苹果(Malus domestica)属于双子叶蔷薇科植物,是世界上广泛栽培的水果之一。中国苹果栽培面积及产量均居世界首位,苹果产业在中国农村经济振兴、促进农民增收和出口创汇中发挥着重要的作用[1]。利用现代分子生物学技术开展苹果种质改良与创新,能够进一步提高苹果生产效率及品质[2]。建立稳定的苹果遗传转化体系是关键基因发掘及功能验证的必要条件之一,目前主要通过根癌农杆菌转化法、发根农杆菌转化法实现[3]。根癌农杆菌转化方法具有表达稳定等优点,但其依赖于组培无菌条件下进行,操作过程繁琐,转化周期长、成本高[4-6]。发根农杆菌转化方法相比根癌农杆菌转化法具有转化效率高、操作步骤简单、用时短等优点,尤其在土壤逆境相关抗性基因的挖掘及功能验证中发挥重要作用[7-9]。因此建立一个简单、快速、高效的苹果发根农杆菌转化体系具有重要的意义。
发根农杆菌(Agrobacterium rhizogenes)属于根瘤菌科农杆菌属,是一种侵染性强的好氧型革兰氏阴性细菌,具有感染大多数双子叶植物能力,其携带Ri 质粒中T-DNA 片段在完成侵染植物后可整合至植物基因组中,从而诱导植物形成毛状根(hairy root)[10-11]。毛状根是具有分枝多、生长快、遗传特性稳定等特性的不定根[12],通常用来进行植株基因过表达、基因沉默和基因编辑等[13-15]。发根农杆菌介导的遗传转化技术在草本植物如甘薯[16]、大豆[17]、菠菜[18]、花生[19]、大白菜[20-21]中运用较为普遍,而在部分木本植物中仍存在转化率低的问题。随着发根农杆菌转化技术的不断进步,目前在核桃[22]、苹果[2,23]、梨[24]、茶树[11]、光皮桦[25]、银杏树和桉树[26]等材料研究中的研究结果表明,使用不同方法进行发根农杆菌侵染毛状根诱导率存在较大差异,木本植物毛状根的诱导率为2%~88.3%[27-28]。
目前苹果中常规的发根农杆菌转化体系以注射方法为主[9,13,29],对苹果幼苗进行发根农杆菌侵染虽具有一定的毛状根诱导效率,但在注射实际操作过程中仍存在效率低下问题,例如幼苗注射后难以存活,大苗注射后毛状根诱导率较低,同时也因物理损伤及后期剪断主根等操作极大地延长遗传转化时间。笔者在本研究中拟利用发根农杆菌K599 建立一种高效转化体系,通过对不同苗龄材料及不同侵染方法的发根率统计,筛选苹果砧木平邑甜茶(Ma‐lus hupehensis var. mengshanensis G.Z.Qian & W.H.Shao)发根农杆菌转化效率高、操作简单、用时较短的转化方法;此外鉴定发根农杆菌K599在转化株系中的迁移性,以期为平邑甜茶中基因功能的分析提供更为有效、便捷的方法,同时为发根农杆菌技术的进一步利用提供理论依据。
1 材料和方法
1.1 植物材料
以平邑甜茶(具有无融合生殖特性)实生苗为试验材料。幼苗培养于光照时间16 h·d-1、光照度100 μmol·m-2·s-1、(25±2)℃的苗木培养室。发根农杆菌K599 感受态购自北京庄盟国际生物基因科技有限公司。用于遗传转化载体基于pCAMBIA1305载体骨架改造,同时携带β-葡萄糖苷酸酶基因(βglucuronidase,GUS)及绿色荧光蛋白基因(green fluorescent protein,GFP),由西北农林科技大学园艺学院李征教授惠赠。
1.2 试验方法
1.2.1 催芽与播种 将平邑甜茶种子用纱布包裹好,放置于水龙头下冲洗1 d,将冲洗后的种子同灭菌后粗砂混合后置于玻璃皿中,随后放置于4 ℃冰箱,每隔3 d 检查粗砂湿度,挑除发霉种子。待种子萌发露白后直接播种在装有灭菌基质(草炭∶蛭石∶珍珠岩=3∶1∶1,体积比)穴盘中,喷水覆膜后放置于苗木培养室中培养。幼苗生长至三叶龄(木质化程度较低)、五叶龄(木质化程度增加)、八叶龄(木质化程度高)时用于后续侵染试验。
1.2.2 发根农杆菌转化 从-80 ℃冰箱中取出发根农杆菌K599感受态迅速置于冰上融化,每100µL感受态加入1µg 载体质粒,缓慢吹打混匀后分别在冰上静置5 min、液氮中5 min、37 ℃水浴锅中5 min,冰上静置5 min。随后加入500µL TY 培养液,置于28 ℃恒温摇床中震荡培养2 h 后,均匀涂布于含50 mg·L-1链霉素、50 mg·L-1卡那霉素的固体TY 培养基,28 ℃倒置培养2 d。挑取单克隆接种于600µL含链霉素和卡那霉素的液体LB 培养基中,28 ℃200 r·min-1振荡培养过夜。菌液经PCR 鉴定后进行50%甘油保菌,保存于-80 ℃冰箱备用。
1.2.3 菌株活化与侵染液的制备 将-80 ℃冰箱保存的菌株在TY 固体培养基上均匀涂布后倒置活化培养2 d,用无菌的枪头挑取单菌落接种于500 μL TY 液体培养基中,28 ℃、200 r·min-1 振荡培养12 h,吸取100 μL 活化的菌液接种于100 mL TY 液体培养基中继续过夜振荡培养,待菌液OD600值为0.8时,集菌后重悬于MES缓冲液,静止3 h后进行注射。
1.2.4 毛状根诱导 注射法侵染:利用2 mL无菌注射器吸取阳性菌株重悬液,注射于平邑甜茶幼苗根颈处。
菌液浸染法:在平邑甜茶幼苗根颈处将主根系剪除,随后直接浸入活化菌液中,浸入深度3 cm 左右,浸染时间30 min。
菌株涂抹法:在平邑甜茶幼苗根颈处将主根系剪除,利用无菌涂布器将平板上的菌株收集起来,涂抹于平邑甜茶幼苗剪口处。
侵染后把幼苗移栽到基质中,使用基质覆盖侵染部位,随后盖上育苗盘透明保湿罩,在黑暗条件下共培养2 d。共培养结束后转移至正常光照条件下培养,注意通过定期喷水保湿,而不是通过托盘中大量浇水保湿。培养30 d后统计成功诱导出毛状根的株数,计算诱导率。
1.2.5 毛状根和转基因根的鉴定及统计分析 为了鉴定平邑甜茶遗传转化株系毛状根有效性,使用便携式荧光蛋白激发光源(LUYOR-3415,美国)鉴定诱导出毛状根GFP信号并拍照。为了鉴定目的基因是否整合到平邑甜茶根系基因组中,进一步提取毛状根DNA,通过PCR 克隆GUS 标签基因,有目的条带的即为阳性(引物见表1)。采取成功转化株系根系及叶片进行GUS 染色,采用北京华越洋生物科技有限公司GUS 染色试剂盒(CAT:GT0391),染色方法见试剂盒说明书。提取转化株系根和叶的RNA,反转录后进行GUS基因表达量分析,相关引物见表1。选取3株阳性转化株系的根和叶片进行蛋白印迹分析,平邑甜茶根叶组织总蛋白提取采用碧云天植物Western 及IP 细胞裂解液(P0043)提取,蛋白印迹检测一抗为Biolinkedin@ Anti-GFP 鼠单克隆抗体(LMAb06),二抗为SIGMA-ALDRICH 公司抗小鼠IgG(Fc特异性,CAT:A1418),采用北京庄盟国际生物基因科技有限公司碱性磷酸酶底物显色试剂盒(CAT:ZD315)进行显色,具体操作步骤见试剂盒说明书。
表1 引物列表
Table 1 Primer list
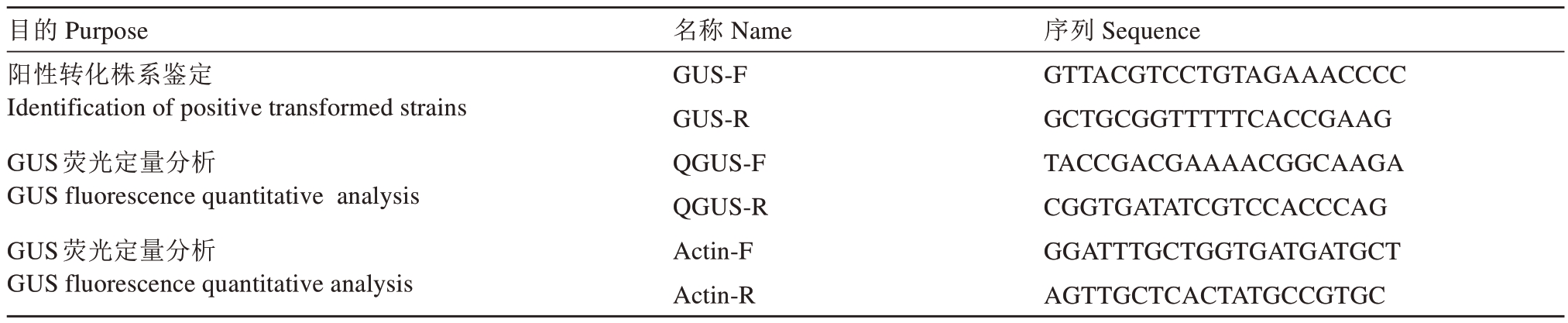
目的Purpose阳性转化株系鉴定Identification of positive transformed strains GUS荧光定量分析GUS fluorescence quantitative analysis GUS荧光定量分析GUS fluorescence quantitative analysis名称Name GUS-F GUS-R QGUS-F QGUS-R Actin-F Actin-R序列Sequence GTTACGTCCTGTAGAAACCCC GCTGCGGTTTTTCACCGAAG TACCGACGAAAACGGCAAGA CGGTGATATCGTCCACCCAG GGATTTGCTGGTGATGATGCT AGTTGCTCACTATGCCGTGC
1.2.6 数据统计与分析 采用Excel 记录试验数据,运用Prism8.0进行数据分析,使用Adobe illustrator作图。
2 结果与分析
2.1 平邑甜茶注射法发根农杆菌侵染体系建立
选取三叶龄、八叶龄的平邑甜茶幼苗为材料,进行注射法进行侵染(图1-A)。在培养30 d 后统计发现,以八叶龄幼苗为材料的毛状根诱导率为39%(表2),以三叶龄幼苗侵染材料的毛状根诱导率为35%,需要注意的是在注射后3 d 左右发现三叶龄平邑甜茶幼苗不断萎蔫(图1-B),经观察为注射创伤较大导致伤口溃烂,进而引起植株萎蔫死亡,在去除萎蔫株系后生根率为67.9%。对发根农杆菌诱导出的毛状根进行荧光检测,发现所有毛状根均具有GFP 荧光。综合以上结果表明,利用注射法进行发根农杆菌转化时,总体上以八叶龄幼苗为材料要优于三叶龄幼苗,而抛除萎蔫死亡株系后,三叶龄幼苗诱导毛状根能力更强。此外,注射法转化株系,剪去主根后毛状根通常分布在注射孔一侧(图1-C、D、E)。
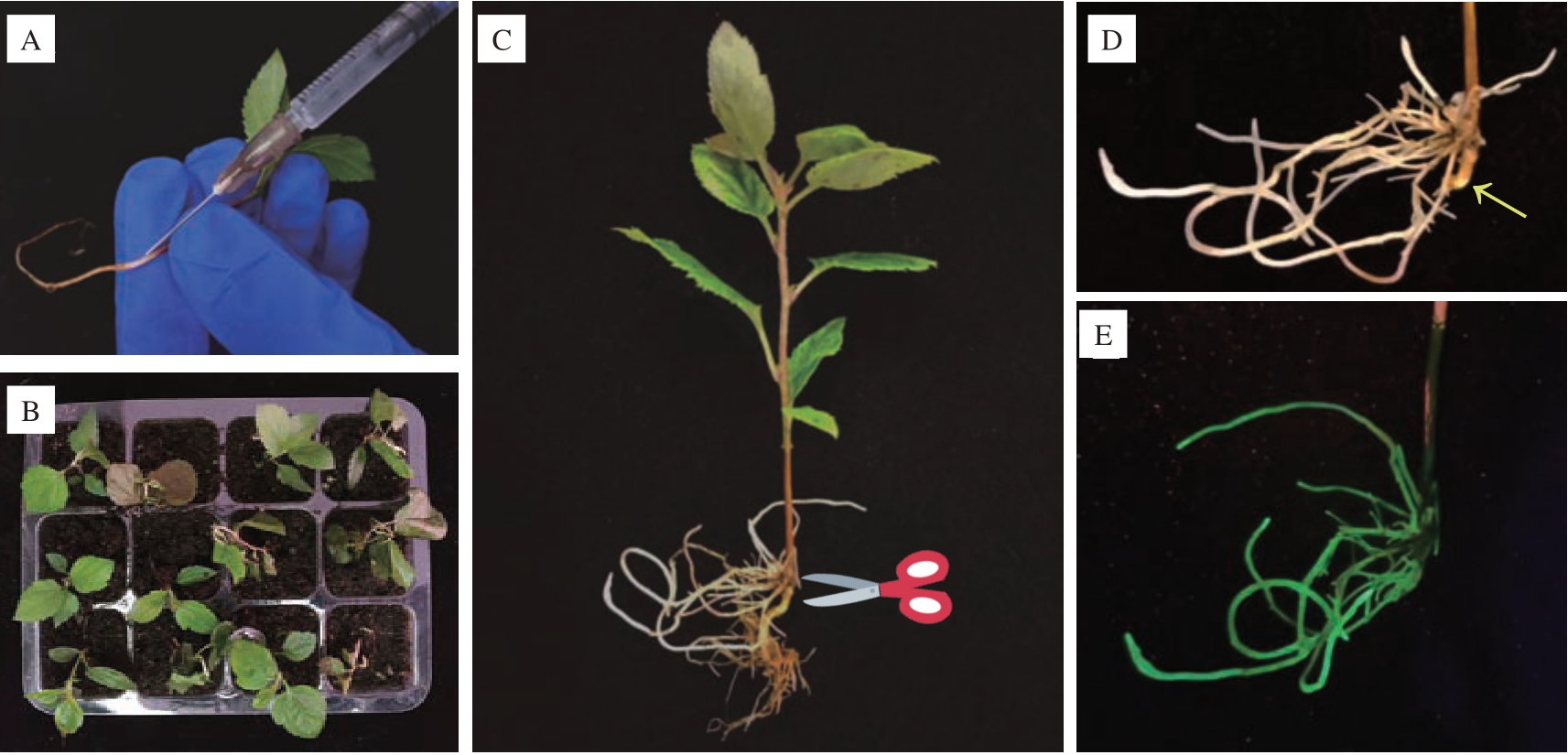
图1 平邑甜茶注射法转化的关键步骤
Fig.1 Key steps for transformation of A.rhizogenes by injection method in M.hupehensis var.mengshanensis
A.发根农杆菌菌液注射;B.三叶龄平邑甜茶幼苗侵染7 d 后状态;C~D.剪去成功转化株系主根;E.毛状根荧光检测。
A.A.rhizogenes injection;B.State of M.hupehensis var.mengshanensis seedlings at three-leaf stage after one week of injection;C-D.Cut off the main root of the successfully transformed strain;E.Hairy root fluorescence detection.
表2 采用注射法侵染不同苗龄平邑甜茶幼苗毛状根诱导率
Table 2 Hairy root induction rate of apple seedlings of different ages infected by injection
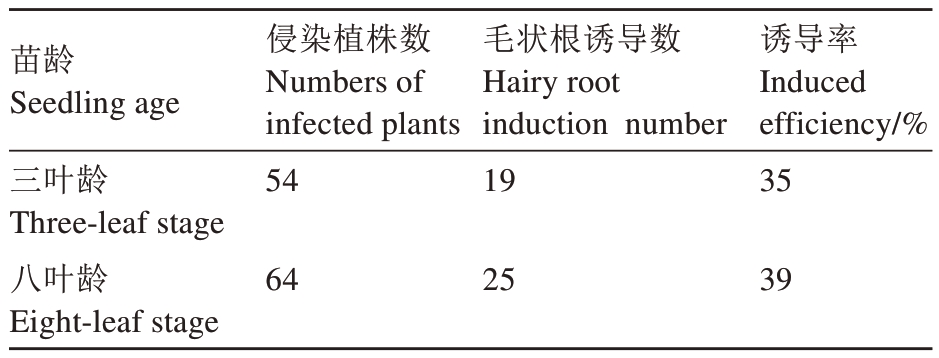
苗龄Seedling age三叶龄Three-leaf stage八叶龄Eight-leaf stage侵染植株数Numbers of infected plants 54毛状根诱导数Hairy root induction number 19诱导率Induced efficiency/%35 64 25 39
2.2 不同苗龄对平邑甜茶毛状根诱导率的影响
苹果幼苗注射法存在转化效率整体较低、操作步骤繁琐、用时较长等问题。近几年随着发根农杆菌技术的不断发展,发现使用幼嫩植株直接浸泡菌液或刮取细菌菌株涂抹方法可以更为简单、快速、高效地获得转基因株系[28,30-31]。为了缩短遗传转化时间,简化操作步骤,接下来分别采用菌液浸染法和菌株涂抹法对平邑甜茶发根农杆菌遗传转化体系进行优化。选取三叶龄、五叶龄、八叶龄平邑甜茶幼苗为材料,使用菌液浸染法转化后培养30 d,对毛状根诱导株数进行统计(图2)。
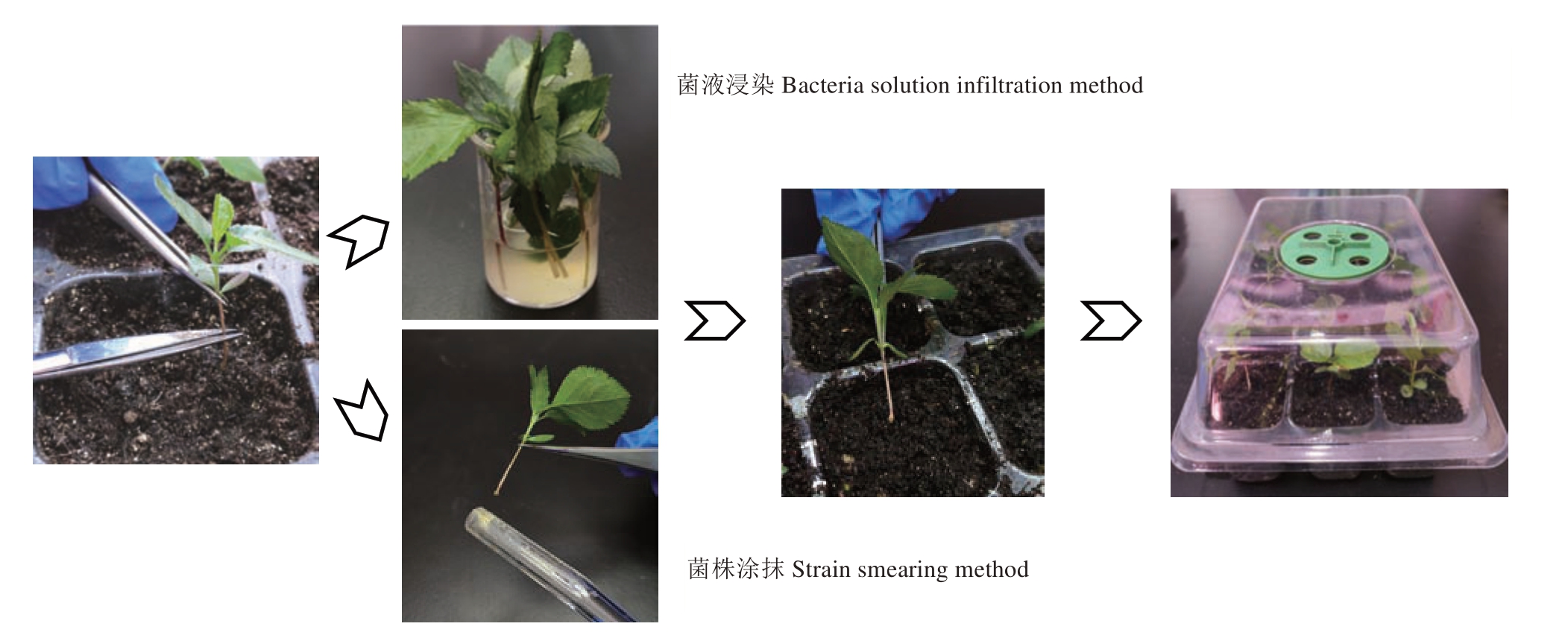
图2 菌液浸染法与菌株涂抹法关键步骤
Fig.2 Key steps of bacteria solution infiltration method and strain smearing method
结果表明,不同苗龄平邑甜茶的毛状根的诱导数存在差异,三叶龄平邑甜茶苗的毛状根诱导率最高,为84%,五叶龄、八叶龄平邑甜茶幼苗的诱导率分别为47%、52.5%,因此三叶龄平邑甜茶苗更适宜作为菌液浸染法的转化材料(表3)。为了进一步优化毛状根诱导流程,选取三叶龄、八叶龄平邑甜茶幼苗为材料,使用菌株涂抹法进行侵染后扦插于穴盘中培养30 d,毛状根诱导率统计结果表明,不同苗龄平邑甜茶苗都能成功诱导毛状根,其中三叶龄直接涂抹菌株处理毛状根诱导率为96%,八叶龄毛状根诱导率为60%(表3)。此外对比以上途径诱导出的毛状根后,发现三叶龄平邑甜茶苗菌株涂抹所诱导出根系均匀分布于茎段基部,其根系更为丰富,且同一批转化幼苗整齐度较高(图3)。通过GFP 荧光观察发现毛状根系均有荧光信号。除此之外,对比毛状根诱导率较高的转化方法后发现,从种植幼苗开始算起注射法整个转化周期要60~90 d才能获取转基因株系,而以三叶龄平邑甜茶幼苗为材料采用菌株涂抹法可以将转基因株系获取时间缩短至45 d,并且拥有更高的毛状根发根整齐度及根系结构(图3)。综合以上结果表明,选择三叶龄平邑甜茶幼苗作为转化材料,采用菌株涂抹方式去侵染可以较快、简单、高效地获得整齐度高的转化株系。
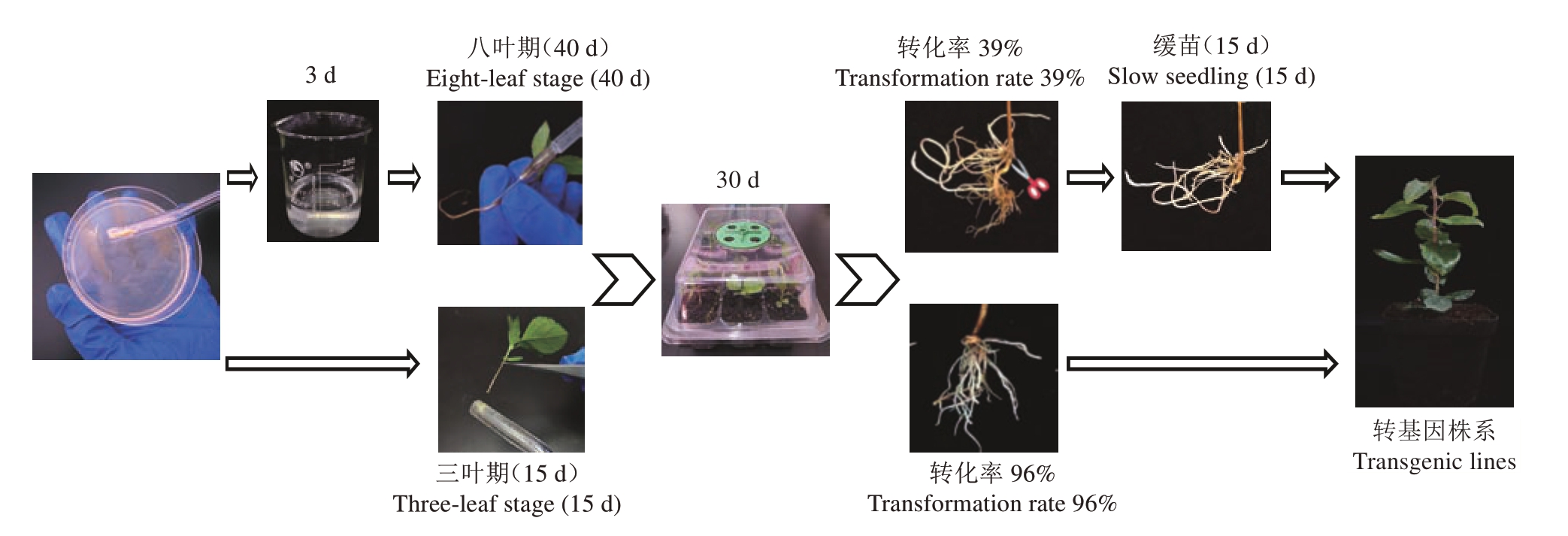
图3 不同平邑甜茶幼苗发根农杆菌转化体系对比
Fig.3 Comparison of different A.rhizogenes transformation systems in M.hupehensis var.mengshanensis seedlings
表3 不同侵染方式的平邑甜茶幼苗毛状根诱导率
Table 3 Hairy root induction rate of M.hupehensis var.mengshanensis seedlings with different infection methods
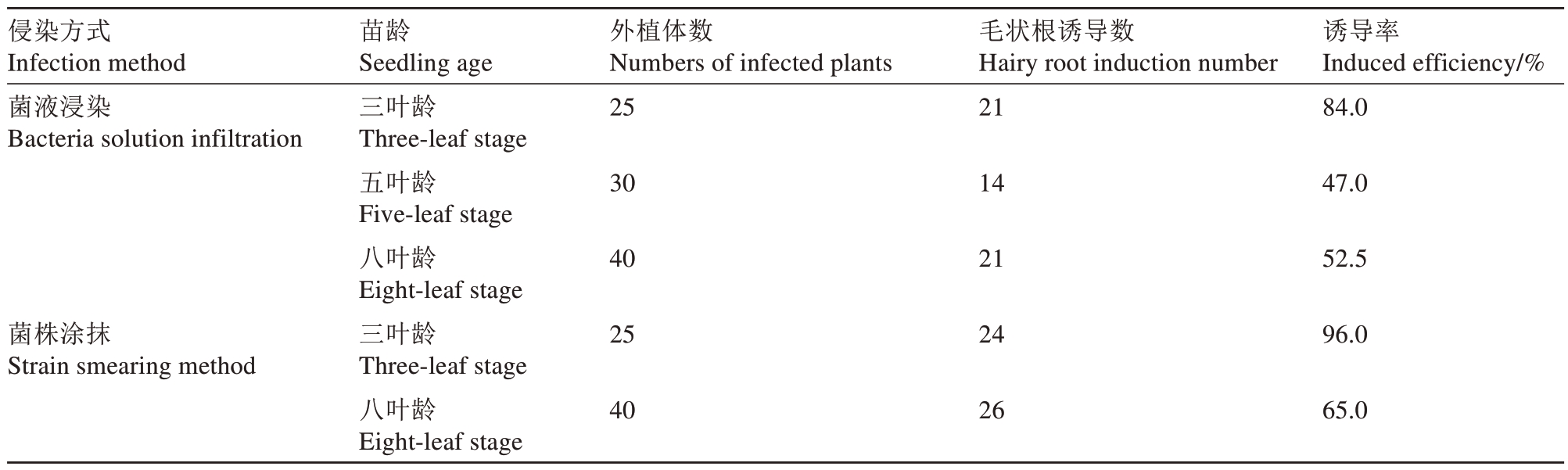
侵染方式Infection method菌液浸染Bacteria solution infiltration外植体数Numbers of infected plants 25毛状根诱导数Hairy root induction number 21苗龄Seedling age三叶龄Three-leaf stage五叶龄Five-leaf stage八叶龄Eight-leaf stage三叶龄Three-leaf stage八叶龄Eight-leaf stage诱导率Induced efficiency/%84.0 30 14 47.0 40 21 52.5菌株涂抹Strain smearing method 25 24 96.0 40 26 65.0
2.3 平邑甜茶发根农杆菌转化植株的鉴定
对平邑甜茶遗传转化株系毛状根进行荧光检测,结果表明,所有成功诱导出的毛状根系均有绿色荧光信号(图4-A)。为了鉴定目的基因是否整合到平邑甜茶根系基因组中,从不同组合中随机选取3株转化植株,进一步提取毛状根DNA,通过PCR 克隆GUS 标签基因后发现,所有毛状根均有阳性信号(图4-B)。
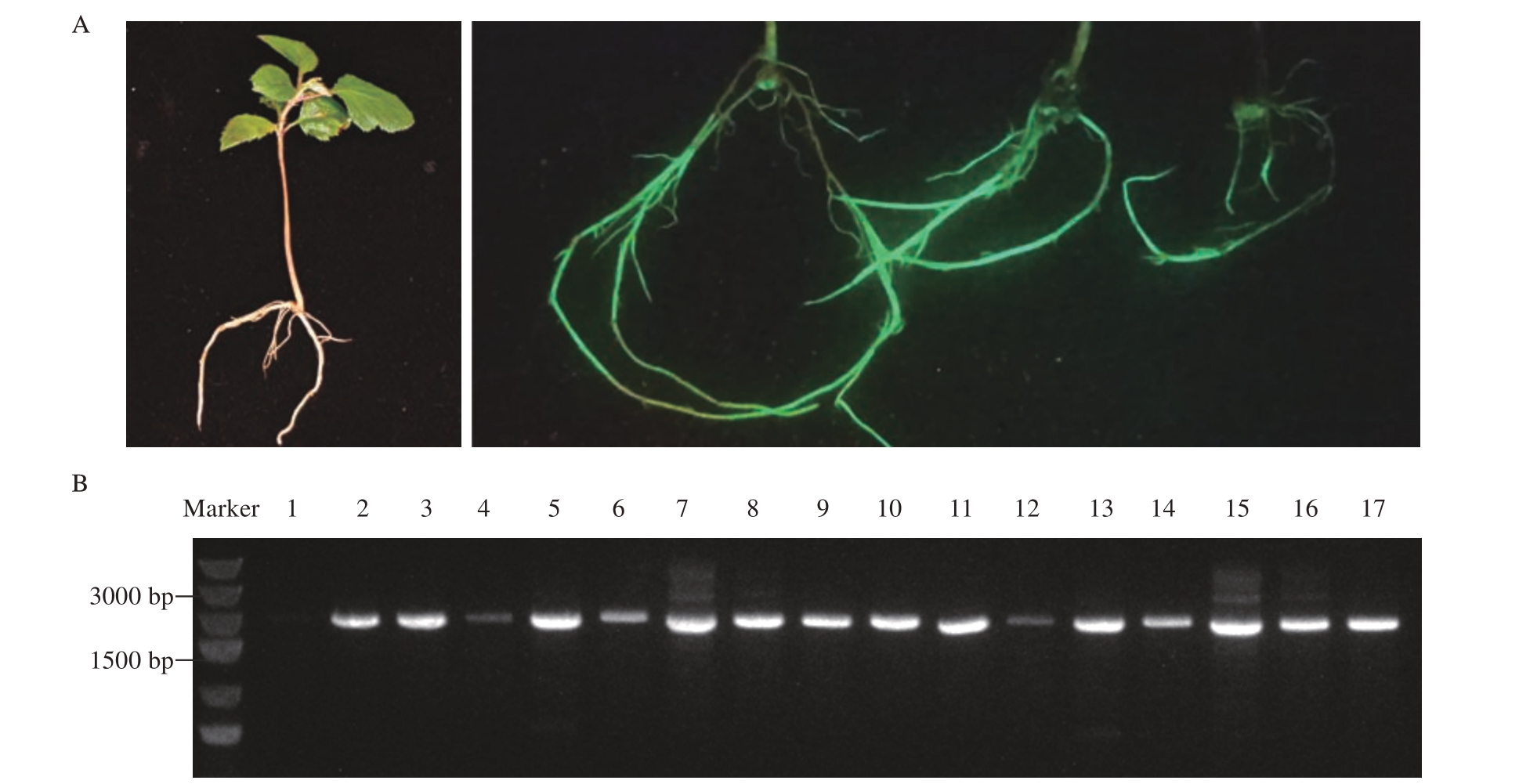
图4 平邑甜茶毛状根荧光检测及DNA 分子鉴定
Fig.4 Fluorescence detection and DNA molecular identification of hairy roots of M.hupehensis var.mengshanensis
A.平邑甜茶毛状根荧光检测;B.毛状根的DNA 分子鉴定,1 为空白对照,2~16 为毛状根DNA 分子检测,17 为阳性对照。
A.Fluorescence detection of apple hairy root;B.The DNA molecular identification of hairy roots showed that 1 was blank control,2-16 was DNA molecular detection of hairy roots,and 17 was positive control.
2.4 发根农杆菌在植株中迁移特性分析
为了鉴定发根农杆菌在转化植株中的迁移性,对转化植株根、茎和叶组织DNA 中GUS 基因进行克隆,发现根、茎和叶中均有GUS 的信号(图5-A)。对转化根系进行根和叶的GUS 染色分析,发现部分株系叶片叶柄、主叶脉中有GUS 信号(图5-B)。进一步对叶片中检测到GUS 信号株系的根、茎、叶样品中GUS 表达量进行检测,结果表明在茎段和叶片中均检测到GUS基因的微弱表达(图5-C)。随后对转化阳性株系根和叶片的总蛋白GFP 蛋白印迹检测发现,根系中检测到GFP 蛋白表达,而叶片中并未检测到GFP 荧光蛋白表达(图5-D)。综合以上结果表明,使用发根农杆菌遗传转化技术获得株系中,发根农杆菌存在随机通过脉管组织向地上部迁移的现象。
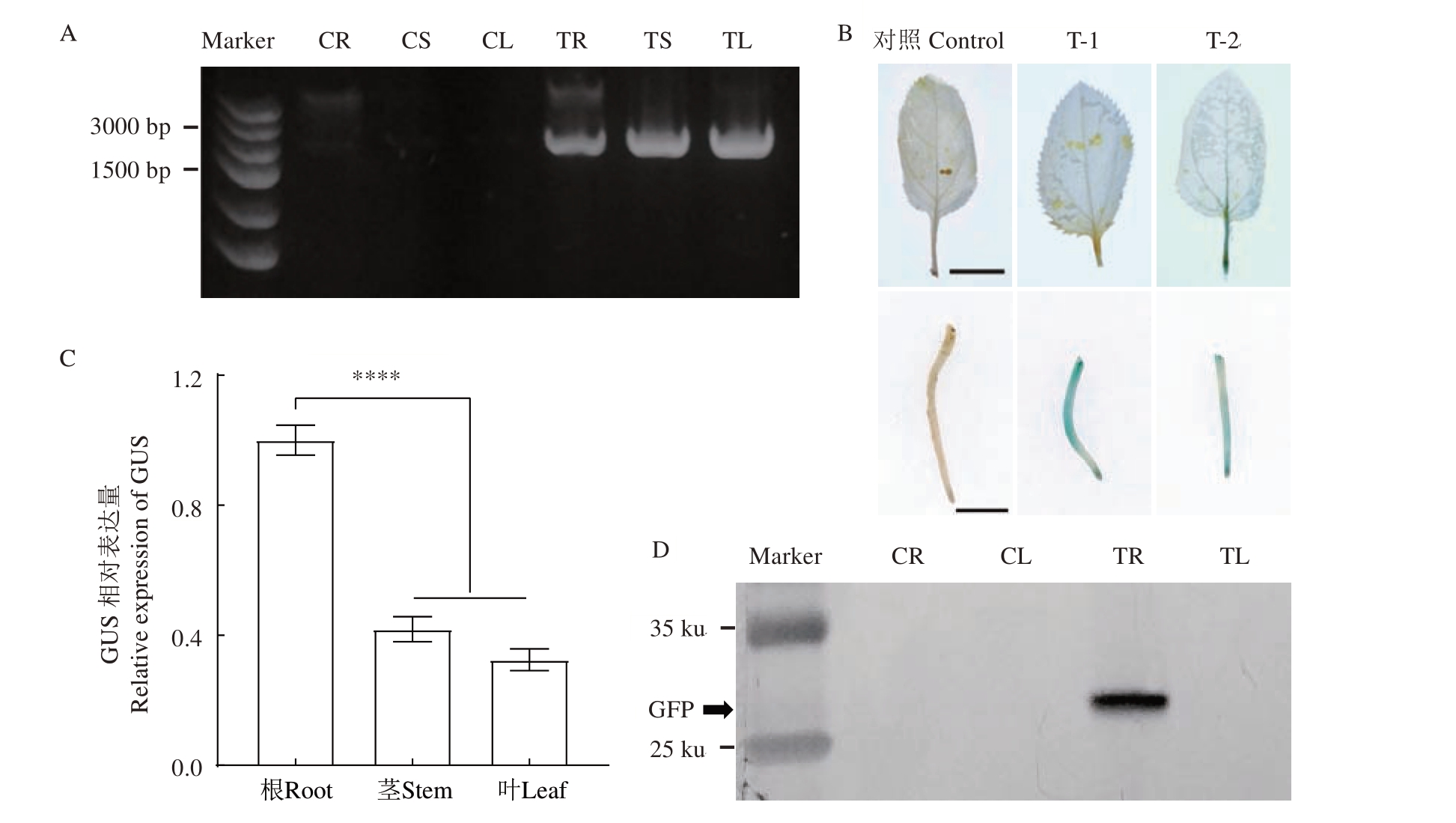
图5 转化植株中发根农杆菌迁移性分析
Fig.5 Analysis of migration of A.rhizogenes in transformed plants
A.平邑甜茶转基因株系根茎叶间DNA 分子检测,CR 为未转化株系的根系,CS 为未转化株系的茎段,CL 为未转化株系的叶片,TR 为转化株系的根系,TS 为转化株系的茎段,TL 为转化株系的叶片。B.发根农杆菌转化株系根和叶GUS 染色检测,对照为未转化株系根和叶,T-1、T-2 为转化株系根和叶。叶片比例尺为1.0 cm,根系比例尺为0.5 cm。C.发根农杆菌转化株系根、茎和叶中GUS 基因相对表达量分析。****,p<0.000 1。D.平邑甜茶转基因株系根叶间GFP 蛋白印迹检测,CR 为未转化株系的根系,CL 为未转化株系的叶片,TR 为转化株系根系,TL 为转化株系叶片。
A.Molecular detection of DNA between roots, stems and leaves of transgenic M.hupehensis var. mengshanensis lines.CR is the root of the untransformed line,CS is the stem segment of the untransformed line,CL is the leaf of the untransformed line,TR is the root system of the transformed line,TS is the stem segment of the transformed line, and TL is the leaf of the transformed line.B.GUS staining of roots and leaves of A.rhizogenes transformed strains,control is the untransformed plant,while T1 and T2 are the root and leaf of the transformed plant.The leaf scale is 1.0 cm and the root scale is 0.5 cm.C.Analysis of relative expression of GUS gene in roots,stems and leaves of A.rhizogenes transformed lines.****, p<0.000 1.D.GFP Western blotting detection between roots and leaves of transgenic M.hupehensis var. mengshanensis lines.CR is the root of the untransformed plant,CL is the leaf of the untransformed plant,TR is the root of the transformed plant,and TL is the leaf of the transformed plant.
3 讨 论
植物转基因技术广泛应用于植物种质改良,也是鉴定基因功能的关键技术[7,12,32]。然而对于木本植物,基于根癌农杆菌稳定转化体系存在着费时、操作繁琐等问题,不能快速获得转化植株进行功能验证[5,33]。而发根农杆菌遗传转化体系的建立能够快速得到具备稳定转化的根系,从而快速进行一些基因功能的验证,尤其在土传病害抗性基因的挖掘与营养元素高效吸收相关基因的鉴定方面[3,34-35]。例如使用发根农杆菌转化技术在苹果幼苗中过表达Md‐NRT2.4 可以显著提高对低氮的耐受性[10],过表达MdWRKY75可增强苹果根系对F.solani的抗性[7],过表达CHS促进了类黄酮的积累和对氮的吸收[30]。然而目前苹果发根农杆菌体系往往需要60~90 d 才能获得转基因植物。此外在毛状根诱导的过程中,如注射法,采用木质化程度较低的三叶龄幼苗进行注射时很容易将茎段刺穿从而导致幼苗萎蔫死亡,采用木质化程度较高的八叶龄平邑甜茶幼苗注射法存在毛状根诱导率较低、根系分布不均匀等问题。随着植物发根农杆菌转化方法的不断改进,直接浸泡菌液或刮取细菌菌株涂抹方法被证明是具有更为快速、简单、高效等优点的转化方法[28,31,36]。笔者在本研究中使用菌液直接浸泡法和涂抹菌株法对不同苗龄平邑甜茶幼苗进行侵染,结果表明,以菌液涂抹法毛状根诱导率最高(96%)。通过GFP 荧光观察和PCR 分析发现,采用菌株涂抹法诱导毛状根根系均具有转化阳性信号,该结果与先前在山丁子中得到的结果相似[23]。本研究中首次使用菌株涂抹法对木质化程度较低的三叶龄平邑甜茶幼苗进行侵染,对比传统注射法可将转化周期由60~90 d缩短至45 d,转化率相比传统注射法明显增高,并且已达到相同方法在草本植物的转化率水平[37],同时拥有更好的毛状根发根整齐度及根系结构。
先前报道表明,农杆菌在植株中具有一定的迁移性,通过发根农杆菌转化株系通常会引起烟草叶片卷曲等表型,在通常情况下是无害的[10],柑橘发根农杆菌转化株系中也发现农杆菌从根系到茎中的转移[38],但目前发根农杆菌在植株中迁移及转化规律尚不明确。为了探明在发根农杆菌在苹果植株中的迁移性,对成功转化植株根、叶样品进行检测,发现在平邑甜茶中存在着随机向上迁移的现象,然而这种传递仅在叶柄及部分主叶脉处有表达,对整个叶片进行蛋白水平上检测并未检出明显阳性信号,可能是培养时间较短导致表达量较低,或者表达部位仅局限在叶柄和部分叶脉所致。以上结果可为苹果发根农杆菌转化体系的应用提供理论基础,例如在鉴定根、茎、叶间长距离传递信号中存在一定的假阳性,需要通过增加空载质粒转化株系的数量来验证目的传递基因及蛋白信号的可信度。
4 结 论
笔者在本研究中建立了快速、简单、高效的发根农杆菌K599 介导的平邑甜茶转化体系。利用菌株涂抹法侵染三叶龄幼苗,可在45 d 左右获得整齐度较高的阳性毛状根植株,诱导率高达96%。验证了发根农杆菌在转化植株内随机向地上部迁移的现象。
[1] 王忆,杨晓光,谢红江,王春良,王继勋,张建军,郝淑英,马钧,于文全,张宏,高建国,韩振海.基于品质和效益的我国苹果栽培新区区划初见[J].中国果树,2022(11):1-5.WANG Yi,YANG Xiaoguang,XIE Hongjiang,WANG Chunliang,WANG Jixun,ZHANG Jianjun,HAO Shuying,MA Jun,YU Wenquan,ZHANG Hong,GAO Jianguo,HAN Zhenhai.Preliminary views of regional plantation for apple production in China referred to fruit quality and orchard income[J].China Fruits,2022(11):1-5.
[2] 武姣,孔瑾,王忆,韩振海,许雪峰.发根农杆菌介导山定子遗传转化及发根再生植株[J].园艺学报,2008,35(7):959-966.WU Jiao,KONG Jin,WANG Yi,HAN Zhenhai,XU Xuefeng.Agrobacterium rhizogenes-mediated transformation and hairy root regeneration of Malus baccata (L.) Borkh.[J].Acta Horticulturae Sinica,2008,35(7):959-966.
[3] 王玉珠.发根农杆菌介导苹果毛状根遗传转化体系的建立[D].杨凌:西北农林科技大学,2023.WANG Yuzhu.Establishment of Agrobacterium rhizogenes mediated hairy root genetic transformation system in Malus domes‐tica[D].Yangling:Northwest A&F University,2023.
[4] WANG S J,WANG G,LI H L,LI F,WANG J B.Agrobacteri‐um tumefaciens-mediated transformation of embryogenic callus and CRISPR/Cas9-mediated genome editing in‘Feizixiao’litchi[J].Horticultural Plant Journal,2023,9(5):947-957.
[5] 王忆.苹果再生体系建立与MxIrt1 基因功能的初步研究[D].北京:中国农业大学,2005.WANG Yi.The establishment of apple regeneration system and functional characterization of MxIrt1 gene[D].Beijing:China Agricultural University,2005.
[6] 秦玲.苹果(Malus domestica Borkh.)遗传转化体系的建立及ipt 基因的导入[D].杨凌:西北农林科技大学,2002.QIN Ling.Establishment of the genetic transformation system and transferring ipt genes into apple (Malus domestica Borkh.)[D].Yangling:Northwest A&F University,2002.
[7] LIU Y S,LIU Q W,LI X W,ZHANG Z J,AI S K,LIU C,MA F W,LI C.MdERF114 enhances the resistance of apple roots to Fusarium solani by regulating the transcription of MdPRX63[J].Plant Physiology,2023,192(3):2015-2029.
[8] CHAI X F,WANG X N,PI Y,WU T,ZHANG X Z,XU X F,HAN Z H,WANG Y.Nitrate transporter MdNRT2.4 interacts with rhizosphere bacteria to enhance nitrate uptake in apple rootstocks[J].Journal of Experimental Botany,2022,73(18):6490-6504.
[9] SMOLKA A,LI X Y,HEIKELT C,WELANDER M,ZHU L H.Effects of transgenic rootstocks on growth and development of non-transgenic scion cultivars in apple[J].Transgenic Research,2010,19(6):933-948.
[10] TEPFER D.Transformation of several species of higher plants by Agrobacterium rhizogenes:Sexual transmission of the transformed genotype and phenotype[J].Cell,1984,37(3):959-967.
[11] ALAGARSAMY K,SHAMALA L F,WEI S.Protocol:High-efficiency in- planta Agrobacterium- mediated transgenic hairy root induction of Camellia sinensis var. sinensis[J].Plant Methods,2018,14:17.
[12] STANIŠIĆ M,ĆOSIĆ T,SAVIĆ J,KRSTIĆ-MILOŠEVIĆ D,MIŠIĆ D,SMIGOCKI A,NINKOVIĆ S,BANJAC N.Hairy root culture as a valuable tool for allelopathic studies in apple[J].Tree Physiology,2019,39(5):888-905.
[13] 张月乔,葛洁,田树娟,袁黎.利用发根农杆菌体系检测西瓜CRISPR/Cas9 系统的靶位点[J].中国瓜菜,2020,33(4):7-11.ZHANG Yueqiao,GE Jie,TIAN Shujuan,YUAN Li.Detection of target sites of CRISPR/Cas9 system in watermelon by Agro‐bacterium rhizogenes system[J].China Cucurbits and Vegetables,2020,33(4):7-11.
[14] HU Q Y,DONG J H,YING J L,WANG Y,XU L,MA Y B,WANG L,LI J X,LIU L W.Functional analysis of RsWUSb with Agrobacterium-mediated in planta transformation in radish(Raphanus sativus L.) [J].Scientia Horticulturae,2024,323:112504.
[15] QIN T J,WANG S,YI X F,YING J L,DONG J H,YAO S Q,NI M,LIU L W,XU L,WANG Y.Development of a fast and efficient root transgenic system for exploring the function of RsMYB90 involved in the anthocyanin biosynthesis of radish[J].Scientia Horticulturae,2024,323:112490.
[16] 陶娜,李茂兴,郭华春.发根农杆菌介导的甘薯遗传转化体系优化[J].生物技术通报,2023,39(10):175-183.TAO Na,LI Maoxing,GUO Huachun.Optimization of sweet potato genetic transformation system mediated by Agrobacterium rhizogenes[J].Biotechnology Bulletin,2023,39(10):175-183.
[17] CHENG Y Y,WANG X L,CAO L,JI J,LIU T F,DUAN K X.Highly efficient Agrobacterium rhizogenes-mediated hairy root transformation for gene functional and gene editing analysis in soybean[J].Plant Methods,2021,17(1):73.
[18] 徐悦,曹英萍,王玉,付春祥,戴绍军.发根农杆菌介导的菠菜毛状根遗传转化体系的建立[J].植物学报,2019,54(4):515-521.XU Yue,CAO Yingping,WANG Yu,FU Chunxiang,DAI Shaojun. Agrobacterium rhizogenes-mediated transformation system of Spinacia oleracea[J].Chinese Bulletin of Botany,2019,54(4):515-521.
[19] SHARMA K K,ANJAIAH V.An efficient method for the production of transgenic plants of peanut (Arachis hypogaea L.)through Agrobacterium tumefaciens-mediated genetic transformation[J].Plant Science,2000,159(1):7-19.
[20] WANG H Y,ZHENG Y S,ZHOU Q,LI Y,LIU T K,HOU X L.Fast,simple,efficient Agrobacterium rhizogenes-mediated transformation system to non-heading Chinese cabbage with transgenic roots[J/OL].Horticultural Plant Journal,[2023-11-13].https://doi.org/10.1016/j.hpj.2023.03.018.
[21] LI X N,LI H Y,ZHAO Y Z,ZONG P X,ZHAN Z X,PIAO Z Y.Establishment of A simple and efficient Agrobacterium-mediated genetic transformation system to Chinese cabbage (Brassi‐ca rapa L.ssp.pekinensis)[J].Horticultural Plant Journal,2021,7(2):117-128.
[22] 谢晓婷,黄巧宇,温广超,袁虎威,何漪,闫道良,黄坚钦,王晓飞,郑炳松.非组培依赖的发根农杆菌介导的薄壳山核桃转化体系构建[J].果树学报,2022,39(1):131-140.XIE Xiaoting,HUANG Qiaoyu,WEN Guangchao,YUAN Huwei,HE Yi,YAN Daoliang,HUANG Jianqin,WANG Xiaofei,ZHENG Bingsong.Construction of Agrobacterium rhizogenesmediated transformation system of Carya illinoinensis without dependence on tissue culture[J].Journal of Fruit Science,2022,39(1):131-140.
[23] WU J,WANG Y,ZHANG L X,ZHANG X Z,KONG J,LU J,HAN Z H.High-efficiency regeneration of Agrobacterium rhizo‐genes-induced hairy root in apple rootstock Malus baccata (L.)Borkh.[J].Plant Cell,Tissue and Organ Culture,2012,111(2):183-189.
[24] XIAO Y X,ZHANG S C,LIU Y,CHEN Y,ZHAI R,YANG C Q,WANG Z G,MA F W,XU L F.Efficient Agrobacterium-mediated genetic transformation using cotyledons,hypocotyls and roots of‘Duli’(Pyrus betulifolia Bunge)[J].Scientia Horticulturae,2022,296:110906.
[25] 刘雪羽,杜笑雪,陈思源,胡现铬,黄华宏,童再康.发根农杆菌介导的光皮桦毛状根高频诱导体系及遗传转化[J].农业生物技术学报,2021,29(3):495-505.LIU Xueyu,DU Xiaoxue,CHEN Siyuan,HU Xiange,HUANG Huahong,TONG Zaikang. Agrobacterium rhizogenes mediated high frequency hairy root induction system and genetic transformation in Betula luminifera[J].Journal of Agricultural Biotechnology,2021,29(3):495-505.
[26] 罗萍,张昊楠,徐建民,胡冰,王晓萍,李光友,范春节.发根农杆菌介导的尾巨桉遗传转化体系的建立[J].植物研究,2022,42(3):512-520.LUO Ping,ZHANG Haonan,XU Jianmin,HU Bing,WANG Xiaoping,LI Guangyou,FAN Chunjie.Establishment of Agro‐bacterium rhizogenes-mediated genetic transformation system of Eucalyptus urophylla × E.grandis[J].Bulletin of Botanical Research,2022,42(3):512-520.
[27] MENG D,YANG Q,DONG B Y,SONG Z H,NIU L L,WANG L T,CAO H Y,LI H H,FU Y J.Development of an efficient root transgenic system for pigeon pea and its application to other important economically plants[J].Plant Biotechnology Journal,2019,17(9):1804-1813.
[28] YING W,WEN G C,XU W Y,LIU H X,DING W N,ZHENG L Q,HE Y,YUAN H W,YAN D L,CUI F Q,HUANG J Q,ZHENG B S,WANG X F. Agrobacterium rhizogenes:Paving the road to research and breeding for woody plants[J].Frontiers in Plant Science,2023,14:1196561.
[29] YAMASHITA H,DAIMON H,AKASAKA- KENNEDY Y,MASUDA T.Plant regeneration from hairy roots of apple rootstock,Malus prunifolia Borkh.var. ringo Asami,strain Nagano No.1,transformed by Agrobacterium rhizogenes[J].Journal of the Japanese Society for Horticultural Science,2004,73(6):505-510.
[30] WANG X N,CHAI X F,GAO B B,DENG C,GÜNTHER C S,WU T,ZHANG X Z,XU X F,HAN Z H,WANG Y.Multiomics analysis reveals the mechanism of bHLH130 responding to low-nitrogen stress of apple rootstock[J].Plant Physiology,2023,191(2):1305-1323.
[31] FAN Y L,XU F L,ZHOU H Z,LIU X X,YANG X Y,WENG K X,SUN X L,LYU S H.A fast,simple,high efficient and onestep generation of composite cucumber plants with transgenic roots by Agrobacterium rhizogenes-mediated transformation[J].Plant Cell,Tissue and Organ Culture,2020,141(1):207-216.
[32] NUCCIO M L,WU J,MOWERS R,ZHOU H P,MEGHJI M,PRIMAVESI L F,PAUL M J,CHEN X,GAO Y,HAQUE E,BASU S S,LAGRIMINI L M.Expression of trehalose-6-phosphate phosphatase in maize ears improves yield in well-watered and drought conditions[J].Nature Biotechnology,2015,33(8):862-869.
[33] LIU L F,GU Q S,IJAZ R,ZHANG J H,YE Z B.Generation of transgenic watermelon resistance to cucumber mosaic virus facilitated by an effective Agrobacterium-mediated transformation method[J].Scientia Horticulturae,2016,205:32-38.
[34] XU S L,LAI E H,ZHAO L,CAI Y M,OGUTU C,CHERONO S,HAN Y P,ZHENG B B.Development of a fast and efficient root transgenic system for functional genomics and genetic engineering in peach[J].Scientific Reports,2020,10:2836.
[35] BAHRAMNEJAD B,NAJI M,BOSE R,JHA S.A critical review on use of Agrobacterium rhizogenes and their associated binary vectors for plant transformation[J].Biotechnology Advances,2019,37(7):107405.
[36] FAN Y L,ZHANG X H,ZHONG L J,WANG X Y,JIN L S,LYU S H.One-step generation of composite soybean plants with transgenic roots by Agrobacterium rhizogenes-mediated transformation[J].BMC Plant Biology,2020,20(1):208.
[37] 王平勇,徐永阳,赵光伟,贺玉花,李明,孔维虎,张健,刘水苗,刘梦丽,徐志红.发根农杆菌介导甜瓜转基因过表达体系的建立[J].中国瓜菜,2019,32(12):15-18.WANG Pingyong,XU Yongyang,ZHAO Guangwei,HE Yuhua,LI Ming,KONG Weihu,ZHANG Jian,LIU Shuimiao,LIU Mengli,XU Zhihong.Establishment of Agrobacterium rhizo‐genes-mediated gene overexpression system in melon[J].China Cucurbits and Vegetables,2019,32(12):15-18.
[38] XIAO Y X,DUTT M,MA H J,XIAO C,TONG Z,WANG Z Q,HE X J,SUN Z H,QIU W M.Establishment of an efficient root mediated genetic transformation method for gene function verification in citrus[J].Scientia Horticulturae,2023,321:112298.