随着城市化的高速发展以及化工生产、开矿采矿、化肥农药和生活固体废物堆积等因素的影响,重金属流入土壤[1-2],致使中国土壤重金属污染不断加重[3]。中国农田土壤重金属污染源有铅、镉、汞、砷等元素,其中镉(Cd)为主要的污染元素之一,土壤受重金属污染导致肥力下降,影响作物的品质和产量[4],也会通过食物链对人体产生危害[5-6]。有研究表明,镉与女性乳腺癌发病存在关联性[7],因此,重金属镉污染防治已迫在眉睫。
土壤中的镉会被作物根系吸收,再通过转运途径运输到茎、叶和果实中并富集,导致农产品镉超标[8]。葫芦科的蔬菜植物,包括葫芦、西瓜、甜瓜、南瓜等,是重要的食用作物,部分品种也可作为嫁接砧木使用。西瓜在镉污染地区种植,其生长受到抑制[9],镉胁迫会影响甜瓜生长、降低产量[10]。嫁接栽培技术是通过更换根系来提高植物抗土传病害、抗有毒物质危害、抗重茬的能力[11],提高作物产量和品质等[12-13],已广泛应用于瓜类作物生产。
嫁接能缓解重金属对植物的影响,选择嫁接低镉积累的砧木,通过调控含硫化合物的合成与代谢,来减少镉向作物地上部运输和富集[14];有学者研究表明,利用不同品种砧木嫁接西瓜,其果实中重金属的含量存在差异[15-16];还有学者研究表明,南瓜类型砧木嫁接西瓜,果实的镉含量显著降低[17],目前学者们的研究主要集中在重金属对嫁接苗和实生苗的影响方面,而砧木实生苗对重金属的响应却鲜见报道,因此,研究不同类型砧木对镉的生理响应,对嫁接栽培研究及砧木育种具有重要意义。
笔者利用4 种不同类型砧木进行水培处理,对地上部鲜质量、根长、叶片和根部的丙二醛(MDA)含量、超氧化物歧化酶(SOD)活性、过氧化物酶(POD)活性以及叶绿素荧光参数进行测定和分析,研究不同类型砧木对镉胁迫的生理反应,分析镉对不同类型砧木生理特性的影响,为砧木育种及在镉污染土壤中瓜类嫁接栽培安全生产提供参考。
1 材料和方法
1.1 试验材料
选用野生西瓜类型砧木(YZ1)、中国南瓜类型砧木(SZ111)、印度南瓜与中国南瓜杂交类型砧木(SZ7)、葫芦类型砧木(LZS),种子由宁波市丰登种业科技有限公司提供。试验在宁波市高新农业技术试验园区进行。
1.2 试验方法
1.2.1 材料培养 将YZ1、SZ111、SZ7、LZS 浸种催芽,播入50 孔穴盘,待其生长到1 叶1 心期,洗苗,将幼苗移栽至水培槽内,水槽内放置气泡石连接换气泵,为根系输送氧气,营养液配制参照黄芸萍等[18],以0.6 mg·L-1的CdCl2溶液进行处理[根据土壤环境质量标准(GB 15618—2018)农用地土壤污染风险值设定]。对照(清水)和处理各3 次重复,每重复10株,处理15 d。
1.2.2 根长测定 将露白后的种子放置到种子萌发袋后放入智能人工气候箱(宁波江南仪器,型号:RXZ-1000C)进行培养,设定昼夜16 h/8 h、25 ℃/18 ℃温度,22 000 lx/0 lx光/暗,每萌发袋加入20 mL 0.6 mg·L-1的CdCl2溶液,对照以清水代替,每处理3次重复,处理7 d,测量其根部长度。
1.2.3 叶绿素荧光参数测定 先将植株移至暗处,等待20 min 后,利用Junior-PAM 调制叶绿素荧光仪检测第4枚真叶的光合作用相关参数,测得最小荧光Fo,最大荧光Fm,光合电子传递速率ETR(μmol·m-2·s-1),光系统Ⅱ(PSⅡ)最大光化学效率Fv/Fm,以及通过公式计算的Fv=Fm-Fo,PSⅡ实际光化学效率Y(Ⅱ)=(Fm’-F’)/Fm’,PSⅡ潜在光化学效率Fv/Fo=(Fm-Fo)/Fo。
1.2.4 生理指标的测定 测量地上部鲜质量(g)后,对根部和叶片进行取样,使用苏州格锐思生物科技有限公司的试剂盒对叶片和根部样品提取,利用南京菲勒G-9 紫外可见分光光度计对丙二醛(MDA)含量(b,nmol·g-1)、超氧化物歧化酶(SOD)活性(U·g-1)、过氧化物酶(POD)活性(△OD470·g-1·min-1)进行测定,并通过公式计算影响程度[(处理-对照)/对照×100]。
1.3 统计分析
数据的分析、处理和作图使用SPSS 26.0 和WPS 表格,作图数据采用样品检测结果平均值,误差线采用±标准误差。其组间差异采用单因素方差分析ANOVA,采用Duncan法进行显著性检验。
2 结果与分析
2.1 镉胁迫对不同类型砧木地上部鲜质量的影响
经0.6 mg·L-1 CdCl2处理,不同砧木地上部鲜质量均低于对照(图1)。其中,YZ1、SZ111 和SZ7 地上部鲜质量分别比对照极显著降低26.75%、59.35%、45.72%,LZS 地上部鲜质量降低9.06%,与对照差异不显著,表明SZ111 受影响程度最大,LZS 最小,受影响程度依次为SZ111>SZ7>YZ1>LZS。
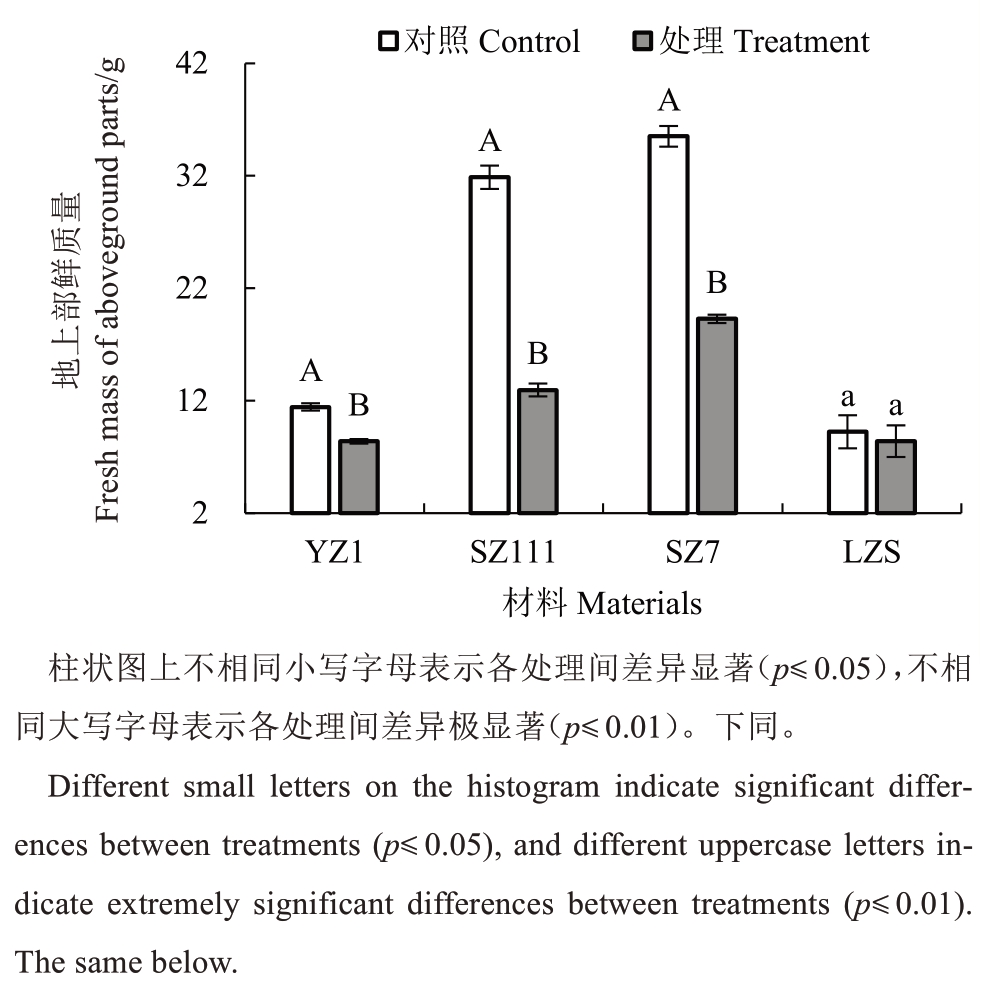
图1 镉胁迫对不同类型砧木地上部鲜质量的影响
Fig.1 Effects of cadmium stress on the fresh mass of aboveground plants of different types of rootstocks
2.2 镉胁迫对不同类型砧木主根长度的影响
通过测量不同类型砧木主根长度(图2)发现,4 种类型砧木的根长均低于对照。其中YZ1 显著降低50.20% ;SZ111、SZ7 和LZS 分别降低了17.71%、29.39%、4.41%,与对照差异不显著,说明YZ1 根长受影响程度最大,LZS 最小。
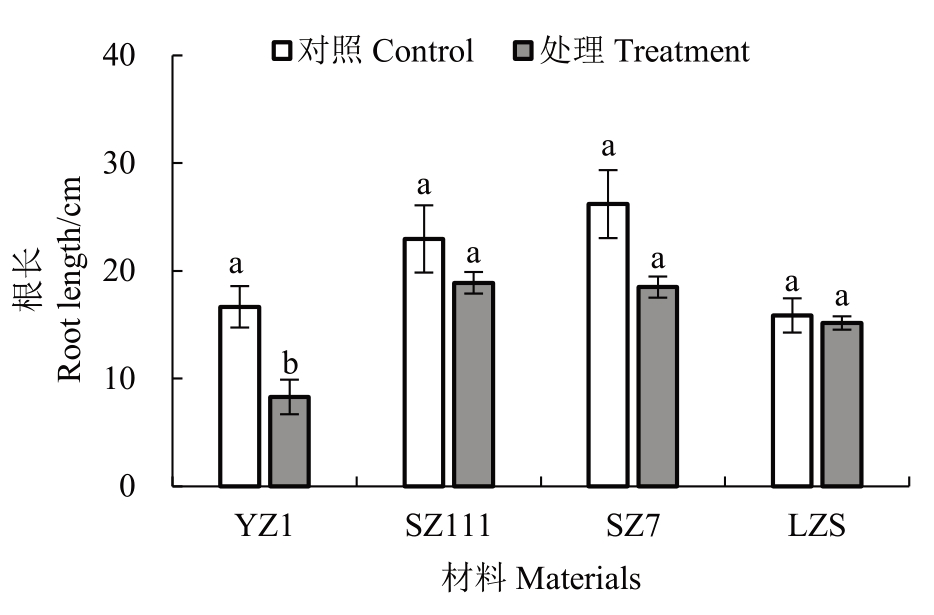
图2 镉胁迫对不同类型砧木根长的影响
Fig.2 Effects of cadmium stress on root length of different types of rootstocks
2.3 镉胁迫对不同类型砧木MDA含量的影响
与对照相比,经镉处理后的砧木叶片MDA 含量不同(图3-A)。其中,YZ1 叶片MDA 含量极显著升高22.68% ;SZ111 和LZS 分别升高5.68% 、22.84%,SZ7 降低5.91%,与对照差异均不显著。说明LZS受影响程度最大,SZ111最小。
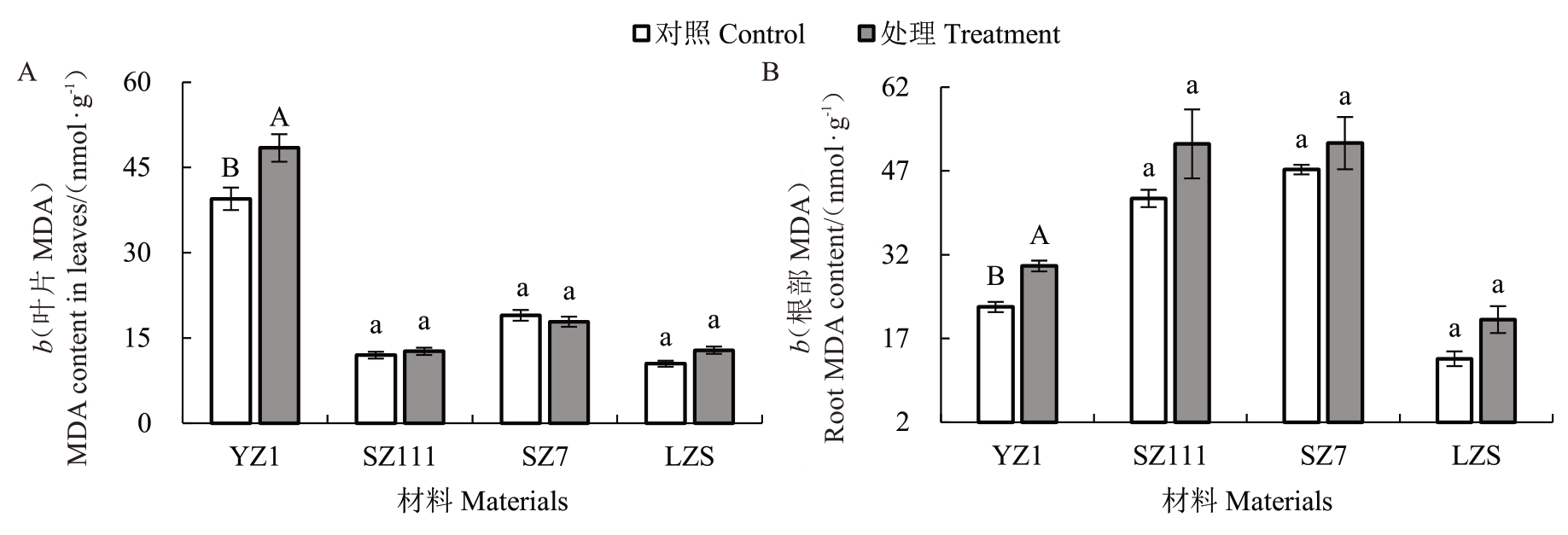
图3 镉胁迫对不同类型砧木MDA 含量的影响
Fig.3 Effects of cadmium stress on MDA content in different types of rootstocks
经镉处理,各砧木根部MDA 含量均比对照高(图3-B)。其中,YZ1 根部MDA 含量极显著升高32.45% ;SZ111、SZ7 和LZS 分别升高23.22% 、10.03%、52.46%,与对照差异不显著。说明LZS 受影响程度最大,SZ7最小。
2.4 镉胁迫对不同类型砧木SOD活性的影响
与对照相比,经镉处理后的4 种砧木叶片SOD活性均降低(图4-A)。其中YZ1 降低27.54%,与对照差异极显著;SZ111 和SZ7 分别降低10.00%、19.29%,与对照差异不显著;LZS 降低34.90%,与对照差异显著。说明LZS 受影响程度最大,SZ111 最小。
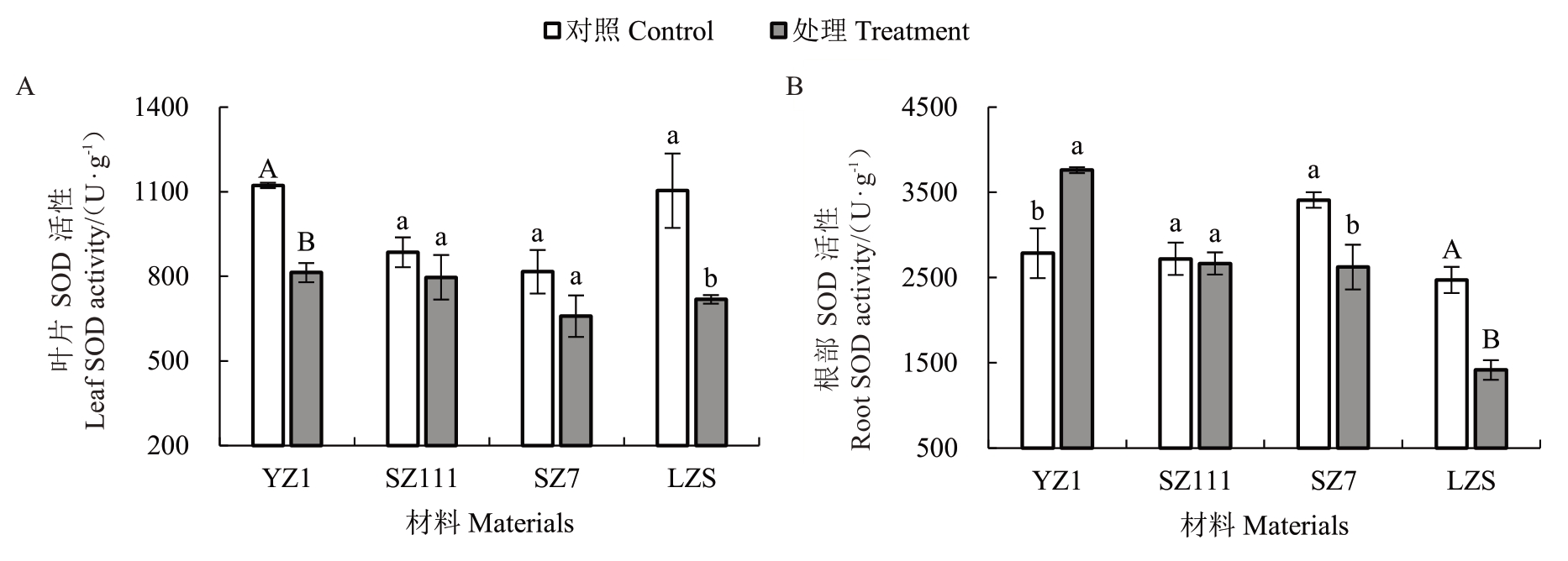
图4 镉胁迫对不同类型砧木SOD 活性的影响
Fig.4 Effects of cadmium stress on SOD activity in different types of rootstocks
与对照相比,经镉处理后的4 种砧木根部SOD活性各不相同(图4-B)。其中YZ1 显著提高34.99%,SZ7 显著降低23.06%;SZ111 降低2.01%,差异不显著;LZS 极显著降低42.72%。说明受影响程度最大的是LZS,最小是SZ111。
2.5 镉胁迫对不同类型砧木POD活性的影响
经镉处理后,与对照相比,不同类型砧木叶片POD 活性差异不同(图5-A)。其中,YZ1 叶片POD活性极显著升高142.55%;SZ111 活性显著降低41.02%;SZ7 和LZS 的活性分别降低31.78%、40.48%,差异均不显著。说明YZ1 受影响程度最大,最小是SZ7。
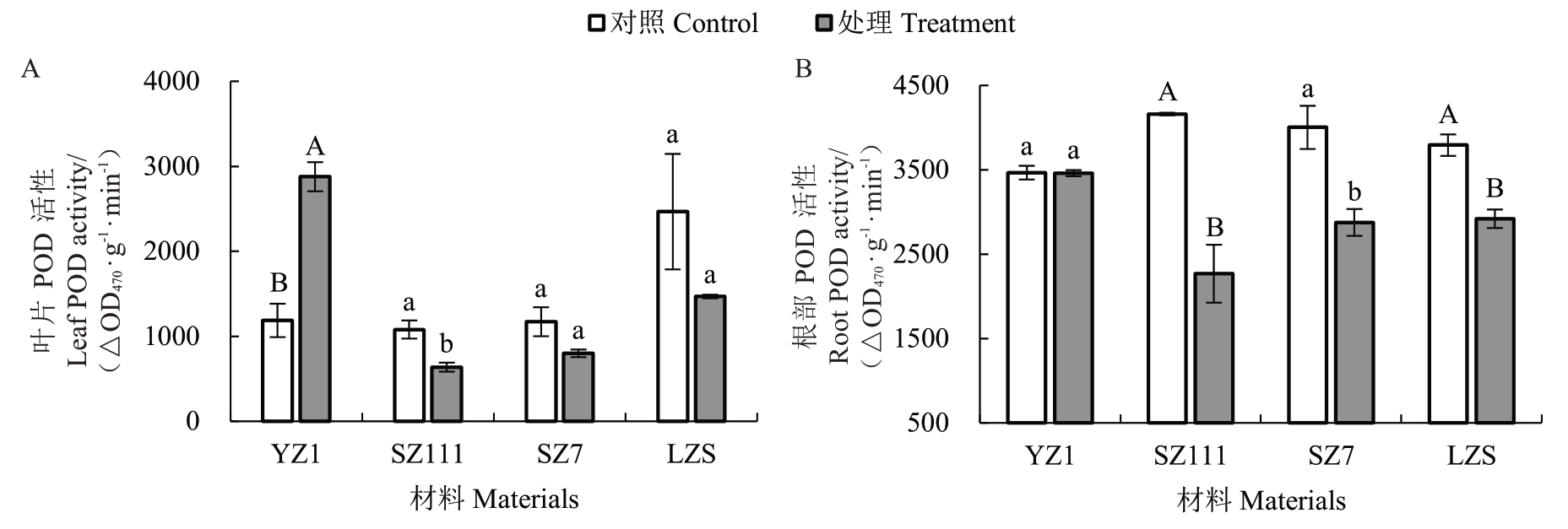
图5 镉胁迫对不同类型砧木POD 活性的影响
Fig.5 Effects of cadmium stress on POD activity of different types of rootstocks
经镉处理后,不同类型砧木根部POD 活性差异不同(图5-B)。与对照相比,YZ1 根部POD 活性降低0.18%,差异不显著;SZ111 和LZS 分别极显著降低45.48%、23.01%;SZ7 活性显著降低28.16%。说明受影响程度最大的是SZ111,最小是YZ1。
2.6 镉胁迫对不同类型砧木光合参数的影响
镉胁迫下,4 种类型砧木对光合作用Y(Ⅱ)影响不同(图6-A)。与对照相比,SZ111 与SZ7 的Y(Ⅱ)分别极显著下降51.23%和57.83% ;YZ1 降低5.82%,LZS 升高24.62%,差异均不显著。说明受影响程度最大的是SZ7,最小的是YZ1。
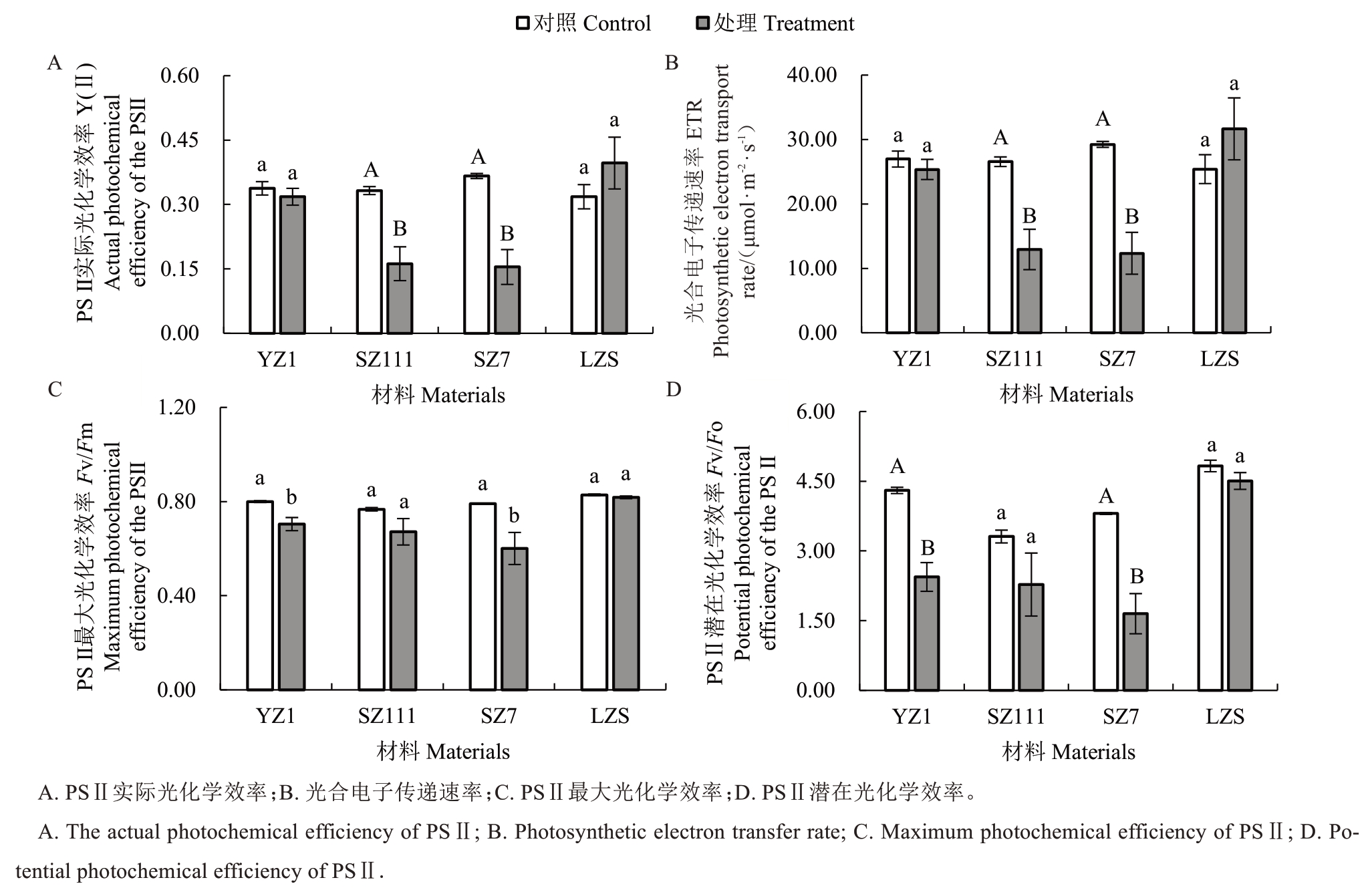
图6 镉胁迫对不同类型砧木叶绿素荧光参数的影响
Fig.6 Effect of cadmium stress on chlorophyll fluorescence parameters of different types of rootstocks
镉胁迫下,与对照相比,4 种类型砧木的ETR 差异显著性不同(图6-B),其中,SZ111 与SZ7 的ETR分别极显著降低51.29%、57.81%;YZ1 降低6.00%,LZS 升高24.61%,差异均不显著。说明受影响程度最大的是SZ7,最小的是YZ1。
与对照相比,4 种砧木光合作用Fv/Fm参数差异显著性表现不同,但均表现为降低(图6-C)。YZ1和SZ7 分别显著降低11.96%、24.13%;SZ111 和LZS分别降低12.47%、1.25%,差异不显著。其中受影响程度最大的是SZ7,受影响程度最小的是LZS。
与对照相比,4 种砧木光合作用Fv/Fo参数差异显著性呈现不同水平,但均呈现降低(图6-D)。YZ1和SZ7 分别极显著降低43.29%、56.63%;SZ111 和LZS 分别降低31.23%、6.67%,差异均不显著。其中受影响程度最大的是SZ7,其次是YZ1,最小的是LZS。
3 讨 论
金属镉对植物生长有抑制作用,当土壤里的Cd2+进入植物体内并累积时,植物会出现细胞氧化应激反应,影响植物生理平衡[19],导致植物生长不良。在本研究中,YZ1、SZ111 和SZ7 这3 种砧木的地上部鲜质量极显著降低,LZS降低不显著,这与田传玉等[20]对高Cd2+浓度导致多肉植物康平寿鲜质量显著降低的研究结果一致,这可能是因为镉进入植物体内后,对叶绿素传递产生影响,抑制光合作用[21],光合产物减少,生长受到抑制,导致地上部鲜质量降低。
植物的根是最先接触到镉的部位,根长的生长因镉的胁迫而受到抑制。在本研究中,YZ1 砧木根长比对照显著降低,其他3种砧木根长降低,但与对照差异不显著,此结果与孟琦涛等[22]和李倩等[23]对南瓜和黑豆幼苗的研究结果相似,原因可能是镉胁迫导致植物生理代谢紊乱,降低细胞壁的可塑性[24],导致植物对水分和养分吸收减少,最终抑制根系生长。
膜脂过氧化可作为植物氧化损伤的标志,而MDA 含量的高低反映了植物膜脂过氧化程度、ROS含量的多少和遭受逆境伤害程度的高低[25-26]。在本研究中,与对照相比,YZ1 叶片和根部处理的MDA含量极显著升高,其他3 种砧木均未发生显著变化,吕小娜等[27]的研究表明,不同植物叶片MDA 含量不同,根部MDA 含量升高,与笔者在本研究中的结果相似,这说明4 种砧木应对镉胁迫的抗性不同,YZ1耐镉胁迫性最弱。
在镉胁迫下,植物产生大量ROS 并累积,导致体内ROS含量升高,诱导抗氧化酶活性升高[28],POD与SOD 是植物体内清除ROS 的主要抗氧化酶[29]。在本研究中,经处理后的叶片SOD 活性均比对照低,其中YZ1 极显著降低,LZS 显著降低;YZ1 的根部SOD活性显著升高,SZ7显著降低,LZS极显著降低;YZ1的叶片POD 活性极显著升高,SZ111显著降低;SZ11 和LZS 的根部POD 活性极显著降低,SZ7显著降低,而张秀娟等[30]对3 种草坪草的生理研究表明,SOD 和POD 活性总体趋势为上升,这与笔者在本研究中的结果不同,其原因可能是不同植物对镉的反应机制不同,YZ1 受镉胁迫导致体内酶活系统反应激烈,抗氧化系统可能遭到破坏,而SZ111和LZS可能由于根部是直接接触镉的部位而受影响严重,地上部可能已稳定内部镉胁迫的生理环境,有一定的抗性机制。
在重金属胁迫下,植物氧化应激会抑制光合作用,致使叶绿体膜系统损伤[31],光合速率下降,导致植株发育滞缓甚至萎蔫死亡[32-33]。植物叶绿素荧光参数反应了PSⅡ特性及光能使用效率,进而体现外界逆境对PSⅡ产生的危害[34]。Y(Ⅱ)值的变化反映了PSⅡ反应中心在部分关闭下的实际原初光能捕获效率,在本试验中,SZ111、SZ7 在镉胁迫下Y(Ⅱ)值极显著降低,说明镉胁迫导致PSⅡ反应中心关闭,从而降低光能捕获效率,这与姜永雷等[35]对镉胁迫水蕨幼苗的研究结果一致。ETR 数值反映电子传递速率的快慢,在本试验中,SZ111 和SZ7 两个砧木与对照相比极显著降低,说明其光合反应速率降低,可能是由于光化学反应效率被镉影响[36],减慢电子传递速率,促使吸收的光能散失,避免因光抑制或光呼吸对光系统产生危害,提高了这两组砧木的光保护能力,这与张栋栋等[37]的研究结果一致。Fv/Fm可作为原初光能转换效率高低的依据,可以反映植物损伤程度[38]。在本试验中,YZ1 和SZ7 的Fv/Fm值均显著降低,可能是由于PSⅡ反应中心关闭数量增多,植物光合活性降低,生长受抑制,这与刘金秀等[39]的研究结果一致。Fv/Fo值反映有活性的PSⅡ反应中心的数量和捕获转化所需光能的能力。在本试验中,YZ1 和SZ7 的Fv/Fo比对照均极显著降低,这与张培等[40]的研究结果相同,说明在镉胁迫下有活性的PSⅡ反应中心数量减少,捕获及转化所需光能的能力减弱。
4 结 论
以4 种类型砧木进行镉胁迫研究,发现不同类型砧木的地上部鲜质量、根长、MDA 含量、SOD 和POD 活性及叶绿素荧光参数响应镉胁迫的程度不同,可为相关育种及栽培研究提供参考,同时发现了SZ111和LZS这2个抗镉胁迫的砧木,可为西瓜安全生产保驾护航。
[1] 乔晓芳,刘奇志,刘松忠,刘军,刘聪鹤.梨园常规施药与土壤重金属潜在污染风险评价[J].果树学报,2018,35(8):947-956.QIAO Xiaofang,LIU Qizhi,LIU Songzhong,LIU Jun,LIU Cong‐he.The evaluation of the potential risk of soil heavy metal pollu‐tion with conventional pesticide application in pear orchards[J].Journal of Fruit Science,2018,35(8):947-956.
[2] 王月.农业土壤重金属污染成因及治理[J].农业科技与装备,2022(6):20-21.WANG Yue.Cause and treatment of heavy metal pollution in ag‐ricultural soil[J].Agricultural Science & Technology and Equip‐ment,2022(6):20-21.
[3] 章海波,骆永明,李远,周倩,刘兴华.中国土壤环境质量标准中重金属指标的筛选研究[J].土壤学报,2014,51(3):429-438.ZHANG Haibo,LUO Yongming,LI Yuan,ZHOU Qian,LIU Xinghua. Screening of criteria for heavy metals for revision of the national standard for soil environmental quality of China[J].Acta Pedologica Sinica,2014,51(3):429-438.
[4] LU A X,LI B R,LI J,CHEN W,XU L. Heavy metals in paddy soil-rice systems of industrial and township areas from subtropi‐cal China:Levels,transfer and health risks[J]. Journal of Geo‐chemical Exploration,2018,194:210-217.
[5] 赵杨阳. 食品中重金属超标危害与检测[J]. 现代食品,2018(7):111-113.ZHAO Yangyang. Heavy metal excessive hazard and detection in food[J].Modern Food,2018(7):111-113.
[6] YOUSAF B,LIU G J,WANG R W,IMTIAZ M,ZIA-URREHMAN M,MUNIR M A M,NIU Z Y.Bioavailability evalua‐tion,uptake of heavy metals and potential health risks via di‐etary exposure in urban-industrial areas[J]. Environmental Sci‐ence and Pollution Research,2016,23(22):22443-22453.
[7] 孙可望,刘娣,潘风山,韦燕燕,候丹迪,杨肖娥.重金属暴露与乳腺癌发病的关系初探[J].浙江农业学报,2017,29(8):1353-1357.SUN Kewang,LIU Di,PAN Fengshan,WEI Yanyan,HOU Dan‐di,YANG Xiao’e. Study on relationship between heavy metals exposure and breast cancer[J].Acta Agriculturae Zhejiangensis,2017,29(8):1353-1357.
[8] 秦晓明. 硒对冬小麦镉吸收、转运及生理特性的影响[D]. 郑州:河南农业大学,2019.QIN Xiaoming. Effects of selenium on cadmium uptake,trans‐port and physiological characteristics of winter wheat[D].Zhengzhou:Henan Agricultural University,2019.
[9] 卢敏敏,孙胜.镉胁迫对小型西瓜幼苗生长及脂膜过氧化的影响[J].山西农业科学,2008,36(12):64-66.LU Minmin,SUN Sheng. Effect of cadmium stress on growth and membrane- lipid peroxidation of small watermelon seed‐lings[J]. Journal of Shanxi Agricultural Sciences,2008,36(12):64-66.
[10] 万凤婷.镉污染对甜瓜生理特性及甜瓜蒂有效成分含量的影响研究[D].武汉:武汉理工大学,2021.WAN Fengting. Study on the effect of cadmium pollution on the physiological characteristics of muskmelon and the contents of active components in the pedicellus muskmelo[D]. Wuhan:Wu‐han University of Technology,2021.
[11] 黄成东,李宝深,刘全清.西瓜嫁接育苗好处多[J].现代农村科技,2010(19):20.HUANG Chengdong,LI Baoshen,LIU Quanqing. Watermelon grafting seedling many benefits[J]. Modern Rural Science and Technology,2010(19):20.
[12] 孔富军.温室瓜菜嫁接栽培好处多[J].西北园艺,2009(7):55.KONG Fujun.Greenhouse melon and vegetable grafting cultiva‐tion benefits[J].Northwest Horticulture,2009(7):55.
[13] 余昌.西瓜嫁接好处多[N].河南科技报,2009-02-20(8).YU Chang. Watermelon grafting has many benefits[N]. Henan Science and Technology News,2009-02-20(8).
[14] 高天晗,孙梨宗,台培东,郭橙.嫁接诱导大豆低镉富集性状及遗传稳定性[J].生态学杂志,2021,40(1):140-147.GAO Tianhan,SUN Lizong,TAI Peidong,GUO Cheng. Genet‐ic stability of low cadmium accumulation induced by grafting in soybean[J].Chinese Journal of Ecology,2021,40(1):140-147.
[15] 王志伟,李涵,孙波,孙小武,梁志怀,谢汉忠.不同砧木对西瓜镉积累和品质的影响[J]. 果树学报,2017,34(10):1309-1315.WANG Zhiwei,LI Han,SUN Bo,SUN Xiaowu,LIANG Zhi‐huai,XIE Hanzhong. Effects of different stocks on the accumu‐lation of cadmium and fruit quality in watermelon[J]. Journal of Fruit Science,2017,34(10):1309-1315.
[16] 夏睿,夏新发,王元龙,田红梅,王爱听,王朋成.葫芦砧木优良亲本筛选试验[J].现代农业科技,2023(19):77-79.XIA Rui,XIA Xinfa,WANG Yuanlong,TIAN Hongmei,WANG Aiting,WANG Pengcheng. Excellent parent screening test of gourd rootstock[J]. Modern Agricultural Science and Technology,2023(19):77-79.
[17] 王志伟,李涵,孙波,邹甜,孙小武.不同砧木嫁接对西瓜果实Cd 含量和品质的影响[J].长江蔬菜,2018(2):66-70.WANG Zhiwei,LI Han,SUN Bo,ZOU Tian,SUN Xiaowu. Ef‐fect of different kinds of grafting rootstock on cadmium content and quality of watermelon fruit[J]. Journal of Changjiang Vege‐tables,2018(2):66-70.
[18] 黄芸萍,邢乃林,付玉婧,王迎儿,严蕾艳,应泉盛,王毓洪.不同类型砧木嫁接对西瓜果实镉含量的影响[J].上海农业学报,2020,36(4):60-64.HUANG Yunping,XING Nailin,FU Yujing,WANG Ying’er,YAN Leiyan,YING Quansheng,WANG Yuhong. Influences of cadmium content in watermelon fruit grafted by different type of rootstocks[J].Acta Agriculturae Shanghai,2020,36(4):60-64.
[19] 芦建国,彭河忠.鸢尾属植物的抗性研究进展[J].江西农业学报,2012,24(2):48-51.LU Jianguo,PENG Hezhong. Research progress in resistance of plants in iris[J].Acta Agriculturae Jiangxi,2012,24(2):48-51.
[20] 田传玉,陈建中,李露园,李红丽,张梦雅,王锋敏,朱婧婧,洽丹. 镉胁迫对多肉植物康平寿生长和增殖的影响[J]. 湖州师范学院学报,2020,42(2):33-38.TIAN Chuanyu,CHEN Jianzhong,LI Luyuan,LI Hongli,ZHANG Mengya,WANG Fengmin,ZHU Jingjing,QIA Dan.Effects of cadmium stress on growth and proliferation of Hawor‐thia emelyae[J]. Journal of Huzhou University,2020,42(2):33-38.
[21] GRAJEK H,RYDZYŃSKI D,PIOTROWICZ-CIEŚLAK A,HERMAN A,MACIEJCZYK M,WIECZOREK Z. Cadmium ion-chlorophyll interaction-Examination of spectral properties and structure of the cadmium-chlorophyll complex and their rel‐evance to photosynthesis inhibition[J].Chemosphere,2020,261:127434.
[22] 孟琦涛,徐颖超,朱吉童,曾霖根,傅曼琴,聂呈荣,钟玉娟.镉对广蜜二号南瓜幼苗生长与镉累积特性的影响[J/OL]. 中国瓜菜:1-13[2024-04-27]. https://doi.org/10.16861/j.cnki.zg‐gc.202423.0715.MENG Qitao,XU Yingchao,ZHU Jitong,ZENG Lingen,FU Mangin,NIE Chengrong,ZHONG Yujuan. Effects of cadmium on the growth and cadmium accumulation characteristics of Guangmi 2 miben pumpkin seedlings[J]. China Cucurbits and Vegetables:1-13[2024-04-27].https://doi.org/10.16861/j.cnki.zg‐gc.202423.0715.
[23] 李倩,赵英旭,刘浩,吕彦,周鑫.硫酸铜对黑豆种子萌发和幼苗生长的影响[J/OL].烟台大学学报(自然科学与工程版):1-8[2024-04-27].https://doi.org/10.13951/j.cnki.37-1213/n.230615.LI Qian,ZHAO Yingxu,LIU Hao,LÜ Yan,ZHOU Xin. Effect of copper sulfate on germination and seedling growth of black bean seeds[J/OL]. Journal of Yantai University (Natural Science and Engineering Edition):1- 8[2024- 04- 27]. https://doi.org/10.13951/j.cnki.37-1213/n.230615.
[24] HU Y F,ZHOU G Y,NA X F,YANG L J,NAN W B,LIU X,ZHANG Y Q,LI J L,BI Y R. Cadmium interferes with mainte‐nance of auxin homeostasis in Arabidopsis seedlings[J]. Journal of Plant Physiology,2013,170(11):965-975.
[25] 乔新燕,吴仁杰,曹少飞,李建国,原寒.铀对植物的胁迫效应研究进展[J].核农学报,2023,37(1):207-215.QIAO Xinyan,WU Renjie,CAO Shaofei,LI Jianguo,YUAN Han.Advances in stress effects of uranium on plants[J]. Journal of Nuclear Agricultural Sciences,2023,37(1):207-215.
[26] MONTEIRO M S,SANTOS C,SOARES A M V M,MANN R M. Assessment of biomarkers of cadmium stress in lettuce[J].Ecotoxicology and Environmental Safety,2009,72(3):811-818.
[27] 吕小娜,昝亚玲,原红娟. 不同植物对镉土壤污染的修复作用及分子机制[J/OL]. 分子植物育种,1-13[2024-02-18].https://kns- cnki- net.webvpn.zafu.edu.cn/kcms/detail/46.1068.s.20230328.1124.008.html.LÜ Xiaona,ZAN Yyanling,YUAN Hongjuan.The remediation ef‐fect and molecular mechanism of different plants on cadmium soil pollution [J/OL]. Molecular Plant Breeding,1-13[2024-02-18].https://kns- cnki- net.webvpn.zafu.edu.cn/kcms/detail/46.1068.s.20230328.1124.008.html.
[28] 孟凡茹,郭卫丽,赵戴军,陈碧华,李新峥.瓜类蔬菜砧木耐镉研究进展[J]. 河南科技学院学报(自然科学版),2020,48(5):15-21.MENG Fanru,GUO Weili,ZHAO Daijun,CHEN Bihua,LI Xinzheng. Research progress of cadmium tolerance in gourd vegetables rootstocks[J]. Journal of Henan Institute of Science and Technology(Natural Science Edition),2020,48(5):15-21.
[29] 李源恒,赵春莉,郭宏亮,陈元晖,刘翰升,王晟旭.镉胁迫对黄秋英生理及富集特性的影响[J/OL]. 山东农业科学,1-16[2024-02-18]. https://kns-cnki-net.webvpn.zafu.edu.cn/kcms/de‐tail/37.1148.S.20231103.0940.002.html.LI Yuanheng,ZHAO Chunli,GUO Hongliang,CHEN Yuanhui,LIU Hansheng,WANG Chengxu. Effects of cadmium stress on physiological and enrichment characteristics of Cosmos sulphu‐reus[J/OL]. Shandong Agricultural Sciences,1-16[2024-02-18].https://kns- cnki- net.webvpn.zafu.edu.cn/kcms/detail/37.1148.S.20231103.0940.002.html.
[30] 张秀娟,范玟,杨乐,吴楚,杨永胜.镉胁迫对3 种草坪草生理特性及叶片超微结构的影响[J].草地学报,2023,31(9):2663-2670.ZHANG Xiujuan,FAN Men,YANG Le,WU Chu,YANG Yong‐sheng. Effects of cadmium stress on physiological activities and leaf ultrastructures of three turfgrass species[J].Acta Agrestia Si‐nica,2023,31(9):2663-2670.
[31] 赵晶晶,詹万龙,周浓.非生物胁迫下植物体内活性氧和丙酮醛代谢的研究进展[J].南方农业学报,2022,53(8):2099-2113.ZHAO Jingjing,ZHAN Wanlong,ZHOU Nong. Research prog‐ress on the metabolisms of reactive oxygen species and methylg‐lyoxal in plants under abiotic stresses[J]. Journal of Southern Agriculture,2022,53(8):2099-2113.
[32] CHI Z X,HONG B W,TAN S W,WU Y C,LI H J,LU C H,LI W G.Impact assessment of heavy metal cations to the character‐istics of photosynthetic phycocyanin[J]. Journal of Hazardous Materials,2020,391:122225.
[33] CHEN L,YANG J Y,WANG D. Phytoremediation of uranium and cadmium contaminated soils by sunflower (Helianthus ann‐uus L.) enhanced with biodegradable chelating agents[J]. Jour‐nal of Cleaner Production,2020,263:121491.
[34] LIU L Y,ZHANG Y J,JIAO Q J,PENG D L.Assessing photo‐synthetic light-use efficiency using a solar-induced chlorophyll fluorescence and photochemical reflectance index[J]. Interna‐tional Journal of Remote Sensing,2013,34(12):4264-4280.
[35] 姜永雷,唐探,陈嘉裔,冯程程,黄晓霞.镉胁迫对水蕨幼苗叶绿素荧光参数和生理指标的影响[J].江苏农业科学,2015,43(9):357-360.JIANG Yonglei,TANG Tan,CHEN Jiayi,FENG Chengcheng,HUANG Xiaoxia. Effect of cadmium stress on chlorophyll fluo‐rescence parameters and physiological indicators of Ceratopter‐is thalictroides seedlings[J]. Jiangsu Agricultural Sciences,2015,43(9):357-360.
[36] KÜPPER H,PARAMESWARAN A,LEITENMAIER B,TRTÍLEK M,ŠETLÍK I. Cadmium-induced inhibition of photo‐synthesis and long-term acclimation to cadmium stress in the hy‐peraccumulator Thlaspi caerulescens[J].New Phytologist,2007,175(4):655-674.
[37] 张栋栋,李萌,甘龙,李晓玲,任东,胥焘,黄应平.重金属Cd-Cu 复合污染对苍耳生长及叶绿素荧光特性的影响[J].武汉大学学报(理学版),2019,65(1):66-76.ZHANG Dongdong,LI Meng,GAN Long,LI Xiaoling,REN Dong,XU Tao,HUANG Yingping. Effects of heavy metal Cd-Cu combined pollution on the growth and fluorescence charac‐teristics of Xanthium sibiricum Patrin ex Widder[J]. Journal of Wuhan University(Natural Science Edition),2019,65(1):66-76.
[38] FROM N,RICHARDSON K,MOUSING E A,JENSEN P E.Removing the light history signal from normalized variable fluo‐rescence (Fv/Fm) measurements on marine phytoplankton[J].Limnology and Oceanography:Methods,2014,12(11):776-783.
[39] 刘金秀,张松彦,周建.镉胁迫对刺槐幼苗生长与光合生理特性的影响[J].林业科学研究,2023,36(3):168-178.LIU Jinxiu,ZHANG Songyan,ZHOU Jian. Effects of cadmium stress on growth and photosynthetic physiological characteris‐tics of Robinia pseudoacacia seedlings[J]. Forest Research,2023,36(3):168-178.
[40] 张培,庞圣江,刘士玲,谌红辉,段润梅,曾琪瑶.遮阴对江南油杉幼苗生长和叶绿素荧光参数的影响[J]. 西北植物学报,2023,43(10):1716-1722.ZHANG Pei,PANG Shengjiang,LIU Shiling,CHEN Honghui,DUAN Runmei,ZENG Qiyao.Effects of shading on growth and chlorophyll fluorescence characteristics of Keteleeria fortunei var. cyclolepis seedlings[J]. Acta Botanica Boreali-Occidentalia Sinica,2023,43(10):1716-1722.