丁香假单胞菌猕猴桃致病变种(Pseudomonas syringae pv.actinidiae,Psa)引起的猕猴桃细菌性溃疡病严重影响猕猴桃产业发展[1],该病害发生前期隐蔽性强,不易发现,且传播迅速、感染植株死亡率高,防治极其困难[2-3]。全国各猕猴桃产区均受到猕猴桃溃疡病的危害,受害严重园区发病率超过80%,甚至全园发病导致毁园[4-6]。近年来,各国研究者通过指纹图谱、多位点序列分析和全基因组序列分析等方法将Psa 分为5 种不同的生物型(biovar),分别为biovar 1、2、3、5 和6。其中biovar 1 最早在日本发现,意大利也曾有零星发现;biovar 5 和6 仅在日本发现;biovar 2仅在韩国发现;biovar 3为全球流行群体,其群体遗传结构十分复杂[7],中国截至目前所分离到的Psa 均属于biovar 3。对Psa 群体结构和分布特征开展系统分析有助于认识猕猴桃溃疡病的传播和流行趋势,为病害防控策略的制定提供科学依据[8-10]。
多位点可变数目串联重复序列分析(multiple loci variable number of tandem repeats analysis,MLVA)广泛应用于医学病原细菌及植物病原细菌群体遗传结构研究,具有成本低、分辨率高、数据易于整合、通量高等优点[11],Osanloo 等[12]采用ERIC-PCR分型方法和MLVA分型方法对鲍曼不动杆菌开展分型研究,使用rep-PCR分型方法将伊朗100株鲍曼不动杆菌分为4个类群,MLVA 分型方法则分为9个类群,表明MLVA 技术分辨率较ERIC-PCR 更高;Siarkou 等[13]采用MLST 分型和MLVA 分型方法对流产衣原体进行了分型研究,发现MLST 将流产衣原体分为6 个类群,MLVA 技术则分为7 个类群,表明MLVA 分型技术的分辨率高于MLST。已有研究者采用此方法对Psa 群体遗传结构进行了研究,Ciarroni等[14]设计了13对MLVA 引物,对142株Psa进行群体遗传结构研究,发现来自中国、日本、新西兰和法国等不同国家或地区的142 株Psa 可分为10 个不同的亚群,并发现中国的Psa 菌株具有广泛的遗传多样性;Cunty等[15]设计了11对MLVA引物对340株Psa 的群体遗传结构进行了分析,发现中国Psa 具有丰富的遗传多样性;赵志博等[16]设计了10 对MLVA引物用以建立中国Psa 群体遗传结构分型技术,并应用于来自贵州修文县7 个代表性果园62 株Psa 群体结构研究中,发现62 株Psa 可分为3 个不同的亚群。至此,MLVA 引物数量多达34 对。使用过多MLVA 引物会使该技术失去低成本和便捷的优势。笔者在本研究中对已报道的MLVA引物进行筛选和分析,力求减少引物的使用而不降低分型效果,筛选出可以精准快速地进行Psa 分型的引物组合。该引物组合可用于Psa 群体遗传结构研究,探索Psa 的传播和流行规律,为猕猴桃溃疡病防控策略的制定提供科学依据。
1 材料和方法
1.1 MLVA引物PCR扩增效率验证
1.1.1 MLVA引物 MLVA引物如表1所示。
表1 MLVA 引物
Table 1 MLVA primer

注:a.该序列为原文献序列的反向互补序列;b.该引物对串联重复单元长度不唯一;c.TR14=TR11II,TR19=Psa-01 仅使用1 对。
Note:a.This sequence is the reverse complementary chain sequence of the original document sequence;b.The length of tandem repeat unit of this primer pair is not unique;c.TR14=TR11II,TR19=Psa-01 using only one pair of primers.
引物名称Primer name Primer sequenceTm/℃重复单元长度/侧翼长度TR length/Flank length/bp引物序列来源Reference TR2 60 7/163[15]TR5 60 7/108[15]TR7 60 8/45[15]TR8 60 9/140[15]TR14c 60 11/136[15]TR16 60 23/147[15]TR17 60 12/121[15]TR19c 60 7/146[15]TR22 60 9/143[15]TR23 60 5/182[15]Psa-01c 58 7/139[13]Psa-03 58 7/145[13]Psa-04 58 33/124[13]Psa-05 58 7/147[13]Psa-06 58 8/123[13]Psa-07 58 8/56[13]Psa-08 58 9/145[13]Psa-09 58 99-112b/64[13]Psa-10 58 7/148[13]GM-254 58 8/327[13]GM-1553 58 7/164[13]GM-1834 TGAAAGAACTGTGCCAATTTGTG TTCTGGTAGTTTACACCGCCTC GTAGCGTAGGAACGATATTCAAGTT GCGCAAGGTTTACGGGTTT GGTACGCATTCCAATCAACC AAGGGCAAATGATCGCTAACT GTGCGTTAAGTATGTAGCGTCTT CGCTGGAGTTGCGAAGG GATTGGTGACGTTGCGATGA TTGTTGCCCTACACGCTCTA GCCTGAACCGTCCGTGG CGACACCCAGTTCATTACGAAT AATCTGCACCTCGCCGACTC CAAGAAGGTCAACCCGTCCC CATGCGGGCAATCTGATAGT CAAGCAGGAGATGGAAGAGC CTGCACCGAAGCGATGACC CGCCAACATTGCCCTGCTA AAGTCGGCGAGCGAAGATAA GTTGCACGATAGCACAACCTCT CAAGCAGGAGATGGAAGAGC CATGCGGGCAATCTGATAGT TTATCGGCGGGATGTGTATT ACTGCGTCTGGTCGATAACC ACGAGTCCGCTCCTACAAAA TACAACCAAGGTGGCCTGTT GTAGGCCGCGCTTTCAAT TGCACTTCTTTTTCGCCTCT GCCTACCTTTTACGCCATGA TTAACGCAAGCAATCCTAACC TGTGCAATAAATGCGGGTTA CCGCCTCCAGTCAGGTTAAT GTCATTGGCGAACTGATCCT CTTTTCATGCTGAAAGTCATGC CGCTGTCTGGCTTTGAAAAT TAGGACGGCCGAAGGTTTAT AAGCCTGAGTAAGCGGTTCA GCCCCAGTCCCAGTTGTAAT CGTGTCACTGAAGTCACCAT TATTTACCCGGTGTTGAGGC CTGGCACGAGACGAGTCC GCTGAGCTTGAAGGAGACG CGAGTTCTATTTGCGTCAGG TGTCCAGCGTAATCTTGCTC 58 6/170[13]
表1 (续) Table 1 (Continued)
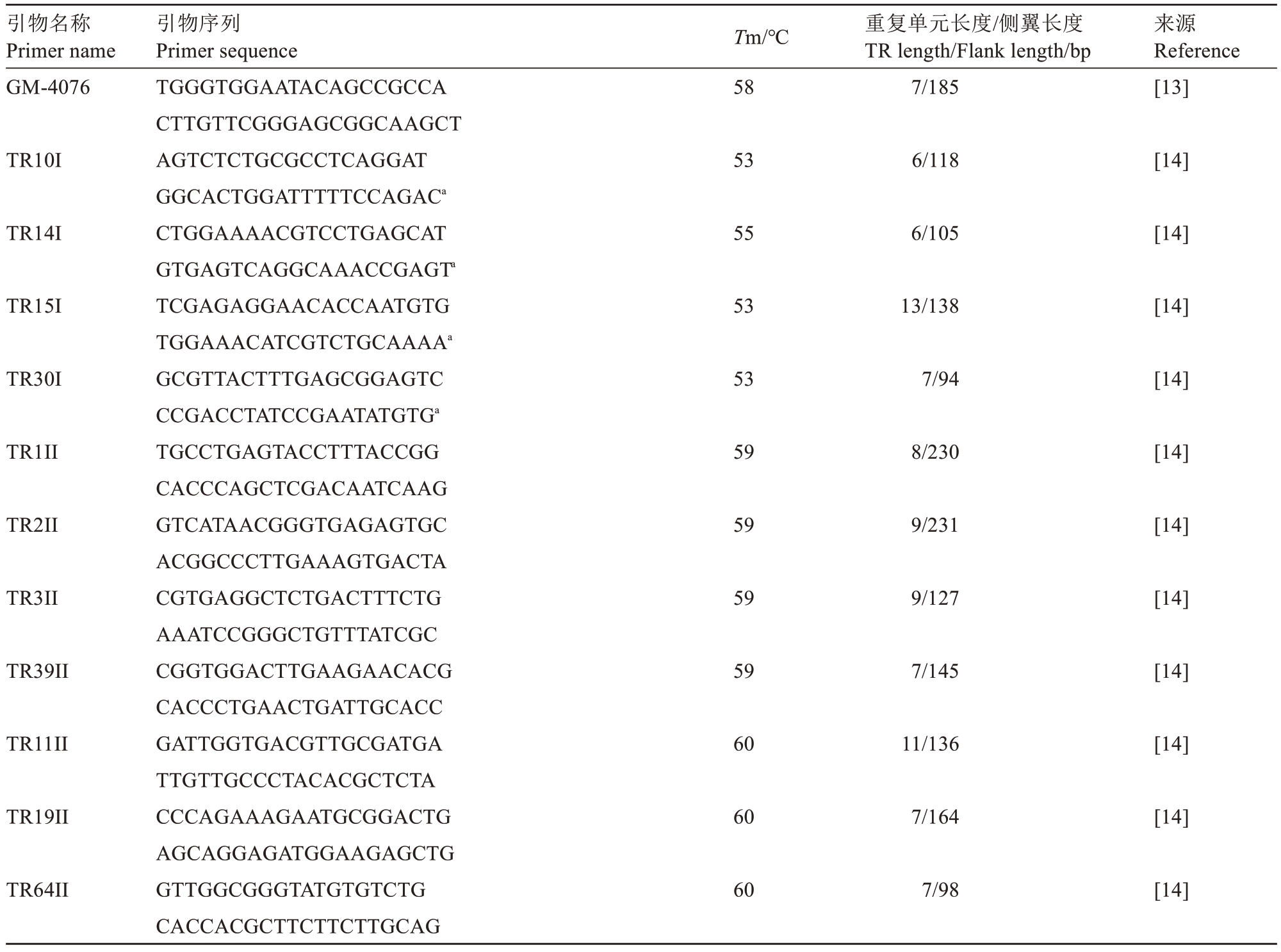
引物名称Primer name Primer sequenceTm/℃重复单元长度/侧翼长度TR length/Flank length/bp引物序列来源Reference GM-4076 58 7/185[13]TR10I 53 6/118[14]TR14I 55 6/105[14]TR15I 53 13/138[14]TR30I 53 7/94[14]TR1II 59 8/230[14]TR2II 59 9/231[14]TR3II 59 9/127[14]TR39II 59 7/145[14]TR11II 60 11/136[14]TR19II 60 7/164[14]TR64II TGGGTGGAATACAGCCGCCA CTTGTTCGGGAGCGGCAAGCT AGTCTCTGCGCCTCAGGAT GGCACTGGATTTTTCCAGACa CTGGAAAACGTCCTGAGCAT GTGAGTCAGGCAAACCGAGTa TCGAGAGGAACACCAATGTG TGGAAACATCGTCTGCAAAAa GCGTTACTTTGAGCGGAGTC CCGACCTATCCGAATATGTGa TGCCTGAGTACCTTTACCGG CACCCAGCTCGACAATCAAG GTCATAACGGGTGAGAGTGC ACGGCCCTTGAAAGTGACTA CGTGAGGCTCTGACTTTCTG AAATCCGGGCTGTTTATCGC CGGTGGACTTGAAGAACACG CACCCTGAACTGATTGCACC GATTGGTGACGTTGCGATGA TTGTTGCCCTACACGCTCTA CCCAGAAAGAATGCGGACTG AGCAGGAGATGGAAGAGCTG GTTGGCGGGTATGTGTCTG CACCACGCTTCTTCTTGCAG 60 7/98[14]
1.1.2 供试菌株 挑选10株具有代表性的Psa菌株验证34对引物对中国菌株的扩增效率(表2)。
表2 Psa 菌株信息
Table 2 Psa Strains Information
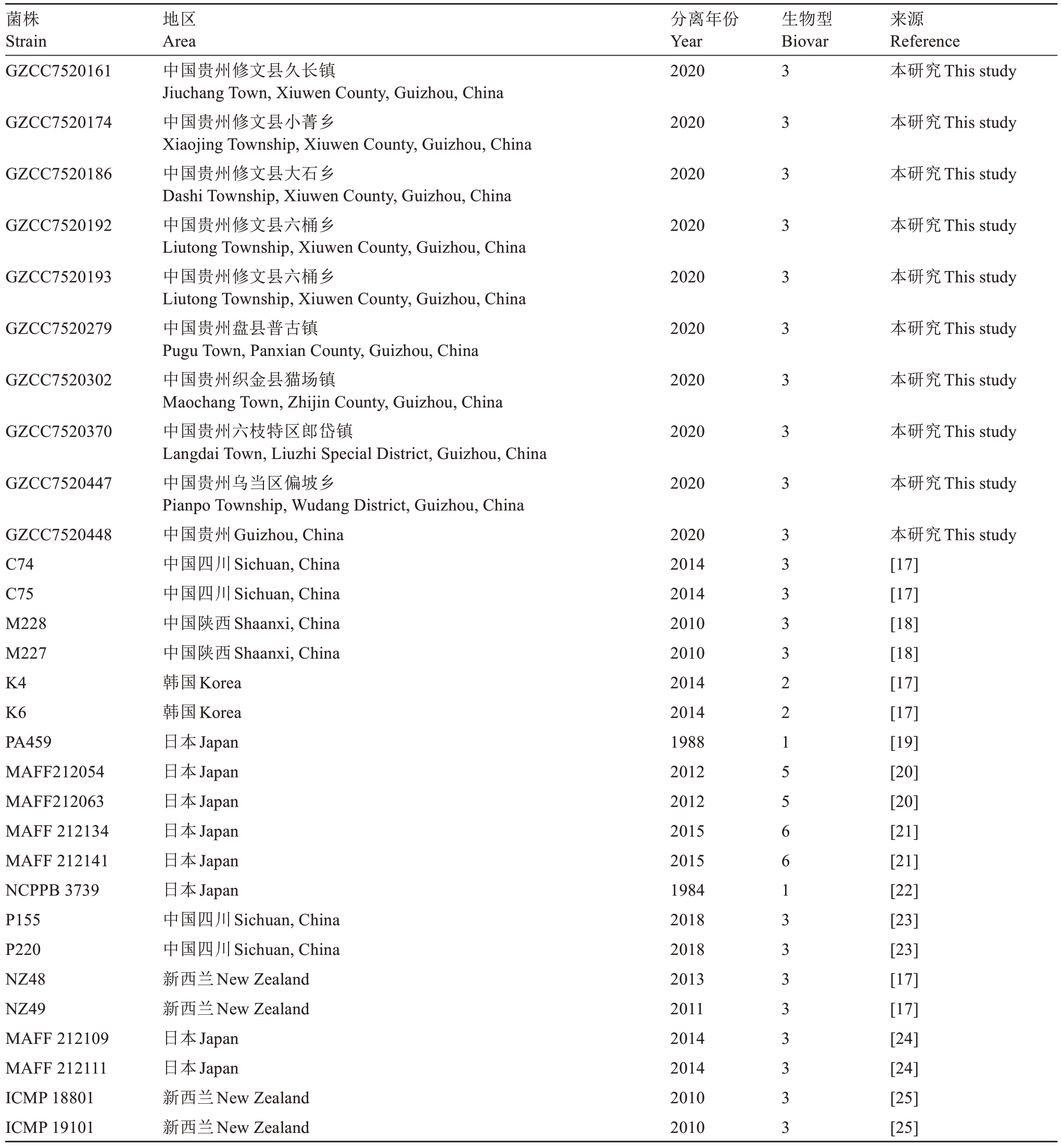
注:本研究菌株使用biovar 3 特异性引物P0F/P6R 进行生物型鉴定,其余菌株的生物型等信息根据文献报道获得。
Note:Biovar 3 specific primers P0F/P6R were used for biovar identification of strains in our laboratory,and the biovar information of other strains was obtained based on literature reports.
菌株Strain GZCC7520161分离年份Year 2020生物型Biovar来源Reference本研究This study GZCC7520174 2020本研究This study GZCC7520186 2020本研究This study GZCC7520192 2020本研究This study GZCC7520193 2020本研究This study GZCC7520279 2020本研究This study GZCC7520302 2020本研究This study GZCC7520370 2020本研究This study GZCC7520447 2020本研究This study本研究This study[17][17][18][18][17][17][19][20][20][21][21][22][23][23][17][17][24][24][25][25]GZCC7520448 C74 C75 M228 M227 K4 K6 PA459 MAFF212054 MAFF212063 MAFF 212134 MAFF 212141 NCPPB 3739 P155 P220 NZ48 NZ49 MAFF 212109 MAFF 212111 ICMP 18801 ICMP 19101地区Area中国贵州修文县久长镇Jiuchang Town,Xiuwen County,Guizhou,China中国贵州修文县小菁乡Xiaojing Township,Xiuwen County,Guizhou,China中国贵州修文县大石乡Dashi Township,Xiuwen County,Guizhou,China中国贵州修文县六桶乡Liutong Township,Xiuwen County,Guizhou,China中国贵州修文县六桶乡Liutong Township,Xiuwen County,Guizhou,China中国贵州盘县普古镇Pugu Town,Panxian County,Guizhou,China中国贵州织金县猫场镇Maochang Town,Zhijin County,Guizhou,China中国贵州六枝特区郎岱镇Langdai Town,Liuzhi Special District,Guizhou,China中国贵州乌当区偏坡乡Pianpo Township,Wudang District,Guizhou,China中国贵州Guizhou,China中国四川Sichuan,China中国四川Sichuan,China中国陕西Shaanxi,China中国陕西Shaanxi,China韩国Korea韩国Korea日本Japan日本Japan日本Japan日本Japan日本Japan日本Japan中国四川Sichuan,China中国四川Sichuan,China新西兰New Zealand新西兰New Zealand日本Japan日本Japan新西兰New Zealand新西兰New Zealand 2020 2014 2014 2010 2010 2014 2014 1988 2012 2012 2015 2015 1984 2018 2018 2013 2011 2014 2014 2010 2010 3 3 3 3 3 3 3 3 3 3 3 3 3 3 2 2 1 5 5 6 6 1 3 3 3 3 3 3 3 3
1.1.3 PCR、电泳条件 PCR 条件:95 ℃3 min,95 ℃30 s,退火温度30 s,72 ℃1.5 min,35 个循环,72 ℃5 min,最后保持12 ℃(表1);毛细管电泳采用安捷伦科技有限公司生产的毛细管电泳仪,试剂采用DNF900 试剂盒;毛细管电泳程序设置:5.0 kV 30 s,5.0 kV 10 s注入Marker,5.0 kV 10 s注入样品及Ladder,最后5.0 kV运行80 min。
1.2 模拟PCR筛选MLVA引物
以Genbank 中下载127 株Psa 全基因组序列为模板,表1 所列引物对为引物,使用SPCR 3.0 进行模拟PCR,模拟PCR 参数为:Up Threshold:0.8;Down Threshold:0.8;Pa Threshold:0.8;Max Product:500 bp;Min Product:35 bp(其中Psa-09 Max Product设置为1000 bp)[26]。
1.3 数据分析
1.3.1 串联重复数(TRs)计算 将模拟PCR 扩增结果的长度信息导入Excel中,整理后按照下列公式进行串联重复数(tandem repeats,TRs)的计算,统计引物的扩增情况。
1.3.2 引物组合筛选 以辛普森指数(Simpson’s index,SI)作为筛选引物组合的标准,计算所有引物组合的SI,并根据SI 的大小筛选引物组合[27]。基于R version 4.0.2 开发程序进行引物组合的筛选,采用poppr软件包的df2genind()函数将模拟PCR结果矩阵转化为genind 格式,利用diversity_stats[mlg.table()]函数进行引物组合SI 的计算,从2 对引物组合的SI开始计算,然后计算3对引物组合的SI,直到所计算引物组合的SI 等于全部引物组合下的SI,该引物组合即为最优引物组合[28]。
1.3.3 引物组合效果验证 采用poppr 软件包的genotype_curve()函数对所有的引物组合下Psa 的多位点基因型数(Multilocus genotypes,MLGs)进行统计;以10 株Psa 的TR 数据结合Genbank 下载20 株Psa全基因组序列(表2),采用bruvo.boot()函数基于筛选获得的引物组合构建UPGMA 聚类树,验证所筛选引物组合的分型效果[29]。
2 结果与分析
2.1 PCR扩增效果验证
除TR10I、TR14I、TR15I、TR30I 等4 对引物外,Psa-10(见图1 左)、TR2、TR5 等30 对引物对供试的10 个Psa 菌株均能扩增出清晰明亮的单一条带,进一步分析发现,TR10I(见图1 右)、TR14I、TR15I、TR30I 等4 对引物的下游引物采用反向互补序列后可扩增出单一清晰条带。结果表明34 对引物均对供试Psa菌株表现良好扩增效果,详细结果见表3。
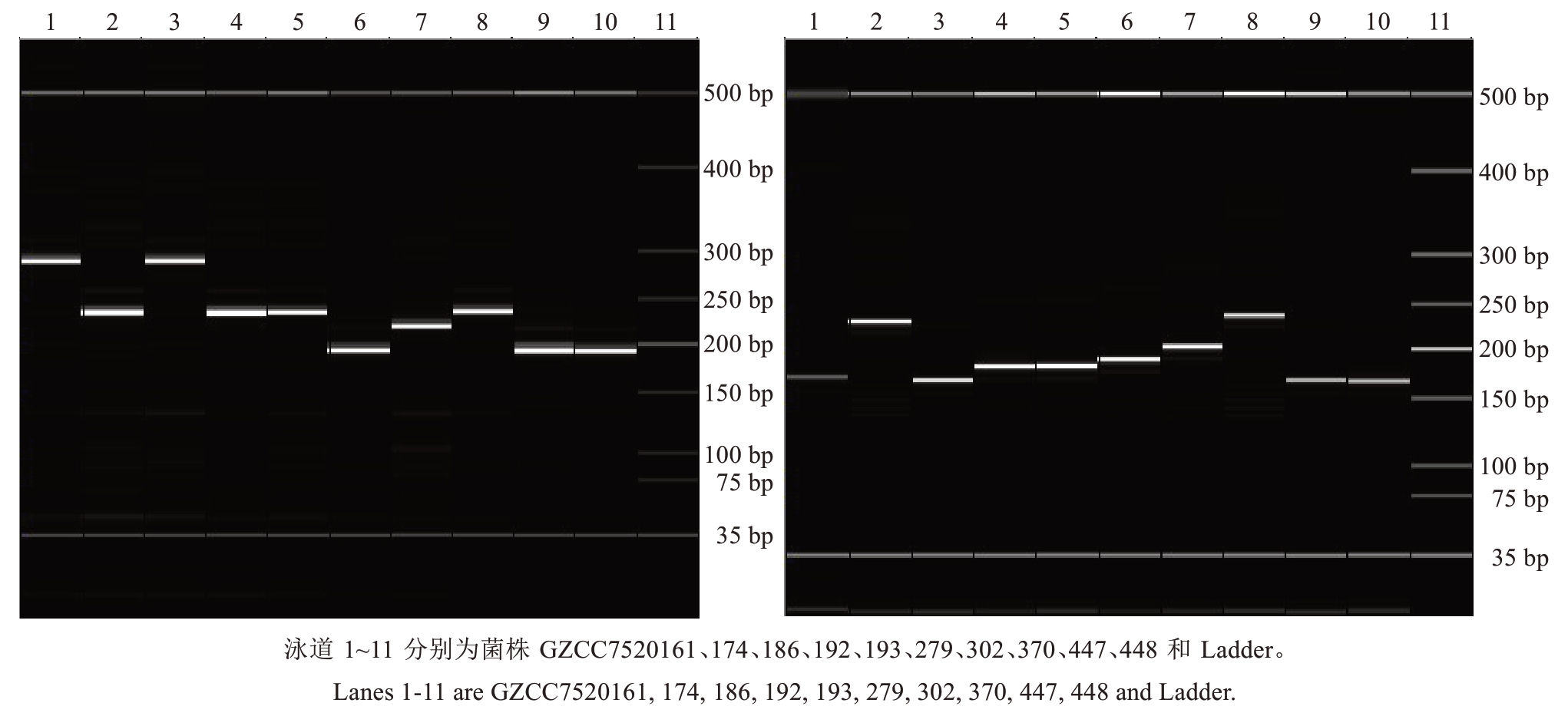
图1 引物Psa-10(左)和TR10I(右)分别对10 株Psa 进行PCR 扩增的电泳胶图
Fig.1 Electrophoretic gel images of PCR amplification of 10 Psa isolates using primers Psa-10(left)and TR10I(right)
表3 模拟PCR 情况
Table 3 Simulated PCR situation
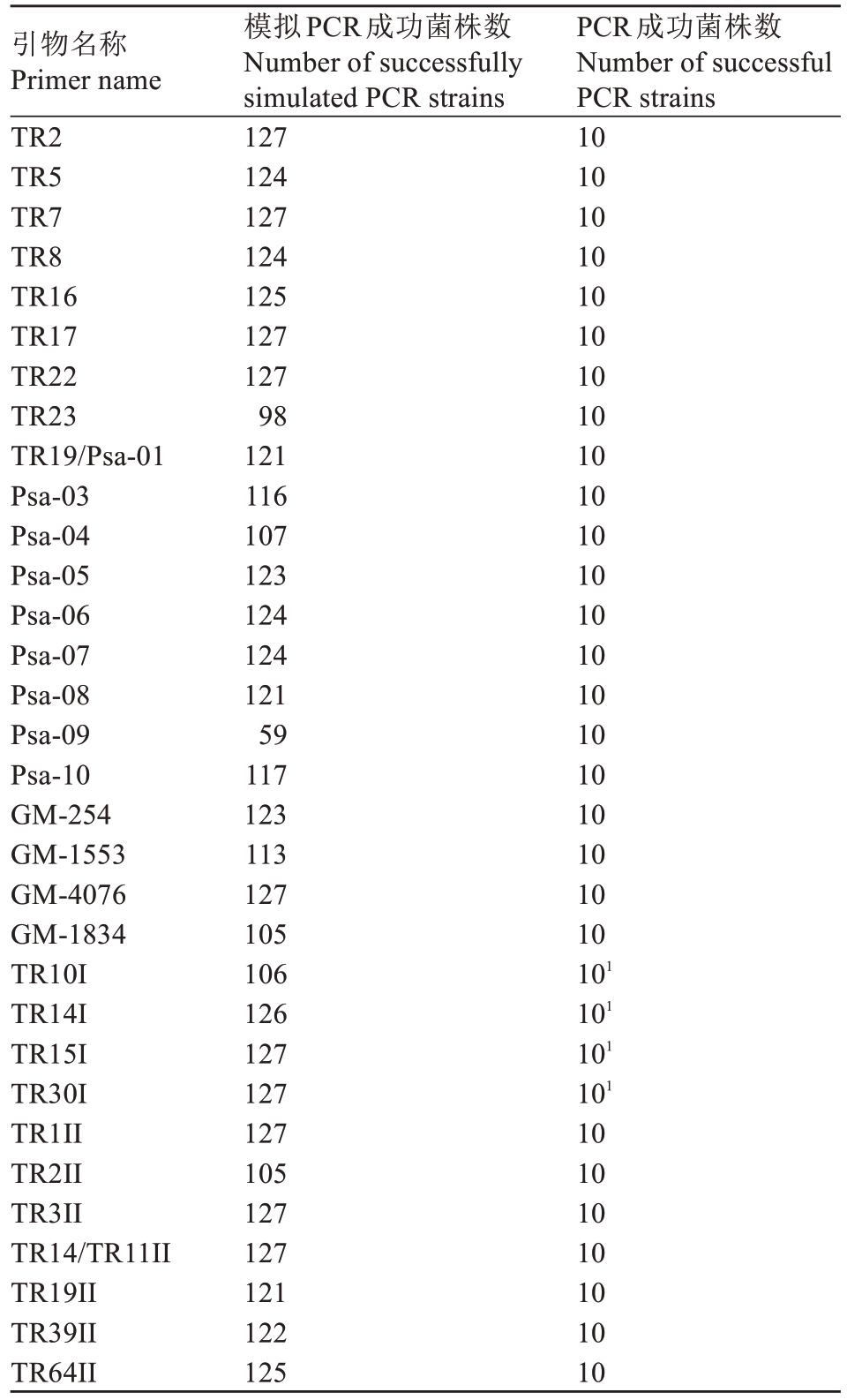
注:1.下游引物序列反向互补。
Note:1.Downstream primer sequences are reverse complementary.
引物名称Primer name TR2 TR5 TR7 TR8 TR16 TR17 TR22 TR23 TR19/Psa-01 Psa-03 Psa-04 Psa-05 Psa-06 Psa-07 Psa-08 Psa-09 Psa-10 GM-254 GM-1553 GM-4076 GM-1834 TR10I TR14I TR15I TR30I TR1II TR2II TR3II TR14/TR11II TR19II TR39II TR64II PCR成功菌株数Number of successful PCR strains 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 101 101 101 101 10 10 10 10 10 10 10模拟PCR成功菌株数Number of successfully simulated PCR strains 127 124 127 124 125 127 127 98 121 116 107 123 124 124 121 59 117 123 113 127 105 106 126 127 127 127 105 127 127 121 122 125
2.2 模拟PCR
模拟PCR 结果如表3 所示,TR2 等10 对引物能将127 株Psa 完全扩增成功,GM-1834 等22 对引物扩增的菌株数大于105 株,Psa-09 和TR23 引物扩增的菌株数最少,分别为59株和98株。
通过对模拟PCR 扩增的序列文件分析,TR8与Psa-08、TR39II 与Psa-10、GM-1834 与TR10I、GM1553 与TR64II、TR19Psa-01 与TR19II 扩增同一TR;Psa-09 TR 长度不固定,TR2II 侧翼长度变异较大,不能通过电泳准确推断TR数。将序列相同的引物、扩增同一TR 的引物仅保留1 对,不能通过电泳判断TRs 的引物被剔除,剩余25 对引物进行后续的分析。
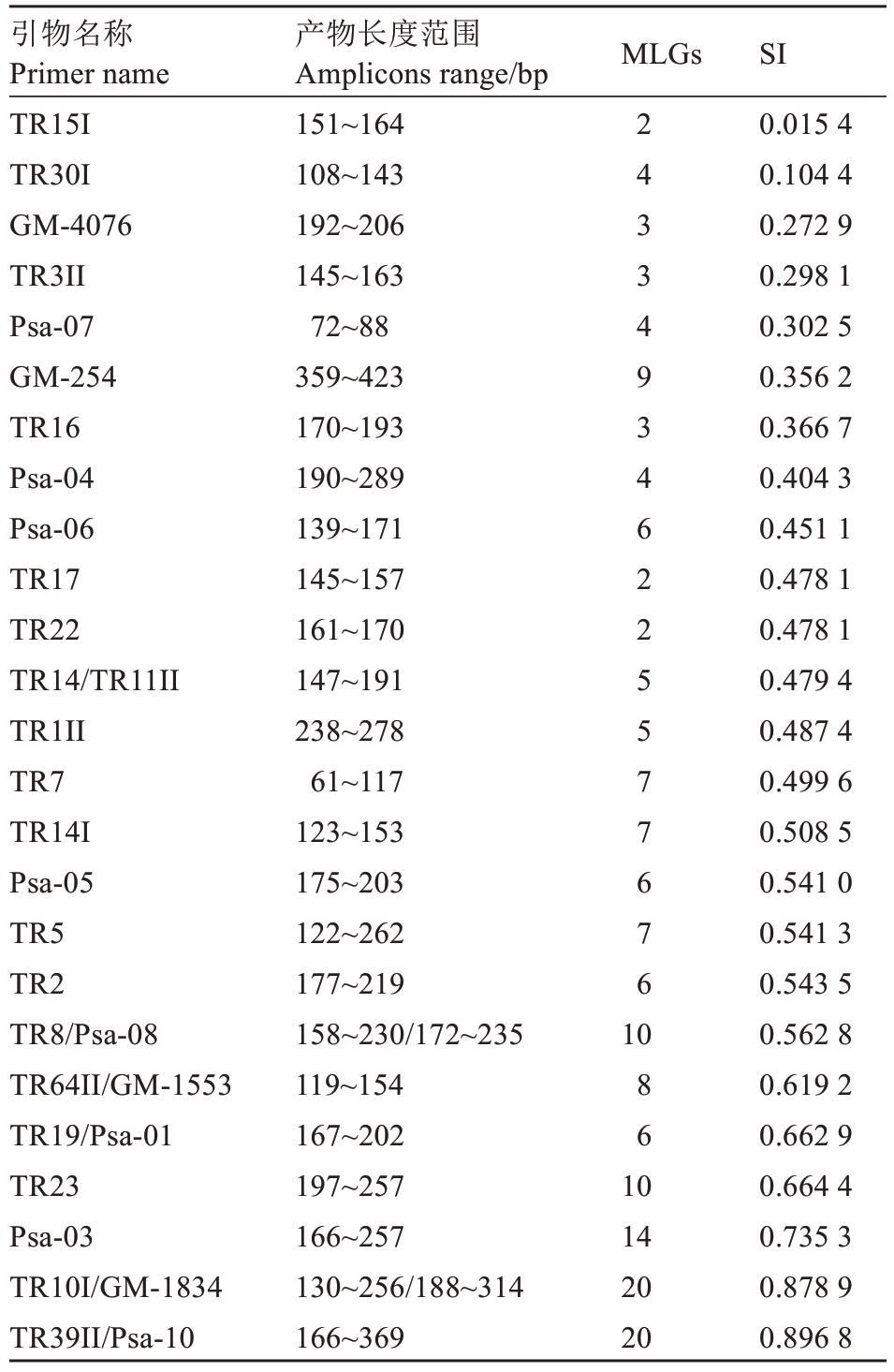
25 对引物扩增的产物长度、多位点基因型数(MLGs)、辛普森指数(SI)结果各不相同。从MLGs来看TR10I、GM-1834、Psa-10 和TR39II 等4 对引物扩增的MLGs 最多,为20 个;TR15I、TR17 和TR22等3 对引物扩增的MLGs 最少,为2 个;从SI 来看,TR39IIPsa-10、TR10I、GM-1834 和Psa-03 等4 对引物扩增基因型的SI 较高,分别为0.896 8、0.878 9、0.878 9 和0.735 3;TR15I、TR30I、GM-4076 和TR3II 等4 对引物扩增基因型的SI 较低,均小于0.3。25 对引物中,GM-254 扩增的MLGs 较多,为9,其SI 为0.356 2,均匀度较低;TR17 和TR22 扩增的MLGs较少,为2,SI为0.478 1,均匀度较高(表4)。
表4 模拟PCR 信息
Table 4 Simulation PCR information
注:参照Paul R.Hunter and Michael A.Gaston 的Simpson’s Index 计算公式。
Note:Refer to the Simpson’s index calculation formula of Paul R.hunter and Michael A.Gaston.
引物名称Primer name TR15I TR30I GM-4076 TR3II Psa-07 GM-254 TR16 Psa-04 Psa-06 TR17 TR22 TR14/TR11II TR1II TR7 TR14I Psa-05 TR5 TR2 TR8/Psa-08 TR64II/GM-1553 TR19/Psa-01 TR23 Psa-03 TR10I/GM-1834 TR39II/Psa-10产物长度范围Amplicons range/bp 151~164 108~143 192~206 145~163 72~88 359~423 170~193 190~289 139~171 145~157 161~170 147~191 238~278 61~117 123~153 175~203 122~262 177~219 158~230/172~235 119~154 167~202 197~257 166~257 130~256/188~314 166~369 SI 0.015 4 0.104 4 0.272 9 0.298 1 0.302 5 0.356 2 0.366 7 0.404 3 0.451 1 0.478 1 0.478 1 0.479 4 0.487 4 0.499 6 0.508 5 0.541 0 0.541 3 0.543 5 0.562 8 0.619 2 0.662 9 0.664 4 0.735 3 0.878 9 0.896 8 MLGs 2 4 3 3 4 9 3 4 6 2 2 5 5 7 7 6 7 6 10 8 6 10 14 20 20
2.3 引物组合筛选
使用R 进行25 对引物所有组合的查找及SI 值的计算结果表明:选择25 对引物时,其组合仅有1 个,SI值为0.984 7;选择9对引物时,引物组合有2 042 975个,其SI 值最大的前3 个组合的SI 值分别为0.984 7、0.984 7 和0.984 5,最大SI 值与25 对引物的SI 值相同。选择9 对以上引物组合时,其最大SI 值也达到了0.984 7,最大SI 值未随引物增加而增加(表5、图2)。从MLGs 来看,使用2 对引物组合时最大MLGs 为65,使用9 对引物组合最大MLGs 与25 对引物的MLGs相同,均为99。
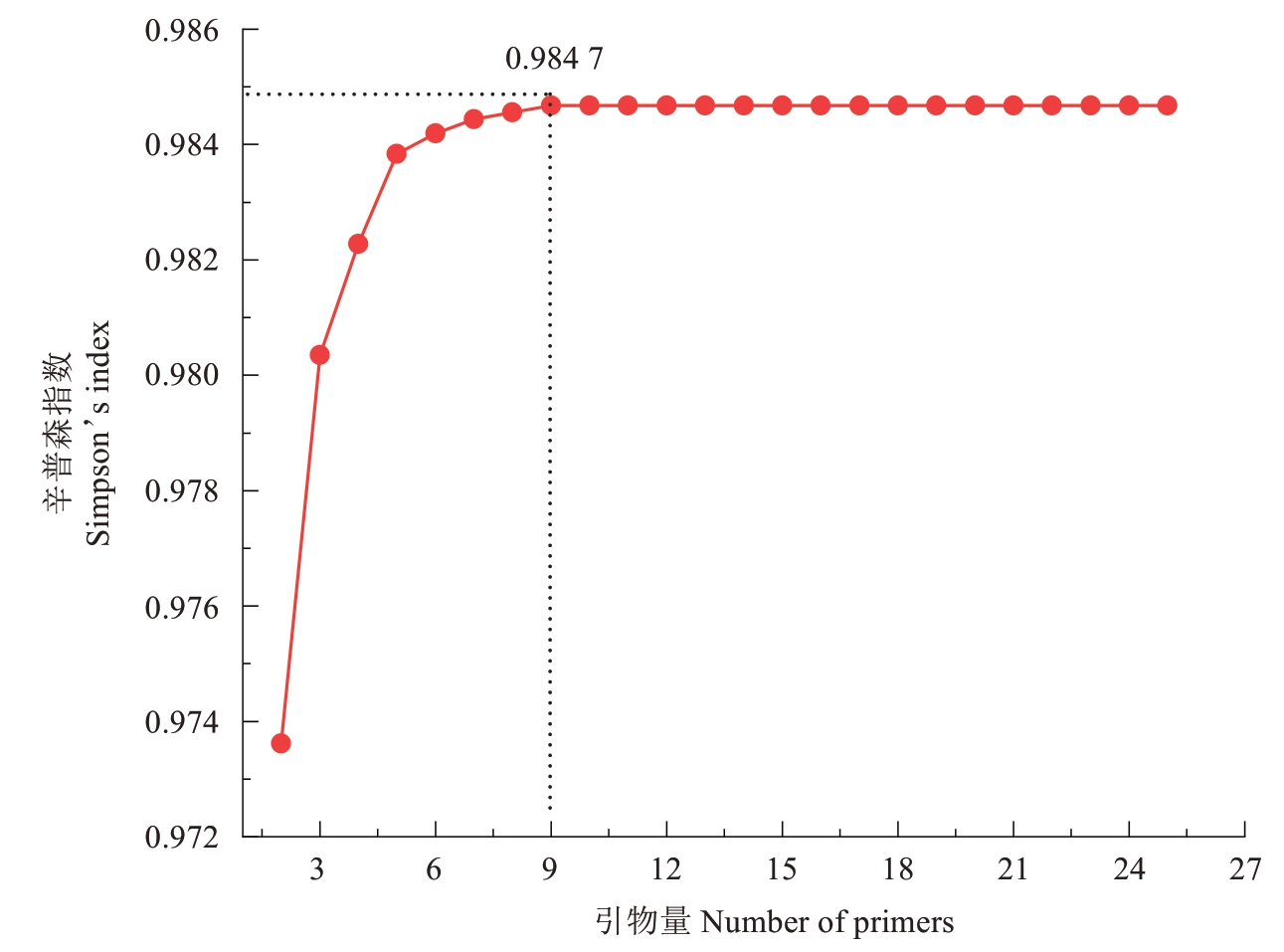
图2 最优引物组合SI 曲线
Fig.2 SI curve of the optimal primer combination
表5 引物组合SI 表
Table 5 SI Table of primer combination
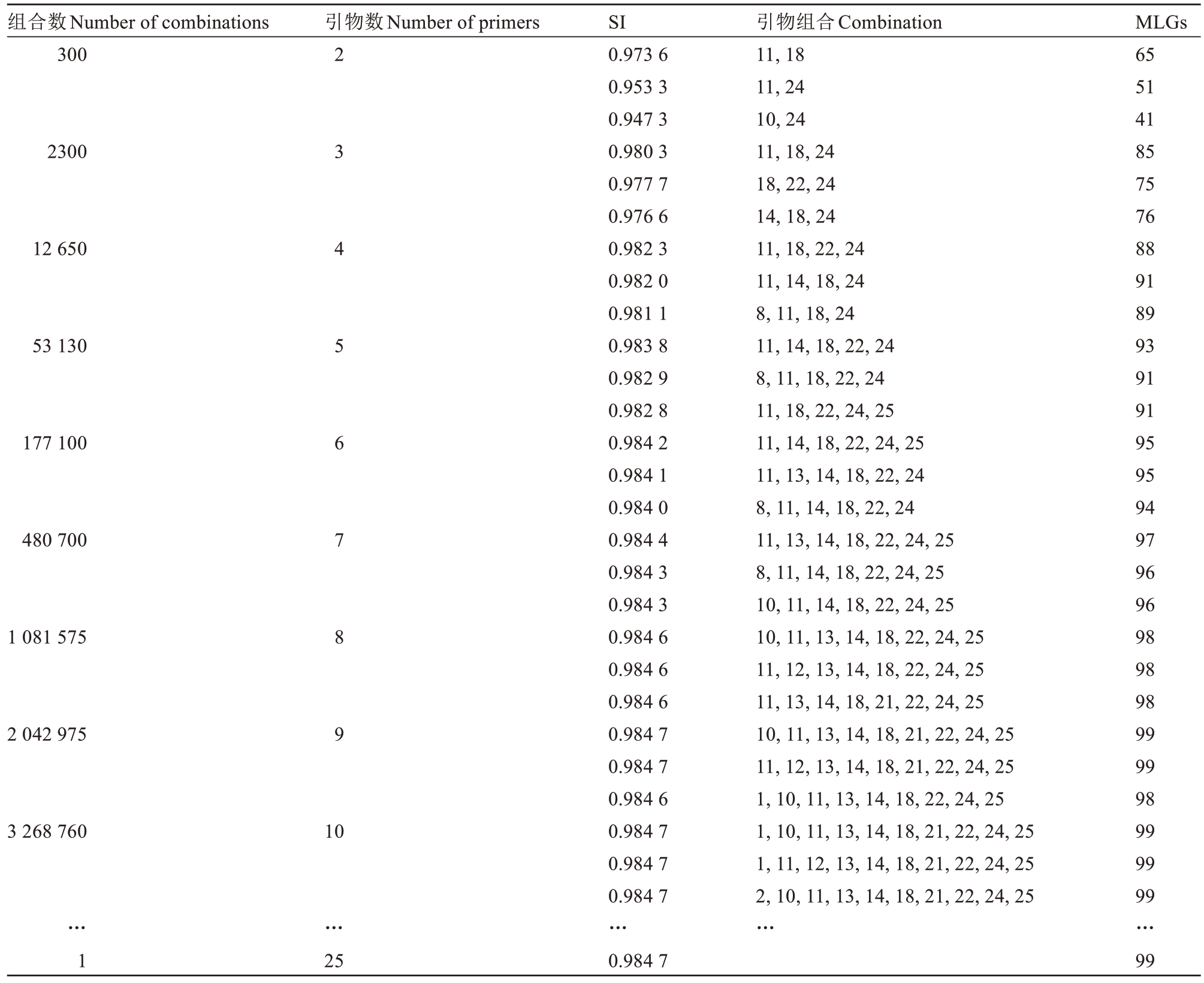
注:引物编号:1.TR2;2.TR5;3.TR7;4.TR8/Psa-08;5.TR14/TR11II;6.TR16;7.TR17;8.TR19/Psa-01;9.TR22;10.TR23;11.Psa-03;12.Psa-04;13. Psa-05;14. Psa-06;15. Psa-07;16. GM-254;17. GM-4076;18. TR10/IGM-1834;19. TR14I;20. TR15I;21. TR30I;22. TR1II;23. TR3II;24.TR39II/Psa10;25.TR64II。只显示SI 值最大的前3 个组合。
Note:Primer No.:1. TR2;2. TR5;3. TR7;4. TR8/Psa-08;5. TR14/TR11II;6. TR16;7. TR17;8. TR19/Psa-01;9. TR22;10. TR23;11. Psa-03;12.Psa-04;13.Psa-05;14.Psa-06;15.Psa-07;16.GM-254;17.GM-4076;18.TR10/IGM-1834;19.TR14I;20.TR15I;21.TR30I;22.TR1II;23.TR3II;24.TR39II/Psa10;25.TR64II.Only the largest three combinations are displayed.
MLGs 65 51 41 85 75 76 88 91 89 93 91 91 95 95 94 97 96 96 98 98 98 99 99 98 99 99 99…99组合数Number of combinations 300引物数Number of primers 2300 12 650 53 130 177 100 480 700 1 081 575 2 042 975 2 3 4 5 6 7 8 9 3 268 760 10…1…引物组合Combination 11,18 11,24 10,24 11,18,24 18,22,24 14,18,24 11,18,22,24 11,14,18,24 8,11,18,24 11,14,18,22,24 8,11,18,22,24 11,18,22,24,25 11,14,18,22,24,25 11,13,14,18,22,24 8,11,14,18,22,24 11,13,14,18,22,24,25 8,11,14,18,22,24,25 10,11,14,18,22,24,25 10,11,13,14,18,22,24,25 11,12,13,14,18,22,24,25 11,13,14,18,21,22,24,25 10,11,13,14,18,21,22,24,25 11,12,13,14,18,21,22,24,25 1,10,11,13,14,18,22,24,25 1,10,11,13,14,18,21,22,24,25 1,11,12,13,14,18,21,22,24,25 2,10,11,13,14,18,21,22,24,25…25 SI 0.973 6 0.953 3 0.947 3 0.980 3 0.977 7 0.976 6 0.982 3 0.982 0 0.981 1 0.983 8 0.982 9 0.982 8 0.984 2 0.984 1 0.984 0 0.984 4 0.984 3 0.984 3 0.984 6 0.984 6 0.984 6 0.984 7 0.984 7 0.984 6 0.984 7 0.984 7 0.984 7…0.984 7
以上结果表明,使用TR23/Psa-04、Psa-03、Psa-05、Psa-06、TR10IGM-1834、TR30 Ⅰ、TR1II、Psa-10TR39II、TR64IIGM-1553 等9 对引物的组合可以达到25 对引物的效果,其中TR23 和Psa-04 可以相互替换,任选其一使用即可。
2.4 引物组合分型效果验证
获得的9 对引物组合可将Psa 的5 种生物型(biovar)准确分开;10 株代表性Psa 均为biovar 3,其中GZCC7520447 和GZCC7520448 来自乌当区偏坡乡,独立聚为一支;GZCC7520193 和GZCC7520192均来自修文县六桶乡,以相近的遗传距离聚在一起;GZCC7520186 和GZCC7520161 均来自修文县,以相近的遗传距离聚在一起;biovar 3菌株间存在一定差异(图3)。上述结果表明,所获得的9对引物组合可用于Psa群体遗传结构研究。

图3 30 株Psa 基于9 对引物组合构建的UPGMA 树
Fig.3 UPGMA tree constructed using 30 Psa strains based on 9 primer combinations
3 讨 论
各国学者采用MLVA 技术开展Psa 群体遗传结构研究共设计了34对MLVA引物[14-16],34对引物中,有些是相同序列(TR14=TR11Ⅱ、TR19=Psa-01);有些是扩增同一TR(TR8 与Psa-08、TR39Ⅱ与Psa-10、GM1834 与TR10Ⅰ、GM1553 与TR64Ⅱ、TR19Psa-01与TR19II);有些序列(TR10I、TR14I、TR15I、TR30I)不能直接被引用,Mazzaglia 等[30]的研究中也提到了该不足。有必要针对该34 对引物开展系统的分析和筛选,以获得一组可以直接使用、快速精准地对Psa进行分型分析的引物组合。
笔者在本研究中在不降低分辨率的前提下进行MLVA 引物组合的筛选,将34 对引物减少到只需9对(TR23/Psa-04、Psa-03、Psa-05、Psa-06、TR10IGM-1834、TR30I、TR1II、Psa-10TR39II、TR64IIGM-1553),大大降低了研究的时间和资源成本。该9 对引物的组合经验证可准确、快速地对Psa 进行分型分析,进行Psa 群体遗传结构研究,探索Psa 传播和流行规律,为猕猴桃溃疡病防控策略的制定提供科学依据。
笔者在本研究中需查找引物的所有组合并计算SI 值,计算量达百万级以上。Optimal Combination Finder(OCF)是一个专门用来找出引物所有可能的组合,并进行引物所有可能组合的SI 计算的程序[18]。但由于其操作比较复杂,非开发者很难自行利用该程序进行引物组合的查找和SI 的计算,因此笔者基于R 开发了一段计算程序,进行所有可能引物组合的查找和SI 的计算,它是利用R 编写并依托于poppr 软件包使用。R 语言平台使用方式简单快捷,可多线程运行,提高计算速度,直接采用poppr软件包计算SI 值则大大简化了计算步骤,如笔者在本研究中所有引物组合的SI 值的计算仅需几个小时便可以完成,这极大地提高了引物组合筛选的效率。该计算程序可以应用于其他微生物MLVA引物的筛选。
笔者在本研究中所引用的34 对引物对127 株Psa 菌株的全基因组扩增信息中,存在部分引物扩增不出条带的情况,但整体来说,供试引物对大多数菌株能扩增出条带,仅Psa-09和TR23引物能扩增出条带的菌株数量较少。不能扩增出条带的原因可能是:(1)菌株序列中本身不存在此位点;(2)GenBank中下载的127 株Psa 全基因组序列中有118 株未组装,未组装的Psa 菌株的全基因组信息由几十到几百条序列组成,如果序列截断的位置刚好是位点所在,模拟PCR 便无法扩增。无法准确判断扩增不出条带的原因,若将缺失信息的菌株舍弃,样本量将会大大减少,不利于引物组合的筛选。其他的研究者在面对缺失信息时,一般是使用字符将其标记出来,如:Ikawaty 等[31]使用“999”表示PCR 无扩增信息;Conceição等[32]使用“99”表示未获得PCR扩增信息;Ciarroni 等[14]使用“-1”来表示扩增信息的缺失。笔者在本研究中将缺失信息统一使用“0”表示,一方面便于SI 的计算,另一方面也使所有菌株都参与到了引物组合的筛选中,获得了适用于Psa 群体遗传结构研究的引物组合。
[1] TAKIKAWA Y,SERIZAWA S,ICHIKAWA T,TSUYUMU S,GOTO M. Pseudomonas syringae pv. actinidiae pv. nov.:The causal bacterium of canker of kiwifruit in Japan[J]. Japanese Journal of Phytopathology,1989,55(4):437-444.
[2] SAWADA H,FUJIKAWA T. Genetic diversity of Pseudomonas syringae pv.actinidiae,pathogen of kiwifruit bacterial canker[J].Plant Pathology,2019,68(7):1235-1248.
[3] 张迪,高小宁,赵志博,秦虎强,黄丽丽.不同猕猴桃品种对溃疡病的抗性差异及其机制研究[J]. 果树学报,2019,36(11):1549-1557.ZHANG Di,GAO Xiaoning,ZHAO Zhibo,QIN Huqiang,HUANG Lili. Differences in resistance to Pseudomonas syringae pv. actinidiae and acting mechanism of different kiwifruit varieties[J].Journal of Fruit Science,2019,36(11):1549-1557.
[4] 虞江,李渺,张文娟,冯双,卢永仲,徐志华,张善淇,龚子雄,何鹏,魏娴.贵州省贵阳市修文县猕猴桃溃疡病发生现状调查[J].贵州农机化,2022(1):41-43.YU Jiang,LI Miao,ZHANG Wenjuan,FENG Shuang,LU Yongzhong,XU Zhihua,ZHANG Shanqi,GONG Zixiong,HE Peng,WEI Xian. Investigation on the current situation of kiwifruit canker disease in Xiuwen County,Guiyang City,Guizhou Province[J]. Guizhou Agricultural Mechaniation,2022(1):41-43.
[5] 马利.四川猕猴桃溃疡病发生区划和综合防控技术研究[D].雅安:四川农业大学,2018.MA Li. Occurrence regionalization and integrated management of kiwifruit canker caused by Pseudomonas syringae pv.actinidiae in Sichuan[D].Yaan:Sichuan Agricultural University,2018.
[6] 王茹琳,刘原,李庆,沈沾红,陆兴利,赵金鹏,王闫利,王明田.气候变化情景下四川省猕猴桃溃疡病菌潜在地理分布模拟[J].植物保护,2020,46(2):38-47.WANG Rulin,LIU Yuan,LI Qing,SHEN Zhanhong,LU Xingli,ZHAO Jinpeng,WANG Yanli,WANG Mingtian. Analysis of geographical distribution of Pseudomonas syringae pv. actinidiae in Sichuan under climate change[J]. Plant Protection,2020,46(2):38-47.
[7] FUJIKAWA T,SAWADA H. Draft genome sequences of nine Japanese strains of the kiwifruit bacterial canker pathogen Pseudomonas syringae pv. actinidiae biovar 3[J]. Microbiology Resource Announcements,2020,9(45):e01007-e01020.
[8] 朱海云,马瑜,柯杨,李勃.陕西省猕猴桃溃疡病病原菌分离鉴定及分型研究[J].微生物学杂志,2023,43(4):74-83.ZHU Haiyun,MA Yu,KE Yang,LI Bo. Isolation,identification and typing the pathogen of Chinese gooseberry or kiwifruit (Actinidia chinensis) canker in Shaanxi Province[J]. Journal of Microbiology,2023,43(4):74-83.
[9] HE R,LIU P,JIA B,XUE S Z,WANG X J,HU J Y,AL SHOFFE Y,GALLIPOLI L,MAZZAGLIA A,BALESTRA G M,ZHU L W.Genetic diversity of Pseudomonas syringae pv.actinidiae strains from different geographic regions in China[J]. Phytopathology,2019,109(3):347-357.
[10] 代玉立,兰成忠,甘林,刘晓菲,龚国淑,杨秀娟.中国4 省猕猴桃细菌性溃疡病菌的生物型检测和遗传多样性分析[J].植物保护,2022,48(6):58-68.DAI Yuli,LAN Chengzhong,GAN Lin,LIU Xiaofei,GONG Guoshu,YANG Xiujuan. Detection of Pseudomonas syringae pv. actinidiae biovars from four provinces in China and genetic diversity analysis[J].Plant Protection,2022,48(6):58-68.
[11] VAN BELKUM A.Tracing isolates of bacterial species by multilocus variable number of tandem repeat analysis (MLVA)[J].FEMS Immunology & Medical Microbiology,2007,49(1):22-27.
[12] OSANLOO L,ZEIGHAMI H,HAGHI F,SHAPOURI R,SHOKRI R. Molecular typing of multidrug-resistant Acinetobacter baumannii isolates from clinical specimens by ERICPCR and MLVA[J].Current Microbiology,2023,80(11):355.
[13] SIARKOU V I,VORIMORE F,VICARI N,MAGNINO S,RODOLAKIS A,PANNEKOEK Y,SACHSE K,LONGBOTTOM D,LAROUCAU K. Diversification and distribution of ruminant Chlamydia abortus clones assessed by MLST and MLVA[J].PLoS One,2015,10(5):e0126433.
[14] CIARRONI S,GALLIPOLI L,TARATUFOLO M C,BUTLER M I,POULTER R T M,POURCEL C,VERGNAUD G,BALESTRA G M,MAZZAGLIA A. Development of a multiple loci variable number of tandem repeats analysis (MLVA) to unravel the intra-pathovar structure of Pseudomonas syringae pv. actinidiae populations worldwide[J]. PLoS One,2015,10(8):e0135310.
[15] CUNTY A,CESBRON S,POLIAKOFF F,JACQUES M A,MANCEAU C. Origin of the outbreak in France of Pseudomonas syringae pv. actinidiae biovar 3,the causal agent of bacterial canker of kiwifruit,revealed by a multilocus variable-number tandem-repeat analysis[J]. Applied and Environmental Microbiology,2015,81(19):6773-6789.
[16] 赵志博,杜淑凤,李月,杨文,樊荣,王勇,龙友华.猕猴桃溃疡病菌biovar 3 群体MLVA 分型技术的建立与应用[J].植物病理学报,2019,49(4):445-455.ZHAO Zhibo,DU Shufeng,LI Yue,YANG Wen,FAN Rong,WANG Yong,LONG Youhua. Establishment and application of MLVA typing for Pseudomonas syringae pv. actinidiae biovar 3 populations[J]. Acta Phytopathologica Sinica,2019,49(4):445-455.
[17] MCCANN H C,LI L,LIU Y F,LI D W,PAN H,ZHONG C H,RIKKERINK E H A,TEMPLETON M D,STRAUB C,COLOMBI E,RAINEY P B,HUANG H W. Origin and evolution of the kiwifruit canker pandemic[J]. Genome Biology and Evolution,2017,9(4):932-944.
[18] 赵志博.猕猴桃细菌性溃疡病菌群体结构与致病机制研究[D].杨凌:西北农林科技大学,2016.ZHAO Zhibo. Population composition and pathogenic mechanism in Pseudomonas syringae pv. actinidiae[D]. Yangling:Northwest A&F University,2016.
[19] MAZZAGLIA A,STUDHOLME D J,TARATUFOLO M C,CAI R M,ALMEIDA N F,GOODMAN T,GUTTMAN D S,VINATZER B A,BALESTRA G M. Pseudomonas syringae pv.actinidiae (PSA) isolates from recent bacterial canker of kiwifruit outbreaks belong to the same genetic lineage[J].PLoS One,2012,7(5):e36518.
[20] SAWADA H,MIYOSHI T,IDE Y. Novel MLSA group (Psa5)of Pseudomonas syringae pv. actinidiae causing bacterial canker of kiwifruit (Actinidia chinensis) in Japan[J]. Japanese Journal of Phytopathology,2014,80(3):171-184.
[21] FUJIKAWA T,SAWADA H. Genome analysis of Pseudomonas syringae pv. actinidiae biovar 6,which produces the phytotoxins,phaseolotoxin and coronatine[J]. Scientific Reports,2019,9:3836.
[22] MARCELLETTI S,FERRANTE P,PETRICCIONE M,FIRRAO G,SCORTICHINI M. Pseudomonas syringae pv. actinidiae draft genomes comparison reveal strain-specific features involved in adaptation and virulence to Actinidia species[J].PLoS One,2011,6(11):e27297.
[23] PAN X,ZHAO S Y,WANG Y Z,LI M Z,HE L Q,ZHUANG Q G. Complete genome sequencing of Pseudomonas syringae pv.actinidiae Biovar 3, P155, kiwifruit pathogen originating from China[J].Bioscience Journal,2020,36(6):2220-2228.
[24] FUJIKAWAT,SAWADAH.Genome analysis of the kiwifruit canker pathogen Pseudomonas syringae pv. actinidiae biovar 5[J].Scientific Reports,2016,6:21399.
[25] MCCANN H C,RIKKERINK E H A,BERTELS F,FIERS M,LU A,REES-GEORGE J,ANDERSEN M T,GLEAVE A P,HAUBOLD B,WOHLERS M W,GUTTMAN D S,WANG P W,STRAUB C,VANNESTE J L,RAINEY P B,TEMPLETON M D. Genomic analysis of the kiwifruit pathogen Pseudomonas syringae pv. actinidiae provides insight into the origins of an emergent plant disease[J]. PLoS Pathogens,2013,9(7):e1003503.
[26] CAO Y F,WANG L J,XU K X,KOU C H,ZHANG Y L,WEI G F,HE J J,WANG Y F,ZHAO L P. Information theory-based algorithm for in silico prediction of PCR products with whole genomic sequences as templates[J]. BMC Bioinformatics,2005,6:190.
[27] WANG X,HUANG B X,BLAIR B,EGLEZOS S,BATES J.Selection of optimal combinations of loci by the Optimal Combination Finder computer program from a group of variable number tandem repeat loci for use in Staphylococcus aureus food poisoning case investigations[J]. Journal of Medical Microbiology,2012,61(Pt 5):631-639.
[28] R Core Team.R:A language and environment for statisticalcomputing[CP]. R Foundation for Statistical Computing,Vienna,Austria.URL.2020.https://www.R-project.org/.
[29] KAMVAR Z N,TABIMA J F,EVERHART S E,BROOKS J C,KRUEGER-HADFIELD S A. Package‘poppr’[CP]. 2020.https://grunwaldlab.github.io/poppr.
[30] MAZZAGLIA A,TURCO S,TARATUFOLO M C,TATÌ M,RAHI Y J,GALLIPOLI L,BALESTRA G M. Improved MLVA typing reveals a highly articulated structure in Pseudomonas syringae pv. actinidiae populations[J]. Physiological and Molecular Plant Pathology,2021,114:101636.
[31] IKAWATY R,WILLEMS R J L,BOX A T A,VERHOEF J,FLUIT A C. Novel multiple-locus variable-number tandem-repeat analysis method for rapid molecular typing of human Staphylococcus aureus[J]. Journal of Clinical Microbiology,2008,46(9):3147-3151.
[32] CONCEIÇÃO T,DE SOUSA M A,DE LENCASTRE H.Staphylococcal interspersed repeat unit typing of Staphylococcus aureus:Evaluation of a new multilocus variable-number tandem-repeat analysis typing method[J]. Journal of Clinical Microbiology,2009,47(5):1300-1308.