桃是我国重要的栽培果树之一,其味甜多汁、香气浓郁、营养物质丰富,深受广大消费者喜爱。类胡萝卜素是植物体次生代谢产物中的一类重要物质,是人体维生素A 生物合成的前体,并且具有抗氧化作用[1]。随着人民生活水平不断提高,人们更加注重健康,黄肉桃因富含类胡萝卜素而受到追捧。类胡萝卜素裂解加氧酶基因家族(carotenoid cleavage dioxygenases,CCDs)是类胡萝卜素降解的关键基因[2]。白肉桃中由于PpCCD4 高表达而引起类胡萝卜素降解,导致果实中不能积累类胡萝卜素;而黄肉桃中由于PpCCD4 基因突变,类胡萝卜素降解受阻而大量积累,果肉呈现黄色[3-5]。深入研究类胡萝卜素生物合成和积累的代谢调控网络,对富含类胡萝卜素桃品种改良具有重要意义。
WRKY转录因子是植物中特有的一类转录因子超家族[6]。WRKY 基因以高度保守的WRKY 结构域而命名,其包含一个七肽序列(WRKYGQK),并紧邻一个Cx4 - 5Cx22 - 23HxH 或Cx7Cx23HxC 的锌指蛋白基序[7]。WRKY 转录因子根据其DNA 结合结构域的数量和锌指蛋白基序的特征可分为三类。第Ⅰ类具有两个WRKY 结构域。第Ⅱ类只有一个WRKY 结构域,其锌指蛋白基序与第Ⅰ类相同,为Cys(2)-His(2),其包含的WRKY 基因最多;基于氨基酸序列差异,Ⅱ类可进一步划分为Ⅱa、Ⅱb、Ⅱc、Ⅱd 和Ⅱe 5个亚群。第Ⅲ类WRKY 转录因子锌指蛋白基序为Cx7Cx23HxC[8]。WRKY 家族成员在植物中被相继发现和鉴定,其在不同植物中数量各不相同,如在水稻(Oryza sative)中存在109 个[9]、玉米中(Zea mays)119 个[10]、拟南芥中(Arabidopsis thaliana)74个[11]、苹果中(Malus domestica)56 个[12]、番茄中(So‐lanum lycopersicum)81 个[13]和桃中(Prunus persica)58 个[14]。
WRKY 蛋白通常作为抑制因子或激活因子,参与植物多种发育过程,如生物胁迫、非生物胁迫、发育过程以及生物合成等[7-8,15]。过表达小麦TaWRKY2 转录因子调节抗旱相关基因表达,进而增强小麦植株抗旱性[16]。甘蔗ScWRKY3 负调控烟草茄病镰刀菌蓝色变种的抗性[17]。OsWRKY45 的过表达除了增强抗病能力外,还增强了耐盐性和耐旱性[18]。WRKY 转录因子调控植物对温度胁迫的抗性,包括对高温[19]和冷胁迫[20-21]。越来越多的研究证明,WRKY 转录因子参与植物次生物质代谢过程。桂花中OfWRKY3 通过调控OfCCD4 表达进而可能影响挥发性物质β-ionone合成[22]。番茄WRKY32基因调控YELLOW FRUITED-TOMATO 1 基因表达,调控类胡萝卜素积累,进而影响果实颜色[23]。Sl‐WRKY35 通过激活番茄果实MEP(2-C-methyl-Derythritol 4-phosphate)通路正向调控类胡萝卜素生物合成[24]。虽然WRKY 参与植物代谢调控逐渐增多,但是,WRKY 基因是否参与调控桃果实类胡萝卜素降解尚不清楚。基于此,笔者在本研究中测定了中油桃14号果实四个时期(S1、S2、S3、S4)类胡萝卜素含量变化和PpCCD4 基因表达趋势,PpCCD4基因启动子元件分析发现其包含WRKY 转录因子结合元件,进一步克隆了PpWRKY4,并初步验证了PpWRKY4 能够调控PpCCD4 基因表达。研究结果将有助于对桃类胡萝卜素积累分子机制的理解,同时为桃果肉颜色改良提供理论基础。
1 材料和方法
1.1 试验材料
中油桃14 号果实取自河南农业大学科教园区,分别采集果实的S1、S2、S3 和S4 四个时期的果肉组织进行类胡萝卜素提取和检测,提取DNA 用于PpCCD4基因启动子克隆,提取RNA用于PpWRKY4基因克隆和qRT-PCR分析。
1.2 类胡萝卜素提取和检测
称取5 g 研磨成粉的桃果实样品进行类胡萝卜素提取。在样品中加入色素提取液(正己烷、丙酮、无水酒精体积比2∶1∶1,含0.01%BHT)。采用UV755B型紫外可见分光光度计在波长450 nm下进行扫描,测定桃果实总类胡萝卜素含量。类胡萝卜素的提取与测定参考朱运钦等[25]的方法。
1.3 PpCCD4基因启动子克隆和转录因子结合位点预测
以中油桃14 号的DNA 为模板,使用引物(PpCCD4-p-F:TGGTAGTTACTAGGGTGTTGTT‐GCC,PpCCD4-p-R:AAAAAGGTAGTGAGGTGT‐GGGA)进行启动子序列扩增。将克隆得到的PpCCD4 启动子序列利用PlantRegMap(https://plantregmap.gao-lab.org/)网站预测转录因子结合位点,并利用GSDS(https://gsds.gao-lab.org/)绘制结合位点图谱。
1.4 PpWRKY4基因克隆及蛋白序列分析
以中油桃14 号的cDNA 为模板,使用高保真酶2×Phanta Flash Master Mix(P520,诺唯赞生物科技)和引物(PpWRKY4- G- F:ATGGACGCAACCA‐CACTC,PpWRKY4- G- R:CTATGGACCTGTTAG‐TACCCTT)进行编码区段(coding sequence,CDS)扩增;使用MEGA11 软件,将拟南芥AtWRKY20、葡萄VvWRKY20 和桂花OfWRKY3 蛋白氨基酸序列进行比对分析。PCR扩增和载体构建参照谭彬等[26]的方法进行。
1.5 基因表达分析
将中油桃14 号发育期果实的RNA 进行反转录合成cDNA,利用实时荧光定量PCR 仪检测PpCCD4 和PpWRKY4 基因相对表达量。定量引物序列:PpCCD4-F:GGCTAGAGAGCCCGAGA‐ATC,PpCCD4-R:GAGGAGACTTGGCATCCATC;PpWRKY4-F:GATCGGCCGTGATGACAAGC,Pp-WRKY4-R:CCAACATCCATCCTCCTCCTT,内参基因PpEF2(Elongation factor 2),PpEF2-F:GGTGT‐GACGATGAAGAGTGATG,PpEF2- R:TGAAGGAGAGGGAAGGTGAAAG。利用2-ΔΔCT法计算基因相对表达量[26],每个样品3次重复。
1.6 亚细胞定位
将PpWRKY4编码区序列(不含终止密码子)克隆到含有绿色荧光蛋白的载体pSAK277-GFP上,构建PpWRKY4-GFP 融合表达载体,将融合表达载体转入GV3101 农杆菌菌株中。将阳性菌液注射烟草叶片,48 h 后取样,使用激光共聚焦显微镜(尼康A1R HD25)观察荧光信号。
1.7 酵母单杂交验证PpWRKY4 结合PpCCD4 启动子
将PpCCD4 基因起始密码子上游2000 bp 片段(PpCCD4-promoter)连接到pAbAi载体上,之后转化到Y1H Gold酵母菌株中,在SD/-Ura琼脂板上培养2~3 d,筛选阳性菌株。随后,筛选PpAbAi-PpCCD4-promoter背景抑制质量浓度200 ng·mL-1的AbA。
将PpWRKY4 的编码区序列连接到pGADT7 载体上,将pGADT7- PpWRKY4 质粒转化进含PpAbAi-PpCCD4-promoter 质粒的酵母菌株中。将阳性对照(pAbAi-p53+ pGADT7-rec-53)、阴性对照(pAbAi-PpCCD4-promoter+pGADT7-EMPTY)和试验 组(pAbAi- PpCCD4- promoter + pGADT7-PpWRKY4),分别在SD-leu和SD-leu+200 ng·mL-1 AbA培养基中观察酵母生长情况。
1.8 双荧光素酶报告系统检测PpWRKY4 对PpCCD4的调控作用
将PpCCD4-promoter 连接到pGreenII 0800-LUC 载体中作为报告质粒,将PpWRKY4 编码序列连接到pSAK277载体上作为效应质粒。将两种质粒分别转化农杆菌,并进行扩繁(OD600=0.9~1.1)。离心后(10 min,4000 r·min-1),使用重悬液(0.5 mol·L-1 MES,10 mmol·L-1 MgCl2,100 mmol·L-1 AS)重悬菌体并调整菌液OD600=0.8 左右。注射烟草叶片,培养48 h 后,按照Promega 双荧光素酶报告基因检测试剂盒实验方法,测定酶活性。
1.9 数据统计与分析
利用SPSS 17.0软件对数据进行显著性分析,采用Excel 2010进行数据统计和作图。
2 结果与分析
2.1 中油桃14 号果实类胡萝卜素含量及PpCCD4表达模式分析
对不同发育阶段中油桃14 号果实类胡萝卜素含量进行测定,结果显示,在果实发育S1 时期类胡萝卜素含量为1.97 μg·g-1,随后类胡萝卜素含量急剧下降,S2 时期为0.64 μg·g-1,S2-S4 时期果实中类胡萝卜素含量差异不显著(图1-A)。对类胡萝卜素裂解加氧酶PpCCD4 基因在中油桃14 号果实不同发育时期表达分析显示,其在S1 时期低表达,随后急剧上升,说明类胡萝卜素的降解是由PpCCD4 的高表达所导致的。对PpCCD4 上游2000 bp 序列进行转录因子结合元件分析,发现区域内包含多种转录因子结合基序,包括Dof、MADS、MYB、bHLH 以及WRKY 等(图1-B)。由此推测,PpCCD4 可能会受到WRKY、MYB等转录因子的调控。
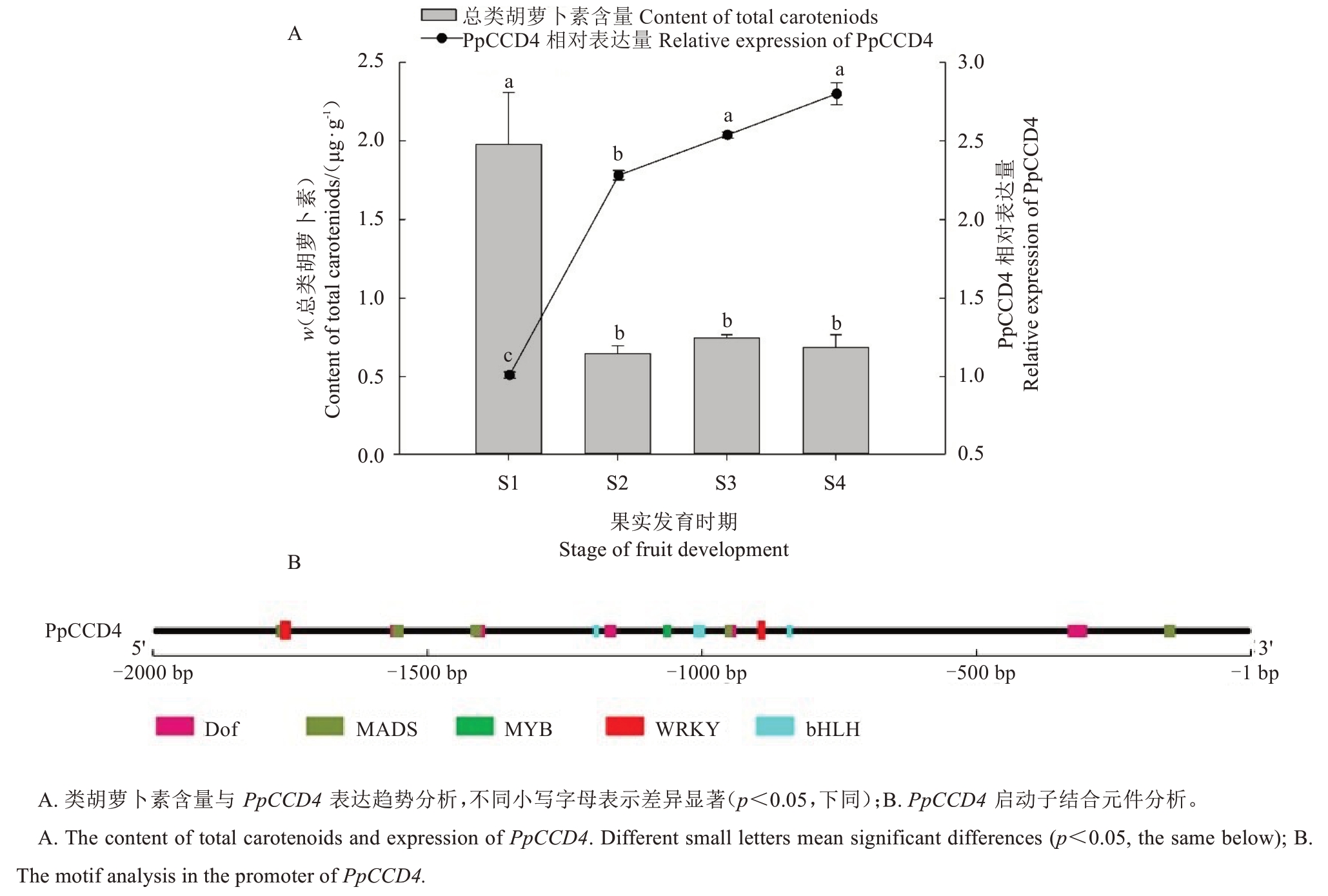
图1 中油桃14 号不同时期果实类胡萝卜素含量与PpCCD4 表达及启动子结合元件分析
Fig.1 The content of total caroteniods and expression pattern of PpCCD4 in the fruit of Zhongyoutao14 at different stages and motif analysis in the promoter of PpCCD4
2.2 PpWRKY4基因克隆与表达分析
以中油桃14 号果肉组织cDNA 为模板克隆PpWRKY基因,测序分析发现,其CDS区共1764 bp,编码587 个氨基酸,预测PpWRKY4 蛋白分子质量为64.06 ku,等电点为6.00。序列比对结果显示PpWRKY4 与AtWRKY20 和OfWRKY3 高度同源,含有两个WRKY 保守结构域,属于Ⅰ类WRKY 蛋白(图2-A)。对PpWRKY4 基因在中油桃14 号果实不同发育期表达趋势分析发现PpWRKY4 在S1 时期高表达,随后急剧下降(图2-B),与PpCCD4 表达趋势相反,且PpCCD4 基因启动子区有WRKY 结合元件,据此推测PpWRKY4 可能负调控PpCCD4表达。
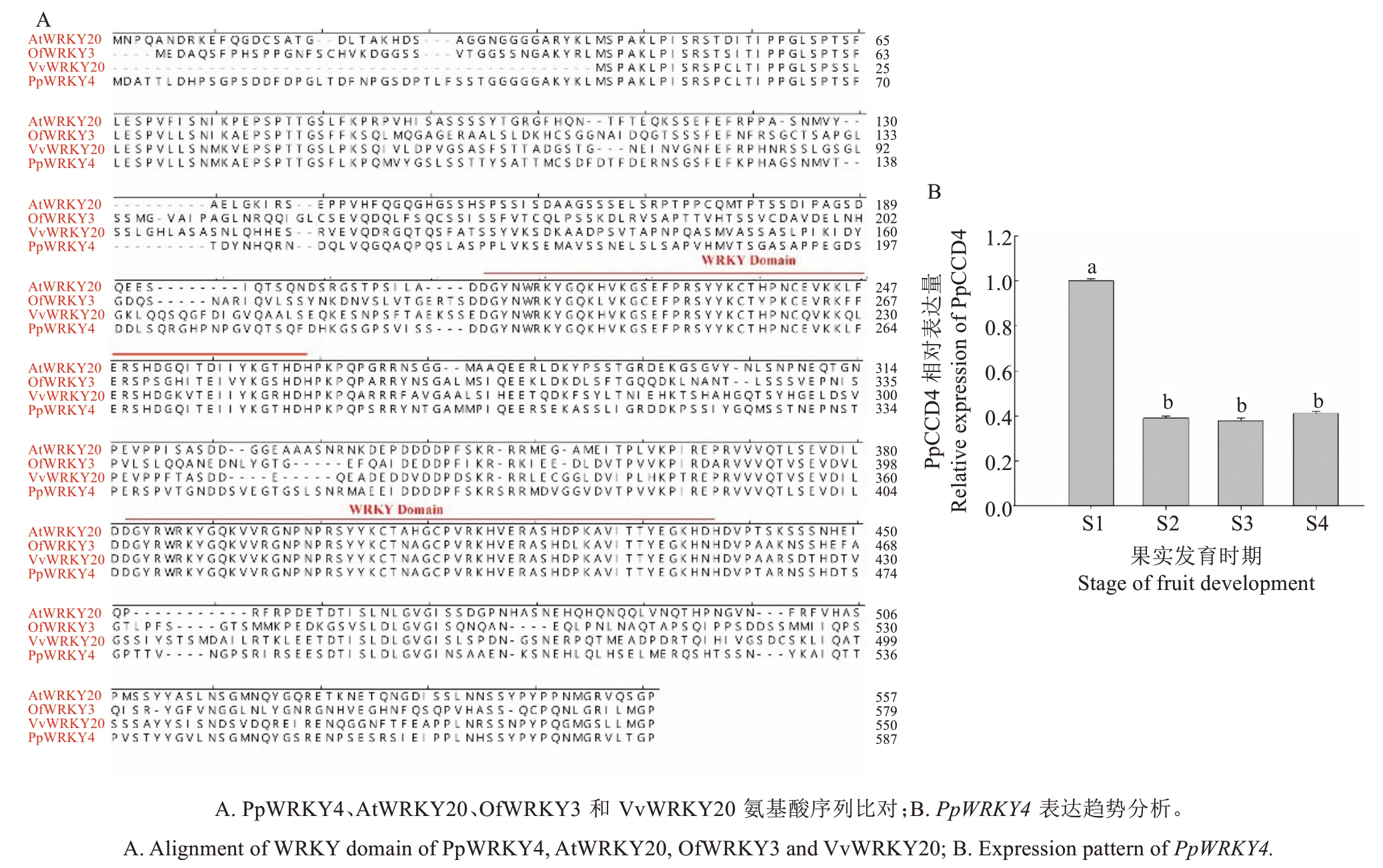
图2 PpWRKY4 结构域及表达趋势分析
Fig.2 Characterization and expression of PpWRKY4
2.3 PpWRKY4亚细胞定位
为了确认PpWRKY4蛋白在细胞内可能发挥作用的部位,构建了pSAK277-PpWRKY4-GFP 融合表达载体和pSAK277-GFP 空载,通过农杆菌侵染烟草。亚细胞定位结果显示PpWRKY4-GFP 融合蛋白仅在烟草表皮细胞的细胞核中观察到绿色荧光(图3),表明PpWRKY4定位于细胞核。
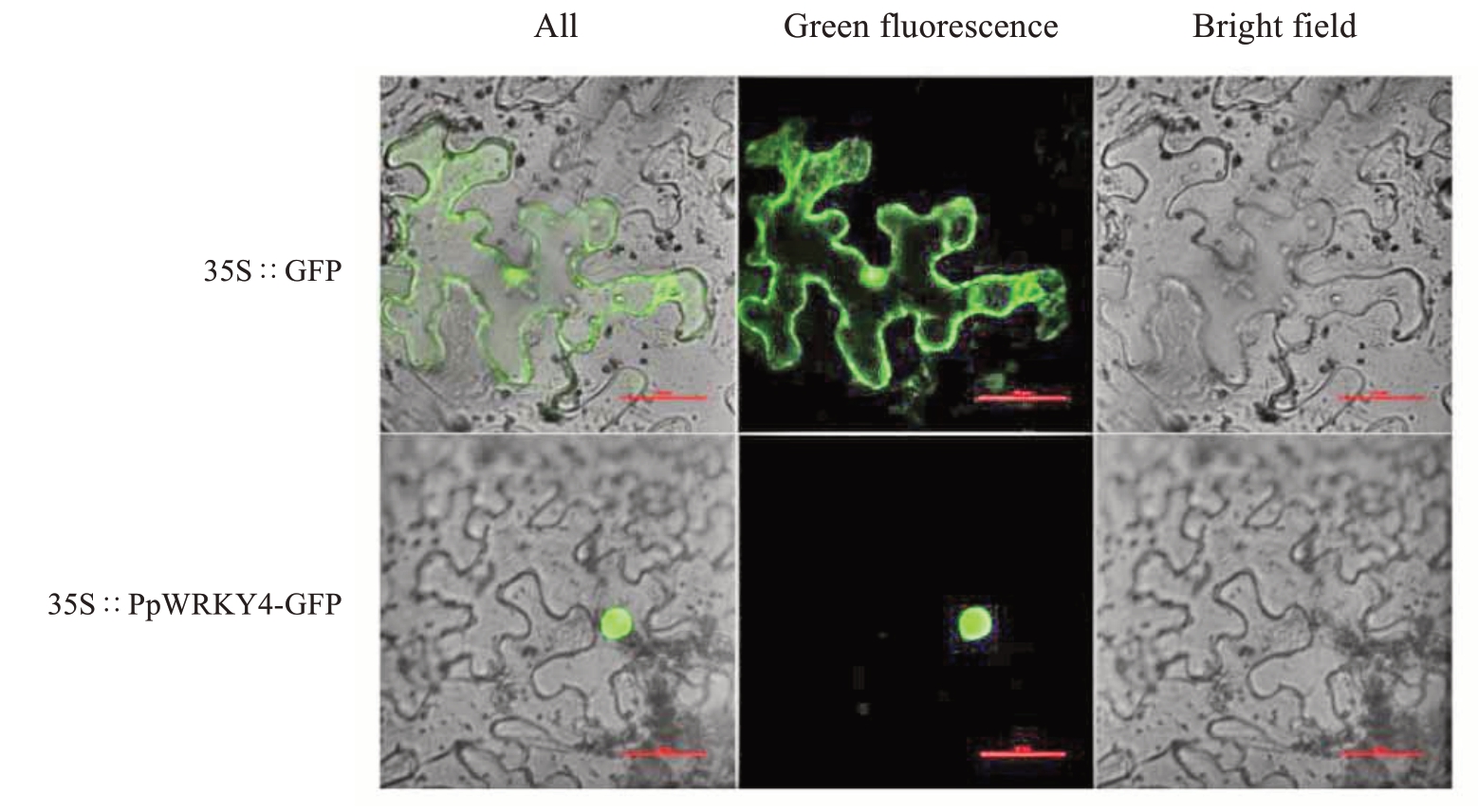
图3 PpWRKY4 蛋白亚细胞定位
Fig.3 Subcellular localization of PpWRKY4
2.4 PpWRKY4与PpCCD4启动子的互作分析
为了验证候选的PpWRKY4 是否可以直接结合在PpCCD4 启动子上,将PpCCD4 启动子片段(PpCCD4P)连接到PAbAi载体上,并转化到酵母中,PAbAi-PpCCD4-promoter质粒在AbA为200 ng·mL-1下被抑制生长。将PpWRKY4 的CDS 全长连接到pGADT7 载体上,与PAbAi-PpCCD4-promoter 载体共转,验证PpWRKY4 是否可以结合PpCCD4 启动子。结果显示,当SD-leu培养基中AbA 质量浓度为0时,阳性对照p53-promoter+AD-Rec-p53、阴性对照PpCCD4-promoter+AD-empty、PpCCD4-promoter+AD-PpWRKY4均可以正常生长;当培养基中AbA质量浓度为200 ng·mL-1时,仅有阳性对照和PpCCD4-promoter+AD-PpWRKY4 可以正常生长,阴性对照酵母的生长受到严重的抑制,表明PpWRKY4 可以结合PpCCD4启动子(图4)。
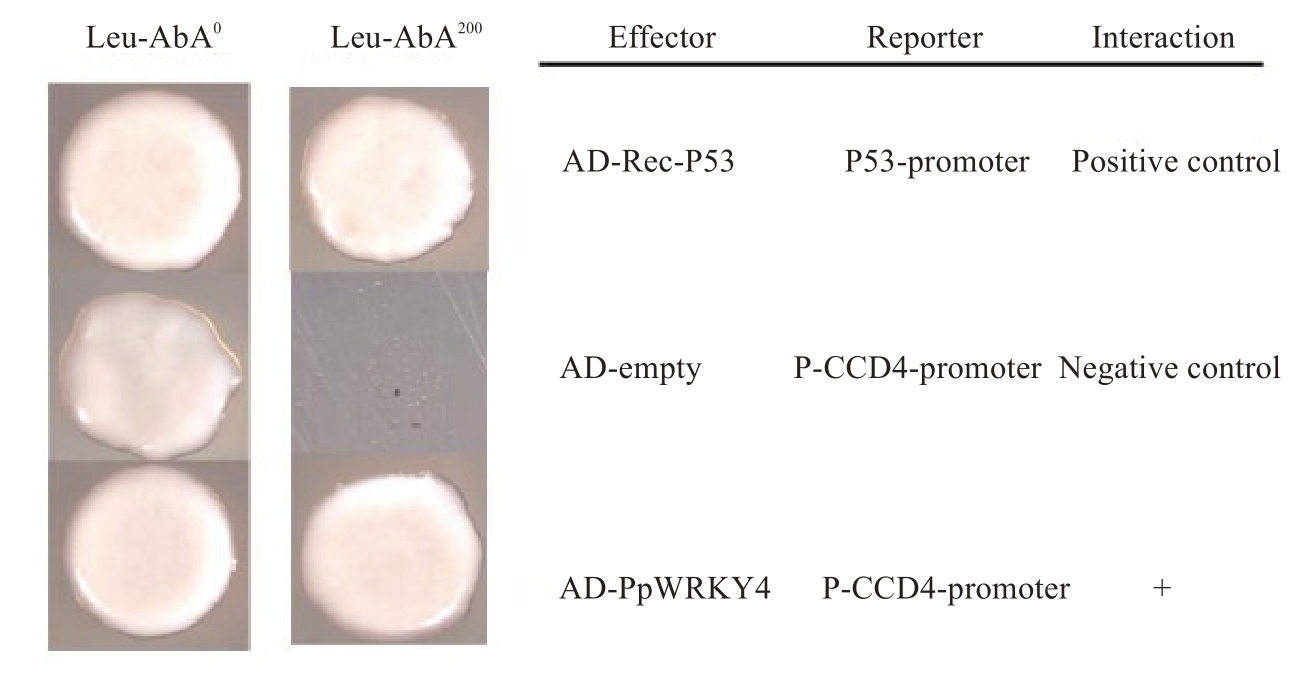
图4 酵母单杂交揭示PpWRKY4 可以结合PpCCD4 启动子
Fig.4 PpWRKY4 binds to the promoter of PpCCD4 by yeast one-hybrid
2.5 PpWRKY4抑制PpCCD4表达
为了进一步明确PpWRKY4 对PpCCD4 基因的转录调控活性,利用双荧光素酶系统,构建pSAK277-PpWRKY4 过表达载体,以及将PpCCD4启动子片段连接到pGreenII0800-LUC 载体上。利用农杆菌介导方法,将pSAK277-PpWRKY4 和pGreenII0800-LUC-PpCCD4-promoter共转化烟草叶片。结果显示,阴性对照的LUC/REN 值是试验组PpWRKY4 的4 倍左右,表明PpWRKY4 可显著抑制PpCCD4启动子活性(图5)。
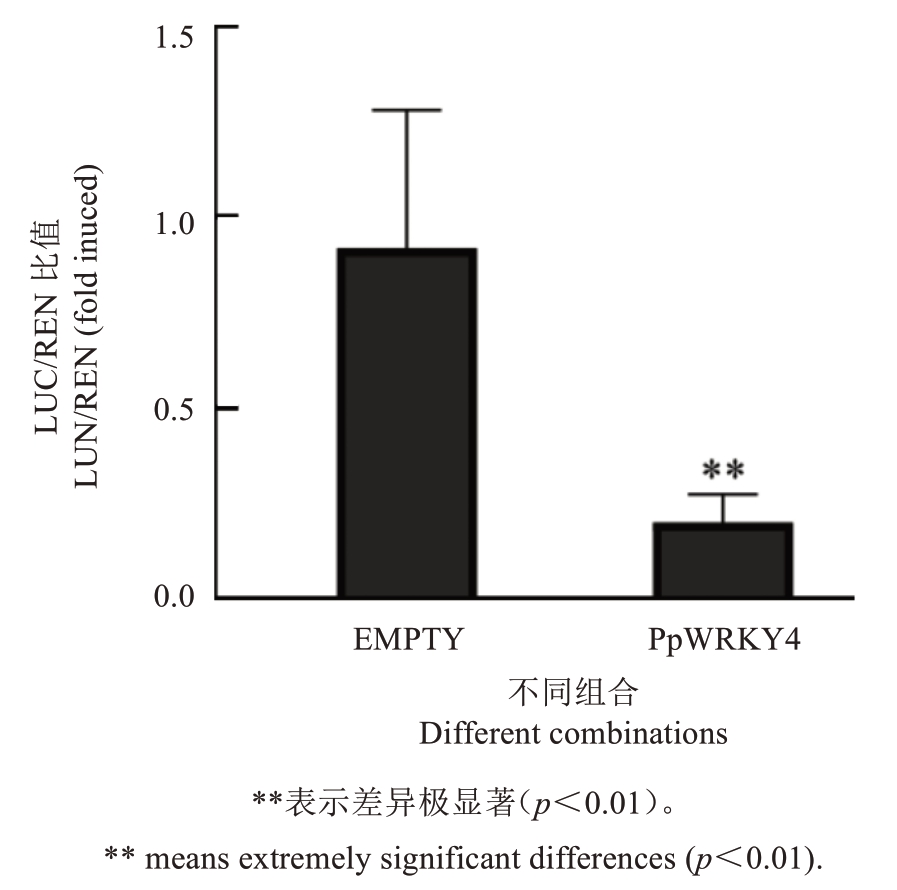
图5 双荧光素酶试验证明PpWRKY4 抑制PpCCD4基因转录
Fig.5 PpWRKY4 repress transcription of PpCCD4 by Dual-Luciferase reporter assay
3 讨 论
桃果肉颜色是一个重要的农艺性状,其类胡萝卜素含量变异丰富[27-29]。桃果肉颜色(黄肉/白肉)是受一对等位基因控制的质量性状,白肉对黄肉为显性,PpCCD4为控制该性状的关键基因[3,5]。PpCCD4的表达与类胡萝卜素含量具有显著相关性,在白肉桃中随着果实成熟PpCCD4 表达量逐渐升高,而类胡萝卜素含量逐渐降低;黄肉桃果实中,由于PpCCD4基因突变,导致其功能丧失或表达量降低,类胡萝卜素积累,果肉呈现黄色[25,30]。目前,关于桃果实类胡萝卜素积累的研究主要集中在类胡萝卜素含量的差异以及合成通路相关结构基因表达分析[3-5,25,31]方面,而对类胡萝卜素合成通路相关结构基因的转录调控因子研究还不清楚。本研究中,在白肉桃中油桃14 号果实发育过程中,PpCCD4 表达量随着果实成熟而提高,导致类胡萝卜素降解,果肉呈现白色。对PpCCD4 启动子元件分析发现,共检测到多种转录因子结合位点,其中包含两个WRKY 转录因子结合基序。
WRKY 转录因子超家族是植物中最大的转录因子家族之一[6]。WRKY 蛋白在调节多种形态、发育、生理和生化过程,以及对生物/非生物胁迫的反应中起着重要作用[32-35]。目前,桃中鉴定到WRKY家族基因58 个,其根据基因保守结构域特性和聚类分析,可将其分为3 类;通过表达趋势分析,共有36个基因在休眠芽中表达,其中6个PpWRKYs可能参与桃休眠过程[14]。在桃干旱胁迫响应分子机制研究中,PpWRKY18 基因表达受干旱胁迫诱导,而复水后,其表达量下降,推测其可能参与干旱胁迫的响应[36]。本研究中克隆了桃PpWRKY4 基因,对其氨基酸序列分析发现为Ⅰ类WRKY 蛋白,且与桂花Of‐WRKY3 高度同源。桂花中,OfWRKY3 调控Of‐CCD4 表达,推测其可能通过调控PpCCD4 基因表达参与类胡萝卜素代谢[22]。
在植物中,许多代谢物的生物合成或降解受到与这些通路相关的结构基因的转录调控,而这种调控作用主要依赖于特定转录因子的调节[37]。植物中类胡萝卜素合成和积累受到类胡萝卜素生物合成、降解酶及存储相关基因的影响,其受众多转录因子调控[38]。在柑橘中,CsERF61(ethylene response fac‐tor)能够结合至CsLCYB2(lycopene β-cyclase)基因的启动子激活其表达,参与类胡萝卜素合成[39]。在番茄中,通过对类胡萝卜素生物合成途径相关结构基因SlDXS1(1-deoxy-D-xylulose 5-phosphate syn‐thase)和SlPSY1(phytoene synthase)表达模式与已知调控基因表达模式分析,发现SlWRKY35 可以控制MEP(2-C-methyl-D-erythritol 4-phosphate)途径的初级代谢,从而诱导下游类胡萝卜素的生物合成[24]。甜瓜CmWRKY49 调控CmPSY1 进而促进β-胡萝卜素积累[40]。本研究分析发现,PpWRKY4 基因在桃果实发育过程中的表达趋势与类胡萝卜素合成关键基因PpCCD4 的表达趋势相反,且亚细胞定位结果显示PpWRKY4 蛋白定位在细胞核,与转录因子在细胞核中发挥的作用相符。进一步通过酵母单杂交技术、LUC 试验,证明PpWRKY4 负调控PpCCD4。以上研究结果表明,PpWRKY4可能具有调控类胡萝卜素积累的功能,在桃果实颜色调控中发挥重要作用,为桃果实颜色形成研究提供更多理论基础。
4 结 论
笔者在研究中克隆了中油桃14 号中PpWRKY4基因,为Ⅰ类WRKY 转录因子。随着果实成熟,中油桃14 号果实的类胡萝卜素含量显著降低,PpWRKY4 表现出与类胡萝卜素降解基因PpCCD4相反的表达模式。进一步研究证明,PpWRKY4 可直接与PpCCD4 基因启动子结合并抑制其表达,因此,PpWRKY4 通过影响PpCCD4 表达参与调节桃类胡萝卜素的积累,为调节桃的肉色提供了理论依据。
[1] MIKI W. Biological functions and activities of animal carot‐enoids[J].Pure and Applied Chemistry,1991,63(1):141-146.
[2] YUAN H,ZHANG J X,NAGESWARAN D,LI L. Carotenoid metabolism and regulation in horticultural crops[J]. Horticulture Research,2015,2:15036.
[3] ADAMI M,DE FRANCESCHI P,BRANDI F,LIVERANI A,GIOVANNINI D,ROSATI C,DONDINI L,TARTARINI S.Identifying a carotenoid cleavage dioxygenase (ccd4) gene con‐trolling yellow/white fruit flesh color of peach[J]. Plant Molecu‐lar Biology Reporter,2013,31(5):1166-1175.
[4] BRANDI F,BAR E,MOURGUES F,HORVÁTH G,TURCSI E,GIULIANO G,LIVERANI A,TARTARINI S,LEWINSOHN E,ROSATI C.Study of‘Redhaven’peach and its white-fleshed mutant suggests a key role of CCD4 carotenoid dioxygenase in carotenoid and norisoprenoid volatile metabolism[J]. BMC Plant Biology,2011,11:24.
[5] FALCHI R,VENDRAMIN E,ZANON L,SCALABRIN S,CIPRIANI G,VERDE I,VIZZOTTO G,MORGANTE M.Three distinct mutational mechanisms acting on a single gene underpin the origin of yellow flesh in peach[J]. The Plant Jour‐nal,2013,76(2):175-187.
[6] CHI Y J,YANG Y,ZHOU Y,ZHOU J,FAN B F,YU J Q,CHEN Z X. Protein-protein interactions in the regulation of WRKY transcription factors[J]. Molecular Plant,2013,6(2):287-300.
[7] RUSHTON P J,SOMSSICH I E,RINGLER P,SHEN Q J.WRKY transcription factors[J]. Trends in Plant Science,2010,15(5):247-258.
[8] RUSHTON D L,TRIPATHI P,RABARA R C,LIN J,RING‐LER P,BOKEN A K,LANGUM T J,SMIDT L,BOOMSMA D D,EMME N J,CHEN X F,FINER J J,SHEN Q J,RUSHTON P J.WRKY transcription factors:Key components in abscisic ac‐id signalling[J].Plant Biotechnology Journal,2012,10(1):2-11.
[9] ROSS C A,LIU Y,SHEN Q J. The WRKY gene family in rice(Oryza sativa)[J]. Journal of Integrative Plant Biology,2007,49(6):827-842.
[10] WEI K F,CHEN J,CHEN Y F,WU L J,XIE D X. Molecular phylogenetic and expression analysis of the complete WRKY transcription factor family in maize[J]. DNA Research,2012,19(2):153-164.
[11] ÜLKER B,SOMSSICH I E. WRKY transcription factors:From DNA binding towards biological function[J]. Current Opinion in Plant Biology,2004,7(5):491-498.
[12] 许瑞瑞,张世忠,曹慧,束怀瑞.苹果WRKY 转录因子家族基因生物信息学分析[J].园艺学报,2012,39(10):2049-2060.XU Ruirui,ZHANG Shizhong,CAO Hui,SHU Huairui. Bioin‐formatics analysis of WRKY transcription factor genes family in apple[J].Acta Horticulturae Sinica,2012,39(10):2049-2060.
[13] HUANG S X,GAO Y F,LIU J K,PENG X L,NIU X L,FEI Z J,CAO S Q,LIU Y S. Genome-wide analysis of WRKY tran‐scription factors in Solanum lycopersicum[J]. Molecular Genet‐ics and Genomics,2012,287(6):495-513.
[14] CHEN M,TAN Q P,SUN M Y,LI D M,FU X L,CHEN X D,XIAO W,LI L,GAO D S. Genome- wide identification of WRKY family genes in peach and analysis of WRKY expres‐sion during bud dormancy[J]. Molecular Genetics and Genom‐ics,2016,291(3):1319-1332.
[15] ZHANG W W,ZHAO S Q,GU S,CAO X Y,ZHANG Y,NIU J F,LIU L,LI A R,JIA W S,QI B X,XING Y.FvWRKY48 binds to the pectate lyase FvPLA promoter to control fruit softening in Fragaria vesca[J].Plant Physiology,2022,189(2):1037-1049.
[16] GAO H M,WANG Y F,XU P,ZHANG Z B.Overexpression of a WRKY transcription factor TaWRKY2 enhances drought stress tolerance in transgenic wheat[J]. Frontiers in Plant Science,2018,9:997.
[17] WANG L,LIU F,ZHANG X,WANG W J,SUN T T,CHEN Y F,DAI M J,YU S X,XU L P,SU Y C,QUE Y X. Expression characteristics and functional analysis of the ScWRKY3 gene from sugarcane[J]. International Journal of Molecular Sciences,2018,19(12):4059.
[18] QIU Y P,YU D Q. Over-expression of the stress-induced Os‐WRKY45 enhances disease resistance and drought tolerance in Arabidopsis[J]. Environmental and Experimental Botany,2009,65(1):35-47.
[19] WANG C T,RU J N,LIU Y W,LI M,ZHAO D,YANG J F,FU J D,XU Z S. Maize WRKY transcription factor ZmWRKY106 confers drought and heat tolerance in transgenic plants[J]. Inter‐national Journal of Molecular Sciences,2018,19(10):3046.
[20] ZHANG Y,YU H J,YANG X Y,LI Q,LING J,WANG H,GU X F,HUANG S W,JIANG W J. CsWRKY46,a WRKY tran‐scription factor from cucumber,confers cold resistance in trans‐genic-plant by regulating a set of cold-stress responsive genes in an ABA-dependent manner[J].Plant Physiology and Biochemis‐try,2016,108:478-487.
[21] SUN X M,ZHANG L L,WONG D C J,WANG Y,ZHU Z F,XU G Z,WANG Q F,LI S H,LIANG Z C,XIN H P.The ethyl‐ene response factor VaERF092 from Amur grape regulates the transcription factor VaWRKY33,improving cold tolerance[J].The Plant Journal,2019,99(5):988-1002.
[22] HAN Y J,WU M,CAO L Y,YUAN W J,DONG M F,WANG X H,CHEN W C,SHANG F D. Characterization of Of‐WRKY3,a transcription factor that positively regulates the ca‐rotenoid cleavage dioxygenase gene OfCCD4 in Osmanthus fra‐grans[J].Plant Molecular Biology,2016,91(4/5):485-496.
[23] ZHAO W H,LI Y H,FAN S Z,WEN T J,WANG M H,ZHANG L D,ZHAO L X.The transcription factor WRKY32 af‐fects tomato fruit colour by regulating YELLOW FRUITED-TO‐MATO 1,a core component of ethylene signal transduction[J].Journal of Experimental Botany,2021,72(12):4269-4282.
[24] YUAN Y,REN S Y,LIU X F,SU L Y,WU Y,ZHANG W,LI Y,JIANG Y D,WANG H H,FU R,BOUZAYEN M,LIU M C,ZHANG Y. SlWRKY35 positively regulates carotenoid biosyn‐thesis by activating the MEP pathway in tomato fruit[J]. The New Phytologist,2022,234(1):164-178.
[25] 朱运钦,曾文芳,牛良,蔡祖国,鲁振华,潘磊,崔国朝,王志强.‘中油桃9 号’及其黄肉突变体花和叶片中类胡萝卜素代谢及相关基因的时空表达[J].园艺学报,2018,45(10):1869-1880.ZHU Yunqin,ZENG Wenfang,NIU Liang,CAI Zuguo,LU Zhenhua,PAN Lei,CUI Guochao,WANG Zhiqiang. Temporal and spatial expression of carotenoid metabolism and relative genes in flowers and leaves of CN9 nectarine and its yellowfleshed mutant[J]. Acta Horticulturae Sinica,2018,45(10):1869-1880.
[26] 谭彬,魏鹏程,栗焕楠,连晓东,陈谭星,郑先波,程钧,王伟,王小贝,冯建灿.桃PEBP 基因家族全基因组鉴定及桃TFL1 基因功能分析[J].果树学报,2020,37(10):1443-1454.TAN Bin,WEI Pengcheng,LI Huannan,LIAN Xiaodong,CHEN Tanxing,ZHENG Xianbo,CHENG Jun,WANG Wei,WANG Xiaobei,FENG Jiancan. Genome-wide identification of PEBP gene family and functional analysis of TFL1 gene in peach (Prunus persica)[J]. Journal of Fruit Science,2020,37(10):1443-1454.
[27] WU J L,FAN J Q,LI Y,CAO K,CHEN C W,WANG X W,FANG W C,ZHU G R,WANG L R. Characterizing of carot‐enoid diversity in peach fruits affected by the maturation and va‐rieties[J].Journal of Food Composition and Analysis,2022,113:104711.
[28] ZHAO B T,SUN M,LI J Y,SU Z W,CAI Z X,SHEN Z J,MA R J,YAN J,YU M L. Carotenoid profiling of yellow-flesh peach fruit[J].Foods,2022,11(12):1669.
[29] CAO S F,LIANG M H,SHI L Y,SHAO J R,SONG C B,BI‐AN K,CHEN W,YANG Z F.Accumulation of carotenoids and expression of carotenogenic genes in peach fruit[J].Food Chem‐istry,2017,214:137-146.
[30] 范家琪,吴金龙,李勇,别航灵,王蛟,郭健,曹珂,王力荣.桃类胡萝卜素合成关键基因PpCCD4 的表达与启动子活性分析[J].果树学报,2020,37(9):1271-1280.FAN Jiaqi,WU Jinlong,LI Yong,BIE Hangling,WANG Jiao,GUO Jian,CAO Ke,WANG Lirong. Expression and promoter activity analysis of PpCCD4 closely related to carotenoid synthe‐sis in peach[J].Journal of Fruit Science,2020,37(9):1271-1280.
[31] BAI S L,TUAN P A,TATSUKI M,YAEGAKI H,OHMIYA A,YAMAMIZO C,MORIGUCHI T. Knockdown of carotenoid cleavage dioxygenase 4 (CCD4) via virus-induced gene silenc‐ing confers yellow coloration in peach fruit:Evaluation of gene function related to fruit traits[J]. Plant Molecular Biology Re‐porter,2016,34(1):257-264.
[32] WANG X,LI J J,GUO X F,MA Y,QIAO Q,GUO J.PlWRKY13:A transcription factor involved in abiotic and biotic stress responses in Paeonia lactiflora[J]. International Journal of Molecular Sciences,2019,20(23):5953.
[33] MAO G H,MENG X Z,LIU Y D,ZHENG Z Y,CHEN Z X,ZHANG S Q. Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosyn‐thesis in Arabidopsis[J].The Plant Cell,2011,23(4):1639-1653.
[34] WANG L N,ZHU W,FANG L C,SUN X M,SU L Y,LIANG Z C,WANG N,LONDO J P,LI S H,XIN H P. Genome-wide identification of WRKY family genes and their response to cold stress in Vitis vinifera[J].BMC Plant Biology,2014,14:103.
[35] GOVARDHANA M,KUMUDINI B S. In-silico analysis of cu‐cumber (Cucumis sativus L.) Genome for WRKY transcription factors and cis-acting elements[J]. Computational Biology and Chemistry,2020,85:107212.
[36] 李森,王庆杰,陈修德,张蕊,李玲,杜桂英,付喜玲.桃WRKY转录因子PpWRKY18 克隆及表达分析[J].核农学报,2021,35(9):1987-1993.LI Sen,WANG Qingjie,CHEN Xiude,ZHANG Rui,LI Ling,DU Guiying,FU Xiling.Cloning and expression analysis of tran‐scription factor PpWRKY18 in peach[J].Journal of Nuclear Agri‐cultural Sciences,2021,35(9):1987-1993.
[37] LACCHINI E,GOOSSENS A. Combinatorial control of plant specialized metabolism:Mechanisms,functions,and conse‐quences[J].Annual Review of Cell and Developmental Biology,2020,36:291-313.
[38] 陆晨飞,高月霞,黄河,戴思兰.植物类胡萝卜素代谢及调控研究进展[J].园艺学报,2022,49(12):2559-2578.LU Chenfei,GAO Yuexia,HUANG He,DAI Silan. Carotenoid metabolism and regulation in plants[J]. Acta Horticulturae Sini‐ca,2022,49(12):2559-2578.
[39] ZHU K J,SUN Q,CHEN H Y,MEI X H,LU S W,YE J L,CHAI L J,XU Q,DENG X X.Ethylene activation of carotenoid biosynthesis by a novel transcription factor CsERF061[J]. Jour‐nal of Experimental Botany,2021,72(8):3137-3154.
[40] DUAN X Y,JIANG C,ZHAO Y P,GAO G,LI M,QI H Y.Transcriptome and metabolomics analysis revealed that CmWRKY49 regulating CmPSY1 promotes β-carotene accumu‐lation in orange fleshed oriental melon[J]. Horticultural Plant Journal,2022,8(5):650-666.