柑橘是世界和中国南方第一大水果。中国是柑橘起源中心[1],拥有丰富多样的柑橘种质资源,合理保存和利用中国优异的柑橘种质具有重要意义。柑橘胚性愈伤组织由败育胚珠诱导而来,其遗传上与亲本保持一致;且胚性愈伤组织具有细胞全能性,能通过体细胞胚发生途径再生植株,是柑橘种质资源离体保存的主要方式。笔者课题组多年来诱导保存了100多个柑橘品种的胚性愈伤组织[2],为利用原生质体融合、基因编辑等生物技术改良柑橘提供了丰富的起始材料[3],为柑橘体细胞胚发生[4]、品质调控[5]和抗性研究[6]提供了离体体系。然而,柑橘胚性愈伤组织均从二倍体种质诱导获得,柑橘多倍体种质胚性愈伤组织诱导未见报道。
多倍体普遍具有器官巨大型、植株矮化和适应性强等特征。柑橘同源四倍体相比二倍体对盐胁迫、干旱、营养缺乏等非生物逆境抗性更强[7-9],柑橘四倍体相比二倍体果实品质也发生显著变异[10-11],为柑橘倍性育种提供了优良亲本。诱导柑橘多倍体的胚性愈伤组织,将为柑橘多倍体倍性和代谢等研究提供周期短、状态稳定、易获得的离体材料,加速柑橘多倍体基础研究和利用。
无核是柑橘鲜食品种选育的重要目标性状[12]。但无核品种常年通过嫁接繁殖,累积多种病毒,导致果实品质和产量下降。中国较常见且对柑橘生产危害较大的病毒和细菌性类病害主要有柑橘黄龙病、溃疡病、裂皮病、衰退病和黄脉病等。柑橘无核品种没有种子,无法通过珠心苗实生繁殖脱毒;柑橘茎尖培养难以生根,微芽嫁接操作要求高且成活率低,脱毒效率有待提高。胚性愈伤组织可通过体细胞胚发生途径再生植株,在稳定遗传亲本性状的同时,能培育无病毒植株,操作相对简单,再生苗数量多,结构完整,尤其适合多胚无核柑橘品种提纯复壮[13]。
笔者课题组利用柑橘多胚品种存在珠心细胞自然加倍的现象,从柑橘60 多个接穗和砧木类型中发掘获得了一大批同源四倍体植株[14-16],丰富了中国柑橘多倍体种质资源,为通过倍性杂交培育柑橘三倍体无核新种质提供了优良亲本[17]。笔者在本研究中通过离体培养二倍体和四倍体柑橘的败育胚珠,诱导胚性愈伤组织,将为多倍体抗性和代谢相关研究提供倍性成对的离体材料;无核品种诱导胚性愈伤组织并通过体细胞胚发生途径再生不携带病毒的植株,将为中国主栽的脐橙和温州蜜柑等无核品种提纯复壮提供种苗。
1 材料和方法
1.1 试验材料
用于诱导胚性愈伤组织的柑橘二倍体及其同源四倍体(2x/4x)成对倍性材料,包括红橘(Citrus retic‐ulata Blanco)2x/4x、W.默科特橘橙(C.reticulata Blanco × C.sinensis L.Osbeck)2x/4x、鄂柑1 号椪柑(C.reticulata Blanco)2x/4x、日辉橘(C.reticulata Blanco)2x/4x;无核柑橘品种,包括早红脐橙(C.si‐nensis L.Osbeck)、伦晚脐橙(C.sinensis L.Osbeck)、国庆1号温州蜜柑(C.unshiu Blanco)、大分4号温州蜜柑(C.unshiu Blanco)和兴津温州蜜柑(C.unshiu Blanco)。
1.2 柑橘胚性愈伤组织离体诱导
将成熟果实浸泡于75%乙醇20 min 后,超净工作台内用酒精灯灼烧果实表面消毒。在无菌滤纸上,将果实切开后,用镊子将败育胚珠挑出,接种于3 种愈伤诱导培养基[18](MES:MT[19]+0.5 g·L-1 ME+40 mg·L-1 SAD;MGS:MT+0.5 g·L-1 ME+40 mg·L-1 SAD +1 mg·L-1 GA3;MK:MT+0.5 mg·L-1 KT)进行暗培养。离体培养约1 个月后,待败育胚珠萌发产生胚状体或胚性愈伤组织后,将胚性愈伤组织挑出并继代于MT 培养基,光下培养至愈伤组织状态稳定并持续增殖,即完成愈伤组织驯化。将驯化完成的愈伤组织保存在MT 培养基中光照培养,每月继代1 次长期保存。胚性愈伤组织诱导率/%=形成胚性愈伤组织的败育胚珠数/离体培养的败育胚珠总数×100,胚状体发生率/%=形成胚状体的败育胚珠数/离体培养败育胚珠总数×100。
1.3 胚性愈伤组织胚状体增殖、生芽和试管嫁接
新诱导获得的柑橘胚性愈伤组织在MT 培养基上驯化和继代培养过程中,会再生胚状体,将胚状体转移至增殖培养基(MT+1.5 g·L-1 ME+50 g·L-1蔗糖),待胚状体膨大后转移至生芽培养基(MT+0.5 mg·L-1 6-BA + 0.5 mg·L-1 KT + 0.1 mg·L-1 NAA)诱导生芽。待丛生芽长至2~3 cm 高时,采用试管嫁接技术将其嫁接至枳橙黄化砧木上;待嫁接苗成活并新长出3~4 枚真叶后,将其转移到生长室炼苗;待幼苗长至6~7枚叶片时转移至温室。
1.4 倍性测定和InDel标记鉴定
利用流式细胞仪(Cyflowspace,Sysmex,Japan)检测胚性愈伤组织及再生植株的染色体倍性,详情参考解凯东等[17]的方法。制备待测愈伤组织或植株叶片混合液,比较待测样品与二倍体,参照峰值横坐标位置关系,确定待测样品倍性。分子鉴定参考谢善鹏等[14]的方法,InDel 标记引物[20(]表1)由生工生物工程(上海)股份有限公司合成。用ProFlex PCR 仪(ABI,USA)进行PCR 反应,PCR 扩增体系为10 μL:2×Rapid Taq Master Mix 5 μL,ddH2O 3.5 μL,DNA 1 μL,Primer F、R 各0.25 μL(10 μmol·L-1)。扩增程序:95 ℃预变性5 min,95 ℃变性30 s,55 ℃退火30 s,72 ℃延伸10 s,35 个循环,72 ℃延伸5 min,4 ℃保存。
表1 早红脐橙InDel 标记引物序列
Table 1 InDel marker primer sequences for Zaohong navel orange
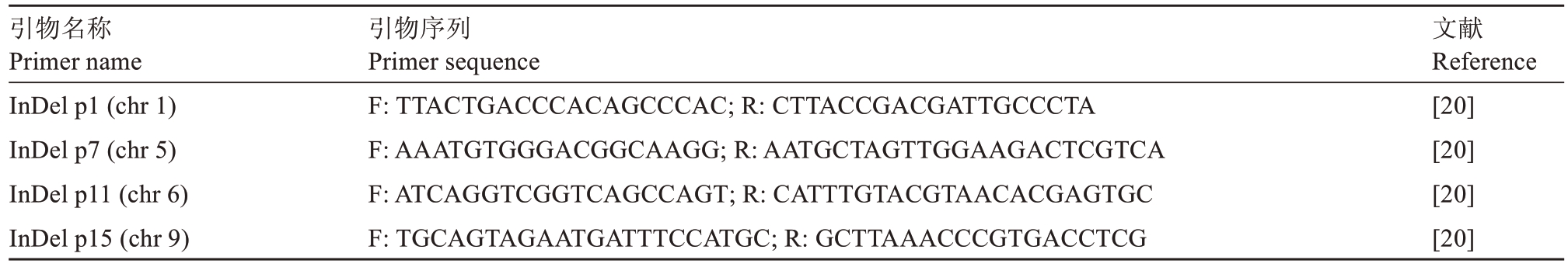
引物名称Primer nameInDel p1(chr 1)InDel p7(chr 5)InDel p11(chr 6)InDel p15(chr 9)引物序列Primer sequence F:TTACTGACCCACAGCCCAC;R:CTTACCGACGATTGCCCTA F:AAATGTGGGACGGCAAGG;R:AATGCTAGTTGGAAGACTCGTCA F:ATCAGGTCGGTCAGCCAGT;R:CATTTGTACGTAACACGAGTGC F:TGCAGTAGAATGATTTCCATGC;R:GCTTAAACCCGTGACCTCG文献Reference[20][20][20][20]
1.5 病毒PCR检测
取嫁接苗植株叶片,使用液氮冷冻后磨样。叶片RNA 提取方法参照HiPure HP Plant RNA Mini Kit(Magen)说明书;反转录方法参照HiScripte Ⅱ Q RT SuperMix for qPCR(+gDNA wiper)(Vazyme)说明书。PCR反应体系和程序同InDel标记鉴定,设置阴性(ddH2O 模板)和阳性对照(感染黄脉病和衰退病的夏橙叶片DNA)。衰退病[21]和黄脉病检测引物[22](表2)由生工生物工程(上海)股份有限公司合成。
表2 病毒检测引物序列
Table 2 Primer sequences for virus detection
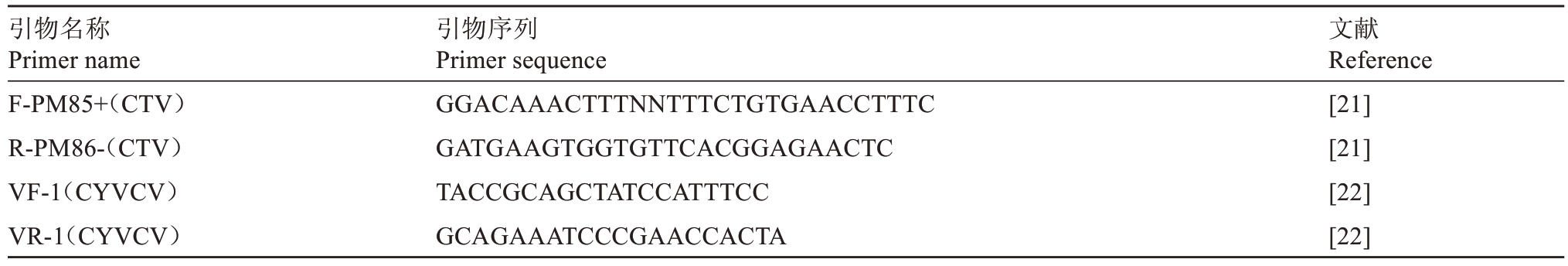
引物名称Primer name F-PM85+(CTV)R-PM86-(CTV)VF-1(CYVCV)VR-1(CYVCV)引物序列Primer sequence GGACAAACTTTNNTTTCTGTGAACCTTTC GATGAAGTGGTGTTCACGGAGAACTC TACCGCAGCTATCCATTTCC GCAGAAATCCCGAACCACTA文献Reference[21][21][22][22]
2 结果与分析
2.1 柑橘胚性愈伤组织诱导和驯化
从柑橘成熟果实中挑出败育胚离体培养,暗培养0.7~3.0 个月后在败育胚珠附近形成胚性愈伤组织。将胚性愈伤组织转移至MT 培养基多次继代培养进行驯化,耗时5~9 个月。13 个材料均诱导出胚性愈伤组织,经过多次继代培养,其中8个材料的胚性愈伤组织状态稳定并能持续增殖,包括红橘2x/4x、W.默科特橘橙2x/4x、鄂柑1 号椪柑2x/4x、日辉橘2x 和早红脐橙,说明这些胚性愈伤组织已完成驯化,可常规继代培养;其余5 个材料包括日辉橘4x、伦晚脐橙、国庆1 号温州蜜柑、大分4 号温州蜜柑和兴津温州蜜柑诱导出的愈伤组织胚性较强,经多次继代逐渐分化形成胚状体,虽未能获得稳定增殖的胚性愈伤组织,但通过体细胞胚发生途径可能再生植株。采用流式细胞仪对8 份2x/4x 胚性愈伤组织进行检测,其倍性均与其败育胚珠外植体来源母本植株一致(图1)。
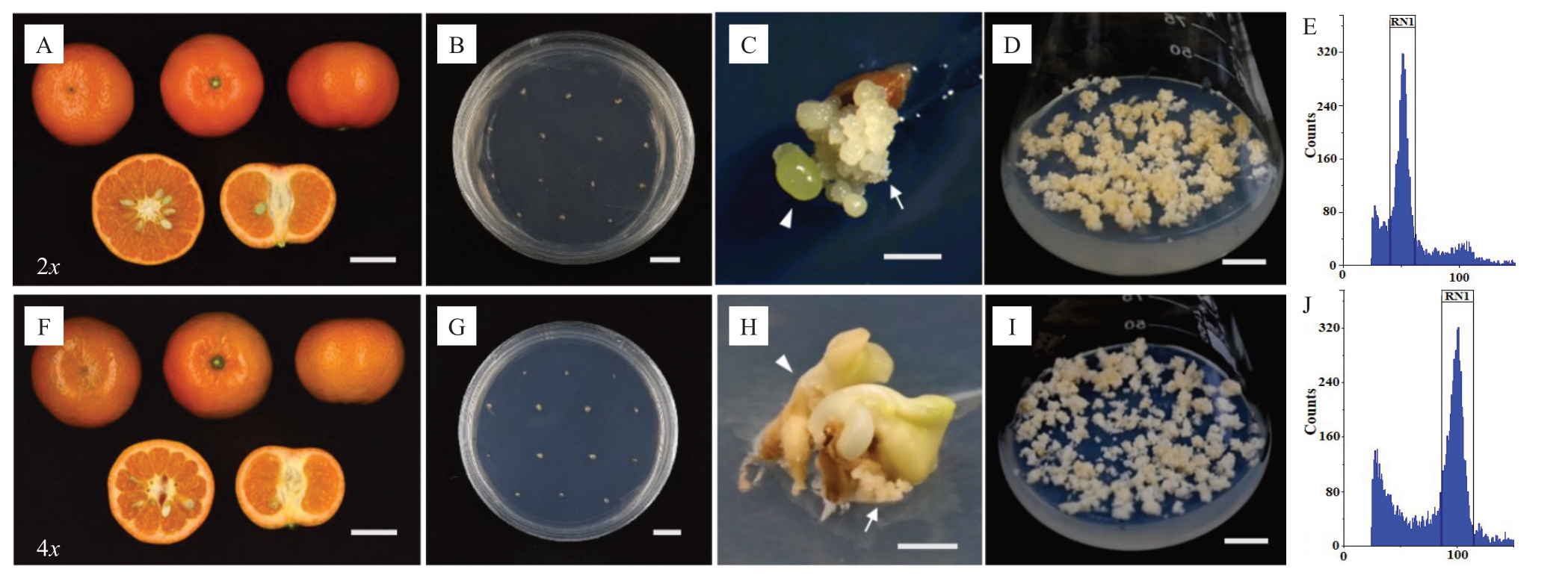
图1 W.默科特橘橙二倍体/四倍体(2x/4x)胚性愈伤组织诱导过程
Fig.1 Induction process of embryogenic callus from diploid/tetraploid(2x/4x)of Nadorcott tangor
A,F.2x/4x 果实;B,G.接种培养2x/4x 败育胚珠;C.2x 败育胚珠直接长出胚性愈伤组织;D,I.2x/4x 胚性愈伤组织完成驯化;E,J.流式细胞仪鉴定愈伤组织倍性为2x/4x;H.4x 瘪种子先长出胚状体,再从胚状体下胚轴长出愈伤组织;箭头指示胚状体,带尾箭头指示胚性愈伤组织;标尺.3 cm(A.F),1 cm(B.D.G~I),1 mm(C)。
A, F.Fruit of diploid/tetraploid (2x/4x); B, G.Inoculation of 2x/4x aborted ovules; C.Embryogenic callus generated directly from 2x shrivelled seed;D,I.The trained 2x/4x embryogenic callus;E,J.Ploidy analysis of 2x/4x embryogenic callus;H.Embryogenic callus generated from the hypocotyl of the embryoid that formed from the aborted ovule;The arrow head points to the embryoid,and the arrow point to embryogenic callus;Bar.3 cm(A.F),1 cm(B.D.G-I),1 mm(C).
不同于其2x 能直接从败育胚珠中长出胚性愈伤组织,W.默科特橘橙4x接种的败育胚珠均未长出愈伤组织或胚状体;而是从较大的瘪种子中长出胚状体,再从胚状体下胚轴长出胚性愈伤组织(图1-H)。相比W.默科特橘橙2x 胚性愈伤组织诱导和驯化分别耗时0.7 和5.5 个月,W.默科特橘橙4x 的愈伤组织诱导和驯化耗时较长,分别为3和5.5 个月。红橘4x 愈伤组织诱导和驯化均相比对应的2x 耗时也较长,但鄂柑1 号椪柑4x 愈伤组织诱导耗时较2x更短。
2.2 基因型和培养基影响胚性愈伤组织诱导效率
柑橘不同基因型的胚性愈伤组织诱导效率不同(表3)。早红脐橙诱导率最高,达74.73%;其次为国庆1 号温州蜜柑、兴津温州蜜柑、大分4 号温州蜜柑和鄂柑1 号椪柑2x,诱导率均>37%;红橘2x、日辉橘2x、伦晚脐橙和W.默科特橘橙2x的诱导率较低,在6.85%~12.61%之间。4 对倍性材料均诱导出胚性愈伤组织(其中日辉橘4x愈伤组织未能完成驯化获得稳定增殖的胚性愈伤组织),二倍体愈伤组织诱导率为6.85%~37.75%,其中鄂柑1号椪柑2x愈伤组织诱导率最高(37.75%),红橘2x 的愈伤组织诱导率最低(6.85%)。四倍体愈伤组织诱导率相比二倍体较低,仅为0.56%~22.61%,其中鄂柑1号椪柑4x愈伤组织诱导率仍最高,达22.61%,而W.默科特橘橙4x 的愈伤组织诱导率最低,仅为0.56%。2x/4x 成对倍性材料中,二倍体的胚状体发生率为16.15%~58.28%,椪柑的胚状体发生率最高,为58.28%。四倍体的胚状体发生率为1.13%~30.65%,椪柑仍为最高,为30.65%,W.默科特橘橙最低,仅为1.13%。总结可知,椪柑2x 和4x 的愈伤组织诱导率和胚状体发生率均为4个品种2x 和4x 材料中最高,W.默科特橘橙4x 的愈伤组织诱导率和胚状体发生率均为4个四倍体品种中最低;同品种二倍体的愈伤组织诱导率和胚状体发生率均比对应的四倍体更高。
表3 柑橘不同品种和配方的胚性愈伤组织诱导率和胚状体发生率
Table 3 The induction rates of embryonic callus and embryoids from different citrus cultivars and medium

注:“-”代表该处理未实施;未标注倍性为二倍体;愈伤组织诱导率/胚状体发生率=愈伤组织或胚状体数/接种败育胚珠数×100%;平均诱导/发生率为3 种培养基诱导/发生率的加权平均;MES、MGS、MK 表示3 种培养基。
Note:“-”represents that the treatment is not implemented,and the ploidy not specified is diploid.Induction rate=number of callus or embryoids/number of inoculated ovules×100%.The average induction rate is the weighted mean of induction rates on three induction medium.MES,MGS,MK represent three mediums.
品种Cultivars外植体接种数Number of explants胚性愈伤组织诱导率Induction rate of embryogenic callus/%胚状体发生率Induction rate of embryoids/%MES MGS MK MES MGS MK MES MGS MK红橘2x Red tangerine 2x红橘4x Red tangerine 4x W.默科特橘橙2x Nadorcott tangor 2x W.默科特橘橙4x Nadorcott tangor 4x鄂柑1号椪柑2x Ponkan mandarin Egan No.1 2x鄂柑1号椪柑4x Ponkan mandarin Egan No.1 4x日辉橘2x Sunburst mandarin 2x日辉橘4x Sunburst mandarin 4x早红脐橙Zaohong navel orange伦晚脐橙Lane Late navel orange国庆1号温州蜜柑Satsuma mandarin Guoqing No.1大分4号温州蜜柑Satsuma mandarin Oita 4兴津温州蜜柑Satsuma mandarin Okitsu 79 105 35 109 124 40 104 126 44 0.00 0.95 5.71 1.83 2.42 17.50 17.31 10.32 13.64平均诱导率Average induction rate 6.85 4.79 12.61 40.51 19.05 20.00 36.70 24.19 22.50 46.15 17.46 27.27平均发生率Average induction rate 41.10 20.28 23.53 72 50 55 1.39 0.00 0.00 0.56 2.78 0.00 0.00 1.13 48 56 47 25.00 21.43 70.21 37.75 52.08 44.64 80.85 58.28 59 74 66 30.51 13.51 25.76 22.61 30.51 32.43 28.79 30.65 101 92 98 6.93 14.13 11.22 10.65 8.91 20.65 19.39 16.15 70 81-1.43 3.70-2.65 21.43 9.88-15.23 83 112 82 77.11 76.79 69.51 74.73 86.75 89.29 64.63 81.23 66 72 58 15.15 9.72 10.34 11.73 28.79 20.83 20.69 23.47 108 73 62 42.59 63.01 37.10 47.33 47.22 68.49 41.94 52.26 60 58 45 36.67 44.83 33.33 38.65 46.67 72.41 35.56 52.76 39 39 37 51.28 53.85 21.62 42.61 33.33 61.54 21.62 39.13
不同培养基的胚性愈伤组织诱导效率不同(表3)。国庆1 号温州蜜柑、兴津温州蜜柑、大分4 号温州蜜柑、W.默科特橘橙2x和日辉橘2x共5个品种在MGS 培养基的胚性愈伤组织诱导率均高于MK 和MES 培养基。早红脐橙、国庆1 号温州蜜柑、大分4号温州蜜柑和兴津温州蜜柑这4 个品种在MK 培养基的愈伤组织诱导率最低;但MK 培养基对鄂柑1号椪柑2x和红橘2x的愈伤组织诱导率最高,且明显高于MES 和MGS 培养基的诱导率。椪柑2x 在MK培养基的愈伤组织诱导率高达70.21%,约为MES、MGS 培养基的3 倍;红橘2x 在MK 培养基的愈伤组织诱导率高达17.31%,约为MGS培养基的15倍。
2.3 柑橘无核品种胚性愈伤组织再生植株及其来源和病毒检测
早红脐橙、伦晚脐橙、国庆1号温州蜜柑、大分4号温州蜜柑、兴津温州蜜柑5 个无核品种诱导出的胚性愈伤组织胚性较强,诱导过程产生大量胚状体;挑选胚状体进行增殖培养,胚状体膨大后转移至生芽培养基诱导生芽。约2 个月后再生大量丛生芽,将再生芽切下进行试管嫁接,待嫁接苗长出3~4 枚真叶移栽至基质炼苗,1~2 个月后移栽至温室(图2)。早红脐橙愈伤组织再生苗经流式细胞仪鉴定均为二倍体。分别获得无核品种早红脐橙、伦晚脐橙、国庆1号温州蜜柑、大分4号温州蜜柑和兴津温州蜜柑的离体再生苗23、22、20、10、15株。

图2 早红脐橙胚性愈伤组织诱导及植株再生
Fig.2 Induction of embryogenic callus from Zaohong navel orange and regeneration of plantlets
A.早红脐橙成熟果实;B.接种培养败育胚珠;C.愈伤组织形成;D.胚性愈伤组织分化胚状体;E.胚状体生芽;F.丛生芽试管嫁接;G.再生苗倍性鉴定;H.嫁接苗转移至基质炼苗;I.移栽至温室;标尺:5 cm(H),3 cm(A),1 cm(B.D~F),1 mm(C)。
A.Fruit of Zaohong navel orange;B.Inoculation of aborted ovules in callus induction medium;C.Callus formation;D.The embryoid regenerated from embryogenic callus; E.Plants regenerated from the embryoids; F.The grafted plantlet; G.Ploidy analysis of the regenerated plants by flow cytometry;H.The transplanted plantlets;I.Plantlets in greenhouse;Bar=5 cm(H),3 cm(A),1 cm(B.D-F),1 mm(C).
早红脐橙是罗伯逊脐橙(Robertson navel orange)高接于温州蜜柑产生的嫁接嵌合体[23]。利用前人筛选的4 对InDel 标记引物[20]鉴定早红脐橙胚性愈伤组织再生苗的遗传组成。结果(图3)表明,早红脐橙23 株嫁接苗与其亲本罗伯逊脐橙带型相同,认为嵌合突变体早红脐橙胚性愈伤组织再生苗实为罗伯逊脐橙。
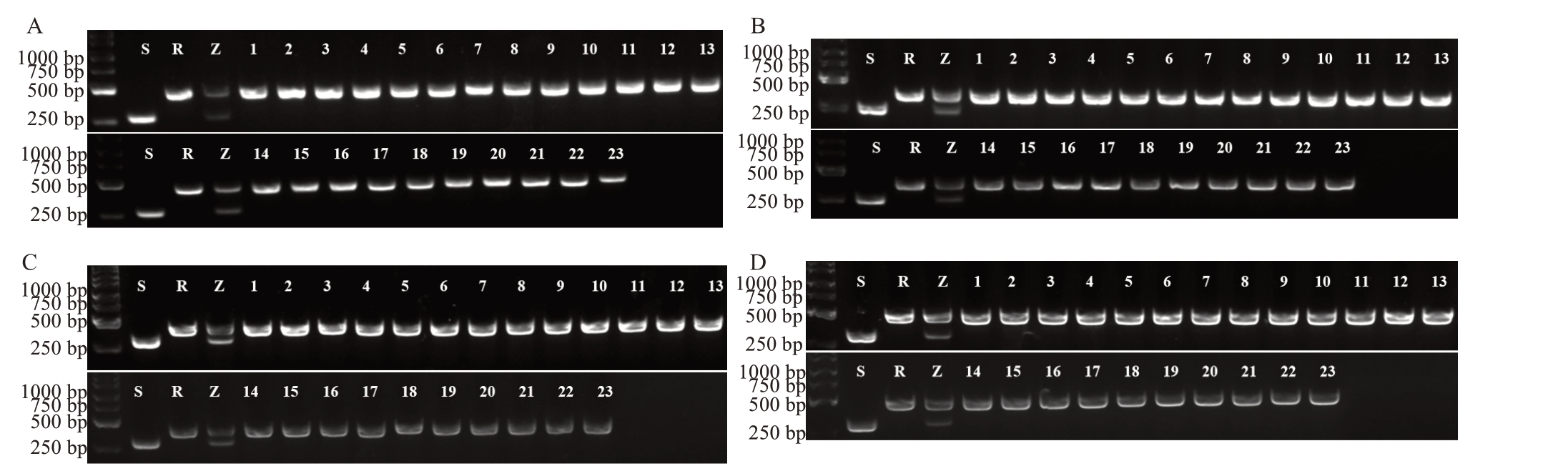
图3 早红脐橙再生苗InDel 标记鉴定
Fig.3 The InDel marker profile of the regenerated plantlets of Zaohong navel orange
S.温州蜜柑;R.罗伯逊脐橙;Z.早红脐橙;1~23.早红脐橙再生苗。引物:A.InDel p1;B.InDel p7;C.InDel p11;D.InDel p15。
S.Satsuma mandarin;R.Robertson navel orange;Z.Zaohong navel orange;1~23.The regenerated plants of Zaohong navel orange.Primers:A.In-Del p1;B.InDel p7;C.InDel p11;D.InDel p15.
柑橘衰退病(Citrus tristeza virus,CTV)和黄脉病(Citrus yellow vein clearing virus,CYVCV)是柑橘果园最常见的两种病毒。对国庆1 号温州蜜柑、伦晚脐橙和早红脐橙再生苗进行PCR 病毒检测,结果(图4)显示阳性样本(带有衰退病和黄脉病的夏橙叶片)泳道有明显条带,3 个品种的再生苗与阴性对照(水模板)均未扩增出目的条带,证明国庆1 号温州蜜柑、伦晚脐橙和早红脐橙再生苗均不含可检出量的衰退病和黄脉病病毒,无毒率达100%。

图4 柑橘胚性愈伤组织再生苗病毒PCR 检测结果
Fig.4 PCR-based virus detection for citrus regenerated plantlets
A~C,D~F.衰退病(CTV)、黄脉病(CYVCV)病毒检测结果;A,D.国庆1 号温州蜜柑再生苗;B,E.伦晚脐橙再生苗;C,F.早红脐橙再生苗。
A-C,D-F.Virus detection results for CTV and CYVCV;A,D.Regenerated plantlets of Satsuma mandarin Guoqing No.1;B,E.Regenerated plantlets of Lane Late navel orange;C,F.Regenerated plantlets of Zaohong navel orange.
3 讨 论
柑橘胚性愈伤组织诱导效率受基因型影响,9个二倍体品种的胚性愈伤组织诱导率为6.85%~74.73%。由柑橘基本种杂交而来的橙类(脐橙)和柑类(温州蜜柑)的胚性愈伤组织诱导率较古老的橘类(椪柑、红橘)更高;其中红橘的胚性愈伤组织诱导率最低,且诱导时间长,败育胚珠离体接种3个月后才长出少量愈伤组织。这可能是由于较古老的橘类次生代谢更丰富,影响了胚性愈伤组织诱导。桃野生近缘种光核桃大量积累次生代谢物,其子叶外植体的愈伤组织诱导率相比一般品种也较低[24],与本研究结果相似。
笔者在本研究中发现二倍体品种的胚性愈伤组织诱导率和胚状体发生率均高于其对应的同源四倍体,如W.默科特橘橙2x 的愈伤组织诱导率是其4x的25 倍。以败育胚珠为外植体诱导获得的胚性愈伤组织由胚性珠心组织增殖而来,而柑橘二倍体加倍成四倍体后果实种子数减少[10],且种子的珠心胚数减少(数据未发表),可能是四倍体胚性愈伤组织诱导率及体细胞胚发生率均低于二倍体的原因。
笔者在本研究中采用3 种不同培养基对柑橘败育胚珠进行离体培养,培养基类型影响不同品种的愈伤组织诱导率。前人研究中表明柑橘MGS 培养基广泛适用于柑橘品种的胚性愈伤组织诱导[18,25],本研究中同样发现MGS 培养基诱导7 个柑橘品种的胚性愈伤组织诱导率均为最高。除温州蜜柑外,利用MK 培养基不经过胚状体发生途径直接诱导出胚性愈伤组织的诱导率高于其他两种培养基,并且愈伤组织状态更为松软,颜色偏白,可能0.5 mg·L-1 KT 更有利于诱导柑橘形成疏松的胚性愈伤组织。对于3个温州蜜柑品种,MK培养基诱导的愈伤组织结构较为致密、颜色偏黄,而MGS 培养基诱导的愈伤组织质地疏松、颜色偏白、增殖旺盛。前人研究发现温州蜜柑愈伤组织生长状态与植物生长调节剂有密切关系,添加GA3和麦芽提取物(ME)有利于温州蜜柑愈伤组织生长[26],本研究在添加了GA3和ME的MGS 培养基中诱导出的温州蜜柑的愈伤组织的状态良好,与前人报道结果相似。
柑橘无核品种由于长期嫁接繁殖累积多种病害。无核品种没有种子,不能利用珠心胚实生苗脱毒,而茎尖微芽嫁接操作要求高,脱毒可能不完全[27]。笔者在本研究中通过诱导胚性愈伤组织并再生国庆1 号温州蜜柑、伦晚脐橙和早红脐橙植株均未检测出果园最常见的衰退病和黄脉病病毒,说明胚性愈伤组织通过体细胞胚发生途径再生植株能达到无毒的效果,为无核品种提纯复壮的无毒种苗。早红脐橙是由罗伯逊脐橙和温州蜜柑构成的周缘嵌合体,其L1层来源于温州蜜柑(果肉),L2/L3层来源于罗伯逊脐橙(果皮和生殖细胞)[23]。根据“原套-原体”学说,果实的生殖细胞由L2层细胞衍生而来,可遗传给后代;早红脐橙愈伤组织再生苗由多胚的珠心组织发育而来,属于L2 层,因此其再生植株应为罗伯逊脐橙。InDel 标记检测证明早红脐橙胚性愈伤组织再生植株确为罗伯逊脐橙。
4 结 论
笔者在本研究中通过离体培养柑橘成熟果实的败育胚珠,经诱导和驯化获得3 个品种的二倍体/四倍体的胚性愈伤组织,为柑橘多倍体研究提供了稳定易获得的离体材料;二倍体的胚性愈伤组织诱导率和胚状体发生率均高于对应品种的四倍体。利用体细胞胚发生诱导获得5个多胚无核品种的愈伤组织再生苗,经鉴定不携带果园常见的黄脉病和衰退病,为品种提纯复壮提供种苗。嫁接嵌合体早红脐橙的愈伤组织再生苗经过InDel 标记鉴定为罗伯逊脐橙。
[1] 郭文武,叶俊丽,邓秀新.新中国果树科学研究70 年:柑橘[J].果树学报,2019,36(10):1264-1272.GUO Wenwu,YE Junli,DENG Xiuxin.Fruit scientific research in New China in the past 70 years:Citrus[J].Journal of Fruit Science,2019,36(10):1264-1272.
[2] 邓秀新.中国柑橘育种60 年回顾与展望[J].园艺学报,2022,49(10):2063-2074.DENG Xiuxin.A review and perspective for citrus breeding in China during the last six decades[J].Acta Horticulturae Sinica,2022,49(10):2063-2074.
[3] GUO W W,XIE K D,WU X M,XIE Z Z,XU Q,DENG X X.Ploidy manipulation via cell engineering for citrus improvement facilitated by application of molecular markers[J].Acta Horticulturae,2018(1203):105-110.
[4] FENG M Q,LU M D,LONG J M,YIN Z P,JIANG N,WANG P B,LIU Y,GUO W W,WU X M.miR156 regulates somatic embryogenesis by modulating starch accumulation in citrus[J].Journal of Experimental Botany,2022,73(18):6170-6185.
[5] SUN Q,HE Z C,WEIR R,ZHANG Y,YE J L,CHAIL J,XIE Z Z,GUO W W,XU J,CHENG Y J,XU Q,DENG X X.The transcriptional regulatory module CsHB5-CsbZIP44 positively regulates abscisic acid-mediated carotenoid biosynthesis in citrus (Citrus spp.)[J].Plant Biotechnology Journal,2024,22(3):722-737.
[6] SONG X,DUAN Y Y,TAN F Q,REN J,CAO H X,XIE K D,WU X M,GUO W W.Comparative transcriptome analysis of salt tolerance of roots in diploid and autotetraploid citrus rootstock(C.junos cv.Ziyang Xiangcheng)and identification of salt tolerance-related genes[J].Scientia Horticulturae,2023,317:112083.
[7] TAN F Q,TU H,WANG R,WU X M,XIE K D,CHEN J J,ZHANG H Y,XU J,GUO W W.Metabolic adaptation following genome doubling in citrus doubled diploids revealed by nontargeted metabolomics[J].Metabolomics,2017,13(11):143.
[8] WEIT L,WANG Y,XIE Z Z,GUO D Y,CHEN C W,FAN Q J,DENG X D,LIU J H.Enhanced ROS scavenging and sugar accumulation contribute to drought tolerance of naturally occurring autotetraploids in Poncirus trifoliata[J].Plant Biotechnology Journal,2019,17(7):1394-1407.
[9] OUSTRIC J,HERBETTE S,QUILICHINIY,MORILLON R,GIANNETTINIJ,BERTIL,SANTINIJ.Tetraploid Citrumelo 4475 rootstocks improve diploid common clementine tolerance to long-term nutrient deficiency[J].Scientific Reports,2021,11(1):8902.
[10] TAN F Q,ZHANG M,XIE K D,FAN Y J,SONG X,WANG R,WU X M,ZHANG H Y,GUO W W.Polyploidy remodels fruit metabolism by modifying carbon source utilization and metabolic flux in Ponkan mandarin (Citrus reticulata Blanco)[J].Plant Science,2019,289:110276.
[11] ZHANG M,TAN F Q,FAN Y J,WANG T T,SONG X,XIE K D,WU X M,ZHANG F,DENG X X,GROSSER J W,GUO W W.Acetylome reprograming participates in the establishment of fruit metabolism during polyploidization in citrus[J].Plant Physiology,2022,190(4):2519-2538.
[12] 邓秀新,王力荣,李绍华,张绍铃,张志宏,丛佩华,易干军,陈学森,陈厚彬,钟彩虹.果树育种40 年回顾与展望[J].果树学报,2019,36(4):514-520.DENG Xiuxin,WANG Lirong,LIShaohua,ZHANG Shaoling,ZHANG Zhihong,CONG Peihua,YIGanjun,CHEN Xuesen,CHEN Houbin,ZHONG Caihong.Retrospection and prospect of fruit breeding for last four decades in China[J].Journal of Fruit Science,2019,36(4):514-520.
[13] CARDOSO J C,CURTOLO M,LATADO R R,MARTINELLIA P.Somatic embryogenesis of a seedless sweet orange [Citrus sinensis (L.) Osbeck][J].In Vitro Cellular & Developmental Biology-Plant,2017,53(6):619-623.
[14] 谢善鹏,解凯东,夏强明,周锐,张成磊,郑浩,伍小萌,郭文武.柑橘6 个地方品种资源四倍体高效发掘及分子鉴定[J].果树学报,2022,39(1):1-9.XIE Shanpeng,XIE Kaidong,XIA Qiangming,ZHOU Rui,ZHANG Chenglei,ZHENG Hao,WU Xiaomeng,GUO Wenwu.Efficient exploration and SSR identification of 53 doubled diploid seedlings from six local citrus cultivars and germplasm resources[J].Journal of Fruit Science,2022,39(1):1-9.
[15] 陈昊,谢善鹏,解凯东,肖公傲,周锐,伍小萌,吴群,邓家锐,敖义俊,刘高平,郭文武.柑橘13 个多胚品种同源四倍体高效发掘与分子鉴定[J].果树学报,2023,40(11):2297-2306.CHEN Hao,XIE Shanpeng,XIE Kaidong,XIAO Gongao,ZHOU Rui,WU Xiaomeng,WU Qun,DENG Jiarui,AO Yijun,LIU Gaoping,GUO Wenwu.Efficient exploration and SSR identification of autotetraploids from the seedlings of thirteen apomictic Citrus genotypes[J].Journal of Fruit Science,2023,40(11):2297-2306.
[16] GUO W W,LIANG W J,XIE K D,XIA Q M,FU J,GUO D Y,XIE Z Z,WU X M,XU Q,YIH L,DENG X X.Exploitation of polyploids from 39 citrus seedling populations[J].Acta Horticulturae,2016(1135):11-16.
[17] 解凯东,彭珺,袁东亚,强瑞瑞,谢善鹏,周锐,夏强明,伍小萌,柯甫志,刘高平,GROSSER J W,郭文武.以本地早橘和槾橘为母本倍性杂交创制柑橘三倍体[J].中国农业科学,2020,53(23):4961-4968.XIE Kaidong,PENG Jun,YUAN Dongya,QIANG Ruirui,XIE Shanpeng,ZHOU Rui,XIA Qiangming,WU Xiaomeng,KE Fuzhi,LIU Gaoping,GROSSER J W,GUO Wenwu.Production of Citrus triploids based on interploidy crossing with bendizao and man tangerines as female parents[J].Scientia Agricultura Sinica,2020,53(23):4961-4968.
[18] 朱世平.柑橘胚性愈伤组织诱导和LEAFY COTYLEDON 1-LIKE(CsL1L)基因的克隆和分析[D].武汉:华中农业大学,2009.ZHU Shiping.Embryogenic callus induction and the cloning and analysis of LEAFY COTYLEDON 1-LIKE (CsL1L) gene in citrus[D].Wuhan:Huazhong Agricultural University,2009.
[19] 杨雯惠,解凯东,郭文武.柑橘组织培养常用培养基配制方法[DB].(2018- 07- 10).Bio- 101:e1010184.DOI:10.21769/BioProtoc.1010184.YANG Wenhui,XIE Kaidong,GUO Wenwu.Medium preparation for citrus tissue culture[DB].(2018- 07- 10).Bio- 101:e1010184.DOI:10.21769/BioProtoc.1010184.
[20] 徐远涛.柑橘无融合生殖和嫁接嵌合的分子基础研究[D].武汉:华中农业大学,2019.XU Yuantao.Molecular basis of apomixis and grafting chimerism in citrus[D].Wuhan:Huazhong Agricultural University,2019.
[21] SAMBADE A,LÓPEZ C,RUBIO L,FLORES R,GUERRIJ,MORENO P.Polymorphism of a specific region in gene p23 of citrus tristeza virus allows discrimination between mild and severe isolates[J].Archives of Virology,2003,148(12):2325-2340.
[22] CHEN H M,LIZ A,WANG X F,ZHOU Y,TANG K Z,ZHOU C Y,ZHAO X Y,YUE J Q.First Report of citrus yellow vein clearing virus on lemon in Yunnan,China[J].Plant Disease,2014,98(12):1747.
[23] 张敏.柑橘果实扇形嵌合体的分离及两组嫁接嵌合体的遗传研究[D].武汉:华中农业大学,2006.ZHANG Min.Separation of citrus fruit sector chimeras and genetic analysis of two graft chimeras[D].Wuhan:Huazhong Agricultural University,2006.
[24] GAO L L,LIU J J,LIAO L,GAO A Q,NJUGUNA B N,ZHAO C P,ZHENG B B,HAN Y P.Callus induction and adventitious root regeneration of cotyledon explants in peach trees[J].Horticulturae,2023,9(8):850.
[25] 霍合强,郝玉金,邓秀新.宽皮柑橘品种的胚性愈伤组织诱导[J].实验生物学报,1999,32(3):289-295.HUO Heqiang,HAO Yujin,DENG Xiuxin.Induction of embryogenic callus of loose skin mandarins[J].Acta Biologiae Experimentalis Sinica,1999,32(3):289-295.
[26] LING J T,NITO N,IWAMASA M,KUNITAKE H.Plant regeneration from protoplasts isolated from embryogenic callus of Satsuma[J].HortScience,1990,25(8):970-972.
[27] 祁鹏志.柑橘茎尖微芽嫁接脱毒及黄龙病和衰退病分子检测的研究[D].武汉:华中农业大学,2007.QIPengzhi.Studies on elimination of pathogen of citrus huanglongbing and citrus tristeza virus with shoot-tip grafting and molecular determination[D].Wuhan:Huazhong Agricultural University,2007.