芦柑(Citrus reticulata Blanco‘Ponkan’)属于芸香科(Rutaceae)柑橘属(Citrus)植物,果实色泽橘黄,果肉多汁,是福建省优良果树品种。芦柑裂果是果实发育过程中常见的一种生理病害,常在果实膨大期出现。裂果后会导致果实水分的损失,病菌侵入发生霉变,加快病虫害的传播,影响果实外观等[1]。芦柑裂果现象严重时高达70%,已成为芦柑产业发展中的一大难题。
Ca 作为一种重要的常量元素,在植物生长发育中起着多种作用,能够维持并提高生物膜的稳定性和完整性[2],促进果胶酸钙的形成,提高细胞耐压力、延展性,降低裂果的发生率。Nie等[3]在对杏(Ar‐meniaca vulgaris Lam.)的研究中发现,Ca 元素含量与裂果率呈负相关。在园艺植物实际生产中,葡萄(Vitis vinifera L.)[4]、石榴(Punica granatum Linn.)[5]等果实都可能因缺Ca 而造成裂果,因此喷施Ca 肥是生产上减少裂果的重要措施。在对脐橙[C.sinen‐sis(L.)Osbeck.]等的研究中发现钙处理能够缓解原果胶和纤维素降解,增加果实硬度和细胞壁厚度,降低裂果率[6]。近年来,有报道指出外源赤霉素(gib‐berellin,GA3)对裂果有调控作用。GA3能改变果实表皮的厚度和蜡质含量[7]。喷施GA3能够维持稳定果皮细胞层次,增强细胞之间的连接性,减少裂果的发生[8]。在对柑橘的研究中,GA3在一定程度上能缓解胞间裂隙的发展,从而防控裂果的发生[9]。生产上应用外源GA3处理能够降低脐橙[10]、石榴[11]的裂果率。
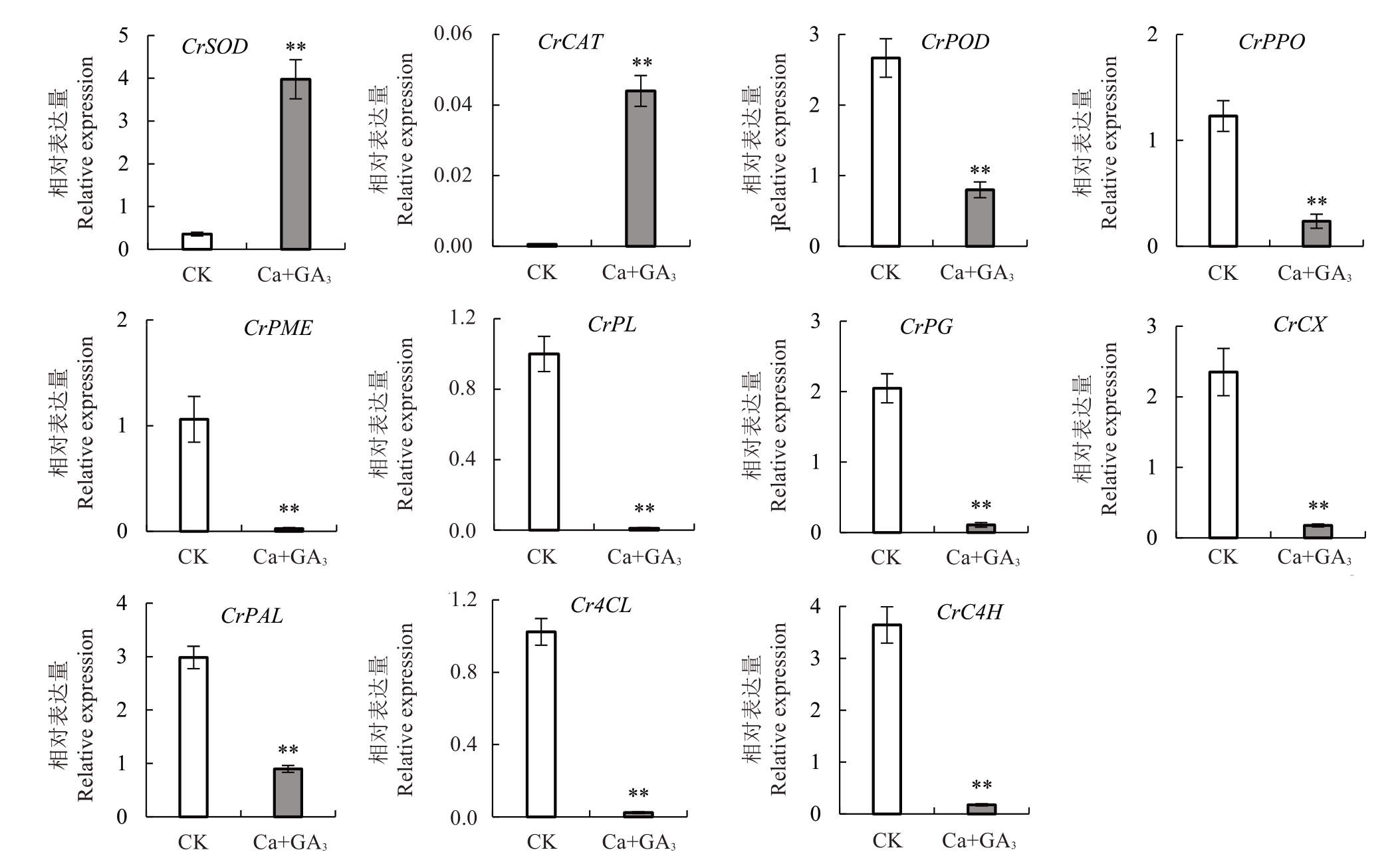
活性氧(reactive oxygen species,ROS)代谢平衡与果实裂果密切相关。ROS 包括过氧化氢(H2O2)、超氧阴离子自由基(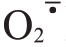 )等,ROS清除系统中有抗氧化酶和抗氧化物质[12]。超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、过氧化物酶(POD)、多酚氧化酶(PPO)等都是植物应对外界胁迫的关键抗氧化酶类[13-14]。ROS 的清除可协调细胞膜系统状态稳定,维持果皮细胞的完整性。Yang 等[15]在番茄(Sola‐num lycopersicum L.)中发现,易裂品种果皮各部位SOD、CAT 活性均显著或极显著低于不易裂品种,而POD 活性则显著高于不易裂品种。在对枇杷[Er‐iobotrya japonica(Thunb.)Lindl.]的研究中发现,裂果中SOD、CAT 活性低于正常果,而POD、PPO 活性高于正常果[16]。李建国等[17]研究发现,易裂品种荔枝(Litchi chinensis Sonn.)果皮细胞壁中的POD、PPO 活性显著高于抗裂品种。蜜广橘(C.reticulata Blanco.)正常果的抗氧化酶相关基因CsSOD、CsCAT表达量高于裂果品种,而CsPOD 低于裂果品种[18]。易裂番荔枝在贮藏过程中PPO 基因表达量明显高于不裂果品种[19]。
)等,ROS清除系统中有抗氧化酶和抗氧化物质[12]。超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、过氧化物酶(POD)、多酚氧化酶(PPO)等都是植物应对外界胁迫的关键抗氧化酶类[13-14]。ROS 的清除可协调细胞膜系统状态稳定,维持果皮细胞的完整性。Yang 等[15]在番茄(Sola‐num lycopersicum L.)中发现,易裂品种果皮各部位SOD、CAT 活性均显著或极显著低于不易裂品种,而POD 活性则显著高于不易裂品种。在对枇杷[Er‐iobotrya japonica(Thunb.)Lindl.]的研究中发现,裂果中SOD、CAT 活性低于正常果,而POD、PPO 活性高于正常果[16]。李建国等[17]研究发现,易裂品种荔枝(Litchi chinensis Sonn.)果皮细胞壁中的POD、PPO 活性显著高于抗裂品种。蜜广橘(C.reticulata Blanco.)正常果的抗氧化酶相关基因CsSOD、CsCAT表达量高于裂果品种,而CsPOD 低于裂果品种[18]。易裂番荔枝在贮藏过程中PPO 基因表达量明显高于不裂果品种[19]。
果皮抗裂性与其细胞壁韧性及延伸性有关。植物细胞壁主要是由多糖、纤维素、半纤维素、果胶以及木质素等构成的复杂动态结构[20]。在初生壁中纤维素微纤维镶嵌在半纤维素果胶中,影响着细胞壁强度与完整性。果胶可以促进细胞之间的黏附和沟通,维持细胞壁的水分和弹性[21]。木质素主要位于纤维素纤维之间,通过形成交织网来硬化细胞壁,纤维素微纤维的定向排列和木质素含量决定着次生壁的强度和硬度[22]。细胞壁结构成分含量变化决定着果皮的机械强度。果实裂果一般伴随着细胞壁、果胶和纤维素等多糖类物质的降解[23-24]。果胶甲酯酶(PME)、多聚半乳糖醛酸酶(PG)、果胶裂解酶(PL)为果胶类降解酶[25-26],纤维素酶(CX)则参与纤维素的降解[27]。苯丙氨酸解氨酶(PAL)、4-香豆酸辅酶A连接酶(4CL)和肉桂酸-4-羟基化酶(C4H)是参与木质素合成的重要酶[28]。在番荔枝[29](Annona squamo‐sa L.)、蜜广橘[30]、番茄[31]中发现,PME、PG 和CX 活性高,原果胶、纤维素含量降低,可溶性果胶含量提高,果皮开裂。抑制草莓(Fragaria×ananassa Duch.)果实中PL 活性可维持细胞壁中果胶含量,增加果实硬度[32]。在葡萄生长期喷施钙能降低果皮中PME、PG 的表达量,降低裂果率[33]。易裂番茄中SIPL 的表达量显著高于耐裂品种[34]。抑制红江橙[C.sinensis(L.)Osbeck.]果实细胞壁代谢酶基因中PG、PME 和CX 的表达,能减少果实开裂[35]。Liu等[36]在研究辣椒(Capsicum annuum Linn.)裂果与木质素的关系时发现严重裂果的木质素含量显著高于不裂果果实。柚果实中木质素合成相关酶PAL、C4H、4CL 活性及基因表达量升高,木质素含量增加[28]。
笔者在本研究中以易裂早熟芦柑为试验材料,研究果面喷施Ca+GA3对芦柑裂果率、外观形态、活性氧代谢、细胞壁代谢相关酶活性和相关代谢物质含量,以及相关基因表达水平的影响。探讨Ca+GA3处理对减轻果实开裂的相关生理和分子机制,为生产上预防芦柑裂果提供理论依据。
1 材料和方法
1.1 试验材料与处理
以永春县天马芦柑果园10 年生早熟芦柑(易裂品种)为试验材料,选择营养条件和树体长势相对一致的果树18株,统一管理。
根据2021 年预试验得到预防芦柑裂果最佳Ca+GA3质量浓度。2022年正式试验以喷施0.3 g·L-1螯合钙(美国丰利惠)和10 mg·L-1 GA3(北京索莱宝)为处理组(Ca+GA3),并以喷施清水为对照组(CK),于果实膨大期开始对树体喷施,共喷施2 次(2022 年6 月15 日、7 月15 日),以果实、叶片滴水为度。处理组和对照组各9 株树。于8 月15 日芦柑开始裂果后每隔15 d 采样1 次(盛花期后105、120、135、150和165 d)。取样部位为果树外围东、南、西、北及内膛中部5 个方位大小一致的果实,每次每株按裂果率采摘对应比例数量的正常果和裂果共40个,每3 株为1 个生物学重复,共120 个果。试验共设3 个生物学重复。裂果取裂开部位周边的果皮,用手术刀沿果顶到果蒂方向,取两边宽各0.5 cm 果皮,长度2 cm;正常果取相同部位同等大小的果皮,混样后用锡箔纸分装。液氮处理0.5 h,置于-80 ℃超低温冰箱保存,用于后续生理与分子试验。另取部分果皮烘干保存,用于后续纤维素、木质素含量测定。
1.2 试验方法
1.2.1 芦柑裂果率、形态指标测定 统计每株总果数和每株裂果数,计算裂果率。3株为1个生物学重复,共设3个生物学重复。
分别在采摘的处理组和对照组果实中随机挑选10 个果皮无损伤的正常果,用电子游标卡尺测定横纵径、果顶处果皮厚度,用百分之一天平测定单果质量,计算果形指数(纵径/横径)。
1.2.2 芦柑果皮过氧化氢(H2O2)、超氧阴离子(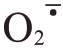 )、丙二醛(MDA)含量及抗氧化酶活性的测定 芦柑果皮过氧化氢(H2O2)、超氧阴离子(
)、丙二醛(MDA)含量及抗氧化酶活性的测定 芦柑果皮过氧化氢(H2O2)、超氧阴离子(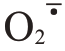 )和丙二醛(MDA)含量及多酚氧化酶(PPO)活性分别参考检测试剂盒(北京索莱宝生物公司)说明测定。
)和丙二醛(MDA)含量及多酚氧化酶(PPO)活性分别参考检测试剂盒(北京索莱宝生物公司)说明测定。
超氧化物歧化酶(SOD)活性参照Wang 等[37]的氮蓝四唑光化还原法测定;过氧化氢酶(CAT)活性参照Wang 等[37]的紫外吸收法测定;过氧化物酶(POD)活性参照Jiang等[38]的愈创木酚法测定。
1.2.3 芦柑果皮细胞壁代谢酶和结构成分的测定芦柑果皮果胶甲酯酶(PME)、多聚半乳糖醛酸酶(PG)、果胶裂解酶(PL)、纤维素酶(CX)、苯丙氨酸解氨酶(PAL)、4-香豆酸辅酶A 连接酶(4CL)和肉桂酸-4-羟基化酶(C4H)活性分别参考检测试剂盒(北京索莱宝生物公司)说明测定。
原果胶与可溶性果胶的提取和含量参考曹建康等[39]的方法测定。
纤维素含量测定:取果皮干样0.05 g加入30 mL 60% H2SO4 30 min 后定容至50 mL,摇匀,过滤,取2.5 mL 滤液至25 mL 容量瓶中,用蒸馏水定容。然后从中取2 mL 依次加入5 mL 2%蒽酮、H2SO4,震荡,沸水浴10 min,取出冰浴1 min 后在620 nm 处测OD值。
木质素含量采用乙酰溴法[40]测定。
1.2.4 芦柑果皮RNA 提取及相关基因表达分析参照天根公司多糖多酚植物总RNA 提取试剂盒(无DNA 残留型)说明书提取盛花期后135 d 裂果最严重时期的CK和Ca+GA3处理的芦柑果皮总RNA;采用Vazyme 公司Hiscript II Q RT SuperMix for qPCR试剂盒合成cDNA。
基因表达分析采用实时荧光定量PCR(qRTPCR)方法,在Roche实时荧光定量PCR仪(LightCy‐cler 96)上完成。利用NCBI 设计引物,以柑橘Actin为内参基因,由福州易禾基因科技有限公司合成引物,引物信息详见表1。基因扩增体系为:10 μL 2×ChamQ Universal SYBR qPCR Master Mix,上下游引物各0.4 μL,1 μL cDNA、8.2 μL ddH2O。基因扩增体系总体积为20 μL。反应程序为①预变性95 ℃30 s;②循环反应95 ℃10 s,60 ℃30 s(45 个循环);③溶解曲线:95 ℃30 s,60 ℃60 s,95 ℃15 s。每个样品3 次重复,采用2-ΔΔCT法计算基因的相对表达量并制图。
表1 芦柑果皮qRT-PCR 引物
Table 1 qRT-PCR primers of ponkan peel

引物名称Primer name SOD-F SOD-R CAT-F CAT-R POD-F POD-R PPO-F PPO-R PME-F PME-R PL-F PL-R PG-F PG-R CX-F CX-R PAL-F PAL-R 4CL-F 4CL-R C4H-F C4H-R Actin-F Actin-R引物序列(5'—3')Primer sequence(5'—3')GTGATGCTTCTTTCGCTGTTGG GCTAGGGAGATTTTCAGCCAGG CACAGGGATGAGGAGGTCG GGTATCTCTCTCCAGGCTGC TTATGCAGAGAGGCGATGGG GAGGCATCACATCCCACGAT TCCCTACTCACAAAGCCCAAG GACCTCCAAGACCAAGAAGCA TCTGCAACCTTCATCGTGGT TGTAGACAAATCAGCCGCACT CACAAGATGTGCACAGGAGCAT TGGGTCACATCTCCAGCAATC TAATCGACGGACAAGGCTCC CATAGAACCTCAGCGCCGTT TGTGGCAATATGGTAGTCAA CCAATGAGGGTAAAGAAGAT GAGCTCATTAGATTGAACGC GCTGCAACTCAAAGAACCCG GGGAGTTCGACATAGCGGTT TGAAATAACCCGGCGTCACA CAGTGTAGGAGGTTGGCTTTCT TGTTGTTTCTATTGCCGCC ATCTGCTGGAAGGTGCTGAG CCAAGCAGCATGAAGATCAA碱基数The number of bases 22 22 19 20 20 20 21 21 20 21 22 21 20 20 20 20 20 20 20 20 22 19 20 20
1.3 数据分析
使用Microsoft Excel 2021 进行统计处理及作图,采用IBM SPSS Statistics 25.0进行差异显著性分析。
2 结果与分析
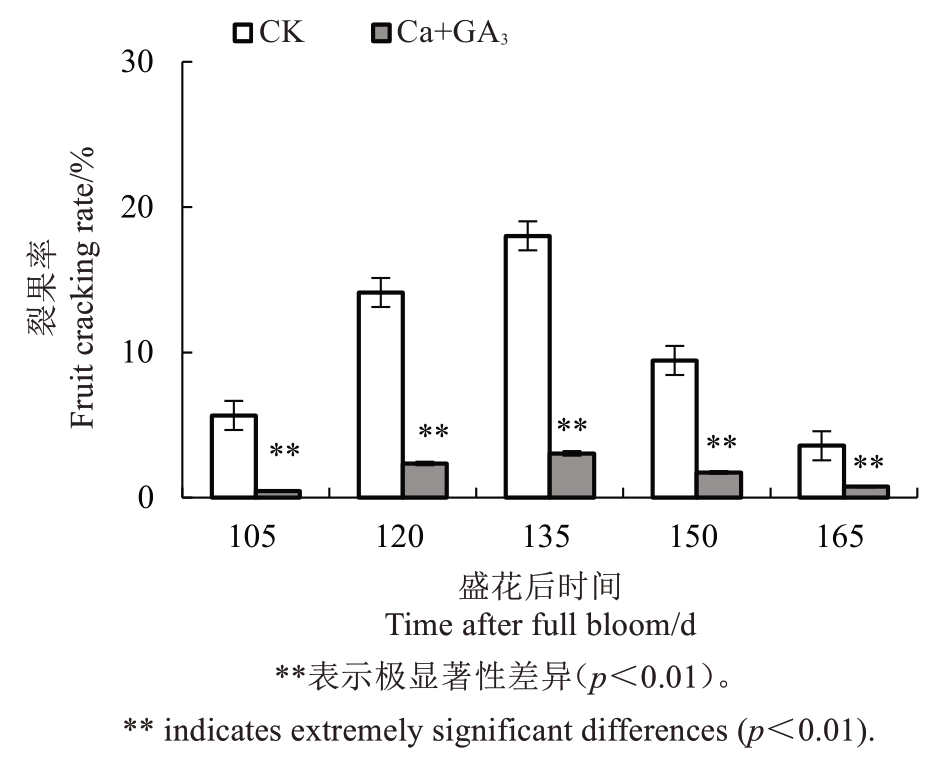
2.1 Ca+GA3处理对芦柑果实裂果率的影响
图1 显示,芦柑盛花后135 d 是裂果发生的高峰期。从盛花后105 d 开始,Ca+GA3处理明显降低了裂果率,与CK 比较达到极显著差异水平。由此可见,在芦柑生长过程中喷施外源Ca+GA3可显著降低果实的裂果率,有效减少芦柑裂果的发生。
2.2 Ca+GA3处理对芦柑果实外观品质的影响
随着芦柑果实的生长发育,果实横径、纵径均逐渐增加,经Ca+GA3 处理后果实横、纵径均大于CK。从芦柑盛花后135 d开始,Ca+GA3处理的果形指数大于CK。果形指数小,皮层组织生长受到遏制,易发生裂果。单果质量随着芦柑果实的生长发育而持续上升,在果实不同发育阶段呈对数型增长,Ca+GA3处理的单果质量大于CK。说明Ca+GA3处理能提高芦柑的单果质量。芦柑果实的果顶果皮厚度呈先上升后下降再上升的趋势,从芦柑盛花后120 d 达到峰值,在盛花后第135 天果顶果皮厚度达到谷值。此外,Ca+GA3处理可显著增加芦柑的果顶果皮厚度(表2)。
表2 Ca+GA3处理对芦柑果实外观品质的影响
Table 2 Effects of Ca+GA3 treatment on the appearance quality of ponkan fruits

注:不同小写字母表示在0.05 水平差异显著。
Note:The different small letters indicate significant differences at the 0.05 level.
盛花后时间Time after full bloom/d 105 120 135 150 165横径Transverse diameter/mm CK 39.36±0.64 46.57±0.80 50.14±2.59 52.81±2.49 b 54.22±0.90 b Ca+GA3 1.75±0.25 a 1.92±0.16 a 1.28±0.11 a 1.52±0.07 a 1.81±0.06 a Ca+GA3 40.90±3.41 48.81±3.90 51.42±1.18 55.66±0.82 a 57.29±2.05 a纵径Vertical diameter/mm CK 34.89±0.34 39.17±3.52 40.71±1.53 b 42.19±2.12 b 43.80±1.89 b Ca+GA3 35.63±1.41 41.59±0.63 45.24±0.68 a 47.20±1.34 a 49.47±1.68 a果形指数Fruit shape index CK 0.87±0.02 0.89±0.09 0.84±0.07 0.81±0.07 0.80±0.04 Ca+GA3 0.86±0.04 0.87±0.08 0.86±0.03 0.88±0.03 0.85±0.06单果质量Single fruit mass/g CK 29.75±0.78 42.36±2.20 55.73±2.35 b 63.23±1.66 b 79.95±0.61 b Ca+GA3 33.67±1.50 46.39±0.70 67.77±1.98 a 77.13±2.87 a 89.17±1.12 a果皮厚度Peel thickness/mm CK 1.56±0.08 b 1.68±0.05 b 0.82±0.10 b 1.10±0.04 b 1.35±0.06 b
2.3 Ca+GA3处理对芦柑果皮活性氧代谢的影响
在盛花后105~165 d 期间,芦柑果皮H2O2含量呈先上升后下降的趋势,且Ca+GA3 处理后果皮H2O2含量显著低于对照。芦柑果皮H2O2含量在盛花后135 d 达到峰值,此时Ca+GA3 比对照减少了29.59%。Ca+GA3处理明显减少了果皮H2O2含量,并有效抑制后期H2O2的积累,降低芦柑的裂果率。芦柑果皮 产生速率总体呈先下降后上升再下降的趋势,芦柑盛花后135 d 果皮O2产生速率达到峰值。Ca+GA3处理的芦柑果皮显著抑制了O2的积累。芦柑果皮的MDA 含量呈先上升后下降的趋势,Ca+GA3处理的果皮MDA含量一直显著低于CK(图2)。Ca+GA3处理降低了芦柑果皮细胞膜脂过氧化程度。
产生速率总体呈先下降后上升再下降的趋势,芦柑盛花后135 d 果皮O2产生速率达到峰值。Ca+GA3处理的芦柑果皮显著抑制了O2的积累。芦柑果皮的MDA 含量呈先上升后下降的趋势,Ca+GA3处理的果皮MDA含量一直显著低于CK(图2)。Ca+GA3处理降低了芦柑果皮细胞膜脂过氧化程度。
如图3 所示,芦柑果皮的SOD 活性在盛花后105~165 d期间呈先上升后下降的趋势,在135 d达到峰值,之后缓慢下降。Ca+GA3处理的果皮SOD活性始终高于CK。从芦柑盛花后105 d开始,Ca+GA3处理的果皮CAT 活性均显著高于CK。果皮CAT 活性也呈先上升后下降的趋势,在盛花后135 d 达到峰值。Ca+GA3处理可以显著提高果皮CAT 活性。果皮POD活性呈先上升后下降的趋势,在盛花后135 d达到峰值,Ca+GA3处理的果皮POD 活性均显著低于CK;果皮PPO 活性也呈现先上升后下降的趋势,Ca+GA3处理的果皮PPO 活性始终显著低于CK,说明Ca+GA3处理降低了芦柑果皮POD和PPO活性。
2.4 Ca+GA3处理对芦柑果皮细胞壁结构成分和相关酶活性的影响
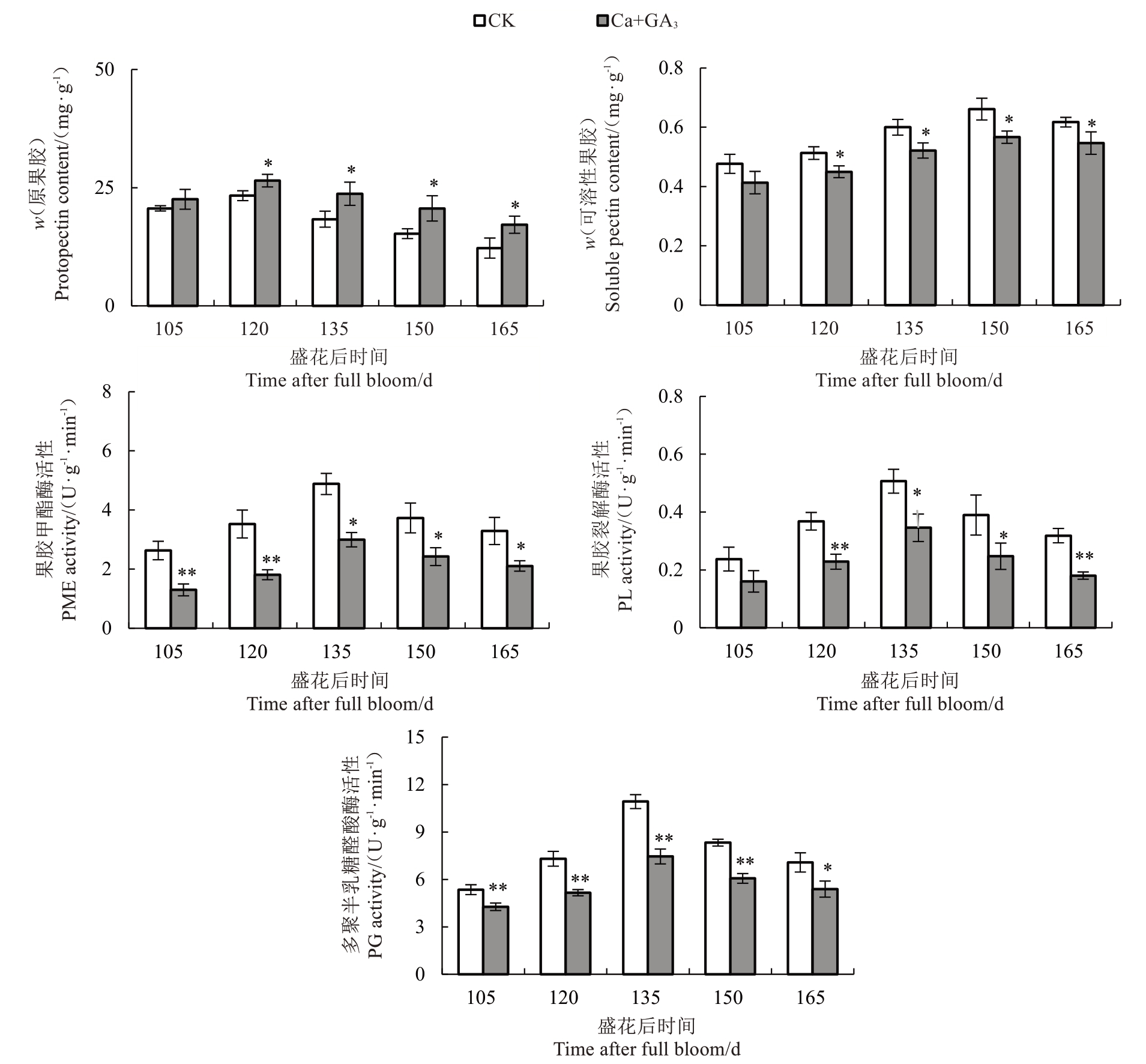
在盛花后105~165 d 期间,随着芦柑果实的生长发育,果皮原果胶含量呈先上升后下降的趋势,在芦柑盛花后第120 天达到峰值。Ca+GA3处理后果皮的原果胶含量始终高于CK。芦柑果皮的可溶性果胶含量也为先上升后下降的趋势,在芦柑盛花后的第150 天达到峰值,此时已在裂果后期。经Ca+GA3处理后的可溶性果胶含量始终低于CK。Ca+GA3处理延缓了原果胶降解成可溶性果胶的速度。芦柑果皮PME 活性呈先上升后下降的趋势。Ca+GA3处理后的果皮PME 活性始终低于CK。且处理后PME 活性上升幅度明显小于CK。Ca+GA3处理降低了果皮PME 活性。在芦柑生长发育期果皮中PL 活性均维持较低水平。随着果实生长发育,果皮中PL活性均逐渐升高,但Ca+GA3处理的果皮PL活性始终低于CK。芦柑果皮PG 活性均呈现先上升后下降的趋势,Ca+GA3处理始终低于CK,在盛花后135 d达到峰值,此时Ca+GA3处理的果皮PG活性与CK差异最大(图4)。
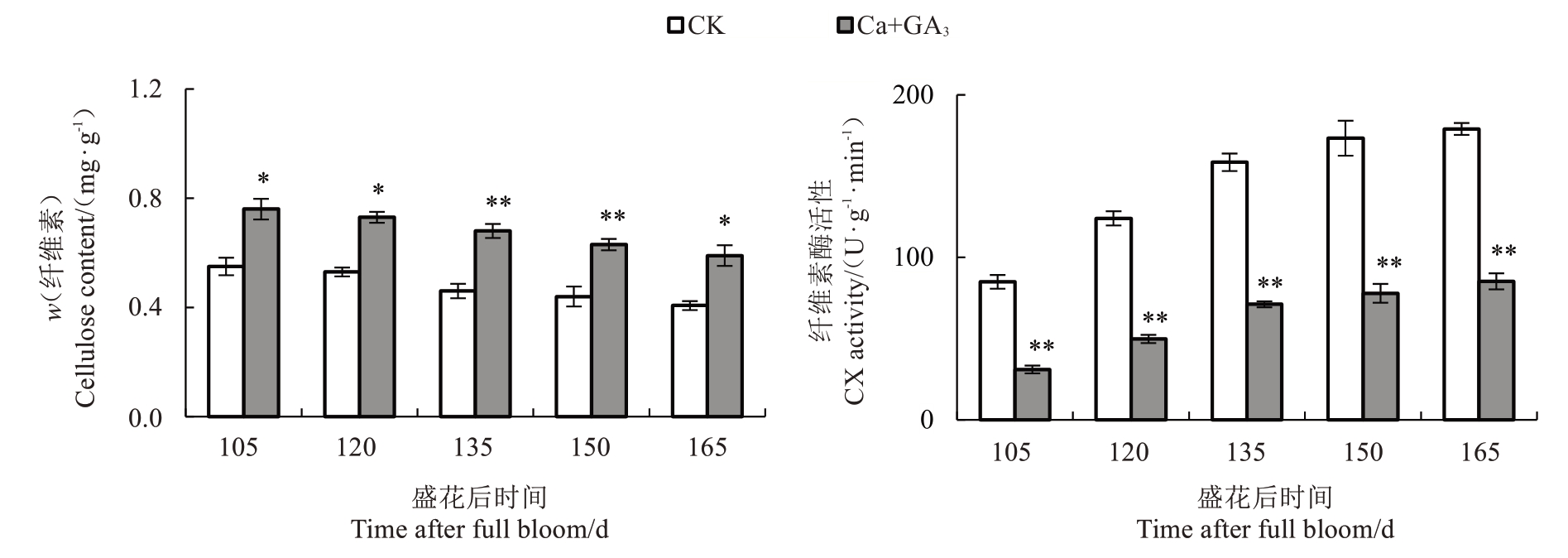
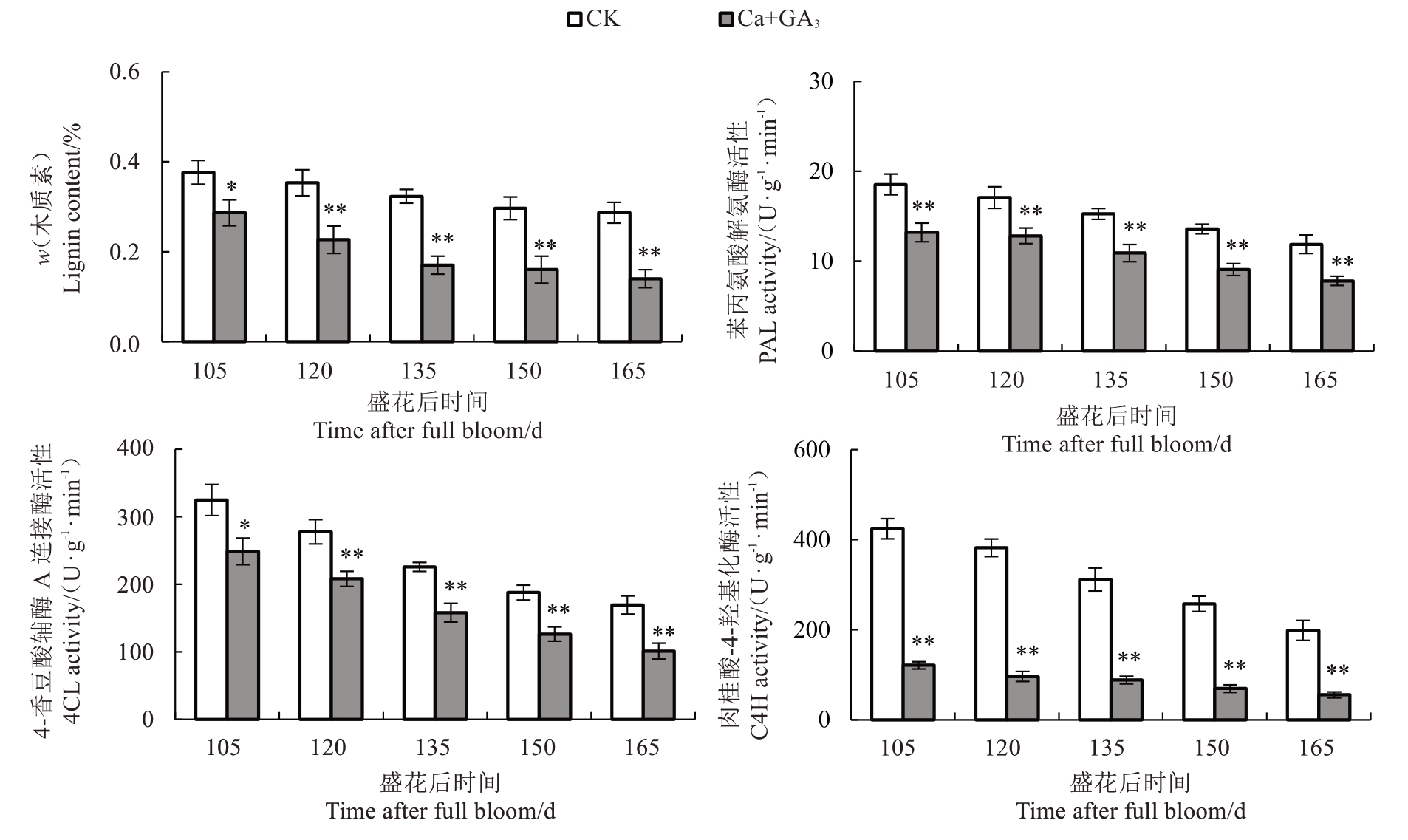
由图5 所示,芦柑果皮中纤维素含量在盛花后105~165 d 期间呈缓慢下降的趋势,Ca+GA3处理的果皮纤维素含量始终高于CK。Ca+GA3处理可以有效地增加芦柑果皮的纤维素含量。随着果实的生长发育,芦柑果皮中CX 活性逐渐上升。在整个生长发育期,Ca+GA3处理的果皮CX 活性明显降低,并达到极显著差异。说明Ca+GA3处理可以有效地降低芦柑果皮的CX活性。
随着果实的生长发育,芦柑果皮的木质素含量呈下降的趋势,在盛花后105~165 d 期间Ca+GA3处理的果皮木质素含量均低于CK。作为木质素合成中的关键酶,芦柑果皮PAL、4CL和C4H活性在整个生长发育期间均呈下降趋势。说明Ca+GA3处理果皮能够有效降低木质素合成关键酶的活性(图6)。
2.5 Ca+GA3处理对芦柑果皮抗氧化酶和细胞壁代谢酶相关基因表达的影响
如图7 所示,Ca+GA3处理后芦柑果皮抗氧化酶相关基因CrSOD、CrCAT 表达量显著上调,而CrPOD、CrPPO 显著下调。在细胞壁代谢酶相关基因中,果胶降解酶基因CrPME、CrPL、CrPG 和纤维素酶基因CrCX 以及木质素合成酶基因CrPAL、Cr4CL、CrC4H 在Ca+GA3处理后表达量明显下调,呈极显著差异。这些结果表明Ca+GA3处理后芦柑果皮抗氧化酶、细胞壁代谢酶相关基因的表达量发生改变。
3 讨 论
裂果是果实发育过程中的一种生理性病害,受果皮发育过程中细胞多种生理生化代谢的调控。Ca 能提高细胞耐压力、延展性,降低裂果的发生率[2]。GA3能维持稳定的果皮细胞层次,增强细胞之间的连接性[8]。因此外源Ca和GA3协同作用对减缓芦柑裂果效果更佳。当前,芦柑裂果相关的研究多集中在果形指数、果实大小[41-42]以及一些生理代谢酶活性[16,29]方面,但总体研究还不够深入和完整。因此,笔者在本试验中系统地研究了Ca+GA3处理对芦柑果实活性氧代谢和细胞壁代谢的影响,以揭示其缓解芦柑裂果的生理和分子机制。
果实外观品质和果皮组织结构与果实抗裂能力密切相关。与易裂番茄相比,不易裂品种番茄的角质层更厚[15]。开裂柠檬[C. ×limon(L.)Osbeck.]的果皮厚度较薄[43],红江橙[C.sinensis(L.)Osbeck.]的裂果果形指数小,果皮通常较薄、弹性差且硬度低[42]。Ca 作为细胞壁的重要组分,能增加细胞壁强度,改变果皮的组织结构,增强果皮伸长性和破裂应力[44]。有报道指出外源钙和EBR 处理下番茄果实的单果质量、横径和纵径均显著高于对照[45]。在对枣(Ziziphus jujuba Mill.)[46]和樱桃[Cerasus pseudoc‐erasus(Lindl.)G.Don.][7]的研究中发现,GA3处理增加了果皮角质层的厚度,从而降低了裂果率。笔者在本研究中发现,Ca+GA3处理增加了芦柑果皮厚度和果形指数,显著降低了芦柑裂果率。
果皮活性氧代谢异常是果实开裂的重要原因。前人研究报道,易裂果番茄果皮的H2O2、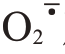 产生速率和MDA 含量以及膜脂过氧化程度均高于耐裂果番茄[47]。在对枇杷的研究中发现,裂果果皮中的POD 活性高于正常果,而SOD、CAT 活性则相反[16]。易裂俊枣果皮POD活性高于不易裂枣[48]。崔守尧等[49]研究发现,CaCl2处理可明显降低易裂果番茄品种的裂果率,降低果皮中H2O2和MDA 含量。另有研究指出,Ca处理后降低了蜜广橘果皮POD活性和基因表达量,提高了SOD、CAT 活性和相关基因表达水平[18]。GA3 处理延缓了冬枣果皮H2O2、MDA 和
产生速率和MDA 含量以及膜脂过氧化程度均高于耐裂果番茄[47]。在对枇杷的研究中发现,裂果果皮中的POD 活性高于正常果,而SOD、CAT 活性则相反[16]。易裂俊枣果皮POD活性高于不易裂枣[48]。崔守尧等[49]研究发现,CaCl2处理可明显降低易裂果番茄品种的裂果率,降低果皮中H2O2和MDA 含量。另有研究指出,Ca处理后降低了蜜广橘果皮POD活性和基因表达量,提高了SOD、CAT 活性和相关基因表达水平[18]。GA3 处理延缓了冬枣果皮H2O2、MDA 和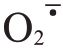 积累,提高了CAT 活性,延缓了组织受活性氧的伤害时间[50]。值得注意的是,POD 在氧化还原反应过程中具有多种功能。一方面,它在NAD(P)H 的帮助下,催化H2O2与
积累,提高了CAT 活性,延缓了组织受活性氧的伤害时间[50]。值得注意的是,POD 在氧化还原反应过程中具有多种功能。一方面,它在NAD(P)H 的帮助下,催化H2O2与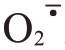 生成羟基自由基(·OH),而·OH 是一种高活性分子,已被证实在非酶促反应中促进细胞壁多糖的裂解[51-53]。另一方面,POD 又与H2O2反应,将酚类物质转化为苯氧自由基(Phe·)。Phe·随后受化学氧化和交叉耦合影响,生成木质素,并在细胞壁结构组分(结构蛋白、半纤维素和果胶)之间形成酚醛交联,导致细胞壁硬化并降低其延展性[54-56]。在逆境胁迫下,植物细胞膜质过氧化,生物膜系统完整性受损,细胞质中的PPO与液泡中酚类物质接触会引发褐变,产生醌类有害物质,从而损伤细胞[57],这与裂果部位常常出现棕褐色颜色变化密切相关。GA3处理后降低了枣果果皮PPO 活性,有效抑制枣果果皮酶促褐变和减轻细胞损伤[58]。易裂的番荔枝中PPO 基因表达量明显增高[19]。笔者在本研究中发现Ca+GA3处理能降低果皮H2O2含量和
生成羟基自由基(·OH),而·OH 是一种高活性分子,已被证实在非酶促反应中促进细胞壁多糖的裂解[51-53]。另一方面,POD 又与H2O2反应,将酚类物质转化为苯氧自由基(Phe·)。Phe·随后受化学氧化和交叉耦合影响,生成木质素,并在细胞壁结构组分(结构蛋白、半纤维素和果胶)之间形成酚醛交联,导致细胞壁硬化并降低其延展性[54-56]。在逆境胁迫下,植物细胞膜质过氧化,生物膜系统完整性受损,细胞质中的PPO与液泡中酚类物质接触会引发褐变,产生醌类有害物质,从而损伤细胞[57],这与裂果部位常常出现棕褐色颜色变化密切相关。GA3处理后降低了枣果果皮PPO 活性,有效抑制枣果果皮酶促褐变和减轻细胞损伤[58]。易裂的番荔枝中PPO 基因表达量明显增高[19]。笔者在本研究中发现Ca+GA3处理能降低果皮H2O2含量和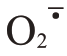 产生速率,提升SOD、CAT 活性,降低了POD、PPO 活性,从而限制了羟基自由基和苯氧自由基生成以及细胞氧化褐变损伤,减缓了对芦柑果皮韧性和硬度的影响。
产生速率,提升SOD、CAT 活性,降低了POD、PPO 活性,从而限制了羟基自由基和苯氧自由基生成以及细胞氧化褐变损伤,减缓了对芦柑果皮韧性和硬度的影响。
果皮细胞壁代谢改变与果实开裂紧密相关。果胶和纤维素等细胞壁多糖的降解影响果皮的韧性、黏附性和硬度,造成果皮和果肉的开裂[59]。而细胞壁多糖的降解又与细胞壁代谢酶活性相关。枣的裂果率与原果胶和纤维素含量呈显著负相关[44]。果皮细胞壁水解酶活性越高,细胞壁多糖降解越快,果实更容易开裂。Ca2+能促进果胶酸钙的形成,而果胶酸钙有利于维持细胞壁结构的完整及稳定,提高果皮的机械强度,减少裂果的发生[60-61]。在对葡萄[4]、苹果(Malus pumila Mill.)[62]的研究中发现,钙处理降低了PG、CX活性,使果皮原果胶和纤维素降解减缓。GA3可以通过调节果胶的甲酯化,改变Ca2+桥形成相互交联的多聚体影响细胞壁结构,从而影响PME、PG 等活性[63]。喷施GA3 可以降低柑橘果皮PME和PG活性[64]。在对葡萄的研究中发现,钙处理后裂果率降低,PME、PG、PL、CX活性和相关基因表达量会下调[65]。交联的酚类化合物和木质素沉积会导致细胞壁变硬,降低细胞壁的延展性,制约果皮的生长。果实进入膨大期,内部果肉生长速度远大于细胞壁的延展速度,造成果皮开裂。研究砂梨(Py‐rus pyrifolia Nakai.)果皮性状形成机制时发现木质素的增加能引起果皮的异常加厚,促进果皮的木质化,降低果皮的韧性[66]。严重裂果的辣椒木质素含量和木质素合成酶基因PAL、4CL 的表达量均显著高于不裂果果实[36]。在本研究中,笔者发现Ca+GA3处理降低了芦柑果皮PME、PL、PG、CX 活性,减缓了果胶和纤维素的降解。同时,降低了芦柑果皮中PAL、4CL、C4H 活性,从而抑制了木质素合成,提高了果皮的韧性和延展性,降低了芦柑裂果率。
4 结 论
Ca+GA3处理降低芦柑果皮中H2O2含量、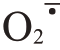 产生速率和MDA 含量,提高抗氧化酶SOD、CAT 活性,降低POD、PPO 活性,增强活性氧的清除能力。同时,Ca+GA3处理降低果皮果胶降解酶PME、PL、PG 和纤维素酶CX,以及木质素合成酶PAL、4CL、C4H 等细胞壁代谢酶的活性,防止细胞壁中果胶、纤维素的降解,以及木质素的过多积累,从而提高了果皮的韧性和延展性,显著降低了裂果率。qRTPCR分析证实,Ca+GA3处理下芦柑果皮抗氧化酶和细胞壁代谢酶相关基因的差异表达与相关酶活性以及代谢产物积累相一致。在生理和分子水平上,系统地揭示了Ca+GA3处理下芦柑果实生长发育过程中果皮活性氧代谢、细胞壁代谢与裂果的关系,为进一步深入解析芦柑裂果机制奠定了理论基础,并明确了芦柑果面喷施Ca+GA3是预防和减轻裂果发生的有效生产措施。
产生速率和MDA 含量,提高抗氧化酶SOD、CAT 活性,降低POD、PPO 活性,增强活性氧的清除能力。同时,Ca+GA3处理降低果皮果胶降解酶PME、PL、PG 和纤维素酶CX,以及木质素合成酶PAL、4CL、C4H 等细胞壁代谢酶的活性,防止细胞壁中果胶、纤维素的降解,以及木质素的过多积累,从而提高了果皮的韧性和延展性,显著降低了裂果率。qRTPCR分析证实,Ca+GA3处理下芦柑果皮抗氧化酶和细胞壁代谢酶相关基因的差异表达与相关酶活性以及代谢产物积累相一致。在生理和分子水平上,系统地揭示了Ca+GA3处理下芦柑果实生长发育过程中果皮活性氧代谢、细胞壁代谢与裂果的关系,为进一步深入解析芦柑裂果机制奠定了理论基础,并明确了芦柑果面喷施Ca+GA3是预防和减轻裂果发生的有效生产措施。
参考文献References:
[1] GONG Y,YANG Y T,LI L,ZENG X,BIE Y H,MAO H Q,WANG L X Y,XIONG B. Study on the effect difference of two kinds of control techniques on cracking fruit of Qingcui plum[J].IOP Conference Series:Earth and Environmental Science,2021,792(1):012042.
[2] ZHU M T,YU J,WANG R,ZENG Y X,KANG L F,CHEN Z Y. Nano-calcium alleviates the cracking of nectarine fruit and improves fruit quality[J]. Plant Physiology and Biochemistry,2023,196:370-380.
[3] NIE G W,LI K,TIAN Y Q,DAI G L,YANG X H,SONG Y H,LI J J,ZHANG X P,LÜ J G. Correlation analysis between cracking and mineral element content of apricot fruit[J].Agricul‐tural Science&Technology,2017,18(10):1852-1855.
[4] SHI H,ZHOU X Y,QIN M L,WANG W L,HE X E,ZHOU W H. Effect of CaCl2 sprays in different fruit development stages on grape berry cracking[J].Frontiers in Plant Science,2022,13:870959.
[5] DROGOUDI P,PANTELIDIS G E. Comparative effects of gib‐berellin A3,glycine betaine,and Si,Ca,and K fertilizers on physi‐ological disorders and yield of pomegranate cv. Wonderful[J].Journal of the Science of Food and Agriculture,2022,102(1):259-267.
[6] PHAM T T M,SINGH Z,BEHBOUDIAN M H. Different sur‐factants improve calcium uptake into leaf and fruit of‘Washing‐ton navel’sweet orange and reduce albedo breakdown[J]. Jour‐nal of Plant Nutrition,2012,35(6):889-904.
[7] CORREIA S,SANTOS M,GLIŃSKA S,GAPIŃSKA M,MA‐TOS M,CARNIDE V,SCHOUTEN R,SILVA A P,GONÇALVES B. Effects of exogenous compound sprays on cherry cracking:Skin properties and gene expression[J]. Journal of the Science of Food and Agriculture,2020,100(7):2911-2921.
[8] YILMAZ C,ÖZGÜVEN A I. The effects of some plant nutri‐ents,gibberellic acid and pinolene treatments on the yield,fruit quality and cracking in pomegranate[J].Acta Horticulturae Sini‐ca,2009,818:205-212.
[9] 李永杰,金国强,淳长品,朱潇婷,邱晓莹.柑橘果皮的发育特征及GA3的防裂效果[J].果树学报,2021,38(7):1092-1101.LI Yongjie,JIN Guoqiang,CHUN Changpin,ZHU Xiaoting,QIU Xiaoying. Developmental characteristics of citrus peel and the effect of gibberellic acid on fruit cracking[J].Journal of Fruit Science,2021,38(7):1092-1101.
[10] 叶正文,叶兰香,张学英.‘朋娜’等脐橙的裂果规律及赤霉素防裂效果[J].上海农业学报,2002,18(4):52-57.YE Zhengwen,YE Lanxiang,ZHANG Xueying.The fruit crack‐ing rules of navel orange varieties such as‘Pengna’and the ef‐fect of gibberellin (GA) preventing fruits from cracking[J].Acta Agriculturae Shanghai,2002,18(4):52-57.
[11] ZAHEDI S M,HOSSEINI M S,MEYBODI N D H,ABADÍA J,GERM M,GHOLAMI R,ABDELRAHMAN M. Evaluation of drought tolerance in three commercial pomegranate cultivars us‐ing photosynthetic pigments,yield parameters and biochemical traits as biomarkers[J]. Agricultural Water Management,2022,261:107357.
[12] ZHANG C,ZHAO Y J,JIANG F L,WU Z,CUI S Y,LV H M,YU L. Differences of reactive oxygen species metabolism in top,middle and bottom part of epicarp and mesocarp influence tomato fruit cracking[J]. The Journal of Horticultural Science and Biotechnology,2020,95(6):746-756.
[13] JIANG Y L,YIN H,WANG D F,ZHONG Y,DENG Y. Explor‐ing the mechanism of Akebia trifoliata fruit cracking based on cell- wall metabolism[J]. Food Research International,2022,157:111219.
[14] XUE L Z,SUN M T,WU Z,YU L,YU Q H,TANG Y P,JIANG F L. LncRNA regulates tomato fruit cracking by coordinating gene expression via a hormone- redox- cell wall network[J].BMC Plant Biology,2020,20(1):162.
[15] YANG Z E,WU Z,ZHANG C,HU E M,ZHOU R,JIANG F L.The composition of pericarp,cell aging,and changes in water absorption in two tomato genotypes:Mechanism,factors,and potential role in fruit cracking[J].Acta Physiologiae Plantarum,2016,38(9):215.
[16] 王引,倪海枝,颜帮国,陈方永.白沙枇杷裂果的钙素调控生理研究[J].果树学报,2022,39(5):826-835.WANG Yin,NI Haizhi,YAN Bangguo,CHEN Fangyong. Influ‐ence of calcium on fruit cracking and its physiological mecha‐nism in white flesh loquats[J].Journal of Fruit Science,2022,39(5):826-835.
[17] 李建国,黄旭明,黄辉白.裂果易发性不同的荔枝品种果皮中细胞壁代谢酶活性的比较[J]. 植物生理与分子生物学学报,2003,29(2):141-146.LI Jianguo,HUANG Xuming,HUANG Huibai. Comparison of the activities of enzymes related to cell-wall metabolism in peri‐carp between litchi cultivars susceptible and resistant to fruit cracking[J].Journal of Plant Physiology and Molecular Biology,2003,29(2):141-146.
[18] 张蓓,刘林婷,刘若南,高志键,葛聪,周秋蓉,李江波,李延,王平.钙与IAA 对易裂果蜜广橘果皮活性氧代谢和相关抗氧化基因表达的影响[J].果树学报,2021,38(12):2034-2044.ZHANG Bei,LIU Linting,LIU Ruonan,GAO Zhijian,GE Cong,ZHOU Qiurong,LI Jiangbo,LI Yan,WANG Ping. Ef‐fects of calcium and IAA treatments on active oxygen metabo‐lism and related antioxidant gene expressions in the peel of cracking sensitive fruit of Miguang tangerine[J].Journal of Fruit Science,2021,38(12):2034-2044.
[19] 陈晶晶,段雅婕,莫亿伟,胡玉林,胡会刚,谢江辉.裂果性不同的番荔枝品种果皮中细胞壁代谢相关基因的表达分析[J].果树学报,2015,32(5):769-776.CHEN Jingjing,DUAN Yajie,MO Yiwei,HU Yulin,HU Hui‐gang,XIE Jianghui.Expression analysis of cell wall metabolism gene in pericarp of custard apple cultivars with different fruit cracking characters[J]. Journal of Fruit Science,2015,32(5):769-776.
[20] RONGPIPI S,YE D,GOMEZ E D,GOMEZ E W. Progress and opportunities in the characterization of cellulose-an important regulator of cell wall growth and mechanics[J]. Frontiers in Plant Science,2019,9:1894.
[21] PEDROSA L F,RAZ A,FABI J P. The complex biological ef‐fects of pectin:Galectin-3 targeting as potential human health improvement?[J].Biomolecules,2022,12(2):289.
[22] KINNAERT C,DAUGAARD M,NAMI F,CLAUSEN M H.Chemical synthesis of oligosaccharides related to the cell walls of plants and algae[J].Chemical Reviews,2017,117(17):11337-11405.
[23] ROSE J K,COSGROVE D J,ALBERSHEIM P,DARVILL A G,BENNETT A B. Detection of expansin proteins and activity during tomato fruit ontogeny[J].Plant Physiology,2000,123(4):1583-1592.
[24] GIOVANNONI J.Molecular biology of fruit maturation and rip‐ening[J].Annual Review of Plant Physiology and Plant Molecu‐lar Biology,2001,52:725-749.
[25] WANG D D,YEATS T H,ULUISIK S,ROSE J K C,SEY‐MOUR G B. Fruit softening:Revisiting the role of pectin[J].Trends in Plant Science,2018,23(4):302-310.
[26] WANG X,LIN L J,TANG Y,XIA H,ZHANG X C,YUE M L,QIU X,XU K,WANG Z H. Transcriptomic insights into citrus segment membrane’s cell wall components relating to fruit sen‐sory texture[J].BMC Genomics,2018,19(1):280.
[27] SHEN Y H,LU B G,FENG L,YANG F Y,GENG J J,MING R,CHEN X J.Isolation of ripening-related genes from ethylene/1-MCP treated papaya through RNA-seq[J]. BMC Genomics,2017,18(1):671.
[28] 刘鹿宁,赵秋月,葛聪,田志娇,周晓俐,阮翥龙,庄木来,李延,王平.木质素生物合成途径相关基因调控琯溪蜜柚汁胞粒化的研究[J].果树学报,2023,40(3):432-441.LIU Luning,ZHAO Qiuyue,GE Cong,TIAN Zhijiao,ZHOU Xiaoli,RUAN Zhulong,ZHUANG Mulai,LI Yan,WANG Ping.The genes related to lignin biosynthesis pathway regulate juice sac granulation in Guanxi pomelo[J]. Journal of Fruit Science,2023,40(3):432-441.
[29] 李伟明,陈晶晶,段雅婕,胡会刚,庞振才,胡玉林.番荔枝果实后熟过程多糖代谢与果实软化和采后裂果的关系[J].植物生理学报,2018,54(11):1727-1736.LI Weiming,CHEN Jingjing,DUAN Yajie,HU Huigang,PANG Zhencai,HU Yulin. Effects of anti-ethylene treatments on poly‐saccharide metabolism,fruit softening and their relationship with postharvest cracking of atemoya fruit[J]. Plant Physiology Journal,2018,54(11):1727-1736.
[30] 吴维林,张蓓,田志娇,杜茜,黄嘉康,李江波,李延,王平.Ca+IAA对易裂果蜜广橘果皮细胞壁成分及水解酶活性的影响[J/OL].分子植物育种,2023:1-14(2023-07-21).https://kns.cnki.net/kc‐ms/detail/46.1068.S.20230721.1453.004.html.WU Weilin,ZHANG Bei,TIAN Zhijiao,DU Xi,HUANG Jiakang,LI Jiangbo,LI Yan,WANG Ping. Effects of Ca +IAA treatment on cell wall components and hydrolase activities in the peel of cracking sensitive fruit of miguang tangerine[J/OL].Molecular Plant Breeding,2023:1-14(2023-07-21). https://kns.cnki.net/kcms/detail/46.1068.S.20230721.1453.004.html.
[31] 苏敬,乜兰春,齐迎斌,王苗苗.番茄叶面喷施硅和钙对果实硬度及相关生理代谢的影响[J].园艺学报,2016,43(4):789-795.SU Jing,NIE Lanchun,QI Yingbin,WANG Miaomiao. Effect of foliar application of silicon and calcium on the firmness and related physiological metabolism of tomato fruits[J].Acta Horti‐culturae Sinica,2016,43(4):789-795.
[32] 周鹤莹,张玮,张卿,沈元月,秦岭,邢宇. 森林草莓‘Ruegen’果胶裂解酶基因的克隆及荧光定量表达分析[J]. 园艺学报,2015,42(3):455-461.ZHOU Heying,ZHANG Wei,ZHANG Qing,SHEN Yuanyue,QIN Ling,XING Yu. The cloning and quantitative expression analysis of pectate lyase gene in Fragaria vesca[J]. Acta Horti‐culturae Sinica,2015,42(3):455-461.
[33] MARTINS V,GARCIA A,ALHINHO A T,COSTA P,LANCEROS-MÉNDEZ S,COSTA M M R,GERÓS H. Vine‐yard calcium sprays induce changes in grape berry skin,firm‐ness,cell wall composition and expression of cell wall-related genes[J].Plant Physiology and Biochemistry,2020,150:49-55.
[34] 仲钊江,吴震,周蓉,朱为民,杨学东,于筱薇,徐艳,高扬杨,蒋芳玲.番茄果胶裂解酶基因SlPL 参与调控裂果机制研究[J].园艺学报,2024,51(2):295-308.ZHONG Zhaojiang,WU Zhen,ZHOU Rong,ZHU Weimin,YANG Xuedong,YU Xiaowei,XU Yan,GAO Yangyang,JIANG Fangling. Study on the regulatory mechanism of SlPL gene af‐fecting tomato fruit cracking[J]. Acta Horticulturae Sinica,2024,51(2):295-308.
[35] 怀斌.钙影响‘红江橙’果皮陷痕发生及机理的研究[D].广州:华南农业大学,2016.HUAI Bin.The effect of calcium on fruit creasing of‘Hongjiang’orange[D]. Guangzhou:South China Agricultural University,2016.
[36] LIU Y L,CHEN S Y,LIU G T,JIA X Y,HAQ S U,DENG Z J,LUO D X,LI R,GONG Z H. Morphological,physiochemical,and transcriptome analysis and CaEXP4 identification during pepper (Capsicum annuum L.) fruit cracking[J]. Scientia Horti‐culturae,2022,297:110982.
[37] WANG Y S,TIAN S P,XU Y,QIN G Z,YAO H J. Changes in the activities of pro- and anti-oxidant enzymes in peach fruit in‐oculated with Cryptococcus laurentii or Penicillium expansum at 0 or 20 ℃[J]. Postharvest Biology and Technology,2004,34(1):21-28.
[38] JIANG A L,TIAN S P,XU Y. Effects of controlled atmo‐spheres with high-O2 or high-CO2 concentrations on postharvest physiology and storability of‘Napoleon’sweet cherry[J]. Jour‐nal of Integrative Plant Biology,2002,44(8):925-930.
[39] 曹建康,姜微波,赵玉梅.果蔬采后生理生化实验指导[M].北京:中国轻工业出版社,2007.CAO Jiankang,JIANG Weibo,ZHAO Yumei. Experiment guid‐ance of fostharvest physiology and biochemistry of fruits and vegetables[M].Beijing:China Light Industry Press,2007.
[40] FUKUSHIMA R S,KERLEY M S. Use of lignin extracted from different plant sources as standards in the spectrophotometric acetyl bromide lignin method[J]. Journal of Agricultural and Food Chemistry,2011,59(8):3505-3509.
[41] RICHARDSON A C,MARSH K B,MACRAE E A. Tempera‐ture effects on satsuma mandarin fruit development[J]. Journal of Horticultural Science,1997,72(6):919-929.
[42] 高飞飞,黄辉白,许建楷.红江橙裂果原因的探讨[J].华南农业大学学报,1994,15(1):34-39.GAO Feifei,HUANG Huibai,XU Jiankai. Exploration of the causes of fruit cracking in‘Hongjiang’orange[J]. Journal of South China Agricultural University,1994,15(1):34-39.
[43] KAUR R,KAUR N,SINGH H. Pericarp and pedicel anatomy in relation to fruit cracking in lemon (Citrus limon L.Burm.)[J].Scientia Horticulturae,2019,246:462-468.
[44] 郭红彦,白晋华,段风琴,郗鑫,李涛,郭晋平.钙处理对‘壶瓶枣’裂果细胞壁降解酶活性及组织结构的影响[J].园艺学报,2019,46(8):1486-1494.GUO Hongyan,BAI Jinhua,DUAN Fengqin,XI Xin,LI Tao,GUO Jinping. Effect of CaCl2 treatment on cell wall degrading enzymes activities and microstructure of fruit cracking of Ziziphus jujuba‘Huping Zao’[J]. Acta Horticulturae Sinica,2019,46(8):1486-1494.
[45] 姚棋,韩天云,梁祎,石玉,侯雷平,张毅.外源钙和EBR 处理对番茄果实品质特性的影响[J].中国瓜菜,2021,34(10):74-79.YAO Qi,HAN Tianyun,LIANG Yi,SHI Yu,HOU Leiping,ZHANG Yi. Effects of exogenous calcium and EBR on fruit quality characteristics of tomato[J]. China Cucurbits and Vegeta‐bles,2021,34(10):74-79.
[46] OZTURK B,BEKTAS E,AGLAR E,KARAKAYA O,GUN S F. Cracking and quality attributes of jujube fruits as affected by covering and pre-harvest Parka and GA3 treatments[J]. Scientia Horticulturae,2018,240:65-71.
[47] 张川,王亚晨,崔守尧,杨泽恩,吴震,蒋芳玲.耐裂果与易裂果番茄果实发育过程中果实组织衰老与裂果的关系[J].南京农业大学学报,2016,39(4):534-542.ZHANG Chuan,WANG Yachen,CUI Shouyao,YANG Zeen,WU Zhen,JIANG Fangling. The relationship between fruit tis‐sue senescence and fruit cracking in cracking-resistant and sus‐ceptible tomato during fruit ripening[J].Journal of Nanjing Agri‐cultural University,2016,39(4):534-542.
[48] 曹一博,李长江,孙帆,张凌云.抗裂与易裂枣内源激素含量和细胞壁代谢相关酶活性比较[J]. 园艺学报,2014,41(1):139-148.CAO Yibo,LI Changjiang,SUN Fan,ZHANG Lingyun. Com‐parison of the endogenous hormones content and the activities of enzymes related to cell-wall metabolism between jujube culti‐vars susceptible and resistant to fruit cracking[J].Acta Horticul‐turae Sinica,2014,41(1):139-148.
[49] 崔守尧,吴震,吕海萌,薛灵姿,蒋芳玲.外源CaCl2 缓解番茄裂果的生理机制[J].南京农业大学学报,2019,42(1):59-65.CUI Shouyao,WU Zhen,LÜ Haimeng,XUE Lingzi,JIANG Fangling. The physiological mechanism of exogenous CaCl2 re‐lieving tomato fruit cracking[J]. Journal of Nanjing Agricultural University,2019,42(1):59-65.
[50] 李红卫,韩涛,李丽萍,冯双庆,赵玉梅.ABA、GA3处理对冬枣采后果肉活性氧代谢的影响[J]. 园艺学报,2005,32(5):793-797.LI Hongwei,HAN Tao,LI Liping,FENG Shuangqing,ZHAO Yumei. Effect of ABA and GA3 treatments on the metabolism of active oxygen species in cold stored‘Brumal jujube’flesh[J].Acta Horticulturae Sinica,2005,32(5):793-797.
[51] FRY S C. Oxidative scission of plant cell wall polysaccharides by ascorbate- induced hydroxyl radicals[J]. The Biochemical Journal,1998,332(Pt 2):507-515.
[52] CHEN S X,SCHOPFER P.Hydroxyl-radical production in phys‐iological reactions.A novel function of peroxidase[J]. European Journal of Biochemistry,1999,260(3):726-735.
[53] LISZKAY A,KENK B,SCHOPFER P.Evidence for the involve‐ment of cell wall peroxidase in the generation of hydroxyl radi‐cals mediating extension growth[J]. Planta,2003,217(4):658-667.
[54] VANHOLME R,DEMEDTS B,MORREEL K,RALPH J,BOERJAN W. Lignin biosynthesis and structure[J]. Plant Physi‐ology,2010,153(3):895-905.
[55] CAMPA A. Biological roles of plant peroxidases:Known and potential function[M]//EVERSE J,GRISHAM M B. Peroxidas‐es in Chemistry and Biology. New York:CRC Press,1991:25-50.
[56] JIANG F L,LOPEZ A,JEON S,DE FREITAS S T,YU Q H,WU Z,LABAVITCH J M,TIAN S K,POWELL A L T,MIT‐CHAM E. Disassembly of the fruit cell wall by the ripening-as‐sociated polygalacturonase and expansin influences tomato cracking[J].Horticulture Research,2019,6:17.
[57] BOONSIRI K,KETSA S,VAN DOORN W G. Seed browning of hot peppers during low temperature storage[J]. Postharvest Biology and Technology,2007,45(3):358-365.
[58] 薛梦林,张平,张继澍,王莉.‘脆枣’采后赤霉素处理对其生理生化的影响[J].园艺学报,2003,30(2):147-151.XUE Menglin,ZHANG Ping,ZHANG Jishu,WANG Li. Effect of postharvest treatment with GA3 on physiological and bio‐chemical changes of‘Cui jujube’fruit during cold storage[J].Acta Horticulturae Sinica,2003,30(2):147-151.
[59] ABELES F B,TAKEDA F. Cellulase activity and ethylene in ripening strawberry and apple fruits[J]. Scientia Horticulturae,1990,42(4):269-275.
[60] SILVEIRA A C,AGUAYO E,CHISARI M,ARTÉS F. Calcium salts and heat treatment for quality retention of fresh-cut‘Ga‐lia’melon[J]. Postharvest Biology and Technology,2011,62(1):77-84.
[61] WHITE P J,BROADLEY M R. Calcium in plants[J].Annals of Botany,2003,92(4):487-511.
[62] BLANCO A,FERNÁNDEZ V,VAL J. Improving the perfor‐mance of calcium-containing spray formulations to limit the inci‐dence of bitter pit in apple(Malus×domestica Borkh.)[J].Scien‐tia Horticulturae,2010,127(1):23-28.
[63] 陈光辉,高艳,陈秀娟,谢丽琼.植物激素在植物细胞壁扩展中的作用[J].生命的化学,2012,32(5):464-470.CHEN Guanghui,GAO Yan,CHEN Xiujuan,XIE Liqiong. The role of phytohormones in plant cell wall expansion[J]. Chemis‐try of Life,2012,32(5):464-470.
[64] EL-HAMMADY A,ABDEL-HAMID N,SALEH M,SALAH A. Effect of gibberellic acid and calcium chloride treatments on delaying maturity,quality and storability of‘Balady’mandarin fruits[J]. Arab Universities Journal of Agricultural Sciences,2000,8:755-766.
[65] 余俊.葡萄裂果及其外源钙调控机理研究[D].长沙:湖南农业大学,2020.YU Jun. Research on the mechanism of fruit cracking and its regulation by exogenous calcium in Vitis vinifera[D].Changsha:Hunan Agricultural University,2020.
[66] 施泽彬. 砂梨果皮性状形成机制研究[D]. 南京:南京农业大学,2011.SHI Zebin. The mechanisms of fruit appearance characters for‐mation in sand pear (Pyrus pyrifolia Nakai.)[D]. Nanjing:Nan‐jing Agricultural University,2011.
Physiological and molecular mechanism of Ca+GA3 treatment alleviating fruit cracking incidence in ponkan
WU Weilin1, ZHOU Qiurong1#, HUANG Jiakang1, REN Huaqing1, CHEN Yiran1, ZHANG Jiacheng1,DU Xi1,ZHANG Yuping2,WANG Ping1*
(1College of Horticulture, Fujian Agriculture and Forestry University/Key Laboratory of Ministry of Education for Genetics, Breeding and Multiple Utilization of Crops, Fuzhou 350002, Fujian, China;2Tianma Citrus Farm, Yongchun County, Fujian Province, Yongchun 362600,Fujian,China)
Abstract: 【Objective】Fruit cracking is a physiological disease, which seriously affects the appearance and quality of fruit. Ponkan fruit cracking rate can be as high as 70% in serious cases, which has be‐come a major problem in the development of the ponkan production.Fruit cracking is regulated by mul‐tiple cell physiological and biochemical metabolisms during peel development. Therefore, the physio‐logical and molecular mechanisms of ponkan fruit cracking was investigated in order to provide theoret‐ical basis for the prevention and alleviation of the fruit cracking in production.【Methods】In this study,10-year-old early-maturing ponkan fruit (cracking-susceptible cultivar) in Tianma Citrus Farm in Yong‐chun County, Fujian, China, were used as the experimental material. Eighteen trees with relatively con‐sistent nutrition level and growth state under conventional cultivation management were selected for the experiment.At the expansion period of ponkan fruit in June and July, the fruit sprayed with 0.3 g·L-1 chelated calcium and 10 mg·L-1 GA3 (Ca+GA3) were used as the treatment group, and the fruit sprayed with water as the control(CK).Ponkan fruit started to crack on August 15,2022,and the fruit were col‐lected at 15 d intervals (105, 120, 135, 150 and 165 d after full bloom).The fruit cracking rate and ap‐pearance quality were investigated, including transverse diameter, vertical diameter, fruit shape index,single fruit weight and peel thickness. In addition, H2O2 and MDA contents, and 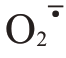 production rate as well as antioxidant enzyme activities(SOD,CAT,POD,and PPO)in ponkan peel were determined.Fur‐thermore, the cell wall metabolic enzyme activities (PME, PL, PG, CX, PAL, 4CL, and C4H) and the structural components (protopectin, soluble pectin, cellulose, and lignin) were measured. And the ex‐pression of the related genes of antioxidant enzymes and cell wall metabolic enzymes in the peel were analyzed.【Results】Ca+GA3 treatment significantly reduced the cracking rate of ponkan fruit. The transverse diameter and vertical diameter of the fruit increased, and fruit shape index, single fruit weight and top peel thickness in Ca+GA3 treatment were greater than those of CK.Abnormal metabo‐lism of ROS is one of the most important causes of fruit cracking. During the period of 105-165 days after full bloom,the contents of H2O2,MDA and the rate of
production rate as well as antioxidant enzyme activities(SOD,CAT,POD,and PPO)in ponkan peel were determined.Fur‐thermore, the cell wall metabolic enzyme activities (PME, PL, PG, CX, PAL, 4CL, and C4H) and the structural components (protopectin, soluble pectin, cellulose, and lignin) were measured. And the ex‐pression of the related genes of antioxidant enzymes and cell wall metabolic enzymes in the peel were analyzed.【Results】Ca+GA3 treatment significantly reduced the cracking rate of ponkan fruit. The transverse diameter and vertical diameter of the fruit increased, and fruit shape index, single fruit weight and top peel thickness in Ca+GA3 treatment were greater than those of CK.Abnormal metabo‐lism of ROS is one of the most important causes of fruit cracking. During the period of 105-165 days after full bloom,the contents of H2O2,MDA and the rate of 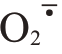 production in ponkan peel showed a gen‐eral trend of increasing and then decreasing and reached a peak at 135 d after full bloom.Ca+GA3 treat‐ment reduced the degree of lipid peroxidation in cell membrane of the peel.The activities of antioxidant enzymes SOD and CAT in ponkan peel peaked at 135 d after full bloom, which was significantly high‐er,while POD and PPO activities significantly lower in Ca+GA3 treatment than in CK.The results indi‐cated that Ca+GA3 treatment increased SOD and CAT activities,and decreased POD and PPO activities in ponkan peel.The higher activities of peel cell wall hydrolases, the faster the cell wall polysaccharide degradation, and the higher fruit cracking incidence. The Ca+GA3 treatment resulted in a consistently higher protopectin and lower soluble pectin contents in ponkan peel than those of CK. In addition,PME,PL and PG in ponkan peel were significantly reduced after Ca+GA3 treatment.The results indicat‐ed that Ca+GA3 treatment reduced the rate of degradation of protopectin into soluble pectin.The content of cellulose in ponkan peel decreased slowly during 105-165 days after full bloom. Ca+GA3 treatment effectively increased the content of cellulose in ponkan peel, while decreased CX activity. Lignin con‐tent in ponkan peel treated with Ca+GA3 was lower than that of CK during the period of 105-165 d after full bloom, and the activities of PAL, 4CL, and C4H, key enzymes in lignin synthesis, decreased in the peel throughout the whole period of growth and development of ponkan fruit.These results indicate that Ca+GA3 treatment can effectively reduce the activities of key enzymes in lignin synthesis in the peel.Ca+GA3 treatment significantly increased the relative expression of antioxidant enzyme genes CrSOD and CrCAT, and decreased the relative expressions of CrPOD and CrPPO, as well as cell wall metabo‐lism related genes CrPME, CrPL, CrPG, CrCX, CrPAL, Cr4CL, and CrC4H in ponkan peel. Ca+GA3 treatment improved the ductility and toughness of the peel and reduced the occurrence of the fruit crack‐ing by influencing the ROS content, antioxidant capacities, cell wall metabolic enzyme activities and structural components in the peel.【Conclusion】In summary, Ca+GA3 treatment increased SOD and CAT activities,decreased
production in ponkan peel showed a gen‐eral trend of increasing and then decreasing and reached a peak at 135 d after full bloom.Ca+GA3 treat‐ment reduced the degree of lipid peroxidation in cell membrane of the peel.The activities of antioxidant enzymes SOD and CAT in ponkan peel peaked at 135 d after full bloom, which was significantly high‐er,while POD and PPO activities significantly lower in Ca+GA3 treatment than in CK.The results indi‐cated that Ca+GA3 treatment increased SOD and CAT activities,and decreased POD and PPO activities in ponkan peel.The higher activities of peel cell wall hydrolases, the faster the cell wall polysaccharide degradation, and the higher fruit cracking incidence. The Ca+GA3 treatment resulted in a consistently higher protopectin and lower soluble pectin contents in ponkan peel than those of CK. In addition,PME,PL and PG in ponkan peel were significantly reduced after Ca+GA3 treatment.The results indicat‐ed that Ca+GA3 treatment reduced the rate of degradation of protopectin into soluble pectin.The content of cellulose in ponkan peel decreased slowly during 105-165 days after full bloom. Ca+GA3 treatment effectively increased the content of cellulose in ponkan peel, while decreased CX activity. Lignin con‐tent in ponkan peel treated with Ca+GA3 was lower than that of CK during the period of 105-165 d after full bloom, and the activities of PAL, 4CL, and C4H, key enzymes in lignin synthesis, decreased in the peel throughout the whole period of growth and development of ponkan fruit.These results indicate that Ca+GA3 treatment can effectively reduce the activities of key enzymes in lignin synthesis in the peel.Ca+GA3 treatment significantly increased the relative expression of antioxidant enzyme genes CrSOD and CrCAT, and decreased the relative expressions of CrPOD and CrPPO, as well as cell wall metabo‐lism related genes CrPME, CrPL, CrPG, CrCX, CrPAL, Cr4CL, and CrC4H in ponkan peel. Ca+GA3 treatment improved the ductility and toughness of the peel and reduced the occurrence of the fruit crack‐ing by influencing the ROS content, antioxidant capacities, cell wall metabolic enzyme activities and structural components in the peel.【Conclusion】In summary, Ca+GA3 treatment increased SOD and CAT activities,decreased  production rate and H2O2 and MDA contents,as well as POD and PPO ac‐tivities in ponkan peel.Ca+GA3 treatment reduced PME,PL,PG,PAL,4CL and C4H activities and lig‐nin content, increased the protopectin and cellulose contents in ponkan peel. The expression levels of CrSOD, CrCAT, CrPOD, CrPPO, CrPME, CrPL, CrPG, CrCX, CrPAL, Cr4CL and CrC4H in the peel of ponkan fruit treated with Ca+GA3 were consistent with the activities of related enzymes and the accu‐mulation of the metabolites. Ca+GA3 treatment is effective to reduce fruit cracking rate by affecting ROS metabolism and cell wall metabolism in fruit peel at both molecular and physiological levels.
production rate and H2O2 and MDA contents,as well as POD and PPO ac‐tivities in ponkan peel.Ca+GA3 treatment reduced PME,PL,PG,PAL,4CL and C4H activities and lig‐nin content, increased the protopectin and cellulose contents in ponkan peel. The expression levels of CrSOD, CrCAT, CrPOD, CrPPO, CrPME, CrPL, CrPG, CrCX, CrPAL, Cr4CL and CrC4H in the peel of ponkan fruit treated with Ca+GA3 were consistent with the activities of related enzymes and the accu‐mulation of the metabolites. Ca+GA3 treatment is effective to reduce fruit cracking rate by affecting ROS metabolism and cell wall metabolism in fruit peel at both molecular and physiological levels.
Key words: Ponkan;Fruit cracking;Ca+GA3 treatment;ROS metabolism;Cell wall metabolism
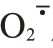 产生速率和MDA含量以及POD、PPO活性。同时,Ca+GA3处理提高了芦柑果皮果胶、纤维素含量,降低木质素含量以及PME、PL、PG、CX和PAL、4CL、C4H 活性;提高了果皮的延展性和韧性,减少了裂果的发生。Ca+GA3处理显著提高了抗氧化酶相关基因CrSOD、CrCAT在芦柑果皮中的相对表达量,降低了CrPOD、CrPPO以及细胞壁代谢相关基因CrPME、CrPL、CrPG、CrCX、CrPAL、Cr4CL、CrC4H 的相对表达量。【结论】 在生理与分子水平上明确了Ca+GA3处理可以通过调控果皮活性氧代谢与细胞壁代谢来降低裂果率。研究结果对生产上预防和减轻芦柑裂果具有一定理论意义和应用价值。
产生速率和MDA含量以及POD、PPO活性。同时,Ca+GA3处理提高了芦柑果皮果胶、纤维素含量,降低木质素含量以及PME、PL、PG、CX和PAL、4CL、C4H 活性;提高了果皮的延展性和韧性,减少了裂果的发生。Ca+GA3处理显著提高了抗氧化酶相关基因CrSOD、CrCAT在芦柑果皮中的相对表达量,降低了CrPOD、CrPPO以及细胞壁代谢相关基因CrPME、CrPL、CrPG、CrCX、CrPAL、Cr4CL、CrC4H 的相对表达量。【结论】 在生理与分子水平上明确了Ca+GA3处理可以通过调控果皮活性氧代谢与细胞壁代谢来降低裂果率。研究结果对生产上预防和减轻芦柑裂果具有一定理论意义和应用价值。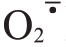 )等,ROS清除系统中有抗氧化酶和抗氧化物质
)等,ROS清除系统中有抗氧化酶和抗氧化物质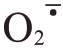 )、丙二醛(MDA)含量及抗氧化酶活性的测定 芦柑果皮过氧化氢(H
)、丙二醛(MDA)含量及抗氧化酶活性的测定 芦柑果皮过氧化氢(H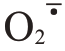 )和丙二醛(MDA)含量及多酚氧化酶(PPO)活性分别参考检测试剂盒(北京索莱宝生物公司)说明测定。
)和丙二醛(MDA)含量及多酚氧化酶(PPO)活性分别参考检测试剂盒(北京索莱宝生物公司)说明测定。

 产生速率总体呈先下降后上升再下降的趋势,芦柑盛花后135 d 果皮O
产生速率总体呈先下降后上升再下降的趋势,芦柑盛花后135 d 果皮O
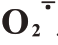 产生速率(B)和MDA 含量(C)的影响
产生速率(B)和MDA 含量(C)的影响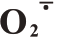 production rate(B)and MDA content(C)in peel of ponkan fruit at different developmental stages
production rate(B)and MDA content(C)in peel of ponkan fruit at different developmental stages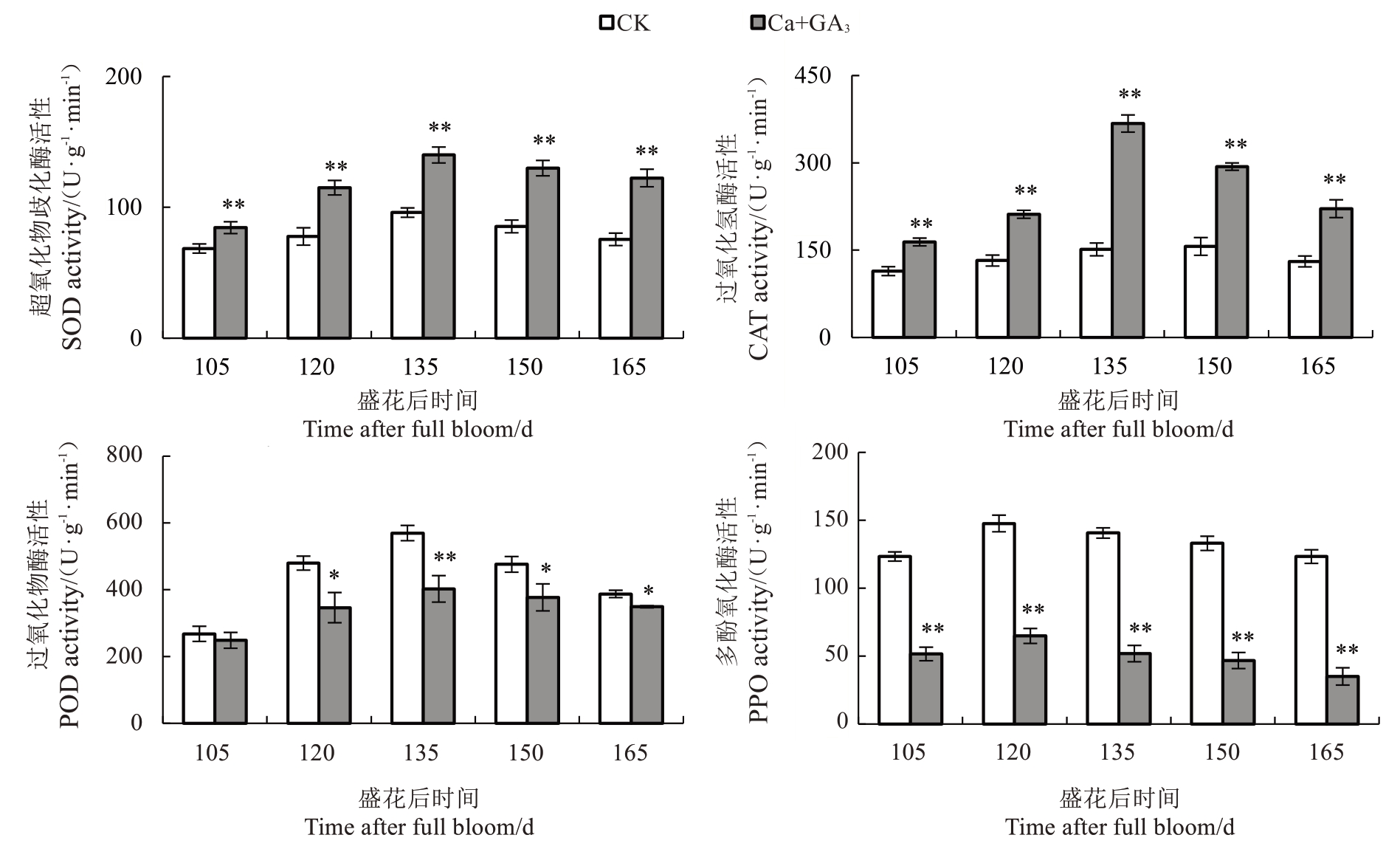
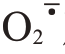 产生速率和MDA 含量以及膜脂过氧化程度均高于耐裂果番茄
产生速率和MDA 含量以及膜脂过氧化程度均高于耐裂果番茄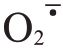 积累,提高了CAT 活性,延缓了组织受活性氧的伤害时间
积累,提高了CAT 活性,延缓了组织受活性氧的伤害时间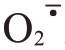 生成羟基自由基(·OH),而·OH 是一种高活性分子,已被证实在非酶促反应中促进细胞壁多糖的裂解
生成羟基自由基(·OH),而·OH 是一种高活性分子,已被证实在非酶促反应中促进细胞壁多糖的裂解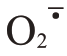 产生速率,提升SOD、CAT 活性,降低了POD、PPO 活性,从而限制了羟基自由基和苯氧自由基生成以及细胞氧化褐变损伤,减缓了对芦柑果皮韧性和硬度的影响。
产生速率,提升SOD、CAT 活性,降低了POD、PPO 活性,从而限制了羟基自由基和苯氧自由基生成以及细胞氧化褐变损伤,减缓了对芦柑果皮韧性和硬度的影响。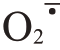 产生速率和MDA 含量,提高抗氧化酶SOD、CAT 活性,降低POD、PPO 活性,增强活性氧的清除能力。同时,Ca+GA
产生速率和MDA 含量,提高抗氧化酶SOD、CAT 活性,降低POD、PPO 活性,增强活性氧的清除能力。同时,Ca+GA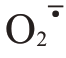 production rate as well as antioxidant enzyme activities(SOD,CAT,POD,and PPO)in ponkan peel were determined.Fur‐thermore, the cell wall metabolic enzyme activities (PME, PL, PG, CX, PAL, 4CL, and C4H) and the structural components (protopectin, soluble pectin, cellulose, and lignin) were measured. And the ex‐pression of the related genes of antioxidant enzymes and cell wall metabolic enzymes in the peel were analyzed.【Results】Ca+GA
production rate as well as antioxidant enzyme activities(SOD,CAT,POD,and PPO)in ponkan peel were determined.Fur‐thermore, the cell wall metabolic enzyme activities (PME, PL, PG, CX, PAL, 4CL, and C4H) and the structural components (protopectin, soluble pectin, cellulose, and lignin) were measured. And the ex‐pression of the related genes of antioxidant enzymes and cell wall metabolic enzymes in the peel were analyzed.【Results】Ca+GA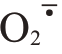 production in ponkan peel showed a gen‐eral trend of increasing and then decreasing and reached a peak at 135 d after full bloom.Ca+GA
production in ponkan peel showed a gen‐eral trend of increasing and then decreasing and reached a peak at 135 d after full bloom.Ca+GA production rate and H
production rate and H