玉露香梨以果肉酥脆、汁多香甜[1]等特性作为近年来发展迅速的优新梨品种,在山西及华北地区的栽培面积及产量均不断上升。但玉露香梨受采收成熟度[2]和贮藏条件[3-4]等因素的影响,在中长期贮藏中果心部位易发生褐变,成为影响其贮后品质的主要因素。梨果褐变目前仍是贮藏领域研究的重点方向,酶促褐变是影响梨果褐变的主要因素[5-6],且受品种易褐变敏感性[7]、成熟度[8-9]、贮藏环境温度[10]及气体组分[11-12]等诸多因素的影响。冰温贮藏是将果品贮藏于0 ℃至冰点之间的贮藏方式,可有效延缓果实代谢,减轻果实褐变的发生。-0.5~-0.2 ℃的冰温贮藏抑制了多酚氧化酶(polyphenol oxidase,PPO)活性,减缓了磨盘柿和次郎甜柿总酚含量的下降,减轻了果实褐变[13]。冰温贮藏结合1-MCP 处理提高乍娜葡萄采后贮藏品质和果实抗氧化性,降低了果梗褐变指数[14]。-0.7 ℃抑制了冷藏期间库尔勒香梨果实呼吸和乙烯代谢,保持了香梨品质,减少了货架期果心褐变[15]。冰温贮藏虽可有效缓解玉露香梨果实生理代谢及色泽变化[3,16],但对玉露香梨果心褐变的影响未见深入研究,因此探讨冰温对玉露香梨果心褐变的影响及其机制,对玉露香梨采后商品品质的保持有一定意义。
代谢组学是系统生物学的重要组成部分,可利用代谢产物积累、变化寻找特定生理时期、特定条件下其与表型及生理的关系[17],已广泛应用于采后果实品质及生理病害分析中。Gong 等[18]研究了科特兰(Cortland)和红元帅(Red Delicious)两个苹果品种在0~1 ℃下虎皮病发生过程中果皮的代谢组学变化,发现59 种差异代谢物在DPA 和1-MCP 处理中发生了显著变化。易褐变丝瓜品种35D-7酶促褐变前后的差异代谢物分析表明,229 种差异代谢物中,松柏醛、紫丁香苷和异绿原酸A 参与主要褐变过程[19]。王森[20]利用广靶次生代谢组学在石榴褐变与健康假种皮中共鉴定出89 种显著差异代谢物,分析认为黄酮类、黄酮醇和异黄酮类的生物合成,尤其是苯丙烷类的生物合成途径是参与假种皮褐变的主要途径。非靶向代谢技术具有快速、检测范围广等特点,可分析代谢物质的整体变化及其对内外在影响因素的响应规律[21-22],为此采用液相色谱-质谱非靶向技术结合代谢轮廓分析方法可对玉露香梨果实褐变过程中代谢物变化进行分析,进一步探讨冰温技术对梨果品质及褐变发生的影响,为玉露香梨采后品质控制提供技术参考。
1 材料和方法
1.1 材料
2021 年9 月15 日于山西省隰县采收玉露香梨,为防止冰温对果实造成低温伤害,利用NCS001 近红外水果无损伤检测仪(SACMI,意大利)筛选成熟度一致的果实[可溶性固形物含量(w,后同)为11.5%~12.5%,采用冻结法测定果实冰点温度-1.74~-1.68 ℃,冰温贮藏果温控制在-1.0 ℃),0~2 ℃预冷12 h 后高渗出CO2 保鲜袋(770 mm × 950 mm,0.018~0.020 mm 厚,山西龙田保鲜技术开发有限公司]包装,设置(-1.0±0.5)℃(冰温)、(0.0±0.5)℃(冷藏)贮藏,每个处理设3 个生物学重复,定期取样观察褐变发生情况及测定生理指标。组学样品分别为:Y-C(采后0 d,健康组织);Y-S-C[(0.0±0.5)℃贮藏240 d,褐变组织];Y-I-C[(-1.0±0.5)℃贮藏240 d,未褐变组织],共3 组果心,取样部位:果心线内去除种子后组织,液氮速冻,-80 ℃超低温储存用于组学分析。
1.2 指标测定
果心褐变指数:果实沿横径切开,褐变分级标准及指数计算参照张微等[16]的方法略有调整。
总酚含量:取0.2 g 果心冻样加入2.5 mL 提取液,60 ℃,300 W,12 000 r·min-1超声提取30 min,25 ℃离心10 min,取上清液采用总酚含量检测试剂盒测定,结果以mg·g-1表示。
总黄酮含量:提取方法同总酚含量,测定参照苏艳丽等[7]的方法略作调整,结果以mg·g-1表示。
PPO 活性:采用邻苯二酚比色法测定[2],结果以U·g-1表示。
代谢物提取:称取0.1 g 果心样品(4 ℃解冻),加入300 μL 冷乙腈,研磨。冰水浴超声提取30 min,4 ℃,12 000 r·min-1离心10 min。取100 μL,37 ℃真空离心浓缩至干,100 μL 乙腈溶解残渣,4 ℃、12 000 r·min-1离心10 min,取10 μL 上清液进样,用UPLC-MS检测。
色谱条件:Waters BEH C18 Column(100 mm×2.1 mm,1.7 μm),柱温:35 ℃。流动相:A:0.1%甲酸,1 mmol·L-1乙酸铵,B:乙腈,流速:0.3 mL·min-1。梯度洗脱程序见表1。
表1 色谱梯度洗脱程序
Table 1 Chromatographic gradient elution procedures
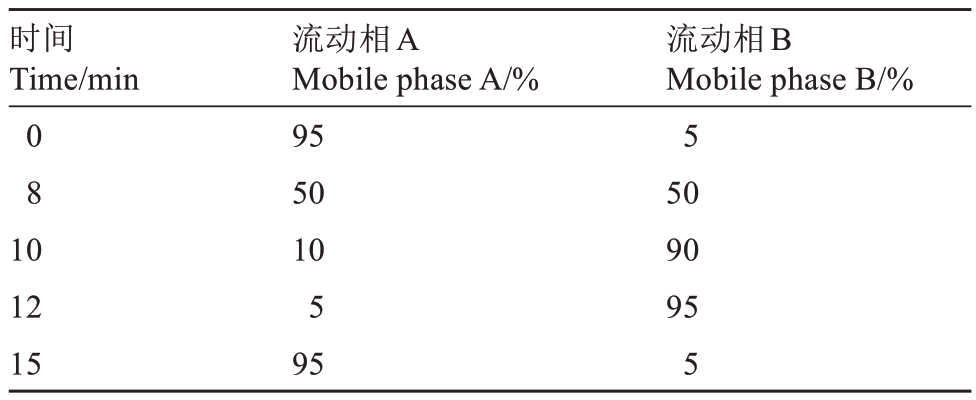
时间Time/min 0 8 10 12 15流动相A Mobile phase A/%95 50 10 5 95流动相B Mobile phase B/%5 50 90 95 5
质谱条件:正、负离子模式检测。离子源:气体1∶50 psi,气体2∶50 psi。离子源温度:500 ℃(正离子)和450 ℃(负离子)。喷射电压:5500 V(正离子)和4400 V(负离子)。飞行时间质量扫描范围:100~1200 Da,0.2 s,产品离子扫描范围:50~1000 Da,0.01 s。二级质谱采用information dependent acqui‐sition(IDA)获得。
1.3 数据分析
代谢物组学数据预处理:利用北京百泰派克生物科技有限公司提供的实验平台进行分析,利用Analysis Base File Converter 软件、MS-DIAL 4.60 软件进行预处理,获得的峰信息在MassBank、Re‐spect、GNPS 数据库检索匹配,根据质谱信息分析代谢产物。使用主成分分析(principal component anal‐ysis,PCA)、偏最小二乘判别分析(partial least squares-discriminant analysis,PLS-DA)方法进行代谢物的差异分析,依据投影重要度(variable impor‐tance in the projection,VIP)(VIP>1)、t 检验(p<0.05)筛选差异代谢物。利用KEGG数据库进行代谢通路注释分析。
2 结果与分析
2.1 不同贮藏温度下玉露香梨果心褐变指数的变化
由图1可知,贮藏120 d时,0 ℃下玉露香梨果心发生褐变,褐变指数为1.11%。-1 ℃果心褐变出现在180 d。贮藏中后期,不同贮藏温度下玉露香梨果心褐变指数均呈上升的趋势,180 d、240 d 时0 ℃果心褐变指数显著升高,极显著高于-1 ℃贮藏下的果心褐变指数。贮藏240 d时,0 ℃与-1 ℃下玉露香梨果心褐变指数分别为15.56%、9.70%。整个贮藏期不同温度下果肉色泽虽因衰老泛黄,但均未发生褐变。
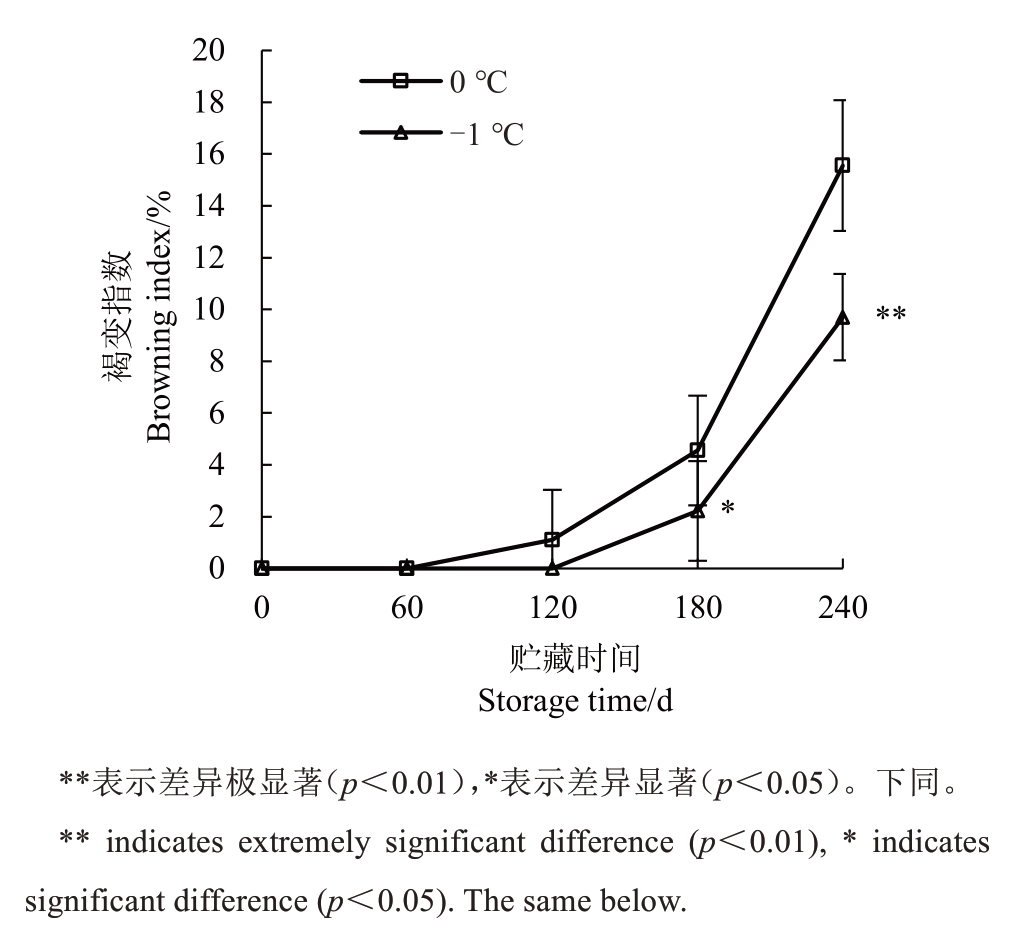
图1 不同贮藏温度下果心褐变指数的变化
Fig.1 Changes in browning index of pear core under different storage temperatures
2.2 不同贮藏温度下玉露香梨果心总酚含量的变化
不同温度贮藏期间玉露香梨果心总酚含量均呈逐渐下降的趋势(图2)。贮藏前期,2 个温度下果心总酚含量差异不显著。120 d 后0 ℃果心总酚含量下降幅度增加,-1 ℃果心总酚含量在180 d 降幅增加,但仍显著高于0 ℃果心总酚含量。贮藏240 d时,0 ℃和-1 ℃下玉露香梨果心总酚含量分别较入贮时下降了40.13%、23.66%。
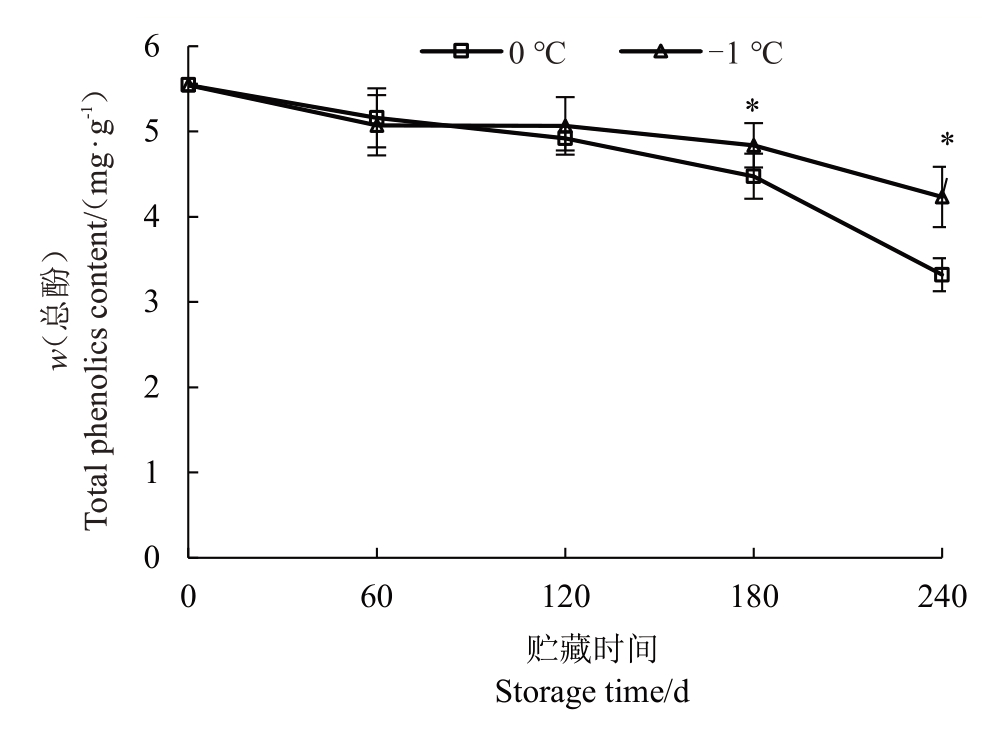
图2 不同贮藏温度下果心总酚含量的变化
Fig.2 Changes in total phenolic content of pear core under different storage temperatures
2.3 不同贮藏温度下玉露香梨果心总黄酮含量的变化
入贮时玉露香梨果心中总黄酮含量为12.03 mg·g-1(图3)。随贮藏时间的延长,不同温度处理下果心总黄酮含量变化趋势相同,均在入贮初期60 d 上升后逐渐下降。贮藏期间-1 ℃果心总黄酮含量在60 d、120 d 和240 d 显著高于0 ℃果心总黄酮含量,且贮藏后期下降的趋势也较为缓慢。
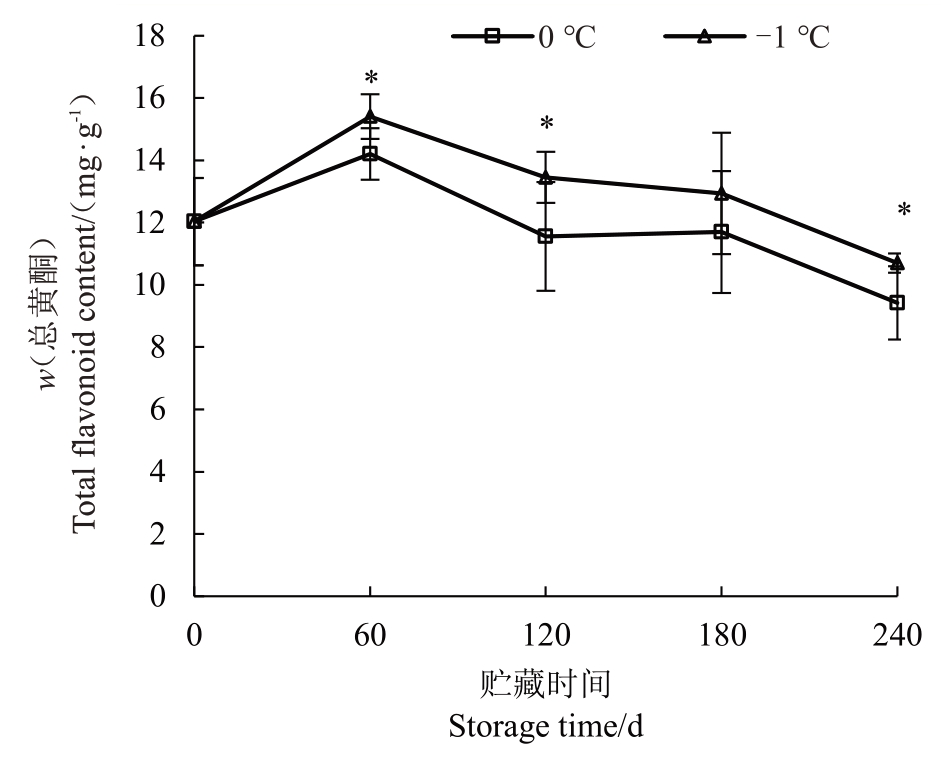
图3 不同贮藏温度下果心总黄酮含量的变化
Fig.3 Changes in total flavonoid content of pear core under different storage temperatures
2.4 不同贮藏温度下玉露香梨果心多酚氧化酶活性的变化
不同贮藏温度下玉露香梨果心PPO 活性的变化如图4 所示,果心PPO 活性在入贮后呈上升的趋势,0 ℃下果心PPO 活性峰出现在120 d,达到74.29 U·g-1,此时0 ℃果心褐变出现并快速发展。-1 ℃下果心PPO 活性峰值出现在180 d,为68.06 U·g-1,-1 ℃下延缓了果心在贮藏中褐变的发生及症状的发展。
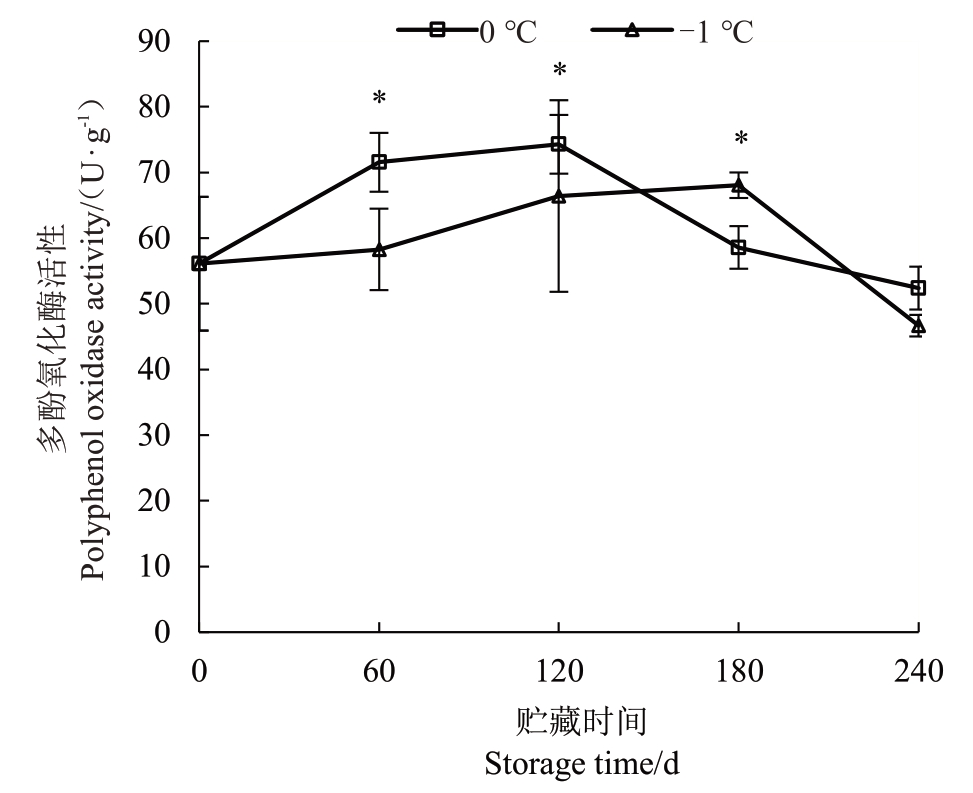
图4 不同贮藏温度下玉露香梨果心多酚氧化酶活性的变化
Fig.4 Changes in PPO activity of pear core under different storage temperatures
2.5 玉露香梨果心代谢产物鉴定分析
利用UPLC-MS 对玉露香梨果心进行非靶标代谢组学分析检测,发现不同温度处理贮藏前后果心组织均含有不同相对含量的331种代谢产物(图5),其中包括72 种黄酮,56 种苯丙烷类,44 种萜类,40种糖及糖醇,34 种生物碱,15 种有机酸,13 种脂质,10 种氨基酸,4 种单宁,4 种醌类,2 种核苷酸和37 种其他未归类化合物。
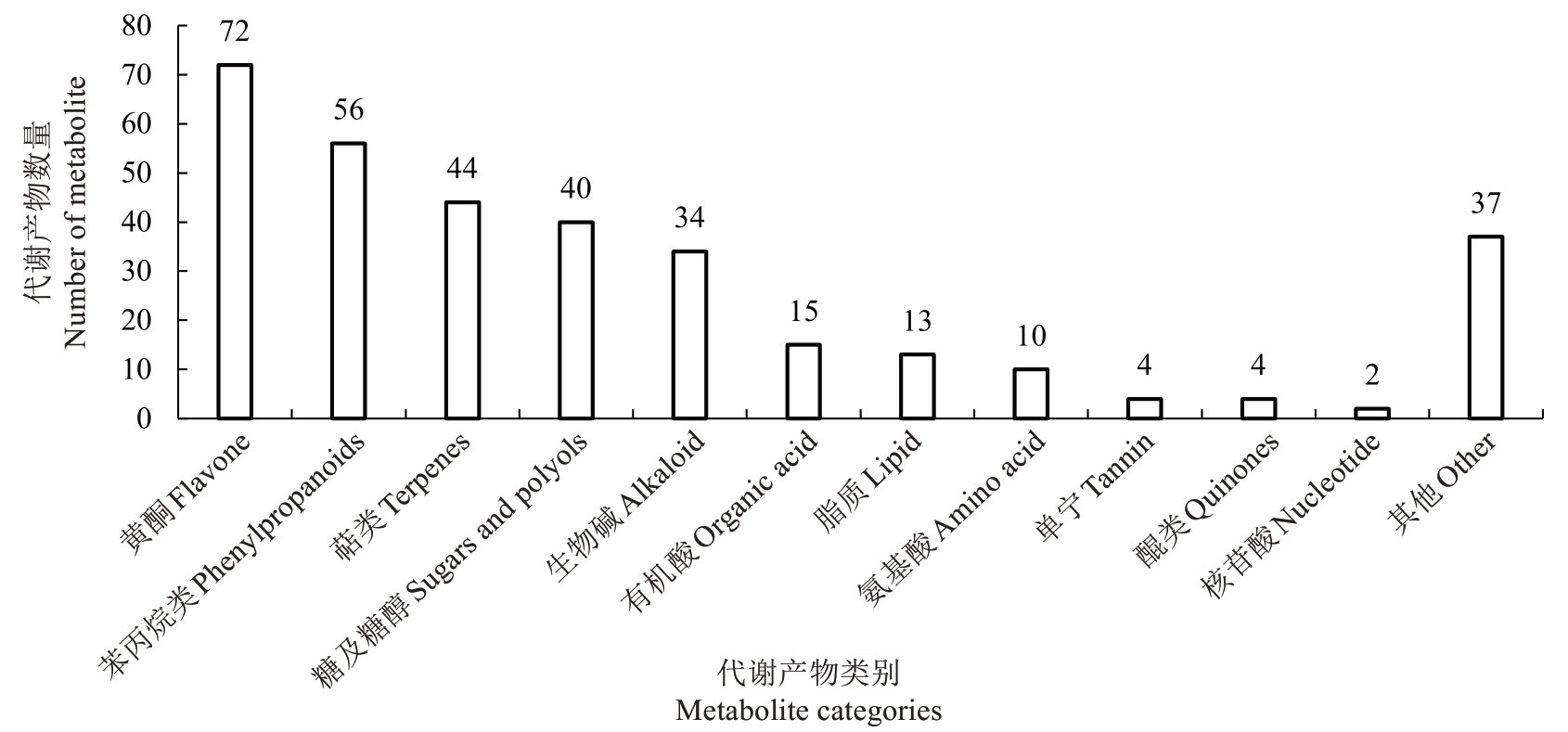
图5 玉露香梨果心代谢产物类别
Fig.5 Metabolites categories in the core of Yuluxiang pear
对鉴定出的代谢产物种类分布情况进行分析,对数据进行归一化处理后,按物质种类对主要代谢物进行了分类,图6为玉露香梨采后果心(Y-C)代谢产物类别及相对含量,其中相对含量较高的物质为有机酸、糖及糖醇、生物碱、苯丙烷类及黄酮类物质等。有机酸类代谢物质中柠檬酸在果心中相对含量较高。糖及糖醇类物质中D-甘露糖醇、半乳糖醇相对含量较高。苯丙烷类物质中邻苯二酚、2,6-二叔丁基对甲苯酚、2,5-二羟基苯甲酸、原儿茶酸相对含量较高。黄酮类物质中黄杞苷、豆苷、槲皮素等物质相对含量较高。氨基酸类代谢产物中L-天门冬氨酸相对含量较高。

图6 玉露香梨果心代谢产物相对含量
Fig.6 Metabolites relative content in the core of Yuluxiang pear
2.6 玉露香梨果心代谢物PCA及PLS-DA分析
主成分分析可判别数据组内的重复性和组间的差异性,对3 组果心代谢产物进行主成分分析,从PCA 得分图(图7-A)可以看出,Y-C、Y-S-C 及Y-I-C处理果心代谢物具有不同的空间,虽在Y-S-C 处理中1 个重复偏离程度较大,但各组代谢产物之间的分离趋势明显。尤其是Y-S-C 与Y-I-C 处理数据轮廓可以清晰地分离开,说明0 ℃和-1 ℃贮藏后果心之间的代谢产物存在一定的差异。其中横坐标PC1和纵坐标PC2 分别代表着主成分1 和主成分2 的得分。PC1 解释72.5%总方差变量,PC2 解释了19.7%总方差变量。所有样品信息均落于95%置信区间中,数据重复性较好、可信度较高。
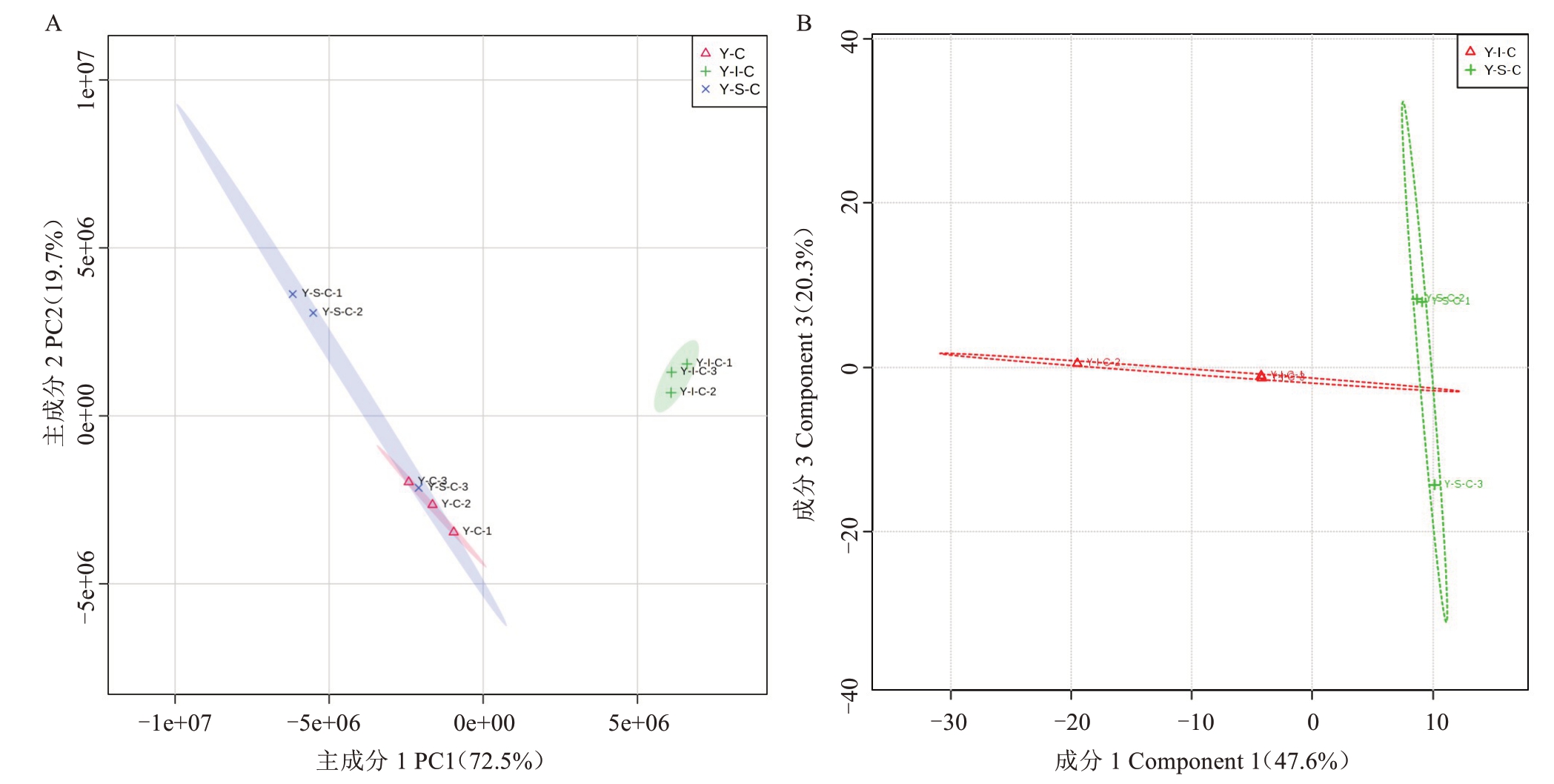
图7 Y-C/Y-S-C/Y-I-S 组间PCA(A)和Y-S-C/Y-I-S 组间PLS-DA(B)得分图
Fig.7 PCA score diagram of Y-C/Y-S-C/Y-I-S treatment groups and PLS-DA score diagram of Y-S-C/Y-I-S treatment groups
PCA 分析虽可提取大部分数据信息,但对组间差异不敏感。为进一步探讨不同温度处理组间的差异,采用PLS-DA 可选择区分各组特征变量,以确定Y-I-C 与Y-S-C 处理之间的关系。图7-B 显示,两组处理区分效果明显,具有显著的差异。该模型的解释率为67.9%,2 个主成分的解释率分别为47.6%、20.3%。基于PLS-DA 建立了代谢物表达量与分组关系之间的模型,可有效提取组间变异信息,但也会导致数据模型存在过拟合。为此进行模型交叉验证,主要参考R(2代表分组的预测率)、Q(2代表模型预测的准确率)等参数。结果表明,R2=0.992 7、Q2=0.711 6,说明该评估模型可靠有效,不存在过拟合,确认PLS-DA模型的建立具有有效性。
2.7 玉露香梨果心差异代谢产物筛选
依据VIP>1和p值<0.05筛选各组间显著差异代谢物。在Y-S-C 与Y-C 处理组间发现31种差异显著代谢产物(表2),4 种物质上调,27 种物质下调。显著差异代谢物中包括苯丙烷类物质6 种,糖及糖醇类5 种,有机酸3 种,黄酮类物质3 种,萜类物质3种,脂质2 种,生物碱3 种,氨基酸1 种,核苷酸1 种,其他类4 种。与采后初始(Y-C)相比,邻苯二酚、石斛酚、阿魏酸和原儿茶酸等苯丙烷类物质在0 ℃冷藏后下调,且差异倍数较大(表3)。三氟乙酸、2-异丙基苹果酸、甲基丁二酸等有机酸糖及糖醇、黄酮类物质在0 ℃冷藏后均下调。推测与有机酸、糖类等物质随果实衰老代谢发生降解,酚类物质作为底物发生氧化有关。
表2 不同处理组间果心差异代谢产物统计
Table 2 Statistics of differential metabolites in pear core between treatment groups
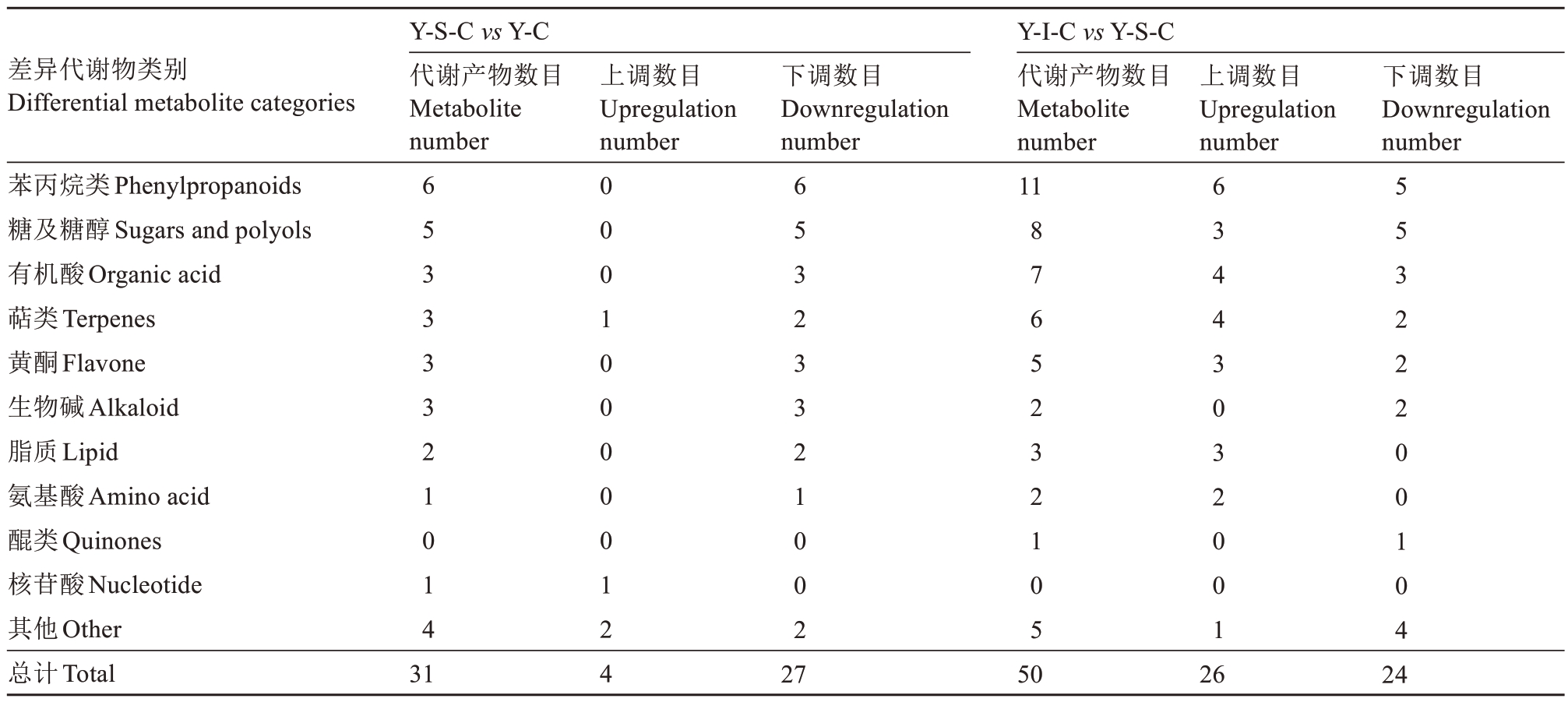
差异代谢物类别Differential metabolite categories Y-S-C vs Y-C代谢产物数目Metabolite number上调数目Upregulation number下调数目Downregulation number上调数目Upregulation number下调数目Downregulation number苯丙烷类Phenylpropanoids糖及糖醇Sugars and polyols有机酸Organic acid萜类Terpenes黄酮Flavone生物碱Alkaloid脂质Lipid氨基酸Amino acid醌类Quinones核苷酸Nucleotide其他Other总计Total 6 5 3 3 3 3 2 1 0 1 4 6 5 3 2 3 3 2 1 0 0 2 Y-I-C vs Y-S-C代谢产物数目Metabolite number 11 8 7 6 5 2 3 2 1 0 5 6 3 4 4 3 0 3 2 0 0 1 5 5 3 2 2 2 0 0 1 0 4 31 0 0 0 1 0 0 0 0 0 1 24 27 50 26 24
表3 Y-S-C 与Y-C 主要差异代谢物
Table 3 The main differential metabolites between Y-S-C and Y-C

代谢物类别Metabolite categories苯丙烷类Phenylpropanoids糖及糖醇Sugars and polyols有机酸Organic acid黄酮Flavone萜类Terpenes脂质Lipid生物碱Alkaloid氨基酸Amino acid核苷酸Nucleotide差异代谢物Differential metabolites鬼臼毒素Podophyllotoxin 2,5-二羟基苯甲酸2,5-Dihydroxybenzoic acid邻苯二酚Catechol原儿茶酸Protocatechuic acid阿魏酸Ferulic acid石斛酚Dendrophenol龙胆苦苷Gentiopicrin芦荟宁Aloenin梓醇Catalpol半乳糖二酸Mucic acid D-甘露糖醇D-mannitol三氟乙酸Trifluoroacetic acid 2-异丙基苹果酸2-Isopropylmalic acid甲基丁二酸Methylsuccinic acid棕矢车菊素4,5,7-Trihydroxy-3,6-dimethoxyflavone金丝桃苷Hyperoside 4-乙烯基氯苄Isokaempferide土荆皮乙酸Pseudolaric acid B水杨醛2-Hydroxybenzaldehyde马钱子苷Loganin全氟辛基磷酸酯Perfluorooctyl phosphate脂肪酸FA 18:2去氢毛钩藤碱Hirsuteine L-天门冬氨酸Aspartate 5-氟脲嘧啶5-Fluorouracil差异倍数对数log2FC-1.046 9-2.247 4-2.762 2-3.337 5-3.398 4-4.346 8-3.262 7-1.867 1-1.879 4-1.762 7-1.201 6-5.995 1-2.051 4-1.843 8-2.492 6-2.492 6-2.111 8 3.926 9-1.565 3-1.820 5-1.201 6-1.688 2-2.389 6-1.307 1 2.172 9调控模式Regulatory mode下调Down下调Down下调Down下调Down下调Down下调Down下调Down下调Down下调Down下调Down下调Down下调Down下调Down下调Down下调Down下调Down下调Down上调Up下调Down下调Down下调Down下调Down下调Down下调Down上调Up
Y-I-C 与Y-S-C 处理果心代谢物筛选发现了50种差异代谢物(表2)。-1 ℃冰温贮藏后果心26 种代谢产物上调,24 种物质下调。差异代谢物包括苯丙烷类11种,其中邻苯二酚、反式-4-羟基肉桂酸、熊果苷、奎宁酸、阿魏酸与Y-S-C 相比,Y-I-C 表达量上调。有机酸类物质7 种,其中L-苹果酸、2-异丙基苹果酸、三氟乙酸、柠檬酸与Y-S-C 相比,Y-I-C 表达量上调。其他差异代谢物还包括糖及糖醇类8 种、萜类6 种、黄酮类5 种、脂类3 种、生物碱2 种、氨基酸2种、醌类1种、其他类5种(主要代谢差异物见表4)。
表4 Y-I-C 与Y-S-C 主要差异代谢物
Table 4 The main differential metabolites between Y-I-C and Y-S-C
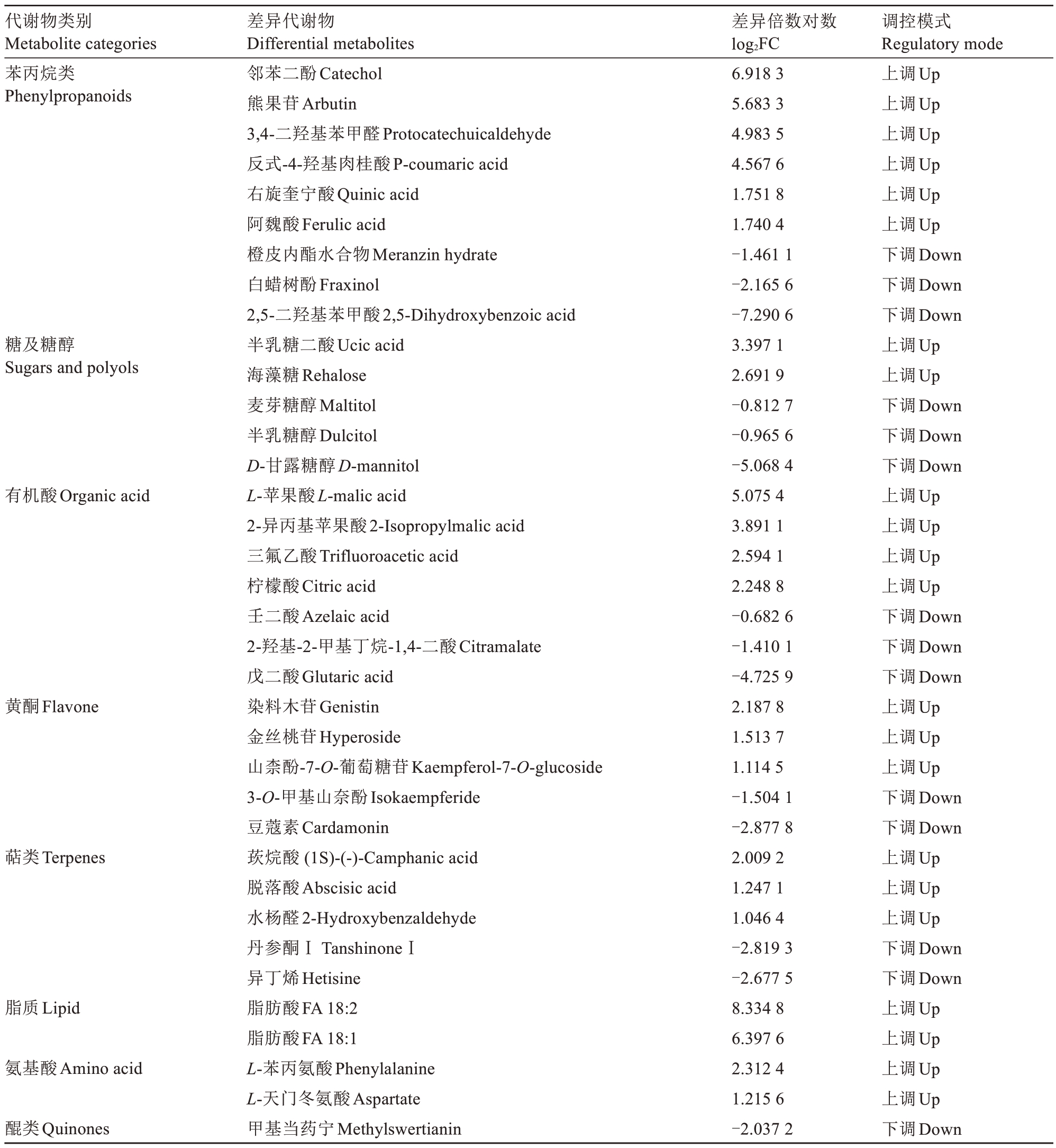
代谢物类别Metabolite categories苯丙烷类Phenylpropanoids糖及糖醇Sugars and polyols有机酸Organic acid黄酮Flavone萜类Terpenes脂质Lipid氨基酸Amino acid醌类Quinones差异代谢物Differential metabolites邻苯二酚Catechol熊果苷Arbutin 3,4-二羟基苯甲醛Protocatechuicaldehyde反式-4-羟基肉桂酸P-coumaric acid右旋奎宁酸Quinic acid阿魏酸Ferulic acid橙皮内酯水合物Meranzin hydrate白蜡树酚Fraxinol 2,5-二羟基苯甲酸2,5-Dihydroxybenzoic acid半乳糖二酸Ucic acid海藻糖Rehalose麦芽糖醇Maltitol半乳糖醇Dulcitol D-甘露糖醇D-mannitol L-苹果酸L-malic acid 2-异丙基苹果酸2-Isopropylmalic acid三氟乙酸Trifluoroacetic acid柠檬酸Citric acid壬二酸Azelaic acid 2-羟基-2-甲基丁烷-1,4-二酸Citramalate戊二酸Glutaric acid染料木苷Genistin金丝桃苷Hyperoside山柰酚-7-O-葡萄糖苷Kaempferol-7-O-glucoside 3-O-甲基山奈酚Isokaempferide豆蔻素Cardamonin莰烷酸(1S)-(-)-Camphanic acid脱落酸Abscisic acid水杨醛2-Hydroxybenzaldehyde丹参酮ⅠTanshinoneⅠ异丁烯Hetisine脂肪酸FA 18:2脂肪酸FA 18:1 L-苯丙氨酸Phenylalanine L-天门冬氨酸Aspartate甲基当药宁Methylswertianin差异倍数对数log2FC 6.918 3 5.683 3 4.983 5 4.567 6 1.751 8 1.740 4-1.461 1-2.165 6-7.290 6 3.397 1 2.691 9-0.812 7-0.965 6-5.068 4 5.075 4 3.891 1 2.594 1 2.248 8-0.682 6-1.410 1-4.725 9 2.187 8 1.513 7 1.114 5-1.504 1-2.877 8 2.009 2 1.247 1 1.046 4-2.819 3-2.677 5 8.334 8 6.397 6 2.312 4 1.215 6-2.037 2调控模式Regulatory mode上调Up上调Up上调Up上调Up上调Up上调Up下调Down下调Down下调Down上调Up上调Up下调Down下调Down下调Down上调Up上调Up上调Up上调Up下调Down下调Down下调Down上调Up上调Up上调Up下调Down下调Down上调Up上调Up上调Up下调Down下调Down上调Up上调Up上调Up上调Up下调Down
2.8 玉露香梨果心差异代谢物KEGG分析
对0 ℃冷藏240 d(Y-S-C)的玉露香梨果心与采后果心(Y-C)相比较呈显著差异的代谢产物进行KEGG 通路富集(图8-A),差异代谢物主要富集在11 条代谢途径上,缬氨酸、亮氨酸和异亮氨酸生物合成,丙酮酸代谢,果糖和甘露糖代谢途径较为显著,其他涉及的代谢途径还包括单萜生物合成、酪氨酸代谢、其他次级代谢产物的生物合成、氨基酸的生物合成、代谢通路、2-氧代羧酸代谢、ABC 转运、次级代谢产物的生物合成。
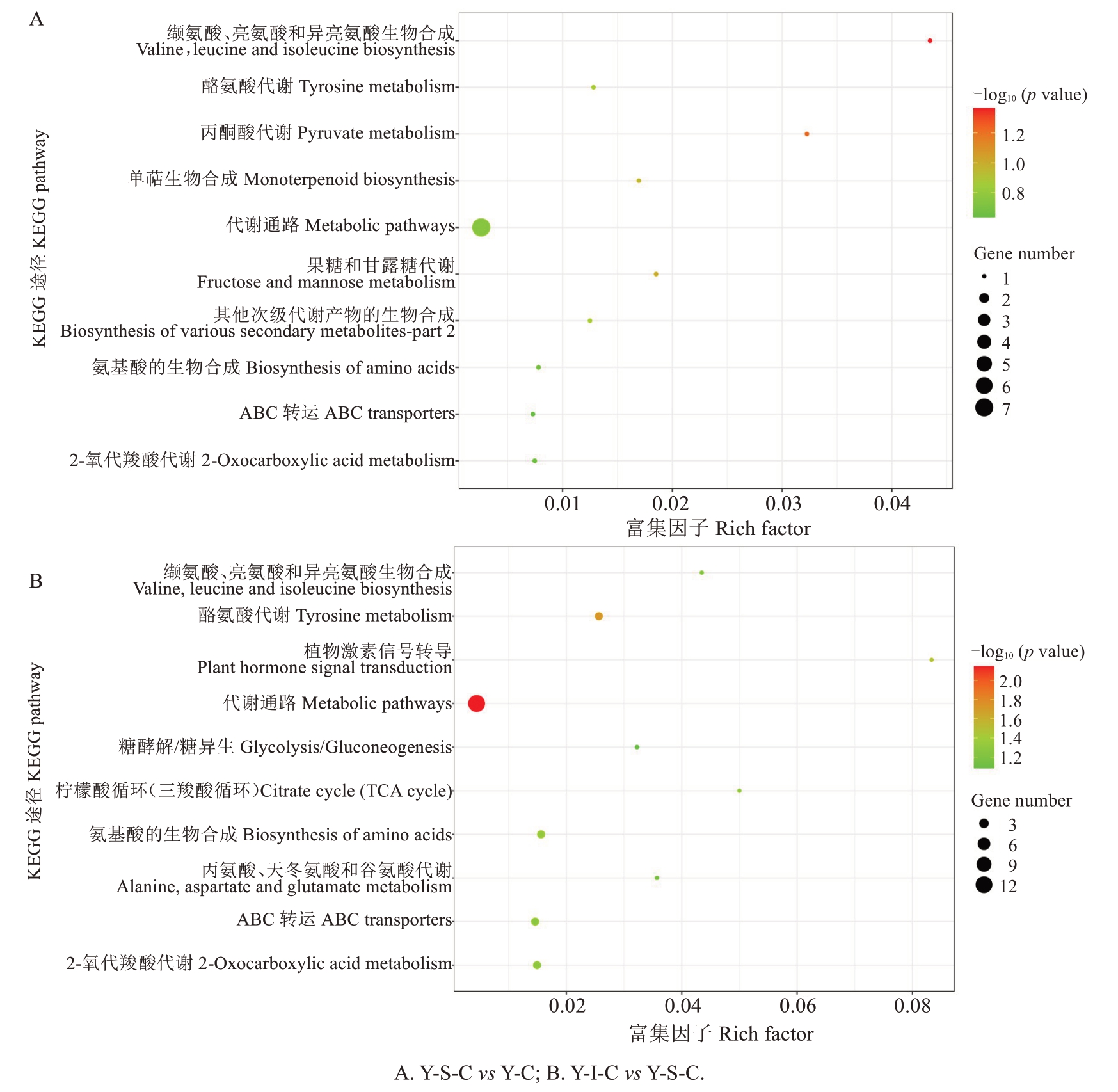
图8 果心差异代谢物KEGG 富集图
Fig.8 KEGG map of different metabolites pear core
不同温度处理(Y-I-C vs Y-S-C)的果心差异代谢物KEGG 注释富集在25 条代谢途径,富集因子排在前10 的途径见图8-B。将代谢途径分为几类,其中较为集中的途径有代谢通路;氨基酸代谢(酪氨酸代谢,缬氨酸、亮氨酸和异亮氨酸生物合成,丙氨酸、天冬氨酸和谷氨酸代谢,苯丙氨酸、酪氨酸和色氨酸生物合成,赖氨酸降解);次生代谢产物的生物合成(苯丙类生物合成途径、异喹啉生物碱生物合成);碳水化合物代谢(TCA 循环、糖酵解、丙酮酸代谢、果糖甘露糖代谢、半乳糖代谢);另外还涉及到脂肪酸降解、辅因子和维生素的代谢、植物激素信号转导、萜类和聚酮的代谢、膜运输等途径。
3 讨 论
果实褐变除与环境胁迫密切相关外,随贮藏期的延长果实衰老导致的褐变[23]亦是主要的因素,且受果实总酚类物质种类、分布及多酚氧化酶活性的影响[24-25]。黄怡等[26]对苹果梨、早酥梨和皇冠梨的研究表明,低温贮藏期间3 种梨果皮总酚和总黄酮含量均呈降低趋势,且抗氧化能力随贮藏期的延长而下降。与0 ℃冷藏相比,-1 ℃冰温贮藏抑制了玉露香梨PPO 活性的上升,延缓总酚、总黄酮含量的下降,保持了一定的抗氧化能力,果心褐变较轻。
代谢组学可反映表型的差异。与采收初始(YC)时相比较,0 ℃冷藏240 d(Y-S-C)后玉露香梨果心褐变指数升高,差异代谢物阿魏酸、邻苯二酚及黄酮类物质均呈下调的趋势。Busatto 等[27]利用靶向代谢组学技术研究发现,Granny Smith 苹果果皮中多酚类物质绿原酸、根皮苷、儿茶素和表儿茶素与果皮褐变的发生密切相关。-1 ℃冰温贮藏(Y-I-C)后果心代谢差异物右旋奎宁酸、熊果苷、阿魏酸、邻苯二酚均呈现上调的趋势,一定程度上表明冰温贮藏减缓了果实多酚类底物的氧化作用,同时黄酮类物质的上调协同了抗氧化作用,减轻了果实褐变。梨果中的有机酸组成根据品种不同主要为苹果酸、柠檬酸、奎宁酸、莽草酸和草酸等[28-29],且大多数梨品种总酸的含量差异都是由苹果酸与柠檬酸主导[30-31]。-1 ℃冰温贮藏促进了柠檬酸、苹果酸等有机酸物质的积累,呈上调的趋势,推测与冰温处理减缓苹果酸、柠檬酸等有机酸在果实成熟过程中作为糖酵解和三羧酸循环等呼吸作用的底物[32]被消耗的速率有关,且糖酵解、TCA 代谢通路在冰温贮藏对其的影响中富集因子水平也较高。有机酸类物质通过降低反应体系pH值,使其远离PPO最适pH值,来实现对PPO活性的抑制[33]。此外,羧基与PPO 活性中心的铜离子螯合限制了其与底物的结合[6],推测这也是冰温贮藏减轻果心褐变的原因之一。另外也有研究发现,苹果贮藏中褐变敏感和不敏感品种中丙酮酸、柠檬酸、延胡索酸、丙氨酸等代谢产物存在着差异[34];褐变菠萝组织中柠檬酸含量减少,且结合关键酶基因表达的分析认为柠檬酸降解与菠萝果肉的褐变过程密切相关[35]。
-1 ℃冰温贮藏(Y-I-C)相对0 ℃贮藏(Y-S-C)的果心差异代谢物富集的代谢通路中发现多项代谢差异物参与到氨基酸合成及氨基酸代谢等通路中。氨基酸代谢除在植物初级和次生代谢中发挥重要功能外,在冷胁迫时也参与渗透调节,增强抗低温胁迫能力[36]。差异代谢物天门冬氨酸、苯丙氨酸也在冰温贮藏后呈现上调。此外遭遇低温胁迫时,细胞膜会通过不饱和脂肪酸组分及含量的变化调节改善膜的流动相、稳定性[37]。玉露香梨-1 ℃冰温贮藏后差异代谢物中脂类物质呈现上调趋势。郝慧慧[38]研究发现,低温和近冰温对灵武长枣贮藏代谢的影响主要体现在脂类及氨基酸代谢。且笔者在本试验中同时发现作为参与果实逆境胁迫信号应答[39]的脱落酸在冰温处理下也呈现上调,推测这些代谢差异与果实在低温下的抗性作用有关。龚意辉等[40]对黄桃果肉褐变的非靶向代谢组学研究表明,PE 包装处理降低黄桃果肉褐变涉及代谢通路主要为其他次生代谢产物的生物合成、氨基酸代谢、辅因子和维生素的代谢通路,其他氨基酸代谢等通路。吴敏等[41]基于代谢组学解析红地球葡萄贮藏品质的研究认为SO2通过调控葡萄果实中类黄酮生物合成、氨基酸代谢和碳水化合物代谢等主要途径有效保持了葡萄果实贮藏品质。为此冰温对氨基酸代谢途径的影响是果实低温胁迫下的抗性反应还是冰温延缓了果实衰老代谢仍有待深入研究。
4 结 论
基于LC-MS/MS 非靶向代谢组学分析方法,在玉露香梨采后果心中共鉴定出331 种代谢产物,主要包括黄酮、苯丙烷类、萜类、糖及糖醇、生物碱、有机酸、脂质、氨基酸、醌类、单宁、核苷酸等物质。相对于采后果心(Y-C),0 ℃冷藏240 d(Y-S-C)果心共有27 种代谢物显著下调,4 种物质上调;有机酸、糖及糖醇等物质随果实衰老代谢发生降解,多酚类物质作为底物参与果心褐变的发生。与0 ℃冷藏相比,-1 ℃冰温贮藏延缓了果心PPO 酶活性的上升,延缓了总酚、总黄酮的氧化,抑制了果心褐变。相对于0 ℃冷藏240 d(Y-S-C),-1 ℃冷藏240 d(Y-I-C)果心中筛选出50种代谢差异产物,上调的26种物质主要为苯丙烷类、黄酮类、有机酸等类物质,通过KEGG代谢通路富集分析,发现其对代谢通路、氨基酸代谢,次生代谢产物的生物合成等途径贡献显著。-1 ℃冰温贮藏后邻苯二酚、熊果苷、柠檬酸等物质的上调增强了果实的抗氧化性,并通过相关途径协同影响果心氧化代谢。
[1] 郭黄萍,郝国伟,张晓伟,杨盛.‘玉露香梨’的性状表现与栽培贮藏保鲜技术[J].落叶果树,2011,43(5):41-43.GUO Huangping,HAO Guowei,ZHANG Xiaowei,YANG Sheng. Character expression and cultivation storage and fresh keeping techniques of‘Yuluxiang’pear[J]. Deciduous Fruits,2011,43(5):41-43.
[2] 赵迎丽,张微,杨志国,王亮,陈会燕,李超.可溶性固形物含量对玉露香梨冷藏品质及果心褐变的影响[J].中国果树,2023(11):27-31.ZHAO Yingli,ZHANG Wei,YANG Zhiguo,WANG Liang,CHEN Huiyan,LI Chao.Effect of soluble solids content on cold storage quality and core browning of‘Yuluxiang’pear[J].China Fruits,2023(11):27-31.
[3] 贾晓辉,王文辉,姜云斌,杜艳民,王志华,佟伟.不同贮藏温度对‘玉露香’梨果实保绿效果和品质维持的影响[J].果树学报,2016,33(S1):166-174.JIA Xiaohui,WANG Wenhui,JIANG Yunbin,DU Yanmin,WANG Zhihua,TONG Wei. Effects of storage temperature on green keeping and quality of‘Yuluxiang’pear[J]. Journal of Fruit Science,2016,33(S1):166-174.
[4] 王春生,赵迎丽,王华瑞,李建华,施俊凤,张晓宇.气调贮藏对玉露香梨品质的影响[J].保鲜与加工,2007,7(5):25-28.WANG Chunsheng,ZHAO Yingli,WANG Huarui,LI Jianhua,SHI Junfeng,ZHANG Xiaoyu. Effect of controlled atmosphere storage on qulity of Yuluxiang pear[J]. Storage and Process,2007,7(5):25-28.
[5] 袁江,张绍铃,曹玉芬,吴俊,田路明,陶书田,董星光.梨果实酚类物质与酶促褐变底物的研究[J].园艺学报,2011,38(1):7-14.YUAN Jiang,ZHANG Shaoling,CAO Yufen,WU Jun,TIAN Luming,TAO Shutian,DONG Xingguang. Polyphenolic com‐pound and substances determination of enzymatic browning in pear[J].Acta Horticulturae Sinica,2011,38(1):7-14.
[6] 白宇皓,李超,杨志国,赵迎丽,张立新.梨多酚氧化酶特性与酶促褐变抑制研究进展[J].食品科技,2022,47(2):75-81.BAI Yuhao,LI Chao,YANG Zhiguo,ZHAO Yingli,ZHANG Lixin.Advances in polyphenol oxidase properties and enzymatic browning inhibition of pear[J]. Food Science and Technology,2022,47(2):75-81.
[7] 苏艳丽,杨健,田永真,王龙,王苏珂,薛华柏,李秀根.梨果实发育过程中褐变相关生理指标的变化[J].果树学报,2018,35(增刊1):118-124.SU Yanli,YANG Jian,TIAN Yongzhen,WANG Long,WANG Suke,XUE Huabai,LI Xiugen. Study on the changes of physio‐logical indexes related to browning during fruit development in pears[J].Journal of Fruit Science,2018,35(Suppl.1):118-124.
[8] 杜艳民,王文辉,贾晓辉,佟伟,王志华.不同可溶性固形物含量‘鸭梨’耐贮性差异比较[J]. 果树学报,2018,35(10):1262-1270.DU Yanmin,WANG Wenhui,JIA Xiaohui,TONG Wei,WANG Zhihua. The comparison of storage ability of‘Yali’pear in dif‐ferent soluble solids contents grades[J].Journal of Fruit Science,2018,35(10):1262-1270.
[9] 韩云云,宋方圆,韩艳文,李玲,闫师杰.不同采收贮藏条件下鸭梨果实LOX 基因表达及其与果心褐变的关系[J]. 食品科学,2016,37(18):216-222.HAN Yunyun,SONG Fangyuan,HAN Yanwen,LI Ling,YAN Shijie. LOX gene expression and its relationship with core browning of Yali pear under different harvest and storage condi‐tions[J].Food Science,2016,37(18):216-222.
[10] 王志华,高剑利,王文辉,贾朝爽,姜云斌.不同贮藏温度对‘红香酥’梨果实品质和相关生理指标的影响[J].中国果树,2020(5):13-19.WANG Zhihua,GAO Jianli,WANG Wenhui,JIA Chaoshuang,JIANG Yunbin. Effects of different storage temperature on fruit quality and related physiological indexes of‘Hongxiangsu’pear[J].China Fruits,2020(5):13-19.
[11] 刘佰霖,王文辉,马风丽,王阳,杜艳民,贾晓辉.自发气调包装和乙烯吸收剂对‘玉露香’梨果实品质及耐贮性的影响[J].果树学报,2019,36(7):911-921.LIU Bailin,WANG Wenhui,MA Fengli,WANG Yang,DU Yan‐min,JIA Xiaohui. Effect of modified atmosphere packaging and ethylene absorbents on postharvest fruit quality and storage per‐formance of‘Yuluxiang’pear[J]. Journal of Fruit Science,2019,36(7):911-921.
[12] 周慧娟,叶正文,骆军,苏明申,卢昆,陈翅宏.气调处理对‘早生新水’梨贮藏品质的影响[J]. 中国农学通报,2018,34(28):143-152.ZHOU Huijuan,YE Zhengwen,LUO Jun,SU Mingshen,LU Kun,CHEN Chihong. Effect of controlled-atmosphere on stor‐age quality of‘Zaoshengxinshui’pear[J]. Chinese Agricultural Science Bulletin,2018,34(28):143-152.
[13] 董长林,李江阔,张鹏,张平,农绍庄.冰温贮藏对柿果褐变以及相关指标的影响[J].果树学报,2011,28(2):263-267.DONG Changlin,LI Jiangkuo,ZHANG Peng,ZHANG Ping,NONG Shaozhuang. Effect of freezing point storage on brown‐ing and physio-quality in persimmon[J]. Journal of Fruit Sci‐ence,2011,28(2):263-267.
[14] 李志文,张平,张昆明,任朝辉.1-MCP 结合冰温贮藏对葡萄果实质地的影响[J].农业机械学报,2011,42(7):176-181.LI Zhiwen,ZHANG Ping,ZHANG Kunming,REN Zhaohui.Ef‐fect of 1-methylcyclopropene combined with controlled freezingpoint storage on texture of grape fruit[J].Transactions of the Chi‐nese Society for Agricultural Machinery,2011,42(7):176-181.
[15] 邓冰,韩云云,韩艳文,梁鹏,刘畅,闫师杰.库尔勒香梨缓慢降温后适宜温湿度条件的研究[J].包装工程,2016,37(7):45-50.DENG Bing,HAN Yunyun,HAN Yanwen,LIANG Peng,LIU Chang,YAN Shijie. Optimal temperature and humidity condi‐tions of Korla fragrant pear under slow cooling[J]. Packaging Engineering,2016,37(7):45-50.
[16] 张微,赵迎丽,王亮,杨志国,陈会燕.冰温贮藏对不同产地玉露香梨果实品质及耐贮性的影响[J].山西农业科学,2020,48(10):1665-1670.ZHANG Wei,ZHAO Yingli,WANG Liang,YANG Zhiguo,CHEN Huiyan.Effect of ice-temperature storage on fruit quality and storability of Yuluxiang pears from different places[J]. Jour‐nal of Shanxi Agricultural Sciences,2020,48(10):1665-1670.
[17] HANHINEVA K,AHARONI A. Metabolomics in fruit develop‐ment[M]//JAIN S M,BRAR D S. Molecular Techniques in Crop Improvement. 2nd ed. Dordrecht:Springer Netherlands,2009:675-693.
[18] GONG Y H,SONG J,PALMER L C,VINQVIST-TYMCHUK M,FILLMORE S,TOIVONEN P,ZHANG Z Q. Tracking the development of the superficial scald disorder and effects of treat‐ments with diphenylamine and 1-MCP using an untargeted me‐tabolomic approach in apple fruit[J]. Food Chemistry:Molecu‐lar Sciences,2021,2:100022.
[19] 王雅慧,刘晓宏,雍明丽,熊爱生,苏小俊.基于代谢组学分析丝瓜果肉褐变过程酚酸类物质变化[J].中国农业科学,2021,54(22):4869-4879.WANG Yahui,LIU Xiaohong,YONG Mingli,XIONG Aisheng,SU Xiaojun. Analysis of changes in phenolic acids of Luffa cy‐lindrica pulp during browning based on metabolomics[J]. Scien‐tia Agricultura Sinica,2021,54(22):4869-4879.
[20] 王森.超声波对石榴果实品质的影响及基于代谢组解析褐变机理[D].郑州:河南农业大学,2023.WANG Sen. Effect of ultrasound on pomegranate fruit quality and analyzing browning mechanism based on metabolome[D].Zhengzhou:Henan Agricultural University,2023.
[21] 高静怡,马浩,刘东贺,方啸宇,刘晓.非靶向代谢组学对砂梨水心病不同代谢物质的差异分析[J].分子植物育种,2021,19(24):8297-8304.GAO Jingyi,MA Hao,LIU Donghe,FANG Xiaoyu,LIU Xiao.The difference analysis of non targeted metabonomics on differ‐ent metabolites in watercore Pyrus pyrifolia[J]. Molecular Plant Breeding,2021,19(24):8297-8304.
[22] FARNETI B,BUSATTO N,KHOMENKO I,CAPPELLIN L,GUTIERREZ S,SPINELLI F,VELASCO R,BIASIOLI F,COSTA G,COSTA F. Untargeted metabolomics investigation of volatile compounds involved in the development of apple super‐ficial scald by PTR-ToF-MS[J].Metabolomics,2015,11(2):341-349.
[23] 程顺昌.不同采收期南果梨采后褐变发生机理及调控技术研究[D].沈阳:沈阳农业大学,2013.CHENG Shunchang. Study of browning mechanisms,regulation and control technology of different harvest date Nanguo pear[D].Shenyang:Shenyang Agricultural University,2013.
[24] 邹丽红,张玉星.砂梨果肉褐变与酚类物质及相关酶活性的相关分析[J].果树学报,2012,29(6):1022-1026.ZOU Lihong,ZHANG Yuxing. Correlation analysis of flesh browning between phenolic compound and relavent enzymatic activity in fruit of Pyrus pyrifolia[J]. Journal of Fruit Science,2012,29(6):1022-1026.
[25] 王海艳,田国奎,王立春,李凤云,潘阳,庞泽,丁凯鑫,郝智勇.鲜切马铃薯加工及货架期品质控制的研究进展[J].中国瓜菜,2023,36(10):10-15.WANG Haiyan,TIAN Guokui,WANG Lichun,LI Fengyun,PAN Yang,PANG Ze,DING Kaixin,HAO Zhiyong. Research progress on processing and shelf-life quality control of fresh cut potatoes[J]. China Cucurbits and Vegetables,2023,36(10):10-15.
[26] 黄怡,高春丽,毕阳,李冬美,董玉鹏,张彦东,李永才.低温贮藏期间梨果皮酚类物质及抗氧化性变化[J].食品与发酵工业,2019,45(19):219-226.HUANG Yi,GAO Chunli,BI Yang,LI Dongmei,DONG Yu‐peng,ZHANG Yandong,LI Yongcai. Changes in phenolic compounds and antioxidant activity of pear peel during cold storage[J].Food and Fermentation Industries,2019,45(19):219-226.
[27] BUSATTO N,FARNETI B,TADIELLO A,VRHOVSEK U,CAPPELLIN L,BIASIOLI F,VELASCO R,COSTA G,COS‐TA F. Target metabolite and gene transcription profiling during the development of superficial scald in apple (Malus × domesti‐ca Borkh.)[J].BMC Plant Biology,2014,14:193.
[28] WU J Y,FAN J B,LI Q H,JIA L T,XU L L,WU X,WANG Z W,LI H X,QI K J,QIAO X,ZHANG S L,YIN H.Variation of organ‐ic acids in mature fruits of 193 pear (Pyrus spp.) cultivars[J].Journal of Food Composition and Analysis,2022,109:104483.
[29] 郑璞帆.梨种质资源评价及果实代谢组学研究[D].天津:南开大学,2022.ZHENG Pufan. Study of evaluation and metabolomics of pear(Pyrus L.)germplasm resources[D].Tianjin:Nankai University,2022.
[30] 殷晨,田路明,曹玉芬,董星光,张莹,霍宏亮,齐丹,徐家玉,刘超. 梨果实糖酸研究进展[J]. 果树学报,2023,40(12):2610-2623.YIN Chen,TIAN Luming,CAO Yufen,DONG Xingguang,ZHANG Ying,HUO Hongliang,QI Dan,XU Jiayu,LIU Chao.Research progress in sugar and acid in pear fruit[J]. Journal of Fruit Science,2023,40(12):2610-2623.
[31] LI Q H,QIAO X,JIA L T,ZHANG Y X,ZHANG S L. Tran‐scriptome and resequencing analyses provide insight into differ‐ences in organic acid accumulation in two pear varieties[J]. In‐ternational Journal of Molecular Sciences,2021,22(17):9622.
[32] 仇岑,晋朝,齐秋爽,樊秀花,陈存坤,闫师杰.降温方法对不同成熟度鸭梨冰温贮藏中有机酸含量的影响[J].食品安全质量检测学报,2023,14(13):284-293.QIU Cen,JIN Zhao,QI Qiushuang,FAN Xiuhua,CHEN Cunkun,YAN Shijie. Effects of cooling method on organic acid content of different maturity of Pyrus bretschneideri Rehd. cv.Yali during ice-temperature storage[J].Journal of Food Safety&Quality,2023,14(13):284-293.
[33] 郑丽静,刘超超,满杰,钱井,韦强,赵立群.鲜榨柠檬汁在即食鲜切生菜上的应用效果[J].中国瓜菜,2023,36(11):86-92.ZHENG Lijing,LIU Chaochao,MAN Jie,QIAN Jing,WEI Qiang,ZHAO Liqun. Application effect of fresh squeezed lem‐on juice on ready-to-eat fresh-cut lettuce[J]. China Cucurbits and Vegetables,2023,36(11):86-92.
[34] VANDENDRIESSCHE T,SCHÄFER H,VERLINDEN B E,HUMPFER E,HERTOG M L A T M,NICOLAÏ B M. Highthroughput NMR based metabolic profiling of Braeburn apple in relation to internal browning[J]. Postharvest Biology and Tech‐nology,2013,80:18-24.
[35] 冼洁文.基于代谢组学的采后菠萝黑心病发病的机制[D].广州:华南农业大学,2019.XIAN Jiewen.The mechanism of internal browning in harvested pineapple fruit based on metabolomics[D]. Guangzhou:South China Agricultural University,2019.
[36] KAZEMI-SHAHANDASHTI S S,MAALI-AMIRI R,ZEINA‐LI H,KHAZAEI M,TALEI A,RAMEZANPOUR S S. Effect of short-term cold stress on oxidative damage and transcript ac‐cumulation of defense-related genes in chickpea seedlings[J].Journal of Plant Physiology,2014,171(13):1106-1116.
[37] 王芳,王淇,赵曦阳.低温胁迫下植物的表型及生理响应机制研究进展[J].分子植物育种,2019,17(15):5144-5153.WANG Fang,WANG Qi,ZHAO Xiyang. Research progress of phenotype and physiological response mechanism of plants un‐der low temperature stress[J]. Molecular Plant Breeding,2019,17(15):5144-5153.
[38] 郝慧慧.贮藏温度对灵武长枣细胞壁代谢及软化特性的影响[D].银川:宁夏大学,2022.HAO Huihui. Effect of storage temperature on cell wall metabo‐lism and softening characteristics of Lingwu long jujube[D].Yinchuan:Ningxia University,2022.
[39] 李皎琪,李美琪,王玉婷,刘秀玲,张新华,李富军,李晓安.果蔬逆境胁迫应答中AP2/ERF 转录因子的激素调控研究进展[J/OL]. 食品科学. [2023-10-11](2024-05-16). https://link.cnki.net/urlid/11.2206.TS.20231010.0834.002.LI Jiaoqi,LI Meiqi,WANG Yuting,LIU Xiuling,ZHANG Xin‐hua,LI Fujun,LI Xiaoan. Research process on hormone regula‐tion of AP2/ERF transcription factors in response to stress in fruit and vegetables[J/OL].Food Science.[2023-10-11](2024-05-16).https://link.cnki.net/urlid/11.2206.TS.20231010.0834.002.
[40] 龚意辉,李丽梅,黄华,李论,田宗琼,张娟,曾永贤,陈致印.基于非靶向代谢组学分析黄桃果肉褐变过程中代谢产物的差异[J].中国食品学报,2023,23(2):265-275.GONG Yihui,LI Limei,HUANG Hua,LI Lun,TIAN Zongqiong,ZHANG Juan,ZENG Yongxian,CHEN Zhiyin.Analysis of me‐tabolite differences during the browning process of yellow peach based on untargeted metabolomics[J]. Journal of Chinese Institute of Food Science and Technology,2023,23(2):265-275.
[41] 吴敏,张健,玛依努尔·扎衣提,翟荣臻,张政,魏佳,吴斌,吴忠红. 基于代谢组学解析SO2 对鲜食葡萄贮藏品质的调控作用[J].食品与发酵工业,2023,49(24):88-96.WU Min,ZHANG Jian,MAYINNER·Zhayiti,ZHAI Rong‐zhen,ZHANG Zheng,WEI Jia,WU Bin,WU Zhonghong.Anal‐ysis of regulation of SO2 on storage quality of fresh grape based on metabolomics[J].Food and Fermentation Industries,2023,49(24):88-96.