根结线虫是一种重要的植物寄生性线虫,主要危害作物根系[1-2],严重影响农业生产。在桃产业中,根结线虫感染会导致树势衰弱、果实产量和品质降低,甚至死树。南方根结线虫是危害我国桃树的主要线虫种类[3]。对比传统化学防治方法,选用抗性砧木是解决根结线虫危害问题的根本途径。前人研究发现野生种质红根甘肃桃1 号(P.kansuensis)对南方根结线虫完全免疫,山桃(P.davidiana)以及寿星桃1号(P.persica)对南方根结线虫高抗[4]。
近年来,分子标记辅助育种在植物中得到广泛应用,显著提高了选择的准确性,缩短了育种周期。李肯等[5]利用indel分子标记检测32份甜瓜基因型,检测结果与表型符合率极高;吴翼等[6]利用分子标记对100株香水椰子的纯度进行检验,发现分子标记结果与表型鉴定完全吻合,可用于鉴定苗期香水椰子的纯度;刘广等[7]利用筛选到的3 个分子标记检测20 份西瓜材料抗枯萎病情况,检测结果与表型基本一致。由于不同研究者利用的遗传群体不同,因此得到的标记与性状连锁距离的远近不同,甚至位于不同的染色体上[8]。在桃上,为获得与桃抗南方根结线虫紧密连锁的标记,刘伟[9]利用分子标记将红根甘肃桃抗南方根结线虫基因定位在LG5(linkage group,LG),紧密连锁M3E15-300 标记;Cao 等[10]、张倩[11]利用多种分子标记如SSR、RGA 等将野生种质红根甘肃桃1 号的抗南方根结线虫基因PkMi定位到2号染色体顶端,位于两个标记NBS29 与NBS3 之间,连锁SSR的标记UDP98-025,随后通过标记加密鉴定到了红根甘肃桃抗南方根结线虫关键基因并加以验证。Duval 等[12]利用[(Pamirskij×Rubira)×(Montclar×Nemared)]的杂交群体,将栽培桃(P.persica)抗性基因定位在2号染色体,但与野生种质红根甘肃桃1 号抗性基因位置不同,位于A20 SNP 和SNP_APP91 标记之间,约92 kb,关键基因尚不明确。
栽培桃是桃砧木的最重要类型。笔者在本研究中基于Duval 等[12]对栽培桃抗南方根结线虫的定位结果,拟通过定位区间内序列差异比较,锁定候选关键基因,开发抗南方根结线虫的相关分子标记,以期在抗南方根结线虫砧木育种中应用。
1 材料和方法
1.1 试验材料
5 个表型为抗南方根结线虫的品种:列玛格、阿克拉娃、筑波2 号、筑波3 号、寿星桃1 号;5 个表型感性品种:贝蕾、喀什1 号、喀什2 号、哈露红、西伯利亚C[13]。杂交F2代群体为筑波3 号(抗)×哈露红(感)。
线虫材料取自中国农业科学院郑州果树研究所桃园,鉴定为南方根结线虫后接种至番茄苗中进行繁殖备用。
1.2 候选基因的确认
通过Duval 等[12]对栽培桃的定位结果,桃抗根结线虫基因在标记A20SNP与SNP_APP91的92 kb区间内,通过GDR 网站在peach genome V2.0 中对该候选序列进行BLAST 找到对应区域包含的所有候选基因共6 个。利用IGV 可视化和Excel表查看10 份种质的基因组重测序结果,挑选具有规律性序列差异的基因进行下一步验证[14]。
1.3 叶片DNA的提取、PCR扩增及测序
采集筑波3 号(抗)×哈露红(感)F2群体(共200株)及10 份种质的叶片,用CTAB 法提取DNA。DNA的质量与浓度用紫外分光光度仪NanoDrop 1000 spectrophotomete(rThemo Scientific)测定,利用无菌水将其稀释至100~200 ng·μL-1后保存至-20 ℃。在10 份种质中对候选基因进行基因组序列扩增(扩增引物见表1),扩增模板为H2O 7 μL,上、下游引物各1 μL,1 μL DNA以及10 μL Mix(南京诺唯赞生物科技股份有限公司,南京)。扩增条件按照Mix 说明书进行。PCR 产物通过凝胶电泳后,参考韦莹华等[15]的方法稍作修改,将产物进行回收、连接载体、挑取单克隆并通过阳性鉴定后将菌液交由生工生物工程(上海)股份有限公司测序、拼接,查看序列差异的软件为DNAMAN。
表1 扩增候选基因所用引物序列信息
Table 1 Primer sequence information used to amplify candidate genes
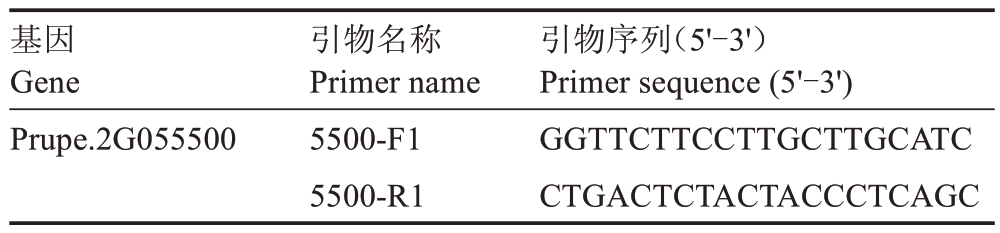
基因Gene Prupe.2G055500引物名称Primer name 5500-F1 5500-R1引物序列(5'-3')Primer sequence(5'-3')GGTTCTTCCTTGCTTGCATC CTGACTCTACTACCCTCAGC
1.4 KASP标记基因分型
竞争性等位基因特异性PCR(KASP)扩增参考吉爽秋等[16]的方法,所用荧光为六氯荧光素(hexachlorouorescein,HEX)和羧基荧光素(carboxy fluorescein,FAM),引物序列见表2。
表2 KASP 分型所用引物序列信息
Table 2 Primer sequence information used for KASP genotyping
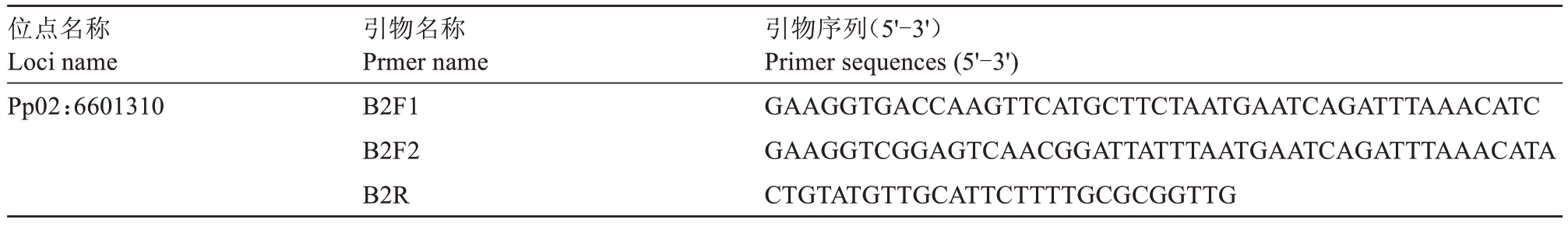
位点名称Loci name Pp02:6601310引物名称Prmer name B2F1 B2F2 B2R引物序列(5'-3')Primer sequences(5'-3')GAAGGTGACCAAGTTCATGCTTCTAATGAATCAGATTTAAACATC GAAGGTCGGAGTCAACGGATTATTTAATGAATCAGATTTAAACATA CTGTATGTTGCATTCTTTTGCGCGGTTG
1.5 2个分子标记的检测
采用前人开发的SCAR分子标记和35 bp indel 分子标记检测200 株实生苗抗南方根结线虫情况[17-18]。利用2个标记分别对200株实生苗进行PCR序列扩增(引物见表3),SCAR 标记检测结果通过凝胶电泳查看,35 bp indel 分子标记检测结果通过聚丙烯酰胺凝胶电泳查看。
表3 分子标记所用引物序列信息
Table 3 Primer sequence information used in molecular markers
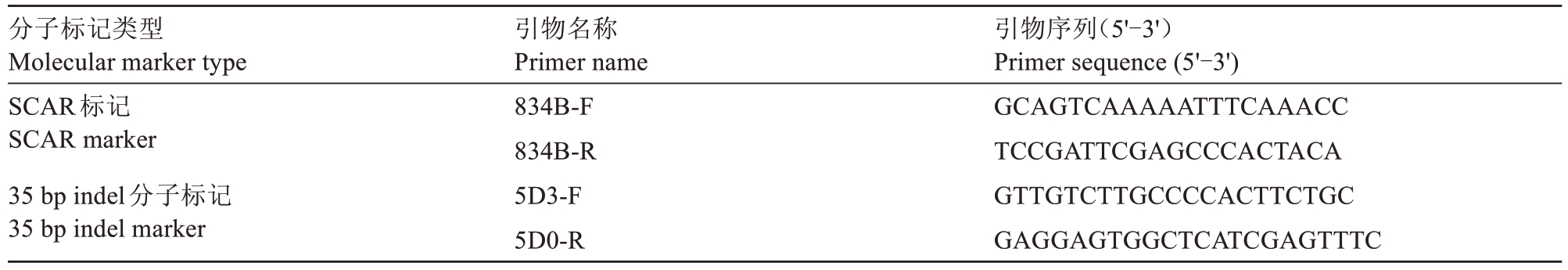
分子标记类型Molecular marker type SCAR标记SCAR marker 35 bp indel分子标记35 bp indel marker引物名称Primer name 834B-F 834B-R 5D3-F 5D0-R引物序列(5'-3')Primer sequence(5'-3')GCAGTCAAAAATTTCAAACC TCCGATTCGAGCCCACTACA GTTGTCTTGCCCCACTTCTGC GAGGAGTGGCTCATCGAGTTTC
1.6 F2群体表型的调查
参考吴波鸿[19]的方法稍作修改,收集番茄根上繁殖的南方根结线虫虫卵,在28 ℃培养箱孵化5 d后收集南方根结线虫二龄幼虫(J2)制成线虫悬浮液于50 mL 离心管中,随后在显微镜下确认该悬浮液浓度为50 头J2·100 μL-1。对20 株桃苗进行南方根结线虫的接种,每盆接种3 mL。接种后定期对温室的桃苗进行管理,3 个月后调查表型,观察桃苗有无根结。
1.7 3个单一标记在杂交群体中的选择符合率
抗性符合率=标记为抗性的F2群体中表型为无根结的个数/标记检测为无根结的群体总数;感性符合率=标记为感性的F2群体中表型为有根结的个数/标记检测为有根结的群体总数。
2 结果与分析
2.1 候选基因的确认及序列差异分析
根据Duval等[12]对栽培桃抗南方根结线虫的定位结果,找到了6 个候选基因即Prupe.2G055500、Prupe.2G055600、Prupe.2G055700、Prupe.2G055800、Prupe.2G055900 和Prupe.2G056000。利用重测序数据查看候选基因的序列差异情况,发现基因Prupe.2G055500 在抗感品种中存在规律性变异位点。为进一步验证,笔者在5个抗性和5个感性品种中对该基因进行扩增、测序,经软件DNAMAN 比对后发现基因Prupe.2G055500 在抗、感品种中确实存在规律性变异,其内含子上存在一个2 bp的ins变异(Pp02:6 601 310 bp,G→GAT),抗性品种存在AT插入,感性品种无(图1);另外,以v2.0.a1 版本为参考基因组,通过IGV 软件查看10 份桃种质材料的重测序数据,并进行序列的比对、分析,发现了同样的结果(图2),表明该插入具有高度准确性。

图1 抗感品种在Prupe.2G055500 中序列差异
Fig.1 Sequence difference of susceptible varieties in Prupe.2G055500
从上到下依次为列玛格、阿克拉娃、筑波2 号、筑波3 号、寿星桃1 号、喀什1 号、贝蕾、喀什2 号、哈露红、西伯利亚C 和Prupe.2G055500 的基因组序列。
From top to bottom, they are Nemaguard, Okinawa,Tsukuba 2,Tsukuba3, Shouxingtao 1, Kashi 1, Bailey, Kashi 2, Harrow Blood, Siberian C and Genome sequence of Prupe.2G055500.
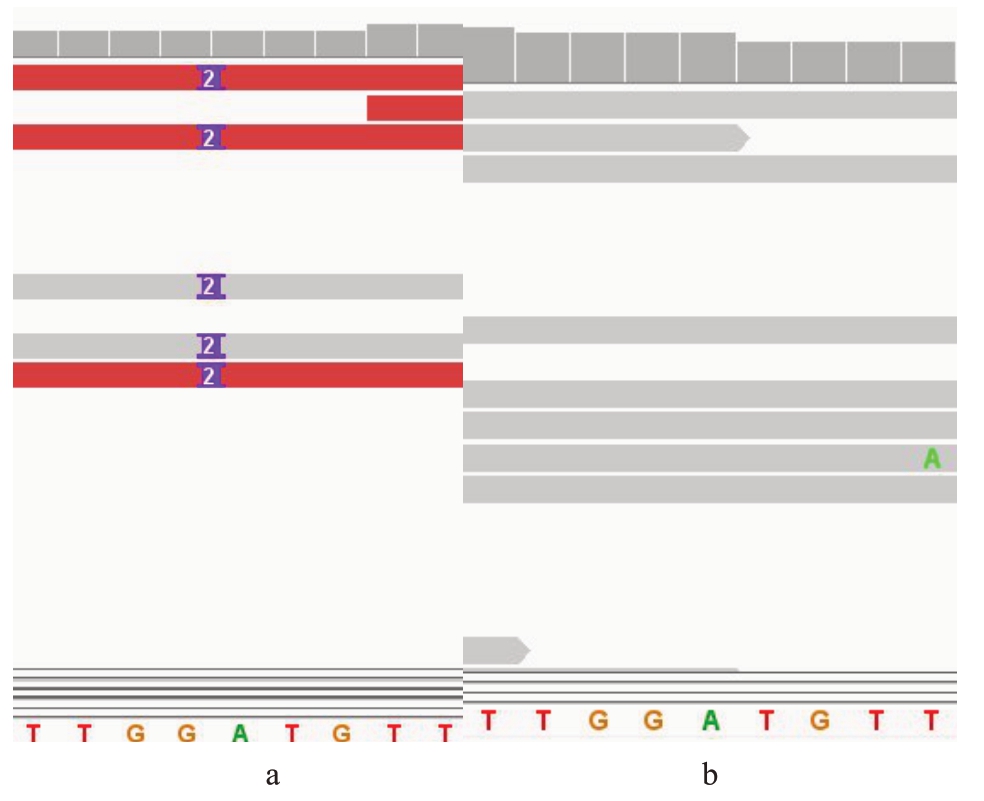
图2 抗感品种IGV 比对结果
Fig.2 Comparison results of IGV resistant varieties
a.抗性品种;b.感性品种。
a.Resistant varieties;b.Susceptible varieties.
2.2 KASP分子标记的开发与检测
结合上述对候选基因序列的比对结果,笔者在该位点开发了一个用于基因分型的KASP 分子标记。利用该标记对5 份抗性种质和5 份感性种质进行目标位点基因型检测,发现抗性种质列玛格同时检测到FAM 和HEX 荧光信号,信号点为红色,基因型为AT/--;抗性种质阿克拉娃、筑波2 号、筑波3 号和红寿星信号点为绿色,聚合在y 轴附近,基因型为AT/AT;感性种质喀什1 号、贝蕾、喀什2 号、哈露红和西伯利亚C 的信号为蓝色,聚合在x 轴附近,基因型为--/--。
为检验KASP 分子标记的适用性和有效性,利用抗性材料筑波3 号和感性材料哈露红的F2代200株个体进行验证。利用KASP 标记对每份单株进行目标位点基因型检测,结果显示该群体有3 种基因型,把荧光信号为绿色的显性纯合类基因型记为A,荧光信号为红色的显性杂合类基因型记为B;荧光信号为蓝色的隐性纯合类基因型记为C(图3)。200株实生苗的基因分型结果如下:A 类的材料有42份,占总群体的21.0%;B 类的材料有94 份,占总群体的47.0%;C 类的材料有64 份,占总群体的32.0%。抗性纯合(A)∶抗性杂合(B)∶感性(C)=42∶94∶64,接近1∶2∶1。经卡方检验可知χ2=5.56,p值>0.05,结果表明内含子的插入与根结线虫抗性显著相关,说明该分子标记符合孟德尔分离定律,且抗南方根结线虫基因为显性遗传,与前人研究结果较一致[20]。
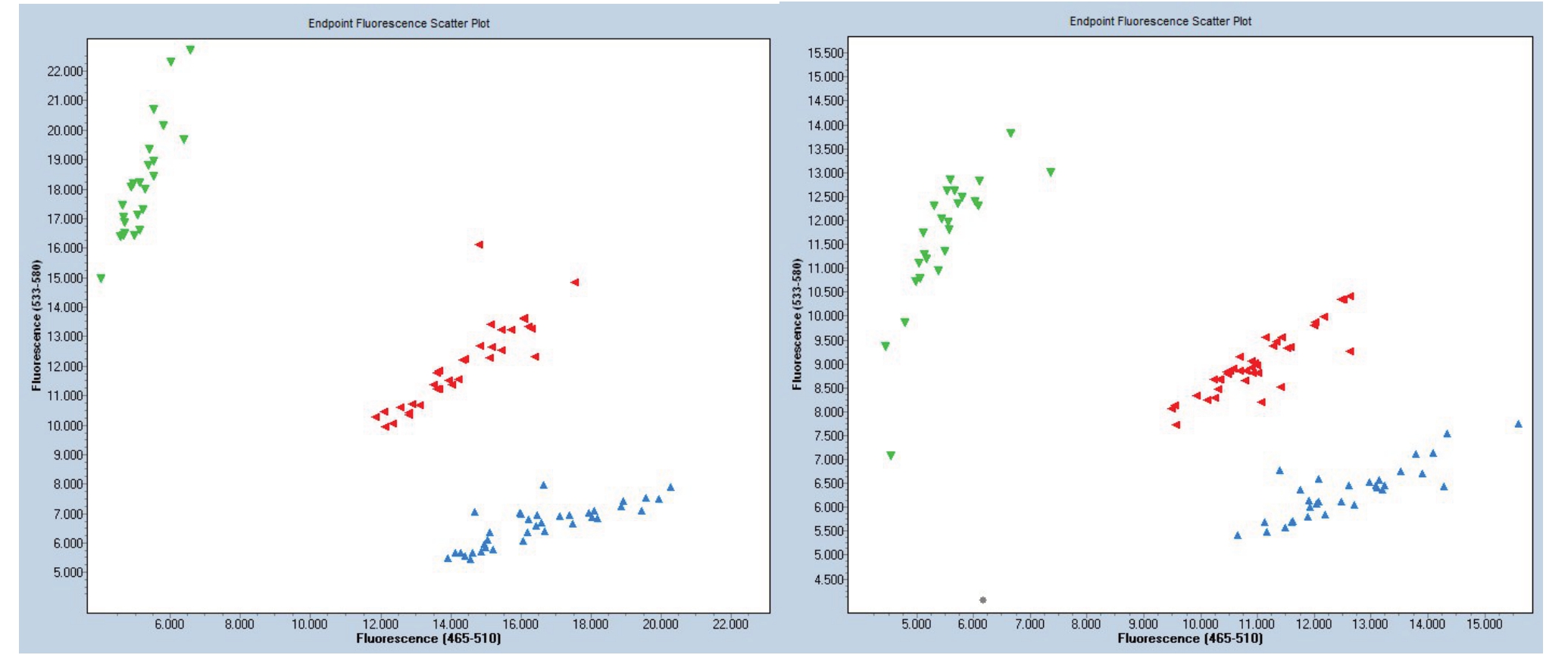
图3 部分KASP 基因分型结果
Fig.3 Partial KASP genotyping results
绿色荧光代表AT/AT;红色荧光代表AT/--;蓝色荧光代表--/--。
Green fluorescence stands for AT/AT;Red fluorescence stands for AT/--;Blue fluorescence stands--/--.
2.3 SCAR、35 bp indel分子标记检测结果
利用前人已开发的SCAR标记[17]、红根甘肃桃35 bp indel[18]分子标记对F2群体200 株实生苗进行基因分型。通过琼脂糖凝胶电泳查看SCAR 标记结果,将结果划分为A1、C1两类。其中A1为抗南方根结线虫,C1为感南方根结线虫。部分SCAR标记结果如图4 所示,扩增出A1 类条带的材料有135 份,占总群体的67.5%;扩增出C1 类条带的材料有65 份,占总群体的32.5%。即SCAR 分子标记检测结果为抗南方根结线虫的植株有135 株,对南方根结线虫感性的植株有65 株,经卡方检验可知χ2=6.00,p 值<0.05,不符合分离定律。
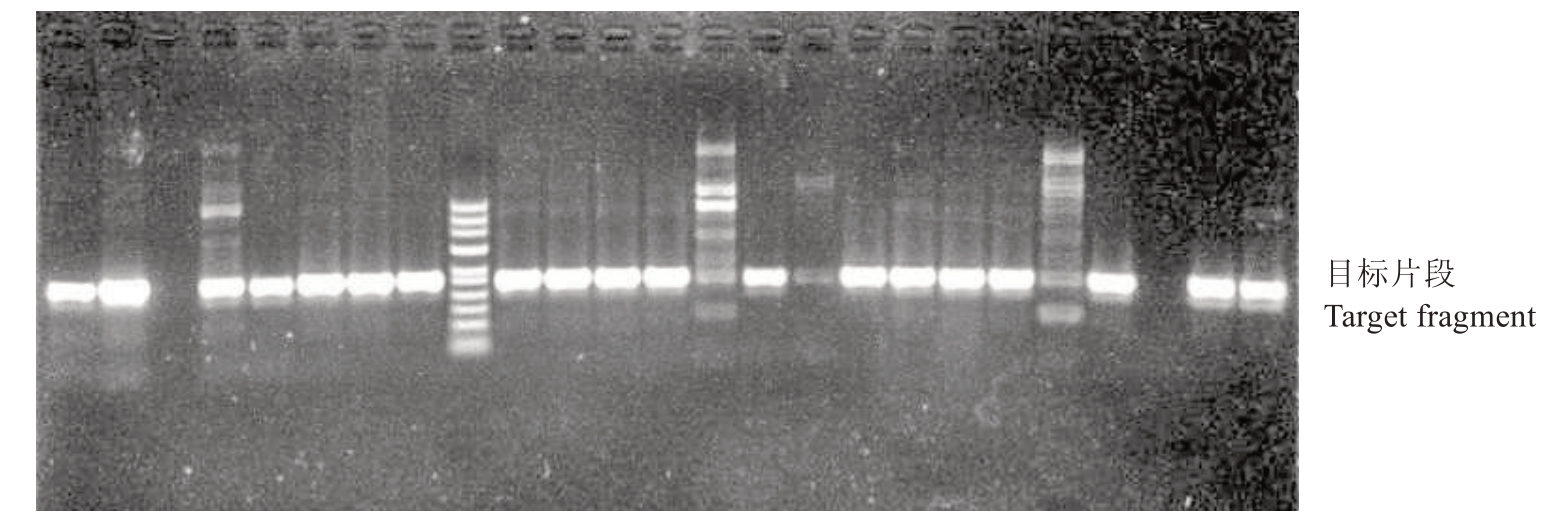
图4 部分SCAR 标记检测结果
Fig.4 Partial SCAR marker detection results
通过聚丙烯酰胺凝胶电泳35 bp indel分子标记扩增出了3种类型的条带,分别记为A2、B2、C2。部分标记检测结果如图5 所示,以抗性材料红根甘肃桃1号为对照,在该位点有1条带记为A2类,在该位点有对应2 条带记为B2 类,在该位点无条带则记为C2 类。结果显示,扩增出A2 类型条带的材料有1份,占总群体的0.5%;扩增出B2 类型条带的材料有154 份,占总群体的77.0%;扩增出C2 类型条带的材料有45 份,占总群体的22.5%,A2∶B2∶C2=1∶154∶45。标记结果表明200 株实生苗中抗根结线虫的有155 株,感性的有45 株,抗∶感≈3∶1,卡方检验显示χ2=0.67,p 值>0.05,该位点的缺失与根结线虫的抗性有显著相关性。在聚丙烯酰胺凝胶电泳结果中显示超过2/3 的植株在该处均有2 条带,说明该群体在此处的基因型大多为杂合。
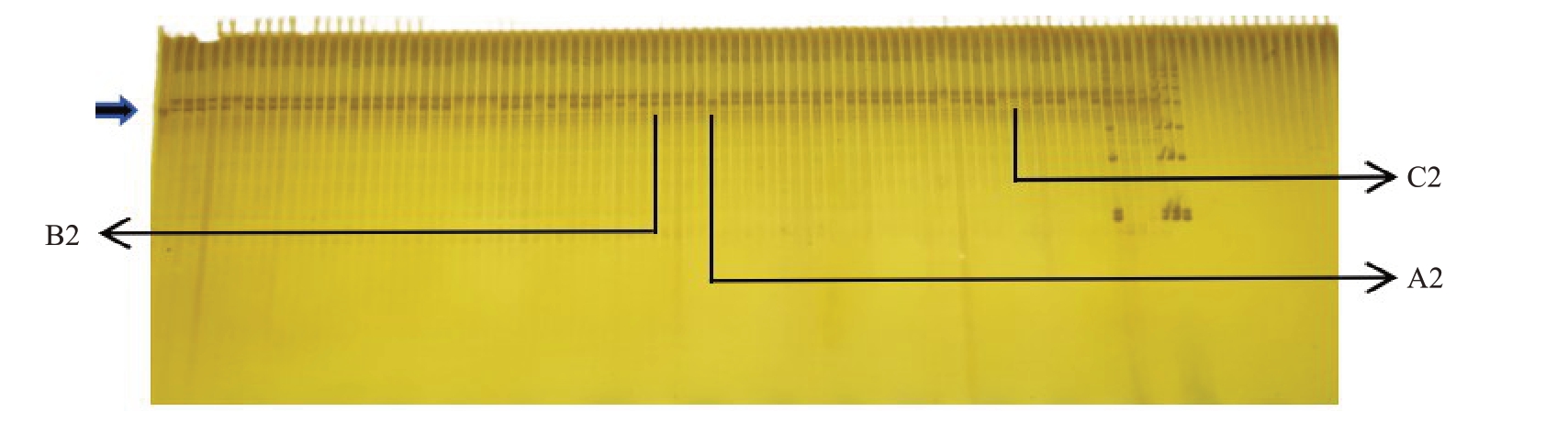
图5 部分35 bp indel 分子标记结果
Fig.5 Partial 35 bp indel marker results
以左一红根甘肃桃1 号为对照。
With Honggen Gansutao 1 as the control.
2.4 3个分子标记的选择效率分析
对F2群体接种南方根结线虫,3 个月后调查该群体对南方根结线虫的抗性情况。结果如表4 所示,无根结与有根结之比为2.77∶1,经卡方检验可知χ2=0.24,p 值>0.05,该群体符合孟德尔遗传定律。基于F2群体对南方根结线虫抗性的表型调查结果,评价3个标记的选择效率。从表5中可以看出,具有KASP 标记的A 类抗性基因型材料有42 份,其中表型鉴定为抗性的材料有41 份,抗性选择符合率为97.6%;具有KASP标记的B类抗性基因型材料有94份,表型鉴定为抗性的材料有89 份,抗性选择符合率为94.7%;具有KASP标记的C类感性基因型材料有64 份,表型鉴定为感性的材料有47 份,感性选择符合率为73.4%,总符合率达到88.5%。具有SCAR标记的A1 类抗性带型的材料有135 份,其中表型鉴定结果为抗性的材料有128 份,抗性选择符合率为94.8%;具有SCAR 标记的C1 类感性带型的材料有65份,表型鉴定为感性的材料有46份,感性选择符合率为70.8%,总符合率也达到87.0%。具有35 bp indel分子标记为A2类抗性带型的材料有1份,无表型鉴定为抗性的材料,抗性表型选择符合率为0;具有35 bp indel分子标记为B2 类的抗性带型材料有154份,表型鉴定为抗性的材料有103份,抗性选择符合率为66.9%;具有35 bp indel 分子标记为C2 类的抗性带型的材料有45份,表型鉴定为感性的材料只有1 份,感性选择符合率为2.2%,总符合率为52.0%。总之,3 个抗南方根结线虫分子标记中,KASP 分子标记检测的正确率最高;SCAR 标记次之,但同样正确率较高;35 bp indel分子标记的正确率最低。
表4 栽培桃杂交后代抗南方根结线虫分离比调查
Table 4 Investigation on the separation ratio of cultivated peach hybrid offspring against southern root-knot nematodes
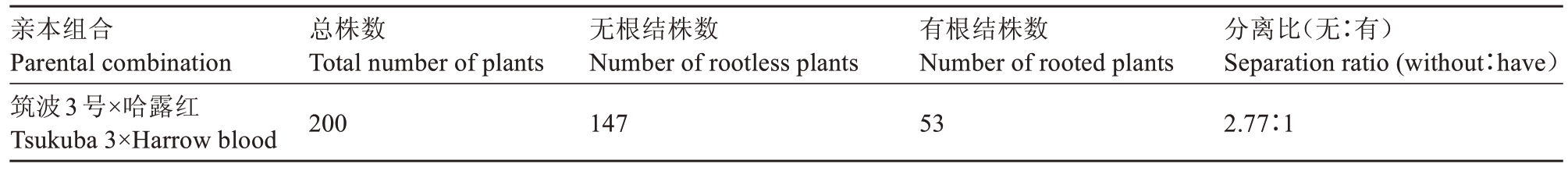
亲本组合Parental combination筑波3号×哈露红Tsukuba 3×Harrow blood总株数Total number of plants 200无根结株数Number of rootless plants 147有根结株数Number of rooted plants 53分离比(无∶有)Separation ratio(without∶have)2.77∶1
表5 分子标记检测与表型比对结果
Table 5 Results of molecular marker detection and phenotype comparison
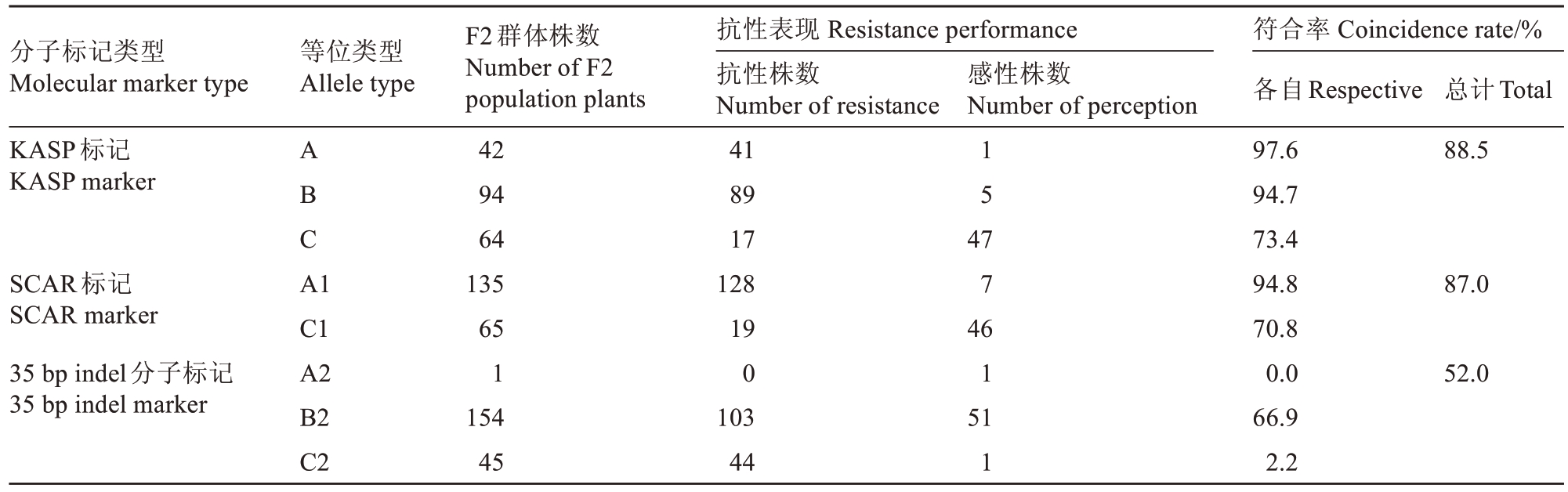
分子标记类型Molecular marker type等位类型Allele type F2群体株数Number of F2 population plants 42 94 64 135 65 1 154 45抗性表现Resistance performance抗性株数Number of resistance 41 89 17 128 19 0 103 44感性株数Number of perception KASP标记KASP marker 1 5总计Total 88.5 SCAR标记SCAR marker 35 bp indel分子标记35 bp indel marker A B C A1 87.0 C1 A2 B2 C2 47 7 46 1 51 1符合率Coincidence rate/%各自Respective 97.6 94.7 73.4 94.8 70.8 0.0 66.9 2.2 52.0
3 讨 论
目前已报道的、能完全用于商业化生产的抗根结线虫基因很有限,野生秘鲁番茄中的Mi基因运用最广泛[21]。在育种改良过程中,研究者利用不同分子标记检测了供试番茄中的Mi基因,发现检测结果差异明显,有的检测方法如CAPS 检测Mi 基因的时候存在明显假阳性,而另一种标记方法即SCAR 标记检测相比之下更稳定、便捷[22-25]。在李属植物中,Ma、Rmia、Rmja 为已知的抗线虫基因。目前桃的抗性基因Rmia 能完全抑制根结线虫繁殖和根结线虫虫瘿的形成,对南方根结线虫、大豆根结线虫都具有抗性[26-27]。Duval等[28]利用分子标记评估该基因对尚未检测过的埃塞俄比亚根结线虫(M.ethiopica)的抗性,发现基因分型结果与表型完全匹配,说明该基因能完全控制M.ethiopica,同时更新了基因Ma、Rmia、Rmja对线虫的抗性谱系,发现Ma基因对线虫具有广谱抗性。笔者在本研究中所用SCAR 标记位于LG2 抗性基因座附近,35 bp indel 分子标记位于红根甘肃桃1 号抗南方根结线虫基因启动子区,KASP 标记位于2 号染色体候选基因Pru‐pr.2G055500 的内含子上。利用不同分子标记检测栽培桃F2群体对南方根结线虫的抗性,发现35 bp indel 分子标记检测结果与另外两个标记结果的准确率相比差异显著,这说明野生种质红根甘肃桃1号与栽培桃的抗性基因不同;SCAR标记与KASP标记准确率较接近,原因可能是所用的遗传群体与样本数量不同。为加快育种进程,利用抗性基因开发分子标记可提高材料中抗性基因筛选的效率,为选育具有综合抗病的新品种奠定基础。范惠冬等[29]利用抗性基因分子标记分析105 份番茄种质资源中7个病害相关的8 个抗性基因的分布情况,为抗性基因的聚合育种提供了参考。笔者通过分析定位区间内的变异,仅在候选基因上找到一处与抗感性显著相关的2 bp indel变异位点,随后开发分子标记并在群体中进行验证准确率为89.0%,较已报道的标记准确率高。但由于标记准确率未达到100%,推测该变异位点为连锁标记,可能并非功能性变异,笔者下一步将对候选区间内结构变异、转座子变异等不同变异类型进行检测,并在群体中开展准确率和功能验证,发掘南方根结线虫抗性关键基因。
在本研究中,群体的表型调查结果符合分离定律,但一定程度上也受环境影响。一方面,南方根结线虫的生长和侵染受土壤温度和湿度影响,适合J2侵染的温度为15 ℃~30 ℃[30-32]。研究发现,温度超过35 ℃或低于5 ℃都会抑制南方根结线虫的生长,最适宜根结线虫生活的土壤湿度为6%,土壤过于干燥或湿润均不利于南方根结线虫的活动[32-33]。另一方面,植物对线虫有一定的趋避性,感病植株在接触线虫时可能会躲避线虫的进攻。Duval等[28]研究发现,易感苗在线虫侵染时偶尔会躲避线虫的进攻而产生假抗性个体,为保证评估表型的准确率,需对植株进行持续性接种根结线虫以降低错评植株的风险。因此,笔者在本试验中改良了抗性鉴定指标,以根结有无替代根结率作为评价指标,显著提高了表型鉴定的准确率。另外,也可通过延长线虫侵染时间和多次接种根结线虫提高表型数据的准确性和稳定性。
4 结 论
基于前人对栽培桃抗南方根结线虫基因的定位结果,开发了一个KASP分子标记,并在杂交F2代群体中进行验证,发现与前人开发的抗南方根结线虫分子标记比较,笔者在本研究中开发的KASP 标记准确率最高。该标记的开发提高了抗性品种的选择效率,为加快分子育种进程提供了资源。
[1] 舒梅,冯绍卫,何志强.甜瓜‘傣姑’冬春栽培技术[J].农业科技通讯,2019(3):270-274.SHU Mei,FENG Shaowei,HE Zhiqiang.Cultivation techniques of melon‘Daigu’in winter and spring[J].Bulletin of Agricultural Science and Technology,2019(3):270-274.
[2] 李红琴.两种高山药材根结线虫病防治药剂筛选[D].昆明:云南农业大学,2023.LIHongqin.Screening of Meloidogyne hapla nematicides for tow alpine medicinal plants[D].Kunming:Yunnan Agricultural University,2023.
[3] 朱更瑞,王力荣,左覃元,张学炜.桃根结线虫种的鉴定及最佳接种方法研究[J].果树科学,2000,17(增刊):30-35.ZHU Gengrui,WANG Lirong,ZUO Qinyuan,ZHANG Xuewei.Identification of peach root-knot nematode species and study on the best inoculation method[J].Journal of Fruit Science,2000,17(Suppl.):30-35.
[4] 朱更瑞,王力荣,左覃元,张学炜.桃砧木资源对南方根结线虫的抗性[J].果树科学,2000,17(增刊):36-39.ZHU Gengrui,WANG Lirong,ZUO Qinyuan,ZHANG Xuewei.Resistance of peach rootstock resources to southern root-knot nematodes[J].Journal of Fruit Science,2000,17(Suppl.):36-39.
[5] 李肯,张伟,武云鹏,潘静怡,彭冬秀,张若纬.甜瓜果肉硬度分子标记的开发与利用[J].华北农学报,2023,38(5):94-101.LI Ken,ZHANG Wei,WU Yunpeng,PAN Jingyi,PENG Dongxiu,ZHANG Ruowei.Development and utilization of molecular markers for identification of pulp firmness in melon[J].Acta Agriculturae Boreali-Sinica,2023,38(5):94-101.
[6] 吴翼,李静,杨耀东.利用SSR 分子标记快速鉴定香水椰子种苗纯度[J].分子植物育种,2023,21(6):1977-1984.WU Yi,LIJing,YANG Yaodong.Rapid identification of aromatic coconut purity by SSR molecular markers[J].Molecular Plant Breeding,2023,21(6):1977-1984.
[7] 刘广,黄晓云,徐锦华,张曼,姚协丰,娄丽娜,徐建,侯茜,朱凌丽,羊杏平.西瓜枯萎病抗性分子标记筛选与应用[J].江苏农业科学,2022,50(18):279-283.LIU Guang,HUANG Xiaoyun,XU Jinhua,ZHANG Man,YAO Xiefeng,LOU Lina,XU Jian,HOU Qian,ZHU Lingli,YANG Xingping.Screening and application of molecular markers for Fusarium wilt resistance in watermelon[J].Jiangsu Agricultural Sciences,2022,50(18):279-283.
[8] 姚晓云,陈春莲,熊运华,黄永萍,彭志勤,刘进,尹建华.水稻加工和外观品质性状QTL 鉴定[J].中国水稻科学,2023,37(5):507-517.YAO Xiaoyun,CHEN Chunlian,XIONG Yunhua,HUANG Yongping,PENG Zhiqin,LIU Jin,YIN Jianhua.Identification of QTL for milling and appearance quality traits in rice (Oryza sativa L.)[J].Chinese Journal of Rice Science,2023,37(5):507-517.
[9] 刘伟.甘肃桃遗传连锁图谱的构建及抗南方根结线虫的分子标记[D].南京:南京农业大学,2010.LIU Wei.Genetic linkage map construction of Prunus kansuen‐sis and molecular markers for resistance to root-kont nematode(Meloidogyne incognita)[D].Nanjing:Nanjing Agricultural University,2010.
[10] CAO K,WANG L R,ZHAO P,ZHU G R,FANG W C,CHEN C W,WANG X W.Identification of a candidate gene for resistance to root-knot nematode in a wild peach and screening of its polymorphisms[J].Plant Breeding,2014,133(4):530-535.
[11] 张倩.红根甘肃桃1 号抗南方根结线虫基因的定位与发掘[D].北京:中国农业科学院,2018.ZHANG Qian.Locating and excavating of genes on Meloido‐gyne incognita resistance in Honggengansutao 1 (Prunus kan‐suensis L.)[D].Beijing:Chinese Academy of Agricultural Sciences,2018.
[12] DUVAL H,HOERTER M,POLIDORIJ,CONFOLENT C,MASSE M,MORETTIA,VAN GHELDER C,ESMENJAUD D.High-resolution mapping of the RMia gene for resistance to root-knot nematodes in peach[J].Tree Genetics & Genomes,2014,10(2):297-306.
[13] 王力荣,朱更瑞,方伟超.中国桃遗传资源[M].北京:中国农业出版社,2012.WANG Lirong,ZHU Gengrui,FANG Weichao.Peach genetic resource in China[M].Beijing:China Agriculture Press,2012.
[14] LIY,CAO K,ZHU G R,FANG W C,CHEN C W,WANG X W,ZHAO P,GUO J,DING T Y,GUAN L P,ZHANG Q,GUO W W,FEIZ J,WANG L R.Genomic analyses of an extensive collection of wild and cultivated accessions provide new insights into peach breeding history[J].Genome Biology,2019,20(1):36.
[15] 韦莹华,丁盛,董娟,杜凤,崔欣,唐卓.基于LAMP 建立非洲猪瘟病毒的快速可视化检测方法[J].应用与环境生物学报,2022,28(5):1325-1330.WEIYinghua,DING Sheng,DONG Juan,DU Feng,CUIXin,TANG Zhuo.Rapid visual detection of African swine fever virus based on LAMP[J].Chinese Journal of Applied and Environmental Biology,2022,28(5):1325-1330.
[16] 吉爽秋,王力荣,李勇,朱更瑞,曹珂,方伟超,陈昌文,王新卫,张琦,吴金龙.桃花花型(铃形/蔷薇形)基因型鉴定、分子标记开发与利用[J].果树学报,2023,40(3):422-431.JIShuangqiu,WANG Lirong,LIYong,ZHU Gengrui,CAO Ke,FANG Weichao,CHEN Changwen,WANG Xinwei,ZHANG Qi,WU Jinlong.Identification of peach flower genotype (Nonshowy/Showy),development of flower-type related molecular markers[J].Journal of Fruit Science,2023,40(3):422-431.
[17] YAMAMOTO T,HAYASHIT.New root-knot nematode resistance genes and their STS markers in peach[J].Scientia Horticulturae,2002,96(1/2/3/4):81-90.
[18] CAO K,PENG Z,ZHAO X,LIY,LIU K Z,ARUS P,FANG W C,CHEN C W,WANG X W,WU J L,FEIZ J,WANG L R.Chromosome-level genome assemblies of four wild peach species provide insights into genome evolution and genetic basis of stress resistance[J].BMC Biology,2022,20(1):139.
[19] 吴波鸿.转录因子HY5 调控南方根结线虫侵染发育机理研究[D].沈阳:沈阳农业大学,2022.WU Bohong.Study on the mechanism of HY5 regulating nematode infection and development[D].Shenyang:Shenyang Agricultural University,2022.
[20] SHARPE R H,HESSE C O,LOWNSBERY B F,PERRY V G,HANSEN C J.Breeding peaches for root-knot nematode Resistance1[J].Journal of the American Society for Horticultural Science,1969,94(3):209-212.
[21] WILLIAMSON V M.Plant nematode resistance genes[J].Current Opinion in Plant Biology,1999,2(4):327-331.
[22] LI Q,XIE Q G,SMITH- BECKER J,NAVARRE D A,KALOSHIAN I.Mi-1-Mediated aphid resistance involves salicylic acid and mitogen-activated protein kinase signaling cascades[J].Molecular Plant-Microbe Interactions,2006,19(6):655-664.
[23] BHATTARAIK K,XIE Q G,POURSHALIMID,YOUNGLOVE T,KALOSHIAN I. Coi1-dependent signaling pathway is not required for Mi-1-mediated potato aphid resistance[J].Molecular Plant-Microbe Interactions,2007,20(3):276-282.
[24] HU C L,ZHAO W C,FAN J W,LIZ L,YANG R,ZHAO F K,WANG J L,WANG S H.Protective enzymes and genes related to the JA pathway are involved in the response to root-knot nematodes at high soil temperatures in tomatoes carrying Mi-1[J].Horticulture,Environment,and Biotechnology,2015,56(4):546-554.
[25] 戴均涛,张慎璞,王暄,丁修恒,李红梅.3 种检测番茄抗根结线虫Mi 基因分子标记法的比较[J].南京农业大学学报,2018,41(5):848-853.DAIJuntao,ZHANG Shenpu,WANG Xuan,DING Xiuheng,LIHongmei.Comparison of three molecular markers for detecting Mi gene of resistance to root-knot nematode in tomato cultivars[J].Journal of Nanjing Agricultural University,2018,41(5):848-853.
[26] ESMENJAUD D,MINOT J C,VOISIN R,PINOCHET J,SI-MARD M H,SALESSES G.Differential response to root-knot nematodes in Prunus species and correlative genetic implications[J].Journal of Nematology,1997,29(3):370-380.
[27] ESMENJAUD D,VOISIN R,VAN GHELDER C,BOSSELUT N,LAFARGUE B,DI VITO M,DIRLEWANGER E,POËSSEL J L,KLEINHENTZ M.Genetic dissection of resistance to root-knot nematodes Meloidogyne spp.in plum,peach,almond,and apricot from various segregating interspecific Prunus progenies[J].Tree Genetics&Genomes,2009,5(2):279-289.
[28] DUVAL H,VAN GHELDER C,PORTIER U,CONFOLENT C,MEZA P,ESMENJAUD D.New data completing the spectrum of the Ma,RMia,and RMja genes for resistance to root-knot nematodes (Meloidogyne spp.) in Prunus[J].Phytopathology,2019,109(4):615-622.
[29] 范惠冬,许世霖,郑士金,田松,王宇微,宫庆锐.105 份番茄种质资源的抗性基因分子标记检测[J/OL].北方园艺,2024:1-10.(2024-01-19).https://kns.cnki.net/kcms/detail/23.1247.S.20240119.1246.004.html.FAN Huidong,XU Shilin,ZHENG Shijin,TIAN Song,WANG Yuwei,GONG Qingrui.Molecular marker detection of resistance genes in 105 tomato germplasm resources[J/OL].Northern Horticulture,2024:1-10.(2024-01-19).https://kns.cnki.net/kcms/detail/23.1247.S.20240119.1246.004.html.
[30] 王袁,郭泽,李晓辉,徐世晓,邢学霞,张思琦,何佳,刘超,陈芳,杨铁钊.不同温度条件下根结线虫侵染对烟草根系的影响[J].作物杂志,2018(4):161-166.WANG Yuan,GUO Ze,LIXiaohui,XU Shixiao,XING Xuexia,ZHANG Siqi,HE Jia,LIU Chao,CHEN Fang,YANG Tiezhao.Effects of Meloidogyne incognita infection on tobacco root system under different temperatures[J].Crops,2018(4):161-166.
[31] 魏佩瑶,潘嵩,彭德良,张锋,陈志杰,张淑莲,李英梅.低温胁迫对南方根结线虫存活的影响及在北方温室的应用[J].应用生态学报,2023,34(7):1981-1987.WEIPeiyao,PAN Song,PENG Deliang,ZHANG Feng,CHEN Zhijie,ZHANG Shulian,LIYingmei.Effect of low-temperature stress on the survival of Meloidogyne incognita and its application in greenhouse of Northern China[J].Chinese Journal of Applied Ecology,2023,34(7):1981-1987.
[32] 陈立杰,魏峰,段玉玺,白春明,霍璟珣,朱晓峰.温湿度对南方根结线虫卵孵化和二龄幼虫的影响[J].植物保护,2009,35(2):48-52.CHEN Lijie,WEIFeng,DUAN Yuxi,BAIChunming,HUO Jingxun,ZHU Xiaofeng.Effects of temperature and moisture on egg hatching and the second instars of Meloidogyne incognita[J].Plant Protection,2009,35(2):48-52.
[33] 曹素芳,漆永红,杜蕙,陈书龙.温度和湿度对南方根结线虫存活的影响[J].植物保护,2012,38(6):108-111.CAO Sufang,QIYonghong,DU Hui,CHEN Shulong.Effects of temperature and moisture on the survival of Meloidogyne incog‐nita[J].Plant Protection,2012,38(6):108-111.