果实角质层位于表皮细胞外缘,紧贴细胞壁的果胶层,是由羟基和环氧羟基脂肪酸经酯化形成的疏水性保护结构,作为化学和物理屏障可防止真菌孢子萌发和侵染果实[1]。角质层的外缘分布有蜡质层,蜡质又可分为内表皮蜡质和外表皮蜡质,前者在角质层形成中填充在其致密的网状结构内,后者则堆积在角质层外缘并且组装成各种形态的晶体[2]。表皮蜡质主要由超长链脂肪酸及烷烃等各类衍生物组成,作为疏水保护层可以防止果实水分蒸腾、病原物在表面黏附以及在表面形成水膜[3]。研究表明,果实表面的蜡质层和角质层作为物理和化学屏障,可以抑制果实呼吸,减少水分蒸腾,从而降低贮藏期间的果实失重率[4]。
不同苹果品种果实角质层和蜡质层的厚度和成分存在差异。金冠的蜡质层要明显厚于元帅,金冠含有更多的9,10-环氧-12-十八碳烯酸和9,10-环氧-18-羟基-12-十八碳烯酸[5]。比较皇家嘎啦、澳洲青苹、粉红女士和元帅4个苹果品种的蜡质成分,发现壬二十烷和五十五烷在澳洲青苹中含量最高;三萜酸、十七醇、α-法尼烯、法尼乙酸酯以及一些饱和脂肪酸在皇家嘎啦中含量较高;粉红女士和元帅中扁桃酸和β-谷甾醇含量较高。与元帅相比,粉红女士含有更多的十六烷酸、γ-亚麻酸和五十七烷[6]。奎里纳的表皮蜡质层和角质层比普利玛更厚,奎里纳还含有更多的9,10,16-二羟基十六烷酸、熊果酸和油酸[7]。角质和蜡质成分含量的差异对果实的贮藏性具有重要影响。对10 个苹果品种果实的蜡质研究表明,红星果实的蜡质含量显著低于其他品种,贮藏期间的失重率最高[8]。
不同砧木也会影响果实的贮藏品质、抗氧化能力和抗病性。与SH3 相比,SH6 作中间砧的富士果实含有较高的果糖、山梨醇、葡萄糖、蔗糖以及草酸、酒石酸、苹果酸和柠檬酸含量[9]。与M9 相比,G213作砧木的Fuji Suprema 果实贮藏期间具有较高的硬度和可溶性固形物含量[10]。与M4 和MM106 相比,M9 T337 砧木可以提高Red Chief® Camspur 苹果果实的总酚、总黄酮含量及总抗氧化能力[11]。与A1和C3 相比,B1 砧木可提高嘎拉和富士果实的总酚和类黄酮含量,也可提高果皮的花青苷和类胡萝卜素含量[12]。与M26 相比,损伤接种Penicillium expan‐sum 和Botrytis cinerea 的SH6 果实的病斑直径显著小于M26果实[13]。
矮化砧木具备使树体矮化、早熟、高产、便于管理等优点,在苹果栽培中具有重要作用[14]。SH 系矮化砧由山西省农业科学院果树所选育,是我国广泛使用的苹果矮化中间砧,具有抗逆性较强,嫁接后易开花结果、果实品质良好等特点[15]。M 系砧木由英国东茂林试验站选育,是目前全球广泛使用的苹果矮化中间砧,但抗逆性不及SH 系矮化砧[16]。笔者近期发现,与M26 相比,SH6 作中间砧的富士苹果的贮藏品质更好,抗氧化活性更高,采后抗病性也更强,但两种砧木是否通过影响贮藏期间苹果果实表皮蜡质和角质的结构和成分,从而影响果实的贮藏性还未见报道。笔者以SH6 和M26 两种中间砧的富士苹果为试材,测定常温贮藏期间两种砧木果实的失重率和呼吸强度变化,观察表皮角质层和蜡质层形态,分析角质和蜡质总量以及各种单体成分的含量,以期比较两种砧木间果实角质、蜡质形态和成分的差异对果实失重率和呼吸强度的影响。
1 材料和方法
1.1 材料
富士苹果于2023年9月底采于甘肃省庆阳市宁县中村15年生树,以八棱海棠为基砧,SH6和M26分别为矮化中间砧。当地属暖温带湿润半湿润气候,年均降水量为572 mm,平均气温8.7 ℃,无霜期170 d左右,田间管理按当地苹果生产标准进行。挑选外观良好、大小一致、无病虫害、无机械损伤的果实,单果套网套装入包装箱,于2023年10月初运抵甘肃农业大学食品科学与工程学院采后生物学与技术实验室,常温下[(25±2)℃,RH 45%~65%]贮藏待用。
1.2 方法
1.2.1 失重率的测定 采用Wang 等[17]的质量法测定常温贮藏0、10、20、30 和40 d 的果实失重率(%)。每组每个时间点用果实3个,3次重复。
1.2.2 呼吸强度的测定 参考Li 等[18]的方法,用果蔬呼吸测定仪(GH-3051H,北京托普云农有限公司,中国)测定呼吸强度。取贮藏0、10、20、30 和40 d 的果实,先分别测定空罐内CO2的浓度,待罐内CO2的质量浓度低于600 mg·L-1时,迅速放入3个果实后密封,待罐内CO2浓度平衡后,每隔5 min 记录红外线吸收的CO2浓度。结果以mg·kg-1·h-1表示。每组每个时间点用果实3个,3次重复。
1.2.3 表皮蜡质和角质形态结构观察 参照徐秀萍等[19]的方法,分别在贮藏0 和40 d,从果实赤道部位随机切取1 mm 厚的正方形(1 cm×1 cm)表皮,置于2.5%戊二醛中常温固定2 h,然后用pH 7.4的磷酸缓冲溶液(PBS)漂洗3 次,每次15 min。再用30%、50%、70%、80%、90%、95%和100%的梯度酒精分别脱水15 min。将完全脱水后的样品放入临界点干燥仪(K850,Quorum,英国)内进行干燥。干燥后的样品紧贴于导电碳膜双面胶上放入离子溅射仪(MC1000,HITACHI,日本)样品台上喷金。在扫描电子显微镜(Regulus 8100,HITACHI,日本)下观察果皮表面蜡质形态和厚度。
1.2.4 角质成分的提取 参考陈剑婷等[20]的方法提取角质成分。用1%次氯酸钠浸泡果实1 min,清水冲洗晾干。分别在果实贮藏第0 天、第20 天和第40 天,从果实赤道部位随机用打孔器取10 个圆片(直径1.5 cm,厚1 mm)。将圆片置于100 mL 柠檬酸缓冲液(20 mmol·L-1,pH 3.7,其中含2%果胶酶和0.1%纤维素酶)中,在37 ℃下酶解3~4 d,酶解后的果皮用去离子水充分冲洗,得到角质提取样品。将上述样品用氯仿∶甲醇(1∶1)混合溶剂进行索氏抽提6~8 h,随后用甲醇洗去样品上的残留氯仿。将获得物在55 ℃烘箱中加热干燥1~2 d,将完全干燥的样品研磨成粉。取样品粉末30 mg,加入8 mL 3 mol·L-1甲醇盐酸溶液(其中含7.5%乙酸甲酯),60 ℃水浴反应16 h,冷却至室温,加入2 mL 饱和NaCI 溶液终止反应。再加入10 mL 二氯甲烷萃取2 次,将萃取所得溶液转移至10 mL 离心管,在温和氮气下吹扫富集,充分干燥后得角质粗提物。每一处理每个时间点用果实3个,3次重复。
取5 mg上述角质粗提物,加入100 μg十七烷酸甲酯作内标,加入200 μL 吡啶:双(三甲基硅烷基)三氟乙酰胺(BSTFA)(1∶1)试剂,100 ℃水浴反应15 min。残余的衍生化试剂在温和氮气下吹干,用1.5 mL庚烷∶甲苯(1∶1)重新溶解样品,转移至1.5 mL进样瓶后待测。
1.2.5 蜡质成分的提取 参考唐瑛[21]的方法提取蜡质成分。用自来水清洗果实表面,自然晾干。取1000 mL 烧杯,倒入600 mL 氯仿,分别在果实贮藏第0、20 和40 天时,将果实浸没在氯仿中60 s,用玻璃棒均匀搅拌。所得溶液经过滤后旋转蒸发,浓缩至约5 mL,转入10 mL 离心管,在稳定氮气下吹扫富集,充分干燥后称质量,置于4 ℃冰箱保存待测。每处理每个时间点用果实3个,3次重复。
取上述样品5 mg于10 mL离心管中,加入100 μg正二十四碳烷作为内标,加200 μL BSTFA 试剂,在100 ℃水浴下衍生反应15 min。衍生后的溶液在温和氮气下吹干,随后加入1.5 mL 氯仿溶解,转移至1.5 mL进样瓶后待测。
1.2.6 角质和蜡质成分的测定 采用气相色谱/质谱联用仪(GC/MS-QP2020NX,岛津公司,日本)测定角质和蜡质成分含量。升温程序:初始温度80 ℃,保持2 min;角质成分以15 ℃·min-1 升至200 ℃,保持2 min;2 ℃·min-1升至280 ℃,保持3 min。蜡质成分以40 ℃·min-1升高至200 ℃,保持2 min;3 ℃·min-1升至320 ℃,保持20 min。高纯He 作载气,流速1 mL·min-1,四极杆温度150 ℃,离子源温度230 ℃。根据检测物峰面积和内标物峰面积计算含量。最终角质单体含量以μg·g-1表示,蜡质单体含量以μg·cm-2表示。
1.3 数据分析
上述每项测定至少3 次重复。采用Microsoft Excel 2010 软件计算平均值和标准偏差,采用SPSS 19.0 软件进行Duncan’s 多重差异显著性分析(p<0.05)和Pearson 相关性分析,采用Origin 2021 软件作图。
2 结果与分析
2.1 SH6 和M26 富士苹果果实常温贮藏期间的失重率和呼吸速率的变化
失重率和呼吸强度是评价苹果采后品质和生理状态的重要指标。贮藏期间,SH6 和M26 果实的失重率均逐渐升高,M26果实的失重率显著高于SH6,第20 天时高出SH6 果实36.33%(图1-A)。SH6 和M26 果实的呼吸速率均呈单峰型变化,均在20 d 时达到峰值,M26果实的呼吸速率显著高于SH6果实,峰值时高出SH6果实53.16%(图1-B)。上述结果表明,常温贮藏期间SH6 果实的失重率和呼吸强度显著低于M26果实。
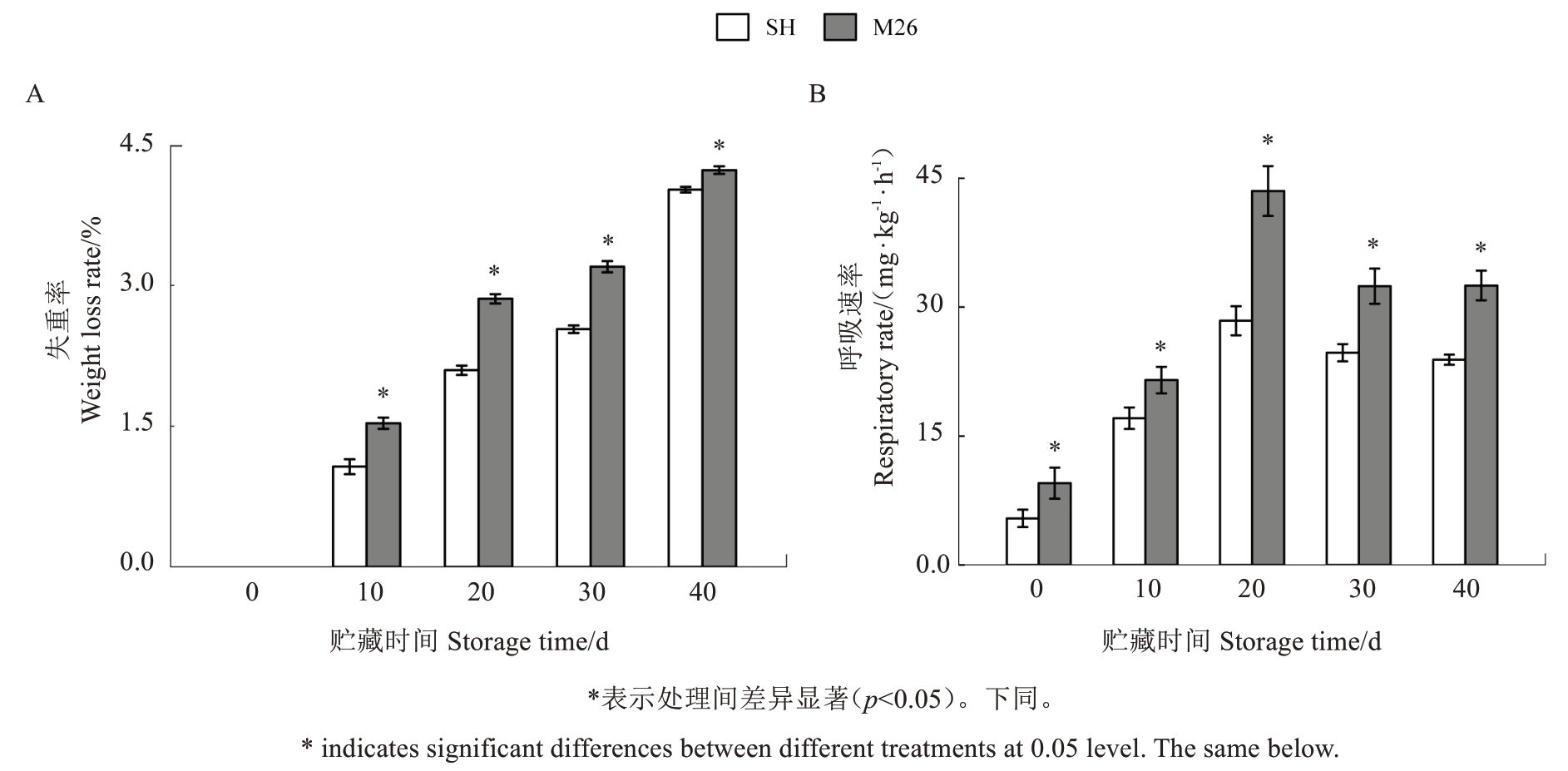
图1 SH6 和M26 富士苹果果实常温贮藏期间失重率(A)和呼吸速率(B)的变化Fig.1 Changes in weight loss rate(A)and respiration rate(B)of SH6 and M26 Fuji apples during ambient storage
2.2 SH6 和M26 富士苹果果实常温贮藏期间角质和蜡质总量的变化
采收时及贮藏期间,SH6 果实的角质总量均显著高于M26 果实,随着贮藏时间的延长,角质总量呈现单峰型变化,贮藏20 d 时达到峰值,峰值时SH6 果实角质含量(w,后同)为(9 683.12±249.58)μg·g-1,M26果实为(6295.88±183.45)μg·g-1,SH 果实显著高出M26 果实53.8%(图2-A)。采收时及贮藏期间,SH6 果实的蜡质总量也显著高于M26 果实,随着贮藏时间的延长,蜡质含量也呈单峰型变化,贮藏20 d时SH 果实蜡质含量为(643.94±31.89)μg·cm-2,M26果实为(503.55±23.04)μg·cm-2,SH6果实显著高出M26果实27.88%(图2-B)。上述结果表明,采收时及贮藏期间SH6 比M26 果实积累了更多的角质和蜡质。
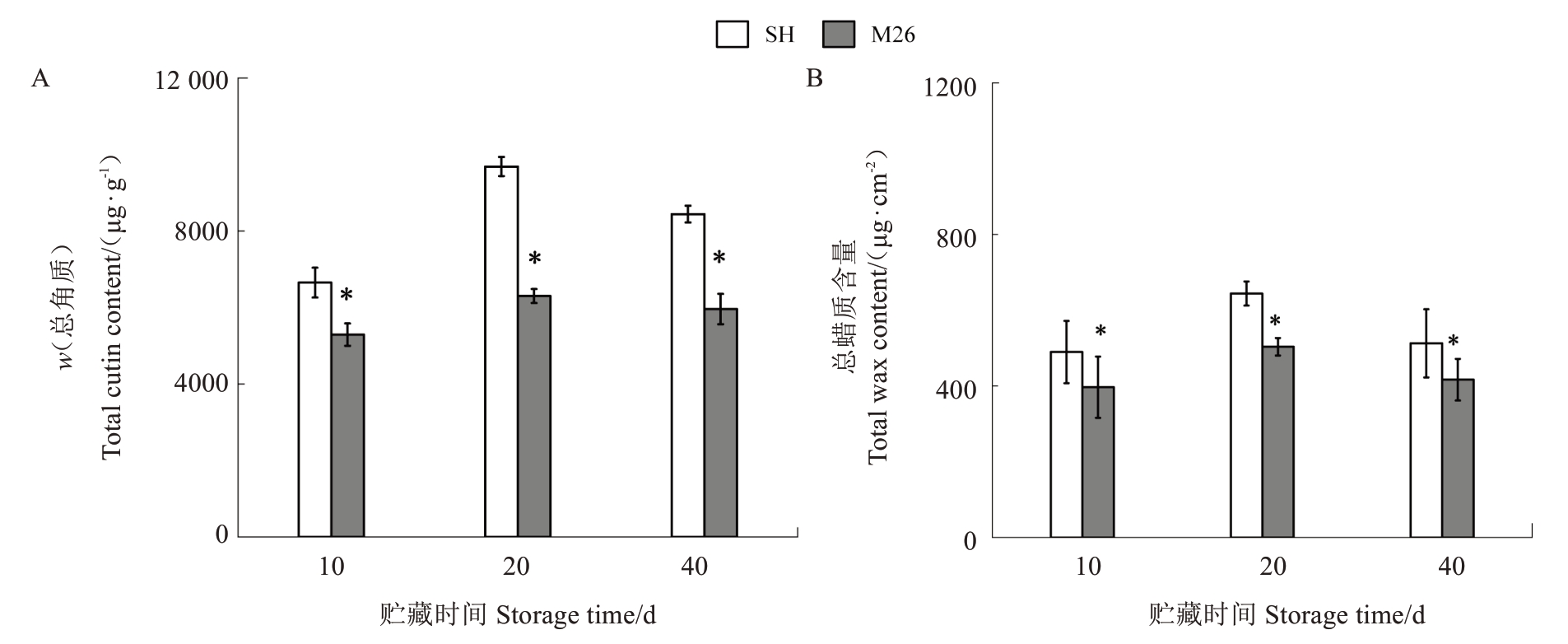
图2 SH6 和M26 富士苹果果实常温贮藏期间角质总量(A)和蜡质总量(B)的变化
Fig.2 Changes in total cutin(A)and total wax(B)content of SH6 and M26 Fuji apples during ambient storage
2.3 SH6 和M26 富士苹果果实常温贮藏期间表皮蜡质层形态的变化
SH6和M26果实表面蜡质形态和厚度存在明显差异。采收时,SH6果实表面蜡质较为平整,有少量较宽的缝隙和凹陷;M26 果实表面蜡质粗糙,凹陷密布。常温贮藏40 d 时,SH6 果实表面蜡质变得较为粗糙,缝隙和凹陷增多,缝隙间距缩小;M26 果实表皮蜡质凹陷更加明显,可见较大较宽的缝隙(图3)。
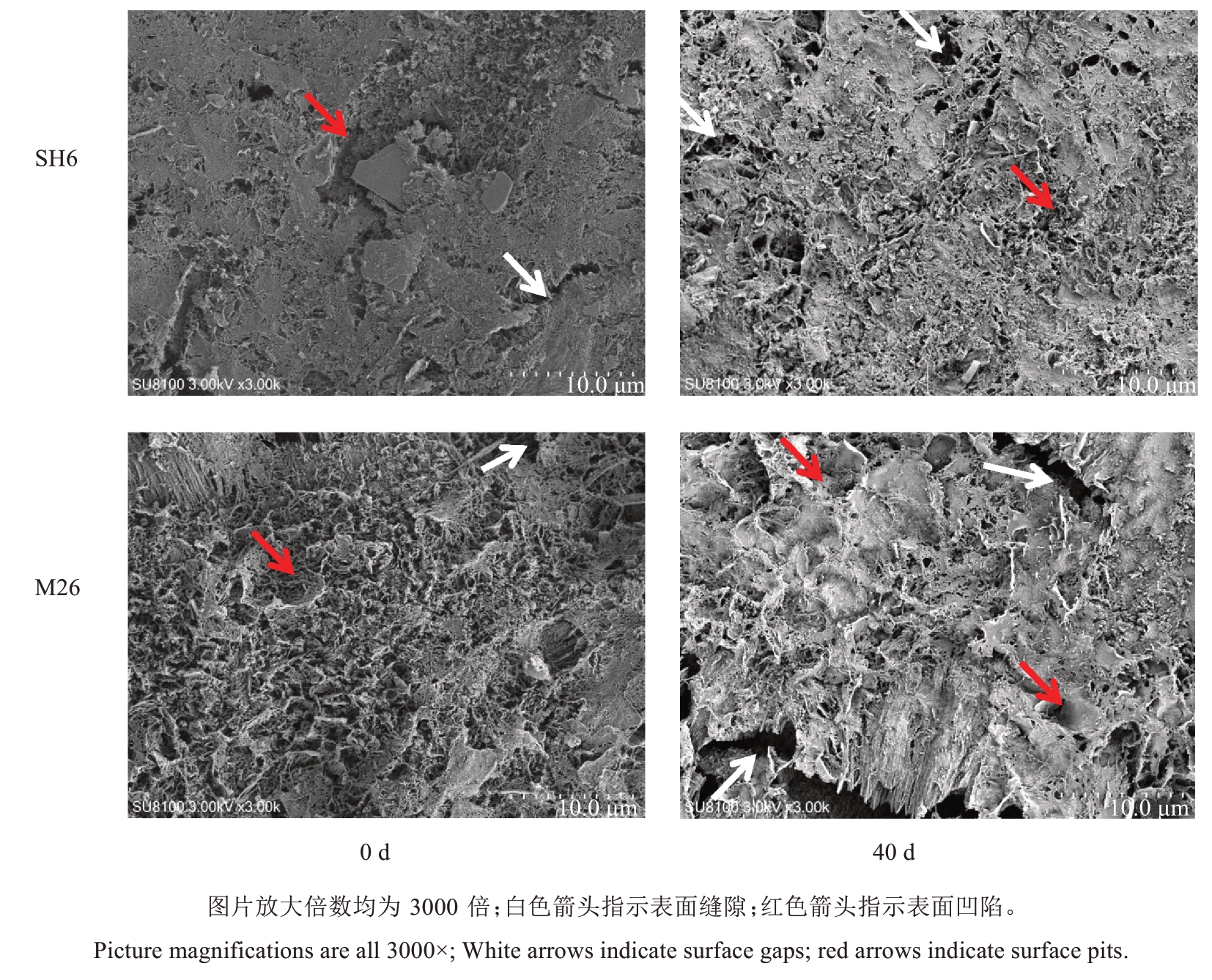
图3 SH6 和M26 富士苹果果实常温贮藏期间表皮蜡质形态的变化
Fig.3 Changes in epidermal wax morphology of SH6 and M26 Fuji apples during ambient storage
采收时,SH6 果实表皮蜡和角质层蜡的厚度均显著大于M26 果实,分别高出M26 果实36.83%和14.02%;贮藏40 d时,SH6果实的表皮蜡和角质层蜡厚度依然显著大于M26 果实,分别高出M26 果实44.1%和72.78%(图4)。
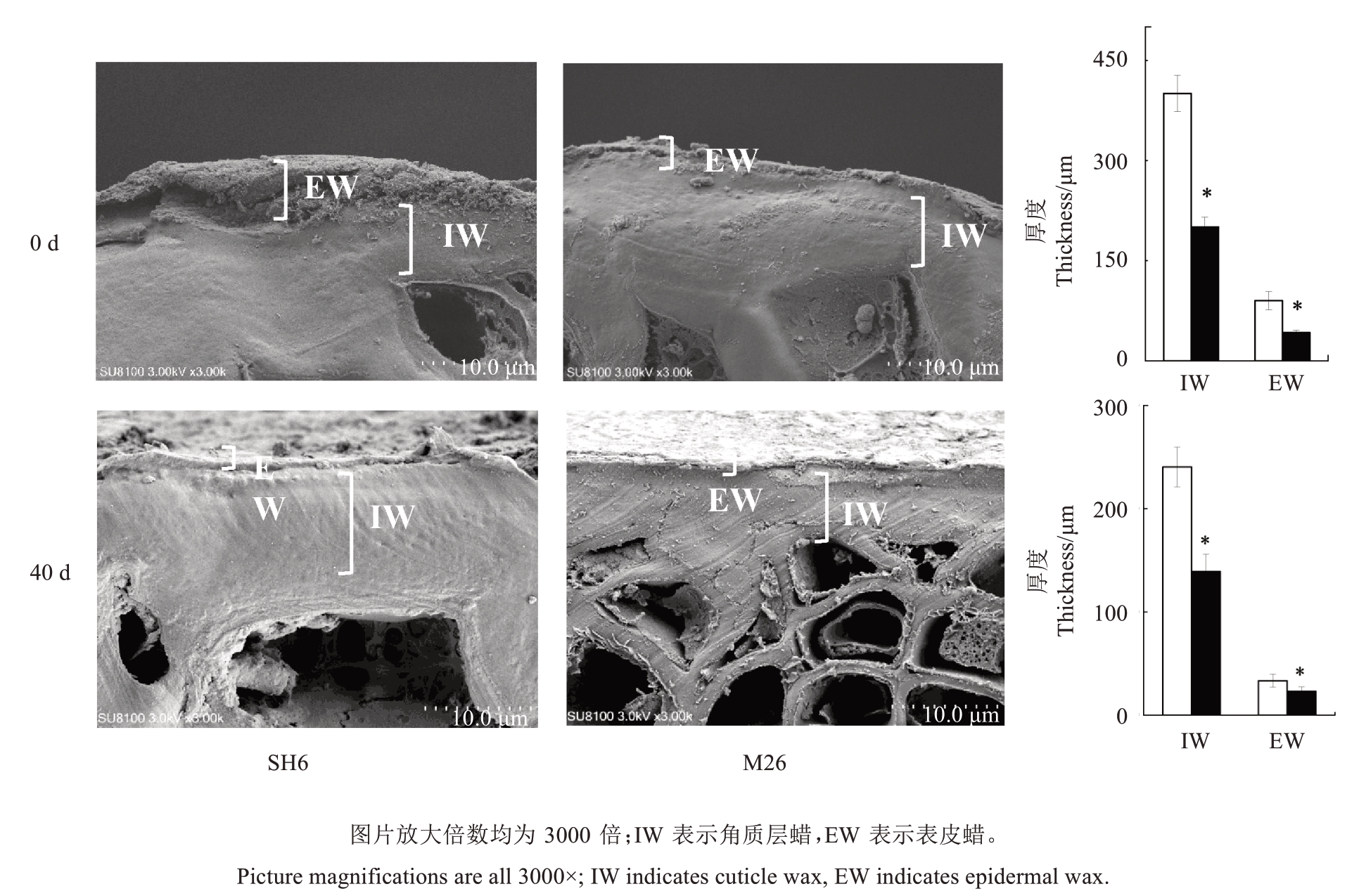
图4 SH6 和M26 富士苹果果实常温贮藏期间蜡质层厚度的变化
Fig.4 Changes in wax layer thickness during ambient storage of SH6 and M26 Fuji apple fruit
2.4 SH6 和M26 富士苹果果实常温贮藏期间角质单体含量的变化
从SH6和M26果皮中共鉴定出37种角质单体,其中的28 种为共有组分,主要包括脂肪酸(14 种)、羟基脂肪酸(7 种)、二羧酸(2 种)和醇(5 种)四大类(表1,表2)。脂肪酸在共有组分中占比最大(54.42%),碳原子数变化范围为C16~C31。此外,SH6果实还有5种特有组分,包括花生四烯酸、顺式-8,11,14-二十烷三烯酸、18-羟基-9,12-十八碳二烯酸、22-羟基二十二烷酸和二十四烷醇。M26 果实有4种特有组分,包括十八烷酸、(3β)-3-三羟基-齐墩果-12-烯-28-酸、10,13-二十碳二烯酸和7,10,13-二十碳三烯酸。
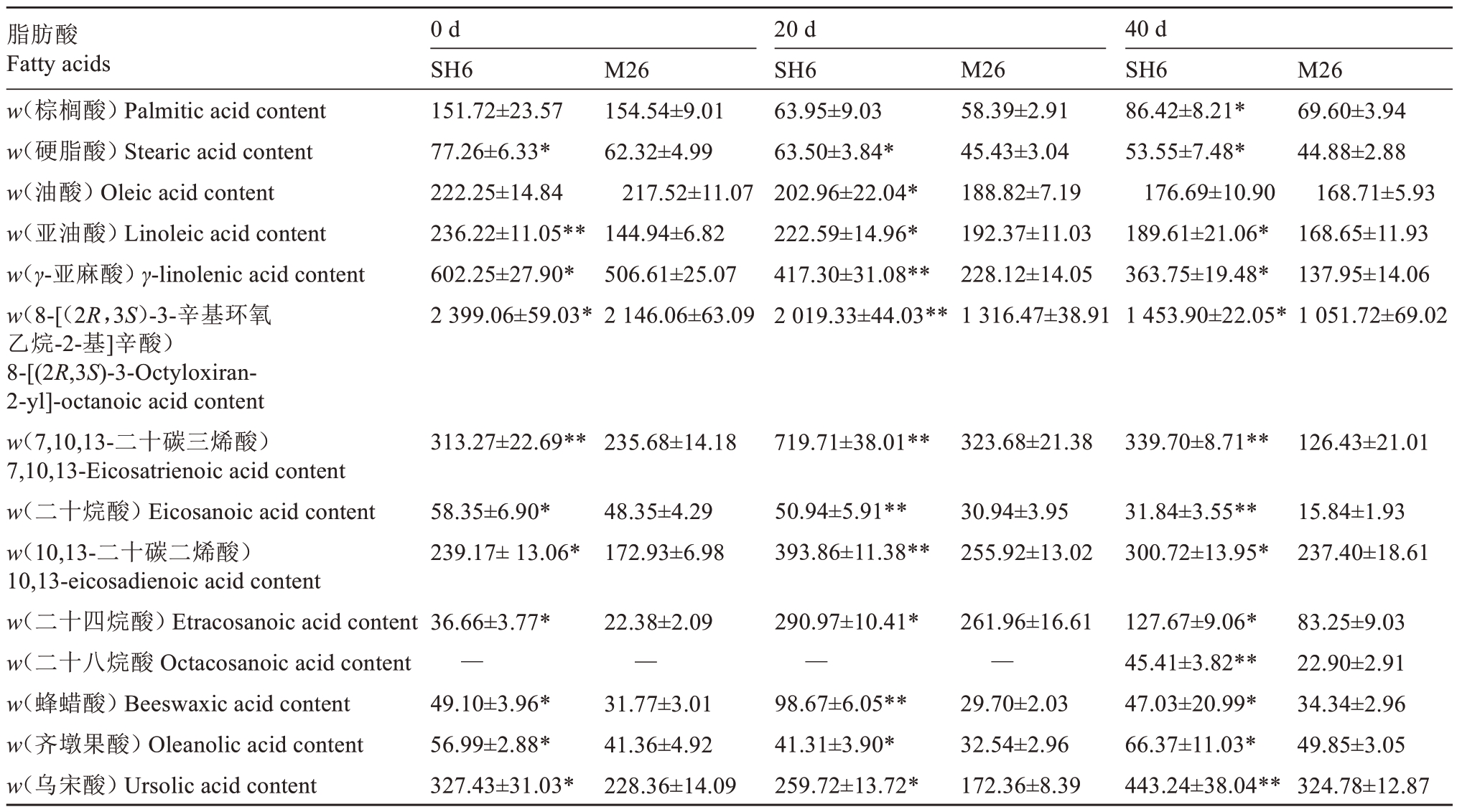
表1 SH6 和M26 富士苹果果实常温贮藏期间脂肪酸含量的变化
Table 1 Changes in fatty acids content of SH6 and M26 Fuji apple fruits during ambient storage(μg·g-1)
注:SH6 为试验组,M26 为对照组:试验组与对照组相比;**表示差异极显著(p<0.01),*表示差异显著(p<0.05);不标注者则为不显著(p>0.05)。下同。
Note:SH6 is the test group and M26 is the control group;**indicates a highly significant difference(p<0.01)and*indicates a significant differ‐ence(p<0.05)when the test group is compared to the control group;those not labeled are non-significant(p>0.05).The same below.
脂肪酸Fatty acids w(棕榈酸)Palmitic acid content w(硬脂酸)Stearic acid content w(油酸)Oleic acid content w(亚油酸)Linoleic acid content w(γ-亚麻酸)γ-linolenic acid content w(8-[(2R,3S)-3-辛基环氧乙烷-2-基]辛酸)8-[(2R,3S)-3-Octyloxiran-2-yl]-octanoic acid content w(7,10,13-二十碳三烯酸)7,10,13-Eicosatrienoic acid content w(二十烷酸)Eicosanoic acid content w(10,13-二十碳二烯酸)10,13-eicosadienoic acid content w(二十四烷酸)Etracosanoic acid content w(二十八烷酸Octacosanoic acid content w(蜂蜡酸)Beeswaxic acid content w(齐墩果酸)Oleanolic acid content w(乌宋酸)Ursolic acid content 0 d SH6 151.72±23.57 77.26±6.33*222.25±14.84 236.22±11.05**602.25±27.90*2 399.06±59.03*M26 154.54±9.01 62.32±4.99 217.52±11.07 144.94±6.82 506.61±25.07 2 146.06±63.09 20 d SH6 63.95±9.03 63.50±3.84*202.96±22.04*222.59±14.96*417.30±31.08**2 019.33±44.03**M26 58.39±2.91 45.43±3.04 188.82±7.19 192.37±11.03 228.12±14.05 1 316.47±38.91 40 d SH6 86.42±8.21*53.55±7.48*176.69±10.90 189.61±21.06*363.75±19.48*1 453.90±22.05*M26 69.60±3.94 44.88±2.88 168.71±5.93 168.65±11.93 137.95±14.06 1 051.72±69.02 313.27±22.69**235.68±14.18 719.71±38.01**323.68±21.38 339.70±8.71**126.43±21.01 58.35±6.90*239.17±13.06*48.35±4.29 172.93±6.98 50.94±5.91**393.86±11.38**30.94±3.95 255.92±13.02 31.84±3.55**300.72±13.95*15.84±1.93 237.40±18.61 83.25±9.03 22.90±2.91 34.34±2.96 49.85±3.05 324.78±12.87 36.66±3.77*—49.10±3.96*56.99±2.88*327.43±31.03*22.38±2.09—31.77±3.01 41.36±4.92 228.36±14.09 290.97±10.41*—98.67±6.05**41.31±3.90*259.72±13.72*261.96±16.61—29.70±2.03 32.54±2.96 172.36±8.39 127.67±9.06*45.41±3.82**47.03±20.99*66.37±11.03*443.24±38.04**
表2 SH6 和M26 富士苹果果实常温贮藏期间羟基脂肪酸、二羧酸和醇类含量的变化
Table 2 Changes of hydroxy fatty acid,dicarboxylic acid and alcohol contents in SH6 and M26 Fuji apple fruits during ambient storage(μg·g-1)
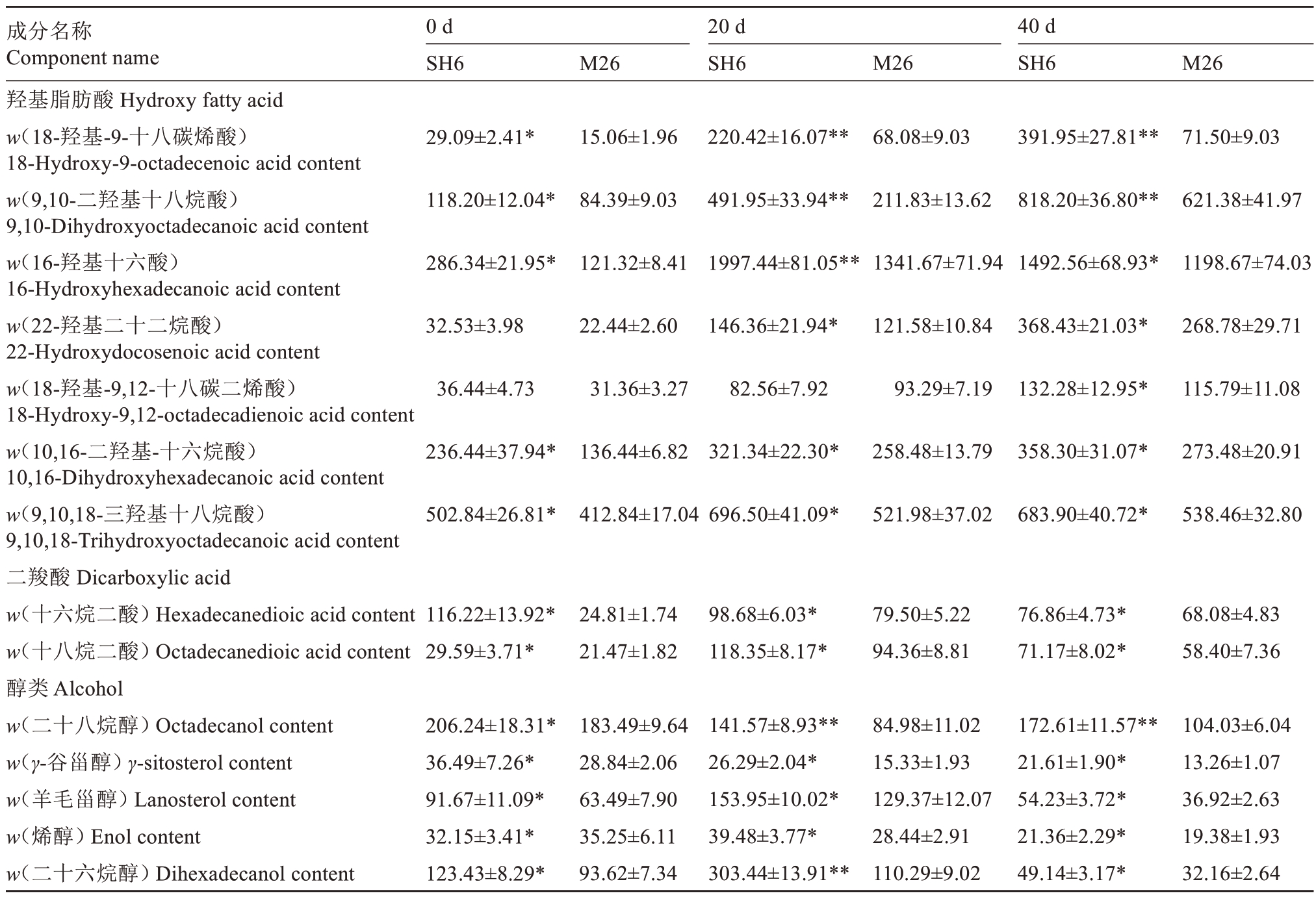
成分名称Component name羟基脂肪酸Hydroxy fatty acid w(18-羟基-9-十八碳烯酸)18-Hydroxy-9-octadecenoic acid content w(9,10-二羟基十八烷酸)9,10-Dihydroxyoctadecanoic acid content w(16-羟基十六酸)16-Hydroxyhexadecanoic acid content w(22-羟基二十二烷酸)22-Hydroxydocosenoic acid content w(18-羟基-9,12-十八碳二烯酸)18-Hydroxy-9,12-octadecadienoic acid content w(10,16-二羟基-十六烷酸)10,16-Dihydroxyhexadecanoic acid content w(9,10,18-三羟基十八烷酸)9,10,18-Trihydroxyoctadecanoic acid content二羧酸Dicarboxylic acid w(十六烷二酸)Hexadecanedioic acid content w(十八烷二酸)Octadecanedioic acid content醇类Alcohol w(二十八烷醇)Octadecanol content w(γ-谷甾醇)γ-sitosterol content w(羊毛甾醇)Lanosterol content w(烯醇)Enol content w(二十六烷醇)Dihexadecanol content 0 d SH6 M26 20 d SH6 M26 40 d SH6 M26 29.09±2.41*15.06±1.96 220.42±16.07**68.08±9.03 391.95±27.81**71.50±9.03 118.20±12.04*84.39±9.03 491.95±33.94**211.83±13.62 818.20±36.80**621.38±41.97 286.34±21.95*121.32±8.41 1997.44±81.05**1341.67±71.94 1492.56±68.93*1198.67±74.03 32.53±3.98 22.44±2.60 146.36±21.94*121.58±10.84 368.43±21.03*268.78±29.71 36.44±4.73 31.36±3.27 82.56±7.92 93.29±7.19 132.28±12.95*115.79±11.08 236.44±37.94*136.44±6.82 321.34±22.30*258.48±13.79 358.30±31.07*273.48±20.91 502.84±26.81*412.84±17.04 696.50±41.09*521.98±37.02 683.90±40.72*538.46±32.80 116.22±13.92*29.59±3.71*24.81±1.74 21.47±1.82 98.68±6.03*118.35±8.17*79.50±5.22 94.36±8.81 76.86±4.73*71.17±8.02*68.08±4.83 58.40±7.36 206.24±18.31*36.49±7.26*91.67±11.09*32.15±3.41*123.43±8.29*183.49±9.64 28.84±2.06 63.49±7.90 35.25±6.11 93.62±7.34 141.57±8.93**26.29±2.04*153.95±10.02*39.48±3.77*303.44±13.91**84.98±11.02 15.33±1.93 129.37±12.07 28.44±2.91 110.29±9.02 172.61±11.57**21.61±1.90*54.23±3.72*21.36±2.29*49.14±3.17*104.03±6.04 13.26±1.07 36.92±2.63 19.38±1.93 32.16±2.64
采收时及贮藏期间,SH6果实硬脂酸、亚油酸和γ-亚麻酸含量显著高于M26 果实,随着贮藏时间延长,SH6和M26果实中硬脂酸、油酸和γ-亚麻酸含量呈下降趋势,贮藏20 d 时SH6 的γ-亚麻酸含量高出M26 果实82.93%。此外,SH6 果实的8-[(2R,3S)-3-辛基环氧乙烷-2-基]辛酸、二十烷酸、7,10,13-二十碳三烯酸、10,13-二十碳二烯酸、二十四烷酸、二十八烷酸、蜂蜡酸、齐墩果酸和乌宋酸含量在采收时及贮藏期间也显著高于M26果实;随着贮藏时间延长,8-[(2R,3S)-3-辛基环氧乙烷-2-基]辛酸和二十烷酸含量逐渐降低,而7,10,13-二十碳三烯酸、10,13-二十碳二烯酸、二十四烷酸、蜂蜡酸、齐墩果酸和乌宋酸含量均呈单峰型变化;其中,二十八烷酸在采收时和贮藏20 d时未检出,SH6果实中的7,10,13-二十碳三烯酸和蜂蜡酸含量在20 d 时分别是M26 果实的2.22倍和3.32倍(表1)。采收时及贮藏期间,SH6和M26 果实中5 种羟基脂肪酸含量呈上升趋势,除18-羟基-9,12-十八碳二烯酸外,SH6 果实的6 种羟基脂肪酸含量均高于M26 果实,包括18-羟基-9-十八碳烯酸、9,10-二羟基十八烷酸、22-羟基二十二烷酸、16-羟基十六烷酸、10,16-二羟基-十六烷酸和9,10,18-三羟基十八烷酸;其中,SH6果实中的18-羟基-9-十八碳烯酸和9,10-二羟基十八烷酸在贮藏20 d 时分别是M26 果实3.24 倍和2.32 倍。此外,SH6 果实在采收时和贮藏期间十六烷二酸含量呈下降趋势,而十八烷二酸含量呈单峰型变化趋势,且SH6 果实十六烷二酸和十八烷二酸含量显著高于M26 果实(表2)。采收时及贮藏期间,SH6 果实的5 种醇含量显著高于M26 果实,包括二十六烷醇、二十八烷醇、烯醇、羊毛甾醇和γ-谷甾醇,SH6和M26果实中二十六烷醇和羊毛甾醇含量呈先升高再降低的趋势,γ-谷甾醇含量呈下降趋势,其中SH6 果实中的二十六烷醇含量在贮藏20 d 时是M26 果实的2.75 倍(表2)。
2.5 SH6 和M26 富士苹果果实常温贮藏期间蜡质单体组分含量的变化
从SH6 和M26 果实中鉴定出了40 种蜡质化合物,其中34 种为共有组分,主要包括烷烃(5 种)、醇(6 种)、脂肪酸(4 种)、酯类(12 种)、醛类(2 种)和其他物质(4 种)六大类。烷烃在共有组分中所占比例最大(17.47%),碳原子数变化范围为C21~C54。其次是脂肪酸和其他萜类化合物。此外,SH6 果实还有3种特有组分,包括熊果醛、二十烷酸丁酯和熊果酸甲酯。M26 果实也有3 种特有组分,包括二十烷酸、棕榈酸-3-甲基丁酯和山嵛酸乙酯。
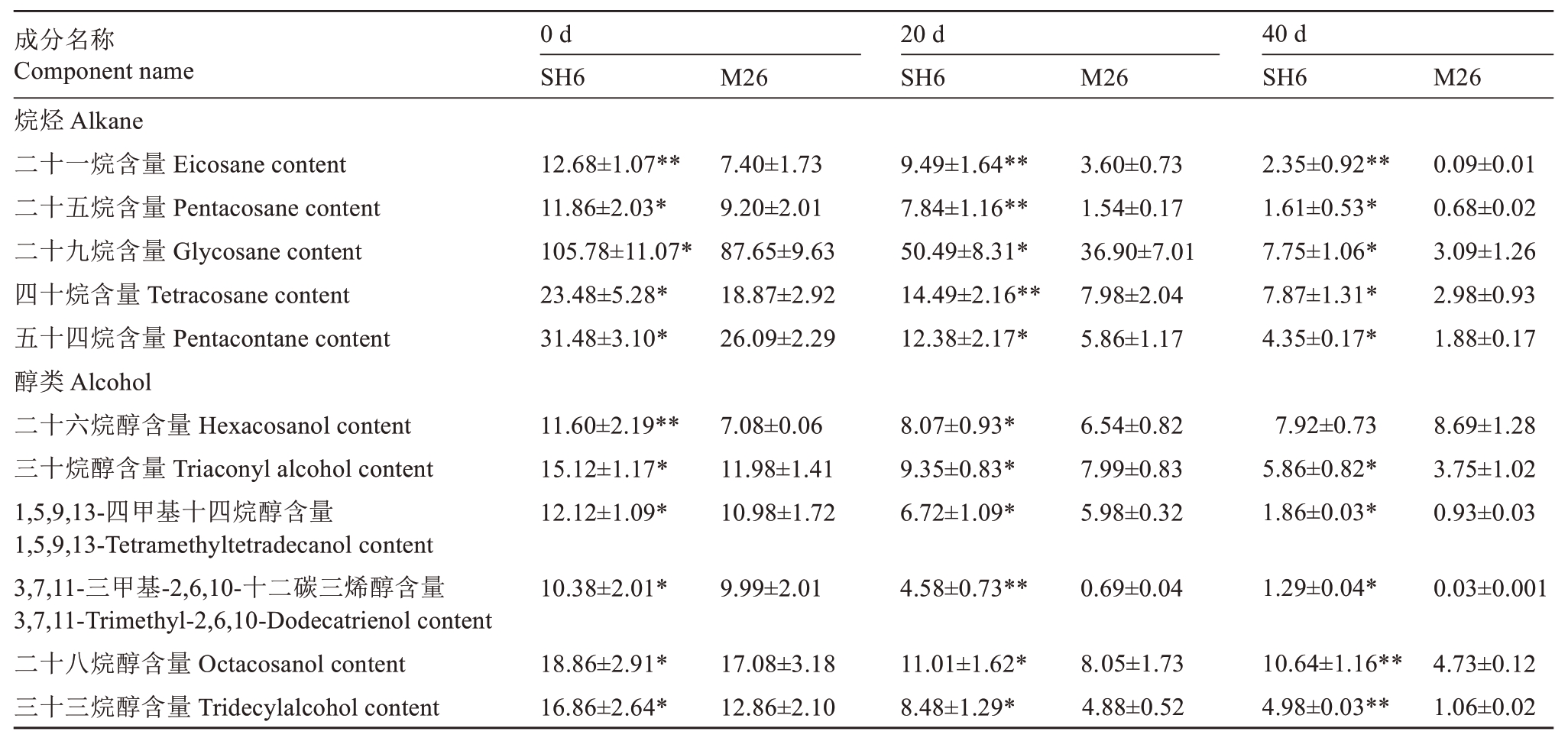
采收时及贮藏期间,SH6 果实的5 种烷烃含量显著高于M26 果实,包括二十一烷、二十五烷、二十九烷、四十烷和五十四烷;SH6 和M26 果实中烷烃含量呈下降趋势,SH6 果实贮藏20 d 时的二十一烷和二十五烷含量分别是M26 果实的2.64 倍和5.09倍(表3)。采收时及贮藏期间,SH6果实的5种醇类物质含量显著高于M26 果实,包括三十烷醇、1,5,9,13-四甲基十四烷醇、二十八烷醇、三十三烷醇和3,7,11-三甲基-2,6,10-十二碳三烯醇;除M26 果实的二十六烷醇含量外,SH6 和M26 果实中醇类物质含量均呈下降趋势,其中,SH6果实中的二十六烷醇含量在采收时高出M26 果实63.84%,而3,7,11-三甲基-2,6,10-十二碳三烯醇含量在贮藏20 d 时是M26 果实的6.64倍(表3)。
表3 SH6 和M26 富士苹果果实常温贮藏期间烷烃和醇含量的变化
Table 3 Changes in alkane and alcohol content of SH6 and M26 Fuji apple fruits during ambient storage(μg·cm-2)
?
采收时及贮藏期间,SH6 果实的棕榈酸、硬脂酸、亚油酸和二十八烷酸含量高于M26 果实;随着贮藏时间延长,SH6 和M26 果实中脂肪酸含量均呈上升趋势,其中SH6 果实中的二十八烷酸含量在贮藏20 d时是M26果实的2.14倍(表4)。采收时,SH6果实9 种酯类物质含量高于M26 果实,包括己酸丁酯、2-甲基丁酸己酯、亚油酸甘油酯、油酸乙酯、9,12-十八碳二烯酸(9Z,12Z)-戊酯、二十二烷酸丁酯、二十四烷酸丁酯、(2E,6E)-3,7,11-三甲基-2,6,10-十二碳三烯-1-基十八烷酸酯和亚油酸乙酯,随着贮藏时间延长,除SH6 果实的(2E,6E)-3,7,11-三甲基-2,6,10-十二碳三烯-1-基十八烷酸酯含量及M26 果实的二十四烷酸丁酯和亚油酸乙酯含量外,SH6 和M26果实的其余9 种酯类物质含量均呈上升趋势,其中SH6果实中亚油酸甘油酯含量在贮藏20 d时是M26果实的3.91 倍;二十二烷酸丁酯和二十四烷酸丁酯含量在贮藏40 d 时分别高出M26 果实89.27%和85.00%(表4)。
表4 SH6 和M26 富士苹果果实常温贮藏期间脂肪酸和酯类含量的变化
Table 4 Changes of fatty acids and esters content in SH6 and M26 Fuji apple fruits during ambient storage (μg·cm-2)

?
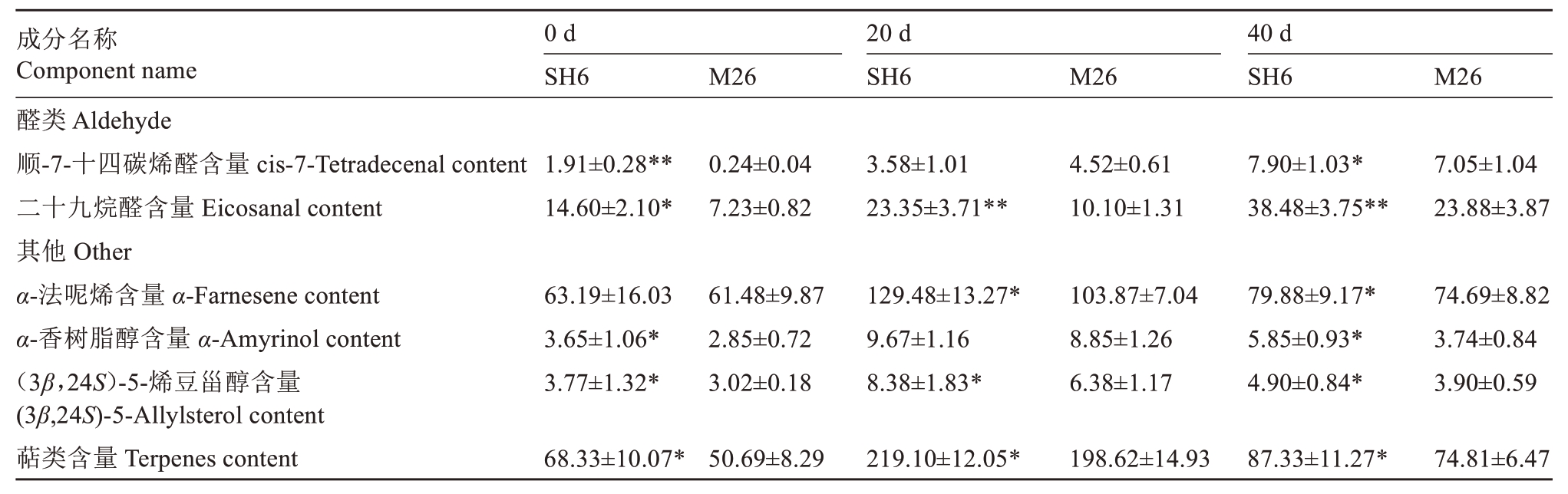
采收及贮藏40 d 时,SH6 果实的2 种醛类物质含量显著高于M26 果实,分别是顺-7-十四碳烯醛和二十九烷醛,其中二十九烷醛含量在贮藏20 d 时是M26 果实的2.31 倍。随着贮藏时间延长,SH6 和M26果实这两种醛含量均呈上升趋势。采收及贮藏40 d 时,SH6 果实的4 种其他物质含量显著高于M26 果实,包括α-法尼烯、α-香树脂醇、(3β,24S)-5-烯豆甾醇和其他萜类化合物,随着贮藏时间延长,这4种物质含量呈先上升后下降趋势(表5)。
表5 SH6 和M26 富士苹果果实常温贮藏期间醛和萜类物质含量的变化
Table 5 Changes of aldehydes and terpenoids content in SH6 and M26 Fuji apple fruits during ambient storage (μg·cm-2)
?
2.6 SH6 和M26 富士苹果果实常温贮藏期间失重率和呼吸强度与果实角质和蜡质成分含量的相关性分析
相关性分析表明(图5),贮藏期间果实的失重率与表皮蜡质和角质层蜡厚度呈显著负相关(r=-0.85,r=-0.96),表明蜡质层和角质层越厚,果实的失重率就越低。失重率与羟基脂肪酸和酯类化合物含量呈极显著正相关(r=0.81,r=0.87),表明这些亲水性物质含量越多就越有利于水分蒸发。呼吸强度与表皮蜡质和角质层蜡厚度呈显著负相关(r=-0.77,r=-0.82),表明蜡质层和角质层越厚,果实的呼吸强度就越弱。呼吸强度与羟基脂肪酸和二羧酸含量呈显著正相关(r=0.59,r=0.50),表明这些物质有利于果实表皮内外气体交换。
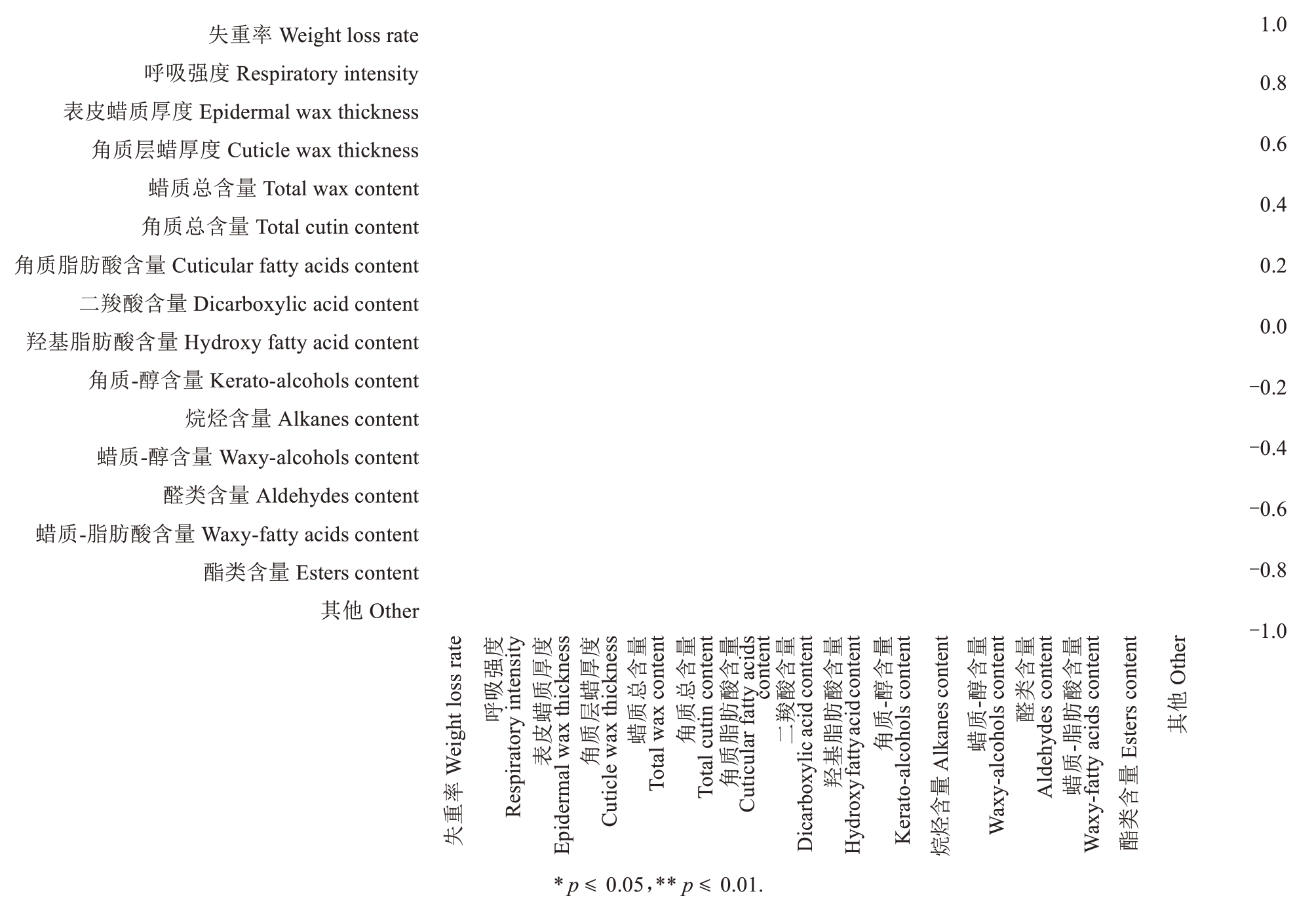
图5 SH6 和M26 富士苹果果实常温贮藏期间失重率和呼吸强度与果实角质和蜡质成分含量的相关性分析
Fig.5 Correlation analysis of weight loss rate and respiration rate of SH6 and M26 Fuji apples with of cutin and wax content during ambient storage
3 讨 论
砧木在果树栽培产业发展过程中起关键作用,不同砧木对接穗的影响不同。植物表皮角质层作为一层疏水性屏障,可以控制水分在表皮细胞外的细胞壁与大气之间的流动[22]。嫁接不同的砧木会影响果实蜡质和角质层的积累,导致果实的角质层和蜡质层在厚度和成分上存在差异。矮化砧木通过影响树体的激素水平达到树体矮化的效果[23]。笔者在本研究中发现,SH6 果实角质总量显著高于M26 果实,SH6果实中的14种脂肪酸、7种羟基脂肪酸、2种二羧酸和5种醇的含量也显著高于M26果实。有研究表明,不同砧木会影响接穗的激素分泌。将黄瓜嫁接到南瓜砧木上,黄瓜植株中的脱落酸(ABA)浓度明显增高[24]。油茶嫁接18 号砧木后体内吲哚乙酸(IAA)、反式玉米素(TZR)、玉米素(ZT)、水杨酸(SA)等多种植物激素含量均显著高于53 号砧木[25]。辣椒嫁接在Atlante 砧木后体内细胞分裂素(CTK)和ABA 含量显著高于Terrano 砧木[26]。另外,ABA 可通过调控角质层代谢基因的表达模式影响角质成分积累,ABA 缺乏会导致甜橙果实成熟过程中角质层透性增强[27]。目前,已证明转录因子MYB、WRKY 和AP2/ERF 等均参与了植物蜡质合成和转运的调控[3]。因此笔者推测,SH6砧木可能通过分泌更多的植物激素调控角质合成相关基因的表达,进而促进角质单体的积累,但详细作用机制还有待进一步研究。
蜡质的成分和含量根据物种、个体发育和环境的变化而变化[28]。表皮蜡质在控制水分散失,提高耐旱性、减轻紫外光伤害、维持植物表面清洁、抵抗细菌和真菌病原体伤害等方面发挥着重要作用[29]。笔者在本研究中发现,SH6 果实的蜡质总量显著高于M26 果实,SH6 果实中5 种烷烃、6 种醇、4 种脂肪酸、12种酯类、2种醛类和4 种其他物质的含量也显著高于M26 果实。有研究表明,苹果嫁接在中间砧SH40 后,体内IAA、GA(赤霉素)和ABA 含量显著高于自根砧嫁接[30]。嫁接在Micro-Tom 上的番茄植株ABA 含量显著高于在Solanum pennellii 上嫁接[31]。以P2 和P16 作为烟富3 号的矮化中间砧时,叶片中ABA含量显著高于其他砧穗组合,IAA/ABA和(ZT+IAA+GA3)/ABA 的比值最小[32]。研究发现,番茄果实中GA 含量过高,会诱导一系列角质层形成相关基因的表达,从而促进番茄果实蜡质的沉积[33]。苹果中MYB 家族成员MdMYB30 与MdKCS1基因启动子结合,激活了MdKCS1基因转录表达,导致果实蜡质积累[34]。此外,其他MYB 家族成员(如MYB16/96/106)可调控梨果实表皮蜡生物合成[35-36]。因此笔者推测,SH6砧木可能通过分泌多种植物激素激活了蜡质合成相关基因的表达,进一步促进了蜡质成分的积累。但详细作用机制还有待进一步研究。
研究表明,角质层中的疏水晶体屏障被认为是高度有序、紧密排列的非环化合物,这些化合物与其他蜡质成分相比更能有效阻止表皮中水分运动,使得果实在采后呈现出低水分蒸腾速率[37]。本研究结果表明,果实的蜡质层和角质层越厚,果实的失重率和呼吸强度就越低。羟基脂肪酸和酯类化合物含量越多就越有利于果实水分蒸发,二羧酸含量有利于果实表皮内外气体交换。此外,笔者还观察到M26 果实表皮蜡质在贮藏期间会产生更多的缝隙和凹陷,更加有助于水分蒸腾和内外气体交换,导致果实贮藏期间更高的失重率和呼吸速率。
4 结 论
与M26果实相比,SH6果实的表皮蜡质层更厚,表面形态更为平整,且表皮蜡质和角质总量显著高于M26 果实,SH6 果实角质单体中的12 种脂肪酸、5种羟基脂肪酸、2 种二羧酸和4 种醇含量,以及蜡质单体中的5 种烷烃、5 种醇、2 种脂肪酸、5 种酯类、1种醛类和2 种其他物质在采收和贮藏期间的含量均显著高于M26 果实。由此可见,SH6 果实具有较厚的表皮蜡质层和更丰富的角质和蜡质含量,使其在贮藏期间具有更低的失重率和呼吸强度。
[1] FICH E A,SEGERSON N A,ROSE J K C. The plant polyester cutin:Biosynthesis,structure,and biological roles[J].Annual Re‐view of Plant Biology,2016,67:207-233.
[2] TRIVEDI P,NGUYEN N,HYKKERUD A L,HÄGGMAN H,MARTINUSSEN I,JAAKOLA L,KARPPINEN K. Develop‐mental and environmental regulation of cuticular wax biosynthe‐sis in fleshy fruits[J].Frontiers in Plant Science,2019,10:431.
[3] WU W J,JIANG B,LIU R L,HAN Y C,FANG X J,MU H L,FARAG M A,SIMAL-GANDARA J,PRIETO M A,CHEN H J,XIAO J B,GAO H Y. Structures and functions of cuticular wax in postharvest fruit and its regulation:A comprehensive re‐view with future perspectives[J]. Engineering,2023,23:118-129.
[4] YANG H B,ZOU Y Q,LI X,ZHANG M F,ZHU Z F,XU R W,XU J,DENG X X,CHENG Y J.QTL analysis reveals the ef‐fect of CER1-1 and CER1-3 to reduce fruit water loss by in‐creasing cuticular wax alkanes in citrus fruit[J]. Postharvest Bi‐ology and Technology,2022,185:111771.
[5] ARRIETA- BAEZ D,PEREA FLORES M J,MÉNDEZMÉNDEZ J V,MENDOZA LEÓN H F,GÓMEZ-PATIÑO M B.Structural studies of the cutin from two apple varieties:Gold‐en delicious and red delicious (Malus domestica)[J].Molecules,2020,25(24):5955.
[6] KLEIN B,THEWES F R,ROGÉRIO DE OLIVEIRA A,BRACKMANN A,BARIN J S,CICHOSKI A J,WAGNER R.Development of dispersive solvent extraction method to deter‐mine the chemical composition of apple peel wax[J]. Food Re‐search International,2019,116:611-619.
[7] LEIDE J,DE SOUZA A X,PAPP I,RIEDERER M. Specific characteristics of the apple fruit cuticle:Investigation of early and late season cultivars‘Prima’and‘Florina’(Malus domesti‐ca Borkh.)[J].Scientia Horticulturae,2018,229:137-147.
[8] 柴奕丰.苹果贮藏期间表皮蜡质变化对生理品质的影响[D].沈阳:沈阳农业大学,2020.CHAI Yifeng. Effects of cuticular wax changes on physiological quality of apple during storage[D]. Shenyang:Shenyang Agri‐cultural University,2020.
[9] 李民吉,张强,李兴亮,周贝贝,杨雨璋,周佳,张军科,魏钦平.SH 系矮化中间砧对‘富士’苹果树体生长、产量和果实品质的影响[J].园艺学报,2018,45(10):1999-2007.LI Minji,ZHANG Qiang,LI Xingliang,ZHOU Beibei,YANG Yuzhang,ZHOU Jia,ZHANG Junke,WEI Qinping. Effects of five different dwarfing interstocks of SH on growth,light distri‐bution,yield and fruit quality in‘Fuji’apple trees[J].Acta Hor‐ticulturae Sinica,2018,45(10):1999-2007.
[10] DE MACEDO T A,DA SILVA P S,SANDER G F,WELTER J F,RUFATO L,DE ROSSI A. Productivity and quality of‘Fuji Suprema’apple fruit in different rootstocks and growing condi‐tions[J].Scientia Horticulturae,2019,256:108651.
[11] MILOŠEVIĆ T,MILOŠEVIĆ N,MLADENOVIĆ J.Role of ap‐ple clonal rootstocks on yield,fruit size,nutritional value and an‐tioxidant activity of‘Red Chief® Camspur’cultivar[J]. Scientia Horticulturae,2018,236:214-221.
[12] 宋伊真,王芝云,沙广利,张玉刚,祝军,戴洪义.不同砧穗组合的苹果果实总酚、类黄酮和果皮色素含量变化的研究[J].青岛农业大学学报(自然科学版),2014,31(3):172-176.SONG Yizhen,WANG Zhiyun,SHA Guangli,ZHANG Yugang,ZHU Jun,DAI Hongyi. Research on the dynamic changes of to‐tal phenols,flavonoids and pericarp pigment content in apples derived from different stock-scion combinations during growth and development period[J].Journal of Qingdao Agricultural Uni‐versity(Natural Science),2014,31(3):172-176.
[13] 贾菊艳.两种中间砧对采后‘富士’苹果品质、抗氧化和抗病性影响的比较[D].兰州:甘肃农业大学,2022.JIA Juyan. Comparison of effects of two intermediate anvils on quality,oxidation resistance and disease resistance of posthar‐vest‘Fuji’apple[D]. Lanzhou:Gansu Agricultural University,2022.
[14] 陈学森,韩明玉,苏桂林,刘凤之,过国南,姜远茂,毛志泉,彭福田,束怀瑞. 当今世界苹果产业发展趋势及我国苹果产业优质高效发展意见[J].果树学报,2010,27(4):598-604.CHEN Xuesen,HAN Mingyu,SU Guilin,LIU Fengzhi,GUO Guonan,JIANG Yuanmao,MAO Zhiquan,PENG Futian,SHU Huairui. Discussion on today’s world apple industry trends and the suggestions on sustainable and efficient development of ap‐ple industry in China[J]. Journal of Fruit Science,2010,27(4):598-604.
[15] JIN W M,ZHANG Q,LIU S Z,WEI Q P,JIN W M,CHENG Z M,XUE X H,YANG T Z. Genetic diversity of 41 apple root‐stocks based on simple sequence repeat markers[J]. Journal of the American Society for Horticultural Science,2012,137(1):51-56.
[16] RABI F,RAB A,RAHMAN K U,MUNIR M,BOSTAN N.Response of apple cultivars to graft take success on apple root‐stock[J]. Journal of Biology,Agriculture and Healthcare,2014,4(3):78-84.
[17] WANG Y,YANG Q,JIANG H,WANG B,BI Y,LI Y C,PRUSKY D. Reactive oxygen species-mediated the accumula‐tion of suberin polyphenolics and lignin at wound sites on musk‐melons elicited by benzo (1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester[J]. Postharvest Biology and Technology,2020,170:111325.
[18] LI X,BI Y,WANG J J,DONG B Y,LI H J,GONG D,ZHAO Y,TANG Y M,YU X Y,SHANG Q. BTH treatment caused physiological,biochemical and proteomic changes of muskmel‐on (Cucumis melo L.) fruit during ripening[J]. Journal of Pro‐teomics,2015,120:179-193.
[19] 徐秀苹,谷丹,冯旻.适用于扫描电镜的拟南芥蜡质样品制备方法[J].电子显微学报,2015,34(1):82-84.XU Xiuping,GU Dan,FENG Min.Comparison of sample prepa‐ration methods for scanning electron microscopy(SEM) of leaf epicuticular waxes in Arabidopsis[J]. Journal of Chinese Elec‐tron Microscopy Society,2015,34(1):82-84.
[20] 陈剑婷,吴坤胜,周磊磊,宋欢,李沆,曲桂芹.苹果果实角质层提取及分析方法的优化[J]. 中国农学通报,2017,33(16):53-58.CHEN Jianting,WU Kunsheng,ZHOU Leilei,SONG Huan,LI Hang,QU Guiqin.Optimization of extraction and analysis meth‐od of apple fruit cuticle[J]. Chinese Agricultural Science Bulle‐tin,2017,33(16):53-58.
[21] 唐瑛. 发育及贮藏期苹果梨果皮蜡质对Alternaria alternata侵染的影响[D].兰州:甘肃农业大学,2016.TANG Ying. Effect of cuticular wax of pingguoli pear from de‐veloping and storage on Alternaria alternata pre-penetration process[D].Lanzhou:Gansu Agricultural University,2016.
[22] SHEPHERD T,WYNNE GRIFFITHS D. The effects of stress on plant cuticular waxes[J].New Phytologist,2006,171(3):469-499.
[23] 隗晓雯,郭静,王菲,张学英,徐继忠.不同矮化砧木叶片酶活性与内源激素含量的差异[J].北方园艺,2013(14):15-18.WEI Xiaowen,GUO Jing,WANG Fei,ZHANG Xueying,XU Jizhong.Difference of enzyme activity and the content of endog‐enous hormones in the leaves of different apple dwarfing root‐stocks[J].Northern Horticulture,2013(14):15-18.
[24] NIU M L,SUN S T,NAWAZ M A,SUN J Y,CAO H S,LU J Y,HUANG Y,BIE Z L. Grafting cucumber onto pumpkin in‐duced early stomatal closure by increasing ABA sensitivity un‐der salinity conditions[J]. Frontiers in Plant Science,2019,10:1290.
[25] 龙伟,姚小华,吕乐燕.基于芽苗砧嫁接油茶砧穗创伤后内源激素动态变化分析[J].植物研究,2021,41(2):232-242.LONG Wei,YAO Xiaohua,LÜ Leyan. Dynamic changes of en‐dogenous hormones in rootstocks and scions within nurse seed‐ling graft in Camellia oleifera under wound[J]. Bulletin of Bo‐tanical Research,2021,41(2):232-242.
[26] GÁLVEZ A,ALBACETE A,MARTÍNEZ-ANDÚJAR C,DEL AMOR F M,LÓPEZ-MARÍN J. Contrasting rootstock-mediat‐ed growth and yield responses in salinized pepper plants (Capsi‐cum annuum L.) are associated with changes in the hormonal balance[J].International Journal of Molecular Sciences,2021,22(7):3297.
[27] ROMERO P,LAFUENTE M T. Abscisic acid deficiency alters epicuticular wax metabolism and morphology that leads to in‐creased cuticle permeability during sweet orange (Citrus sinen‐sis) fruit ripening[J]. Frontiers in Plant Science,2020,11:594184.
[28] BAKER E A. The influence of environment on leaf wax devel‐opment in Brassica oleracea var. gemmifera[J]. New Phytolo‐gist,1974,73(5):955-966.
[29] RAHMAN T,SHAO M X,PAHARI S,VENGLAT P,SOOLA‐NAYAKANAHALLY R,QIU X,RAHMAN A,TANINO K.Dissecting the roles of cuticular wax in plant resistance to shoot dehydration and low-temperature stress in Arabidopsis[J]. Inter‐national Journal of Molecular Sciences,2021,22(4):1554.
[30] 孟红志,姜璇,陈修德,李中勇,徐继忠.SH40 中间砧和自根砧对苹果根系生长和内源激素含量的影响[J].园艺学报,2018,45(6):1193-1203.MENG Hongzhi,JIANG Xuan,CHEN Xiude,LI Zhongyong,XU Jizhong. Effects of SH40 interstocks and scion-roots on ap‐ple root growth and content of endogenous hormones[J]. Acta Horticulturae Sinica,2018,45(6):1193-1203.
[31] PATANÈ C,COSENTINO S L. Effects of soil water deficit on yield and quality of processing tomato under a Mediterranean climate[J]. Agricultural Water Management,2010,97(1):131-138.
[32] 杨生瑞,毛娟,马宗桓,侯应军,李鹏鹏,李建明,冯童,陈佰鸿.不同矮化中间砧对烟富3 号苹果幼树叶片内源激素及糖含量的影响[J].果树学报,2022,39(7):1203-1212.YANG Shengrui,MAO Juan,MA Zonghuan,HOU Yingjun,LI Pengpeng,LI Jianming,FENG Tong,CHEN Baihong.Effects of different dwarfing interstocks on endogenous hormone and sug‐ar contents in leaves of young Yanfu No. 3 apple trees[J]. Jour‐nal of Fruit Science,2022,39(7):1203-1212.
[33] LI R,SUN S,WANG H J,WANG K T,YU H,ZHOU Z,XIN P Y,CHU J F,ZHAO T M,WANG H Z,LI J Y,CUI X. FIS1 en‐codes a GA2-oxidase that regulates fruit firmness in tomato[J].Nature Communications,2020,11(1):5844.
[34] ZHANG Y L,ZHANG C L,WANG G L,WANG Y X,QI C H,ZHAO Q,YOU C X,LI Y Y,HAO Y J. The R2R3 MYB tran‐scription factor MdMYB30 modulates plant resistance against pathogens by regulating cuticular wax biosynthesis[J]. BMC Plant Biology,2019,19(1):362.
[35] WU X,YIN H,CHEN Y Y,LI L,WANG Y Z,HAO P P,CAO P,QI K J,ZHANG S L.Chemical composition,crystal morphol‐ogy and key gene expression of cuticular waxes of Asian pears at harvest and after storage[J]. Postharvest Biology and Technol‐ogy,2017,132:71-80.
[36] WU X,CHEN Y Y,SHI X J,QI K J,CAO P,LIU X Y,YIN H,ZHANG S L. Effects of palmitic acid (16:0),hexacosanoic acid(26:0),ethephon and methyl jasmonate on the cuticular wax composition,structure and expression of key gene in the fruits of three pear cultivars[J].Functional Plant Biology,2020,47(2):156-169.
[37] 潘永贵. 果实表皮组织对采后失水影响研究进展[J]. 广东农业科学,2023,50(10):1-10.PAN Yonggui. Research progress in the effects of epidermal tis‐sue on postharvest fruit water loss[J]. Guangdong Agricultural Sciences,2023,50(10):1-10.