植物表皮蜡质是叶片、茎、花和果实表面细胞覆盖的一道疏水层,具有抵抗外界环境生物和非生物胁迫的作用,如抵抗病虫害、减少非气孔失水、降低高温及紫外线辐射等[1]。植物表皮蜡质主要由脂肪族化合物、环状化合物以及甾醇类化合物构成[2],其中脂肪族化合物包括长链脂肪酸,以及衍生而来的烷烃、醇、醛、酮、酯等。
表皮蜡质含量、结构和组分会随着品种、发育时期和栽培环境的变化而改变,尤其是遭遇外界环境的变化时,植物会通过改变蜡质晶体的微结构形态、调节蜡质含量等适应胁迫。其中高温和光直接改变植物表皮蜡质的形态和性质[3]。据报道,连续的高温可以提高葡萄果实采收时的蜡质总量和萜类化合物含量,并通过增加齐墩果酸与熊果酸的比值来抑制蒸腾作用,减轻高温损伤[4]。小麦叶片蜡质含量在高温胁迫下也显著增加,蜡质的增加可反射辐射,降低气孔导度以减少蒸腾[5]。但是,高温胁迫下韭葱叶表皮蜡质含量明显减少[6]。此外,不同的光照辐射也可改变番茄[7]、樱桃番茄[8]、苹果[9]、黄瓜[10]、大麦[10]、蚕豆[10]的蜡质组分和含量。可见,高温和光会改变蜡质含量和组分,但是不同物种的响应方式不同。
石榴是古老的保健水果,富含花青苷、黄酮、安石榴苷等多酚物质,是国家特色经济林树种之一[11]。石榴成熟期一般在9—10 月,果实在7—9 月暴露于强烈的太阳辐射和高温中,容易发生日灼。据不完全统计,石榴果实日灼发生率为40%~50%,成为危害石榴生产的主要生理性病害之一[12]。目前关于石榴果皮蜡质的研究主要集中在采后储藏方面[13],而关于果皮蜡质对日灼的响应研究较少。笔者以易感日灼的石榴品种红玉石籽为材料,研究日灼对石榴果皮的蜡质含量、形态变化以及组分的影响,以揭示果皮蜡质响应日灼的生理机制,为抗日灼石榴品种选育提供理论依据。
1 材料和方法
1.1 材料
试验所用材料为日灼易感品种红玉石籽[14],该品种种植在安徽省农业科学院岗集示范基地,树龄为8 年生。选择生长势和结果量均匀一致的石榴树,设置3 次重复,每次重复选取10 个果实。按照Liu等[15]制定的石榴日灼分级标准,将果实分为无日灼果实(CK)、轻微日灼果实(SB1)、中度日灼果实(SB2)和重度日灼果实(SB3),取向阳面果皮,用锡箔纸包裹并置于-80 ℃超低温冰箱中冷冻保存备用。
1.2 果皮蜡质的扫描电子显微镜观察
试验材料为商熟期果实,每个处理3 个果实,取向阳面果皮,将取下的果皮用解剖刀切成0.5 cm ×0.5 cm 的小块,放入4%戊二醛的溶液中,用真空泵抽出溶液中的空气,4 ℃固定。扫描电镜样品的制备参考Lufu 等[13]方法,略有改动,将4%戊二醛固定好的样品用pH 6.8 的磷酸缓冲液(PBS)冲洗7~8次,每次20 min,冲洗后经梯度乙醇(30%、50%70%、80%、90%、100%)脱水,每梯度30 min,再经100%丙酮脱水2 次(每次30 min),乙酸异戊酯置换2 次(每次15 min)。处理好的样品进行临界点干燥后粘台,镀膜,采用JEM 6360LV(JEOL)扫描电镜在15 kV条件下观察15次,并拍照记录。
1.3 果皮蜡质组分的测定
用内径为1.9 cm的圆形打孔器在果实赤道部位向阳面打孔,去除果皮圆片的白皮层。每个样品取10个果皮样本称质量,加入10 mL氯仿萃取,在60 ℃萃取5 min。使用玻璃瓶分多次转移,每次转移2 mL,共转移10 mL 萃取液,进行氮气吹干。氮气吹干后的样本置于干燥环境下进行衍生化处理,先加入1 mL氯仿复溶,涡旋1 min,超声5 min;再使用0.22 μm 的有机相针孔过滤器过滤,取500 μL 上清液转移至进样瓶中,加入80 μL BSTFA 试剂(含1%TMCS 试剂),置于70 ℃环境中,反应1 h。衍生化结束后,常温静置30 min,进行GC-MS代谢组学分析。正构烷烃(C7-C40)混标质量浓度20 μg·mL-1,100 μL 质谱上机。
采用气相色谱-质谱联用技术(GC-MS)分析果实果皮蜡质的化学组成成分。色谱条件:DB-5MS毛细管柱(30 m × 0.25 mm × 0.25 μm,Agilent J&W Scientific,Folsom,CA,USA),载气为高纯氦气(纯度>99.999%),流速1.0 mL·min-1,进样口的温度为260 °C。进样量1 μL。程序升温:柱温箱的初始温度为80 °C,保持2 min;以15 °C·min-1程序升温至260 °C,保持10 min;5 °C·min-1升温至315 °C 保持10 min。质谱条件:电子轰击离子源(EI),离子源温度230 °C,四极杆温度150 °C,电子能量70 eV。扫描方式为全扫描模式,质量扫描范围为50~500 m/z。
定性与定量分析方法:利用NIST08谱库(https://webbook.nist.gov/chemistry/)进行匹配,并结合人工图谱解析、标样鉴定及资料分析进行定性。应用归一法进行定量。蜡质含量以μg·cm-2表示。
1.4 数据统计分析
运用SPSS 进行显著性分析,并利用T-test 进行对照和处理间的比较,作图软件使用GraphPad Prism。
2 结果与分析
2.1 日灼对石榴果皮蜡质结构的影响
石榴果皮扫描电子显微镜观测结果表明,日灼果实与正常果实相比蜡质结构存在显著差异(图1)。正常果实(CK)果皮蜡被均匀、致密;轻微日灼(SB1)果实表面明显变得粗糙、不平整,局部区域蜡被凝聚;中度日灼(SB2)果实表面蜡质进一步显著粗糙化,起砂严重;严重日灼(SB3)果实表面凹凸不平,果皮蜡质层呈片状剥落。
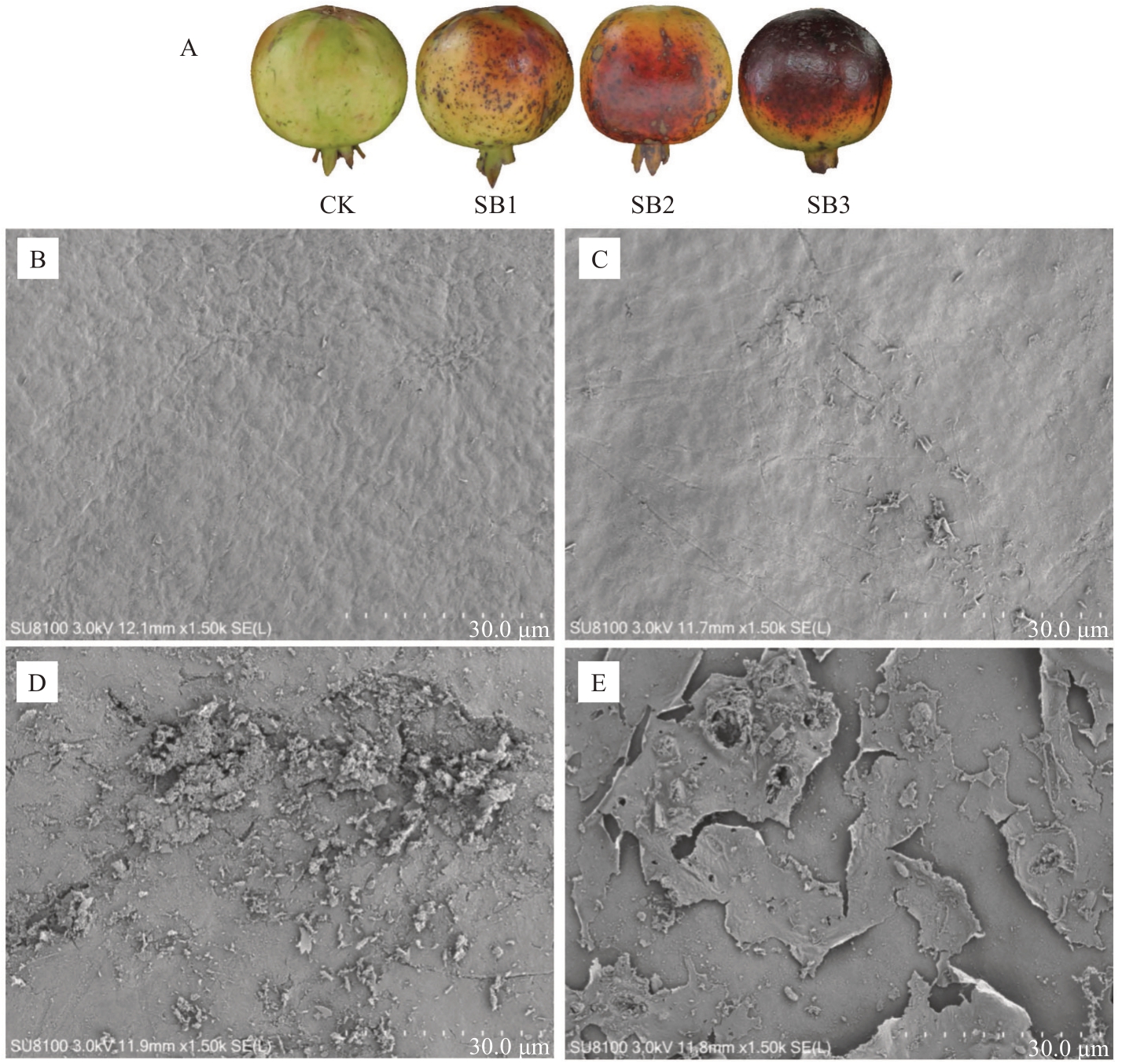
图1 日灼对红玉石籽石榴果皮蜡质的影响
Fig.1 Effect of sunburn on the cuticular wax of Hongyushizi pomegranate
A.不同日灼程度的果实;B.CK;C.SB1;D.SB2;E.SB3。
A.Fruit with diffwerent sunburn degree;B.CK;C.SB1;D.SB2;E.SB3.
2.2 日灼对石榴果皮蜡质组分及含量的影响
为了进一步探明日灼对红玉石籽石榴果皮蜡质的影响,选择红玉石籽中度日灼(SB2)和正常果实(CK)的果皮进行蜡质组分及含量的测定。石榴正常果皮中共检测出67种蜡质成分,主要包括超长链脂肪族化合物(含量26.36 μg·cm-2,占总量的74.95%)和萜类化合物(含量8.81 μg·cm-2,占总量的25.05%),超长链脂肪族化合物主要由烷烃(19.70 μg·cm-2)、脂肪酸(2.49 μg·cm-2)、烯烃(0.19 μg·cm-2)、醇类(3.78 μg·cm-2)和酯类(0.20 μg·cm-2)物质组成。日灼显著提高了蜡质总量(提高了6.96%),尤其是酯类和醇类含量,分别显著提高82.78%和64.71%,但显著降低了萜类物质含量(降低了27.48%)(图2)。
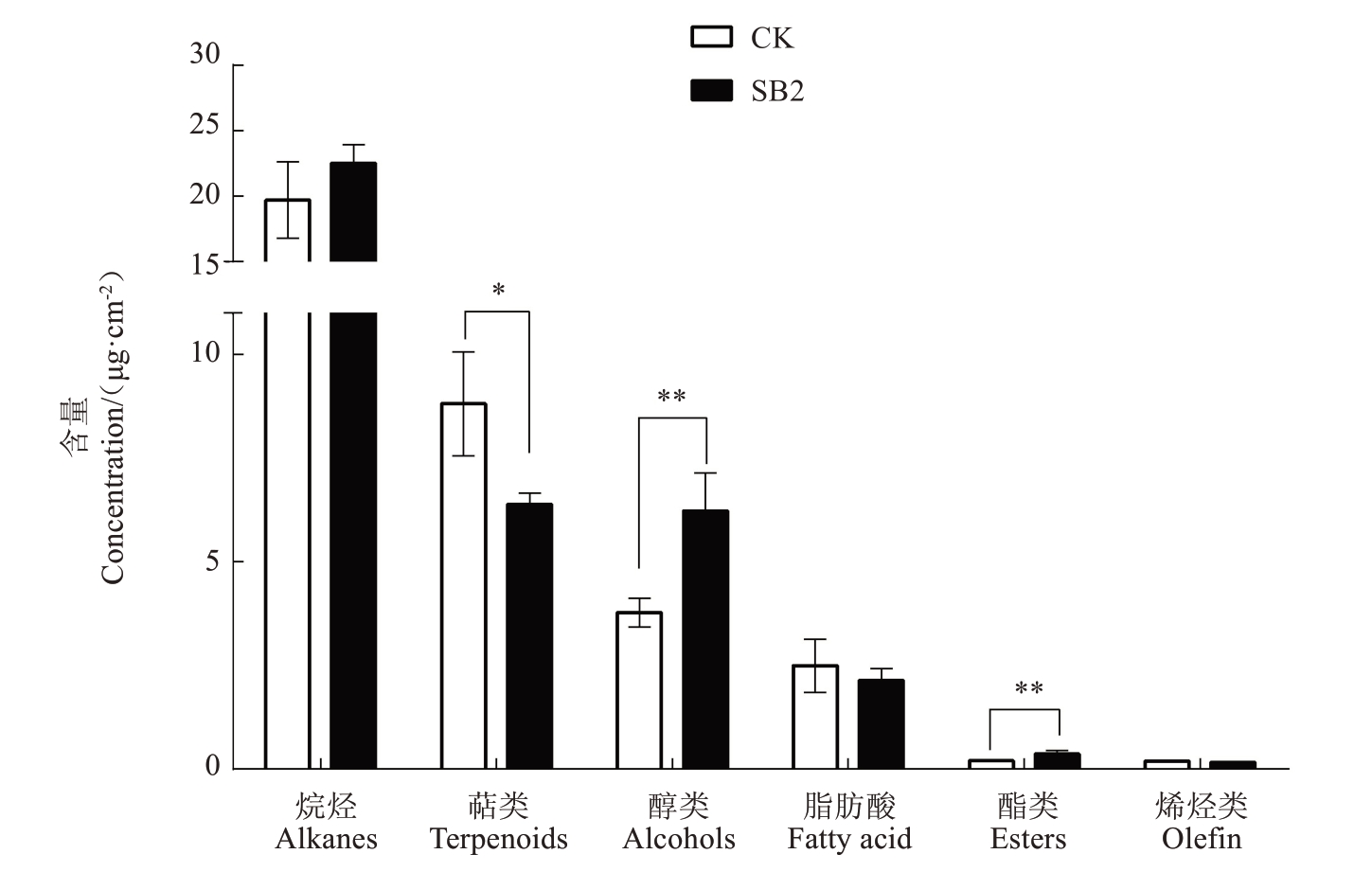
图2 日灼对红玉石籽石榴果皮蜡质组分及含量的影响
Fig.2 Effect of sunburn on compositions and concentration of cuticular wax of Hongyushizi pomegranate
*表示CK 与SB2 在p<0.05 水平上差异显著;**表示CK 与SB2 在p<0.01 水平上差异显著。下同。
*indicate significant difference between the healthy fruit and sunburn fruit at p<0.05;**indicate significant difference between the healthy fruit and sunburn fruit at p<0.01.The same below.
2.3 日灼对石榴果皮萜类化合物含量的影响
石榴果皮中共检测到7 种萜类化合物,分别是熊果酸、α-生育酚、β-谷甾醇、β-香树脂醇、银杏内酯、β-生育酚和白桦脂醇(图3)。正常红玉石籽石榴果皮中萜类化合物的总量为8.81 μg·cm-2,其中熊果酸含量最多,为8.66 μg·cm-2(占总量的98.30%),其次为α-生育酚(含量0.13 μg·cm-2,占总量的1.48%),其余萜类化合物含量较低。果实日灼降低了萜类化合物总量,红玉石籽日灼果皮(SB2)中萜类总量为6.39 μg·cm-2,比正常果实下降了26.21%,其中熊果酸的含量显著降低29.00%。日灼提高了α-生育酚、β-谷甾醇、β-香脂素、银杏内酯、β-生育酚和白桦脂素含量,但差异不显著。

图3 日灼对红玉石籽果皮中萜类化合物的影响
Fig.3 Effect of sunburn on the terpenoid compounds in Hongyushizi pomegranate
2.4 日灼对石榴果皮超长链脂肪族化合物含量及碳链分布的影响
红玉石籽果皮中检测出20种C链长度的烷烃类化合物(图4),正常果皮总量为19.70 μg·cm-2,主要为C29(二十九烷,含量7.32 μg·cm-2)、C31(三十一烷,含量6.70 μg·cm-2)和C40(四十烷,含量4.24 μg·cm-2),三者占烷烃总量的92.69%。日灼使烷烃含量提高至22.53 μg·cm-2,其主要物质C29、C31和C40的含量分别提高了17.98%、13.69%和10.27%。红玉石籽果皮中检测出12 种C 链长度的醇类物质,主要为C30(1-三十烷醇,含量3.06 μg·cm-2),占醇类物质总量的81.04%。日灼使醇类物质含量提高,由3.78 μg·cm-2提高至6.34 μg·cm-2,其主要碳链长度的C30 含量显著提高了49.15%。红玉石籽果皮中脂肪酸的C链长度有11 种,主要为C16(棕榈酸、9-十六碳烯酸)和C18(硬脂酸、亚油酸、异油酸),分别占总脂肪酸含量的50.22%和42.79%,正常果皮总量为2.49 μg·cm-2。日灼降低了脂肪酸的含量至2.13 μg·cm-2(下降了14.46%),其主要物质C16 和C18 的含量分别下降15.23%和23.36%,相反C14、C19、C20、C22 的含量却显著上升。红玉石籽果皮中检测出14 种C 链长度的酯类物质,主要为C19、C34、C22、C10、C12,分别占酯类物质总量的30.36%、11.33%、10.11%、9.64%、8.89%。日灼提高了酯类物质的含量,由0.20 μg·cm-2提高至0.37 μg·cm-2,尤其是C16、C19、C22 和C29,分别显著提高了162.21%、183.53%、66.54%、135.02%。红玉石籽果皮中检测出3 种C 链长度的烯烃,分别是C17(8-十七碳烯)、C30(反式角鲨烯)和C35(17-戊三烯),C17含量最高(0.18 μg·cm-2),占烯烃总量的95.03%。日灼降低了酯类物质的含量,尤其是C17,显著降低了19.20%,但C30 的含量显著提高了100.98%(图4)。
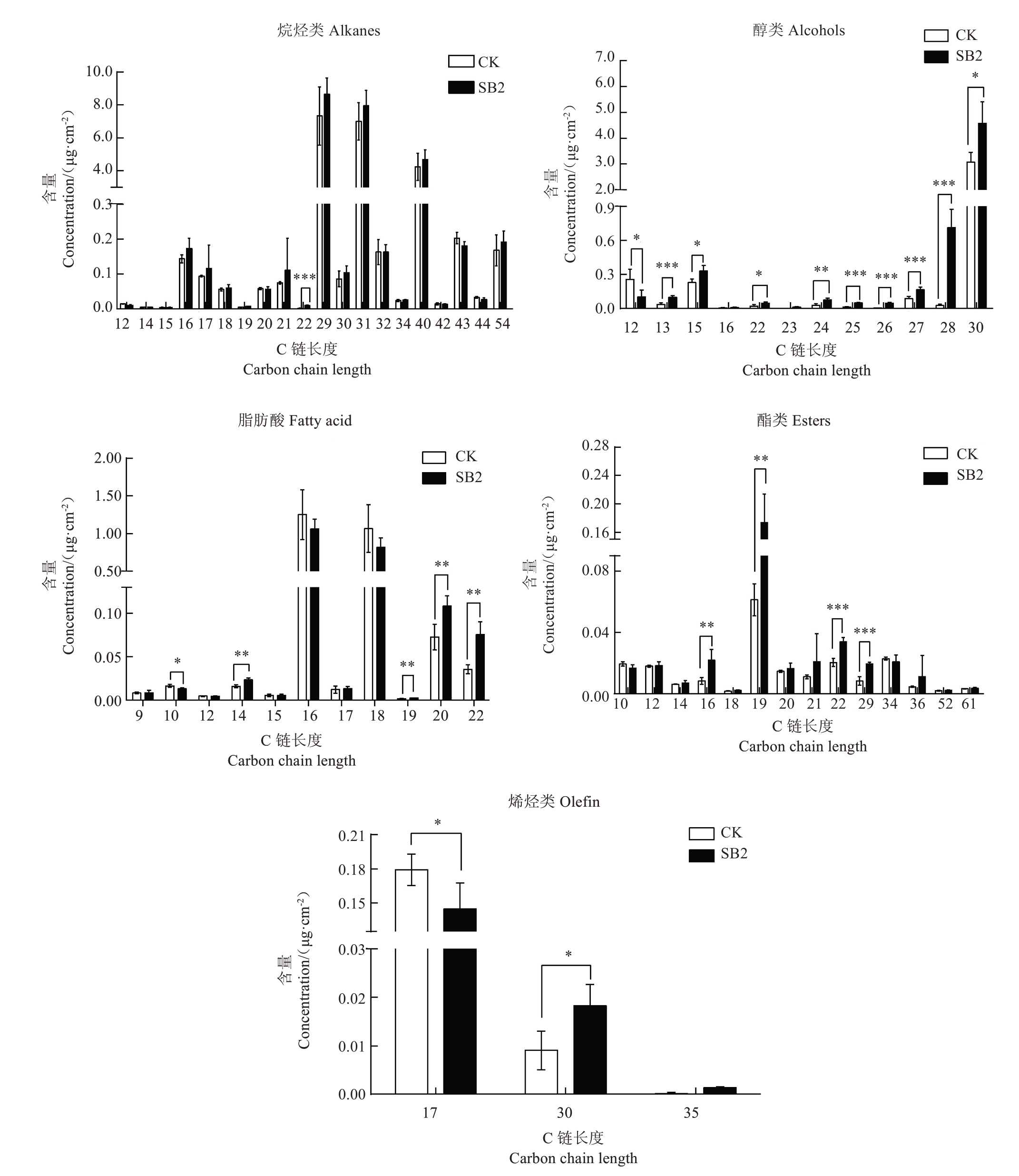
图4 日灼对红玉石籽果皮中超长链脂肪族化合物碳链分布的影响
Fig.4 Effect of sunburn on the chain length distribution of the main VLC aliphatic compoundsin Hongyushizi pomegranate
*表示CK 与SB2 在p<0.05 水平上差异显著;**表示CK 与SB2 在p<0.01 水平上差异显著;***表示CK 与SB2 在p<0.001 水平上差异显著。
* indicate significant difference between the healthy fruit and sunburn fruit at p<0.05; ** indicate significant difference between the healthy fruit and sunburn fruit at p<0.01;***indicate significant difference between the healthy fruit and sunburn fruit at p<0.001.
3 讨 论
果皮蜡质是果实与外界接触的第一道屏障,在果实生长和贮藏过程中有十分重要的作用。本研究中红玉石籽石榴果皮蜡质总量(35.17 μg·cm-2),远低于砂糖橘[16]、蓝莓[17]、梨[18]、苹果[19]等水果,与番茄表皮蜡质含量相当[20]。石榴果实中的主要蜡质组分是烷烃,在瓦伦西亚的黄金帅苹果[19]、Fortune 橘[21]、柠檬[22]、库尔勒香梨[18]果皮中的主要蜡质组分也是烷烃。但是红元帅主要的蜡质组分是脂肪醇[19],蓝莓中主要的蜡质组分是萜类化合物[17],砂糖橘中主要的蜡质组分是长链脂肪醛[16]。由此可见,蜡质的化学组成复杂,且组成成分及含量在不同物种中差异较大。
萜类尤其五环三萜类化合物是表皮蜡质主要的生物活性物质,其化合物的种类及含量在不同植物中也呈现出多样性。蓝莓果皮中主要的萜类物质是熊果酸[17],椪柑中主要是羽扇豆醇[23],柠檬中主要是角鲨烯[23],在库尔勒香梨中主要是δ-生育酚,雪花梨中主要是α 香树脂醇,玉露香梨中主要是乙酸羽扇醇酯[18]。本研究中的红玉石籽中主要的萜类物质是熊果酸,占萜类化合物总量的98.30%,也是表皮蜡质中含量最多的一种物质。熊果酸是植物中一种普遍存在的五环三萜,是石榴、山楂、枇杷等植物中主要的萜类化合物,具有抗氧化、抗菌等重要功能而备受关注[24]。日灼显著降低石榴果皮中熊果酸含量,推测其可能作为抗氧化剂清除日灼胁迫产生的自由基,降低日灼损伤[25]。
日灼是果实在高温和强光条件下引起的一种生理失调症[26],果皮蜡质可以保护果实免受光和热的胁迫。通过对10个白色葡萄品种的表皮去蜡试验,结果表明,去除表皮蜡质加速了日灼的发生[27],表皮蜡质主要是作为保护层发挥运输屏障的功能,此外,蜡质还可以通过散射、反射甚至吸收PAR 和UV 辐射,起保护作用,从而降低组织的暴露水平。蜡质对光的散射能力取决于蜡质晶体的大小、分布和方向。片状蜡晶体比无定形蜡反射和散射光的比例更高,同时还可以促进蒸腾作用。当日灼出现时,葡萄表皮蜡质失去了晶体结构,变得相对无定形[28]。本研究中随着日灼程度的加深,石榴表皮的蜡质结构受损也越来越严重,并且脱落,使得其对光和热胁迫的防护作用减弱。
高温和强光不仅改变蜡质的结构,也影响蜡质的组分和含量。在增强光照或UV-B 紫外辐射的试验中,大部分物种的蜡质含量会随着光照度的增加而增加,如葡萄[4]、小麦[6]。蜡质的增加将有利于反射辐射,降低气孔导度以减少蒸腾。但是,高温条件下敖汉紫花苜蓿的蜡质含量下降,三得利苜蓿蜡质含量无显著变化[29];同样在高温胁迫下,韭葱叶表皮蜡质含量也明显减少[5],在反光膜增强宫川温州蜜柑反射光后,蜡质总量并没有出现显著变化[30]。可见光照和高温对蜡质影响存在物种差异性,可能与植物对光辐射和温度的敏感性以及物种自身生理差异有关[30-31]。
蜡质合成是一个复杂的过程,碳原子数为16 或18 的脂肪酸在多种酶的催化作用下,延伸为碳原子数为20~34 的超长链脂肪酸,接着长链脂肪酸作为蜡质合成的前体物质进入到脱碳基途径和酰基还原途径,在内质网中进行蜡质的合成[3]。据报道,UV-B处理改变了豌豆叶片正面蜡的组成,如醇类转化为酯类和其他碳氢化合物,短链烷基酯与长链烷基酯同源物的比例增大[32]。同样,UV-B 的增加导致糖枫中蜡烷烃含量增加,表明UV-B 诱导了脱碳途径而不是还原途径[33]。本研究中日灼加速了超长链脂肪酸的延伸,以C16 和C18 为主的脂肪酸含量受日灼的影响下降,导致C20 和C22 的脂肪酸含量显著上升,并且进一步导致烷烃、酯类和醇类的含量上升,但是降低了脂肪酸、烯烃以及萜类物质的含量,表明石榴可通过改变特定蜡质组分的含量来响应日灼胁迫。
4 结 论
石榴表皮蜡质有烷烃、萜类、脂肪酸、烯烃、醇类、酯类,以烷烃和萜类化合物为主。日灼导致石榴表皮蜡质结构改变和蜡质总量增加,其中烷烃、酯类和醇类的含量上升,但是降低了脂肪酸、烯烃以及萜类物质的含量。石榴可以通过改变蜡质结构、组分的含量来响应日灼胁迫。研究结果为进一步明晰表皮蜡质响应日灼胁迫的机制提供了理论依据。
[1] KUNST L,SAMUELS L.Plant cuticles shine:Advances in wax biosynthesis and export[J].Current Opinion in Plant Biology,2009,12(6):721-727.
[2] WEN M,JETTER R.Composition of secondary alcohols,ketones,alkanediols,and ketols in Arabidopsis thaliana cuticular waxes[J].Journal of Experimental Botany,2009,60(6):1811-1821.
[3] SHEPHERD T,GRIFFITHS D W.The effects of stress on plant cuticular waxes[J].The New Phytologist,2006,171(3):469-499.
[4] VANDERWEIDE J,YAN Y F,ZANDBERG W F,CASTELLARIN S D.Modulation of grape cuticular wax composition following multiple heatwaves influences grape transpiration[J].Environmental and Experimental Botany,2022,202:105036.
[5] HUGGINS T D,MOHAMMED S,SENGODON P,IBRAHIM A M H,TILLEY M,HAYS D B.Changes in leaf epicuticular wax load and its effect on leaf temperature and physiological traits in wheat cultivars (Triticum aestivum L.) exposed to high temperatures during anthesis[J].Journal of Agronomy and Crop Science,2018,204(1):49-61.
[6] GABRIELA-ANCA MAIER C,POST-BEITTENMILLER D.Epicuticular wax on leek in vitro developmental stages and seedlings under varied growth conditions[J].Plant Science,1998,134(1):53-67.
[7] COZMUTA A M,COZMUTA L M,PETER A,NICULA C,VOSGAN Z,GIURGIULESCU L,VULPOIA,BAIA M.Effect of monochromatic Far-Red light on physical-nutritional-microbiological attributes of red tomatoes during storage[J].Scientia Horticulturae,2016,211:220-230.
[8] CHOID S,PARK S H,CHOIS R,KIM J S,CHUN H H.The combined effects of ultraviolet-C irradiation and modified atmosphere packaging for inactivating Salmonella enterica serovar Typhimurium and extending the shelf life of cherry tomatoes during cold storage[J].Food Packaging and Shelf Life,2015,3:19-30.
[9] BRINGE K,HUNSCHE M,SCHMITZ-EIBERGER M,NOGA G.Retention and rainfastness of mancozeb as affected by physicochemical characteristics of adaxial apple leaf surface after enhanced UV-B radiation[J].Journal of Environmental Science and Health.Part.B,Pesticides,Food Contaminants,and Agricultural Wastes,2007,42(2):133-141.
[10] STEINMÜLLER D,TEVINIM.UV-B-induced effects upon cuticular waxes of cucumber,bean,and barley leaves[C]//Stratospheric Ozone Reduction,Solar Ultraviolet Radiation and Plant Life.Berlin,Heidelberg:Springer,1986:261-269.
[11] 苑兆和.石榴分子生物学研究进展[J].落叶果树,2016,48(5):1-8.YUAN Zhaohe.Research progress in molecular biology of pomegranate[J].Deciduous Fruits,2016,48(5):1-8.
[12] YAZICIK,ERCIŞLIS.Characterization of hybrid pomegranate genotypes based on sunburn and cracking traits related to maturation time[J].Journal of Applied Botany and Food Quality,2017,90:132-139.
[13] LUFU R,AMBAWA,OPARA U L.Functional characterisation of lenticels,micro-cracks,wax patterns,peel tissue fractions and water loss of pomegranate fruit (cv.Wonderful) during storage[J].Postharvest Biology and Technology,2021,178:111539.
[14] 苏颖,刘春燕,张虎平,贾波涛,黎积誉,于晴,杨圆,曹榛,秦改花.120 份石榴种质果实的抗日灼能力评价及抗日灼指标筛选[J].果树学报,2021,38(10):1725-1735.SU Ying,LIU Chunyan,ZHANG Huping,JIA Botao,LIJiyu,YU Qing,YANG Yuan,CAO Zhen,QIN Gaihua.Evaluation of sunburn resistance and screening of physiological indexes related to sunburn of the fruits in 120 pomegranate accessions[J].Journal of Fruit Science,2021,38(10):1725-1735.
[15] LIU C Y,SU Y,LIJ Y,JIA B T,CAO Z,QIN G H.Physiological adjustment of pomegranate pericarp responding to sunburn and its underlying molecular mechanisms[J].BMC Plant Biology,2022,22(1):169.
[16] 徐呈祥,吴秀兰,马艳萍,郑福庆,叶思敏,陈小婷.贮藏温度对砂糖橘果皮表面结构及蜡质的影响[J].园艺学报,2019,46(6):1057-1067.XU Chengxiang,WU Xiulan,MA Yanping,ZHENG Fuqing,YE Simin,CHEN Xiaoting.Effect of storage temperature on the peel surface structure and wax content of‘Shatangju’mandarin(Citrus reticulata) fruit[J].Acta Horticulturae Sinica,2019,46(6):1057-1067.
[17] CHU W J,GAO H Y,CAO S F,FANG X J,CHEN H J,XIAO S Y.Composition and morphology of cuticular wax in blueberry(Vaccinium spp.)fruits[J].Food Chemistry,2017,219:436-442.
[18] WU X,YIN H,CHEN Y Y,LIL,WANG Y Z,HAO P P,CAO P,QIK J,ZHANG S L.Chemical composition,crystal morphology and key gene expression of cuticular waxes of Asian pears at harvest and after storage[J].Postharvest Biology and Technology,2017,132:71-80.
[19] WANG Y X,WANG X J,CAO Y,ZHONG M S,ZHANG J,YU K,LIZ W,YOU C X,LIY Y.Chemical composition and morphology of apple cuticular wax during fruit growth and development[J].Fruit Research,2022,2(1):1-11.
[20] BAUER S,SCHULTE E,THIER H P.Composition of the surface wax from tomatoes:II.Quantification of the components at the ripe red stage and during ripening[J].European Food Research and Technology,2004,219(5):487-491.
[21] SALA J M.Content,chemical composition and morphology of epicuticular wax of Fortune mandarin fruits in relation to peel pitting[J].Journal of the Science of Food and Agriculture,2000,80(13):1887-1894.
[22] 王敏力,刘德春,杨莉,曾琼,王玥辰,吴启,刘山蓓,刘勇.不同种类柑橘的蜡质结构与成分比较[J].园艺学报,2014,41(8):1545-1553.WANG Minli,LIU Dechun,YANG Li,ZENG Qiong,WANG Yuechen,WU Qi,LIU Shanbei,LIU Yong.Comparative analysis of different citrus wax morphological structure and composition[J].Acta Horticulturae Sinica,2014,41(8):1545-1553.
[23] 王金秋,何义仲,徐坤洋,罗怿,盛玲,罗焘,刘欢,程运江.三种类型柑橘成熟果实表面蜡质分析[J].中国农业科学,2016,49(10):1936-1945.WANG Jinqiu,HE Yizhong,XU Kunyang,LUO Yi,SHENG Ling,LUO Tao,LIU Huan,CHENG Yunjiang.Characterization of mature fruit surface waxes of three cultivated citrus species[J].Scientia Agricultura Sinica,2016,49(10):1936-1945.
[24] LÓPEZ- HORTAS L,PÉREZ- LARRÁN P,GONZÁLEZMUÑOZ M J,FALQUÉ E,DOMÍNGUEZ H.Recent developments on the extraction and application of ursolic acid:A review[J].Food Research International,2018,103:130-149.
[25] 杨艳.猕猴桃根中熊果酸的提取及其抗氧化和抑菌性研究[D].杨凌:西北农林科技大学,2013.YANG Yan.Extraction of ursolic acid from the roots of Actinid‐ia chinensis and the antioxidant and antimicrobial activity studies[D].Yangling:Northwest A&F University,2013.
[26] MUNNÉ-BOSCH S,VINCENT C.Physiological mechanisms underlying fruit sunburn[J].Critical Reviews in Plant Sciences,2019,38(2):140-157.
[27] DOMANDA C,PARADISO V M,MIGLIARO D,PAPPACCOGLIG,FAILLA O,RUSTIONIL.Epicuticular waxes:A natural packaging to deal with sunburn browning in white grapes[J].Scientia Horticulturae,2024,328:112856.
[28] GREER D H,ROGIERS S Y,STEEL C C.Susceptibility of Chardonnay grapes to sunburn[J].Vitis,2006,45(3):147-148.
[29] 郭彦军,倪郁,郭芸江,韩龙,唐华,玉永雄.水热胁迫对紫花苜蓿叶表皮蜡质组分及生理指标的影响[J].作物学报,2011,37(5):911-917.GUO Yanjun,NIYu,GUO Yunjiang,HAN Long,TANG Hua,YU Yongxiong.Effect of soil water deficit and high temperature on leaf cuticular waxes and physiological indices in alfalfa(Medicago sativa)leaf[J].Acta Agronomica Sinica,2011,37(5):911-917.
[30] 付沃兴,刘德春,匡柳青,蒙志鑫,刘勇,胡威,杨莉.透湿性反光膜对柑橘果皮蜡质晶体结构和组成成分的影响[J].江苏农业学报,2022,38(4):1062-1069.FU Woxing,LIU Dechun,KUANG Liuqing,MENG Zhixin,LIU Yong,HU Wei,YANG Li.Effects of vapor-permeable reflective film on cuticular wax structure and composition of citrus[J].Jiangsu Journal of Agricultural Sciences,2022,38(4):1062-1069.
[31] 李永才,尹燕,陈松江,毕阳,葛永红.采前套袋对苹果梨表皮蜡质结构和化学组分的影响[J].中国农业科学,2012,45(17):3661-3668.LIYongcai,YIN Yan,CHEN Songjiang,BIYang,GE Yonghong.Effects of preharvest bagging treatment on the microstructure and chemical composition of cuticular wax in Pingguoli pear fruit[J].Scientia Agricultura Sinica,2012,45(17):3661-3668.
[32] GONZALEZ R,PAUL N D,PERCY K,AMBROSE M,MCLAUGHLIN C K,BARNES J D,ARESES M,WELLBURN A R.Responses to ultraviolet-B radiation (280-315 nm)of pea (Pisum sativum) lines differing in leaf surface wax[J].Physiologia Plantarum,1996,98(4):852-860.
[33] GORDON D C,PERCY K E,RIDING R T.Effect of enhanced UV-B radiation on adaxial leaf surface micromorphology and epicuticular wax biosynthesis of sugar maple[J].Chemosphere,1998,36(4/5):853-858.