Ca2+/H+反向转运体(CAXs)是一类低亲合、高容量的转运蛋白,即使胞质中Ca2+水平较低时也可以发挥其生理功能。近年来,在拟南芥、水稻、葡萄等植物中已克隆出多个CAX 基因,这些基因编码的蛋白不仅可以运输Ca2+,还能运输Mn2+[1-2]。将拟南芥的AtCAX4基因超表达载体转入到番茄中,获得转基因番茄苗,其中转基因植株对钙离子的吸收增强,植株体内的钙元素含量增加,甚至延长果实的货架期[3]。
研究CAXs的蛋白结构发现,几乎所有的Ca2+/H+反向转运蛋白都有着相似的结构(图1),400 aa左右的氨基酸,均含有11 个跨膜区域(TMD),都存在着一个Ca2+的结合区(CaD),以此调节CAXs蛋白转运钙离子的能力[4]。Ca2+/H+反向转运蛋白的N 端有亲水性自抑制区(NRR),N末端自抑制区存在于细胞质中,该段序列可直接影响CAX蛋白的活性[5]。利用酵母突变体K667 菌株进行功能互补实验时发现,Ca2+的转运受CAXs蛋白N-末端自抑制区的调控,全长的CAXs 基因编码的蛋白无法有效地转运Ca2+[6-7]。CAXs 的这种转运特性存在于大多数植物的Ca2+/H+反向转运蛋白中,例如拟南芥AtCAX1、AtCAX2、AtCAX3、AtCAX4[8],棉花GhCAX1、GhCAX3[9],水稻OsCAX1、OsCAX3和OsCAX4[10]。
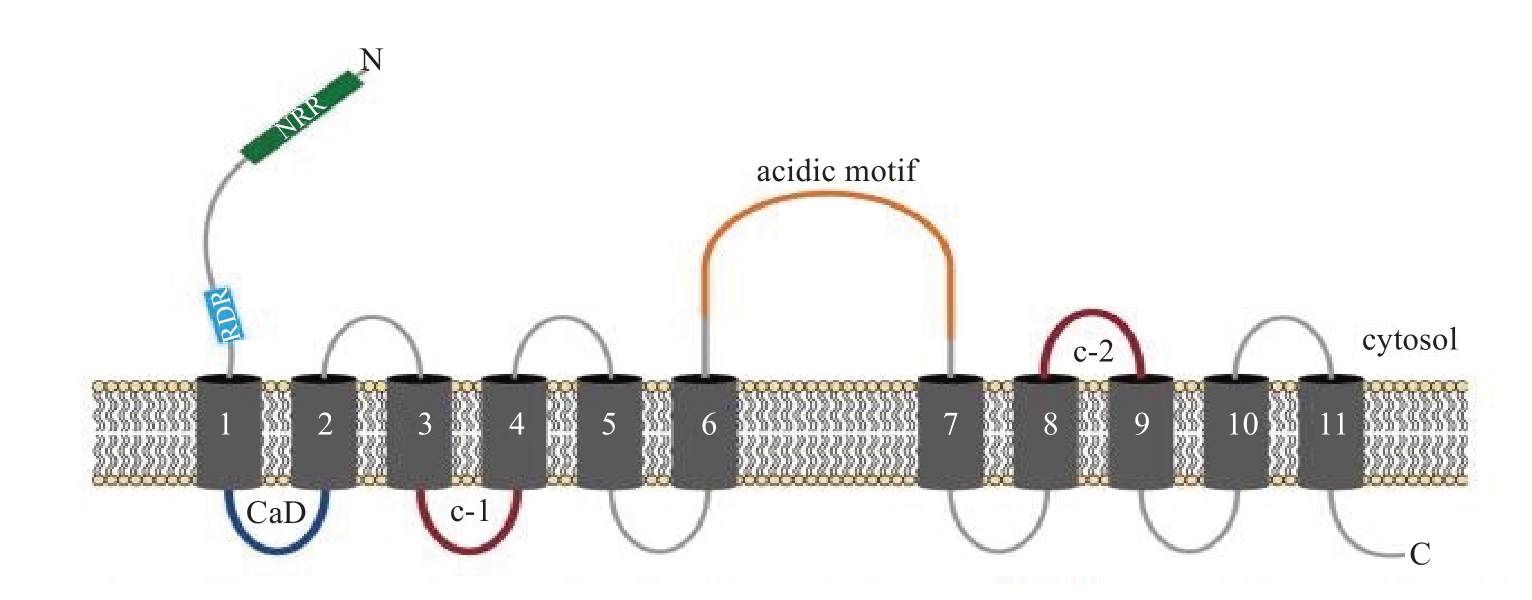
图1 CAXs 保守功能域的拓扑结构
Fig.1 Proposed topological model of CAXs displaying conserved features
蛋白的调控机制在不同植物品种及不同的成员之间不尽相同。例如,绿豆中去NRR 的sVCAX1 可以弥补K667 酵母菌株转运Ca2+的缺陷,同时转化全长MdCAX2L-2 的K667 菌株也可以正常转运Ca2+[11]。相似的情况也多有报道,白菜全长BrCAX蛋白和苹果全长MdCAX5 蛋白在酵母菌株K667 中均可弥补突变株的钙转运缺陷[12-13]。研究去除N-末端自抑制区sPutCAX1 的基因功能时发现,与星星草的全长PutCAX1 基因相比,sPutCAX1 的钙转运能力明显下降[14]。同时也有文献表明,CAXs 的N-末端自抑制区在不同的植物体细胞中有各自不同的调节功能,但目前对这些相关调节机制尚不明了。通过对CAXs 蛋白多样性的研究可以发现,不同物种之间的CAXs蛋白存在着较大的差别。
在前期研究中发现sMdCAX11(去NRR 的Md-CAX11)也表现出较强的钙转运能力,与MdCAX5的基因功能相似[12]。同时sMdCAX11蛋白作为一价阳离子和二价阳离子的转运体,既可以转运Ca2+,也可以转运Na+。因此在本研究中将重点研究sMdCAX11的基因功能,通过观察过表达sMdCAX11 试验材料的表型及分析不同组织的矿质元素含量,研究sMd-CAX11蛋白在植物体内所起到的关键作用。
1 材料和方法
1.1 试验材料
用于瞬时转化的蜜脆苹果果实采收自陕西省宝鸡市西北农林科技大学千阳试验站,选取无病害、机械损伤的苹果,样品采集后迅速带回实验室,1 ℃贮藏冷库存放。用于瞬时转化的本氏烟草放置于光照培养箱进行培养(培养条件:22 ℃/20 ℃,16 h 光照/8 h黑暗),培养至6~8枚叶时用于试验。用于稳定转化的拟南芥为Col-0生态型,光照培养条件为16 h光照(22 ℃)和8 h 黑暗(20 ℃)。
克隆载体pMD19-T Simple vector 购自TaKaRa公司,植物表达载体pVBG2307、pC0390GUS等均由实验室保存。大肠杆菌E.coli DH5α 购自天根公司,农杆菌菌株GV3101 感受态购自上海唯地生物有限公司。
1.2 苹果的瞬时转化
克隆sMdCAX11 基因CDS 序列(去掉终止密码子),将得到的片段插入到融合载体GFP 蛋白的N端,得到新的融合载体35S::sMdCAX11-GFP。将获得的融合载体通过冻融法转入农杆菌GV3101 感受态细胞,获得阳性农杆菌。苹果瞬时转化的方法参考Jiang等[15]方法进行,在果实瞬时转化的第3天、第5天、第9天采样并液氮速冻后保存于-80 ℃冰箱。
1.3 拟南芥的稳定转化方法
利用方法1.2 中获得的含有35S::sMdCAX11-GFP 的农杆菌用于拟南芥的稳定转化,采用浸花序法获得阳性拟南芥植株。
1.4 总Ca、Mg、K、N和P含量的测定
1.4.1 总Ca、Mg和K含量的测定 称取3.00 g果肉冻样置于70 ℃烘箱中烘至恒质量,称取1.00 g 烘干样品并放置于100 mL 消解管中,同时加入3 mL 高氯酸和12 mL硝酸,浸泡过夜后进行高温消解,对消解样品赶酸、定容后稀释一定倍数,利用原子吸收光谱仪(ZA3000)测定样品的总Ca、K和Mg含量。
1.4.2 总N 和P 含量的测定 称取0.20 g 烘干样品与8 mL 硫酸混合后放入100 mL 消解管中浸泡过夜,经高温消解、赶酸、定容、稀释后利用连续流动化学分析仪测定总N、P含量。
总矿质元素含量以干质量表示,每项测定均包括3次生物学重复。
1.5 水溶性Ca、Mg、K、N和P含量的测定
水溶性矿质元素的测定方法参照Pavicic等[16]的报道并有所改动。称取6 g冻样置于研钵中,加20 mL去离子水充分研磨,将研磨后的匀浆10 000 r·min-1离心30 min。收集上清液,将离心管的沉淀用20 mL去离子水重悬后,如上所述再次离心。收集两次离心后的上清液经多次滤纸过滤后定容到50 mL,稀释至一定倍数后利用原子吸收光谱仪测定水溶性Ca、Mg 和K 含量,利用连续流动化学分析仪测定水溶性N 和P含量。水溶性矿质养分含量以鲜质量表示。每项测定均包括3次生物学重复。
1.6 ProCAX11 启动子的克隆及顺式作用元件分析
采用植物基因组DNA 提取试剂盒(AG21011)提取植物总DNA。以MdCAX11 全长在苹果基因组数据库中比对,获得起始密码子上游1500 bp左右的核苷酸序列。随后设计引物,以蜜脆叶片DNA为模板,克隆MdCAX11 基因启动子序列。利用Plant CARE和PLACE在线网站预测ProCAX11启动子存在的转录因子结合位点及顺式作用元件。
1.7 GUS染色方法及蛋白活性分析
克隆MdCAX11 基因启动子ProCAX11 序列,将得到的片段插入到载体pC0390GUS,得到新的融合载体ProCAX11::GUS,对照为pC0390GUS空载。将获得的融合载体采用冻融法转入农杆菌GV3101 感受态细胞。PCR 鉴定阳性的菌液瞬时侵染本氏烟草。GUS 染色、GUS 粗蛋白提取及浓度测定、GUS蛋白荧光值测定等方法参照Chen等[17]的报道。
1.8 ProCAX11 启动子对不同浓度CaCl2的响应分析
为了探究ProCAX11 启动子对CaCl2的响应效果,配制浓度为0、10、20 和40 mmol·L-1的CaCl2溶液。在侵染前24 h 时,对本氏烟草植株喷洒不同浓度的CaCl2溶液,叶片的正反面均匀喷洒,直至叶片两面均被打湿且不断滴水为止,然后放回原来的培养条件下继续培养。利用转化有融合载体Pro-CAX5::GUS 的农杆菌侵染烟草叶片。侵染48 h 后对侵染的烟草叶片进行GUS 染色及GUS 蛋白活性分析。
2 结果与分析
2.1 sMdCAX11 瞬时过表达在苹果果实的表型鉴定及元素分析
利用瞬时转化蜜脆苹果果实的方法,来验证sMdCAX11 蛋白的钙转运功能。对瞬时转化sMd‐CAX11 基因的果实进行切片,观察注射孔附近的果肉组织,发现在侵染第9 天时果肉组织明显变褐,果肉组织皱缩,与对照组相比差异显著(图2-A)。基因相对表达量分析检测侵染第9 天时果肉的sMd‐CAX11 基因表达量显著上调,这也直接说明了瞬时转化试验效果良好,可以用于进一步的分析检测(图2-B)。
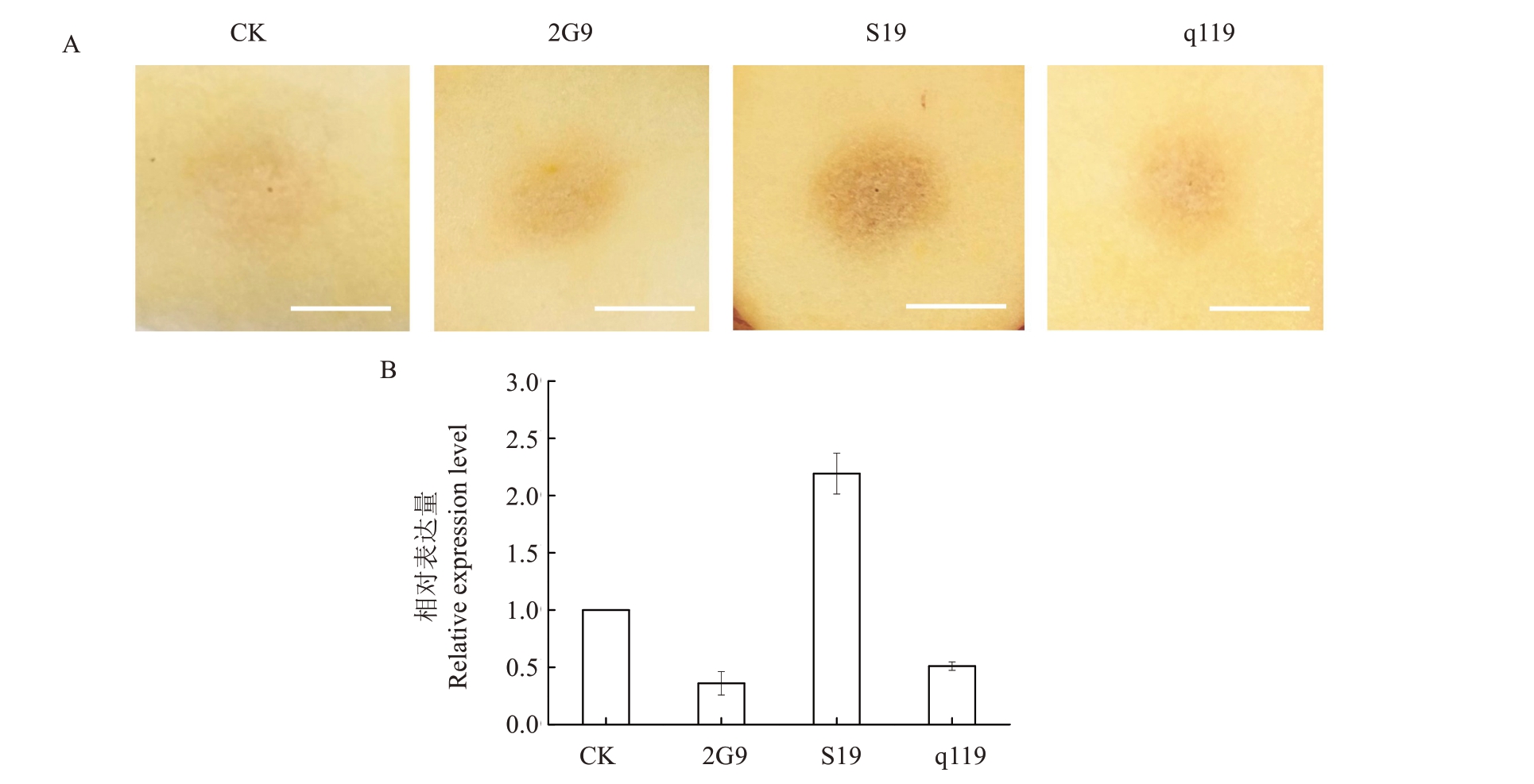
图2 瞬时过表达sMdCAX11 在苹果果实中的表型鉴定
Fig.2 Phenotype identification of transiently overexpressed sMdCAX11 in apple fruits
A.贮藏9 d 后的苹果注射孔附近果肉的表型变化;B.贮藏9 d 后的苹果果肉sMdCAX11 基因相对表达量。CK 为注射无菌侵染液;2G9 为注射空载农杆菌;S19 为注射携带35S::sMdCAX11 农杆菌;q119 为注射携带35S::MdCAX11 农杆菌。比例尺=1 cm。
A.The phenotypic changes of the apple flesh near the injection hole after 9 days of storage;B.The relative expression level of sMdCAX11 gene in the apple flesh after 9 days of storage.CK for injection sterile infection solution; 2G9 for injection of empty vector; S19 is injection carrying 35S::sMdCAX11 Agrobacterium;q119 is injection carrying 35S::MdCAX11 Agrobacterium.Scale bar=1 cm.
2.2 sMdCAX11 瞬时过表达在苹果果实的元素分析
分析瞬时转化sMdCAX11 基因的果肉组织总矿质元素及水溶性矿质元素的含量,对照组为瞬时转化空载的果肉组织,结果发现过表达sMdCAX11 基因的果肉组织总钙含量显著下降,且随着贮藏时间的延长而不断降低(图3-A)。在侵染后第3天时,水溶性Ca含量与对照组相比出现了短暂的上升,但随着贮藏时间的延长而显著下降(图3-B)。分析果肉组织的总Mg、K 含量,这两种元素在侵染后第3 天时出现短暂降低之后迅速升高,在第5 天时达到最高值。而水溶性Mg、K 含量则与总Mg、K 含量的变化趋势相反(图3)。进一步分析果肉组织的元素比值发现,总矿质元素(K+Mg)/Ca 比值在瞬时转化sMdCAX11 的果肉中显著增加,且随着贮藏时间的延长而不断升高。在侵染第5 天时,水溶性矿质元素的(K+Mg)/Ca比值在瞬时转化sMdCAX11基因的果肉组织中明显升高,同时对照组的元素比值无显著变化(图3)。
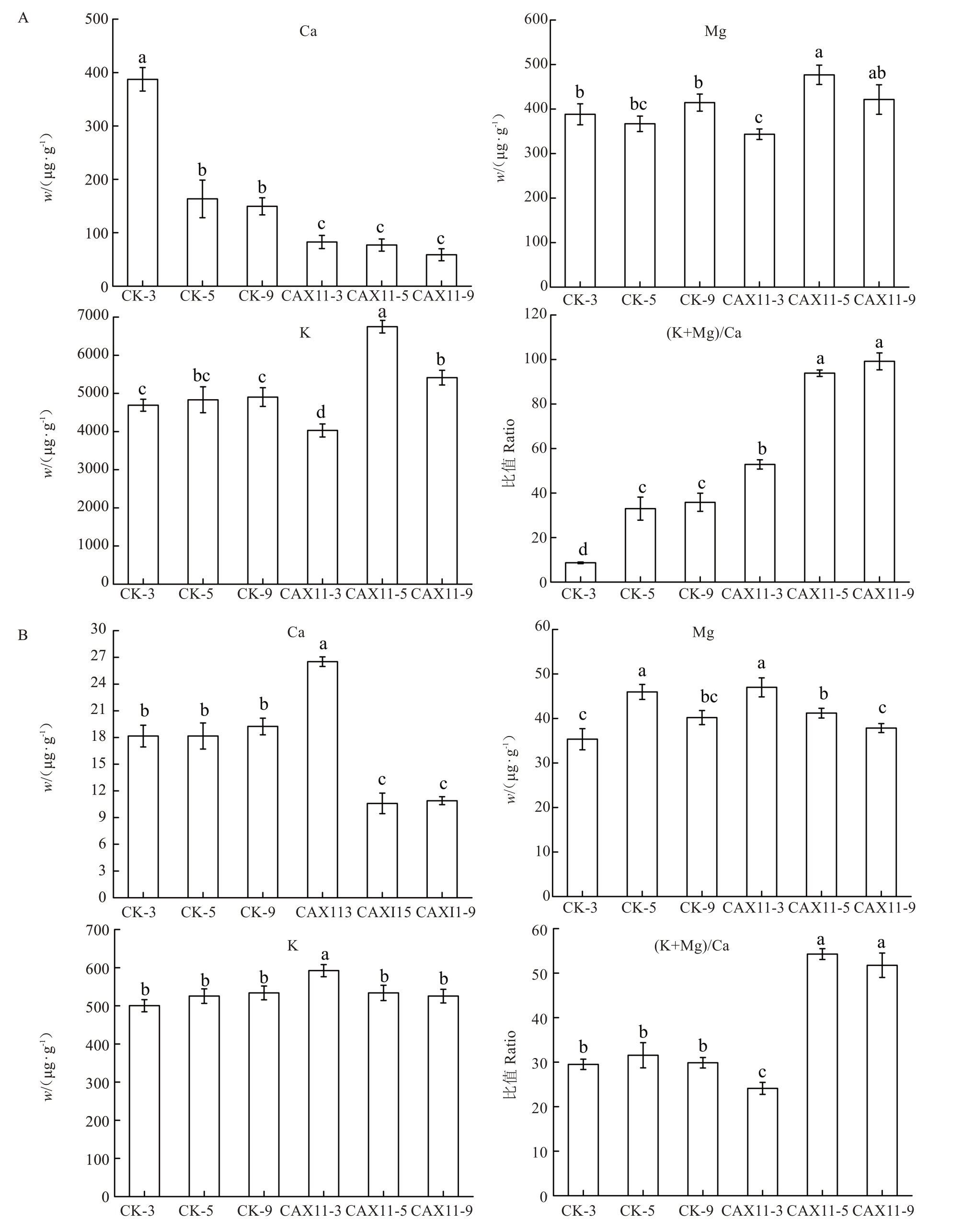
图3 瞬时过表达sMdCAX11 在苹果果实中的元素分析
Fig.3 Element analysis of transiently overexpressed sMdCAX11 in apple fruits
A.总矿质元素含量;B.水溶性矿质元素含量。CK-3、5、9 分别为注射空载农杆菌的果实贮藏3 d、5 d 和9 d,CAX11-3、5、9 分别为瞬时转化sMdCAX11 的果实贮藏3 d、5 d、9 d。
A.Total mineral element content; B.Water-soluble mineral element content.CK-3, 5, 9 are stored for 3 d, 5 d, and 9 d after injection of unloaded Agrobacterium,and CAX11-3,5,and 9 are the apple overexpressed sMdCAX11 storage 3 d,5 d,9 d,respectively.
2.3 过表达sMdCAX11拟南芥的元素分析
为进一步验证sMdCAX11 参与钙转运的功能,将sMdCAX11稳定转化拟南芥Col-0生态型,并成功获得阳性T4代转基因植株。通过DNA 水平及RNA水平的PCR 检验,验证所获得的均为阳性植株,并开展后续试验。对拟南芥叶片进行矿质元素检测分析,结果发现4 个过表达sMdCAX11 拟南芥株系的叶片总Ca 含量与水溶性Ca 含量均出现显著下降,差异极显著(图4)。同时4 个株系的阳性样本间差异不显著,说明转基因植株间的表型稳定,不存在特异性。分析4 个转基因拟南芥株系叶片的总Mg 与水溶性Mg 含量,发现与野生型对照组相比差异不显著(图4)。然而4 个转基因拟南芥株系的总K 含量与水溶性K 含量均高于野生型且差异显著,但在4 个株系间差异不显著(图4)。与瞬时转化sMd‐CAX11的苹果果肉材料相一致的是元素的(K+Mg)/Ca 比值,总元素的比值与水溶性元素的比值在阳性植株中均出现明显增大,且差异显著(图4)。
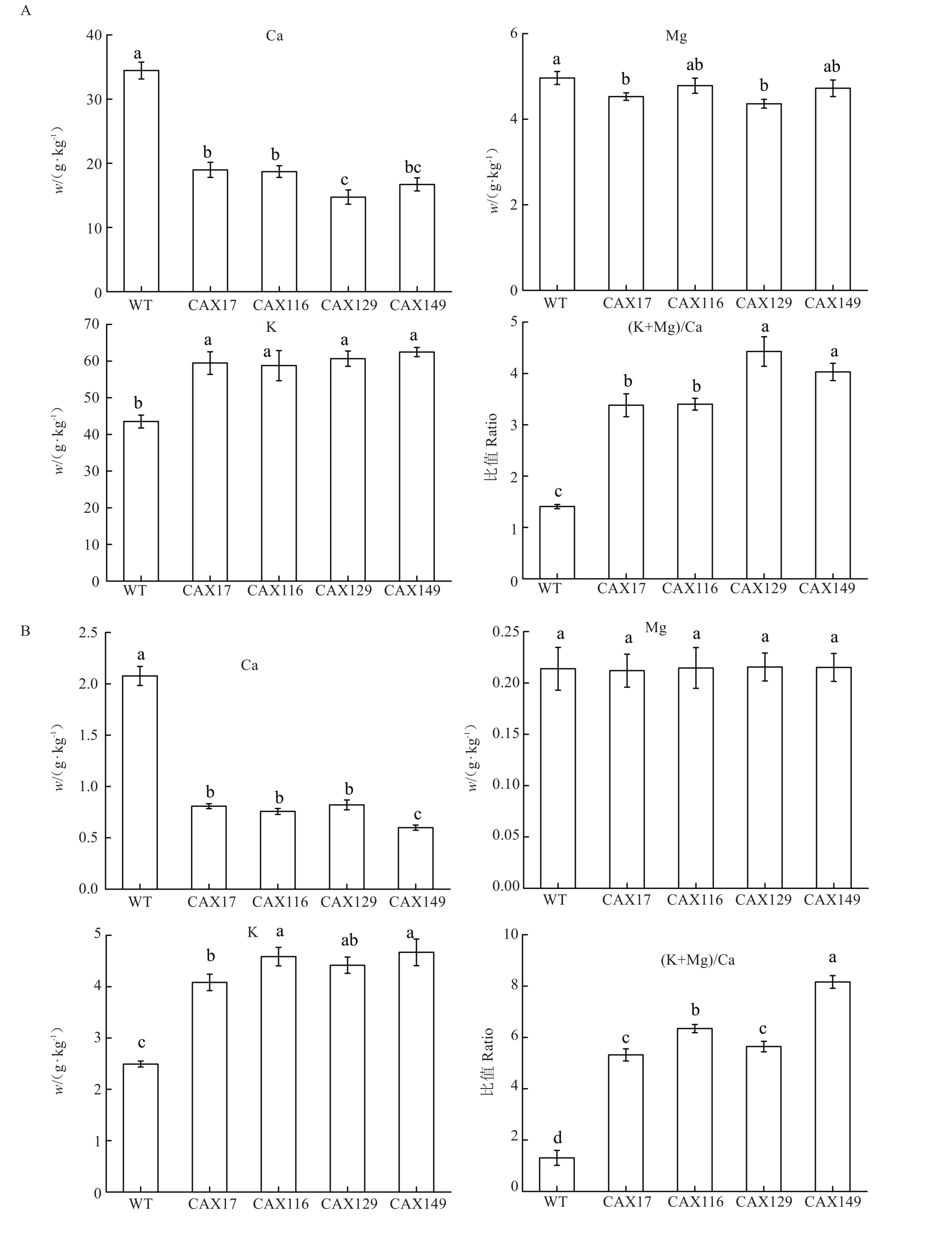
图4 稳定过表达sMdCAX11 的拟南芥叶片元素分析
Fig.4 Elemental analysis of Arabidopsis thaliana leaves overexpressing sMdCAX11
A.总矿质元素含量;B.水溶性矿质元素含量。WT.野生型拟南芥;CAX17~149.阳性植株的不同株系。
A.Total mineral element content;B.Water-soluble mineral element content.WT.Wild-type Arabidopsis thaliana;CAX17-149.The different lines of positive plants.
2.4 ProCAX11启动子顺式作用元件分析
利用Plant CARE 等在线网站,对苹果Md‐CAX11 基因起始密码子上游1500 bp 启动子区域所包含的各类元件进行预测分析(表1)。结果表明,该基因启动子区存在大量响应外界环境条件的顺式作用元件,如参与厌氧诱导的元件ARE,以及参与光响应的元件ATCT-motif、Box 4、G-box、GT1-motif、TCCC-motif和chs-CMA2a(表1)。该基因的启动子也存在参与激素应答的调控元件,如参与脱落酸的ABRE(表1)。另外,ProCAX11启动子区域还存在着多个转录因子结合位点,如WRKY 转录因子结合位点WBOXNTERF3、WBOXATNPR1 和WRKY710S,以及MYB等转录因子的结合位点(表1)。
表1 ProCAX11 启动子顺式作用元件与功能分析
Table 1 Functional analysis of cis-elements of ProCAX11 promoter
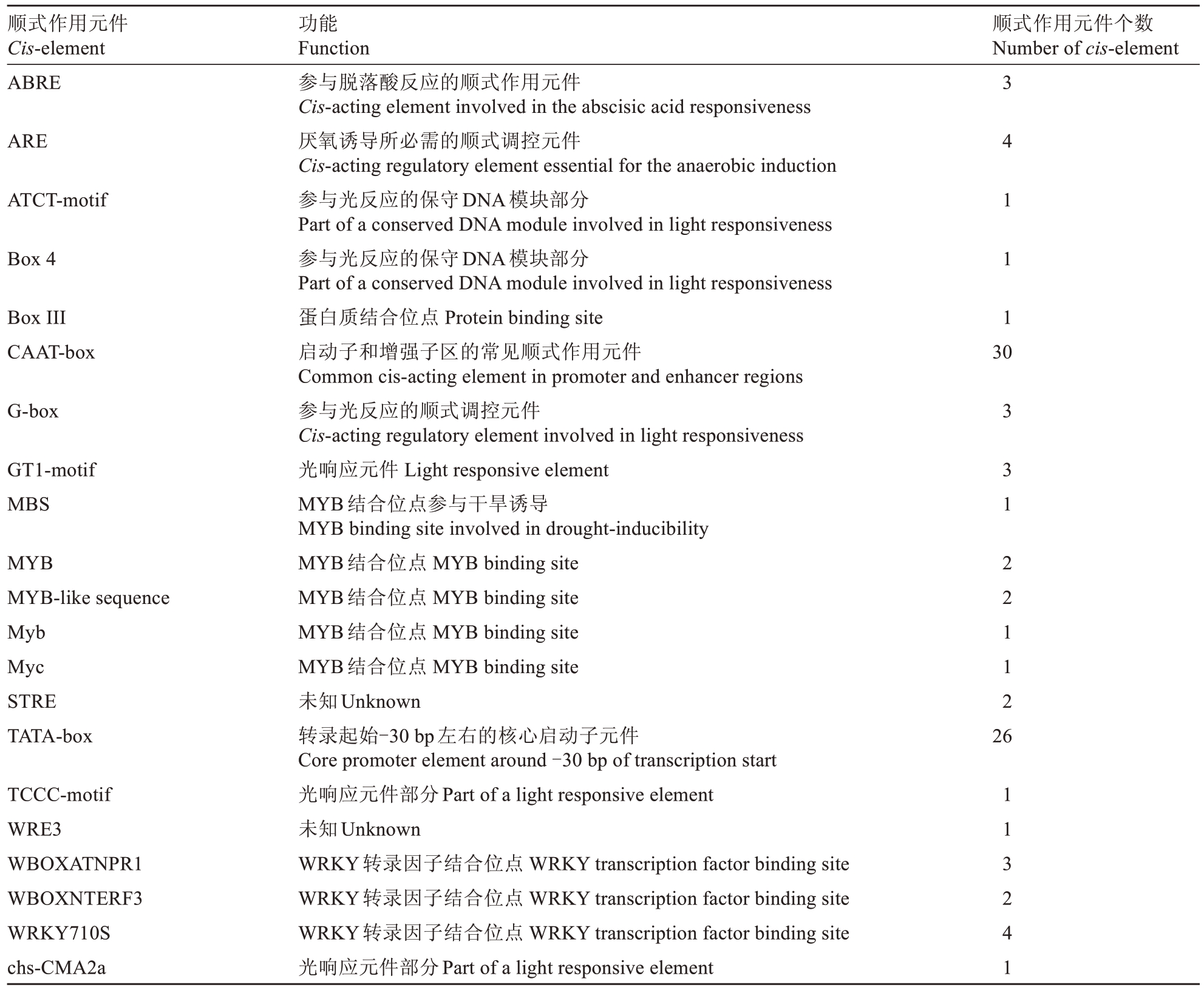
顺式作用元件Cis-element ABRE顺式作用元件个数Number of cis-element ARE ATCT-motif Box 4 Box IIICAAT-box 3 4 1 1 1 30 G-box GT1-motif MBS MYB MYB-like sequence Myb Myc STRE TATA-box 3 3 1 2 2 1 1 2 26 TCCC-motif WRE3 WBOXATNPR1 WBOXNTERF3 WRKY710S chs-CMA2a功能Function参与脱落酸反应的顺式作用元件Cis-acting element involved in the abscisic acid responsiveness厌氧诱导所必需的顺式调控元件Cis-acting regulatory element essential for the anaerobic induction参与光反应的保守DNA模块部分Part of a conserved DNA module involved in light responsiveness参与光反应的保守DNA模块部分Part of a conserved DNA module involved in light responsiveness蛋白质结合位点Protein binding site启动子和增强子区的常见顺式作用元件Common cis-acting element in promoter and enhancer regions参与光反应的顺式调控元件Cis-acting regulatory element involved in light responsiveness光响应元件Light responsive element MYB结合位点参与干旱诱导MYB binding site involved in drought-inducibility MYB结合位点MYB binding site MYB结合位点MYB binding site MYB结合位点MYB binding site MYB结合位点MYB binding site未知Unknown转录起始-30 bp左右的核心启动子元件Core promoter element around-30 bp of transcription start光响应元件部分Part of a light responsive element未知Unknown WRKY转录因子结合位点WRKY transcription factor binding site WRKY转录因子结合位点WRKY transcription factor binding site WRKY转录因子结合位点WRKY transcription factor binding site光响应元件部分Part of a light responsive element 1 1 3 2 4 1
2.5 ProCAX11启动子转录活性及钙元素响应分析
为探究ProCAX11 启动子对CaCl2的响应效果,利用转化融合载体ProCAX11::GUS 的农杆菌侵染喷洒过不同浓度(0、10、20、40 mmol·L-1)CaCl2的烟草叶片,并采用GUS染色及GUS蛋白活性分析的方法来验证钙离子对MdCAX11 基因启动子转录活性的影响(图5)。GUS 染色发现喷洒10 mmol·L-1与20 mmol·L-1 CaCl2的烟草叶片颜色相比于其他组明显更深,CK 为注射空载农杆菌烟草叶片(图5-A)。通过分析烟草叶片的GUS 蛋白活性,发现在高钙的环境下MdCAX11 启动效果明显增强。在喷洒不同浓度的CaCl2时,GUS 蛋白活性显著提高,与对照组相比差异显著。同时在喷洒20 mmol·L-1 CaCl2时,GUS 蛋白活性达到最高值(图5-B)。ProCAX11 启动子转录活性受Ca2+的显著影响。然而,随着钙离子浓度的增加,GUS 蛋白的活性并没有随之增高(图5)。
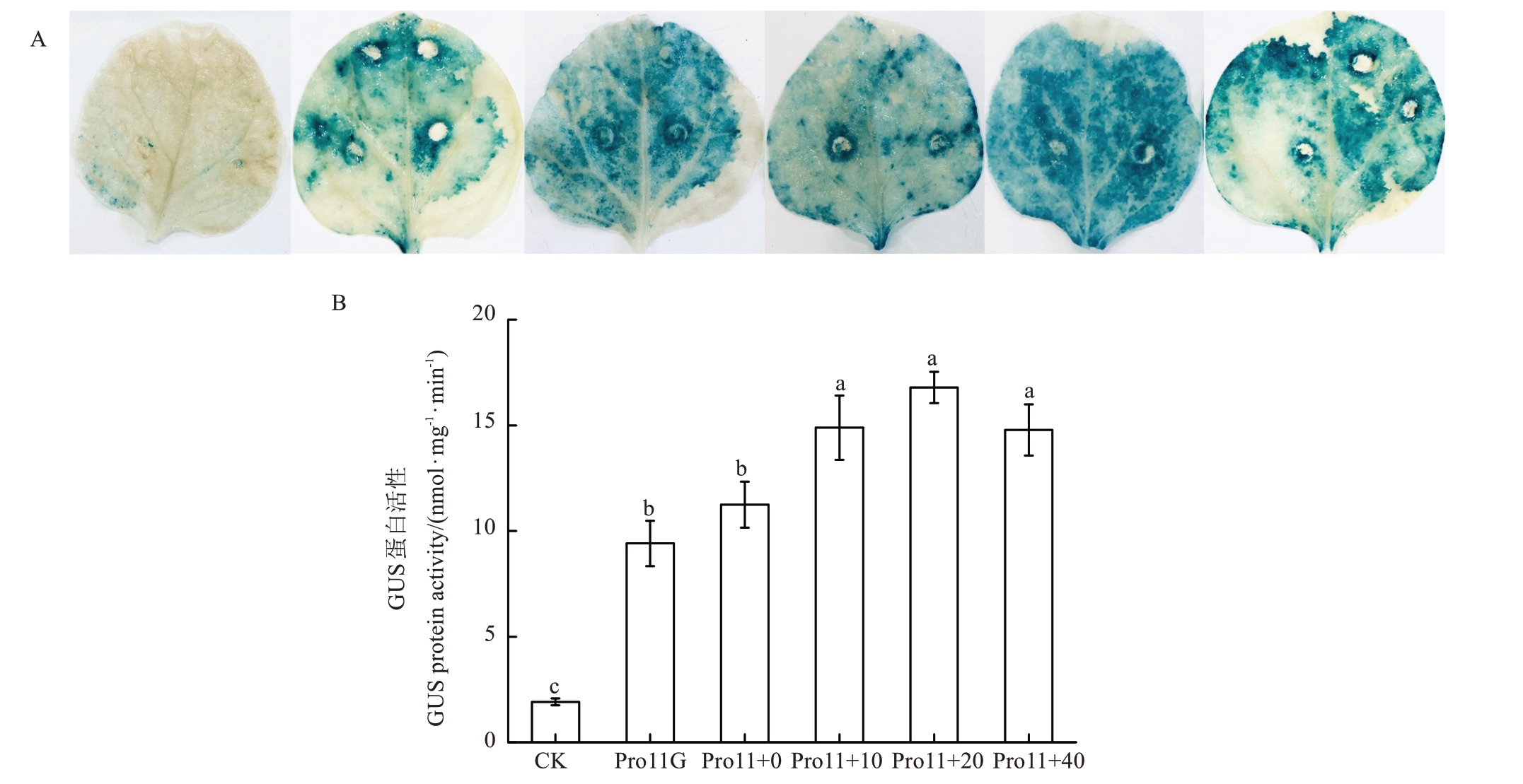
图5 ProCAX11 启动子转录活性及对不同浓度CaCl2的响应分析
Fig.5 ProCAX11 promoter transcriptional activity analysis and response analysis to different concentrations of CaCl2
A.GUS 染色;B.GUS 蛋白活性分析。CK 为注射空载农杆菌烟草叶片;Pro11G.ProCAX11-GUS;0~40 分别代表0、10、20、40 mmol·L-1 的CaCl2。
A.GUS staining; B.GUS protein activity analysis.CK.Inject empty vector tobacco leaves; Pro11G.ProCAX11-GUS; 0-40 represents 0, 10, 20,40 mmol·L-1 CaCl2.
3 讨 论
在前期研究中已经证实sMdCAX11(去NRR 的MdCAX11)可以表现出较强的钙转运能力[12]。同时sMdCAX11蛋白作为一价阳离子和二价阳离子的转运体,既可以转运Ca2+,也可以转运Na+。为验证sMdCAX11 蛋白的功能,笔者在本研究中分别利用了苹果果实和拟南芥材料,采用瞬时过表达和稳定过表达sMdCAX11 的试验手段,观察果肉组织的表型,并测定不同组织的矿质元素含量。
在白菜[13]、番茄[5]和土豆[18]中过表达液泡CAX转运蛋白,植株出现了类似缺钙的症状。瞬时过表达sMdCAX11 的苹果在侵染第9 天时注射孔附近的果肉颜色变褐,并出现皱缩,有明显的死细胞,而这一症状与苦痘病发病部位的果肉表型十分相像。分析瞬时转化sMdCAX11 基因的果肉组织总矿质元素及水溶性矿质元素的含量。过表达sMdCAX11 基因的果肉组织相比于对照组总钙含量显著下降。分析果肉组织的元素比值发现,总矿质元素及水溶性矿质元素(K+Mg)/Ca比值在瞬时转化sMdCAX11的果肉中显著增大,且随着贮藏时间的延长而不断升高。这一结果与苦痘病果实中不同矿质元素的分布及比例极其相似。苦痘病果实中的水溶性Ca 含量显著低于健康果实,且(K+Mg)/Ca 比值显著高于对照果实[19-20]。
稳定过表达sMdCAX11 的拟南芥叶片在矿质元素含量及比值的检测结果上与苹果果实相一致,总Ca 含量与水溶性Ca 含量在阳性植株的叶片中明显下降,且(K+Mg)/Ca 比值在阳性植株中显著升高。这一结果也证实了sMdCAX11 基因过表达可导致植株组织的元素分配不均,不同元素间的比例失衡的结论。但这一结果与先前研究报道并不完全相符[21]。这可能是因为笔者在本研究中对果实的Ca含量检测时并未对细胞膜、细胞质等分别进行检测,所以Ca含量与先前研究不一致。
在先前的研究报道中也表明WRKY 转录因子可能参与了苦痘病的发生与发展[18,22]。对苹果Md‐CAX11 基因启动子区域进行预测分析,发现基因启动子区存在着WRKY 转录因子结合位点WBOXNTERF3、WBOXATNPR1 和WRKY71OS。因此在下一步的工作中将开展WRKY 转录因子与MdCAX11基因启动子的互作分析,以期明确WRKY 转录因子与苦痘病发生的相关性。
4 结 论
MdCAX11 基因的钙转运能力受N 末端自抑制区的影响,sMdCAX11 基因的过表达显著降低植物体内钙含量,打破了矿质元素比例的平衡。
[1] HE F,SHIY J,LIJ L,LIN T T,ZHAO K J,CHEN L H,MIJ X,ZHANG F,ZHONG Y,LU M M,NIU M X,FENG C H,DING S S,PENG M Y,HUANG J L,YANG H B,WAN X Q.Genome-wide analysis and expression profiling of Cation/H+exchanger (CAX) family genes reveal likely functions in cadmium stress responses in poplar[J].International Journal of Biological Macromolecules,2022,204:76-88.
[2] KAMIYA T,AKAHORIT,MAESHIMA M.Expression profile of the genes for rice cation/H+ exchanger family and functional analysis in yeast[J].Plant and Cell Physiology,2005,46(10):1735-1740.
[3] CHENG N H,PITTMAN J K,SHIGAKIT,HIRSCHIK D.Characterization of CAX4,an Arabidopsis H+/cation antiporter[J].Plant Physiology,2002,128(4):1245-1254.
[4] ZOU W L,CHEN J G,MENG L J,CHEN D D,HE H H,YE G Y.The rice cation/H+ exchanger family involved in Cd tolerance and transport[J].International Journal of Molecular Sciences,2021,22(15):8186.
[5] HAN B B,TAIY X,LIS P,SHIJ M,WU X Q,KAKESHPOUR T,WENG J F,CHENG X G,PARK S,WU Q Y.Redefining the N-terminal regulatory region of the Ca2+/H+ antiporter CAX1 in tomato[J].Frontiers in Plant Science,2022,13:938839.
[6] SHIGAKIT,MEIH,MARSHALL J,LIX,MANOHAR M,HIRSCHIK D.The expression of the open reading frame of Arabidopsis CAX1,but not its cDNA,confers metal tolerance in yeast[J].Plant Biology,2010,12(6):935-939.
[7] MARTINS V,CARNEIRO F,CONDE C,SOTTOMAYOR M,GERÓS H.The grapevine VvCAX3 is a cation/H+exchanger involved in vacuolar Ca2+ homeostasis[J].Planta,2017,246(6):1083-1096.
[8] MATHEW IE,RHEIN H S,YANG J,GRADOGNA A,CARPANETO A,GUO Q,TAPPERO R,SCHOLZ-STARKE J,BARKLA B J,HIRSCHIK D,PUNSHON T.Sequential removal of cation/H+ exchangers reveals their additive role in elemental distribution,calcium depletion and anoxia tolerance[J].Plant,Cell&Environment,2024,47(2):557-573.
[9] XU L,ZAHID K R,HE L R,ZHANG W W,HE X,ZHANG X L,YANG X Y,ZHU L F.GhCAX3 gene,a novel Ca2+/H+exchanger from cotton,confers regulation of cold response and ABA induced signal transduction[J].PLoS One,2013,8(6):e66303.
[10] KAMIYA T,AKAHORIT,ASHIKARIM,MAESHIMA M.Expression of the vacuolar Ca2+/H+ exchanger,OsCAX1a,in rice:Cell and age specificity of expression,and enhancement by Ca2+[J].Plant&Cell Physiology,2006,47(1):96-106.
[11] MEIC,YAN P,FENG B B,MAMAT A,WANG J X.The apple Ca2+/H+ exchanger MdCAX2L-2 functions positively in modulation of Ba2+ tolerance[J].Plant Physiology and Biochemistry,2024,207:108314.
[12] LIU J,JIANG Z T,QIY W,LIU Y F,DING Y D,TIAN X N,REN X L.MdCAX affects the development of the‘Honeycrisp’bitter pit by influencing abnormal Ca distribution[J].Postharvest Biology and Technology,2021,171:111341.
[13] CUIS N,LIU H,WU Y,ZHANG L G,NIE S S.Genome-Wide identification of BrCAX genes and functional analysis of BrCAX1 involved in Ca2+ transport and Ca2+ deficiency-induced tip-burn in Chinese cabbage (Brassica rapa L.ssp. pekinensis) [J].Genes,2023,14(9):1810.
[14] LIU H,ZHANG X X,TAKANO T,LIU S K.Characterization of a PutCAX1 gene from Puccinellia tenuiflora that confers Ca2+and Ba2+ tolerance in yeast[J].Biochemical and Biophysical Research Communications,2009,383(4):392-396.
[15] JIANG Y H,LIU C H,YAN D,WEN X H,LIU Y L,WANG H J,DAIJ Y,ZHANG Y J,LIU Y F,ZHOU B,REN X L.Md-HB1 down-regulation activates anthocyanin biosynthesis in the white-fleshed apple cultivar‘Granny Smith’[J].Journal of Experimental Botany,2017,68(5):1055-1069.
[16] PAVICIC N,JEMRIC T,KURTANJEK Z,COSIC T,PAVLOV-IC I,BLASKOVIC D.Relationship between water-soluble Ca and other elements and bitter pit occurrence in‘Idared’apples:A multivariate approach[J].Annals of Applied Biology,2004,145(2):193-196.
[17] CHEN J X,LU C,DELA CRUZ R Y,LIY H,ZHENG J P,ZHANG Y G,WANG Y L.Cloning and functional analysis of the promoter of a UDP-glycosyltransferase gene from Panax quinquefolium L.[J].Plant Cell,Tissue and Organ Culture,2023,153(2):343-356.
[18] LIU Y,HE G D,HE Y Q,TANG Y Y,ZHAO F L,HE T B.Discovery of cadmium-tolerant biomacromolecule(StCAX1/4 transportproteins) in potato and its potential regulatory relationship with WRKY transcription factors[J].International Journal of Biological Macromolecules,2023,228:385-399.
[19] TORRES E,RECASENS I,ÀVILA G,LORDAN J,ALEGRE S.Early stage fruit analysis to detect a high risk of bitter pit in‘Golden Smoothee’[J].Scientia Horticulturae,2017,219:98-106.
[20] SONG J Y,SUN S N,WANG B,CHEN H Y,SHIJ S,ZHANG Y G,KONG X Y.Fruit-stalk supplementing calcium and partition regulation of fruit calcium for prevention of bitter pit of bagged apple[J].Journal of Plant Growth Regulation,2023,42(5):3000-3016.
[21] DE FREITAS S T,PADDA M,WU Q Y,PARK S,MITCHAM E J.Dynamic alternations in cellular and molecular components during blossom- end rot development in tomatoes expressing sCAX1,a constitutively active Ca2+/H+antiporter from Arabidop‐sis[J].Plant Physiology,2011,156(2):844-855.
[22] BUTIM,SARGENT D J,BIANCO L,MAGNAGO P,VELASCO R,COLGAN R J.A study of gene expression changes at the Bp-2 locus associated with bitter pit symptom expression in apple(Malus pumila)[J].Molecular Breeding,2018,38(7):85.