近年来,随着葡萄产业技术的不断成熟和消费市场的多样化,无核葡萄(Vitis vinifera L.)的需求量大幅增加[1],但无核葡萄品种较少,特别是性状优良的无核葡萄品种较少,多数无核品种存在着果粒较小、抗性差等问题[2],急需借助育种手段培育新的无核葡萄品种,满足市场对无核葡萄的需求。传统的无核葡萄育种主要以有核葡萄作母本与无核葡萄父本杂交,杂交后代仅有0%~5.9%的无核率,选育一个无核葡萄新品种需要15~20 a(年),育种效率较低[3]。采用胚挽救技术可对以无核葡萄作母本进行杂交获得的合子胚在其败育前进行人工离体培养,然后促使幼胚发育一定时间后萌发成苗,随着胚挽救技术的发展和不断完善,已经被广泛应用于中国无核葡萄育种中,成为无核葡萄育种的重要手段[4-5]。
影响葡萄胚挽救成苗的因素主要包含亲本基因型、取样时间、胚离体培养时间、胚珠处理方法、培养基类型、培养基添加物、培养条件等[6],其中亲本基因型,特别是母本基因型对无核葡萄胚挽救成苗率有较大的影响,选择合适的母本,有助于提高无核葡萄胚挽救成苗率,Ulaş 等[7]和Liu 等[8]通过选取多种母本进行对比,发现克伦生适合作为胚挽救母本,刘可可等[9]研究认为火焰无核和红宝石无核的胚挽救成苗率较高,适合作为母本。此外,杂交幼果的取样时间对胚挽救成苗率也具有重要影响,史文静等[10]研究认为,火焰无核和昆香无核的最佳采样时间为首次授粉后45 d 和51 d,郝燕等[11]认为火焰无核为母本时最佳采样时间为首次授粉后40 d,因此通过试验筛选出适合不同无核葡萄品种作为母本时的最佳幼果采样时间也具有十分重要的意义。利用分子标记技术,在植物苗期即可对其性状进行鉴定,从而缩短育种周期[12],常用的葡萄无核分子标记有p3-VvAGL11、5U-VviAGL11、SCF27-2000、GSLP1-569、SCC8-1018 以及VMCF7F2-198等[13-17],p3-VvAGL11和SCF27-2000检测无核性状的准确率较高[18]。笔者主要对影响无核葡萄胚挽救成苗率的母本基因型以及取样时间开展研究,辅以p3-VvAGL11、GLSP1-569、SCF27-2000、SCC8-1018 等无核分子标记鉴定,以期对提高无核葡萄新品种培育的效率和创制无核葡萄新种质材料提供一定的参考价值。
1 材料和方法
1.1 试验材料
本试验以欧亚种葡萄(V. vinifera)火焰无核(Flame Seedless)、紫脆无核(Zicui Seedless)、森田尼无核(Centennial Seedless)、红宝石无核(Ruby Seedless)为母本;以欧亚种(V. vinifera)京香玉(Jingxiangyu),欧美杂交种葡萄(V.vinifera×V.labrusca)户太8号(Hutai No.8)、阳光玫瑰(Shine Muscat)、醉金香(Zuijinxiang)等为父本,共9个杂交组合(表1)。
表1 杂交组合配置
Table 1 Hybrid combination settings
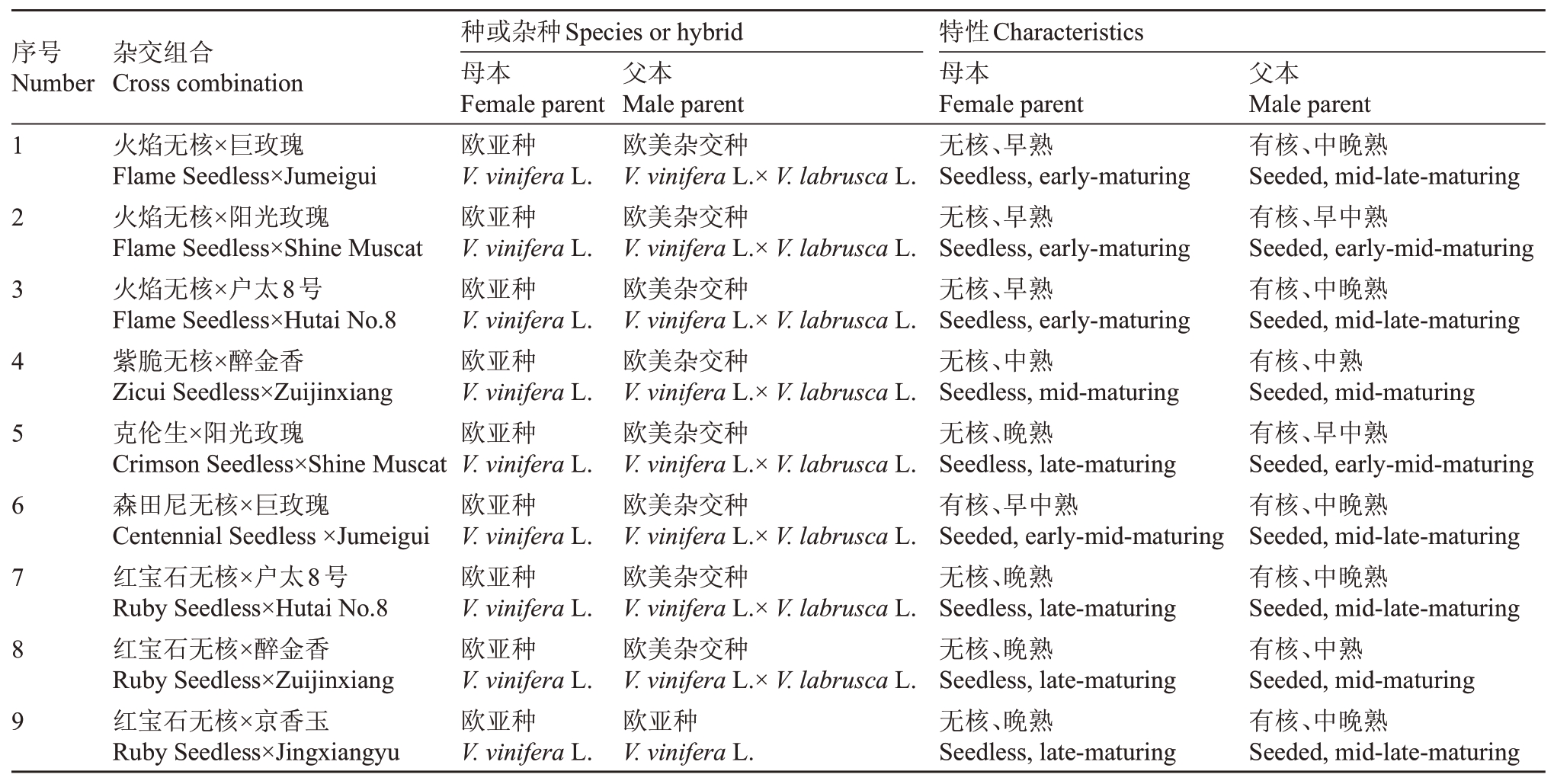
序号Number杂交组合Cross combination 123456789火焰无核×巨玫瑰Flame Seedless×Jumeigui火焰无核×阳光玫瑰Flame Seedless×Shine Muscat火焰无核×户太8号Flame Seedless×Hutai No.8紫脆无核×醉金香Zicui Seedless×Zuijinxiang克伦生×阳光玫瑰Crimson Seedless×Shine Muscat森田尼无核×巨玫瑰Centennial Seedless×Jumeigui红宝石无核×户太8号Ruby Seedless×Hutai No.8红宝石无核×醉金香Ruby Seedless×Zuijinxiang红宝石无核×京香玉Ruby Seedless×Jingxiangyu种或杂种Species or hybrid母本Female parent欧亚种V.vinifera L.欧亚种V.vinifera L.欧亚种V.vinifera L.欧亚种V.vinifera L.欧亚种V.vinifera L.欧亚种V.vinifera L.欧亚种V.vinifera L.欧亚种V.vinifera L.欧亚种V.vinifera L.父本Male parent欧美杂交种V.vinifera L.×V.labrusca L.欧美杂交种V.vinifera L.×V.labrusca L.欧美杂交种V.vinifera L.×V.labrusca L.欧美杂交种V.vinifera L.×V.labrusca L.欧美杂交种V.vinifera L.×V.labrusca L.欧美杂交种V.vinifera L.×V.labrusca L.欧美杂交种V.vinifera L.×V.labrusca L.欧美杂交种V.vinifera L.×V.labrusca L.欧亚种V.vinifera L.特性Characteristics母本Female parent无核、早熟Seedless,early-maturing无核、早熟Seedless,early-maturing无核、早熟Seedless,early-maturing无核、中熟Seedless,mid-maturing无核、晚熟Seedless,late-maturing有核、早中熟Seeded,early-mid-maturing无核、晚熟Seedless,late-maturing无核、晚熟Seedless,late-maturing无核、晚熟Seedless,late-maturing父本Male parent有核、中晚熟Seeded,mid-late-maturing有核、早中熟Seeded,early-mid-maturing有核、中晚熟Seeded,mid-late-maturing有核、中熟Seeded,mid-maturing有核、早中熟Seeded,early-mid-maturing有核、中晚熟Seeded,mid-late-maturing有核、中晚熟Seeded,mid-late-maturing有核、中熟Seeded,mid-maturing有核、中晚熟Seeded,mid-late-maturing
1.2 试验方法
1.2.1 田间杂交 去雄:在花穗有5~10个花蕾开放时,将已开放花朵去除干净,用镊子或指尖掐去花帽,露出雄蕊,然后用镊子或指尖掐去花药,只保留柱头,在操作时要注意不要伤及柱头,以免影响授粉效果。在雄蕊去除干净以后,立即向花穗喷洒纯水进行保湿并套袋,然后用铅笔将去雄时间用吊牌做好标记,悬挂于花穗的枝条上。
人工授粉:在去雄结束后2~3 d授粉,观察柱头上的黏液情况,柱头上出现小水滴式黏液时,为授粉最佳状态,按照每30 穗花穗使用40 mL 花粉,将花粉倒入测序袋中,用测序袋将花穗套住,袋内充满空气后扎紧袋口,轻轻拍击测序袋底部使花粉飞起,均匀散落在柱头上。授粉后,重新将纸袋套好,在吊牌上标记好授粉时间以及花粉品种,在首次授粉结束后的第2天上午重复进行一次授粉,确保授粉充分,提高授粉效果。
1.2.2 设定幼果采样时间 幼果采样时间,按照首次授粉后天数来计算,在本试验中,火焰无核为母本的杂交组合按照36、38、40 d 时间梯度采样;以克伦生为母本的杂交组合按照44、46、48、50 d 时间梯度采样;以红宝石无核为母本的杂交组合按照53、55、57 d 时间梯度采样,其余杂交组合采样时间参考实验室以往采样时期,用剪刀连同果柄一起将果实剪下,放置于塑料袋中,然后将其放置于冰盒之中带回实验室,放置于4 ℃冰箱中待用。
1.2.3 胚珠离体培养 将葡萄果粒放置于网兜中用流水冲洗不少于4 h,冲洗完毕后,将果粒放置于螺口瓶中并在超净工作台中消毒。消毒具体步骤如下:使用75%乙醇冲洗果粒30 s,倒掉废液,用无菌水冲洗果粒2~3 次,将无菌水倒掉;再用1%的次氯酸钠消毒20 min,无菌水冲洗2~3 次,倒掉废液。在超净工作台内,将灭菌的幼果胚珠剥离,并将剥离好的胚珠整齐地摆放在MM3胚发育培养基上,室温下遮光培养8~12 周。MM3 胚发育培养基的成分为:MM3+0.5 g·L-1水解酪蛋白+60 g·L-1蔗糖+3 g·L-1植物凝胶+1.5 g·L-1活性炭+0.1 g·L-1肌醇。
1.2.4 胚萌发培养 胚发育培养结束后,将胚珠放置于解剖镜下,对胚珠尖端进行纵剖,在解剖镜下可以看到明亮的乳白色胚,用手术刀将胚挑出,放置于WPM 胚萌发培养基上,室温光照培养40~60 d。WPM胚萌发培养基的成分为:WPM+3 g·L-1 植物凝胶+0.2 mg·L-1 6-BA+0.1 g·L-1肌醇+1.5 g·L-1活性炭+20 g·L-1蔗糖。胚挽救具体流程见图1。

图1 胚挽救流程
Fig.1 Process of embryo rescue
A.去雄;B.套袋;C.授粉前花穗黏液;D.授粉;E.套袋;F.杂交果穗;G.果实灭菌;H.胚珠离体培养;I.剥取幼胚;J.胚萌发培养;K.胚挽救苗全封盖;L.胚挽救苗半揭盖;M.胚挽救苗全揭盖;N.温室炼苗;O.大田移栽。
A. Emasculation; B. Bagging; C. Inflorescence mucus before pollination; D. Pollination; E. Bagging; F. Hybrid fruit ears; G. Fruit sterilization;H. In vitro culture of ovules; I. Peel young embryos; J. Embryo germination Culture; K. Embryo rescue seedlings fully covered; L. Embryo rescue seedlings partially uncovered;M.Embryo rescue seedlings fully uncovered;N.Greenhouse seedling refining;O.Field transplantation.
1.2.5 杂种后代无核性状分子标记检测 利用CTAB 法提取葡萄杂交亲本及其子代的叶片DNA,利用无核分子标记p3-VvAGL11、GLSP1-569、SCF27-2000、SCC8-1018 进行无核性状检测。引物序列及分子标记长度见表2。
表2 引物序列及分子标记长度
Table 2 Primer sequences and fragment length
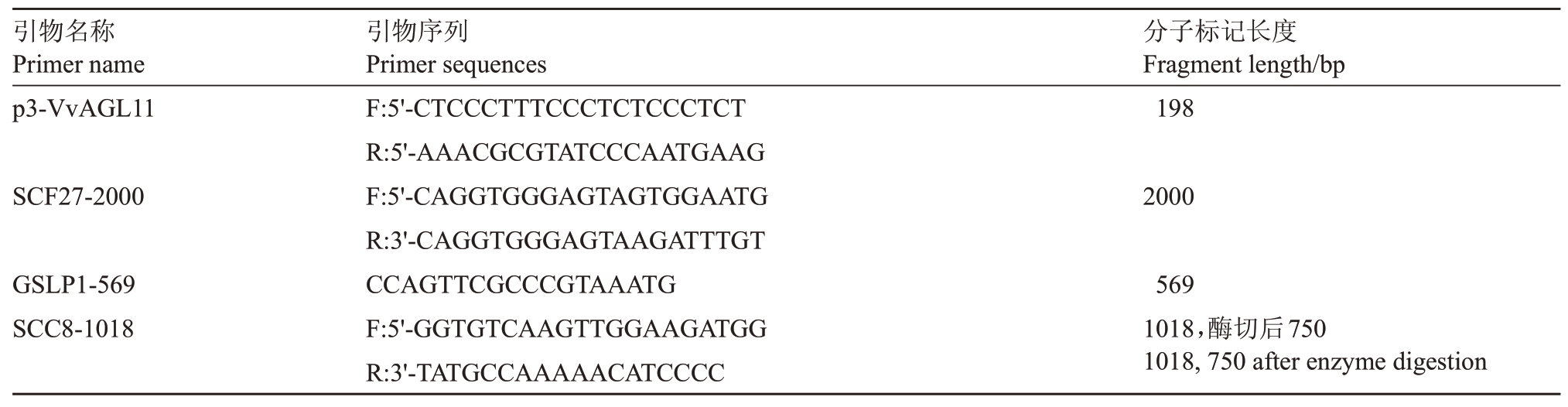
引物名称Primer name p3-VvAGL11分子标记长度Fragment length/bp 198 SCF27-2000 2000 GSLP1-569 SCC8-1018引物序列Primer sequences F:5'-CTCCCTTTCCCTCTCCCTCT R:5'-AAACGCGTATCCCAATGAAG F:5'-CAGGTGGGAGTAGTGGAATG R:3'-CAGGTGGGAGTAAGATTTGT CCAGTTCGCCCGTAAATG F:5'-GGTGTCAAGTTGGAAGATGG R:3'-TATGCCAAAAACATCCCC 569 1018,酶切后750 1018,750 after enzyme digestion
1.3 项目测定
胚发育率/%=发育胚数/接种胚珠数×100;成苗率/%=正常苗数/接种胚珠数×100。
1.4 数据分析
试验数据采用Microsoft Excel 2010软件进行分析。
2 结果与分析
2.1 无核葡萄胚挽救结果
本试验共配置杂交组合9 个,获得杂交果粒4060 个,接种胚珠9404 个,可正常发育胚2092 个,平均胚发育率为22.25%,成苗1059 株,平均胚成苗率11.26%(表3)。其中,红宝石无核×京香玉组合胚发育率和成苗率最高,分别为30.23%和15.46%。在以火焰无核作为母本的3 个组合中,以巨玫瑰作为父本,成苗率最高,为2.78%。在以红宝石无核为母本的3 个组合中,以京香玉作为父本,成苗率最高,为15.46%。在以巨玫瑰为父本的2 个组合中,以火焰无核作为母本成苗率最高,为2.78%。在以阳光玫瑰为父本的两个组合中,以克伦生为母本成苗率最高,为2.60%。在以户太8 号为父本的两个组合中,以红宝石无核为母本成苗率最高,为12.48%。在以醉金香为父本的两个组合中,以红宝石无核为母本成苗率最高,为13.59%。
表3 无核葡萄的胚挽救结果
Table 3 Results of seedless grape embryo rescue
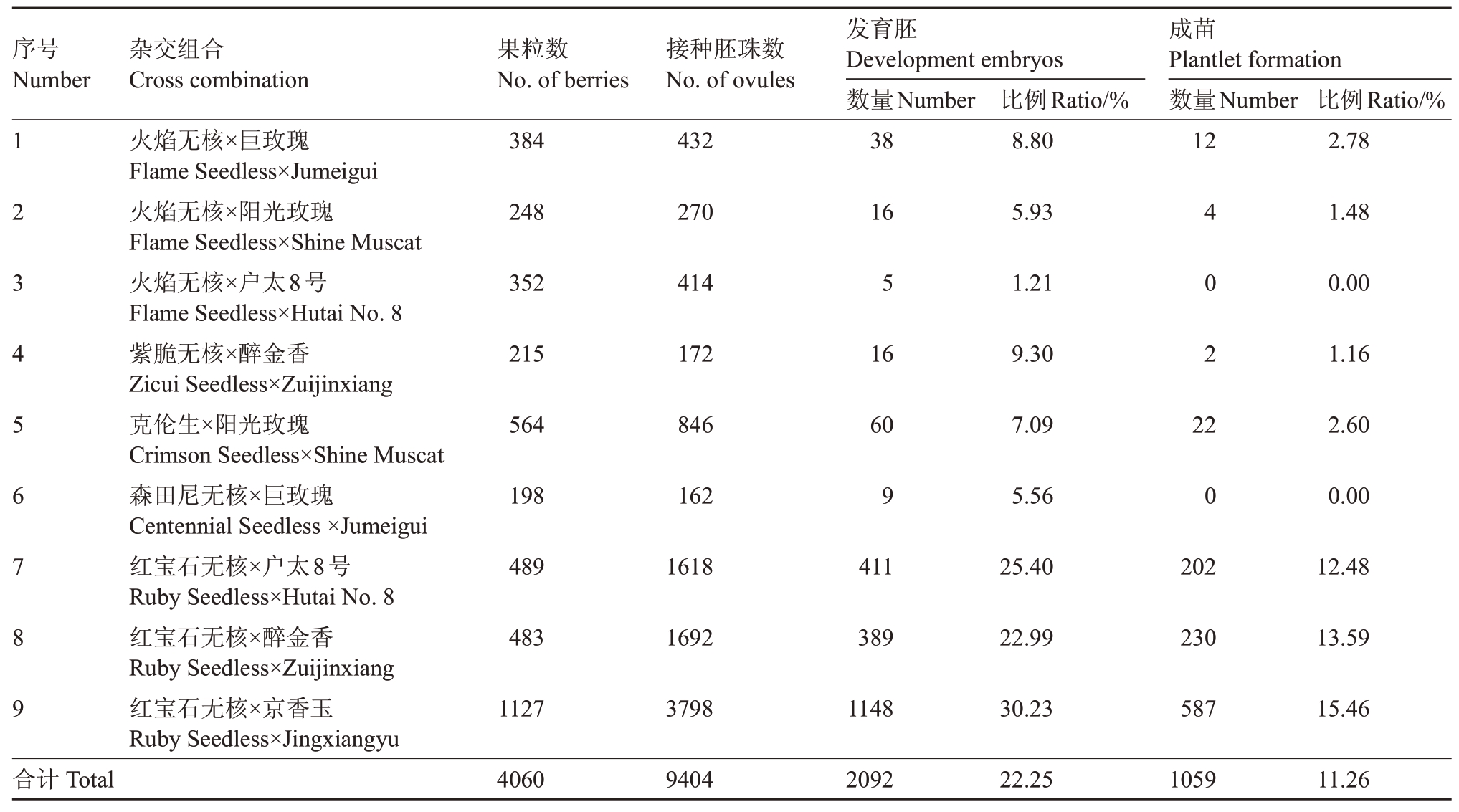
序号Number杂交组合Cross combination果粒数No.of berries接种胚珠数No.of ovules 123456789火焰无核×巨玫瑰Flame Seedless×Jumeigui火焰无核×阳光玫瑰Flame Seedless×Shine Muscat火焰无核×户太8号Flame Seedless×Hutai No.8紫脆无核×醉金香Zicui Seedless×Zuijinxiang克伦生×阳光玫瑰Crimson Seedless×Shine Muscat森田尼无核×巨玫瑰Centennial Seedless×Jumeigui红宝石无核×户太8号Ruby Seedless×Hutai No.8红宝石无核×醉金香Ruby Seedless×Zuijinxiang红宝石无核×京香玉Ruby Seedless×Jingxiangyu 384 432发育胚Development embryos数量Number 38比例Ratio/%8.80成苗Plantlet formation数量Number 12比例Ratio/%2.78 248 270 16 5.93 1.48 352 414 5 1.21 0.00 215 172 16 9.30 402 1.16 564 846 60 7.09 22 2.60 198 162 9 5.56 0 0.00 489 1618 411 25.40 202 12.48 483 1692 389 22.99 230 13.59 1127 3798 1148 30.23 587 15.46合计Total 4060 9404 2092 22.25 1059 11.26
2.2 幼果取样时间对葡萄胚挽救效率的影响
对火焰无核×巨玫瑰和火焰无核×阳光玫瑰两个杂交组合幼果取样时间均设置初次授粉后36、38、40 d 三个时间梯度,两个组合均在初次授粉后38 d 时胚发育率和胚成苗率最高,胚发育率分别为10.74%和8.25%,胚成苗率分别为4.03%和3.10%。对克伦生×阳光玫瑰杂交组合幼果取样时间设置44、46、48、50 d四个幼果取样时间梯度,该组合在初次授粉后48 d 时胚发育率和成苗率最高,分别为7.69%和4.07%。对红宝石无核×户太8 号、红宝石无核×醉金香、红宝石无核×京香玉三个杂交组合幼果取样时间均设置初次授粉后53、55、57 d三个时间梯度,3 个杂交组合均在初次授粉后55 d 时胚发育率和胚成苗率最高,胚发育率分别为28.25%、24.78%、33.88%,胚成苗率分别为17.14%、18.42%、20.09%(表4)。
表4 取样时间对胚挽救的影响
Table 4 Impact of sampling time on embryo rescue

杂交组合Cross combination授粉日期Pollination date果粒数No.of berries成苗Plantlet formation数量Number火焰无核×巨玫瑰Flame Seedless×Jumeigui 5月17日May 17发育胚Development embryos数量Number 12 16 10 38 462 12火焰无核×阳光玫瑰Flame Seedless×Shine Muscat 5月16日May 16 385克伦生×阳光玫瑰Crimson Seedless×Shine Muscat 5月20日May 20 03144693红宝石无核×户太8号Ruby Seedless×Hutai No.8 5月21日May 21红宝石无核×醉金香Ruby Seedless×Zuijinxiang 5月21日May 21红宝石无核×京香玉Ruby Seedless×Jingxiangyu 5月21日May 21授粉后时间Time after pollination/d 36 38 40合计Total 36 38 40合计Total 44 46 48 50合计Total 53 55 57合计Total 53 55 57合计Total 53 55 57合计Total 117 138 129 384 79 85 84 248 135 129 146 154 564 143 188 168 489 149 172 162 483 367 351 409 1127接种胚珠数No.of ovules 136 149 147 432 82 97 91 270 215 207 221 203 846 563 531 524 1618 559 581 552 1692 1173 1160 1465 3798 16 15 15 17 13 60 129 150 132 411 113 144 132 389 379 393 376 1148比例Ratio/%8.82 10.74 6.80 8.80 3.66 8.25 5.49 5.93 6.98 7.25 7.69 6.40 6.94 22.91 28.25 25.19 25.40 20.21 24.78 23.91 22.99 32.31 33.88 25.67 30.23 22 49 91 62 202 53 107 70 230 167 233 187 587比例Ratio/%2.94 4.03 1.31 2.78 0.00 3.10 1.10 1.49 1.86 2.90 4.07 1.48 2.60 8.70 17.14 11.83 12.48 9.48 18.42 12.68 13.59 14.24 20.09 12.76 15.46
2.3 杂种后代无核性状分子标记鉴定
2.3.1 利用四种无核分子标记对亲本进行检测分别利用葡萄无核探针GSLP1-569及无核分子标记SCF27-2000、SCC8-1018、p3-VvAGL11 对10 个亲本进行检测(图2)。利用GSLP1-569 可以检测到火焰无核和阳光玫瑰两个亲本在569 bp处扩增出特异性条带(图2-A),因此GSLP1-569可以用来对火焰无核×巨玫瑰1 个杂交组合的后代进行无核早期鉴定。
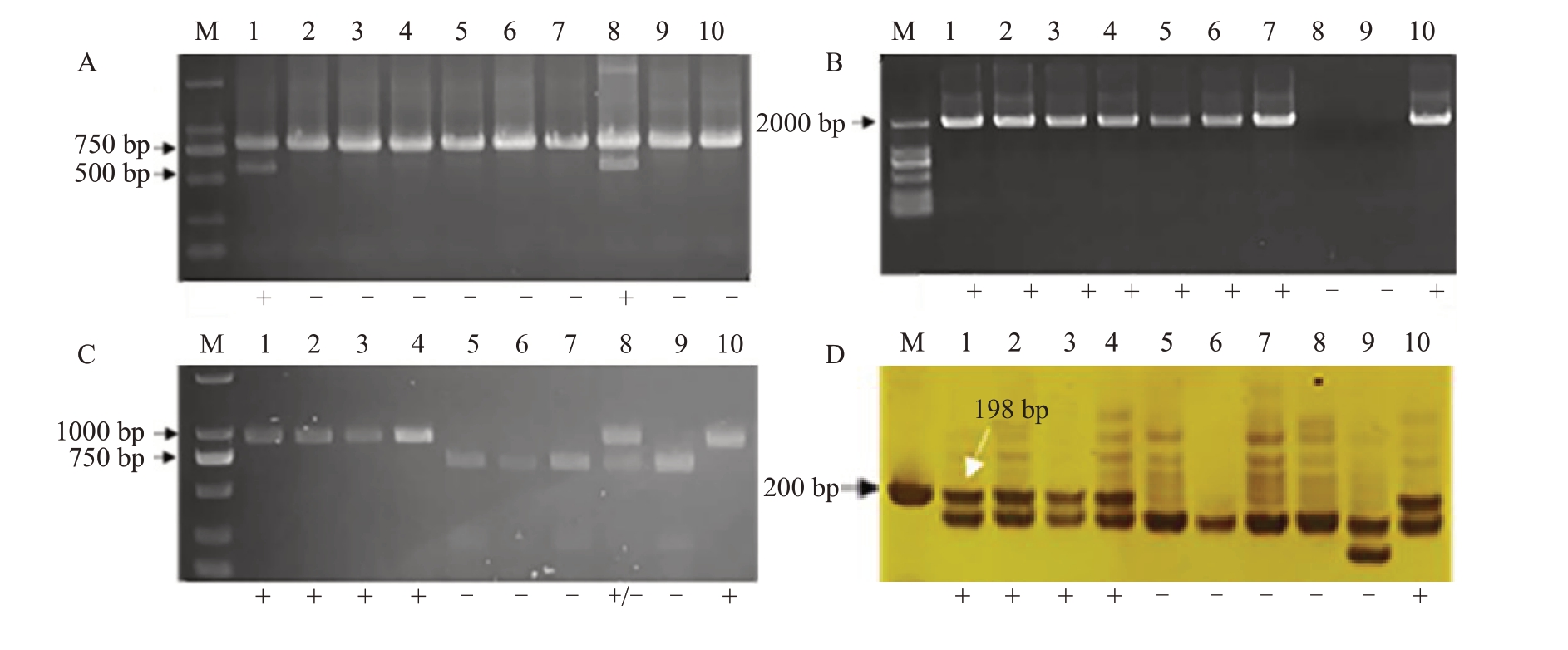
图2 4 种无核分子标记对杂交亲本的鉴定结果
Fig.2 Identification results of hybrid parents using four non nuclear molecular markers
A.GSLP1-569 鉴定结果;B.SCF27-2000 鉴定结果;C.SCC8-1018 鉴定结果;D.p3-VvAGL11 鉴定结果。泳道:M.Marker;1.火焰无核;2.紫脆无核;3.红宝石无核;4.克伦生;5.户太8 号;6.醉金香;7. 京香玉;8.阳光玫瑰;9.巨玫瑰;10.森田尼无核。“+”表示扩增出特异性条带,“-”表示未扩增出特异性条带。
A.Identification results of GSLP1-569;B.Identification results of SCF27-2000;C.Identification results of SCC8-1018;D.Identification results of p3-VvAGL11. Lane: M. Marker; 1. Flame Seedless; 2. Zicui Seedless; 3. Ruby Seedless; 4. Crimson Seedless; 5. Hutai No. 8; 6. Zuijinxiang; 7. Jingxiangyu; 8. Shine Muscat; 9. Jumeigui; 10. Centennial Seedless.“+”indicates the objective bands were present;“-”indicates the objective bands were absent.
利用SCF27-2000 可以检测到火焰无核、紫脆无核、红宝石无核、克伦生、户太8号、醉金香、京香玉、森田尼无核等8 个亲本在2000 bp 处扩增出特异性条带(图2-B),因此SCF27-2000 可以用来对火焰无核×巨玫瑰、火焰无核×阳光玫瑰、克伦生×阳光玫瑰3 个杂交组合的后代进行无核早期鉴定;利用SCC8-1018酶切后可以检测到火焰无核、紫脆无核、红宝石无核、克伦生、森田尼无核等5个亲本在1000 bp附近处扩增出特异性条带,户太8 号、醉金香、京香玉、巨玫瑰只在750 bp处扩增出特异性条带,阳光玫瑰在1000 bp附近和750 bp 处都存在特异性条带(图2-C)。因而SCC8-1018 可以对火焰无核×巨玫瑰、紫脆无核×醉金香、红宝石无核×户太8 号、红宝石无核×醉金香、红宝石无核×京香玉5 个杂交组合后代进行无核早期鉴定;利用p3-VvAGL11可以检测到火焰无核、紫脆无核、红宝石无核、克伦生、森田尼无核等5 个亲本在198 bp处扩增出特异性条带(图2-D),因此p3-VvAGL11可以对火焰无核×巨玫瑰、火焰无核×阳光玫瑰、紫脆无核×醉金香、克伦生×阳光玫瑰、红宝石无核×户太8号、红宝石无核×醉金香、红宝石无核×京香玉7个杂交组合的后代进行无核早期鉴定。
2.3.2 无核分子标记对杂交F1代进行检测 利用无核探针GSLP1-569,无核分子标记SCF27-2000、SCC8-1018、p3-VvAGL11 对火焰无核×巨玫瑰的12株杂交后代进行无核性状检测,其中GSLP1-569 检测出8 株杂种后代在569 bp 处扩增出特异性条带,SCF27-2000检测出10株杂种后代在2000 bp处扩增出特异性条带,SCC8-1018 检测出9 株杂种后代在1000 bp 附近处扩增出特异性条带,p3-VvAGL11 检测出11株杂种后代在198 bp处扩增出特异性条,使用4种无核分子标记都可以扩增出特异性条带的杂种植株有7 株,该杂交组合后代的无核率为58.33%(表5);SCF27-2000、p3-VvAGL11对火焰无核×阳光玫瑰的4 株杂交后代进行无核性状检测,其中SCF27-2000检测出4株杂种后代在198 bp处扩增出特异性条带,p3-VvAGL11检测出4株杂种后代植株在2000 bp处扩增出特异性条带,2种无核分子标记对该杂交组合后代的无核率为100.00%;SCC8-1018、p3-VvAGL11 对紫脆无核×醉金香的2 株杂交后代进行无核性状检测,其中SCC8-1018 检测出2株杂种后代在1000 bp附近处扩增出特异性条带,无核分子标记p3-VvAGL11 检测出2 株杂种后代在198 bp 处扩增出特异性条带,以上2 种无核分子标记都可以扩增出特异性条带的杂种植株有2 株,该杂交组合后代的无核率为100.00%;p3-VvAGL11、SCF27-2000对克伦生×阳光玫瑰的22株杂交后代进行无核性状检测,其中p3-VvAGL11 检测出18 株杂种后代在198 bp 处扩增出特异性条带,SCF27-2000检测出17株杂种后代在2000 bp处扩增出特异性条带,使用2 种无核分子标记都可以扩增出特异性条带的杂种植株有16 株,该杂交组合后代的无核率为72.73%。
表5 利用不同分子标记对胚挽救杂交后代无核性状的检测结果
Table 5 Non nuclear traits detection in embryo rescue hybrid offsprings using different molecular markers
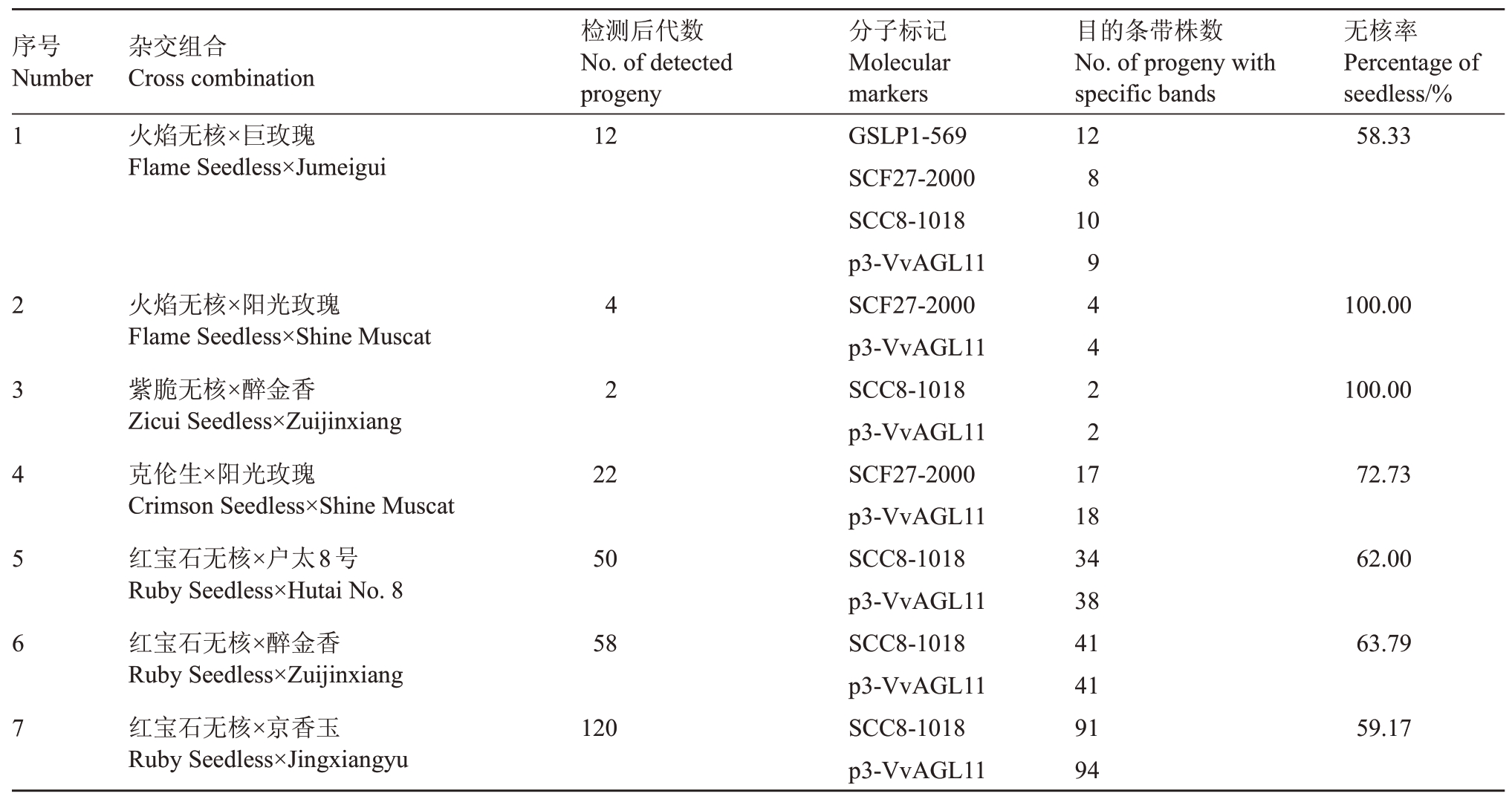
序号Number 1杂交组合Cross combination火焰无核×巨玫瑰Flame Seedless×Jumeigui检测后代数No.of detected progeny 12目的条带株数No.of progeny with specific bands 12 8 10无核率Percentage of seedless/%58.33 234567火焰无核×阳光玫瑰Flame Seedless×Shine Muscat紫脆无核×醉金香Zicui Seedless×Zuijinxiang克伦生×阳光玫瑰Crimson Seedless×Shine Muscat红宝石无核×户太8号Ruby Seedless×Hutai No.8红宝石无核×醉金香Ruby Seedless×Zuijinxiang红宝石无核×京香玉Ruby Seedless×Jingxiangyu 42 94422 100.00 100.00 22 72.73 50 62.00 58 63.79 120分子标记Molecular markers GSLP1-569 SCF27-2000 SCC8-1018 p3-VvAGL11 SCF27-2000 p3-VvAGL11 SCC8-1018 p3-VvAGL11 SCF27-2000 p3-VvAGL11 SCC8-1018 p3-VvAGL11 SCC8-1018 p3-VvAGL11 SCC8-1018 p3-VvAGL11 17 18 34 38 41 41 91 94 59.17
利用SCC8-1018、p3-VvAGL11 对红宝石无核×户太8号的202株杂交后代中随机抽取50株进行无核性状检测,其中SCC8-1018检测出34株杂种后代在1000 bp附近处扩增出无核特异性条带,无核分子标记p3-VvAGL11 检测出38 株杂种后代在198 bp处扩增出无核特异性条带,使用2 种无核分子标记都可以扩增出特异性条带的杂种植株有31株,该杂交组合后代的无核率为62.00%;SCC8-1018、p3-VvAGL11对红宝石无核×醉金香的230株杂交后代中随机抽取58 株进行无核性状检测,其中SCC8-1018检测出41株杂种后代在1000 bp附近处扩增出无核特异性条带,p3-VvAGL11 检测出41 株杂种后代在198 bp 处扩增出无核特异性条带,使用2 种无核分子标记都可以扩增出特异性条带的杂种植株有37 株,该杂交组合后代的无核率为63.79%;SCC8-1018、p3-VvAGL11 对红宝石无核×京香玉的587 株杂交后代中随机抽取120 株进行无核性状检测,其中SCC8-1018检测出91株杂种后代在1000 bp附近处扩增出无核特异性条带,p3-VvAGL11 检测出94株杂种后代在198 bp 处扩增出无核特异性条带,2种分子标记都可以扩增出无核特异性条带的杂种后代有71株,该杂交组合后代的无核率为59.17%。
3 讨论
通过无核葡萄胚挽救技术,打破了假单性结实型葡萄在作为母本进行无核育种时难以获得种子的局限[19-21],使得以无核葡萄作为母本进行杂交育种成为了可能[22],极大地提高了无核葡萄育种效率,笔者以无核葡萄为母本通过胚挽救技术成功获得了1059株杂种植株。
Notsuka等[23]通过试验认为,在影响胚挽救效率中,母本的选择起到了至关重要的作用;Ji等[24]在研究中发现,以红宝石无核作母本,成苗率高达23.00%,明显高于作为母本的粉红无核等其他品种;Ulaş等[7]和Liu等[8]通过选取多种母本对比,发现克伦生适合作为胚挽救母本;贾姗姗[25]等试验表明,红宝石无核适合作母本,胚挽救成苗率较高,而克伦生作母本时,胚挽救成苗率较低;刘可可等[9]研究表明,火焰无核和红宝石无核的胚挽救成苗率较高,适合作母本。笔者在本研究中发现,当父本同为户太8 号时,母本为红宝石无核的成苗率明显高于火焰无核,当父本同为醉金香时,母本为红宝石无核的成苗率明显高于紫脆无核,因此红宝石无核相比火焰无核和醉金香更适合作胚挽救育种的母本,这与前人研究结果一致[24-26];以克伦生为母本时,成苗率仅为2.60%,这与朱佩佩等[27]得出的结论一致,但是与Ulaş 等[7]的研究结论相反;以火焰无核作母本时,成苗率最高仅为2.78%,这与刘可可等[9]等研究发现火焰无核适合作母本的结论相反;以森田尼无核为母本时,没有获得杂种植株,而朱佩佩等[27]在利用森田尼无核为母本时,成苗率为14.40%,并依此认为森田尼无核是胚挽救育种中比较适合的母本材料;以紫脆无核为母本时,成苗率仅为1.16%,成苗率比较低。因此,在无核葡萄胚挽救育种时,笔者建议要谨慎选用克伦生、火焰无核、森田尼无核和紫脆无核葡萄作为母本,其胚挽救成苗率较低甚至没有成苗。
取样时间对无核葡萄胚挽救效率也有很大的影响[10-11],前人研究发现,起初,由于胚在果实内部继续发育,葡萄胚挽救成功率与采样时间呈正相关,而在某一采样时间之后,胚开始急剧败育,胚挽救成功率也急剧下降,与采样时间呈显著负相关[28]。因此,在合子胚发育到最佳状态时对幼果采样,胚挽救的成功率最高。刘可可等[9]和李志瑛等[29]通过研究认为火焰无核作母本时,最佳采样时间为首次授粉后42 d;Giancaspro等[30]和Li等[31]研究发现以红宝石无核为母本时,最佳采样时间为花后55 d。本试验研究结果表明,以火焰无核为母本的杂交组合,在第一次授粉后38 d采样的胚挽救效率最高,这与刘可可等[9]和Li 等[31]的研究结果存在差异;以克伦生为母本的杂交组合,在首次授粉后48 d采样的胚挽救效率最高,以红宝石无核为母本的杂交组合,在首次授粉后55 d采样的胚挽救效率最高,这与前人的研究结果一致[30-31]。
通过无核分子标记辅助育种,可以有效地节约物力和人力,缩短育种周期,提高育种效率[17,29,32]。目前,常用的无核分子标记有p3-VvAGL11、5UVviAGL11、SCF27-2000、GSLP1-569、SCC8-1018 以及VMCF7F2-198[13-17]等。陈豆豆等[18]研究表明,p3-VvAGL11和5U-VviAGL11检测无核性状的准确率较高,Akkurt 等[33]研究表明,无核分子标记SCF27-2000、SCC8-1018 在检测以两个无核葡萄品种作为亲本的杂交后代时准确率较高,无核分子探针GSLP1-569更适合检测与无核白亲缘关系较近品种的杂交后代[8]。李莎莎等[2]利用无核探针GSLP1-569对火焰无核×左优红杂种后代进行鉴定,后代无核率为57.14%,对火焰无核×北冰红杂种后代进行鉴定,后代无核率60.00%;玉赛赛等[34]利用无核分子标记SCF27-2000对克瑞森无核×左优红的杂种后代进行鉴定,后代无核率为76.92%,对红宝石无核×雪兰红杂种后代进行鉴定,后代无核率为18.75%,对美丽无核×木星的杂种后代进行鉴定,后代无核率为50%。笔者利用无核分子标记p3-VvAGL11、GLSP1-569、SCF27-2000、SCC8-1018 分别对火焰无核×巨玫瑰、火焰无核×阳光玫瑰、紫脆无核×醉金香、克伦生×阳光玫瑰、红宝石无核×户太8 号、红宝石无核×醉金香、红宝石无核×京香玉7 个杂交组合的杂种后代进行无核鉴定,无核率分别为58.33%、100%、100%、72.73%、62.00%、63.79%、59.17%。
4 结论
综上所述,以无核葡萄为母本进行胚挽救育种可以显著提高无核葡萄育种效率,红宝石无核是比较理想的母本材料;其次还要根据母本的品种特性选择最佳的幼果采样时间,来提高育种效率;通过无核分子标记p3-VvAGL11、GLSP1-569、SCF27-2000、SCC8-1018进行无核鉴定,可以对杂种后代的无核性状进行早期预测,提高无核葡萄胚挽救育种的效率。
[1] 穆维松,冯建英,田东,牟鑫.我国鲜食葡萄产业的国际贸易与国内需求形势[J].中国果树,2019(2):5-10.MU Weisong,FENG Jianying,TIAN Dong,MU Xin.The international trade and domestic demand of the table grape industry in China[J].China Fruits,2019(2):5-10.
[2] 李莎莎,玉赛赛,傅雨恒,骆强伟,徐炎,王跃进.利用胚挽救与分子标记选育葡萄无核抗寒新种质[J]. 园艺学报,2022,49(4):723-738.LI Shasha,YU Saisai,FU Yuheng,LUO Qiangwei,XU Yan,WANG Yuejin. The embryo rescue and molecular markers are used to breed new seedless,cold-resistant grapes[J].Acta Horticulturae Sinica,2022,49(4):723-738.
[3] 樊秀彩,张颖,姜建福,孙海生,李民,刘崇怀.近20年来国外鲜食葡萄品种选育进展[J]. 中外葡萄与葡萄酒,2012(2):53-59.FAN Xiucai,ZHANG Ying,JIANG Jianfu,SUN Haisheng,LI Min,LIU Chonghuai.Progress in the breeding of fresh grape varieties abroad in the past 20 years[J]. Sino-Overseas Grapevine&Wine,2012(2):53-59.
[4] 蒋爱丽,李世诚,金佩芳,杨天仪,骆军.胚培无核葡萄新品种沪培1 号的选育[J].果树学报,2007,24(3):402-403.JIANG Aili,LI Shicheng,JIN Peifang,YANG Tianyi,LUO Jun.Hupei 1:A new triploid seedless grape cultivar obtained by embryo culture[J].Journal of Fruit Science,2007,24(3):402-403.
[5] 蒋爱丽,李世诚,杨天仪,骆军,张朝轩,金佩芳.无核葡萄新品种沪培2 号的选育[J].果树学报,2008,25(4):618-619.JIANG Aili,LI Shicheng,YIANG Tianyi,LUO Jun,ZHANG Chaoxuan,JIN Peifang. A new seedless grape cultivar:Hupei No.2[J].Journal of Fruit Science,2008,25(4):618-619.
[6] LI T M,LI Z Q,YIN X,GUO Y R,WANG Y J,XU Y. Improved in vitro Vitis vinifera L.embryo development of F1 progeny of‘Delight’בRuby Seedless’using putrescine and marker-assisted selection[J].In Vitro Cellular&Developmental Biology-Plant,2018,54(3):291-301.
[7] ULAŞ S,KESGIN M,DILLI Y. The success of in vitro embryo rescue technique in hybridization of seedless grape varieties[J].BIO Web of Conferences,2015,5:01008.
[8] LIU Q,ZHANG J,WANG Y,YU D,XIA H.Breeding for coldresistant,seedless grapes from Chinese wild Vitis amurensis using embryo rescue[J]. New Zealand Journal of Crop and Horticultural Science,2016,44(2):136-151.
[9] 刘可可,李莎莎,骆强伟,徐炎,王跃进.胚挽救技术创造无核抗病葡萄新种质[J].中国农学通报,2022,38(11):22-29.LIU Keke,LI Shasha,LUO Qiangwei,XU Yan,WANG Yuejin.Breeding new grape germplasm of seedless and disease resistance by embryo rescue technique[J]. Chinese Agricultural Science Bulletin,2022,38(11):22-29.
[10] 史文静,骆强伟,王跃进.无核香味葡萄胚挽救育种研究[J].西北植物学报,2018,38(6):983-993.SHI Wenjing,LUO Qiangwei,WANG Yuejin. Breeding grapevine varieties for seedlessness with flavour using embryo rescue[J]. Acta Botanica Boreali-Occidentalia Sinica,2018,38(6):983-993.
[11] 郝燕,杨瑞,王鸿,王发林.无核葡萄剥胚胚挽救技术关键影响因子[J].西北农业学报,2013,22(9):114-120.HAO Yan,YANG Rui,WANG Hong,WANG Falin.Key factors affecting successful embryo rescue via ovule excision for different crosses of seedless grapes[J].Acta Agriculturae Boreali-Occidentalis Sinica,2013,22(9):114-120.
[12] 方宣钧,吴为人,唐纪良.作物DNA 标记辅助育种[M].北京:科学出版社,2001:2-6.FANG Xuanjun,WU Weiren,TANG Jiliang.Crop DNA markerassisted breeding[M].Beijing:Science Press,2001:2-6.
[13] LAHOGUE F,THIS P,BOUQUET A. Identification of a codominant scar marker linked to the seedlessness character in grapevine[J]. Theoretical and Applied Genetics,1998,97(5):950-959.
[14] MEJÍA N,HINRICHSEN P.A new,highly assertive scar marker potentially useful to assist selection for seedlessness in table grape breeding[J].Acta Horticulturae,2003,603:559-564.
[15] 王跃进,杨英军,周鹏,张剑侠,王西平.用DNA 探针检测我国栽培的无核葡萄及辅助育种初探[J]. 园艺学报,2002,29(2):105-108.WANG Yuejin,YANG Yingjun,ZHOU Peng,ZHANG Jianxia,WANG Xiping. Detecting the seedless characteristics of the grapes in China with DNA probe and DNA marker assistant selection[J].Acta Horticulturae Sinica,2002,29(2):105-108.
[16] CABEZAS J A,CERVERA M T,RUIZ- GARCÍA L,CARREÑO J,MARTÍNEZ-ZAPATER J M. A genetic analysis of seed and berry weight in grapevine[J]. Genome,2006,49(12):1572-1585.
[17] MEJÍA N,SOTO B,GUERRERO M,CASANUEVA X,HOUEL C,MICCONO M D,RAMOS R,LE CUNFF L,BOURSIQUOT J M,HINRICHSEN P,ADAM-BLONDON A F. Molecular,genetic and transcriptional evidence for a role of VvAGL11 in stenospermocarpic seedlessness in grapevine[J].BMC Plant Biology,2011,11:57.
[18] 陈豆豆,贺亮亮,章鹏,关利平,宋银花,刘三军.无核品种中葡萄18 号胚挽救技术体系的建立及其杂交后代早期选择[J].果树学报,2021,38(12):2223-2235.CHEN Doudou,HE Liangliang,ZHANG Peng,GUAN Liping,SONG Yinhua,LIU Sanjun.Construction of embryo rescue technology system for seedless grape cultivar Zhongputao No. 18 and early selection of hybrid offspring[J]. Journal of Fruit Science,2021,38(12):2223-2235.
[19] 李莎莎,王跃进.葡萄无核基因及无核育种研究进展[J].园艺学报,2019,46(9):1711-1726.LI Shasha,WANG Yuejin.Advances in seedless gene researches and seedless breeding in grapevine[J].Acta Horticulturae Sinica,2019,46(9):1711-1726.
[20] 崔梦杰,王晨,张文颖,汤崴,朱旭东,李晓鹏,房经贵.无核葡萄研究进展[J].植物生理学报,2017,53(3):317-330.CUI Mengjie,WANG Chen,ZHANG Wenying,TANG Wei,ZHU Xudong,LI Xiaopeng,FANG Jinggui. Research progress of seedless grape[J].Plant Physiology Journal,2017,53(3):317-330.
[21] 李桂荣,全冉,程珊珊,侯小进,樊秀彩,扈惠灵.无核葡萄离体胚珠发育影响因子及其生理变化[J].中国农业科学,2020,53(22):4646-4657.LI Guirong,QUAN Ran,CHENG Shanshan,HOU Xiaojin,FAN Xiucai,HU Huiling. The influencing factors of in-vitro ovule development in seedless grape and its physiological changes[J].Scientia Agricultura Sinica,2020,53(22):4646-4657.
[22] JI W,WANG Y J. Breeding for seedless grapes using Chinese wild Vitis spp. II. In vitro embryo rescue and plant development[J]. Journal of the Science of Food and Agriculture,2013,93(15):3870-3875.
[23] NOTSUKA K,TSURU T,SHIRAISHI M. Seedless-seedless grape hybridization via in-ovulo embryo culture[J]. Journal of the Japanese Society for Horticultural Science,2001,70(1):7-15.
[24] JI W,LI Z Q,ZHOU Q,YAO W K,WANG Y J. Breeding new seedless grape by means of in vitro embryo rescue[J]. Genetics and Molecular Research,2013,12(1):859-869.
[25] 贾姗姗,骆强伟,李莎莎,王跃进.葡萄胚挽救技术优化及无核和玫瑰香味新种质创制[J].中国农业科学,2020,53(16):3344-3355.JIA Shanshan,LUO Qiangwei,LI Shasha,WANG Yuejin. Optimization of embryo rescue technique and production of potential seedless grape germplasm with rosy aroma[J]. Scientia Agricultura Sinica,2020,53(16):3344-3355.
[26] 赵炅,骆强伟,王跃进. 无核抗病葡萄胚挽救育种与应用[J].北方园艺,2019(3):44-54.ZHAO Jiong,LUO Qiangwei,WANG Yuejin. Breeding for new seedless grapevine varieties with disease-resistance using embryo rescue[J].Northern Horticulture,2019(3):44-54.
[27] 朱佩佩,罗燚佳,向雯,张明磊,张剑侠.抗寒无核葡萄杂种胚挽救及分子标记辅助选择[J]. 中国农业科学,2021,54(6):1218-1228.ZHU Peipei,LUO Yijia,XIANG Wen,ZHANG Minglei,ZHANG Jianxia. Rescue and molecular marker assisted-selection of the cold-resistant seedless grape hybrid embryo[J].Scientia Agricultura Sinica,2021,54(6):1218-1228.
[28] 张爱华,陈虎,陈红梅.‘弗雷无核’胚挽救适宜接种期研究[J].农业科技通讯,2021(1):121-123.ZHANG Aihua,CHEN Hu,CHEN Hongmei. A study on the suitable vaccination period for‘Flame Seedless’free embryo rescue[J].Bulletin of Agricultural Science and Technology,2021(1):121-123.
[29] 李志瑛,骆强伟,王跃进.无核葡萄胚挽救育种与杂种后代分子标记辅助选择[J].果树学报,2019,36(1):31-42.LI Zhiying,LUO Qiangwei,WANG Yuejin. Breeding seedless grapevine via embryo rescue and marker-assisted selection in hybrid progenies[J]. Journal of Fruit Science,2019,36(1):31-42.
[30] GIANCASPRO A,MAZZEO A,CARLOMAGNO A,GADALETA A,SOMMA S,FERRARA G. Optimization of an in vitro embryo rescue protocol for breeding seedless table grapes(Vitis vinifera L.)in Italy[J].Horticulturae,2022,8(2):121.
[31] LI Z Q,LI T M,WANG Y J,XU Y. Breeding new seedless grapes using in ovulo embryo rescue and marker-assisted selection[J]. In Vitro Cellular & Developmental Biology- Plant,2015,51(3):241-248.
[32] OCAREZ N,JIMÉNEZ N,NÚÑEZ R,PERNIOLA R,MARSICO A D,CARDONE M F,BERGAMINI C,MEJÍA N.Unraveling the deep genetic architecture for seedlessness in grapevine and the development and validation of a new set of markers for VviAGL11-based gene-assisted selection[J]. Genes,2020,11(2):151.
[33] AKKURT M,ÇAKIR A,SHIDFAR M,ÇELIKKOL B P,SÖYLEMEZOĞLU G.Using SCC8,SCF27 and VMC7f2 markers in grapevine breeding for seedlessness via marker assisted selection[J]. Genetics and Molecular Research,2012,11(3):2288-2294.
[34] 玉赛赛,李莎莎,骆强伟,徐炎,王跃进.胚挽救创造无核抗寒葡萄新种质[J].北方园艺,2021(22):29-37.YU Saisai,LI Shasha,LUO Qiangwei,XU Yan,WANG Yuejin.Embryo rescue technique create new seedless,cold-resistant grapevines germplasm[J]. Northern Horticulture,2021(22):29-37.