植物会产生一类病程相关蛋白(pathogenesisrelated proteins,PRs)来抵御病原微生物的侵染或非生物因子的刺激[1]。自从在烟草花叶病毒(TMV)侵染烟草叶片试验中首次检测到该蛋白至今[2],PR 蛋白的研究得到了广泛关注。超敏反应(HR)、系统获得性反应(SAR)、信号通路、生物及非生物胁迫都能引起植物体内PR 蛋白的响应和积累[3-5]。PR 蛋白相对分子质量较小,可分为酸性蛋白和碱性蛋白,稳定性较强,能有效地在细胞内和细胞间积累[6],在植物适应不良环境方面具有重要作用。
PR 蛋白可划分为17 个家族,大多数可参与防御信号传导,从而提高植物对病原菌的抗性[7]。如PR-3、PR-4、PR-8 和PR-11 等蛋白具有几丁质酶活性[6];PR-5蛋白具有抵御真菌侵染、激活防御反应的作用[8];PR-6具有蛋白酶抑制剂活性,能够抵抗线虫和昆虫的入侵[9];PR-10 具有核糖核酸酶活性[10];PR-15和PR-16具有细胞重塑、类萌发素等特性[11]。PR-1 具有甾醇结合活性和防御信号肽,可参与植物超敏反应和系统获得性反应,在植物抗病过程中发挥重要作用[12-13]。欧洲花椒SaPR1-like 基因在生物胁迫下显著表达,增强了植株的抗逆性[14];水稻PR-1基因家族成员PR-1-3和PR-1-13在接种水稻纹枯病菌(Thanatephorus cucumeris)[15]和白叶枯病菌(Xan‐thomonas oryzae)后[16]表达量均显著提高;辣椒CAB‐PR1 基因在烟草中过表达后,能够增强转基因烟草对重金属胁迫的耐受性,以及对烟草疫霉菌(Phy‐tophthora nicotianae)、青枯菌(Ralstonia sola‐nacearum)和丁香假单胞杆菌(Pseudomonas syrin‐gae pv. tabaci)的抗性[17]。拟南芥PR-1 基因的积累增强了其对丁香假单胞杆菌(P. syringae pv. tomato DC3000)的抗性;将葡萄VvPR1b1 基因过表达于烟草中,能增强其对烟草野火病病原细菌(P.syringae pv. tabaci)的抗性[18-19]。从上述可见,不同物种的PR-1 蛋白受诱导条件不同,其作用机制尚不一致,但其在防御反应中都具有相似的功能。
丁香假单胞杆菌(P.syringae pv.actinidiae,Psa)是引起猕猴桃(Actinidia Lindl.)细菌性溃疡病的致病菌[20-21],该病害具有传染性强、蔓延快、致病性强、根除难度大等特点,目前尚无有效的根治方法[22]。从1984 年首次在日本发现至今,细菌性溃疡病已成为制约我国乃至世界猕猴桃产业发展的重要病害[23-24]。研究表明,茉莉酸、水杨酸和脱落酸等植物激素能诱导PR-1 基因表达而对病害具有防御作用[25]。迄今为止,PR-1 蛋白在防御反应中的功能在番茄[26]、葡萄[27]、柑橘[28]、核桃[29]、小麦[30]、玉米[31]等多种植物上已有相关研究,但在猕猴桃中未见报道。笔者在前期系统评价鉴定猕猴桃不同品种(系)溃疡病抗性的基础上[32],以筛选的抗病种质为试材,分析了PR-1基因的结构、组织表达及响应溃疡病和外源激素的表达情况,并进一步明确其在植物病原响应过程中的功能,以期为寻找猕猴桃细菌性溃疡病抗性基因及解析抗病机制提供理论基础。
1 材料和方法
1.1 材料
毛花猕猴桃(A.eriantha)品种华特嫁接苗,砧木为对萼猕猴桃(A. valvata)品种中猕抗砧1 号,保存于国家园艺种质资源库猕猴桃分库(郑州);本氏烟草(N. benthamiana)由中国农业科学院郑州果树研究所栽培生理实验室保存;Psa病菌由浙江省农业科学院园艺研究所提供。猕猴桃苗木嫁接后在营养钵中培养3 个月,再移至人工气候箱中培养,条件设置为温度25 ℃、相对湿度80%、12 h 光照/12 h 黑暗。Psa 菌液采用金氏培养基培养,在20 ℃、200 r·min-1摇床振荡24 h,浓度稀释至1×108 cfu·mL-1后进行侵染。使用5 mmol·L-1水杨酸(SA)、250 mg·L-1赤霉素(GA3)、50µmol·L-1脱落酸(ABA)对嫁接苗进行叶面喷施处理,并置于人工气候箱中培养至96 h。试验过程中采集的叶片样本经液氮处理后均置于-80 ℃冰箱保存备用。
1.2 RNA提取、反转录
利用多糖多酚RNA 提取试剂盒(天根生化科技公司)提取样品总RNA,于-80 ℃冰箱保存备用。使用TOYOBO ReverTra Ace qPCR RT Kit(FSQ-101)将RNA 反转录合成cDNA,反应体系为4 µL 5×RT Buffer、1 µL Enzyme Mix、1 µL Primer Mix、4 µL RNA、10 µL ddH2O。先将RNA 和ddH2O 加入PCR管中,采用PCR仪65 ℃变性5 min后置于冰上,再加入其他组分,反应程序:37 ℃、15 min,98 ℃、5 min。反转录后的cDNA保存于-20 ℃冰箱备用。
1.3 PR-1基因克隆及系统发育分析
在前期组学研究的基础上[33],通过在猕猴桃基因组数据库[34]中进行目的序列比对,提取目的基因AePR-1 的CDS 序列,使用Primer Premier 6.0 软件设计引物(上游引物:5'-ATGGGGTGGTTGTGTA-3',下游引物:5'-CTAAATATTTTCTACATAGGTC-3')扩增全长序列;使用2×TransStart® Fast Pfu Master Mix(北京全式金生物技术有限公司)进行PCR 扩增,PCR 产物回收后连接T 载体(pClone007 Blunt Simple Vector Kit,北京擎科生物科技有限公司),并转化至大肠杆菌感受态细胞,挑取单克隆菌落检测后送测序。引物合成和测序在上海生工生物工程有限公司进行。使用Expasy ProtParam工具对AePR-1序列进行相对分子质量、等电点及稳定性等理化特性分析;使用DNAMAN 软件对AePR-1 基因序列进行比对分析;在NCBI 网站分析AePR-1 蛋白序列的保守结构域;用猕猴桃PR-1 蛋白序列在phytozome数据库中Blast检索其他同源序列,并使用MEGA-X软件的邻接法(Neighbor-Joining)构建系统发育树,进行系统发育分析。
1.4 PR-1 基因的表达分析
采用实时荧光定量技术,分析AePR-1 基因在不同组织(根、茎、叶、花、果)和花器官(雌蕊、雄蕊、子房、花瓣、萼片、花托),以及病原菌Psa 和激素(SA、GA3、ABA)处理后0、6、12、24、36、48、72、96 h 的表达情况。荧光定量引物为AePR-1F:5'-AAGACTACCTCAACGCCCACAAC-3',AePR-1R:5'-TTCTTCTCGTCCACCCACATTTT-3';荧光定量试剂为NovoStart SYBR qPCR SuperMix Plus;反应体系为10 µL 2×NovoStart SYBR qPCR SuperMix、2 µL cDNA、1 µL PR1 Primer Forward、1 µL PR1 Primer Reverse、6 µL RNase Free Water;反应程序为95 ℃预变性5 min,95 ℃变性20 s,60℃退火20 s,72 ℃延伸20 s,变性至延伸步骤为40个循环。采用2-ΔΔCT法计算每个样品相对猕猴桃内参基因β-actin的表达量[35]。所有反应均包含3 个独立的生物学重复和技术重复。
1.5 亚细胞定位
使用一步定向克隆试剂盒(上海近岸科技有限公司),将目的片段克隆到pCAM35s-GFP 载体位点中(上游引物:5'-GGGGACGAGCTCGGTACCAT‐GGGGTGGTTGTGTAGGATG- 3',下游引物:5'-CATGGTGTCGACTCTAGAAATATTTTCTACATA‐GGTCTTAAGCTTTAATACATAGG-3'),形成融合表达载体后转化大肠杆菌感受态细胞,单克隆菌落PCR 阳性鉴定后送上海生工生物工程有限公司测序。将测序正确的阳性质粒转化农杆菌GV3101。以空载pCAM35s-GFP 为对照,采用注射方法侵染烟草下表皮[36],置于人工气候箱中培养(条件设置同1.1)。培养2~3 d 后,使用激光共聚显微镜对侵染区域进行亚细胞定位观察。
1.6 过表达载体构建及烟草瞬时转化
采用同源重组的方法将猕猴桃PR-1 基因插入到表达载体PBI121 中(PR-1-121-F:5'-AACACGGGGGACTCTAGAATGGGGTGGTTGTGTA-3',PR-1-121-R:5'-CTGACCACCCGGGGATCCCTAAATATTTTCTACATAGGTC-3'),转化农杆菌GV3101后,以空载体PBI121 作对照,采用烟草下表皮注射的方法进行瞬时表达[36],将处理和对照均放置人工培养箱中培养(条件设置同1.1)。在接种1、2 和3 d时分别检测PR-1 的表达量,筛选Psa 菌液侵染瞬时表达烟草的最佳时间。然后将Psa 菌液注射到瞬时表达的阳性烟草叶片中,处理后放于上述条件的人工气候箱中继续培养,连续观察叶片的表型变化[37]。
1.7 数据分析
采用Excel 和Origin 2023 软件对试验数据进行统计分析及相关图表绘制,利用Dunn-Sidak 多重比较方法进行差异显著性检验,显著水平设定为0.05。
2 结果与分析
2.1 PR-1基因的克隆及序列分析
在华特猕猴桃中克隆获得1 条PR-1 基因,命名为AePR-1。克隆得到cDNA 全长为522 bp,共编码173 个氨基酸。通过在线网站Expasy 对其理化特性分析,表明AePR-1 蛋白相对分子质量为19.28 ku、等电点为9.28、不稳定系数为41.54。将AePR-1 的序列与中华猕猴桃(A. chinensis Planch. var. chinen‐sis)AcPR-1 序列进行同源比对,显示基因CDS 序列中共有12 个碱基位点不同、蛋白序列中有7 个氨基酸不同,但二者皆编码了173 个氨基酸。通过对AePR-1 与AcPR-1、AthPR-1、NtaPR-1 同源蛋白序列分析,发现这些序列都具有6 个保守的半胱氨酸结构基序(实线框表示)和4 个allergen V5/Tpx-1 relat‐ed保守的结构域(虚线框表示)(图1)。
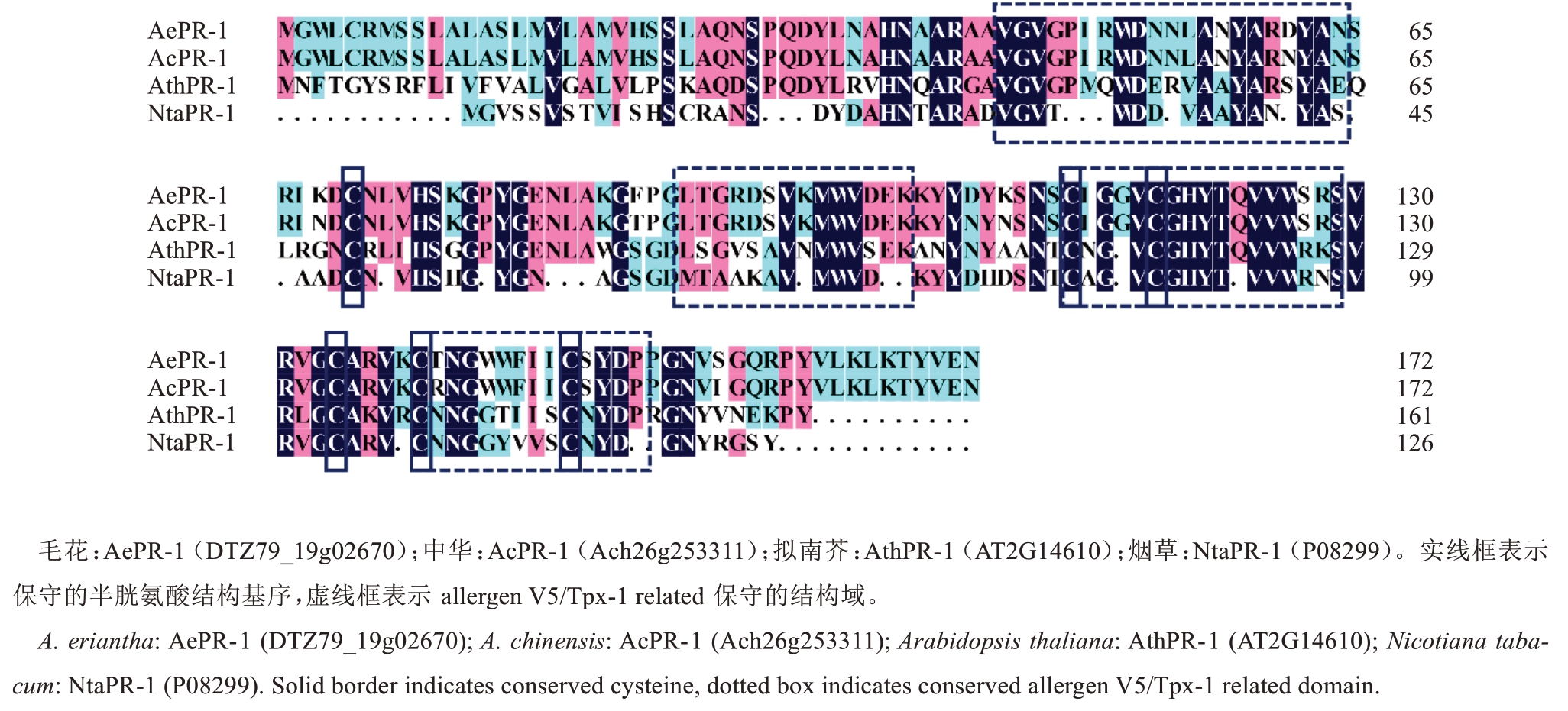
图1 AePR-1 与其他PR-1 蛋白序列同源性分析
Fig.1 Alignment of the predicted amino acid sequences of AePR-1 and other PR-1
构建了猕猴桃AePR-1 与其他植物PR-1 蛋白的系统发育树,发现发育树分为包含了大桉EgrPR-1、大豆GmaPR-1等分支和可可TcaPR-1、葡萄VviPR-1等分支的两个明显大分支(图2)。毛花猕猴桃AePR-1 与中华猕猴桃AcPR-1 及黄瓜CsaPR-1 具有很高的同源性。
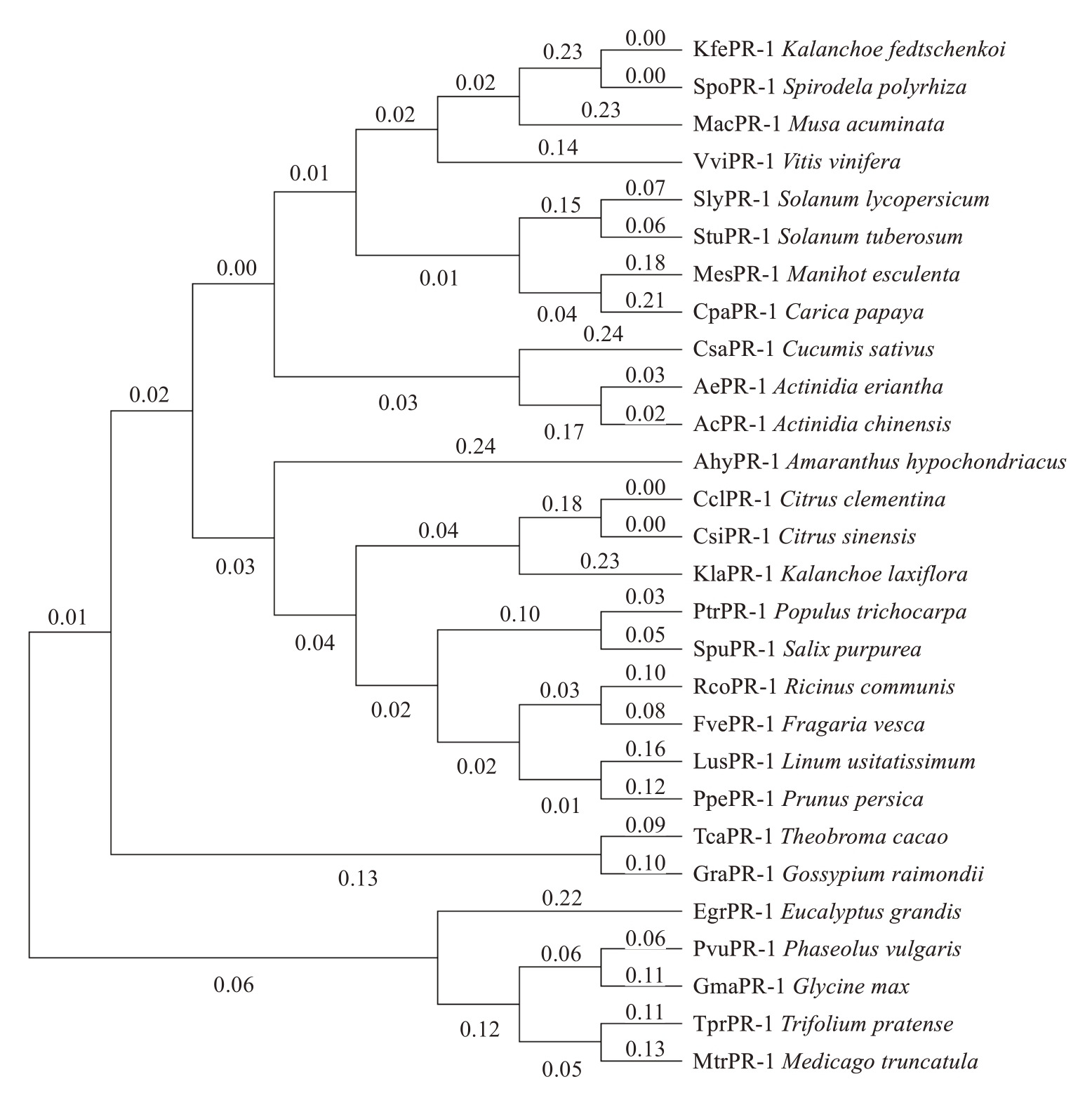
图2 AePR-1 与其他植物PR-1 蛋白系统进化分析
Fig.2 Phylogenetic relationship of AePR-1 with other plant PR-1
2.2 PR-1基因的亚细胞定位分析
为揭示AePR-1 蛋白的定位情况,构建了pCAM35s-PR-1-GFP融合表达载体并转化烟草表皮细胞,并以pCAM35s-GFP 空载体为对照,培养48 h后在激光共聚焦显微镜下观察。如图3 所示,对照组的空载载体在细胞膜、细胞质和细胞核中均能发出绿色荧光,而试验组的烟草叶片荧光信号主要集中在细胞膜上和细胞质中,表明PR-1 主要在细胞膜和细胞质中表达,是功能基因。
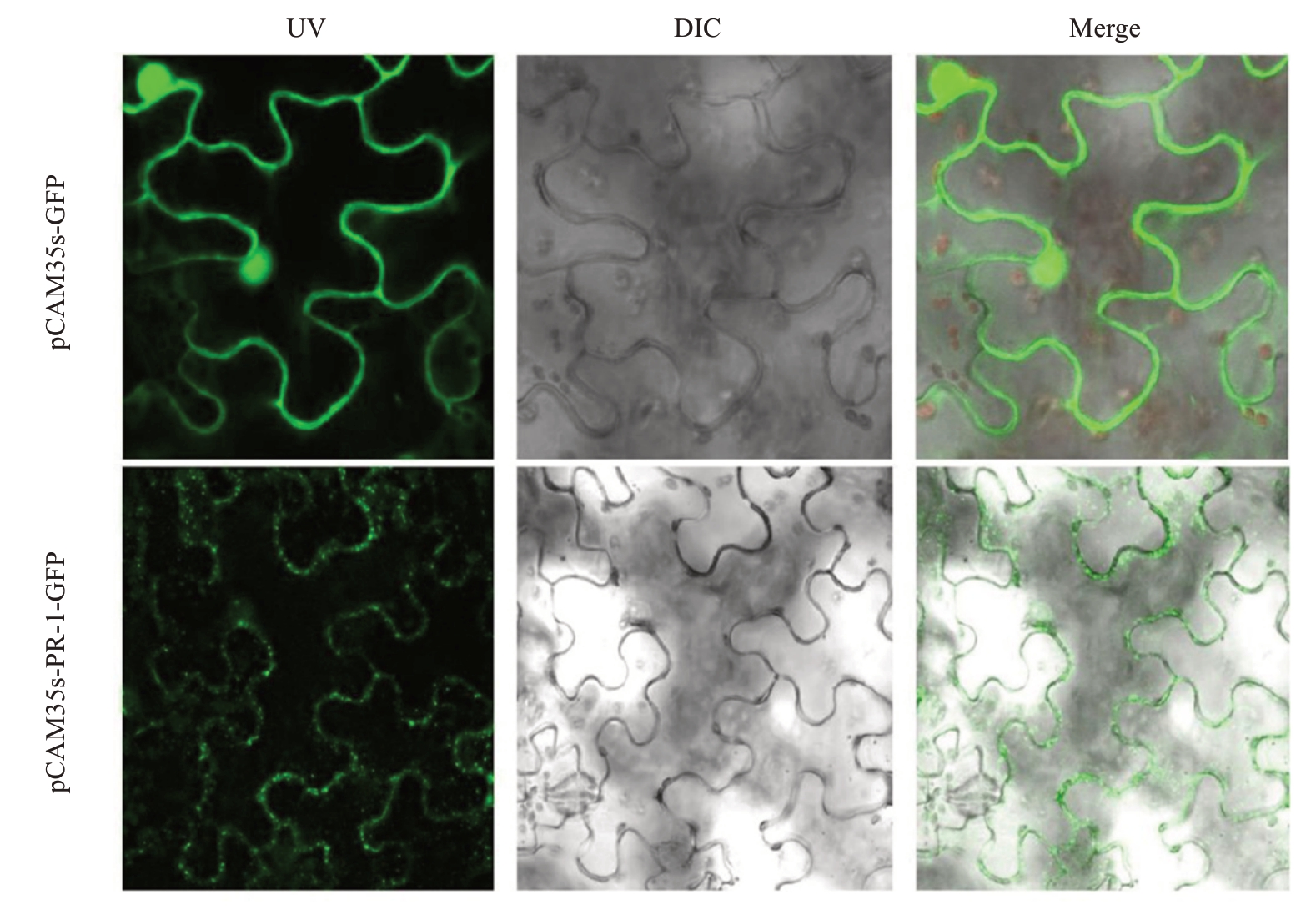
图3 PR-1 基因在烟草表皮细胞中的亚细胞定位
Fig.3 Subcellular localization of PR-1 in tobacco epidermal cells
2.3 PR-1基因的表达分析
为了解AePR-1 基因的时空表达特征,首先对AePR-1 基因在猕猴桃不同组织和花器官中的表达量进行了分析,发现该基因在根和雌蕊中高表达,而在叶、花瓣、雄蕊和子房中低表达(图4-A~B)。
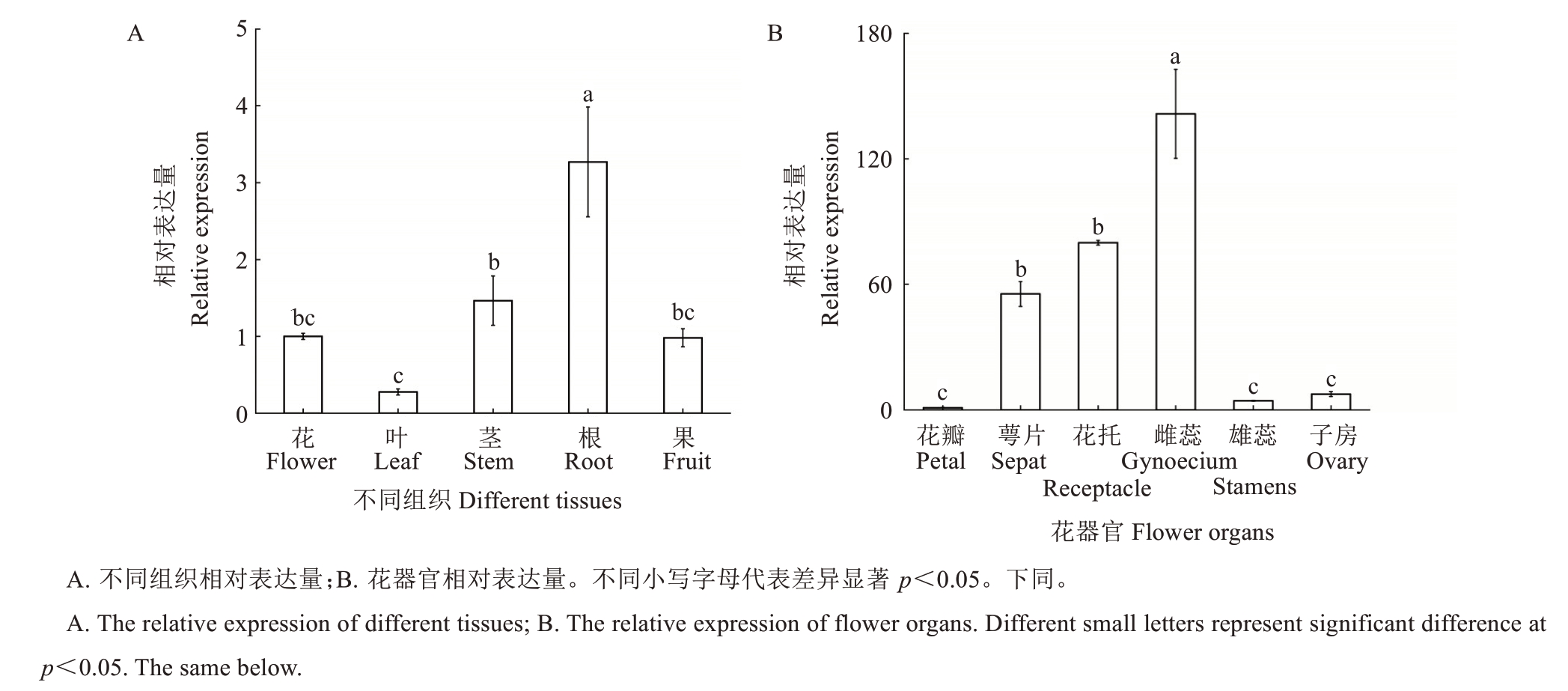
图4 PR-1 基因在不同组织和花器官中的表达分析
Fig.4 Analysis of PR-1 gene expression in different tissues and flower organs
在接种Psa 菌液后,AePR-1 基因相对表达量呈现出先降低后迅速升高的趋势,在接种24 H 时达到最大值,后又逐渐下降(图5-A)。
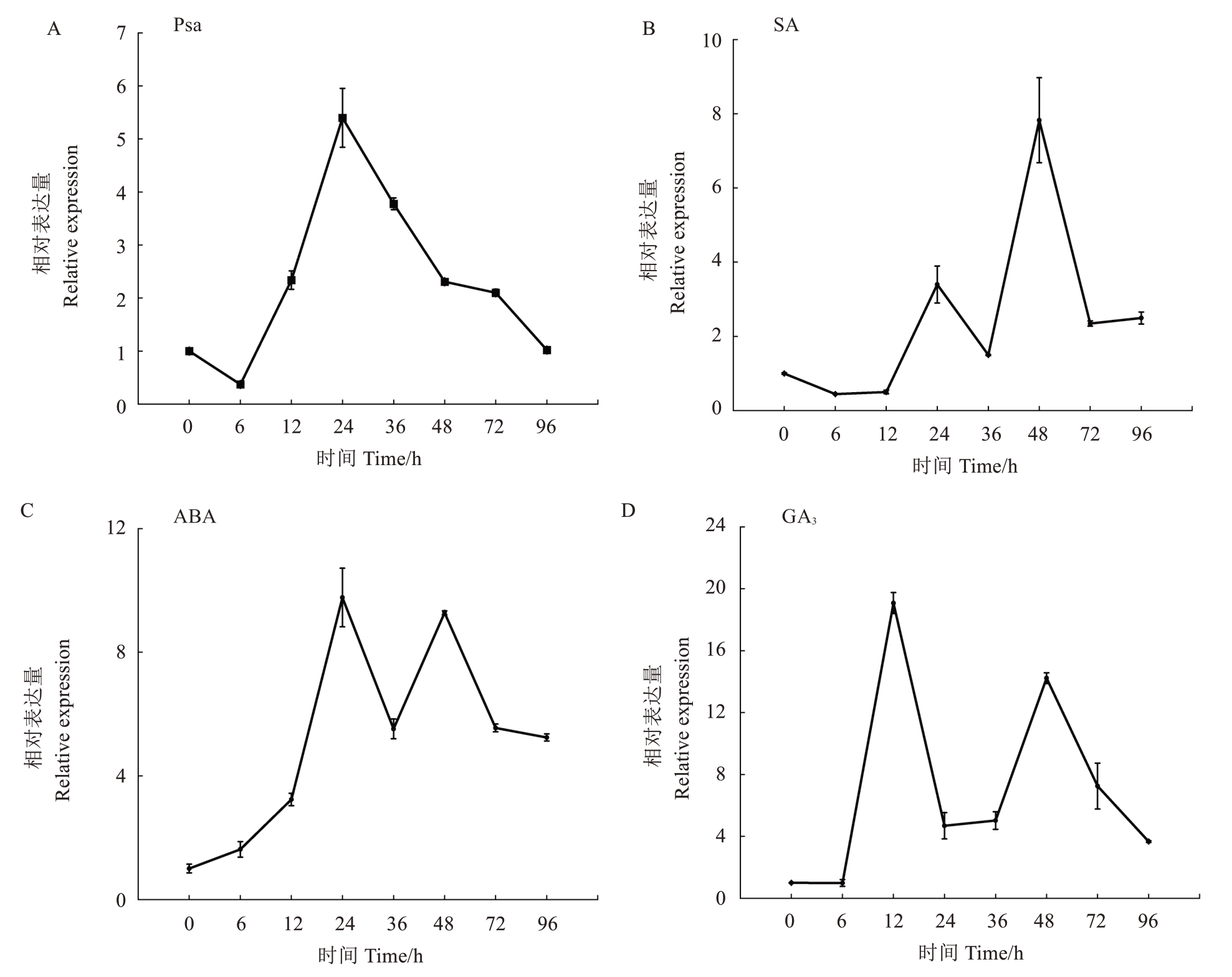
图5 PR-1 基因在Psa 侵染及不同激素处理下的表达分析
Fig.5 Analysis of PR-1 gene expression after Psa infection and hormone treatment
在不同激素处理过程中,随着处理时间的延长,AePR-1 基因相对表达量均呈双峰曲线。在SA 和ABA处理下,AePR-1基因表达量在24 h时达到第一次峰值(图5-B~C);GA3处理可诱导AePR-1 基因迅速表达,接种12 h时达到最大值(图5-D)。3 种激素处理下首次出现峰值的时间存在差异,但在48 h 时均再次达到峰值,表明AePR-1基因对不同激素响应模式存在差异。
2.4 PR-1基因增强了烟草对Psa的抗性
以pBI121 空载体为对照,构建过表达载体pBI121-AePR-1 瞬时表达本氏烟草后发现,AePR-1表达量在2 d 最高(图6-A)。因此,在烟草瞬时表达后的2 d 使用Psa 菌液侵染,将接种后的烟草放入人工气候箱中培养,观察烟草的发病情况。结果表明,在侵染后第14 天,在空载对照组的叶片表面上出现了大量的黄色病斑,而过表达AePR1 基因的叶片表面出现少量黄色斑点(图6-B)。以上结果表明,AePR-1基因能增强本氏烟草对Psa的抗性。
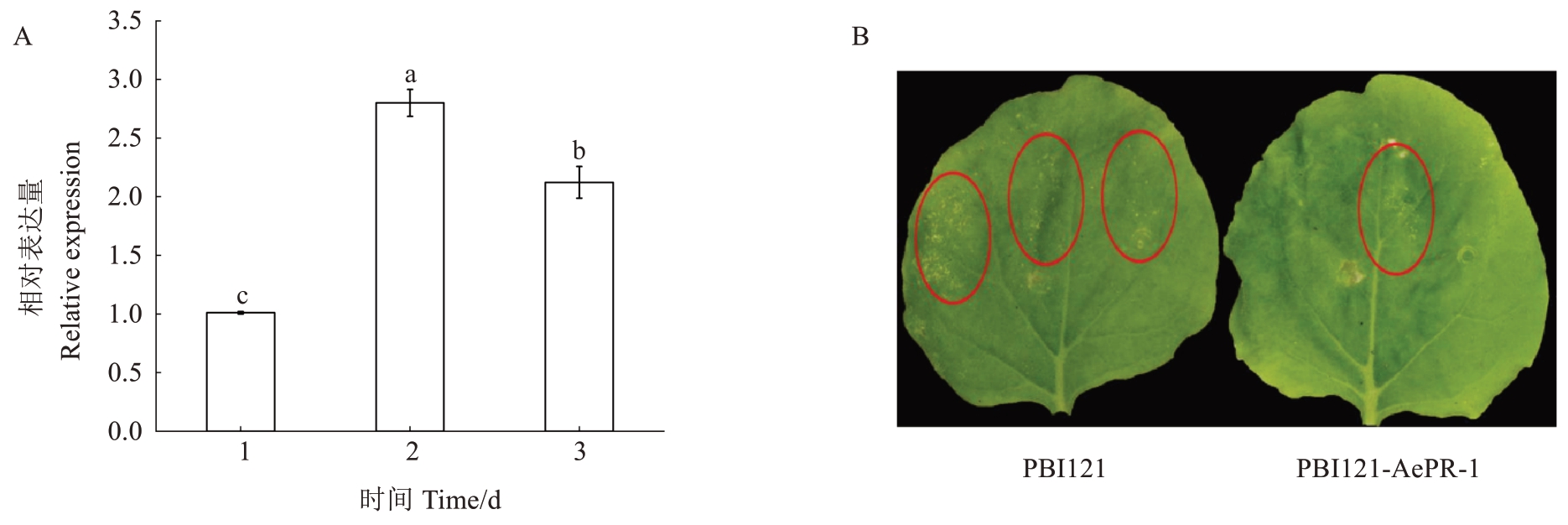
图6 AePR-1 瞬时表达烟草后表达分析(A)及Psa 侵染结果(B)
Fig.6 The expression patterns of AePR-1 after transient expression of tobacco(A)and infection results of Psa(B)
3 讨 论
植物受到生物或非生物胁迫时,会激活其免疫防御反应,而PR-1基因是SAR反应的标志基因,在抗病反应中具有积极作用[38]。牡丹受叶斑病菌(Cylindro‐cladium canadense)侵染后,PsPR-1 基因被显著诱导参与抗病反应,且在24 h达到最高峰[39]。这与本研究结果相似,猕猴桃在接种Psa病菌后,PR-1表达量显著升高,在24 h达峰值,表明其参与了抗病防御反应。
笔者以华特猕猴桃为材料克隆得到了AePR-1基因,通过生物信息学分析发现,该基因的蛋白序列与其他物种的PR-1具有高度的同源性,其理化特性与葡萄[25]、向日葵[40]、核桃[29]等相似且包含了相同的保守结构域。具有该结构域的蛋白积极参与了先天性免疫反应和适应性免疫反应,从而提高植株的抗逆性[41]。这表明不同植物中的PR-1 基因在防御反应中可能存在相似功能。
进一步对PR-1 基因在不同组织和花器官中的表达量检测,发现猕猴桃在根中高表达,而大豆则表现为在叶片中高表达[42],这可能是由于不同物种中PR-1基因表达模式存在差异,猕猴桃PR-1基因的组织特异性表达差异可能与其在植物生长发育及胁迫反应中的不同作用相关。
植物激素能诱导PR-1基因表达量的升高,在防御反应中发挥至关重要的作用[25,43]。在葡萄中,JA、SA 和ABA 均能诱导叶片VvPR1 基因的显著表达[25]。水稻中,OsPR1a 基因受JA、SA 和ET 的诱导而显著表达[44];喷施外源SA 可以显著降低褐斑病(P. oryzicola)的发病率,同时PR-1 和NPR1 的相对表达量均显著升高[45]。笔者采用不同激素诱导处理,发现对猕猴桃PR-1基因表达趋势的整体影响相同,但具体表达量与峰值出现时间存在差异。AePR-1 基因对不同激素的响应差异可能与其启动子中对不同激素的响应元件的有无及数量有关。其中在GA3处理条件下,AePR-1 基因响应表现最早且表达量最高,其次依次为ABA 和SA。本研究结果与前人研究结果相似,在外源激素诱导下,PR-1 基因表达量升高。还有研究发现,干旱、低温、盐等胁迫及H2O2、H2S 等因素也能显著诱导PR-1 基因的表达[46]。
本研究结果表明,猕猴桃PR-1基因在响应生物侵染过程中发挥重要作用,并揭示了过表达AePR-1基因能够增强烟草对Psa 病菌的抗性。今后有必要进一步开展以激素诱导为前提提高猕猴桃植物抗细菌性溃疡病的作用机制研究,以期为科学防控该病害提供理论和实践基础。
4 结 论
笔者探讨了AePR-1 基因在猕猴桃细菌性溃疡病和外源激素侵染中发挥的作用。结果表明,猕猴桃PR-1基因在Psa病菌和外源激素诱导下均可大量表达,参与了免疫应答反应,从而增强了猕猴桃的抗病性。笔者研究证实了PR-1 基因在猕猴桃抗病过程中具有重要作用。
[1] VAN LOON L C,PIERPOINT W S,BOLLER T,CONEJERO V.Recommendations for Naming plant pathogenesis-related pro‐teins[J].Plant Molecular Biology Reporter,1994,12(3):245-264.
[2] VAN LOON L C,VAN KAMMEN A. Polyacrylamide disc elec‐trophoresis of the soluble leaf proteins from Nicotiana tabacum var.‘Samsun’and‘Samsun NN’II. Changes in protein consti‐tution after infection with tobacco mosaic virus[J]. Virology,1970,40(2):199-211.
[3] LIU J J,EKRAMODDOULLAH A K M.The family 10 of plant pathogenesis-related proteins:Their structure,regulation,and function in response to biotic and abiotic stresses[J]. Physiologi‐cal and Molecular Plant Pathology,2006,68(1/2/3):3-13.
[4] VAN LOON L C,VAN STRIEN E A.The families of pathogene‐sis-related proteins,their activities,and comparative analysis of PR-1 type proteins[J].Physiological and Molecular Plant Pathol‐ogy,1999,55(2):85-97.
[5] BALINT- KURTI P. The plant hypersensitive response:Con‐cepts,control and consequences[J]. Molecular Plant Pathology,2019,20(8):1163-1178.
[6] 焦文晓,王晓梅,范新光,姜微波.病程相关蛋白在采后果蔬诱导抗病性中的研究进展[J].保鲜与加工,2020,20(1):206-211.JIAO Wenxiao,WANG Xiaomei,FAN Xinguang,JIANG Weibo.Advances of research on pathogenesis-related protein in induc‐tion of disease resistance of postharvest fruits and vegetables[J].Storage and Process,2020,20(1):206-211.
[7] VAN LOON L C,REP M,PIETERSE C M J.Significance of in‐ducible defense-related proteins in infected plants[J].Annual Re‐view of Phytopathology,2006,44:135-162.
[8] EL-KEREAMY A,EL-SHARKAWY I,RAMAMOORTHY R,TAHERI A,ERRAMPALLI D,KUMAR P,JAYASANKAR S.Prunus domestica pathogenesis-related protein-5 activates the defense response pathway and enhances the resistance to fungal infection[J].PLoS One,2011,6(3):e17973.
[9] SELITRENNIKOFF C P. Antifungal proteins[J]. Applied and Environmental Microbiology,2001,67(7):2883-2894.
[10] 赵琦,崔梦杰,韩锁义,郭腾达,杜培,刘华,徐静,黄冰艳,董文召,张新友.植物病程相关蛋白PR10 及其在花生中的研究进展[J].四川农业大学学报,2024,42(1):1-10.ZHAO Qi,CUI Mengjie,HAN Suoyi,GUO Tengda,DU Pei,LIU Hua,XU Jing,HUANG Bingyan,DONG Wenzhao,ZHANG Xinyou. Plant pathogenesis-related protein PR10 and their research progress in peanut[J]. Journal of Sichuan Agricul‐tural University,2024,42(1):1-10.
[11] PARK C J,AN J M,SHIN Y C,KIM K J,LEE B J,PAEK K H.Molecular characterization of pepper germin-like protein as the novel PR-16 family of pathogenesis-related proteins isolated during the resistance response to viral and bacterial infection[J].Planta,2004,219(5):797-806.
[12] 李爽,熊樱,MÜLLERXING R,邢倩. 转录因子WRKY6 和PR1 在拟南芥胁迫记忆中的表达模式[J].植物研究,2019,39(5):752-759.LI Shuang,XIONG Ying,MÜLLERXING R,XING Qian. Dis‐tinct expression patterns of WRKY6 and PR1 in Arabidopsis stress memory assays[J]. Bulletin of Botanical Research,2019,39(5):752-759.
[13] BREEN S,WILLIAMS S J,OUTRAM M,KOBE B,SOLO‐MON P S.Emerging insights into the functions of pathogenesisrelated protein 1[J]. Trends in Plant Science,2017,22(10):871-879.
[14] 刘亚辉,李佳兴,王升,王铁霖,袁杰,郭兰萍.欧洲花楸病程相关蛋白基因SaPR1-like 的克隆及序列分析[J].中国实验方剂学杂志,2019,25(21):118-123.LIU Yahui,LI Jiaxing,WANG Sheng,WANG Tielin,YUAN Jie,GUO Lanping.Cloning and sequence analysis of pathogene‐sis related protein 1-like gene from Sorbus aucuparia[J].Chinese Journal of Experimental Traditional Medical Formulae,2019,25(21):118-123.
[15] LIU Z Y,LI X J,SUN F,ZHOU T,ZHOU Y J. Overexpression of OsCIPK30 enhances plant tolerance to Rice stripe virus[J].Frontiers in Microbiology,2017,8:2322.
[16] 程雨果,魏炳峥,李春霞,陶均.水稻PR1 基因响应生物胁迫的表达模式[J/OL].分子植物育种,2023:1-24.(2023-06-20).https://kns.cnki.net/kcms/detail/46.1068.s.20230619.1458.002.html.CHENG Yuguo,WEI Bingzheng,LI Chunxia,TAO Jun. Expres‐sion patterns of rice PR1 family genes in response to biotic stress‐es[J/OL]. Molecular Plant Breeding,2023:1-24. (2023-06-20).https://kns.cnki.net/kcms/detail/46.1068.s.20230619.1458.002.html.
[17] SAROWAR S,KIM Y J,KIM E N,KIM K D,HWANG B K,IS‐LAM R,SHIN J S.Overexpression of a pepper basic pathogene‐sis-related protein 1 gene in tobacco plants enhances resistance to heavy metal and pathogen stresses[J]. Plant Cell Reports,2005,24(4):216-224.
[18] LI Z T,DHEKNEY S A,GRAY D J.PR-1 gene family of grape‐vine:A uniquely duplicated PR-1 gene from a Vitis interspecific hybrid confers high level resistance to bacterial disease in trans‐genic tobacco[J].Plant Cell Reports,2011,30(1):1-11.
[19] SEO J S,DILOKNAWARIT P,PARK B S,CHUA N H.elf18-in‐duced long noncoding RNA 1 evicts fibrillarin from mediator sub‐unit to enhance pathogenesis-related gene 1 (Pr1) expression[J].New Phytologist,2019,221(4):2067-2079.
[20] DONATI I,CELLINI A,SANGIORGIO D,VANNESTE J L,SCORTICHINI M,BALESTRA G M,SPINELLI F. Pseudomo‐nas syringae pv.actinidiae:Ecology,infection dynamics and dis‐ease epidemiology[J].Microbial Ecology,2020,80(1):81-102.
[21] 裴艳刚,马利,岁立云,崔永亮,刘晓敏,龚国淑.不同猕猴桃品种对溃疡病菌的抗性评价及其利用[J]. 果树学报,2021,38(7):1153-1162.PEI Yangang,MA Li,SUI Liyun,CUI Yongliang,LIU Xiaomin,GONG Guoshu.Resistance evaluation and utilization of different kiwifruit cultivars to Pseudomonas syringae pv. actinidiae[J].Journal of Fruit Science,2021,38(7):1153-1162.
[22] 钟彩虹,李黎,潘慧,邓蕾,陈美艳.猕猴桃细菌性溃疡病的发生规律及综合防治技术[J].中国果树,2020(1):9-13.ZHONG Caihong,LI Li,PAN Hui,DENG Lei,CHEN Meiyan.Occurrence rule and comprehensive control of kiwifruit bacteri‐al canker disease[J].China Fruits,2020(1):9-13.
[23] VANNESTE J L.The scientific,economic,and social impacts of the new zealand outbreak of bacterial canker of kiwifruit (Pseu‐domonas syringae pv.actinidiae)[J].Annual Review of Phytopa‐thology,2017,55:377-399.
[24] SAWADA H,FUJIKAWA T. Genetic diversity of Pseudomonas syringae pv.actinidiae,pathogen of kiwifruit bacterial canker[J].Plant Pathology,2019,68(7):1235-1248.
[25] 侯丽霞,高超,车永梅,赵方贵,刘新.葡萄病程相关蛋白1 基因的克隆和表达分析[J].植物生理学报,2012,48(1):57-62.HOU Lixia,GAO Chao,CHE Yongmei,ZHAO Fanggui,LIU Xin.Gene cloning and expression analysis of pathogenesis-relat‐ed protein 1 in Vitis vinifera L[J]. Plant Physiology Journal,2012,48(1):57-62.
[26] KARABULUT D,CALIS O.The role of PR1 gene in resistance mechanism to bacterial canker and wilting disease in promising tomato mutant plants[J]. Journal of Tekirdag Agriculture Facul‐ty,2022,19(1):120-131.
[27] LI P,TAN X B,LIU R T,RAHMAN F U,JIANG J F,SUN L,FAN X C,LIU J H,LIU C H,ZHANG Y. QTL detection and candidate gene analysis of grape white rot resistance by interspe‐cific grape (Vitis vinifera L. × Vitis davidii Foex.) crossing[J].Horticulture Research,2023,10(5):uhad063.
[28] 郝晨星.响应柑橘溃疡病菌侵染的枸橼C-05 抗性基因PR4A的鉴定[D].长沙:湖南农业大学,2019.HAO Chenxing. Identification of a defence gene PR4A from citron C-05 in response to Xanthomonas citri subsp. citri infec‐tion[D].Changsha:Hunan Agricultural University,2019.
[29] 李生萍.核桃PR1 与PR4 基因的克隆及表达分析[D].杨凌:西北农林科技大学,2017.LI Shengping. Cloning and expression analysis of PR1 and PR4 genes in walnut[D]. Yangling:Northwest A & F University,2017.
[30] BREEN S,WILLIAMS S J,WINTERBERG B,KOBE B,SOL‐OMON P S. Wheat PR-1 proteins are targeted by necrotrophic pathogen effector proteins[J].The Plant Journal,2016,88(1):13-25.
[31] 石凤梅.病程相关蛋白基因ZmPR-1 和ZmPR-4 的克隆及功能研究[D].哈尔滨:东北林业大学,2019.SHI Fengmei. Cloning and functional study of pathogenesis-re‐lated protein genes ZmPR-1 and ZmPR-4[D]. Harbin:Northeast Forestry University,2019.
[32] 宋雅林,林苗苗,钟云鹏,陈锦永,齐秀娟,孙雷明,方金豹.猕猴桃品种(系)溃疡病抗性鉴定及不同评价指标的相关性分析[J].果树学报,2020,37(6):900-908.SONG Yalin,LIN Miaomiao,ZHONG Yunpeng,CHEN Jin‐yong,QI Xiujuan,SUN Leiming,FANG Jinbao. Evaluation of resistance of kiwifruit varieties (line) against bacterial canker disease and correlation analysis among evaluation indexes[J].Journal of Fruit Science,2020,37(6):900-908.
[33] SONG Y L,SUN L M,LIN M M,CHEN J Y,QI X J,HU C G,FANG J B. Comparative transcriptome analysis of resistant and susceptible kiwifruits in response to Pseudomonas syringae pv.actinidiae during early infection[J]. PLoS One,2019,14(2):e0211913.
[34] YUE J Y,LIU J C,TANG W,WU Y Q,TANG X F,LI W,YANG Y,WANG L H,HUANG S X,FANG C B,ZHAO K,FEI Z J,LIU Y S,ZHENG Y. Kiwifruit Genome Database(KGD):A comprehensive resource for kiwifruit genomics[J].Horticulture Research,2020,7:117.
[35] AMPOMAH- DWAMENA C,MCGHIE T,WIBISONO R,MONTEFIORI M,HELLENS R P,ALLAN A C. The kiwifruit lycopene beta-cyclase plays a significant role in carotenoid accu‐mulation in fruit[J]. Journal of Experimental Botany,2009,60(13):3765-3779.
[36] 欧阳梦真,朱磊,孙治强,李胜利,吴帼秀,李阳,何富豪,李严曼.西瓜ClWRKY54 基因的克隆、亚细胞定位及表达分析[J].中国瓜菜,2019,32(12):8-14.OUYANG Mengzhen,ZHU Lei,SUN Zhiqiang,LI Shengli,WU Guoxiu,LI Yang,HE Fuhao,LI Yanman. Cloning,subcellular localization and expression analysis of ClWRKY54 in Citrullus lanatus[J].China Cucurbits and Vegetables,2019,32(12):8-14.
[37] 宋雅林.猕猴桃应答溃疡病菌侵染的转录组研究及抗性相关基因挖掘[D].武汉:华中农业大学,2019.SONG Yalin.Transcriptome study on kiwifruit response to Pseu‐domonas syringae pv. actinidiae infection and mining of resis‐tance related genes[D].Wuhan:Huazhong Agricultural Universi‐ty,2019.
[38] KINKEMA M,FAN W,DONG X. Nuclear localization of NPR1 is required for activation of PR gene expression[J]. The Plant Cell,2000,12(12):2339-2350.
[39] 杨德翠. Cylindrocladium canadense 侵染对牡丹(Paeonia suf‐fruticosa Andr.)光合特性的影响以及牡丹病程相关蛋白1 基因的克隆和遗传转化[D].太谷:山西农业大学,2013.YANG Decui. Effects on the photosynthetic characteristics of tree peony (Paeonia suffruticosa Andr.) infected by Cylindrocla‐dium canadense and gene cloning and genetic transformation of pathogenesis-related Protein 1 of tree peony[D]. Taigu:Shanxi Agricultural University,2013.
[40] 马立功,张匀华,孟庆林,石凤梅,刘佳,李易初,王志英.向日葵病程相关蛋白HaPR1 基因的克隆与功能[J]. 作物学报,2015,41(12):1819-1827.MA Ligong,ZHANG Yunhua,MENG Qinglin,SHI Fengmei,LIU Jia,LI Yichu,WANG Zhiying. Cloning and function analy‐sis of pathogenesis related protein gene HaPR1 from sunflower(Helianthus annuus)[J].Acta Agronomica Sinica,2015,41(12):1819-1827.
[41] GIBBS G M,ROELANTS K,O’BRYAN M K.The CAP super‐family:Cysteine-rich secretory proteins,antigen 5,and patho‐genesis-related 1 proteins:Roles in reproduction,cancer,and im‐mune defense[J].Endocrine Reviews,2008,29(7):865-897.
[42] CUI H M,QU S,LAMBORO A,JIAO Y L,WANG P W.Bioin‐formatics analysis of disease resistance gene PR1 and its genetic transformation in soybeans and cultivation of multi-resistant ma‐terials[J].Phyton,2022,91(7):1445-1464.
[43] PIETERSE C M J,LEON-REYES A,VAN DER ENT S,VAN WEES S C M.Networking by small-molecule hormones in plant immunity[J].Nature Chemical Biology,2009,5(5):308-316.
[44] AGRAWAL G K,JWA N S,RAKWAL R. A novel rice (Oryza sativa L.) acidic PR1 gene highly responsive to cut,phytohor‐mones,and protein phosphatase inhibitors[J]. Biochemical and Biophysical Research Communications,2000,274(1):157-165.
[45] 尉春雪,苏浩天,张晓宇,何文菡,郑大柽,尹淑霞.外源水杨酸对草地早熟禾抗褐斑病的诱导与抗病基因PR1 和NPR1 的表达的影响[J].草业科学,2019,36(5):1249-1254.WEI Chunxue,SU Haotian,ZHANG Xiaoyu,HE Wenhan,ZHENG Dacheng,YIN Shuxia. Effects of exogenous salicylic acid on the resistance of Kentucky bluegrass to brown patch and expression of PR1 and NPR1 resistance genes[J]. Pratacultural Science,2019,36(5):1249-1254.
[46] 李卓静.H2S 通过CsMYB30 激活黄瓜病程相关蛋白表达[D].太原:山西大学,2021.LI Zhuojing.H2S activates the expression of cucumber pathogen‐esis related protein through CsMYB30[D]. Taiyuan:Shanxi Uni‐versity,2021.