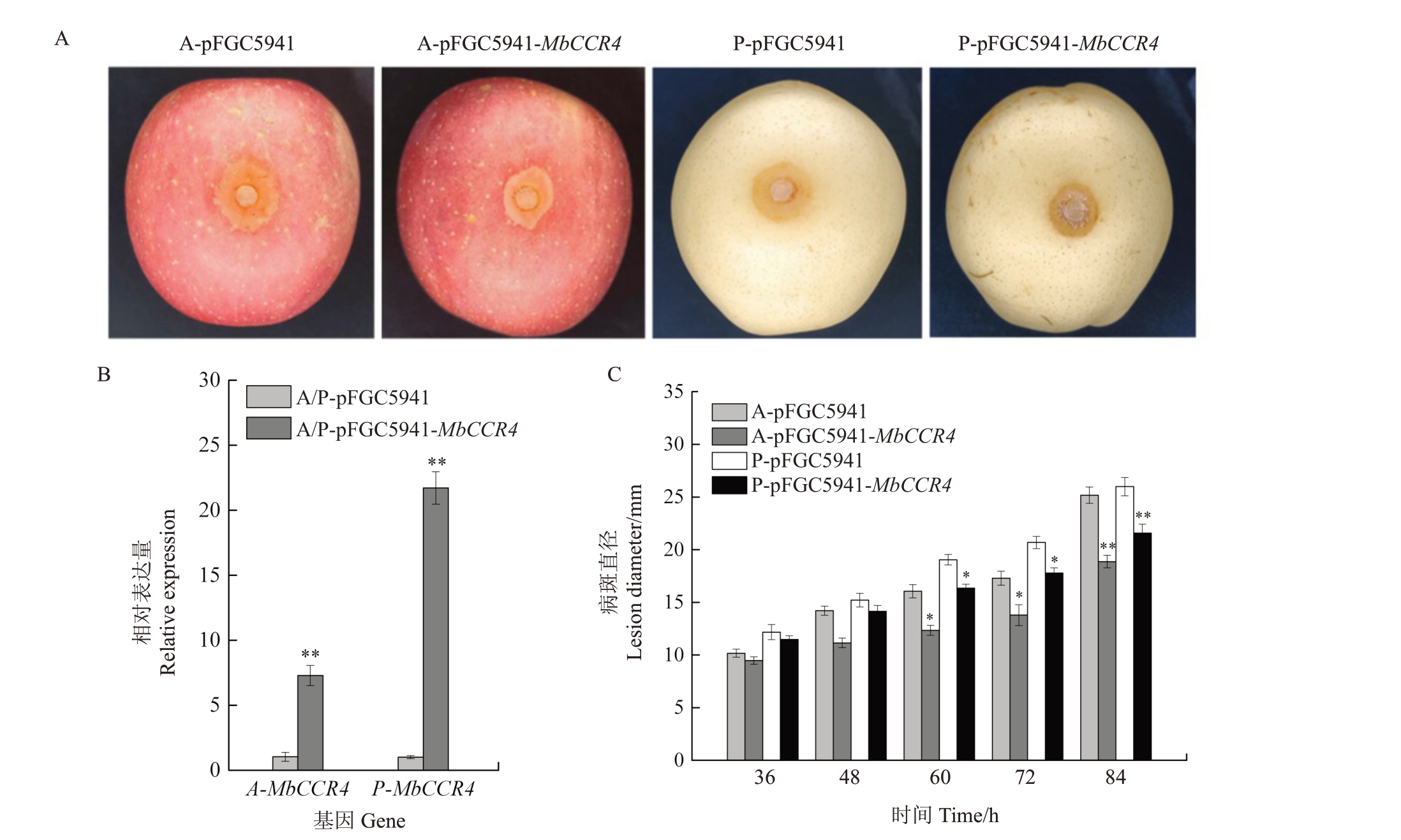
图3 MbCCR4 在苹果和梨果实中的瞬时表达分析
Fig.3 Transient expression analysis of MbCCR4 in apple and pear fruits
A.MbCCR4 过表达60 h 后在黄冠梨和烟富3 号苹果果实上对Vp 和Vm 感染的抗性;B.过表达后MbCCR4 的表达量。C.空载体对照、过表达果实在感染Vp 后的病变直径。数据为平均值(±SD),n=3。*p<0.05,**p<0.01。下同。
A.The resistance of MbCCR4 to Vp and Vm infection at the fruit of Huangguan pear and Yanfu 3 apple after overexpression of MbCCR4 at 60 h;B.The expression of MbCCR4 after over-expression;C.The lesion diameters on the empty-vector controls,overexpression fruits following infection with Valsa.The date were mean(±SD),n=3.*p<0.05,**p<0.01.The same below.
2.4 MbCCR4 正调控杜梨悬浮细胞对腐烂病菌的抗性
利用农杆菌介导的遗传转化,将MbCCR4 转入杜梨-G03悬浮细胞,并获得3个过表达细胞系,分别命名为MbCCR4-OE1、MbCCR4-OE2 和MbCCR4-OE6。与野生型细胞相比,接种Vp 72 h 时,过表达细胞的菌落直径显著小于野生型细胞(WT),MTT染色获得了相似的结果(图4-A)。经病斑统计发现,在接种病原菌48 h时,WT细胞上病斑直径已达1.48 cm,而过表达细胞系MbCCR4-OE1、MbCCR4-OE2 和MbCCR4-OE6 的病斑大小分别为0.48、0.25和0.76 cm,且在72 h 时病斑差异最为明显(图4-B)。qRT-PCR 分析发现,过表达细胞系MbCCR4-OE1、MbCCR4-OE2 和MbCCR4-OE6 中MbCCR4 的表达量分别上调至野生型细胞的11.1、6.8 和5.8 倍(图4-C)。综上,过表达MbCCR4 显著提高了杜梨悬浮细胞对Vp的抗性。
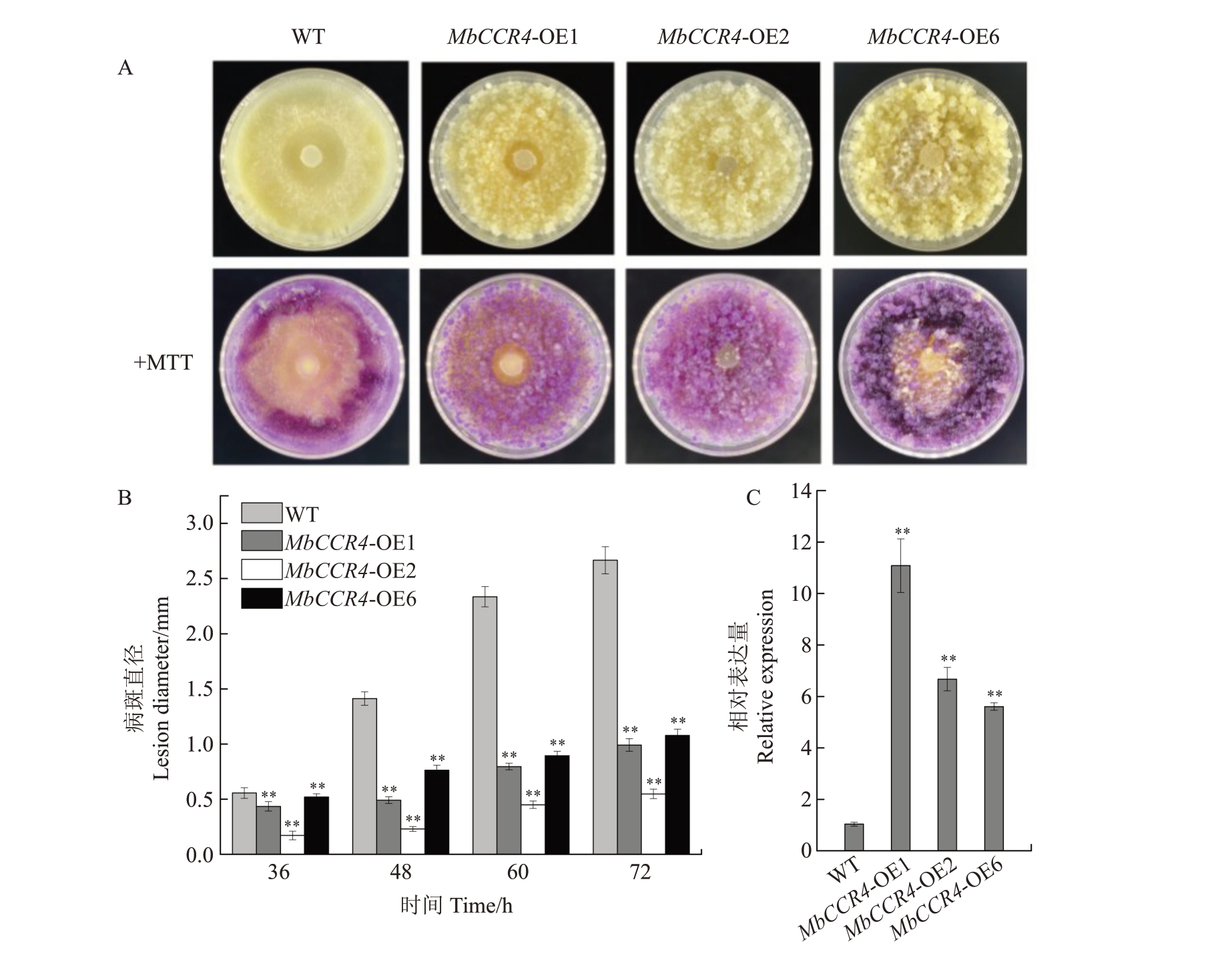
图4 MbCCR4 正调控杜梨-G03 悬浮细胞对腐烂病菌的抗性
Fig.4 MbCCR4 positively regulates the resistance of Duli-G03 suspension cells to Valsa pyri
A.接种Vp 3 d 后,野生型(WT)细胞和3 个MbCCR4 过表达细胞在平板上的病变。平板用MTT 染色液染色以鉴定细胞活性(活细胞呈紫色);B.接种Vp 36、48、60 和72 h 后,WT 和转基因株系上的病斑直径;C.以Actin 为参照物,通过qRT-PCR 测定转基因株系中MbCCR4的相对表达水平。表达量是相对于WT 的,其值设为1。
A.Lesions on plates of wild-type(WT)cells and cells of three MbCCR4-overexpressing lines 3 d after inoculation with Vp;B.The lesion diameters of WT and transgenic lines were observed at 36 h,48 h,60 h and 72 h after inoculation with Vp;C.The relative expression level of MbCCR4 in transgenic lines was determined by qRT-PCR,setting Actin as the reference.The expression level is relative to WT,and its value is set to 1.
2.5 MbCCR4 正调控杜梨悬浮细胞对腐烂病菌代谢物的抗性
通过使用细胞活力跟踪检测MbCCR4-OE2 和WT细胞对VpM的耐受性(图5),结果表明,与野生型相比,当用20%的VpM 处理1、3 和6 h 时,MbCCR4-OE2的细胞活力显著高于野生型细胞,分别比对照高出了1.1、1.09 和1.21 倍。这些结果表明,MbCCR4 的过表达显著增强了杜梨-G03 悬浮细胞对VpM的耐受性。
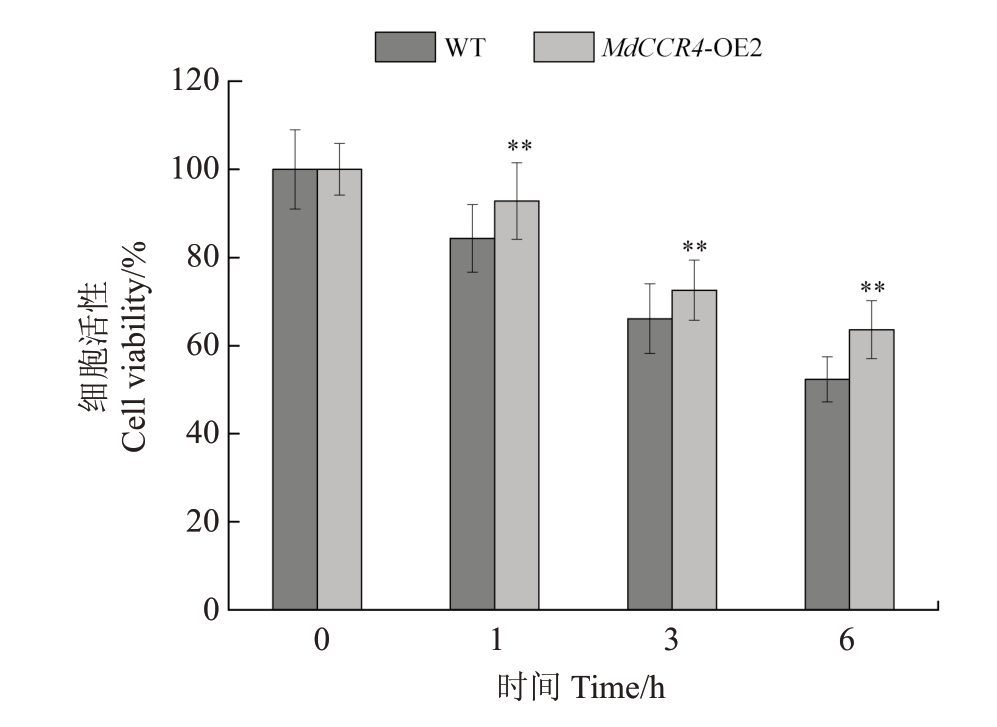
图5 过表达MbCCR4 增强了Duli-G03 悬浮细胞对VpM 的耐受性
Fig.5 The overexpression of MbCCR4 enhanced tolerance of Duli-G03 cells to VpM
野生型细胞和MbCCR4 过表达细胞在20%VpM 处理下的细胞活性。
Viability of wild-type cells and overexpression cells of MbCCR4 with 20%VpM treatment.
2.6 MbCCR4 过表达诱导杜梨悬浮细胞响应腐烂病信号过程中免疫反应相关基因的表达
为了研究MbCCR4 激活的信号通路,分析了与植物免疫直接相关的PTI、ROS、JA 和SA 信号相关的关键基因的表达(图6)。与野生型相比,MbCCR4的过表达导致PTI 相关基因WRKY22、ROS 相关基因OXI1、SA 相关基因PR1 和PR4 以及JA 相关基因CHN50 和LOX1 的上调表达。其中,在VpM 处理后,SA相关基因PR1和PR4的表达在所有时间点都高于野生型细胞。因此,多种免疫信号,包括PTI、ROS、SA和JA参与了MbCCR4调节的防御反应。
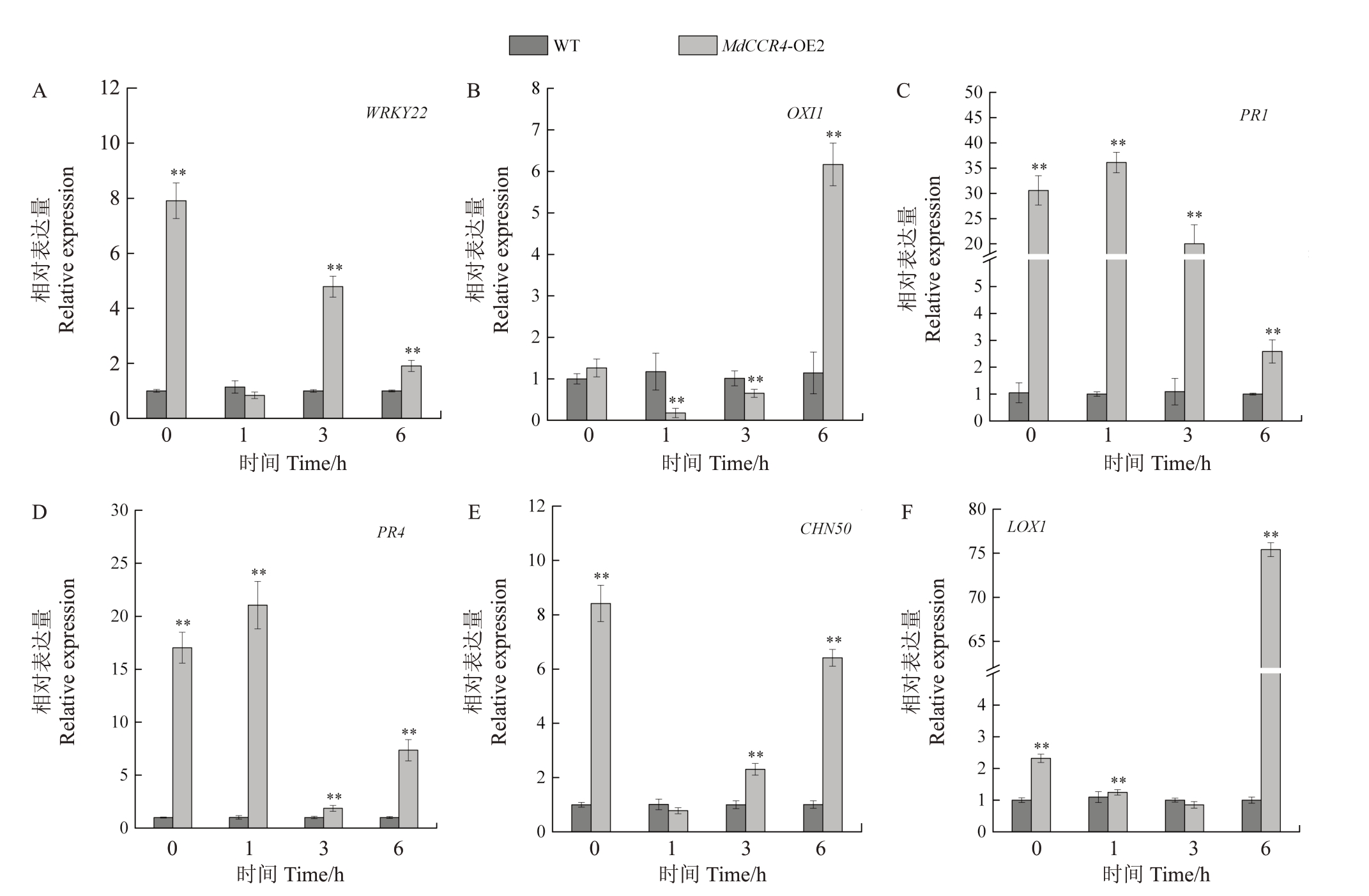
图6 MbCCR4 诱导多个免疫反应相关基因的表达
Fig.6 MbCCR4 induce the expression of multiple immune response related genes
A. PTI 信号通路中的WRKY22;B. ROS 信号通路中的OXI1;C~D. SA 信号通路中的PR1 和PR4;E~F. JA 信号通路中的CHN50 和LOX1。
A.WRKY22 in PTI signaling pathway;B.OXI1 in ROS signaling pathway;C-D.PR1 and PR4 in SA signaling pathway;E-F.CHN50 and LOX1 in JA signaling pathway.
3 讨论
通过对山荆子的CR4 基因进行生物信息学分析、表达分析及关键基因MbCCR4的功能分析,结果表明,MbCCR4与MD08G1217500亲缘关系较近,推测他们可能发挥类似的功能。启动子区域多种胁迫相关顺式调控元件的分布表明,MbCCR4 基因的调控与表达可能受到多种激素共同调控,其中茉莉酸、脱落酸与水杨酸可能在诱导MbCCR4基因表达过程中扮演重要角色,这与之前李婉莹等[21]的研究结果一致。结构域分析表明,MbCCR4 是典型的CR4 家族成员。
RLKs作为模式识别受体的重要组成部分,在信号传导网络中扮演着关键角色,是植物感知环境信号的关键蛋白激酶,也是植物中最大的受体蛋白家族,对植物适应环境变化具有重要意义[22-23]。突出的例子是鞭毛蛋白传感2(Flagellin sensitive 2,FLS2)和延伸因子Tu(elongation factor Tu,EF-Tu)受体(EFR),分别识别细菌鞭毛蛋白和EF-Tu启动植物的防御[24]。此外,凝集素类受体激酶LecRK-I.9 或LecRK-IX.1 可以提高烟草对疫霉菌病原体的抗性[25]。CRINKLY4(CR4)是RLK 亚家族中的一个分支,在拟南芥中主要调控叶片细胞分化、花器官发育和细胞壁发育等过程。其成员ACR4能够感知分泌肽CLE40调节远端根分生组织中的干性稳态[26]。而本研究中的结果表明MbCCR4在调控腐生病原菌的抗性中起着重要的作用,但对其感知的配体还未进行研究,未来可对其配体及共受体展开更深入的研究,这对明确MbCCR4 调控腐烂病抗性的分子机制具有重要的意义。
植物不断受到有害微生物病原体的攻击,为了保护自己免受这些不同的胁迫,植物进化出了高度受调控的防御系统,主要由SA、JA、乙烯(ET)和脱落酸(ABA)等小分子激素协调[27-29]。SA 通常诱导植物对生物营养病原体的防御[30],被认为是诱导植物系统获得抗性(systemic acquired resistance,SAR)的关键信号分子[31]。此外,外施SA可以增强烟草对花叶病毒的抗性[32]。JA和ET是诱导植物防御坏死性病原体的重要激素调节因子[33-35],前期研究证实,CR4在茉莉酸信号中和对灰霉病菌的抗性起着重要的作用[8]。SA对JA途径的影响可以是拮抗、协同或中性,但在拟南芥中的研究结果表明拮抗相互作用似乎占主导地位[36],但笔者在本研究中发现20%VpM处理后,野生型和过表达系中JA信号通路基因CHN50 和LOX1 和SA 信号通路基因PR1 和PR4 的表达都被显著激活,猜测在MbCCR4 调控腐烂病菌的抗性作用中SA 和JA 可能是协同发挥作用,但具体的影响还待笔者更深入地研究。
4 结论
从山荆子中筛选获得了1个响应腐烂病信号的CR4家族成员MbCCR4。进一步的功能分析表明其正调控腐烂病抗性,过表达MbCCR4 主要激活了植物体内的SA和JA相关信号,进而限制了腐生病原菌的进一步入侵。这一研究结果不仅为腐烂病的抗病育种提供了理论依据,也对生产实践具有重要意义。
[1] WANG X L,WEI J L,HUANG L L,KANG Z S.Re-evaluation of pathogens causing Valsa canker on apple in China[J].Mycologia,2011,103(2):317-324.
[2] 张美鑫,翟立峰,周玉霞,陈晓忍,贾娜娜,洪霓,王国平.我国梨树腐烂病菌致病力分化分析[J].果树学报,2013,30(4):657-664.ZHANG Meixin,ZHAI Lifeng,ZHOU Yuxia,CHEN Xiaoren,JIA Nana,HONG Ni,WANG Guoping.Pathogenicity differentiation of Valsa mali var.pyri causing pear stem canker in China[J].Journal of Fruit Science,2013,30(4):657-664.
[3] KE X W,HUANG L L,HAN Q M,GAO X N,KANG Z S.Histological and cytological investigations of the infection and colonization of apple bark by Valsa mali var. mali[J]. Australasian Plant Pathology,2013,42(1):85-93.
[4] DELPLACE F,HUARD-CHAUVEAU C,BERTHOMÉ R,ROBY D. Network organization of the plant immune system:From pathogen perception to robust defense induction[J]. The Plant Journal,2022,109(2):447-470.
[5] MONAGHAN J,ZIPFEL C. Plant pattern recognition receptor complexes at the plasma membrane[J]. Current Opinion in Plant Biology,2012,15(4):349-357.
[6] WU Y,ZHOU J M. Receptor-like kinases in plant innate immunity[J].Journal of Integrative Plant Biology,2013,55(12):1271-1286.
[7] 吕前前,刘钰玺,李静轩,马宗桓,姜雪峰,王宝林,毛娟,褚明宇,陈佰鸿,左存武.苹果CR4 基因家族鉴定与表达分析[J].园艺学报,2018,45(10):2019-2029.LÜ Qianqian,LIU Yuxi,LI Jingxuan,MA Zonghuan,JIANG Xuefeng,WANG Baolin,MAO Juan,CHU Mingyu,CHEN Baihong,ZUO Cunwu. Identification and expression analysis of CR4 in apple[J].Acta Horticulturae Sinica,2018,45(10):2019-2029.
[8] E-ZEREEN J,INGRAM G.A possible involvement of ACR4,a receptor like kinase,in plant defence mechanism[J]. Bangladesh Pharmaceutical Journal,2012,15(2):127-130.
[9] 刘欣颖,吕松,王忆,王昆,李天红,韩振海,张新忠.苹果种质资源对苹果树腐烂病抗性评价[J].果树学报,2011,28(5):843-848.LIU Xinying,LÜ Song,WANG Yi,WANG Kun,LI Tianhong,HAN Zhenhai,ZHANG Xinzhong. Evaluation of resistance of Malus germplasms to apple canker (Valsa ceratosperma) [J].Journal of Fruit Science,2011,28(5):843-848.
[10] YU H Q,SUN E,MAO X,CHEN Z J,XU T,ZUO L G,JIANG D J,CAO Y N,ZUO C W. Evolutionary and functional analysis reveals the crucial roles of receptor-like proteins in resistance to Valsa canker in Rosaceae[J]. Journal of Experimental Botany,2023,74(1):162-177.
[11] MAO X,WANG C,LV Q Q,TIAN Y Z,WANG D D,CHEN B H,MAO J,LI W F,CHU M Y,ZUO C W.Cyclic nucleotide gated channel genes (CNGCs) in Rosaceae:Genome-wide annotation,evolution and the roles on Valsa canker resistance[J]. Plant Cell Reports,2021,40(12):2369-2382.
[12] SUN E,YU H Q,CHEN Z J,CAI M R,MAO X,LI Y Y,ZUO C W. Enhanced Valsa canker resistance conferred by expression of MdLecRK-S. 4.3 in Pyrus betulifolia is largely suppressed by PbePUB36[J]. Journal of Experimental Botany,2023,74(14):3998-4013.
[13] 田丹,朵虎,刘河,吕前前,左存武.苹果L-LEC-RLK 基因家族鉴定及响应病原真菌信号的表达分析[J].园艺学报,2019,46(3):421-432.TIAN Dan,DUO Hu,LIU He,LÜ Qianqian,ZUO Cunwu. Genome wide identification and expression patterns in response to signals from fungal pathogens of L-LEC-RLK gene family in apple[J].Acta Horticulturae Sinica,2019,46(3):421-432.
[14] 孙娥,闫文萍,余宏强,赵丹,朵虎,左存武.苹果和梨抗病相关基因的诱导与表达分析[J]. 西北植物学报,2022,42(9):1468-1476.SUN E,YAN Wenping,YU Hongqiang,ZHAO Dan,DUO Hu,ZUO Cunwu. Induction and expression analysis of the disease resistance related genes in apple and pear[J].Acta Botanica Boreali-Occidentalia Sinica,2022,42(9):1468-1476.
[15] LIVAK K J,SCHMITTGEN T D.Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method[J].Methods,2001,25(4):402-408.
[16] ROZEWICKI J,LI S L,AMADA K M,STANDLEY D M,KATOH K. MAFFT-DASH:Integrated protein sequence and structural alignment[J]. Nucleic Acids Research,2019,47(W1):W5-W10.
[17] PRICE M N,DEHAL P S,ARKIN A P. FastTree:Computing large minimum evolution trees with profiles instead of a distance matrix[J]. Molecular Biology and Evolution,2009,26(7):1641-1650.
[18] LETUNIC I,KHEDKAR S,BORK P.SMART:Recent updates,new developments and status in 2020[J]. Nucleic Acids Research,2021,49(D1):D458-D460.
[19] LESCOT M,DÉHAIS P,THIJS G,MARCHAL K,MOREAU Y,VAN DE PEER Y,ROUZÉ P,ROMBAUTS S.PlantCARE,a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences[J].Nucleic Acids Research,2002,30(1):325-327.
[20] WANG C,MAO X,ZHAO D,YU H Q,DUO H,SUN E,LU Y,ZUO C W. Transcriptomic analysis reveals that cell wall- and hypersensitive response (HR)-related genes are involved in the responses of apple to Valsa mali[J]. Plant Biotechnology Reports,2022,16(5):539-551.
[21] 李婉莹,马乃膺,左存武,毛娟,李文芳,陈佰鸿,褚明宇.葡萄CR4 类受体激酶基因家族的鉴定及表达分析[J].果树学报,2022,39(4):518-531.LI Wanying,MA Naiying,ZUO Cunwu,MAO Juan,LI Wenfang,CHEN Baihong,CHU Mingyu.Identification and bioinformatics analysis of CR4 receptor- like kinase gene family in grapevine[J].Journal of Fruit Science,2022,39(4):518-531.
[22] TANG D Z,WANG G X,ZHOU J M.Receptor kinases in plantpathogen interactions:More than pattern recognition[J]. The Plant Cell,2017,29(4):618-637.
[23] LI X P,ZHANG J J,SHI H Y,LI B,LI J. Rapid responses:Receptor-like kinases directly regulate the functions of membrane transport proteins in plants[J].Journal of Integrative Plant Biology,2022,64(7):1303-1309.
[24] COUTO D,ZIPFEL C. Regulation of pattern recognition receptor signalling in plants[J]. Nature Reviews Immunology,2016,16(9):537-552.
[25] ZHU Y F,HU C,CUI Y W,ZENG L,LI S,ZHU M S,MENG F H,HUANG S T,LONG L,YI J,LI J,GOU X P.Conserved and differentiated functions of CIK receptor kinases in modulating stem cell signaling in Arabidopsis[J]. Molecular Plant,2021,14(7):1119-1134.
[26] WANG Y,NSIBO D L,JUHAR H M,GOVERS F,BOUWMEESTER K. Ectopic expression of Arabidopsis L-type lectin receptor kinase genes LecRK-I.9 and LecRK-IX.1 in Nicotiana benthamiana confers Phytophthora resistance[J]. Plant Cell Reports,2016,35(4):845-855.
[27] PIETERSE C M J,VAN DER DOES D,ZAMIOUDIS C,LEON-REYES A,VAN WEES S C M. Hormonal modulation of plant immunity[J]. Annual Review of Cell and Developmental Biology,2012,28:489-521.
[28] ROBERT-SEILANIANTZ A,GRANT M,JONES J D G. Hormone crosstalk in plant disease and defense:more than just jasmonate-salicylate antagonism[J].Annual Review of Phytopathology,2011,49:317-343.
[29] VOS I A,PIETERSE C M J,VAN WEES S C M.Costs and benefits of hormone-regulated plant defences[J]. Plant Pathology,2013,62(Suppl.1):43-55.
[30] GLAZEBROOK J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens[J]. Annual Review of Phytopathology,2005,43:205-227.
[31] MINE A,SEYFFERTH C,KRACHER B,BERENS M L,BECKER D,TSUDA K. The defense phytohormone signaling network enables rapid,high- amplitude transcriptional reprogramming during effector-triggered immunity[J].The Plant Cell,2018,30(6):1199-1219.
[32] 李国婧,周燮.植物防御反应中水杨酸与茉莉酸的“对话”机制[J].细胞生物学杂志,2002,24(2):101-105.LI Guojing,ZHOU Xie.The“Dialogue”mechanism of salicylic acid and jasmonic acid in plant defense responses[J]. Chinese Journal of Cell Biology,2002,24(2):101-105.
[33] 徐刚,姚银安.水杨酸、茉莉酸和乙烯介导的防卫信号途径相互作用的研究进展[J].生物学杂志,2009,26(1):48-51.XU Gang,YAO Yin’an. The cross-talk between salicylic acid,jasmonic acid and ethylene defense pathway[J].Journal of Biology,2009,26(1):48-51.
[34] PROIETTI S,CAARLS L,COOLEN S,VAN PELT J A,VAN WEES S C M,PIETERSE C M J. Genome-wide association study reveals novel players in defense hormone crosstalk in Arabidopsis[J]. Plant,Cell & Environment,2018,41(10):2342-2356.
[35] BOUWMEESTER K,HAN M,BLANCO- PORTALES R,SONG W,WEIDE R,GUO L Y,VAN DER VOSSEN E A G,GOVERS F. The Arabidopsis lectin receptor kinase LecRK-I. 9 enhances resistance to Phytophthora infestans in Solanaceous plants[J].Plant Biotechnology Journal,2014,12(1):10-16.
[36] TSUDA K,SATO M,STODDARD T,GLAZEBROOK J,KATAGIRI F. Network properties of robust immunity in plants[J].PLoS Genetics,2009,5(12):e1000772.