由欧文氏菌[Erwinia amylovora(Burr)Winslow et al.]侵染所致的梨火疫病是国际检疫性的细菌病害[1-2],该病害传播速度快,传播途径多,寄主范围广,是全球十大植物病原细菌之一[3-5]。库尔勒香梨作为中国国家地理标志产品,是当地农业增效、农民增收的重要产业之一[6]。因受到梨火疫病的影响,库尔勒香梨产业的发展被严重制约。目前防治该病害的主要手段是化学防治,但国内外连续多年使用农用链霉素导致抗药性菌株产生[7],中国于2016年6月已全部停止农用链霉素登记和使用[8],因此尽快寻找安全、有效的替代药剂至关重要。
生物农药四霉素是由辽宁省微生物科学研究院于20 世纪70 年代开始研发,90 年代初实现产品化的纯绿色生物农药[9]。已有研究表明,四霉素通过抑制病原菌生长,促进作物生长,诱导作物产生抗性进而达到防治病害的目的[10-11]。该药剂主要成分为不吸水链霉菌梧州亚种的发酵代谢产物,因是天然产物,故具有高效、低毒的杀菌效果[12]。四霉素对细菌性和真菌性的植物病害均产生抑制作用,如辣椒炭疽病[13]、马铃薯疮痂病[14]、核桃细菌性黑斑病[15]、茭白叶斑病[16]等,其抑菌作用广谱,故应用前景较好。目前中国尚未见梨火疫病菌对四霉素的敏感基线建立和抗药性评价的研究报道。
笔者在本研究中通过建立新疆库尔勒和阿克苏地区(以下简称南疆地区)梨火疫病菌对四霉素的敏感基线,筛选梨火疫病菌对该药剂的抗药突变体,研究抗药突变体的遗传稳定性,以及与其他田间常用化学药剂之间有无交互抗药性,旨在为监测田间梨火疫病菌的四霉素抗药性情况提供理论依据,为该药剂对梨火疫病的田间防治与抗药性治理提供参考。
1 材料和方法
1.1 材料
1.1.1 供试药剂 四霉素标准样品(Tetramycin,北京曼哈格生物科技有限公司)。交互抗性的药剂:84%噻霉酮(Benziothiazolinone,陕西西大华特制药厂);中生菌素标准样品(Zhongshengmycin,北京倍特仁康生物医药科技有限公司)。
1.1.2 供试菌株 2021—2023 年从新疆库尔勒哈拉玉宫乡、铁门关市、阿瓦提乡及阿克苏地区采集的梨火疫病样,经组织分离、鉴定,共获得100 株菌株。
1.1.3 供试培养基 NA 培养基:无菌水1000 mL,牛肉膏3 g,酵母膏1 g,蛋白胨5 g,蔗糖10 g,氯化钠5 g,琼脂17 g,2 g·mL-1 氢氧化钠3000 μL。121 ℃高压灭菌25 min,备用。NB 培养液:去掉琼脂,其余试剂与NA 培养基一致,121 ℃高压灭菌25 min,备用。NA 含药培养基:参考李亚萌[17]“含药培养基”的配比方法,将配好的不同质量浓度四霉素药液按1∶99 的比例加入到NA 培养基中,每个培养皿中药液与培养基体积之和为10 mL。
1.2 试验方法
1.2.1 药剂原药及稀释液配制 四霉素标准样品购自北京曼哈格生物科技有限公司,标准样品质量浓度为100 µg·mL-1,使用时用无菌水将母液稀释制成1 µg·mL-1、2.5 µg·mL-1、5 µg·mL-1、10 µg·mL-1、20µg·mL-1的药液备用。
1.2.2 梨火疫病菌对四霉素的敏感性检测 利用抑菌圈法进行敏感性检测试验。在NA 培养基上将保存菌种进行划线分离,28 ℃恒温培养48 h,将单菌落转入NB 培养液中28 ℃、180 r·min-1摇培12 h,测定OD600为1.0时得到试验菌悬液。将菌悬液稀释涂布于NA 培养基中至干燥。培养皿内放置3 片直径为6 mm的灭菌滤纸片于NA培养基上[18]。吸取6µL的不同浓度的药液滴于滤纸片上,无菌水作为对照。28 ℃正置培养36 h,使用十字交叉法测量抑菌圈直径大小,按公式(1)计算抑菌率。根据抑菌率计算其相关系数及EC50值。依据所有供试菌株的平均EC50值建立新疆库尔勒地区梨火疫病菌对四霉素的敏感基线,按照公式(2)[19]计算各菌株对四霉素的抗性水平。

根据各菌株的抗性水平数值,将各菌株分为敏感、低抗、中抗及高抗类型[20]。具体划分如下:敏感菌株(S):抗性水平≤(敏感基线的95%置信限上限/敏感基线),抗性水平≤1.13 μg·mL-1;低抗菌株(LR):(敏感基线的95%置信限上限/敏感基线)<抗性水平≤敏感基线10 倍,1.13 μg·mL-1<抗性水平≤15.9 μg·mL-1;中抗菌株(MR):敏感基线10倍<抗性水平≤敏感基线100 倍,15.9 μg·mL-1<抗性水平≤159 μg·mL-1;高抗菌株(HR):抗性水平>敏感基线100 倍,抗性水平>159 μg·mL-1。
1.2.3 药剂驯化获得抗药突变体 以4 个地区中最敏感菌株作为诱导抗药突变体的亲本菌株。参考胡白石[1]药剂驯化的方法,将敏感菌株在NA 培养基上进行平板划线分离后获得单菌落,挑单菌落到NB培养液中,28 ℃、180 r·min-1摇培12 h,分光光度计测定OD600值为1.0 的菌悬液作为试验亲本菌悬液。用无菌接种针蘸取亲本菌悬液,划线接种在含有1 µg·mL-1的NA 含药培养基上,按照此方法依次将平板中药剂质量浓度提高为2.5µg·mL-1、5µg·mL-1、10µg·mL-1、20µg·mL-1。
1.2.4 抗药突变体抗性水平测定 采用“抑菌圈法”测定抗药突变体对四霉素的敏感性,按照公式(2)计算抗性水平,公式(3)计算其抗性倍数。
1.2.5 抗药突变体遗传稳定性测定 将药剂驯化得到的抗药突变体在NA 培养基上连续转接10 代,观察其生长情况,待第10 代单菌落生长36 h 后,再次转接到NA 含药培养基上作为第11 代加以验证,并在含药培养基上比较抗药突变体本身和第11 代抗药突变体间的抗药性水平差异,确定抗药突变体继代培养的遗传稳定性。
1.2.6 抗药突变体交互抗药性测定 采用“抑菌圈法”,用50 mL 无菌水将四霉素标准物质样品依次稀释为20、10、5、2.5、1 mg·L-1的质量浓度梯度,将84%噻霉酮依次稀释为1600、800、400、300、200、100 mg·L-1的质量浓度梯度;中生菌素标准物质样品依次稀释为200、100、50、25、10 mg·L-1的质量浓度梯度,测定抗药突变体对3 种药剂的EC50值。以四霉素的EC50浓度对数值为横坐标,分别以84%噻霉酮、中生菌素标准物质样品的EC50浓度对数值为纵坐标,计算相关系数,并以其相关系数的绝对值来判断四霉素与以上2 种杀菌剂之间是否存在交互抗药性[21],如相关系数绝对值大于0.75 时,则该2种药剂之间存在交互抗药性[22]。
1.3 数据处理
使用Microsoft Excel 2019 进行数据统计分析,计算各药剂相对毒力指数,并以各药剂浓度的对数值为横坐标(x),抑菌率对应的概率值为纵坐标(y),计算毒力回归方程及相关系数。使用SPSS 26.0 计算供试药剂的EC50值,以及进行显著性差异分析及其他统计学分析。
2 结果与分析
2.1 梨火疫病菌对四霉素的敏感性分布
通过分离并测定100 株来自新疆不同地区的梨火疫病菌对四霉素的敏感性,发现100 株梨火疫病菌对四霉素的抗性表现不一,但总体表现均较敏感。检测到敏感菌株74 个,占比74%;抗性菌株26个,占比26%,为低抗菌株,未检测到中抗、高抗菌株,具体结果见表1。
表1 100 株梨火疫病菌株对四霉素的敏感性
Table 1 Sensitive types of 100 Erwinia amylovora strains to tetramycin
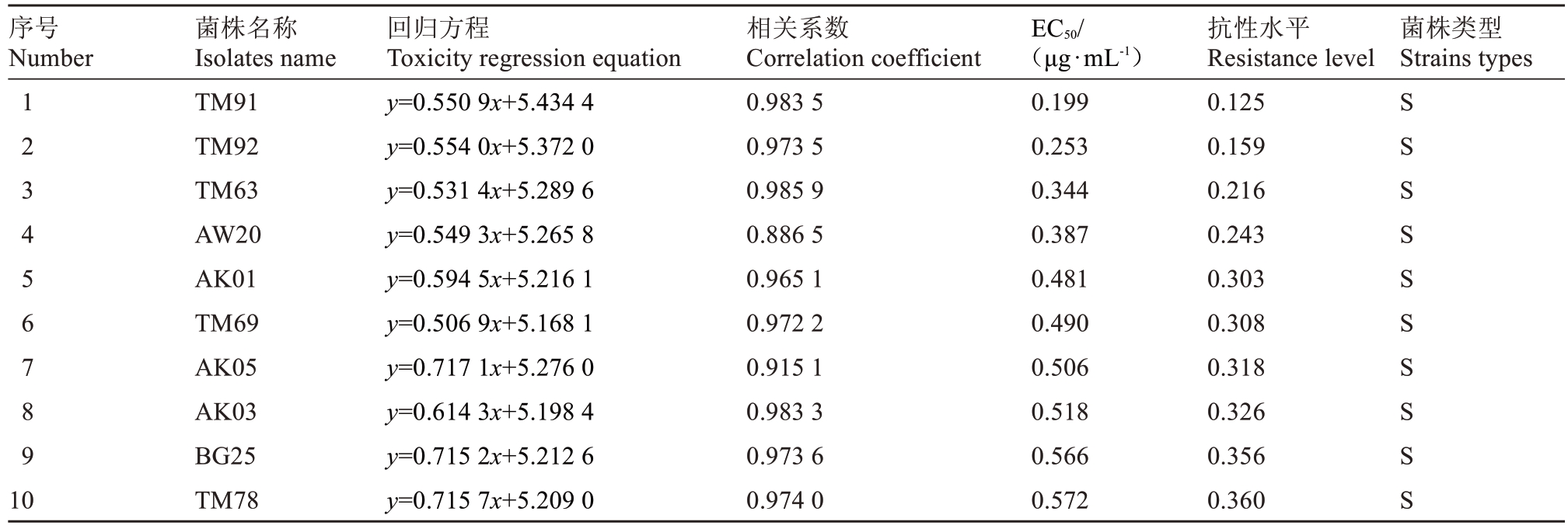
注:按照EC50 值从小到大进行排序。敏感菌株(S),低抗菌株(LR)。
Note:The arrangement was according to the EC50 value from small to large;Sensitive strains(S),low resistance strains(LR).
序号Number菌株名称Isolates name回归方程Toxicity regression equation相关系数Correlation coefficient EC50/(μg·mL-1)抗性水平Resistance level菌株类型Strains types 1 2 3 4 5 6 7 8 9 10 TM91 TM92 TM63 AW20 AK01 TM69 AK05 AK03 BG25 TM78 y=0.550 9x+5.434 4 y=0.554 0x+5.372 0 y=0.531 4x+5.289 6 y=0.549 3x+5.265 8 y=0.594 5x+5.216 1 y=0.506 9x+5.168 1 y=0.717 1x+5.276 0 y=0.614 3x+5.198 4 y=0.715 2x+5.212 6 y=0.715 7x+5.209 0 0.983 5 0.973 5 0.985 9 0.886 5 0.965 1 0.972 2 0.915 1 0.983 3 0.973 6 0.974 0 0.199 0.253 0.344 0.387 0.481 0.490 0.506 0.518 0.566 0.572 0.125 0.159 0.216 0.243 0.303 0.308 0.318 0.326 0.356 0.360 S S S S S S S S S S
表1 (续) Table 1 (Continued)

序号Number菌株名称Isolates name回归方程Toxicity regression equation相关系数Correlation coefficient EC50/(μg·mL-1)抗性水平Resistance level菌株类型Strains types 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 AW11 TM98 AW12 AW09 BG09 AW18 BG22 TM50 TM75 AW08 TM56 TM64 AK02 BG07 TM88 TM53 BG13 BG02 AW15 TM93 TM52 TM68 AW13 BG24 BG08 TM90 TM71 TM96 AW17 TM57 TM74 TM97 TM66 BG21 TM89 TM94 TM54 TM76 TM51 BG04 BG12 BG03 TM58 TM62 TM70 AW06 BG23 TM59 y=0.728 6x+5.189 4 y=0.467 2x+5.110 4 y=0.662 5x+5.148 6 y=0.671 2x+5.134 3 y=0.671 2x+5.134 3 y=0.727 1x+5.148 1 y=0.663 1x+5.107 5 y=0.676 2x+5.081 3 y=0.690 8x+5.085 1 y=0.744 0x+5.075 7 y=0.814 6x+5.069 9 y=0.794 6x+5.059 8 y=0.749 7x+5.051 0 y=0.793 4x+5.050 0 y=0.768 0x+5.050 5 y=0.791 1x+5.044 8 y=0.810 1x+5.049 4 y=0.843 0x+5.023 7 y=0.869 0x+5.020 4 y=0.865 3x+5.004 0 y=0.755 4x+5.000 5 y=0.750 5x+4.995 6 y=0.860 3x+4.991 9 y=0.788 9x+4.979 5 y=0.758 6x+4.971 6 y=0.756 5x+4.951 7 y=0.716 7x+4.958 5 y=0.770 9x+4.959 2 y=0.843 5x+4.958 0 y=0.900 4x+4.943 0 y=0.782 6x+4.934 6 y=0.850 9x+4.937 0 y=0.743 5x+4.935 4 y=0.838 8x+4.933 5 y=0.676 4x+4.931 7 y=0.758 8x+4.909 0 y=0.907 5x+4.908 6 y=0.747 2x+4.908 0 y=0.808 2x+4.913 0 y=0.880 7x+4.902 4 y=0.876 5x+4.902 0 y=0.736 5x+4.906 1 y=0.927 8x+4.890 3 y=0.797 2x+4.901 2 y=0.794 2x+4.901 3 y=0.798 6x+4.899 8 y=0.861 0x+4.890 3 y=0.924 9x+4.877 0 0.970 4 0.964 9 0.978 6 0.978 3 0.978 3 0.986 7 0.967 8 0.978 0 0.965 4 0.968 6 0.975 2 0.970 9 0.983 0 0.972 4 0.959 4 0.959 1 0.959 0 0.945 6 0.942 6 0.985 1 0.959 2 0.973 3 0.928 9 0.975 7 0.962 0 0.974 0 0.971 3 0.957 6 0.955 7 0.957 5 0.998 7 0.949 3 0.958 0 0.962 3 0.998 1 0.998 0 0.898 0 0.977 6 0.902 0 0.942 6 0.944 1 0.978 9 0.983 2 0.962 2 0.962 3 0.963 8 0.966 2 0.930 9 0.604 0.605 0.638 0.670 0.670 0.671 0.734 0.795 0.796 0.846 0.866 0.890 0.901 0.911 0.913 0.926 0.931 0.999 1.009 1.034 1.044 1.053 1.090 1.090 1.123 1.147 1.156 1.161 1.174 1.209 1.227 1.232 1.242 1.245 1.271 1.322 1.328 1.331 1.333 1.334 1.339 1.341 1.346 1.358 1.359 1.362 1.374 1.415 0.380 0.381 0.401 0.421 0.421 0.422 0.462 0.500 0.501 0.532 0.545 0.560 0.567 0.573 0.574 0.582 0.586 0.628 0.635 0.650 0.657 0.662 0.686 0.686 0.706 0.721 0.727 0.730 0.738 0.760 0.772 0.775 0.781 0.783 0.799 0.831 0.835 0.837 0.838 0.839 0.842 0.843 0.847 0.854 0.855 0.857 0.864 0.890 S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S
表1 (续) Table 1 (Continued)
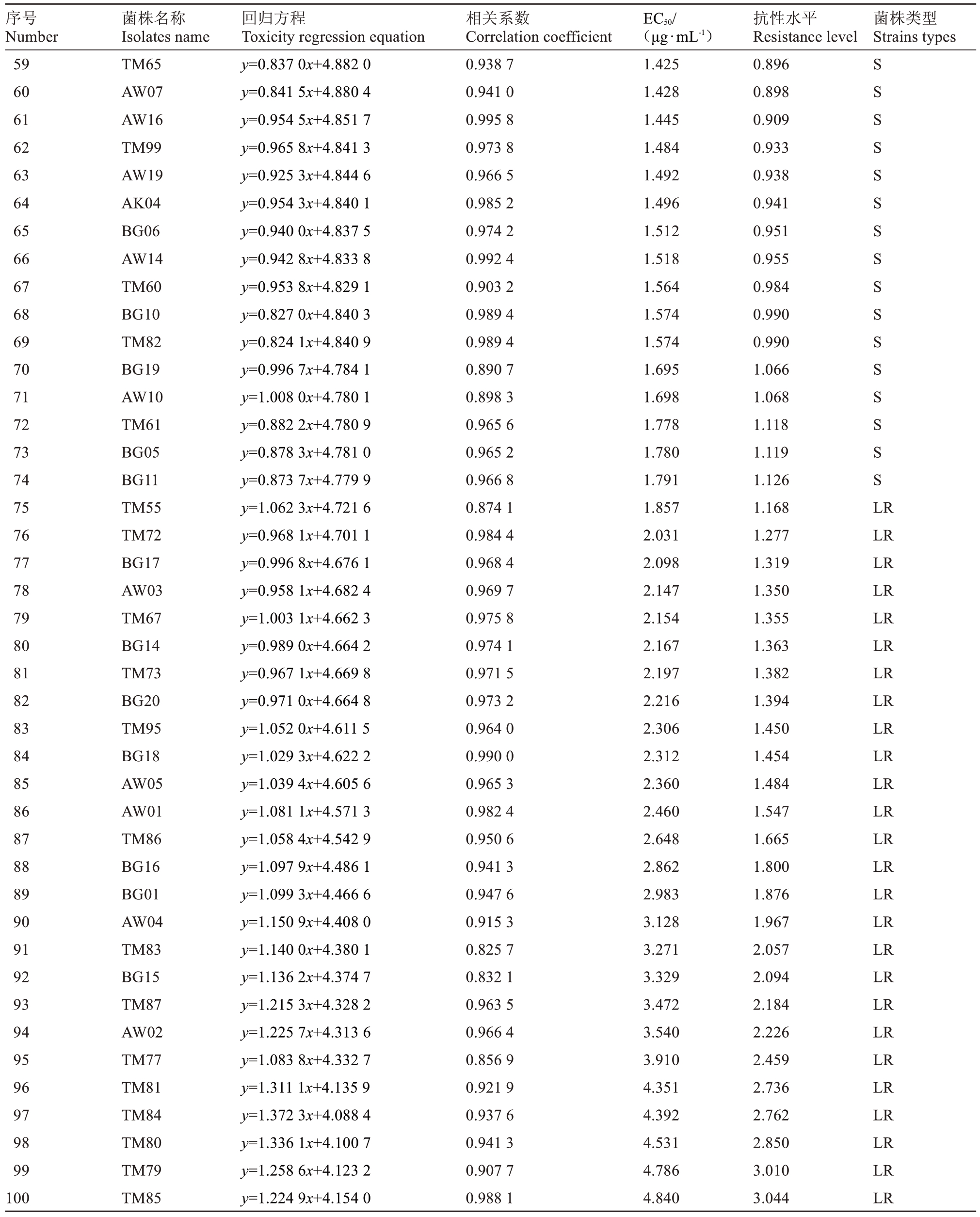
序号Number菌株名称Isolates name回归方程Toxicity regression equation相关系数Correlation coefficient EC50/(μg·mL-1)抗性水平Resistance level菌株类型Strains types 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 TM65 AW07 AW16 TM99 AW19 AK04 BG06 AW14 TM60 BG10 TM82 BG19 AW10 TM61 BG05 BG11 TM55 TM72 BG17 AW03 TM67 BG14 TM73 BG20 TM95 BG18 AW05 AW01 TM86 BG16 BG01 AW04 TM83 BG15 TM87 AW02 TM77 TM81 TM84 TM80 TM79 TM85 y=0.837 0x+4.882 0 y=0.841 5x+4.880 4 y=0.954 5x+4.851 7 y=0.965 8x+4.841 3 y=0.925 3x+4.844 6 y=0.954 3x+4.840 1 y=0.940 0x+4.837 5 y=0.942 8x+4.833 8 y=0.953 8x+4.829 1 y=0.827 0x+4.840 3 y=0.824 1x+4.840 9 y=0.996 7x+4.784 1 y=1.008 0x+4.780 1 y=0.882 2x+4.780 9 y=0.878 3x+4.781 0 y=0.873 7x+4.779 9 y=1.062 3x+4.721 6 y=0.968 1x+4.701 1 y=0.996 8x+4.676 1 y=0.958 1x+4.682 4 y=1.003 1x+4.662 3 y=0.989 0x+4.664 2 y=0.967 1x+4.669 8 y=0.971 0x+4.664 8 y=1.052 0x+4.611 5 y=1.029 3x+4.622 2 y=1.039 4x+4.605 6 y=1.081 1x+4.571 3 y=1.058 4x+4.542 9 y=1.097 9x+4.486 1 y=1.099 3x+4.466 6 y=1.150 9x+4.408 0 y=1.140 0x+4.380 1 y=1.136 2x+4.374 7 y=1.215 3x+4.328 2 y=1.225 7x+4.313 6 y=1.083 8x+4.332 7 y=1.311 1x+4.135 9 y=1.372 3x+4.088 4 y=1.336 1x+4.100 7 y=1.258 6x+4.123 2 y=1.224 9x+4.154 0 0.938 7 0.941 0 0.995 8 0.973 8 0.966 5 0.985 2 0.974 2 0.992 4 0.903 2 0.989 4 0.989 4 0.890 7 0.898 3 0.965 6 0.965 2 0.966 8 0.874 1 0.984 4 0.968 4 0.969 7 0.975 8 0.974 1 0.971 5 0.973 2 0.964 0 0.990 0 0.965 3 0.982 4 0.950 6 0.941 3 0.947 6 0.915 3 0.825 7 0.832 1 0.963 5 0.966 4 0.856 9 0.921 9 0.937 6 0.941 3 0.907 7 0.988 1 1.425 1.428 1.445 1.484 1.492 1.496 1.512 1.518 1.564 1.574 1.574 1.695 1.698 1.778 1.780 1.791 1.857 2.031 2.098 2.147 2.154 2.167 2.197 2.216 2.306 2.312 2.360 2.460 2.648 2.862 2.983 3.128 3.271 3.329 3.472 3.540 3.910 4.351 4.392 4.531 4.786 4.840 0.896 0.898 0.909 0.933 0.938 0.941 0.951 0.955 0.984 0.990 0.990 1.066 1.068 1.118 1.119 1.126 1.168 1.277 1.319 1.350 1.355 1.363 1.382 1.394 1.450 1.454 1.484 1.547 1.665 1.800 1.876 1.967 2.057 2.094 2.184 2.226 2.459 2.736 2.762 2.850 3.010 3.044 S S S S S S S S S S S S S S S S LR LR LR LR LR LR LR LR LR LR LR LR LR LR LR LR LR LR LR LR LR LR LR LR LR LR
2.2 梨火疫病菌对四霉素敏感基线的建立
100 株梨火疫病菌的EC50值分布范围在0.199~4.840 µg·mL-1 之间,EC50 最大值是EC50 最小值的24.32 倍,平均EC50为(1.590±1.029)µg·mL-1,95%置信区间为1.389 2~1.797 4 µg·mL-1。所有梨火疫菌株对四霉素分布呈单峰曲线,近似于正态分布(图1),未发现敏感性显著下降的梨火疫菌群,因此可将平均EC50值(1.590±1.029)µg·mL-1作为新疆南疆地区梨火疫病菌对四霉素的敏感基线。
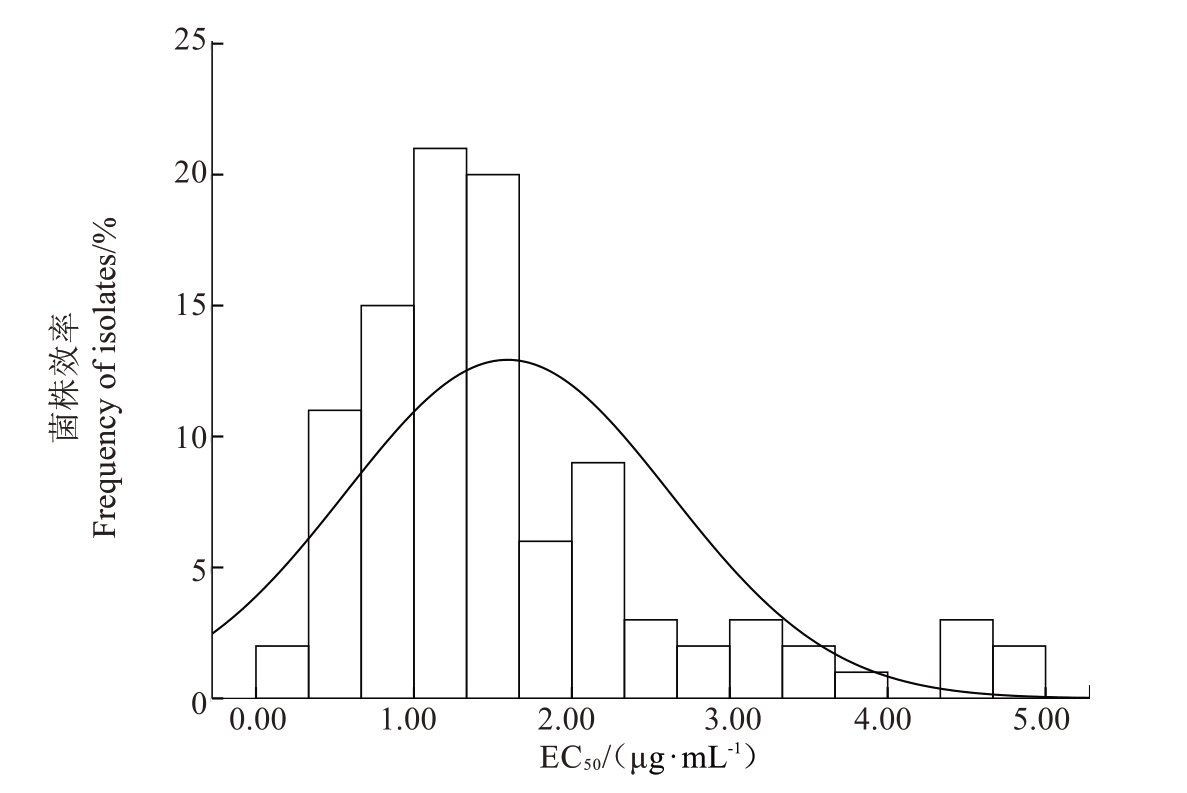
图1 新疆南疆地区100 株梨火疫病菌菌株对四霉素敏感性的频率分布
Fig.1 Frequency distribution of sensitivity to tetramycin on 100 Erwinia amylovora strains from South of Xinjiang
2.3 不同地区梨火疫病菌对四霉素的敏感性差异
4 个地区的100 株梨火疫病菌对四霉素的敏感性存在一定差异。如表2 所示,阿克苏市菌株较为敏感,其EC50平均值为(0.780±0.436)µg·mL-1,与阿瓦提乡菌株、哈拉玉宫乡菌株和铁门关菌株均存在显著差异。
表2 不同地区梨火疫病菌对四霉素的敏感性
Table 2 Sensitivity of Erwinia amylovora to tetramycin from different areas in south of Xinjiang
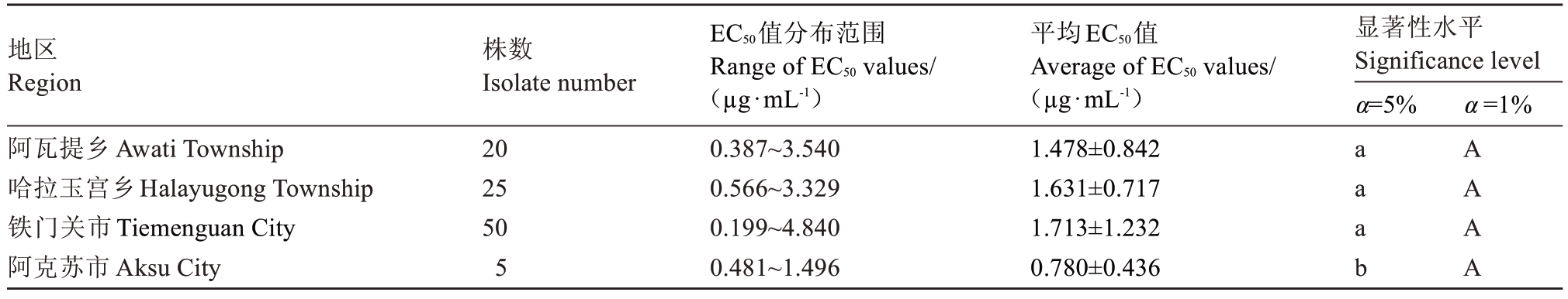
地区Region阿瓦提乡Awati Township哈拉玉宫乡Halayugong Township铁门关市Tiemenguan City阿克苏市Aksu City株数Isolate number 20 25 50 5 EC50值分布范围Range of EC50 values/(µg·mL-1)0.387~3.540 0.566~3.329 0.199~4.840 0.481~1.496平均EC50值Average of EC50 values/(µg·mL-1)1.478±0.842 1.631±0.717 1.713±1.232 0.780±0.436显著性水平Significance level α=5%α=1%Page 1 a a a b A A A A
2.4 抗药突变体筛选
通过药剂驯化试验共获得4 株抗药突变体,见表3。10 µg·mL-1是4 株供试菌株在该药剂下的最高生长质量浓度,因此认定在10 µg·mL-1质量浓度药剂平板上还能继续稳定生长的单菌落为抗药突变体。
表3 室内抗药突变体的获得
Table 3 Information of resistant mutants in laboratory
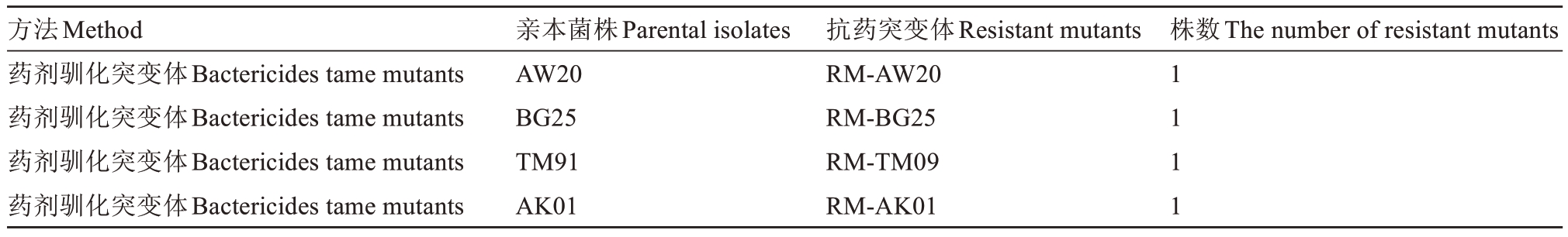
注:菌株名称带有RM 前缀的均为抗药突变体菌株。
Note:All strains with RM prefix were resistant mutants.
方法Method药剂驯化突变体Bactericides tame mutants药剂驯化突变体Bactericides tame mutants药剂驯化突变体Bactericides tame mutants药剂驯化突变体Bactericides tame mutants亲本菌株Parental isolates AW20 BG25 TM91 AK01抗药突变体Resistant mutants RM-AW20 RM-BG25 RM-TM09 RM-AK01株数The number of resistant mutants 1 1 1 1
2.5 抗药突变体抗性水平测定
4 株梨火疫抗药突变体对四霉素抗性水平测定结果见表4。经过室内毒力测定,四霉素对各亲本菌株的EC50值均在1 μg·mL-1以下,而对抗药突变体的EC50值除RM-TM91 外均在2 μg·mL-1以上,表明室内药剂驯化有效。RM-AW20、RM-BG25、RM-TM91、RM-AK01 的EC50 值分别为2.166、2.312、1.352、2.82 μg·mL-1,与其亲本菌株的EC50值相比,抗性倍数分别为5.59 倍、4.08 倍、6.79 倍、5.86 倍,抗性水平逐步提高,按照1.2.1 中的菌株抗药性划分标准,亲本菌株通过药剂驯化后的抗药突变体均已转变为对四霉素产生抗性的低抗菌株(1.13 µg·mL-1<抗性水平≤15.9 µg·mL-1)。
表4 抗药突变体对四霉素的抗性水平
Table 4 Resistance level of the resistant mntants to tetramycin
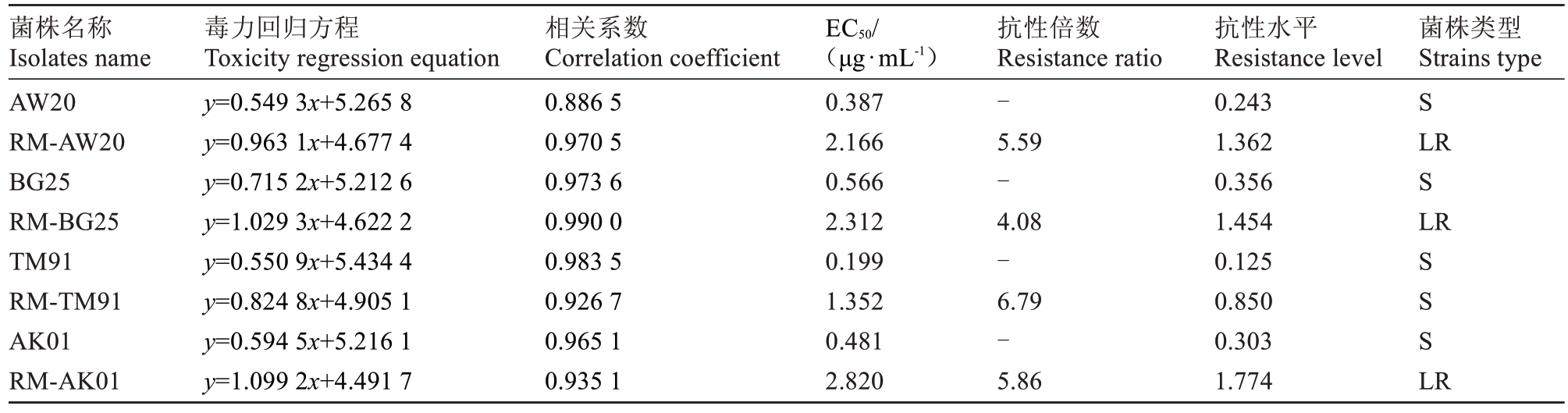
注:菌株名称带有RM 前缀的均为抗药突变体菌株。敏感菌株(S),低抗菌株(LR)。
Note:All strains with RM prefix were resistant mutants;Sensitive strains(S),low resistance strains(LR).
菌株名称Isolates name AW20 RM-AW20 BG25 RM-BG25 TM91 RM-TM91 AK01 RM-AK01毒力回归方程Toxicity regression equation y=0.549 3x+5.265 8 y=0.963 1x+4.677 4 y=0.715 2x+5.212 6 y=1.029 3x+4.622 2 y=0.550 9x+5.434 4 y=0.824 8x+4.905 1 y=0.594 5x+5.216 1 y=1.099 2x+4.491 7相关系数Correlation coefficient 0.886 5 0.970 5 0.973 6 0.990 0 0.983 5 0.926 7 0.965 1 0.935 1菌株类型Strains type S LR S LR EC50/(μg·mL-1)0.387 2.166 0.566 2.312 0.199 1.352 0.481 2.820抗性倍数Resistance ratio-5.59-4.08-6.79-5.86抗性水平Resistance level 0.243 1.362 0.356 1.454 0.125 0.850 0.303 1.774 S S S LR
2.6 抗药突变体交互抗药性测定
交互抗药性测定结果见图2,梨火疫抗药突变体对四霉素的EC50值与84%噻霉酮和中生菌素之间的EC50值相关性较高,相关系数分别为0.765 8与0.900 7,所有相关系数绝对值均高于0.75。由此可见,四霉素与田间常用防治梨火疫药剂84%噻霉酮、中生菌素之间存在交互抗药性问题,故应避免将该三种杀菌剂作为轮换备用农药在田间使用,以免产生交互抗药性问题。
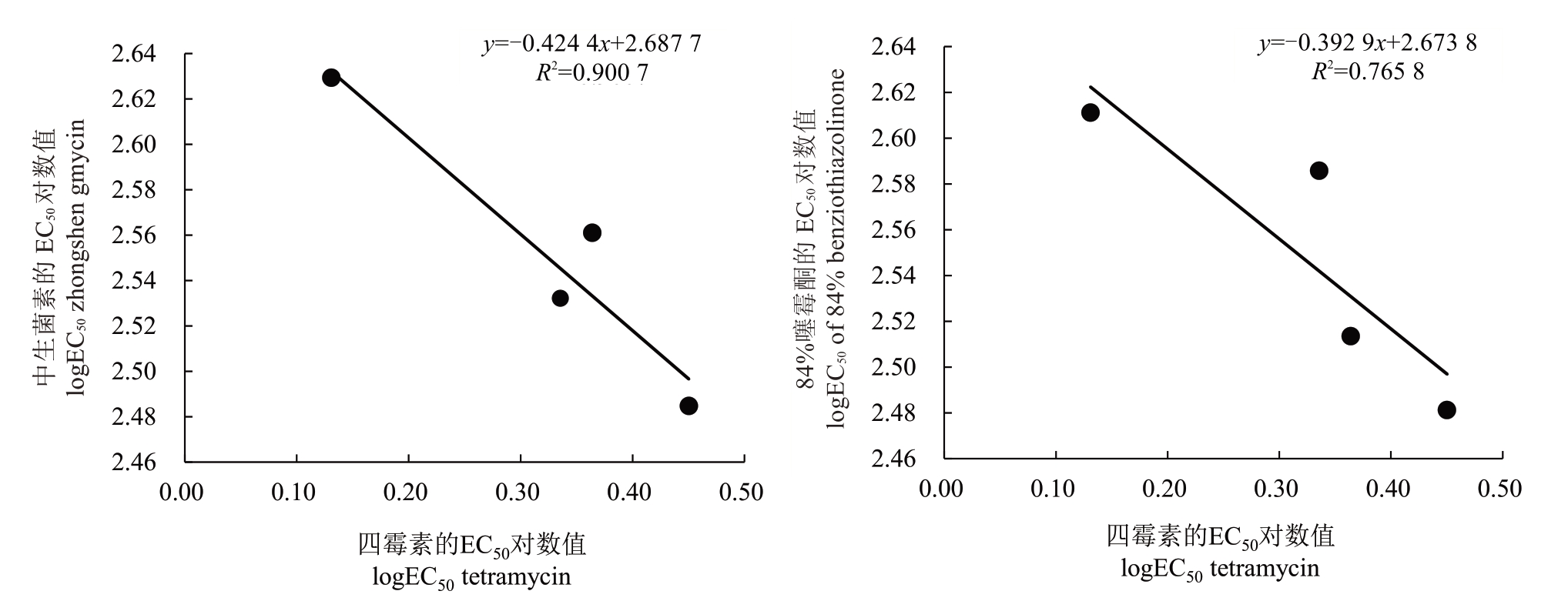
图2 两种杀菌剂对四霉素的交互抗药性
Fig.2 Cross-resistance of 2 bacteriocides to tetramycin
3 讨 论
梨火疫病是危害新疆地区梨树的最主要细菌性病害,该病害对梨果的生长、产量和品质影响极大,传染风险高,因而,对梨火疫病的防治措施研究一直是科研工作者关注的热点[23]。四霉素对多种植物病原菌具有广谱的抑制作用[9],且具有高效、低毒的特点[24],在中国作为对植物真菌与细菌均有防治效果的杀菌剂,该药剂已在各个作物与田间得到大范围、大面积的使用[25-28]。目前有关梨火疫病菌的敏感性检测与抗药性评价研究在国内外鲜有报道,因此笔者在本试验中以100 株新疆库尔勒梨火疫病菌为供试靶标菌株,旨在建立起库尔勒梨火疫病菌对四霉素的敏感基线,以4 株梨火疫病敏感菌株为供试靶标菌株,在室内进行病原菌的抗药性评价试验,旨在为其抗药性监测及田间科学用药提供参考。
在化学杀菌剂长期、单一的使用压力下,病原菌对杀菌剂将产生较高的抗性风险[29]。故在开始使用新型的杀菌剂之前,建立敏感基线对评价其抗药性与制定其抗药性治理策略具有重要意义[30]。本研究结果显示,四霉素对所有供试菌株均有室内抑制作用,抗药性表现不一。检测到敏感菌株74个,抗性菌株26 个,其中低抗菌株26 个,未检测到中抗、高抗菌株。所有EC50值中,未出现明显异常值,表明对四霉素而言,库尔勒地区的梨火疫病菌大部分还处于野生敏感种群阶段。敏感性频率分布呈正态分布,因此可将(1.59±1.029)µg·mL-1作为新疆地区梨火疫病菌对四霉素的敏感基线。从病原菌的角度来看,该敏感基线可作为观测新疆地区是否出现四霉素抗药性群体的依据,从防治药剂角度看,对避免及延缓抗药性的产生具有重要意义。但需要注意的是,不同地区的梨火疫病菌对四霉素的敏感性可能会存在差异,这是因为不同地区使用四霉素防治该病害的用药水平存在差异。
不同地区菌株对杀菌剂的基本抗性是由药剂与病原菌本身两者共同决定的,如防治药剂筛选、杀菌剂的作用机制和病原菌本身的代谢方式、抗药性突变频率、交互抗性等因素,都受菌株自身的遗传性、防治药剂的选择或环境因素影响[31-34]。因此,需进一步加强对新药剂和新防治对象开展抗药性风险评估、制定抗药性管理策略、建立再评价机制等。综上,明确植物病原菌抗药性发生发展特点并制定科学合理的抗性治理策略,对进一步开展植物病害的科学防控具有重要的参考价值[35]。目前在筛选抗药突变体时,根据不同的目标作物、靶标病菌或防治药剂,会采用不同的筛选方法,一般采用紫外诱导与药剂驯化这两种方法[36-37],如王文桥等[38]在诱导葡萄霜霉病菌与马铃薯晚疫病菌对三种药剂的抗性突变体时发现,马铃薯晚疫病菌在药剂驯化条件下,对恶霜灵容易发生抗性变异,而对烯酰吗啉和霜脲氰不易发生抗性变异。王艺烨[39]在诱导辣椒疫霉抗突变菌株时,通过药剂驯化出6株,而采用游动孢子紫外照射仅诱导出2 株,紫外照射菌丝块未诱导出突变菌株,由此说明在该致病菌诱导突变体时,药剂驯化的方法更优于紫外照射。笔者在本研究中共获得4 株抗四霉素的抗药突变体,分别为RM-AW20、RM-BG25、RM-AK01和RM-TM91,因时间限制,未采用紫外照射的方法诱导抗药突变体,因此尚待证明通过不同方法的诱导抗药突变体的抗性水平是否一致。
由4 株抗药突变体经11 代继代培养后,抗性水平值分别为0.275 µg·mL-1、0.430 µg·mL-1、0.145 µg·mL-1和0.564 µg·mL-1,均从低抗菌株的抗性水平下降到敏感菌株抗性水平≤1.13 µg·mL-1的标准范围内,说明这4 株抗药突变体不具有稳定遗传性。由此推断,该致病菌对四霉素不易产生较强抗性,但需要注意,如果在药剂长期、单一的选择压力条件下,田间一旦产生抗药突变体,抗性菌株可能很快上升为优势种群,病害防治问题将会变得尤为棘手。交互抗药性试验结果表明,四霉素与中生菌素、噻霉酮等田间常用杀菌剂之间存在交互抗药性。因此,在四霉素投入生产实践中时,不可与上述杀菌剂轮换使用,否则将产生交互抗性问题[40]。
除此之外,亲本菌株之间的生物学性状差异、遗传多样性和抗药突变体的遗传差异也会导致抗性水平的变化,所以今后研究目标是利用分子生物学技术对抗药性基因进行筛选,深层次地揭示梨火疫病菌抗药性的产生机制,分析抗药性群体的基因型[41-42],进一步揭示该病原菌对四霉素抗药性的遗传本质。
4 结 论
通过测定2021—2023 年新疆分离的100 个梨火疫病菌对四霉素药剂的敏感性,其EC50 均值为(1.59±1.029)µg·mL-1,作为新疆地区梨火疫病菌对四霉素的敏感基线,适用于对四霉素药剂敏感性和抗药性菌株的监测。仅发现少数低抗药性菌株,说明目前该药剂防治梨火疫病的风险低。
[1] 胡白石.梨火疫病菌的风险分析及检测技术研究[D].南京:南京农业大学,2000.HU Baishi.Pest risk analysis and detection techniques of Erwin‐ia amylovora[D].Nanjing:Nanjing Agricultural University,2000.
[2] ABDOLLAHIH,RUGINIE,RUZZIM,MULEO R. In vitro system for studying the interaction between Erwinia amylovora and genotypes of pear[J].Plant Cell,Tissue and Organ Culture,2004,79(2):203-212.
[3] 董欢.梨火疫病检测技术研究与推广应用[J].农业工程技术,2021,41(8):47-48.DONG Huan.Research and application of detection technology of pear fire blight[J].Agricultural Engineering Technology,2021,41(8):47-48.
[4] ZHAO Y Q,TIAN Y L,WANG L M,GENG G M,ZHAO W J,HU B S,ZHAO Y F.Fire blight disease,a fast-approaching threat to apple and pear production in China[J].Journal of Integrative Agriculture,2019,18(4):815-820.
[5] MANSFIELD J,GENIN S,MAGORIS,CITOVSKY V,SRIAR-IYANUM M,RONALD P,DOW M,VERDIER V,BEER S V,MACHADO M A,TOTH I,SALMOND G,FOSTER G D.Top 10 plant pathogenic bacteria in molecular plant pathology[J].Molecular Plant Pathology,2012,13(6):614-629.
[6] 张峰,李养义.库尔勒香梨产业发展现状与对策建议[J].西北园艺(综合),2020(11):3-5.ZHANG Feng,LIYangyi.Development status and countermeasures of Korla pear industry[J].Northwest Horticulture,2020(11):3-5.
[7] CHATTERJEE A.Fire blight:The disease and its causative agent,Erwinia amylovora.edited by J.L.vanneste[J].European Journal of Plant Pathology,2001,107(5):569.
[8] 新华社.农用链霉素已被禁,网售产品均为假药[J].农村百事通,2019(23):20.XINHUA News Agency.Agricultural streptomycin has been banned,and the products sold online are fake pesticides[J].Rural Know It All,2019(23):20.
[9] 韩希军.生物农药四霉素水剂生产研究与应用[Z].沈阳:辽宁省科学技术厅,2014-02-28.HAN Xijun.Study on production and application of biological pesticide tetramycin aqueous agent[Z].Shenyang:Department of Science&Technology of Liaoning province,2014-02-28.
[10] WANG Q P,ZHANG C,LONG Y H,WU X M,SU Y,LEIY,AIQ.Bioactivity and control efficacy of the novel antibiotic tetramycin against various kiwifruit diseases[J].Antibiotics,2021,10(3):289.
[11] MA D C,ZHU J M,HE L M,CUIK D,MU W,LIU F.Baseline sensitivity and control efficacy of tetramycin against Phytoph‐thora capsici isolates in China[J].Plant Disease,2018,102(5):863-868.
[12] 邬劼,王晓琳,黄洁雪,刁春友,闫晓阳,吉沐祥.7 种杀菌剂对草莓胶孢炭疽菌和灰霉病病菌的室内毒力测定[J].江苏农业科学,2019,47(20):129-133.WU Jie,WANG Xiaolin,HUANG Jiexue,DIAO Chunyou,YAN Xiaoyang,JIMuxiang.Determination of indoor virulence of seven fungicides against Colletotrichum gloeosporioides and Botrytis cinerea[J].Jiangsu Agricultural Sciences,2019,47(20):129-133.
[13] 陈鹏宇,杨立辉,翟长兰,田慧迪,张敏,白庆荣,赵廷昌.辣椒炭疽病菌Colletotrichum nigrum 鉴定、生物学特性及药剂敏感性研究[J].中国瓜菜,2023,36(3):27-35.CHEN Pengyu,YANG Lihui,ZHAIChanglan,TIAN Huidi,ZHANG Min,BAIQingrong,ZHAO Tingchang.Identification,biological characteristics and fungicide susceptibility of Colleto‐trichum nigrum in pepper[J].China Cucurbits and Vegetables,2023,36(3):27-35.
[14] 宁楠楠,咸文荣,马永强,郭青云.0.3%四霉素水剂防治马铃薯疮痂病田间药效试验[J].青海农林科技,2020(3):86-88.NING Nannan,XIAN Wenrong,MA Yongqiang,GUO Qingyun.Field efficacy test of 0.3% tetracycline aqueous solution in the control of potato scab[J].Science and Technology of Qinghai Agriculture and Forestry,2020(3):86-88.
[15] 谯天敏,王丽,朱天辉.核桃细菌性黑斑病杀菌剂筛选及药效研究[J].植物保护,2020,46(4):258-263.QIAO Tianmin,WANG Li,ZHU Tianhui.Screening of bactericides and their control effect against bacterial black spot disease of walnut[J].Plant Protection,2020,46(4):258-263.
[16] 蒋冬阳,陈夕军,陈银凤,陈宸,张治平,魏利辉.茭白叶斑病病原鉴定及其对5 种杀菌剂的敏感性测定[J].中国瓜菜,2022,35(10):34-41.JIANG Dongyang,CHEN Xijun,CHEN Yinfeng,CHEN Chen,ZHANG Zhiping,WEILihui.Pathogen identification of cane shoots leaf spot and determination of its sensitivity to five fungicides[J].China Cucurbits and Vegetables,2022,35(10):34-41.
[17] 李亚萌.北京地区番茄灰霉病菌对咯菌腈的抗性风险评估[D].北京:北京农学院,2020.LIYameng.Risk assessment of Botrytis cinerea resistance to fludioxonil in Beijing[D].Beijing:Beijing University of Agriculture,2020.
[18] 陈晓晓,伟力·肉孜,吕振豪,刘琦,陈晶.10 种杀菌剂对梨火疫病的田间药效评价[J].中国果树,2023(7):69-72.CHEN Xiaoxiao,Weili · Rouzi,LÜ Zhenhao,LIU Qi,CHEN Jing.Field efficacy evaluation of 10 bactericides on pear fire blight[J].China Fruits,2023(7):69-72.
[19] 王秋月.辣椒疫霉对氟吡菌胺的抗药性风险研究[D].重庆:西南大学,2016.WANG Qiuyue.Studies on the resistance risk of Phytophthora capsici to fluopicolide[D].Chongqing:Southwest University,2016.
[20] 闫磊.黄瓜霜霉病菌对氟吡菌胺的抗性风险研究[D].保定:河北农业大学,2013.YAN Lei.Studies on risk of resistance in Pseudoperonospora cubensis to fluopicolide[D].Baoding:Hebei Agricultural University,2013.
[21] 侯军.番茄灰霉病菌对丙烷脒的抗药性风险研究[D].杨凌:西北农林科技大学,2011.HOU Jun.Studies on the resistance risk of Botrytis cinerea to fungicide propamidine[D].Yangling:Northwest A& F University,2011.
[22] 王迪,王诗然,杨明佳,卢宝慧,刘丽萍,高洁.吉林省人参黑斑病菌对常用药剂的抗药性监测及交互抗药性测定[J].农药,2018,57(8):603-605.WANG Di,WANG Shiran,YANG Mingjia,LU Baohui,LIU Liping,GAO Jie.Detection of the resistance of Alternaria panax and cross-resistance on ginseng in Jilin Province[J].Agrochemicals,2018,57(8):603-605.
[23] 葛艺欣.梨火疫病菌对春雷霉素的抗性机制以及丁香假单胞菌RsmA 蛋白的功能研究[D].南京:南京农业大学,2019.GE Yixin.Study on mechanism of kasugamycin resistance in Erwinia amylovora and functions of RsmA proteins in Pseudomonas syringae[D].Nanjing:Nanjing Agricultural University,2019.
[24] 殷吉龙.0.3%四霉素水剂对杨树的安全性试验[J].现代农村科技,2020(10):77.YIN Jilong.Safety test of 0.3% tetramycin AS on poplar[J].Modern Rural Science and Technology,2020(10):77.
[25] 梁欢,徐进,王晓宁,张彤,许景升,张昊,冯洁.11 种杀菌剂对马铃薯软腐病的防治效果[J].植物保护,2020,46(5):309-315.LIANG Huan,XU Jin,WANG Xiaoning,ZHANG Tong,XU Jingsheng,ZHANG Hao,FENG Jie.Control effects of eleven bactericides on potato soft rot[J].Plant Protection,2020,46(5):309-315.
[26] 阮宏椿,石妞妞,田佩玉,杜宜新,陈文乐,陈巧红,陈凤平,陈福如.福建省稻瘟病菌对4 种杀菌剂的敏感性分析[J].西北农林科技大学学报(自然科学版),2022,50(2):125-134.RUAN Hongchun,SHINiuniu,TIAN Peiyu,DU Yixin,CHEN Wenle,CHEN Qiaohong,CHEN Fengping,CHEN Furu.Sensitivity of Magnaporthe oryzae in Fujian to 4 fungicides[J].Journal of Northwest A & F University (Natural Science Edition),2022,50(2):125-134.
[27] 朱强兴,周小军,何晓婵,朱丽燕.多种药剂防治水稻白叶枯病田间药效试验[J].上海农业科技,2021(5):109-110.ZHU Qiangxing,ZHOU Xiaojun,HE Xiaochan,ZHU Liyan.Field efficacy trial of several insecticides against rice bacterial blight[J].Shanghai Agricultural Science and Technology,2021(5):109-110.
[28] PAN H W,HE X S,LUX R,LUAN J,SHIW Y.Killing of Esch‐erichia coli by Myxococcus xanthus in aqueous environments requires exopolysaccharide-dependent physical contact[J].Microbial Ecology,2013,66(3):630-638.
[29] URBAN J,LEBEDA A.Fungicide resistance in cucurbit downy mildew-methodological,biological and population aspects[J].Annals of Applied Biology,2006,149(1):63-75.
[30] CHEN Y,YAO J,WANG W X,GAO T C,YANG X,ZHANG A F.Effect of epoxiconazole on rice blast and rice grain yield in China[J].European Journal of Plant Pathology,2013,135(4):675-682.
[31] BASIT M.Status of insecticide resistance in Bemisia tabaci:Resistance,cross-resistance,stability of resistance,genetics and fitness costs[J].Phytoparasitica,2019,47(2):207-225.
[32] ZHANG X M,JIANG H,HAO J J.Evaluation of the risk of development of fluopicolide resistance in Phytophthora erythro‐septica[J].Plant Disease,2019,103(2):284-288.
[33] BIQ Y,MA Z Q.Sensitivity,resistance stability,and cross-resistance of Plasmopara viticola to four different fungicides[J].Crop Protection,2016,89:265-272.
[34] CHEN X D,GILL T A,ASHFAQ M,PELZ-STELINSKIK S,STELINSKIL L.Resistance to commonly used insecticides in Asian citrus psyllid:Stability and relationship to gene expression[J].Journal of Applied Entomology,2018,142(10):967-977.
[35] 刘西莉,苗建强,张灿.植物病原菌抗药性及其抗性治理策略[J].农药学学报,2022,24(5):921-936.LIU Xili,MIAO Jianqiang,ZHANG Can.Fungicide resistance and the management strategies[J].Chinese Journal of Pesticide Science,2022,24(5):921-936.
[36] 李京,纪明山,刘煜财,王广祥,孙中华,郭红霞,王维静.辽宁省野慈姑对氯氟吡啶酯抗性风险评估[J].农药,2020,59(7):541-546.LIJing,JIMingshan,LIU Yucai,WANG Guangxiang,SUN Zhonghua,GUO Hongxia,WANG Weijing.Risk assessment of resistance to Florpyrauxifen-benzyl in Sagittaria trifolia L.of Liaoning Province[J].Agrochemicals,2020,59(7):541-546.
[37] CHEN L,ZHU S S,LU X H,PANG Z L,CAIM,LIU X L.Assessing the risk that Phytophthora melonis can develop a point mutation (V1109L) in CesA3 conferring resistance to carboxylic acid amide fungicides[J].PLoS One,2012,7(7):e42069.
[38] 王文桥,刘国容,张小风,马志强,韩秀英.葡萄霜霉病菌和马铃薯晚疫病菌对三种杀菌剂的抗药性风险研究[J].植物病理学报,2000,30(1):48-52.WANG Wenqiao,LIU Guorong,ZHANG Xiaofeng,MA Zhiqiang,HAN Xiuying.Studies on resistance risk to three fungicides in Plasmopara viticola and Phytophthora infestans[J].Acta Phytopathologica Sinica,2000,30(1):48-52.
[39] 王艺烨.辣椒疫霉对甲霜灵的抗性监测及诱变研究[D].合肥:安徽农业大学,2019.WANG Yiye.Resistance monitoring and mutagenesis of Phy‐tophthora capsici to metalaxyl[D].Hefei:Anhui Agricultural University,2019.
[40] 陈晓晓,艾尼赛·赛米,粟神强,贾玉凤,刘琦,陈晶.梨火疫病病原菌的分离鉴定及室内抑菌药剂筛选[J].西北农业学报,2023,32(3):468-478.CHEN Xiaoxiao,Anisa·Saimi,SU Shenqiang,JIA Yufeng,LIU Qi,CHEN Jing.Separation and identification of pear fire blight pathogens and indoor screening of bactericides[J].Acta Agriculturae Boreali-occidentalis Sinica,2023,32(3):468-478.
[41] 郭爽,黄贞,常绍东,刘玉平,曹翠文.利用分子标记鉴定辣椒抗疫病材料[J].中国农学通报,2012,28(13):163-166.GUO Shuang,HUANG Zhen,CHANG Shaodong,LIU Yuping,CAO Cuiwen.Identification of resistance to Phytophthora blight in hot pepper using molecular marker[J].Chinese Agricultural Science Bulletin,2012,28(13):163-166.
[42] 罗德旭,巩振辉,李大伟.辣椒疫病抗病性分子鉴定技术研究[J].西北农业学报,2008,17(5):76-80.LUO Dexu,GONG Zhenhui,LIDawei.Study on molecular identification technology of pepper resistance to Phytophthora blight[J].Acta Agriculturae Boreali-Occidentalis Sinica,2008,17(5):76-80.