硅是地壳中的第二大元素,已被国际植物营养研究所(IPIN)归类为有益元素[1]。大量研究表明,硅可以提高苹果、葡萄、甜瓜等作物在盐碱、高温等非生物胁迫下的抗性[2-4]。然而,有关硅提高植物抗性的机制较复杂。Zhang等[5]研究发现,硅提高植物的耐盐性是通过沉积在细胞壁周围从而抑制膜系统的退化,维持栅栏组织中叶绿体的形态,进而抵消盐胁迫对叶片解剖结构和超微结构的不利影响。Dhiman 等[6]认为硅可以增加植物叶片的叶绿体大小和叶绿素含量,从而提高盐胁迫下植物的光合效率。Verma等[7]认为硅在应对胁迫时,可以通过提高叶片气孔导度和蒸腾速率来增强植株光合作用。Zhang等[8]发现,在水分胁迫下,硅能够提高番茄PetE、PetF、PsbP、PsbQ、PsbW和Psb28等相关光合基因的转录水平,调节光化学过程。这些研究说明硅可有效调节光合作用的器官,提高光合效率,然而有关其在光能分配中的作用尚不清楚,特别是硅对叶绿体PSⅡ的结构和功能影响还有待研究[9-10]。在光合系统中,PSⅡ负责将光能转化为电能,当逆境发生时PSⅡ也是光损伤的主要部位[11]。因此,笔者在本研究中通过研究光合系统中PSⅡ对硅的响应,探讨硅对植物的光保护机制,为硅在植物生产中的应用提供一定的理论基础。
阳光玫瑰葡萄(Shine Muscat)为日本培育的中晚熟欧美杂交品种,具有高糖、皮薄、粒大、香味浓郁的特点,且抗病性强,不易裂果,耐贮藏[12]。但中国葡萄栽培区大多为盐碱土,影响着葡萄的生长发育,制约着葡萄产业的发展[13]。目前有关葡萄耐盐的研究主要集中在生长调节剂的使用、抗盐砧木的筛选、园区土壤的改良等方面,而有关硅对盐胁迫下葡萄光合特性影响的研究较少[14-15]。因此,笔者在本研究中以阳光玫瑰葡萄扦插苗为材料,通过研究其在碱性盐胁迫下添加硅酸钠对叶片叶绿素荧光相关参数的影响,建立能量响应模型,绘制叶绿体膜模式图,探究硅对葡萄叶片PSⅡ的影响。
1 材料和方法
1.1 试验材料
试验在天津市北辰区天津农学院葡萄研究中心进行。试验于2022 年12 月上旬在葡萄园内采集生长势相对一致且长势健壮、无病虫害的阳光玫瑰葡萄枝条,进行沙藏越冬处理。2023 年3 月下旬取出沙藏枝条,于日光温室内生根催芽,5 月下旬扦插至在含有蛭石基质的塑料花盆中,花盆高16.5 cm,外口径25 cm。后进行不同外源物质的处理试验,处理60 d 后采集叶片。试验在日光温室中进行,环境因素一致,采样数据具有一定代表性。
1.2 试验设计
分别选取长势一致、生长健壮且无病虫害的40 株葡萄作为试验植株。试验设4 个处理,每组处理10 株苗,采用蒸馏水作为空白对照,单一硅处理使用2 mmol·L-1 Na2SiO3溶液,碱性盐处理采用100 mmol·L-1的NaHCO3溶液,硅和碱性盐复合处理采用2 mmol·L-1 Na2SiO3和100 mmol·L-1 NaHCO3的混合液。每株葡萄分别浇灌相应处理溶液1 L,每隔7 d 处理1 次,分4 次达到预定梯度,单次施用量为0.25 L。每次将花盆底部托盘中渗出液均返还基质,以保证试验期内元素含量稳定。其余管理条件保持一致。
1.3 叶绿素荧光诱导动力学曲线(OJIP)及荧光参数的测定
8月下旬利用快速荧光测定仪Handy-PEA(Hansatech,英国)分别测定各处理阳光玫瑰葡萄叶片的快速叶绿素荧光诱导动力学曲线(OJIP)及相关参数(表1),每个处理测定10枚叶片,5次重复。测定前用叶片夹进行暗适应20 min,荧光以3000 μmol·m-2·s-1的饱和光诱导,照射时间为1 s。对OJIP荧光诱导曲线进行JIP-test 分析[16],对相对荧光值进行标准化分析,计算相对可变荧光Vt,Vt=(Ft-Fo)/(Fm-Fo),式中:Ft表示不同时间点的相对荧光强度,计算相对可变荧光的差值ΔVt,ΔVt=Vt(处理)-Vt(对照)。同时对曲线进行OJIP曲线JIP-test分析得到其他荧光参数。
表1 叶绿素荧光诱导动力学曲线OJIP 荧光参数
Table 1 Chlorophyll fluorescence induction kinetics curve OJIP fluorescence parameters
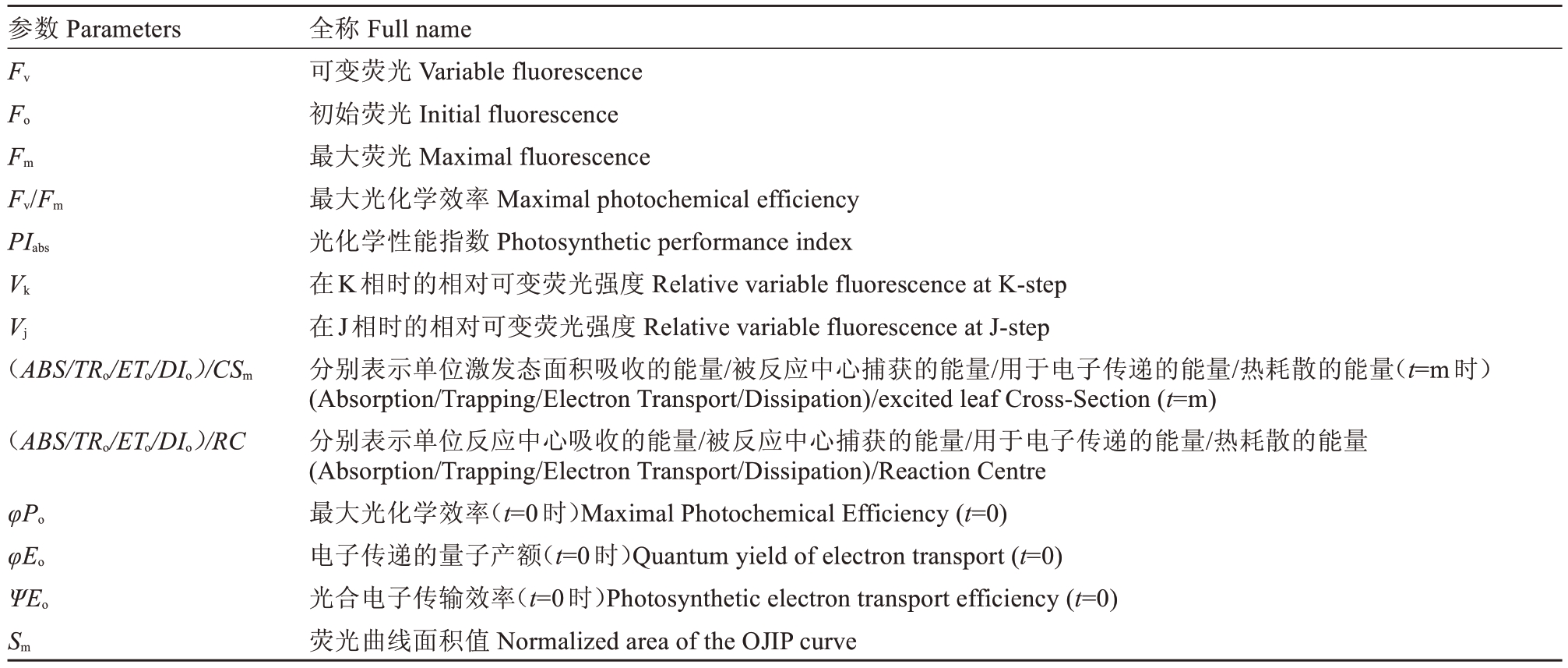
参数Parameters Fv Fo Fm Fv/Fm PIabs Vk Vj(ABS/TRo/ETo/DIo)/CSm(ABS/TRo/ETo/DIo)/RC φPo φEo ΨEo Sm全称Full name可变荧光Variable fluorescence初始荧光Initial fluorescence最大荧光Maximal fluorescence最大光化学效率Maximal photochemical efficiency光化学性能指数Photosynthetic performance index在K相时的相对可变荧光强度Relative variable fluorescence at K-step在J相时的相对可变荧光强度Relative variable fluorescence at J-step分别表示单位激发态面积吸收的能量/被反应中心捕获的能量/用于电子传递的能量/热耗散的能量(t=m时)(Absorption/Trapping/Electron Transport/Dissipation)/excited leaf Cross-Section(t=m)分别表示单位反应中心吸收的能量/被反应中心捕获的能量/用于电子传递的能量/热耗散的能量(Absorption/Trapping/Electron Transport/Dissipation)/Reaction Centre最大光化学效率(t=0时)Maximal Photochemical Efficiency(t=0)电子传递的量子产额(t=0时)Quantum yield of electron transport(t=0)光合电子传输效率(t=0时)Photosynthetic electron transport efficiency(t=0)荧光曲线面积值Normalized area of the OJIP curve
1.4 数据处理
数据统计采用SPSS 22.0进行数据显著性分析,以0.05 水平作为显著性相关的阈值,用Origin 2021制作基本图表。
2 结果与分析
2.1 硅酸钠对碱性盐胁迫下阳光玫瑰PSⅡ光化学效率和性能的影响
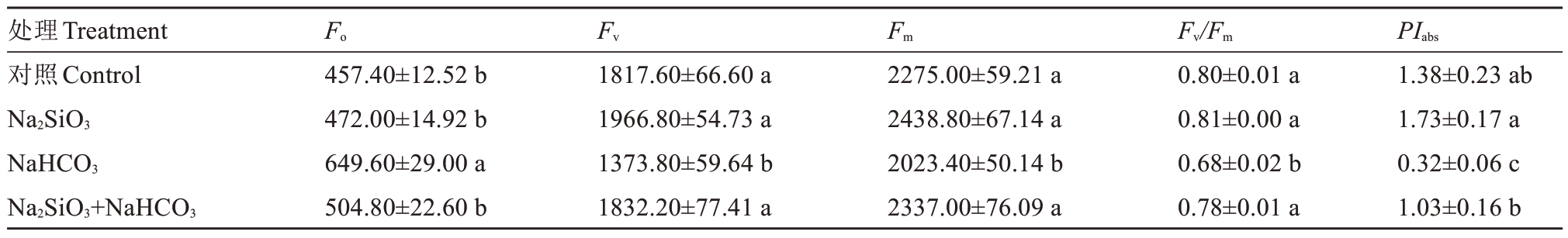
由表2可知,与对照相比,Na2SiO3处理的阳光玫瑰叶片可变荧光(Fv)和最大荧光(Fm)值分别增加了8.21%和7.20%,而NaHCO3处理则降低了24.42%和11.06%,差异显著。与NaHCO3处理相比,Na2SiO3和NaHCO3复合处理的阳光玫瑰叶片Fv和Fm值分别增加了33.37%和15.50%,差异显著。说明Na2SiO3可以有效缓解由碱性盐胁迫而造成植物叶片Fv和Fm值的降低,提高植株叶片PSⅡ的活性。
表2 硅酸钠对碱性盐胁迫下阳光玫瑰Fo、Fv、Fm、Fv/Fm和PIabs的影响
Table 2 Effect of sodium silicate on Fo,Fv,Fm,Fv/Fm of Shine Muscat under alkaline salt stress and PIabs of Shine Muscat under alkaline salt stress
注:不同小写字母表示在p<0.05水平显著差异。
Note:Different small letters indicate significant differences at p<0.05.
处理Treatment对照Control Na2SiO3 NaHCO3 Na2SiO3+NaHCO3 PIabs 1.38±0.23 ab 1.73±0.17 a 0.32±0.06 c 1.03±0.16 b Fo Fv Fm 457.40±12.52 b 472.00±14.92 b 649.60±29.00 a 504.80±22.60 b 1817.60±66.60 a 1966.80±54.73 a 1373.80±59.64 b 1832.20±77.41 a 2275.00±59.21 a 2438.80±67.14 a 2023.40±50.14 b 2337.00±76.09 a Fv/Fm 0.80±0.01 a 0.81±0.00 a 0.68±0.02 b 0.78±0.01 a
PSⅡ最大光化学量子产量(Fv/Fm)是反映PSⅡ活性中心光能转化效率的重要参数[17]。由表2 可知,与对照相比,Na2SiO3处理的阳光玫瑰叶片Fv/Fm值增加了1.03%;NaHCO3处理的叶片Fv/Fm值显著降低了15.06%。Na2SiO3 和NaHCO3 复合处理较NaHCO3 处理的阳光玫瑰叶片Fv/Fm 值显著增加了15.49%。NaHCO3处理后叶片的Fv/Fm值有所上升,且Na2SiO3和NaHCO3复合处理后较NaHCO3处理上升显著,PSⅡ活性中心光能转化效率有所提高,可有效缓解碱性盐胁迫。
如表2 所示,性能指数(PIabs)在Na2SiO3处理时达到最大值。与对照相比,Na2SiO3处理的阳光玫瑰叶片PIabs值增加了24.93%;在NaHCO3处理后PIabs值显著降低了76.66%。与NaHCO3处理相比,Na2SiO3和NaHCO3复合处理后PIabs值增加了217.60%,差异显著。表明Na2SiO3的添加可增强以光能吸收为基础的性能指数,缓解NaHCO3对光系统的胁迫,提高光合系统自身的保护能力,从而提升光合效率。
2.2 硅酸钠对碱性盐胁迫下阳光玫瑰快速叶绿素荧光诱导动力学曲线(OJIP)的影响
如图1 所示,硅酸钠对碱性盐胁迫下阳光玫瑰叶绿素荧光诱导动力学曲线产生了显著的影响。由图1-A 可知,随着时间的延长,NaHCO3 处理后的OJIP 曲线趋于平缓,对照、Na2SiO3和Na2SiO3+NaHCO3处理组的荧光强度随时间的延长逐渐增加。因原始OJIP 曲线变异性受外界影响较大,将其进行标准化处理,随后与Vt(对照)做差,得出图1-B。由图1-B可知,Na2SiO3处理后阳光玫瑰叶片的相对荧光强度和相对可变荧光ΔVt在L点(0.15 ms)、K点(0.3 ms)、J 点(2.0 ms)均低于对照,NaHCO3处理组则在L 点(0.15 ms)、K 点(0.3 ms)、J 点(2.0 ms)显著高于对照。与NaHCO3处理相比,Na2SiO3和NaHCO3复合处理在K 点、J点的相对荧光强度(Vk和Vj)变化幅度较小。Vk和Vj的升高均表明NaHCO3处理后使叶片PSⅡ的电子供体侧和受体侧受到严重损害,而经Na2SiO3和NaHCO3复合处理后Vk和Vj下降,损害程度降低,碱性盐胁迫有所缓解。
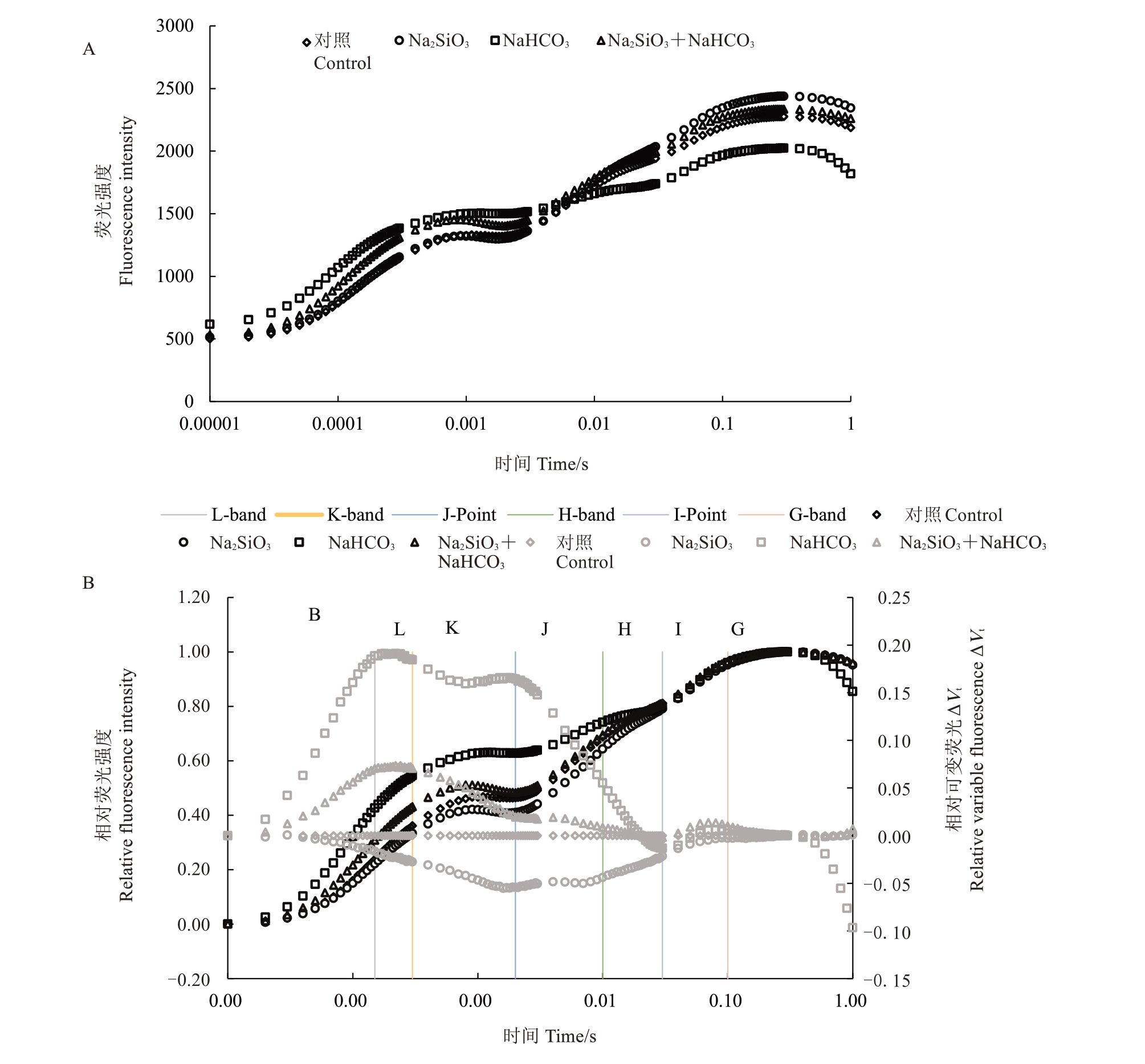
图1 硅酸钠对碱性盐胁迫下阳光玫瑰叶绿素荧光动力学曲线(OJIP)的影响
Fig.1 Effect of sodium silicate on chlorophyll fluorescence kinetics curve(OJIP)of Shine Muscat under alkaline salt stress
2.3 硅酸钠对碱性盐胁迫下阳光玫瑰PSⅡ反应中心量子产额和通量比的影响
如图2 可知,与对照相比,Na2SiO3处理的阳光玫瑰叶片的最大光化学效率(t=0时,φPo)、电子传递的量子产额(t=0 时,φEo)、光合电子传输效率(t=0时,ΨEo)和荧光曲线面积(Sm)值分别增加了1.05%、12.28%、11.31%和11.80%;NaHCO3 处理后叶片的φPo、φEo、ΨEo、Sm 值分别下降了15.04%、38.17%、27.31%和4.44%,且差异显著。Na2SiO3和NaHCO3复合处理较NaHCO3处理的阳光玫瑰叶片φPo、φEo、ΨEo值分别显著增加了15.52%、54.71%、34.18%,Sm值则下降了1.61%,差异不显著。表明Na2SiO3的加入显著抑制了由NaHCO3引起的φEo、ΨEo值的降低。
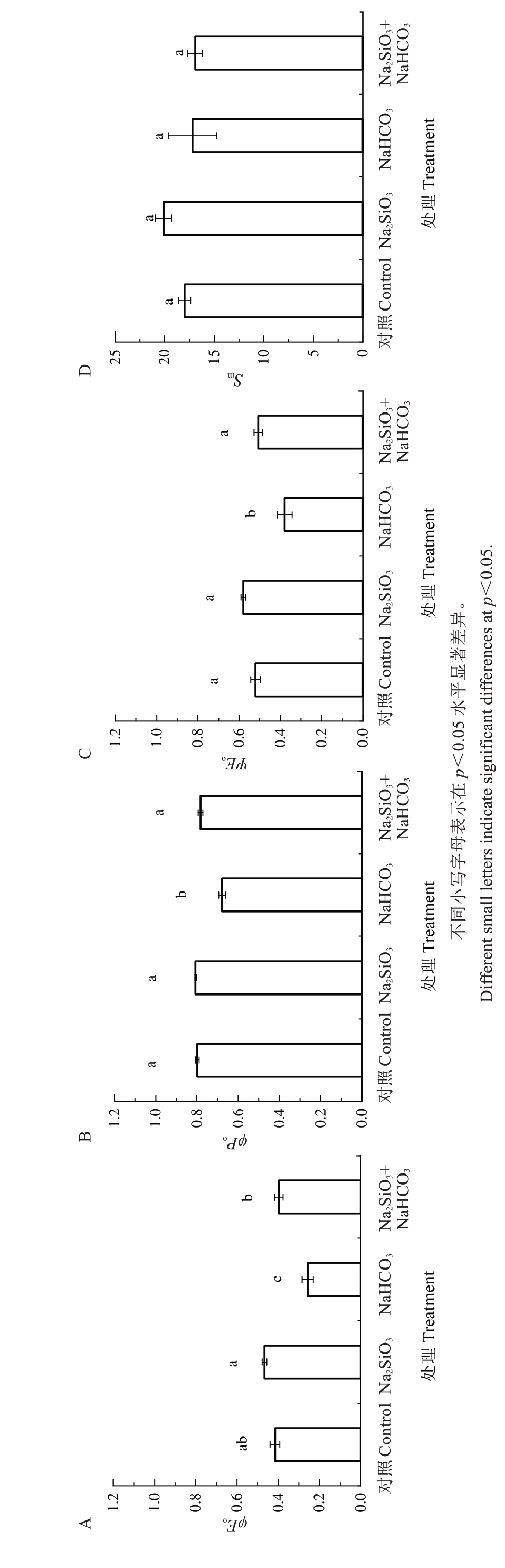
图2 硅酸钠对碱性盐胁迫下阳光玫瑰PSⅡ反应中心量子产额和通量比的影响
Fig. 2 Effect of sodium silicate on quantum yield and flux ratio of the Shine Muscat PSⅡ reaction center under alkaline salt stress
2.4 硅酸钠对碱性盐胁迫下阳光玫瑰叶片光能吸收、捕获和传递的影响
利用叶片模型将叶绿素荧光参数可视化。如图3 所示,与对照相比,Na2SiO3处理的阳光玫瑰叶片的ABS/CSm、TRo/CSm、ETo/CSm、REo/CSm和RC/CSm值分别增加了7.20%、8.21%、19.57%、21.10%和4.05%;NaHCO3处理的ABS/CSm、TRo/CSm、ETo/CSm、REo/CSm和RC/CSm值分别下降了11.06%、24.42%、45.01%、15.17%和31.12%,差异显著。与NaHCO3处理相比,Na2SiO3和NaHCO3复合处理的阳光玫瑰叶片ABS/CSm、TRo/CSm、ETo/CSm、REo/CSm 和RC/CSm 值分别显著增加了15.50%、33.37%、78.44%、21.48%和28.58%。DIo/CSm值在NaHCO3 处理后则显著高于对照、Na2SiO3 和Na2SiO3+NaHCO3,分别为42.02%、37.63%和28.69%,但对照、Na2SiO3以及Na2SiO3和NaHCO3复合处理间无差异。说明Na2SiO3可增加单位叶面积中光合反应中心数量,缓解由碱性盐胁迫导致的光能利用率下降,抑制电子的大量积累,从而导致DIo/CSm的降低。
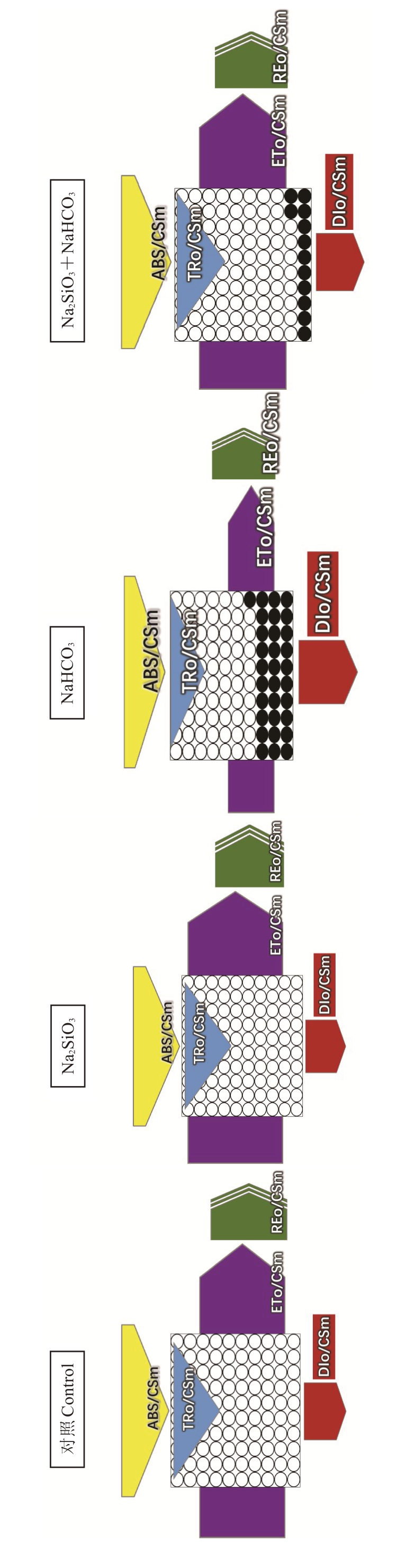
图3 硅酸钠对碱性盐胁迫下阳光玫瑰叶片光能吸收、捕获和传递影响的叶片示意图
Fig. 3 Schematic diagram of sodium silicate on light energy absorption, capture and transfer of light energy from Shine Muscat under alkaline salt stress
如图4所示,与对照相比,Na2SiO3处理的阳光玫瑰叶片的ETo/RC 值增加了15.49%;NaHCO3处理的ETo/RC 值显著下降了20.27%。与NaHCO3 处理相比,Na2SiO3和NaHCO3复合处理的阳光玫瑰叶片ETo/RC 值显著增加了39.37%。阳光玫瑰经NaHCO3处理后,ABS/RC 和DIo/RC 值均显著高于对照、Na2SiO3和Na2SiO3+NaHCO3处理组,ABS/RC 分别高28.72%、25.20%、10.93%,DIo/RC 分别高104.86%、108.28%、64.09%。这说明碱性盐胁迫使RC/CSm 值降低后,Na2SiO3处理主要通过增加ETo/RC 值,迫使剩余有活性的光合反应中心的电子传递速率加快,同时降低热耗散,以此提高光能利用率。
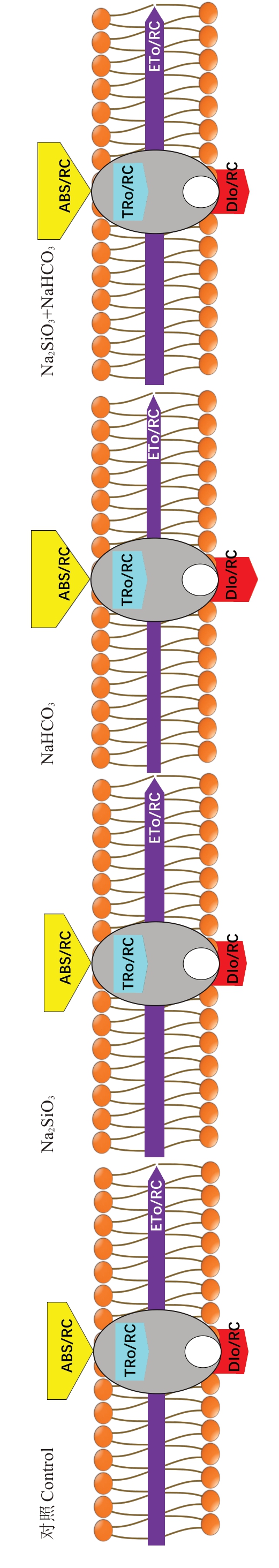
图4 硅酸钠对碱性盐胁迫下阳光玫瑰叶片光能吸收、捕获和传递影响的叶绿体膜示意图
Fig.4 Schematic diagram of chloroplast membrane on light energy absorption, capture and transfer of sodium silicate to the leaves of Shine Muscat under alkaline salt stress
3 讨 论
Fv/Fm和PIabs是研究叶片PSⅡ反应中心光转换效率和电子传递受抑制程度的重要指标[18]。笔者在本研究中发现,与对照相比,NaHCO3处理后阳光玫瑰叶片的Fv、Fm、Fv/Fm和PIabs值显著降低,但在Na2SiO3和NaHCO3复合处理后较NaHCO3处理显著升高。说明Na2SiO3可提高PSⅡ光化学效率,缓解碱性盐胁迫对类囊体造成的损伤。这与Kalaji 等[19]的研究结果相似。说明在碱性盐胁迫下,光合机构功能遭受破坏,Na2SiO3的添加可缓解碱性盐胁迫对类囊体造成的损伤,提高PSⅡ光化学效率。而添加Na2SiO3后光利用效率提高,可能与其对电子转移链产生保护有关。Zhu 等[20]的研究表明硅可增加PSⅡ反应中心开放的数量,更多地用于电子传递,电子传递速率提高。
在快速荧光动力学曲线中,OJIP 的瞬时值可反映叶片PSⅡ中电子传递的状态[21]。笔者在本研究中发现,NaHCO3处理下阳光玫瑰的Vt和ΔVt在L、K、J 点明显升高,φEo、ΨEo 值降低,而Na2SiO3 和NaHCO3 复合处理后表现出相反趋势。该结果与Wang 等[22]的研究结果相似。表明碱性盐胁迫对放氧复合体造成损害,而J 点的升高表明电子从P680至QA受到限制,添加Na2SiO3后,降低了盐碱胁迫对放氧复合体的损害,促进了光合水解,增加H+浓度,改善膜内酸性环境,进而增强ATPase活性,三羧酸循环(TCA)加快,从而促进植物的生长发育[23-24]。同时,盐碱胁迫后PSⅡ受体侧的QA-大量积累,而Na2SiO3可以降低受体侧QA的电子积累,增强受体侧QA下游的电子的传递链将电子传递给QB电子的传递能力[21]。Na2SiO3缓解碱性盐碱胁迫对光合系统的损伤也体现在增加PSⅡ反应中心对光能的捕获(φPo)和电子传递量子产额(ΨEo)、提高有活性的光合反应中心的开放程度和去镁叶绿素(Pheo)到QA的电子传递能力、提高将电子通过QA传递到电子受体的比例(φEo)等方面,从而提高植物耐盐性[22,25-26]。
逆境胁迫条件下,植物可调节能量的重新分配适应外界环境,以达到自我保护的目的[27]。笔者在本研究中发现,与对照相比,阳光玫瑰经NaHCO3处理后,ABS/CSm、TRo/CSm、ETo/CSm、RC/CSm 和ETo/RC值最低,而Na2SiO3和NaHCO3复合处理较NaHCO3处理则显著升高;DIo/CSm、ABS/RC 值则表现出相反的趋势。与鲁倩君等[28]发现在NaCl和NaHCO3的复合胁迫下,不同品种葡萄的叶片ABS/CSm、TRo/CSm、ETo/CSm、RC/CSm值均呈下降趋势,DIo/CSm和ABS/RC值则升高的结果相似,说明碱性盐胁迫可造成单位面积内部分反应中心失活,叶片单位面积内吸收光能、捕获光能的能力低,电子传递能力下降,但为减少光系统的损伤,植物将吸收光能大部分以热耗散的形式消耗。而Na2SiO3可以增加反应中心数量,促进了单位面积内光能的吸收,增强天线色素对光能的捕获能力,电子传递效率加快,同化能力升高,光合能力增强[22,26,29]。
4 结 论
硅显著增加了在碱性盐胁迫下阳光玫瑰叶片的PIabs值、单位叶截面积中光合反应中心的数量,以及对光能的吸收与捕获,增强了电子传递能力,同时减少了植株的热耗散,缓解了盐胁迫造成的光系统的损伤,提高了光利用效率,提升了植物光合能力,从而增强了植物的耐盐性。这为硅在葡萄栽培上的广泛应用提供了较好的理论基础。
[1] COSKUN D,DESHMUKH R,SONAH H,MENZIES J G,REYNOLDS O,MA J F,KRONZUCKER H J,BÉLANGER R R.The controversies of silicon’s role in plant biology[J].New Phytologist,2019,221(1):67-85.
[2] 张瑞,贾旭梅,朱祖雷,张夏燚,赵通,郭爱霞,刘兵,高立杨,王延秀.‘烟富六号’苹果在不同砧木上响应盐碱胁迫的光合及生理特性[J].果树学报,2019,36(6):718-728.ZHANG Rui,JIA Xumei,ZHU Zulei,ZHANG Xiayi,ZHAO Tong,GUO Aixia,LIU Bing,GAO Liyang,WANG Yanxiu.Photosynthesis and physiological characteristics of‘Yanfu 6’apple under saline-alkali stress on different rootstocks[J].Journal of Fruit Science,2019,36(6):718-728.
[3] 窦飞飞,张利鹏,王永康,于坤,刘怀锋.高温胁迫对不同葡萄品种光合作用和基因表达的影响[J].果树学报,2021,38(6):871-883.DOU Feifei,ZHANG Lipeng,WANG Yongkang,YU Kun,LIU Huaifeng.Effects of high temperature stress on photosynthesis and gene expression of different grape cultivars[J].Journal of Fruit Science,2021,38(6):871-883.
[4] 刘月,刘海河,张彦萍,李艳超,王亚伦.外源硅对厚皮甜瓜果实品质及相关酶活性的影响[J].中国瓜菜,2021,34(12):28-32.LIU Yue,LIU Haihe,ZHANG Yanping,LIYanchao,WANG Yalun.Effects of exogenous silicon on fruit quality and related enzyme activities of muskmelon[J].China Cucurbits and Vegetables,2021,34(12):28-32.
[5] ZHANG W J,ZHANG X J,LANG D Y,LIM,LIU H,ZHANG X H.Silicon alleviates salt and drought stress of Glycyrrhiza ura‐lensis plants by improving photosynthesis and water status[J].Biologia Plantarum,2020,64:302-313.
[6] DHIMAN P,RAJORA N,BHARDWAJ S,SUDHAKARAN S S,KUMAR A,RATURIG,CHAKRABORTY K,GUPTA O P,DEVANNA B N,TRIPATHID K,DESHMUKH R.Fascinating role of silicon to combat salinity stress in plants:An updated overview[J].Plant Physiology and Biochemistry,2021,162:110-123.
[7] VERMA K K,SONG X P,ZENG Y,LID M,GUO D J,RAJPUT V D,CHEN G L,BARAKHOV A,MINKINA T M,LIY R.Characteristics of leaf stomata and their relationship with photosynthesis in Saccharum officinarum under drought and silicon application[J].ACS Omega,2020,5(37):24145-24153.
[8] ZHANG Y,SHIY,GONG H J,ZHAO H L,LIH L,HU Y H,WANG Y C.Beneficial effects of silicon on photosynthesis of tomato seedlings under water stress[J].Journal of Integrative Agriculture,2018,17(10):2151-2159.
[9] LIL L,QIQ,ZHANG H H,DONG Q,IQBAL A,GUIH P,KAYOUMU M,SONG M Z,ZHANG X L,WANG X R.Ameliorative effects of silicon against salt stress in Gossypium hirsu‐tum L.[J].Antioxidants,2022,11(8):1520.
[10] 王琰琰,王剑,潘勇,贾志忠,汪鹏,李子玮,杜甫,赵泽玉,谭再钰,吴英乔,方秀,张兴伟,施友志.硅在植物中抵御生物胁迫机制的研究进展[J].植物生理学报,2024,60(1):35-44.WANG Yanyan,WANG Jian,PAN Yong,JIA Zhizhong,WANG Peng,LIZiwei,DU Fu,ZHAO Zeyu,TAN Zaiyu,WU Yingqiao,FANG Xiu,ZHANG Xingwei,SHIYouzhi.Mechanism research advances in plant biotic stress resistance regulated by silicon[J].Plant Physiology Journal,2024,60(1):35-44.
[11] JOHNSON V M,PAKRASIH B.Advances in the understanding of the lifecycle of photosystem Ⅱ [J].Microorganisms,2022,10(5):836.
[12] 傅伟红,夏澜,应永涛,徐杏,王海玮,王三红.5-ALA 对阳光玫瑰葡萄果实品质的影响[J].中国果树,2023(10):45-51.FU Weihong,XIA Lan,YING Yongtao,XU Xing,WANG Haiwei,WANG Sanhong.Effect of 5- ALA on fruit quality of‘Shine Muscat’grape[J].China Fruits,2023(10):45-51.
[13] 刘凤之.中国葡萄栽培现状与发展趋势[J].落叶果树,2017,49(1):1-4.LIU Fengzhi.The current situation and development trend of grape cultivation in China[J].Deciduous Fruits,2017,49(1):1-4.
[14] XU J H,SUIC C,GE J R,REN R J,PANG Y N,GAN H P,DU Y P,CAO H,SUN Q H.Exogenous spermidine improved the salinity-alkalinity stress tolerance of grapevine (Vitis vinifera)by regulating antioxidant system,Na+/K+ homeostasis and endogenous polyamine contents[J].Scientia Horticulturae,2024,326:112725.
[15] ZHAO B L,LIU Z Y,ZHU C M,ZHANG Z J,SHIW C,LU Q J,SUN J L.Saline-alkaline stress resistance of cabernet sauvignon grapes grafted on different rootstocks and rootstock combinations[J].Plants,2023,12(15):2881.
[16] 李书鑫,徐婷,李慧,杨文莹,蔺吉祥,朱先灿.低温胁迫对玉米幼苗叶绿素荧光诱导动力学的影响[J].土壤与作物,2020,9(3):221-230.LIShuxin,XU Ting,LIHui,YANG Wenying,LIN Jixiang,ZHU Xiancan.Effects of low temperature on chlorophyll fluorescence kinetics of maize seedlings[J].Soils and Crops,2020,9(3):221-230.
[17] 贾文飞,魏晓琼,张秋莹,李林宇,王颖,李金英,吴林.盐碱处理对越橘光合特性及叶绿素荧光参数的影响[J].江苏农业科学,2022,50(7):152-158.JIA Wenfei,WEI Xiaoqiong,ZHANG Qiuying,LI Linyu,WANG Ying,LIJinying,WU Lin.Effects of exogenous abscisic acid on stomatal and photosynthetic characteristics of Kraft rhododendron under UV-B stress[J].Jiangsu Agricultural Sciences,2022,50(7):152-158.
[18] 张濛,续高山,滕志远,刘关君,张秀丽.模拟酸雨对小黑杨幼苗生长和光合特性的影响[J].南京林业大学学报(自然科学版),2021,45(6):57-64.ZHANG Meng,XU Gaoshan,TENG Zhiyuan,LIU Guanjun,ZHANG Xiuli.Effects of simulated acid rain on growth and photosynthetic physiological characteristics of Populus simonii×P.nigra[J].Journal of Nanjing Forestry University (Natural Sciences Edition),2021,45(6):57-64.
[19] KALAJIH M,JAJOO A,OUKARROUM A,BRESTIC M,ZIVCAK M,SAMBORSKA IA,CETNER M D,ŁUKASIK I,GOLTSEV V,LADLE R J.Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions[J].Acta Physiologiae Plantarum,2016,38(4):102.
[20] ZHU Y X,GUO J,FENG R,JIA J H,HAN W H,GONG H J.The regulatory role of silicon on carbohydrate metabolism in Cucumis sativus L.under salt stress[J].Plant and Soil,2016,406(1):231-249.
[21] GOVINDJE E.Sixty-three years since Kautsky:Chlorophyll a fluorescence[J].Functional Plant Biology,1995,22(2):131.
[22] WANG X S,DINGXUAN Q Y,SHIM M.Calcium amendment for improved germination,plant growth,and leaf photosynthetic electron transport in oat (Avena sativa) under NaCl stress[J].PLoS One,2021,16(8):e0256529.
[23] DĄBROWSKIP,BACZEWSKA A H,PAWLUŚKIEWICZ B,PAUNOV M,ALEXANTROV V,GOLTSEV V,KALAJIM H.Prompt chlorophyll a fluorescence as a rapid tool for diagnostic changes in PSIIstructure inhibited by salt stress in Perennial ryegrass[J].Journal of Photochemistry and Photobiology B:Biology,2016,157:22-31.
[24] SCHREIBER U,NEUBAUER C.The polyphasic rise of chlorophyll fluorescence upon onset of strong continuous illumination:Ⅱ.Partial control by the photosystem Ⅱdonor side and possible ways of interpretation[J].Zeitschrift Für Naturforschung C,1987,42(11/12):1255-1264.
[25] 丁俊男,张会慧,迟德富.土壤菲胁迫对高丹草幼苗叶片光合机构功能的影响[J].草业科学,2014,31(9):1732-1738.DING Junnan,ZHANG Huihui,CHIDefu.Response of photosynthesis in leaves of Sorghum bicolor × S.sudanense seedlings to phenanthrene polluted soils[J].Pratacultural Science,2014,31(9):1732-1738.
[26] 卢盼玲,杜旋,王颖,张红梅,田守波,王楠,刘娜.碱性盐胁迫对节瓜幼苗叶绿素荧光诱导动力学的影响[J/OL].分子植物育种,2023:1-8.(2023-10-17).https://kns.cnki.net/kcms/detail/46.1068.S.20231016.1123.014.html.LU Panling,DU Xuan,WANG Ying,ZHANG Hongmei,TIAN Shoubo,WANG Nan,LIU Na.Effectsofsalt-alkalinestressonchlorophyll fluorescence characteristics in leaves of Chieh-qua seedlings[J/OL].Molecular Plant Breeding,2023:1-8.(2023-10-17).https://kns.cnki.net/kcms/detail/46.1068.S.20231016.1123.014.html.
[27] 尹赜鹏,鹿嘉智,高振华,齐明芳,孟思达,李天来.番茄幼苗叶片光合作用、PSⅡ电子传递及活性氧对短期高温胁迫的响应[J].北方园艺,2019(5):1-11.YIN Zepeng,LU Jiazhi,GAO Zhenhua,QIMingfang,MENG Sida,LITianlai.Effects of photosynthetic,PS Ⅱelectron transport and reactive oxygen species on short-term high temperature stress in tomato seedlings[J].Northern Horticulture,2019(5):1-11.
[28] 鲁倩君,陈丽靓,马媛媛,刘迎,赵云文,赵宝龙,孙军利.盐碱胁迫对不同葡萄砧木光合及叶绿素荧光特性的影响[J].果树学报,2022,39(5):773-783.LU Qianjun,CHEN Liliang,MA Yuanyuan,LIU Ying,ZHAO Yunwen,ZHAO Baolong,SUN Junli.Effects of saline-alkali stress on photosynthetic and chlorophyll fluorescence characteristics of different grape rootstocks[J].Journal of Fruit Science,2022,39(5):773-783.
[29] 邢庆振,郁松林,牛雅萍,于坤,宋曼曼.盐胁迫对葡萄幼苗光合及叶绿素荧光特性的影响[J].干旱地区农业研究,2011,29(3):96-100.XING Qingzhen,YU Songlin,NIU Yaping,YU Kun,SONG Manman.Effects of salt stress on photosynthetic physiology and chlorophyll fluorescence characteristics of grape (Red Globe)seedlings[J].Agricultural Research in the Arid Areas,2011,29(3):96-100.