桃(Prunus persica L.)是经济价值较高的核果类果树,由于其风味丰富,营养价值较高,而深受世界各国消费者的喜爱,是世界上许多国家的主要消费水果之一。桃起源于中国西部地区,栽培历史可追溯到4000多年前[1]。但目前中国桃产业整体质量水平不高,与发达国家存在差距,正处于数量型向质量型转变的关键时期[2]。目前果实品质已成为影响消费者选择的关键因素[3]。果皮色泽是果实外观品质的重要组成部分,也是消费者在选择水果时最直观的评价因素之一,包括两个方面:果皮着色与果皮底色。有关果皮着色的研究很多,但关于果皮底色的研究则相对较少,其中叶绿素含量对果皮底色有重要影响。
近年来,关于植物中叶绿素主要降解途径的研究结果已经被公认[4]。在叶绿素降解这一途径中叶绿素b首先被还原生成7-羟甲基叶绿素a,该产物由NONYELLOW COLORING1(NYC1)和NYC1- LIKE(NOL)编码的叶绿素b 还原酶催化。随后,7-羟甲基叶绿素a 被7-羟甲基叶绿素a 还原酶(7-hydroxy‐methyl chlorophyll a reductase,HCAR)还原为叶绿素a[5]。叶绿素a可以通过两条不同的途径转化为脱镁叶绿酸a(Pheophorbide a,pheide a)。(1)叶绿素a经叶绿素酶(Chlorophyllase,CLH)和脱镁螯合酶(Mgdechelatase,MDCase)催化生成脱镁叶绿酸a[6]。(2)叶绿素a 在脱镁螯合酶和脱镁叶绿素酶(Pheophytin‐ase,PPH)作用下生成脱镁叶绿酸a[7]。在这两个途径中,脱镁叶绿酸a是共同的中间产物。在脱镁叶绿酸a氧化酶(Pheophorbide a Oxygenase,PAO)和红色叶绿素降解产物还原酶(Red Chlorophyll Catabolites Reductase,RCCR)的催化下降解[4]。PAO 是代谢通路上控制叶绿素降解的重要基因,PAO 催化的卟啉环氧化开环是叶绿素降解的关键步骤。因而叶绿素的这条降解途径被称为PAO 降解途径[8]。SGR(Stay-green)、SGRL 基因的鉴定是近年来叶绿素降解调控研究中的一个里程碑[9]。SGR 与SGRL 基因与叶绿素的降解途径相关,可以通过招募叶绿素降解基因形成复合体,结合到光系统Ⅱ上,形成SGRCCE-LHC Ⅱ复合体,从而导致叶绿素降解[10]。
在春性品种小麦花后旗叶不断衰老的过程中,TaCLH1 的表达量下降、升高再下降[11]。PAO 基因会随着衰老表达上调;在西蓝花采后衰老过程中BoPAO 的表达上调[12];在拟南芥衰老过程中AtPAO表达逐渐升高,并在自然衰老时达到最高峰[13]。在桃果实发育的前期果肉呈现明显绿色时,PpSGR 转录水平较低。随着果实逐步成熟,果肉褪绿,表达量不断升高[14]。
套袋是一项被广泛认可的农业技术,通过人工方式干预果实的光照条件,以改善果实的外观色泽。目前在全球范围内被广泛应用于果树生产。研究显示,套袋能够提升果实表面的光洁度,从而改善果实的整体外观品质[15]。套袋(遮光)通过改变果皮中叶绿素含量来影响果皮底色。在对番石榴、荔枝、猕猴桃的研究中发现,套袋抑制果实叶绿素合成,降低果皮中的叶绿素含量[16-18]。但目前对桃果实套袋后果皮叶绿素降解基因表达等方面还未见相关报道。因此,笔者在本研究中以两个成熟期接近的中油18号和中油19号桃品种为材料,研究套袋对油桃果皮叶绿素降解及叶绿素降解基因表达的影响,对叶绿素降解相关基因的表达进行分析,筛选关键基因,以期探讨套袋影响桃果皮叶绿素降解的机制,寻找果皮底色形成的基础及改进的栽培措施,为深入研究桃果实底色的调控机制提供理论依据,进一步为桃产业套袋生产提供参考。
1 材料和方法
1.1 试验材料
供试材料来自中国农业科学院郑州果树研究所桃品种圃内,株行距为1.0 m×4.0 m,2017年定植,常规管理。以成熟期接近的2 个桃品种中油18 号(CN18)、中油19 号(CN19)为研究材料(图1),均于6月中上旬成熟。2023年5—6月,于果实成熟前44 d(花后45 d)开始使用外黄内黑双层袋套袋并取样,不套袋为对照。之后在果实成熟前30 d采摘树体外围中上部大小均匀、无病虫害、成熟度一致的果实30 个,带回实验室使用尼康700 D 相机于自然光下以黑色植绒布为背景拍照。以10 个果实为1 个样本,取样进行3 次重复,取样间隔7 d,在果实转色期每隔4 d 取样1 次,削取表皮进行液氮速冻,保存至-80 ℃冰箱备用。
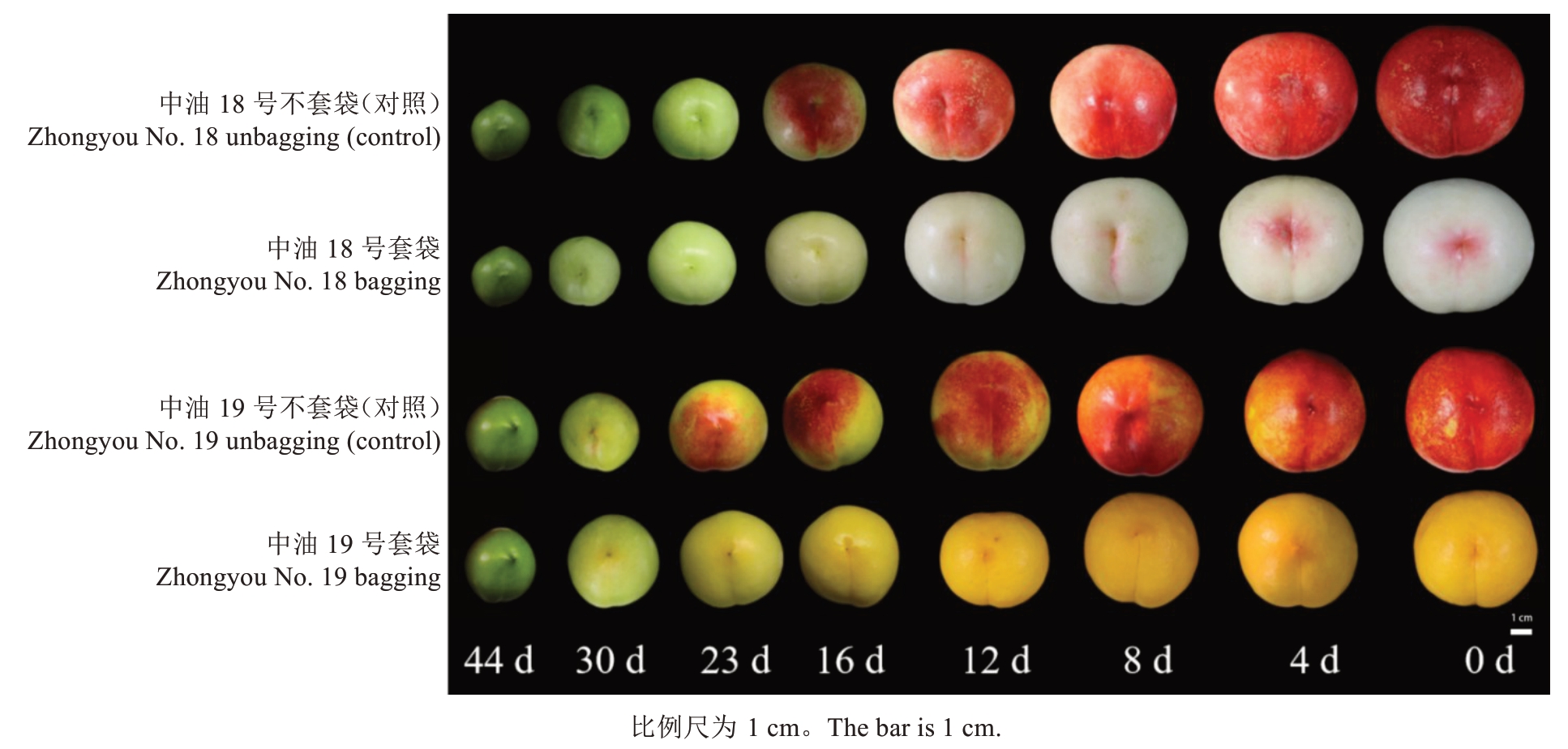
图1 成熟前44~0 d 的果实外观表型
Fig.1 Phenotypes of fruits 44-0 days before maturation
1.2 色差检测
用色差仪(美能达CR-400,柯尼卡美能达)评价果皮底色,颜色用CIE L*、a*、b*标尺表示。随机选取果实赤道区域的四个不同点取平均值,记录L*、a*和b*值[19-20]。
1.3 叶绿素含量测定
桃果皮叶绿素含量采用紫外分光光度法测定[21-22]。
1.4 RNA提取
根据多糖多酚植物总RNA 提取试剂盒(DP441,天根生化科技有限公司,北京,中国)说明书提取桃总RNA。利用1%琼脂糖凝胶电泳检测RNA 质量和纯度,取1 µL RNA 利用微量紫外分光光度计NanoDrop2000(Thermo Scientific,麻省)测定浓度。取1µg RNA参照FastKing cDNA第1链合成试剂盒说明书(天根生化科技有限公司,北京,中国)进行反转录,放置在-20 ℃冰箱保存进行后续的实时荧光定量PCR。
1.5 实时荧光定量检测
从基因组数据库(https://phytozome-next.jgi.doe.gov/)下载相关基因的CDS(Coding sequence,编码序列);利用Primer5.0 设计特异性荧光定量引物,引物长度在20 bp 左右,GC 含量在40%~60%,引物Tm 值在58~62 ℃,扩增片段大小为85~145 bp。选取Actin(ppa007228mg)为桃内参基因[23](表1),最后按照2-ΔΔCT方法计算结果[24]。
表1 实时定量PCR 反应中各基因的引物序列
Table 1 Primers sequence for real-time quantitative PCR reaction

基因名称Gene name Actin登记号Registration number(In GenBank)ppa007228m PpNYC1 ppa010004m PpNOL ppa005304m PpHCAR ppa004221m PpCLH1 ppa009825m PpCLH2 ppa009788m PpPPH ppa019738m PpPAO ppa009783m PpRCCR ppa004339m PpSGR ppa010416m PpSGRL引物序列Primer sequence(5′-3′)GATTCCGGTGCCCAGAAGT CCAGCAGCTTCCATTCCAA ATCGTGTGGTTGTCGCTTCT CAGGTGCTTAGAGGAGGCAC ATACGGGGCAACAAAGCGTA ACCATTCCTGGCGACAAGTT CAGTGGAAATCGCCAACCAT AACTTTGGGGCAGGTTCAGG CATGCCAAAACTGCCCTGTC AGGATATGGGGCCTGGTTCT TCTCACGGCTTCATTGTCGT TGAACATGGGGTGGAAGCAA AGACTCGGGGCTTAGTAGCA CGCTCCGTCTCTGACAAACT AGGCAACCCACGGATTACTG AGTCTTCCCTGGTGCCATTG ACATCCGCAGTGTTGTGTCT ATCCAGCCAAATTCCCAGCA GCTGTTGCTTCCCACCATTG TGTTTCTTGGGTTTGGCCCT TGACGTGGTTGCAGAATGGA GCCAGGTCCAGCATGAGATT ppa014909m
1.6 数据分析
使用SPSS 软件对数据进行分析,用Excel 2003软件制作图表。
2 结果与分析
2.1 套袋对桃果实发育后期果皮色差的影响
2.1.1 L*值的变化 由图2 可知,随着果实生长发育,中油18号不套袋和中油19号不套袋的果皮色差L*值在成熟前44~16 d 呈逐渐上升趋势,中油18 号不套袋的L*值从65.1 变化为69.5,提高6.75%;中油19号不套袋的L*值从63.0变化为68.1,提高8.09%,表明中油18 号不套袋和中油19 号不套袋果皮亮度逐渐升高,且中油19号不套袋亮度比中油18号不套袋升高更快;在成熟前16~0 d,中油18 号不套袋和中油19号不套袋的果皮色差L*值均逐渐降低。
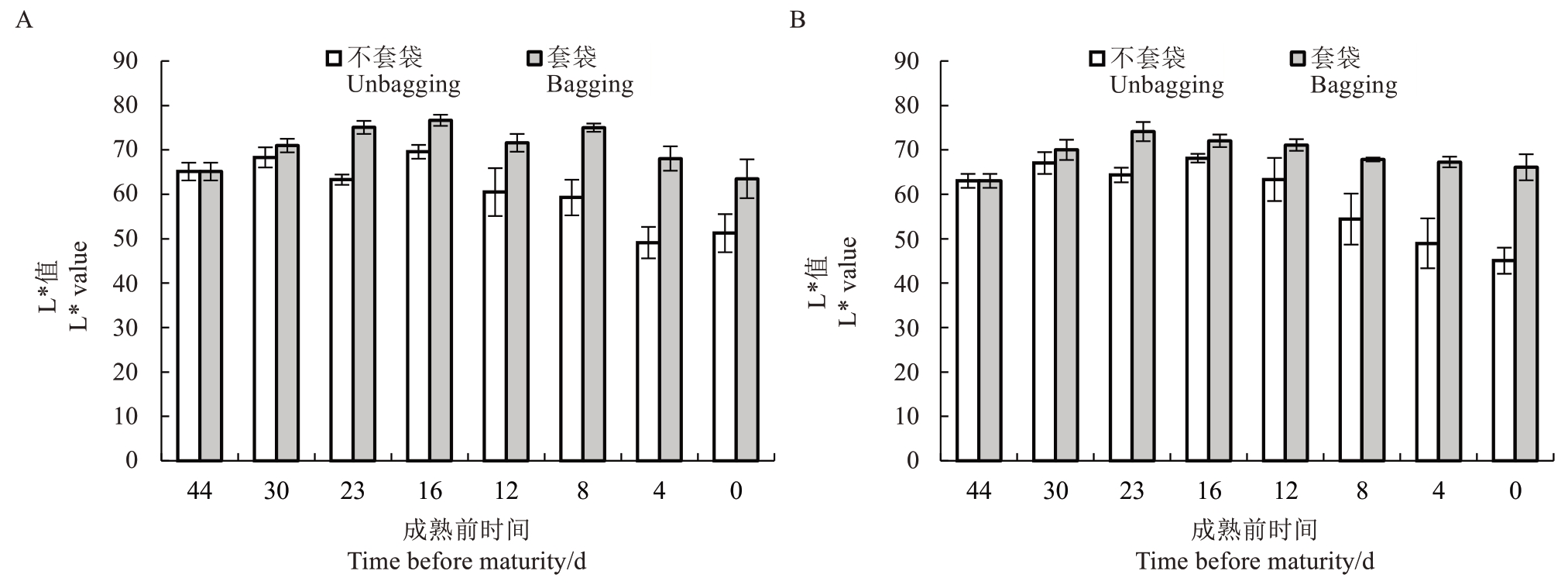
图2 中油18 号(A)、中油19 号(B)果实成熟前果皮色差L*值的变化
Fig.2 Changes in L*value of skin color difference before maturation of Zhongyou No.18(A)and Zhongyou No.19(B)fruits
中油18 号套袋的果皮色差L*值在成熟前44~16 d逐渐上升,中油18号套袋的L*值从65.1变化为76.6,提高18.6%;中油19号套袋的果皮色差L*值在成熟前44~23 d 也呈逐渐上升趋势,中油19 号套袋的L*值从63.0 变化为74.1,提高17.6%,可见套袋会明显提高果实的亮度。在成熟前16~0 d,中油18 号套袋的L*值逐渐降低,在成熟前23~0 d中油19号套袋的L*值逐渐降低。套袋会提升果实亮度,改善果实色泽。
2.1.2 a*值的变化 由图3可知,中油18号不套袋、中油18号套袋、中油19号不套袋和中油19号套袋在成熟前44~12 d 的果皮a*值(红绿色差)快速上升,在成熟前12 d 进入转色期,套袋比不套袋的a*值上升更快,所以套袋会加速果实成熟前44~12 d 的a*值上升。在成熟前12~0 d,相比于套袋,不套袋的a*值上升更快。相较于不套袋,套袋果实的着色更浅。

图3 中油18 号(A)、中油19 号(B)果实成熟前果皮色差a*值的变化
Fig.3 Changes in a*value of skin color difference before maturation of Zhongyou No.18(A)and Zhongyou No.19(B)fruits
2.1.3 b*值的变化 由图4 可知,中油18 号不套袋和中油18 号套袋果皮在成熟前44~0 d 的果皮b*值(黄蓝色差)呈快速下降趋势,中油18号不套袋的b*值在成熟前44~12 d期间下降了43.9%;中油18号套袋的b*值在成熟前44~12 d 期间下降趋势最快,下降了48.7%;并且中油18 号套袋比中油18 号不套袋的b*值下降的更早,可见套袋会加快白肉桃b*值下降。中油19 号不套袋果皮在成熟前44~0 d 的b*值(黄蓝色差)呈缓慢下降趋势,但中油19号套袋的b*值(黄蓝色差)在成熟前44~0 d呈先缓慢上升后逐渐下降趋势。中油19 号不套袋的b*值在成熟前44~12 d 期间变化并不明显,但在成熟前12~0 d 下降了37.2%;中油19号套袋的b*值在成熟前44~12 d期间有缓慢升高,升高了11.1%,之后缓慢下降;可见套袋会导致黄肉桃b*值升高。

图4 中油18 号(A)、中油19 号(B)果实成熟前果皮色差b*值的变化
Fig.4 Changes in b*value of skin color difference before maturation of Zhongyou No.18(A)and Zhongyou No.19(B)fruits
2.2 套袋对桃果实发育后期果皮叶绿素含量的影响
中油18 号套袋的外观表型比中油18 号不套袋提早由绿转白;中油19号套袋的外观表型比中油19号不套袋提早由绿转黄,如图5 所示,从成熟前44~12 d,套袋相较于不套袋的叶绿素含量显著减少,在成熟前12~0 d时,套袋的叶绿素有缓慢降低。
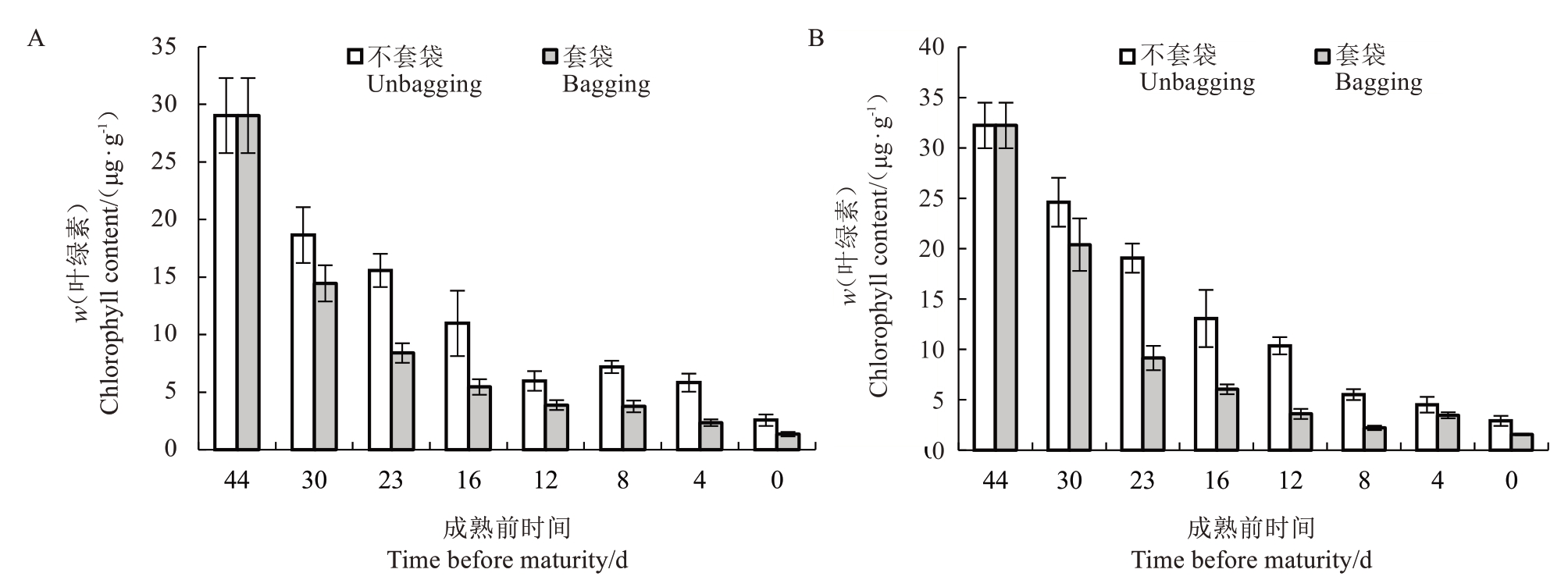
图5 中油18 号(A)、中油19 号(B)果实成熟前果皮叶绿素含量的变化
Fig.5 Changes in chlorophyll content in the peel of Zhongyou No.18(A)and Zhongyou No.19(B)fruits before maturity
2.3 套袋对桃果实发育后期叶绿素降解相关基因表达的影响
通过分析套袋处理2个桃品种中与叶绿素降解相关的10个基因(PpNYC1、PpNOL、PpHCAR、PpCLH1、PpCLH2、PpPPH、PpPAO、PpRCCR、PpSGR、PpS‐GRL)的相对转录水平,结果显示(图6),PpHCAR、PpCLH1、PpPPH、PpPAO、PpSGR在套袋果实中的表达量明显高于不套袋。PpNYC1在中油19 号套袋的成熟前16~12 d 有明显升高,PpNOL、PpHCAR 在中油19 号套袋的成熟前23~16 d 表达量明显上调。PpCLH2在中油18号套袋表达相比较于不套袋有升高,但在中油19 号套袋却被抑制。PpCLH1 在套袋果实的成熟前23 d和成熟前12 d表达量均高于不套袋,中油18号不套袋和中油19号不套袋只在成熟前12 d表达量升高,但在果实成熟时PpCLH1表达量下降。PpPAO、PpRCCR 在套袋的成熟前30~16 d 表达量高于不套袋。PpPPH在2个品种套袋遮光后有显著升高,并且PpPPH在中油19号套袋的成熟前23 d时显著上调。PpSGRL 基因在套袋中显著被抑制,但在中油19 号套袋的成熟前16 d 时表达明显升高,说明PpSGRL 有可能与光照有关。PpSGR 基因在中油18 号套袋和中油19 号套袋的成熟前23 d 的表达量有所升高,随着果实发育,果皮叶绿素降解,果实完全成熟,中油18 号不套袋、中油18 号套袋、中油19 号不套袋和中油19 号套袋中PpSGR 显著高表达。因此推测可能是光引起PpSGR提前表达。
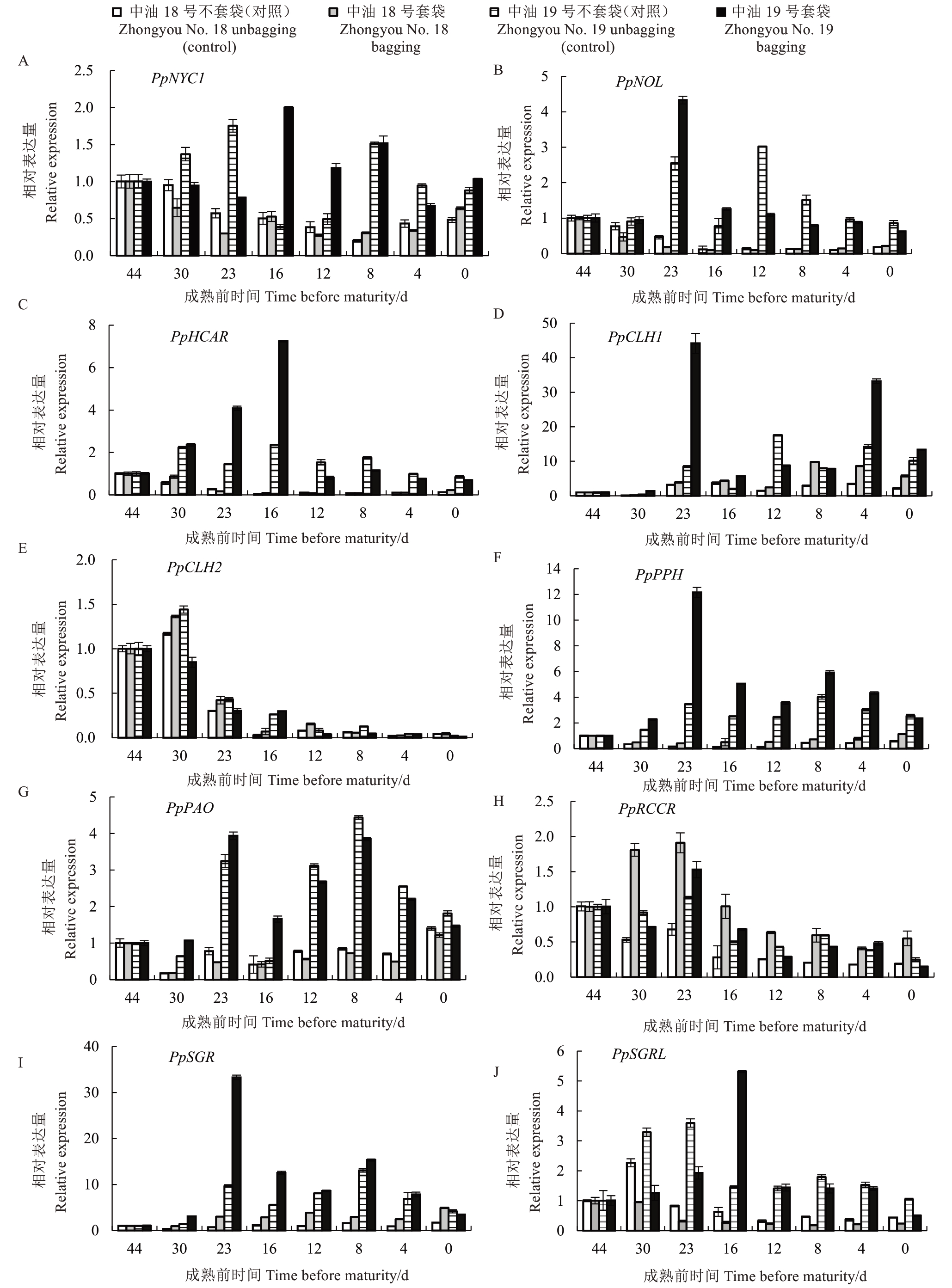
图6 叶绿素降解基因表达量
Fig.6 Expression of chlorophyll degradation gene
聚类分析(图7)显示在中油18 号不套袋中PpCLH1、PpPAO和PpSGR能聚到一类,中油18号套袋中PpCLH1、PpPPH、PpPAO 和PpSGR 能聚到一类。在中油19 号不套袋和中油19 号套袋中PpCLH1、PpPAO、PpPPH 和PpSGR 能聚到一类。在中油18 号不套袋、中油18 号套袋、中油19 号不套袋和中油19 号套袋中,PpNYC1、PpCLH2、PpRCCR、PpSGRL 能聚到一类。说明在两个品种叶绿素降解过程中PpCLH1、PpPAO、PpSGR的表达模式相似。

图7 中油18 号和中油19 号叶绿素降解基因的聚类分析
Fig.7 Cluster analysis of Chlorophyll degradation genes in Zhongyou No.18 and Zhongyou No.19
对叶绿素含量与其降解基因进行相关性分析(表2),发现PpCLH2 基因表达与中油18 号不套袋、中油18 号套袋、中油19 号不套袋、中油19 号套袋中叶绿素含量呈显著正相关,PpNYC1、PpNOL、PpH‐CAR 与中油18 号不套袋、中油18 号套袋的叶绿素含量呈显著正相关,PpSGR 与中油18号套袋的叶绿素含量呈显著负相关。
表2 叶绿素降解基因与叶绿素含量的相关系数
Table 2 The correlation coefficient between chlorophyll degradation genes and chlorophyll
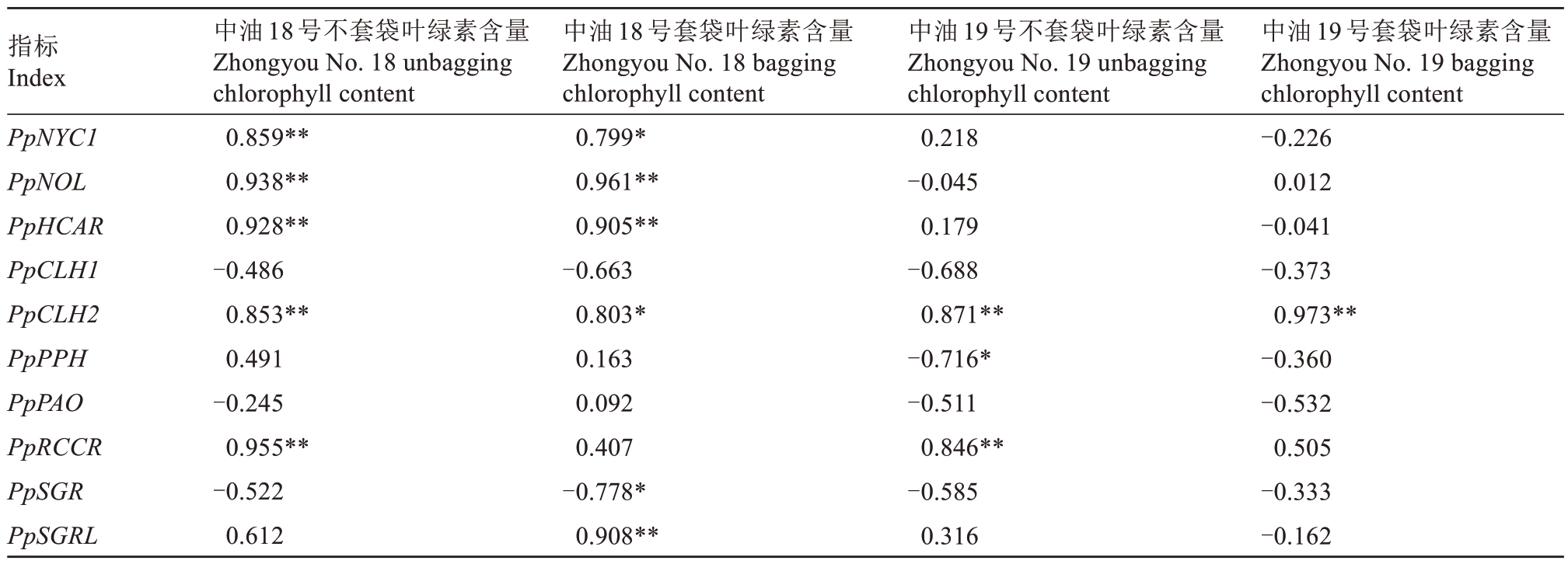
注:*和**分别表示相关性达到0.05 和0.01 显著水平。
Note:*and**respectively indicate that the correlation reaches a significant level of 0.05 and 0.01.
指标Index PpNYC1 PpNOL PpHCAR PpCLH1 PpCLH2 PpPPH PpPAO PpRCCR PpSGR PpSGRL中油18号不套袋叶绿素含量Zhongyou No.18 unbagging chlorophyll content 0.859**0.938**0.928**-0.486 0.853**0.491-0.245 0.955**-0.522 0.612中油18号套袋叶绿素含量Zhongyou No.18 bagging chlorophyll content 0.799*0.961**0.905**-0.663 0.803*0.163 0.092 0.407-0.778*0.908**中油19号套袋叶绿素含量Zhongyou No.19 bagging chlorophyll content-0.226 0.012-0.041-0.373 0.973**-0.360-0.532 0.505-0.333-0.162中油19号不套袋叶绿素含量Zhongyou No.19 unbagging chlorophyll content 0.218-0.045 0.179-0.688 0.871**-0.716*-0.511 0.846**-0.585 0.316
qRT-PCR 结果表明,PpCLH1 在套袋果实成熟前23 d 和12 d 的表达量均高于不套袋,PpPAO 在中油19 号果实成熟过程中表达量升高。PpSGR 基因在中油18 号和中油19 号套袋果实完全成熟时相较于不套袋的显著高表达,而经过套袋处理后在成熟前23 d 表达量显著升高。聚类分析显示在中油18号不套袋中PpCLH1、PpPAO 和PpSGR 能聚到一类,中油18 号套袋中PpCLH1、PpPPH、PpPAO 和PpS‐GR能聚到一类。在中油19号不套袋和中油19号套袋中PpCLH1、PpPAO、PpPPH 和PpSGR 能聚到一类。在中油18 号不套袋、中油18 号套袋、中油19 号不套袋和中油19 号套袋中,PpNYC1、PpCLH2、PpRCCR、PpSGRL 能聚到一类。相关性分析显示PpSGR 与中油18 号套袋的叶绿素含量呈显著负相关。初步表明PpCLH1、PpSGR 基因是果实成熟前果皮叶绿素降解的关键基因。
3 讨 论
果实的褪绿过程是一个复杂的生物学现象,它涉及果实成熟过程中叶绿素的降解过程。这个过程不仅受到果实内部遗传的调控,还受到外部环境的影响。特定的基因和转录因子参与调控叶绿素合成和降解的途径,决定了果实成熟过程中颜色变化的模式[25-27]。外部环境因子,如光照、温度等,也会影响叶绿素的稳定性和降解速率,进而影响果皮的褪绿过程和最终色泽[28-30]。冯静涵等[31]对翠冠梨果实进行套袋,与不套袋相比,套袋后果实的叶绿素含量下降,果面颜色变浅,L*值上升,外观品质提高。桃果实套袋可改变果实生长发育的微环境,使果面洁净,有效防止病虫害对果实的侵害,改善外观品质和内在品质,提高商品价值[32-33]。对陇蜜9 号桃果实进行套袋处理发现,套袋会提高果实的L*值,不套袋的果实L*值最低[34]。笔者在本试验中也发现套袋处理对油桃果实外观的亮度影响较大,套袋会极大地提高果实的亮度,果实成熟时中油18 号套袋和中油19 号套袋亮度分别上升了19.2%和31.8%,这与苹果中报道的研究结果相似[35-36]。李秋利等[37]以映霜红为材料进行套袋处理,发现相比于不套袋处理,套袋会提高果实b*值。笔者在本试验中发现套袋会让油桃果实色差a*值在转色期前快速升高,在黄肉品种中油19中套袋会提高果实b*值,果实成熟时中油19 号套袋的b*值相较于中油19 号不套袋(对照)上升了36.9%。该结果也与前人的研究相符[38-39]。马瑞娟等[40]对油桃进行套袋试验,结果显示,与不套袋相比,果实的L*值提高,色素显著降低,其中果实色素叶绿素a/b 显著降低。姜新等[41]对秋蜜桃1 号进行套袋处理,结果表明外黄内黑双层果袋套袋的果皮色素含量低、果实外观较美观。笔者在本研究中发现,套袋处理均能显著降低中油18号和中油19号果实的叶绿素含量。马英桃等[42]对春艳和春蜜2个桃品种进行套袋处理,结果表明套袋果实中叶绿素含量低于不套袋。
虽然前人对桃套袋后果皮褪绿机制已从生理角度有所探索,但对叶绿素降解基因的表达情况并未进行更深一步的研究,因此,笔者课题组对桃果实成熟过程中叶绿素降解基因的表达情况进行了分析。PpCLH1 在套袋果实成熟前23 d 和12 d 的表达量均高于不套袋,在中油18号不套袋和中油19号不套袋中PpCLH1 在成熟前12 d 表达量升高,但在果实成熟时PpCLH1 表达量下降。杨林先等[43]对苹果梨进行套袋处理,发现盛花后90~120 d,即果实成熟前一个月,叶绿素酶(CLH)的活性明显升高。PpPPH 在套袋遮光后有显著升高,并且PpPPH在中油19号套袋的成熟前23 d时显著上调。陈成等[44]以海沃德猕猴桃为材料进行套袋发现套袋处理后AdPPH 的表达丰富有显著升高。这说明套袋可能会诱导叶绿素酶(CLH)和脱镁叶绿素酶(PPH)的表达。PpSGR 基因在中油18 号和中油19 号果实完全成熟时显著高表达,但在套袋果实中成熟前23 d 的表达量有所升高,并且表达提前。因此推测可能是套袋遮光引起PpCLH1、PpSGR提前表达。
4 结 论
笔者在本试验中选取中油18号和中油19号进行套袋处理,发现套袋处理极大地提高了桃果实外观的亮度(L*值)。在黄肉品种中油19号中套袋会提高果实b*值。荧光定量结果显示,PpCLH1在套袋果实成熟前23 d 和12 d 的表达量均高于不套袋,PpPAO 在中油19号果实成熟过程中表达量升高。PpSGR基因在中油18 号和中油19 号果实完全成熟时相较于不套袋的显著高表达,而经过套袋处理后在成熟前23 d会提前表达,且表达量显著升高。表明PpCLH1、PpPAO、PpSGR的表达导致果皮中叶绿素含量下降。
[1] 俞明亮,王力荣,王志强,彭福田,张帆,叶正文.新中国果树科学研究70 年:桃[J].果树学报,2019,36(10):1283-1291.YU Mingliang,WANG Lirong,WANG Zhiqiang,PENG Futian,ZHANG Fan,YE Zhengwen. Fruit scientific research in New China in the past 70 years:Peach[J]. Journal of Fruit Science,2019,36(10):1283-1291.
[2] 王力荣.我国桃产业现状与发展建议[J].中国果树,2021(10):1-5.WANG Lirong. Current situation and development suggestions of peach industry in China[J].China Fruits,2021(10):1-5.
[3] 徐强,郝玉金,黄三文,邓秀新.果实品质研究进展[J].中国基础科学,2016,18(1):55-62.XU Qiang,HAO Yujin,HUANG Sanwen,DENG Xiuxin. Ad‐vances in fruit quality researches[J].China Basic Science,2016,18(1):55-62.
[4] HÖRTENSTEINER S. Chlorophyll degradation during senes‐cence[J].Annual Review of Plant Biology,2006,57:55-77.
[5] SATO Y,MORITA R,NISHIMURA M,YAMAGUCHI H,KU‐SABA M. Mendel’s green cotyledon gene encodes a positive regulator of the chlorophyll-degrading pathway[J]. Proceedings of the National Academy of Sciences of the United States of America,2007,104(35):14169-14174.
[6] SHEMER T A,HARPAZ-SAAD S,BELAUSOV E,LOVAT N,KROKHIN O,SPICER V,STANDING K G,GOLDSCHMIDT E E,EYAL Y. Citrus chlorophyllase dynamics at ethylene-in‐duced fruit color-break:A study of chlorophyllase expression,posttranslational processing kinetics,and in situ intracellular lo‐calization[J].Plant Physiology,2008,148(1):108-118.
[7] SCHELBERT S,AUBRY S,BURLA B,AGNE B,KESSLER F,KRUPINSKA K,HÖRTENSTEINER S. Pheophytin pheophor‐bide hydrolase(pheophytinase)is involved in chlorophyll break‐down during leaf senescence in Arabidopsis[J]. The Plant Cell,2009,21(3):767-785.
[8] HÖRTENSTEINER S. Update on the biochemistry of chloro‐phyll breakdown[J]. Plant Molecular Biology,2013,82(6):505-517.
[9] HÖRTENSTEINER S. Stay- green regulates chlorophyll and chlorophyll-binding protein degradation during senescence[J].Trends in Plant Science,2009,14(3):155-162.
[10] SAKURABA Y,SCHELBERT S,PARK S Y,HAN S H,LEE B D,ANDRÈS C B,KESSLER F,HÖRTENSTEINER S,PAEK N C. STAY-GREEN and chlorophyll catabolic enzymes interact at light-harvesting complex II for chlorophyll detoxification dur‐ing leaf senescence in Arabidopsis[J]. The Plant Cell,2012,24(2):507-518.
[11] 赵锴. 小麦叶绿素酶1(TaCLH1)基因的关联分析与功能验证[D].太谷:山西农业大学,2022.ZHAO Kai. Association analysis and functional verification of chlorophyllase 1 gene(TaCLH1)in wheat[D].Taigu:Shanxi Ag‐ricultural University,2022.
[12] GOMEZ-LOBATO M E,CIVELLO P M,MARTÍNEZ G A. Ef‐fects of ethylene,cytokinin and physical treatments on BoPaO gene expression of harvested broccoli[J]. Journal of the Science of Food and Agriculture,2012,92(1):151-158.
[13] PRUZINSKÁ A,TANNER G,ANDERS I,ROCA M,HÖRTENSTEINER S. Chlorophyll breakdown:Pheophorbide a oxygenase is a Rieske-type iron-sulfur protein,encoded by the accelerated cell death 1 gene[J]. Proceedings of the National Academy of Sciences of the United States of America,2003,100(25):15259-15264.
[14] 邵允,张蒙蒙,陈云,王晓菲,董康,刘宁,张郎郎,谭彬,王伟,程钧,冯建灿.桃PpSGR 基因功能鉴定及其对乙烯合成的调控[J].果树学报,2023,40(12):2513-2523.SHAO Yun,ZHANG Mengmeng,CHEN Yun,WANG Xiaofei,DONG Kang,LIU Ning,ZHANG Langlang,TAN Bin,WANG Wei,CHENG Jun,FENG Jiancan. Function identification of PpSGR gene and its regulation of ethylene synthesis in peach[J].Journal of Fruit Science,2023,40(12):2513-2523.
[15] 王永博,李勇,李晓,刘国胜,王亚茹,王迎涛.果实套袋对梨果综合品质的影响[J].河北果树,2018(5):30-32.WANG Yongbo,LI Yong,LI Xiao,LIU Guosheng,WANG Ya‐ru,WANG Yingtao. The influence of fruit bagging on the com‐prehensive quality of pear fruit[J].Hebei Fruits,2018(5):30-32.
[16] 王惠聪,黄旭明,黄辉白.‘妃子笑’荔枝果实着色不良原因的研究[J].园艺学报,2002,29(5):408-412.WANG Huicong,HUANG Xuming,HUANG Huibai. A study on the causative factors retarding pigmentation in the fruit of‘Feizixiao’litchi[J]. Acta Horticulturae Sinica,2002,29(5):408-412.
[17] 李平,郑润泉,温华良,陈伟光,吴拥军,罗松.套袋对新世纪番石榴果皮色素及酚类物质的影响[J]. 果树学报,2003,20(2):120-123.LI Ping,ZHENG Runquan,WEN Hualiang,CHEN Weiguang,WU Yongjun,LUO Song. Effects of bagging on pigments and total phenol in Xinshiji guava fruit skin[J]. Journal of Fruit Sci‐ence,2003,20(2):120-123.
[18] 姜新,陈伯伦,刘芸,张晋,黄继魁,王茜,李一伟.不同果袋对红阳猕猴桃果实色泽及花青苷合成相关基因表达的影响[J].福建农业学报,2023,38(9):1054-1063.JIANG Xin,CHEN Bolun,LIU Yun,ZHANG Jin,HUANG Ji‐kui,WANG Xi,LI Yiwei. Effects of various fruit- bagging pouches on coloration and anthocyanin synthesis related gene expression of Hongyang kiwifruits[J]. Fujian Journal of Agricul‐tural Sciences,2023,38(9):1054-1063.
[19] 李桂祥,马瑞娟,张斌斌,俞明亮,倪林箭.套袋对霞晖6 号桃果实发育过程中果皮色素含量和色差的影响[J].江苏农业学报,2012,28(6):1418-1423.LI Guixiang,MA Ruijuan,ZHANG Binbin,YU Mingliang,NI Linjian. Effect of bagging on peel pigment content and fruit chromatism of peach cultivar Xiahui 6[J].Jiangsu Journal of Ag‐ricultural Sciences,2012,28(6):1418-1423.
[20] 郭东花,白红,石佩,杨艳青,李高潮,范崇辉.不同时期套袋对“瑞光19 号”油桃果实挥发性成分及着色的影响[J].食品科学,2016,37(8):242-247.GUO Donghua,BAI Hong,SHI Pei,YANG Yanqing,LI Gao‐chao,FAN Chonghui. Effects of bagging at different stages on volatiles and color of“Ruiguang No. 19”nectarine fruits[J].Food Science,2016,37(8):242-247.
[21] 项倩,吴磊,徐若涵,杨再强.不同温度下染病番茄叶片SPAD和叶绿素含量的相关性[J].北方园艺,2022(18):8-15.XIANG Qian,WU Lei,XU Ruohan,YANG Zaiqiang. Correla‐tion between SPAD and chlorophyll content in infected tomato leaves at different temperatures[J]. Northern Horticulture,2022(18):8-15.
[22] 刘建新,丁华侨,田丹青,王炜勇,刘慧春.擎天凤梨苞片叶绿素代谢关键基因的分离及褪绿的分子机理[J].中国农业科学,2016,49(13):2593-2602.LIU Jianxin,DING Huaqiao,TIAN Danqing,WANG Weiyong,LIU Huichun. Isolation of chlorophyll metabolism key genes and molecular mechanism of green fade in Guzmania bracts dis‐coloration process[J]. Scientia Agricultura Sinica,2016,49(13):2593-2602.
[23] BRANDI F,BAR E,MOURGUES F,HORVÁTH G,TURCSI E,GIULIANO G,LIVERANI A,TARTARINI S,LEWINSOHN E,ROSATI C. Study of‘Redhaven’peach and its white-fleshed mutant suggests a key role of CCD4 carotenoid dioxygenase in carotenoid and norisoprenoid volatile metabolism[J]. BMC Plant Biology,2011,11:24.
[24] SCHMITTGEN T D,LIVAK K J.Analyzing real-time PCR data by the comparative C(T) method[J]. Nature Protocols,2008,3(6):1101-1108.
[25] YIN X R,XIE X L,XIA X J,YU J Q,FERGUSON I B,GIOVANNONI J J,CHEN K S. Involvement of an ethylene re‐sponse factor in chlorophyll degradation during citrus fruit de‐greening[J].The Plant Journal,2016,86(5):403-412.
[26] WU Y Y,WANG L L,LIN Y L,LI X,LIU X F,XU Z H,FU B L,WANG W Q,ALLAN A C,TU M Y,YIN X R.AcHZP45 is a repressor of chlorophyll biosynthesis and activator of chloro‐phyll degradation in kiwifruit[J]. Journal of Experimental Bota‐ny,2024,75(1):204-218.
[27] ZOU S C,ZHUO M G,ABBAS F,HU G B,WANG H C,HUANG X M.Transcription factor LcNAC002 coregulates chlo‐rophyll degradation and anthocyanin biosynthesis in litchi[J].Plant Physiology,2023,192(3):1913-1927.
[28] AIAMLA-OR S,SHIGYO M,YAMAUCHI N. UV-B treatment controls chlorophyll degradation and related gene expression in broccoli(Brassica oleracea L.Italica Group)florets during stor‐age[J].Scientia Horticulturae,2019,243:524-527.
[29] LUO Z D,ZHANG J H,LI J H,YANG C X,WANG T T,OUY‐ANG B,LI H X,GIOVANNONI J,YE Z B. A STAY-GREEN protein SlSGR1 regulates lycopene and β-carotene accumula‐tion by interacting directly with SlPSY1 during ripening process‐es in tomato[J].The New Phytologist,2013,198(2):442-452.
[30] WEI W,YANG Y Y,LAKSHMANAN P,KUANG J F,LU W J,PANG X Q,CHEN J Y,SHAN W. Proteasomal degradation of MaMYB6O mediated by the E3 ligase MaBAH1 causes high temperature-induced repression of chlorophyll catabolism and green ripening in banana[J]. The Plant Cell,2023,35(5):1408-1428.
[31] 冯静涵,徐泽帆,许建锋,常晓晓,刘越飞,马辉.不同果袋对‘翠冠’梨果实品质的影响[J]. 广东农业科学,2023,50(10):110-119.FENG Jinhai,XU Zefam,XU Jianfeng,CHANG Xiaoxiao,LIU Yuefei,MA Hui. Effect of different fruit bags on fruit quality of‘Cuiguan’pear [J]. Guangdong Agricultural Sciences,2023,50(10):110-119.
[32] 马瑞娟,张斌斌,蔡志翔,倪林箭,李桂祥,丁辉.不同类型果袋对霞光油桃果实品质的影响[J]. 江苏农业学报,2012,28(3):627-631.MA Ruijuan,ZHANG Binbin,CAI Zhixiang,NI Linjian,LI Guixiang,DING Hui. Effect of different bags on fruit quality of Xiaguang nectarine[J].Jiangsu Journal of Agricultural Sciences,2012,28(3):627-631.
[33] DE JESUS B LA,ALVARENGA Â A,MALTA M R,GEBERT D,DE LIMA E B. Chemical evaluation and effect of bagging new peach varieties introduced in southern Minas Gerais-Brazil[J].Food Science and Technology,2013,33(3):434-440.
[34] 牛茹萱,赵秀梅,王晨冰,张译文,王发林. 不同套袋处理对陇蜜9 号桃果实品质的影响[J].甘肃农业科技,2020(11):25-29.NIU Ruxuan,ZHAO Xiumei,WANG Chenbing,ZHANG Yiwen,WANG Falin. Effects of different bagging treatments on peach fruit quality of Longmi 9[J]. Gansu Agricultural Science and Technology,2020(11):25-29.
[35] 张小军.‘澳洲青苹’苹果套袋处理后果实着色相关基因的克隆及表达分析[D].杨凌:西北农林科技大学,2013.ZHANG Xiaojun.Cloningin and expression analysis of color re‐lated genes in‘Granny Smith’apple after bagging treatment[D].Yangling:Northwest A&F University,2013.
[36] 景晨娟. 套袋对苹果果皮着色的影响及其相关基因表达分析[D].杨凌:西北农林科技大学,2017.JING Chenjuan. Effect of bagging on apple fruit coloring and the expression of related genes[D]. Yangling:Northwest A & F University,2017.
[37] 李秋利,高登涛,魏志峰,杨文佳,刘军伟,韩园园.不同套袋处理对映霜红桃果实品质的影响[J]. 河南农业科学,2017,46(12):95-102.LI Qiuli,GAO Dengtao,WEI Zhifeng,YANG Wenjia,LIU Jun‐wei,HAN Yuanyuan. Effect of different bagging treatments on fruit quality of yingshuanghong peach[J].Journal of Henan Agri‐cultural Sciences,2017,46(12):95-102.
[38] 刘玉莲.不同色泽类型苹果着色期糖酸变化及花青苷合成特性研究[D].杨凌:西北农林科技大学,2013.LIU Yulian. Study on the changes of sugars,acids and anthocy‐anin biosynthes is in the different apples during coloration[D].Yangling:Northwest A&F University,2013.
[39] SHARMA R R,PAL R K,ASREY R,SAGAR V R,DHIMAN M R,RANA M R. Pre-harvest fruit bagging influences fruit col‐or and quality of apple cv. Delicious[J]. Agricultural Sciences,2013,4(9):443-448.
[40] 马瑞娟,张斌斌,张春华,蔡志翔,严娟.套袋对金陵黄露桃果实品质的影响[J].江苏农业学报,2014,30(5):1127-1131.MA Ruijuan,ZHANG Binbin,ZHANG Chunhua,CAI Zhixiang,YAN Juan.Effect of bagging on quality of Jinlinghuanglu peach[J]. Jiangsu Journal of Agricultural Sciences,2014,30(5):1127-1131.
[41] 姜新,罗瑞鸿,李一伟,阮经宙,刘芸.不同类型果袋对秋蜜桃1 号果实品质形成和果皮色素变化的影响[J].西南农业学报,2021,34(11):2473-2481.JIANG Xin,LUO Ruihong,LI Yiwei,RUAN Jingzhou,LIU Yun. Effects of different bagging on fruit quality and variation of skin pigment of Qiumitao No. 1[J]. Southwest China Journal of Agricultural Sciences,2021,34(11):2473-2481.
[42] 马英桃,赵雪姣,戴茜倩,董佳丽.遮光性套袋对桃果实品质的影响[J].科技视界,2018(3):26-27.MA Yingtao,ZHAO Xuejiao,DAI Xiqian,DONG Jiali. Effects of bagging with opaque paper on peach fruit quality[J]. Science&Technology Vision,2018(3):26-27.
[43] 杨林先,李雄,朴哲虎,金日,刘冰雁.套袋处理对苹果梨品质及果皮色素形成相关酶活性的影响[J]. 延边大学农学学报,2023,45(1):20-24.YANG Linxian,LI Xiong,PIAO Zhehu,JIN Ri,LIU Bingyan.Effects of bagging treatments on quality of Pingguoli and activi‐ties of pigment-formation enzymes in pericarps[J]. Agricultural Science Journal of Yanbian University,2023,45(1):20-24.
[44] 陈成,王依,宋思言,杨勇,万春雁,阎永齐.套袋对海沃德猕猴桃果实品质及叶绿素代谢的影响[J].西北农林科技大学学报(自然科学版),2022,50(7):138-146.CHEN Cheng,WANG Yi,SONG Siyan,YANG Yong,WAN Chunyan,YAN Yongqi. Effect of bagging on fruit quality and chlorophyll metabolism of Hayward kiwifruit[J]. Journal of Northwest A& F University (Natural Science Edition),2022,50(7):138-146.