番石榴(Psidium guajava Linn.)又称芭乐、那拔,果实富含维生素C和膳食纤维,具有较高的营养价值和药用功效,是我国南方地区极为畅销的一种亚热带鲜食果品[1-4]。根据清代泉州知府高拱乾1695年纂修的《台湾府志》记载,番石榴传入中国迄今已有300 多年,台湾、福建、广东、海南、广西是目前我国番石榴主要栽培省份(地区)[5-6]。然而,在高温高湿的采收季节,番石榴果实采后容易发生真菌性病害。其中常见的番石榴真菌性病害包括炭疽病、环斑病、紫腐病、黑斑病、焦腐病等,这些病害的发生严重影响了番石榴果实采后品质及其果实产业的健康发展[7-8]。
2017—2022 年期间,笔者从采摘于福建省漳州诏安、漳州漳浦、漳州华安、泉州洛江、泉州晋江、泉州永春等地区的番石榴果实中发现环斑病。该环斑病症状一般发生于番石榴果实采后常温贮藏1周左右,且在西瓜红番石榴果实中发病率较高(发病率约30%),严重制约果实采后品质的保持。该番石榴果实环斑病的发病症状为果实表面出现水渍状凹陷褐色病斑,病斑逐渐扩大后,病斑边缘有一圈黄褐色环斑,且在病斑中心有大量的黑色分生孢子堆。
为了延长番石榴果实的货架期、控制其采后病害的发生,咪鲜胺等化学杀菌剂能有效起到果实防腐作用,进而稳定果实采后品质[9]。然而,长期使用化学杀菌剂造成化学残留、污染环境和危害人体健康等问题,因而有必要寻找安全的处理技术以控制番石榴果实病害的发生。ε-聚赖氨酸(ε-poly-L-lysine,ε-PL)是一种无毒无害、易溶解、抗真菌的天然防腐剂,在食品工业中得到了广泛应用[10]。另外,水杨酸(salicylic acid,SA)与褪黑素(melatonin,MT)均能延缓果实衰老,保持采后品质[11-12]。因此,笔者在本研究中通过分离鉴定福建省西瓜红番石榴环斑病菌,研究该致病菌侵染对番石榴果实采后品质的影响,探讨ε-PL、SA 和MT 处理对该致病菌体外抑制作用的影响,进而为控制番石榴果实采后环斑病发生、提高果实品质提供理论参考。
1 材料和方法
1.1 材料与仪器
西瓜红番石榴(成熟度为八成熟),福建省漳州市诏安县晓丰农业科技有限公司;马铃薯葡萄糖琼脂(potato dextrose agar,PDA)培养基,青岛高科技工业园海博生物技术有限公司。
JENCO-6173 台式pH 计,上海任氏电子有限公司;TA-XT Plus 质构仪,英国SMS 公司;SQ510C 高压灭菌器,重庆雅马拓科技有限公司;DM2000LED生物显微镜,徕卡显微系统公司;CR400 色差仪,日本柯尼卡美能达公司;PRX-450A恒温人工气候箱,浙江宁波赛福实验室有限公司;SW-CJ-1FD 超净工作台,苏州安泰空气技术有限公司;S230 型电导率仪,梅特勒托利多仪器有限(上海)公司。
1.2 环斑病菌的分离与形态鉴定
1.2.1 分离和纯化 参考陈蓬莲等[13]、亓政良等[14]的方法,用75%乙醇消毒发生环斑病的番石榴果皮病健交界处,用常规组织分离法获得组织块(3 mm×3 mm),经乙醇浸泡30 s、无菌水清洗、晾干后,接种到PDA 培养基(直径为90 mm,下同),在28 ℃下培养。当菌丝长到3 cm时,将边缘菌丝接种于新PDA培养基上,4次重复后获得纯化菌株(命名为F2)。
1.2.2 致病性测定和形态鉴定 参考陈蓬莲等[13]、Chen 等[15]及Chen 等[16]的方法,采用柯赫氏法则,将菌株F2 的菌丝块(直径为5 mm,下同)接种至已消毒的健康果实表面,以接种不含菌株F2的PDA培养基块为对照。将果实装袋后贮藏在28 ℃下,待果实发病后再分离病原菌并培养,与原接种菌株再对比,根据其菌落与分生孢子形态进行初步判定。
1.3 分子生物学鉴定
DNA提取、PCR扩增及病原菌基因测序等委托青岛亿信检测技术服务有限公司完成。利用Gen-Bank 数据库中的Basic Local Alignment Search Tool(BLAST)对菌株F2的ITS、TUB和TEF-1α测序结果进行分析;再通过比较菌株F2的序列与数据库中已有的其他物种序列之间的相似度[17]。最后,参考施俊凤等[18]的方法,采用MEGA 11.0 软件绘制系统发育树(邻接法)和Bootstraps 法检验(1000 次重复数)。
1.4 环斑病菌的生物学特性初探
1.4.1 碳源对菌落生长的影响 参考张居念等[19]、陈南泉等[20]及Cui 等[21]的方法,基于Czapek 固体培养基,将其中的蔗糖换成等质量葡萄糖、可溶性淀粉和D-果糖等碳素,以无蔗糖为对照。将菌株F2菌丝块接种至上述PDA 培养基中,在28 ℃、相对湿度(RH)90%下培养,10 d后测定菌落直径。
1.4.2 氮源对菌落生长的影响 参考张居念等[19]、陈南泉等[20]及Cui 等[21]的方法,基于Czapek 固体培养基,将其中的硝酸钠换成等质量蛋白胨、硝酸钾等氮素,以无硝酸钠为对照。将菌株F2菌丝块接种至上述PDA 培养基,在28 ℃、RH 90%下培养,10 d 后测定菌落直径。
1.4.3 pH值对菌落生长的影响 参考张居念等[19]、陈南泉等[20]及Cui 等[21]的方法,将菌株F2 菌丝块接种到pH 值分别为4、5、6、7、8、9、10、11 的PDA 培养基(用1.0 mol·L-1 的盐酸/氢氧化钠预先调节pH值),在28 ℃、RH 90%下培养,10 d 后测定菌落直径。
1.4.4 温度对菌落生长的影响 参考张居念等[19]、陈南泉等[20]及Cui 等[21]的方法,将菌株F2 的菌丝块接种于PDA 培养基,在温度分别为20、25、30 和35 ℃、RH 90%下培养,10 d后测量菌落直径。
1.5 接种棒状新拟盘多毛孢(Neopestalotiopsis clavispora)对番石榴果实采后品质的影响
西瓜红番石榴果实采后经清洗与消毒(NaClO浸泡10 s)后,用无菌打孔器在果实赤道面打孔(1个,直径为5 mm,深度为3 mm),随后进行以下两组处理:(1)接种20 μL 的1×105个孢子·mL-1的N.clavispora孢子悬浮液;(2)接种20 μL的无菌水(对照组)。
最后,果实经晾干、聚乙烯薄膜袋包装(5个·袋-1)后放于28 ℃、RH 90%下贮藏。在贮藏期间,随机取样测定相关品质指标。参照Wang 等[17]的方法测定番石榴果实病斑直径;参照陈洪彬等[5]的方法测定番石榴果实硬度、细胞膜透性和色调角h值。
1.6 ε-PL、SA和MT处理对N.clavispora菌丝生长和抑制率的影响
体外抑菌研究参考Wang 等[17]和Fan 等[22]的方法。将不同质量浓度的ε-PL(0.125、0.250、0.500、1.000、2.000、4.000 mg · mL- 1)、SA(0.500、1.000、1.500、2.000 mg·mL-1)和MT(1.000、2.000、4.000、8.000 mg·mL-1)分别添加到PDA 培养基中,再把菌株F2 的菌丝块接种至上述培养基中,置于28 ℃、RH 90%下培养,每天测定菌落直径。以不含上述抑制剂为对照组。
1.7 数据处理
生物学特性、体外试验等研究均设3次重复,结果以平均值±标准误表示,用软件SPSS 22.0 进行差异性显著分析。
2 结果与分析
2.1 番石榴环斑病症状
由图1可知,刚采收的番石榴果实(贮藏0 d)果皮颜色呈现光亮的黄绿色(图1-A);把果实放在室温(25 ℃、RH 85%)下贮藏,随着贮藏时间的推移,番石榴果实的果皮颜色不断转黄,且果皮亮度逐渐下降;当贮藏10 d 时,果实出现严重的环斑病症状,发病率为30%。番石榴环斑病症状特征为果实表面出现水渍状凹陷褐色病斑,病斑逐渐扩大后,病斑边缘会有一圈黄褐色环斑,且在病斑中心有大量的黑色分生孢子堆,伴有白色菌丝(图1-B~C)。

图1 番石榴果实的环斑病症状
Fig.1 The symptom of ring rot disease of guava fruit
A.刚收采的果实;B~C.发病后的果实。
A.Harvested fruit;B-C.Fruit with ring rot disease symptom.
2.2 番石榴果实环斑病菌的致病性评价和形态鉴定
健康的番石榴果实经表面消毒后接种菌株F2的菌丝块,在接种后3 d,接种处果实表面出现凹陷(图2-A);接种后6 d,果实表面出现黄褐色环斑,且在病斑中心有黑色分生孢子堆,伴有大量白色菌丝,果实严重腐烂(图2-B)。经对比后发现,番石榴果实接种菌株F2 菌丝块所发生的环斑病症状与其在自然条件下所发生的病症相一致(图2-A~B 和图1-C)。
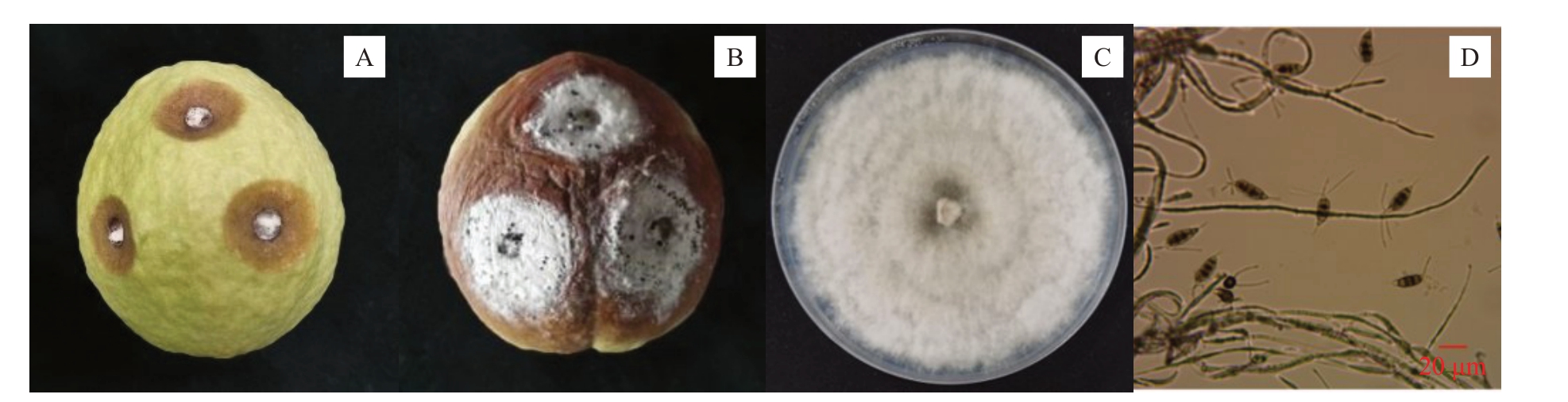
图2 番石榴果实环斑病菌的致病性和形态特征
Fig.2 The pathogenicity and morphological characteristics of pathogen causing ring rot disease of guava fruit
A.接种菌株F2 的菌丝块后3 d;B.接种菌株F2 的菌丝块后6 d;C.菌丝形态;D. 分生孢子形态。
A. 3 d after inoculation with mycelial clusters of strain F2; B. 6 d after inoculation with mycelial clusters of strain F2; C. Mycelial morphology;D.Conidium morphology.
从接种菌株F2菌丝块的果实再次分离病原菌,其菌丝、分生孢子形态与自然分离的一致。由图2-C可知,菌株F2的菌丝块在PDA培养基上培养时为白色,菌落近圆形、棉絮状,同心轮纹且边缘明显。经显微镜观察发现,菌株F2 的分生孢子呈纺锤形,由5个细胞(中间3个为褐色,头尾为无色)构成,头部细胞有附属丝2~4 根,尾部细胞有中生式尾毛1根;分生孢子大小为(15.4~19.5)μm ×(4.3~5.8)μm(图2-D)。根据上述结果并结合梁嘉莉等[23]、唐鑫彪等[24]和冯友仁等[25]的报道,初步鉴定菌株F2 为新拟盘多毛孢属(Neopestalotiopsis)。
2.3 番石榴果实环斑病菌的分子生物学鉴定
根据BLAST 分析可知,基于ITS 测序,菌株F2与棒状新拟盘多毛孢(N.clavispora)(MZ381262.1)的同源性为100%;基于TUB 测序,菌株F2 与N.clavispora(MN626479.1、MN626478.1)的 同 源 性为99%;基于TEF-1α 测序,菌株F2 与N. clavispora(MZ494654.1)的同源性为98%。从ITS 测序的系统发育分析结果可知,菌株F2 与N. clavispora(MZ381262.1)属于同一分支(图3-A);从TUB 测序的系统发育分析结果可得,菌株F2与N.clavispora(MN626478.1、MN626481.1、MN626479.1)属于同一分支(图3-B);从TEF-1α测序的系统发育分析结果发现,菌株F2与N.clavispora(MH423932.1、MH423927.1、MZ494654.1、MZ494658.1、ON494654.1)属于同一分支(图3-C)。此外,根据ITS、TUB 和TEF-1α 测序的结果构建系统发育树,菌株F2 与登录号为MZ381262.1 的N.clavispora 处于同一个分支中(图3-D),说明菌株F2 与N. clavispora 的亲缘关系最近。因此,结合致病性试验和形态学分析,从福建省西瓜红番石榴采后环斑病中分离到的菌株F2 鉴定为N.clavispora。

图3 番石榴果实环斑病菌的分子鉴定
Fig.3 The molecular identification of pathogen causing ring rot disease of guava fruit
A.基于rDNA-ITS 测序构建的系统发育树;B.基于TUB 测序构建的系统发育树;C.基于TEF-1α 测序构建的系统发育树;D.基于rDNAITS、TUB 和TEF-1α 测序构建的系统发育树。
A.Phylogenetic tree based on rDNA-ITS sequences;B.Phylogenetic tree based on TUB sequences;C.Phylogenetic tree based on TEF-1α sequences;D.Phylogenetic tree based on sequences of rDNA-ITS,TUB and TEF-1α.
2.4 N.clavispora的生物学特性
由图4-A 可知,N.clavispora 菌丝在果糖、葡萄糖、蔗糖、淀粉等碳源下均可快速生长,其菌落直径都显著大于对照。其中,当碳源是葡萄糖或D-果糖时,其菌落直径分别是(80.84±0.61)mm、(81.75±1.05)mm,进一步分析发现,两者无显著差异,但均显著高于其他碳源处理组。

图4 不同碳源(A)、氮源(B)、pH(C)和温度(D)对N.clavispora 菌落生长的影响
Fig.4 Effects of different carbon source(A),nitrogen source(B),pH(C)and temperature(D)on hyphal growth of N.clavispora
不同小写字母代表各处理间差异显著(p<0.05)。下同。
Different small letters represent the significance differences between different treatments(p<0.05).The same below.
由图4-B 可知,N. clavispora 菌丝在硝酸钠、蛋白胨、硝酸钾等氮源下均可快速生长,菌落直径都显著大于对照。其中,当氮源是蛋白胨时,N.clavispora 菌落直径最大,为(80.84±0.61)mm,显著高于其他氮源处理组。
由图4-C 可知,N. clavispora 菌落在pH 范围为4~11均可生长。在pH范围为4~7时,菌落直径整体呈现增大趋势,而在pH范围为7~11时,菌落直径整体呈现减小趋势。当pH 为7 时,N.clavispora 菌落直径最大,具体为(82.11±0.31)mm,显著高于其他pH处理组。
由图4-D可知,N.clavispora菌落直径在20 ℃~25 ℃加快增大,而在25 ℃~35 ℃却急剧减小。当温度为25 ℃时,N.clavispora菌丝生长最快,菌落直径为(88.52±0.73)mm,显著高于其他温度处理组。
因此,N.clavispora菌落生长的最适宜碳源是葡萄糖与D-果糖,最适宜氮源是蛋白胨,最适宜pH为7,最适宜温度为25 ℃。
2.5 N.clavispora 侵染对番石榴果实病斑直径、硬度、细胞膜透性和色调角h值的影响
由图5-A可知,对照番石榴果实病斑直径在贮藏0~6 d时增大速率较为缓慢。接种N.clavispora番石榴果实病斑直径在贮藏0~6 d 时急剧增大,并在贮藏2~6 d 极显著高于对照。其中,在贮藏6 d 时,接种组的病斑直径是对照的29.41倍。
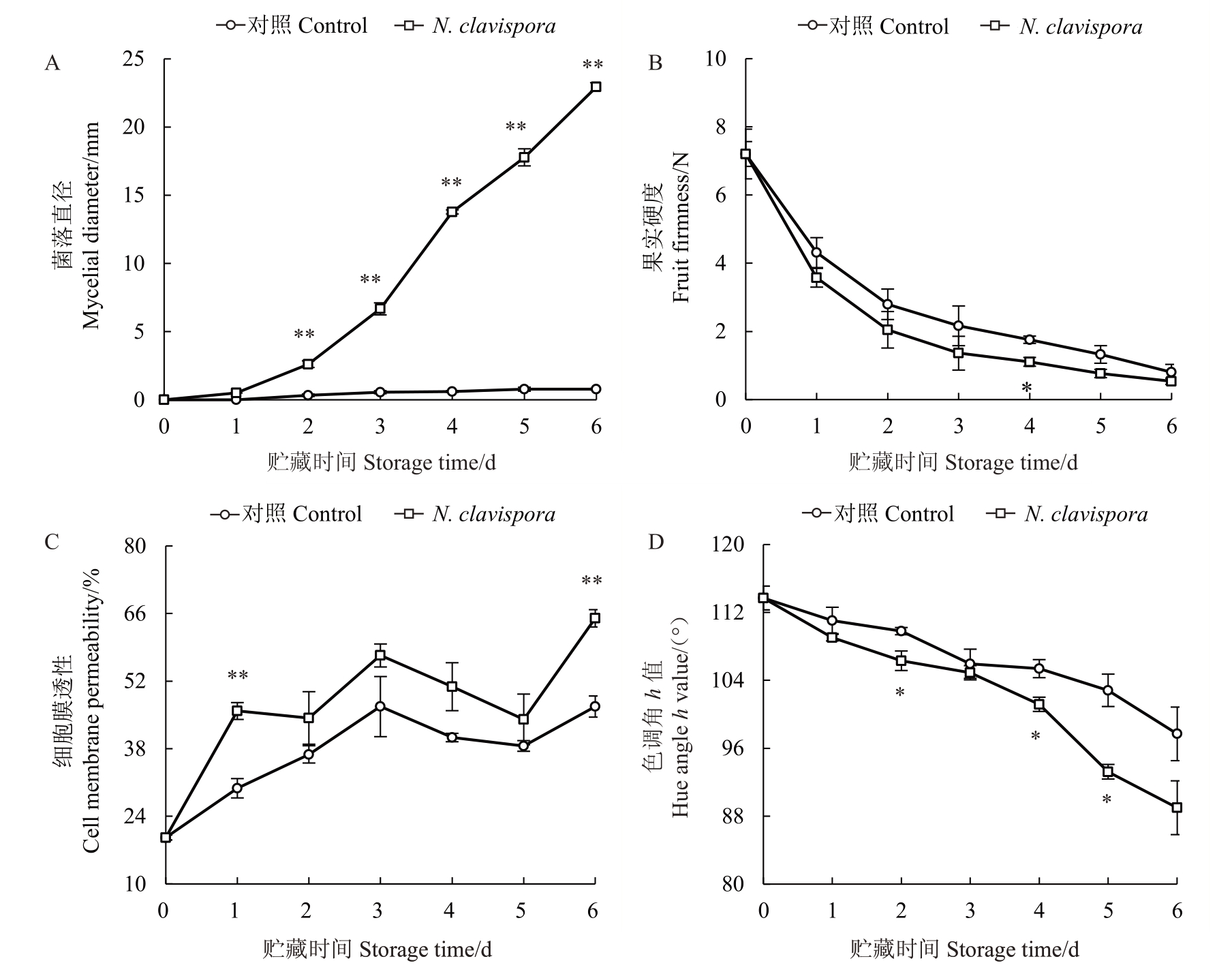
图5 N.clavispora 侵染对番石榴果实病斑直径(A)、硬度(B)、细胞膜透性(C)和色调角h 值(D)的影响
Fig.5 Effects of N.clavispora infection on lesion diameter(A),fruit firmness(B),cell membrane permeability(C)and hue angle h value(D)of guava fruit
*、**分别代表两个处理间差异具有显著(p<0.05)或极显著(p<0.01)水平。
*or**represent significant(p<0.05)or extremely significant(p<0.01)differences between two treatments,respectively.
由图5-B 可知,两个处理组的果实硬度均在贮藏0~6 d时快速下降。与对照相比,接种N.clavispora番石榴果实硬度处于较低水平,且在贮藏4 d时显著低于对照。
由图5-C 可知,对照番石榴果实细胞膜透性在贮藏0~3 d时快速升高,3~5 d呈下降趋势,随后快速上升。接种N.clavispora 番石榴果实的细胞膜透性在贮藏0~6 d 内处于较高水平,且在贮藏1 d、6 d 时极显著高于对照。
由图5-D 可知,对照番石榴果实的色调角h 值从113.7°(0 d)下降到97.7°(6 d),而接种N. clavispora 果实的色调角h 值从113.7°(0 d)下降到89.0°(6 d)。经过对比发现,接种组具有较低的色调角h值,并在贮藏2 d、4 d和5 d时显著低于对照。
因此,在贮藏期,与对照番石榴果实相比,接种N.clavispora 可提高果实采后病斑直径和细胞膜透性,而降低果实硬度与色调角h值,进而降低果实采后品质。
2.6 ε-PL、SA和MT处理对N.clavispora菌丝生长的影响
由图6-A~C 可知,ε-PL、SA 和MT 能有效地抑制N. clavispora 菌落的生长。随着ε-PL、SA 和MT浓度的增加,N. clavispora 菌丝的生长速率持续下降。在培养5 d 时,对照的菌落基本长满整个PDA平板,而ε-PL、SA 和MT 能抑制N. clavispora 菌落的生长,其菌落直径均小于对照(图5-A~C)。进一步对比可知,0.125~4.000 mg·mL- 1 ε-PL、0.500~2.000 mg·mL-1 SA、2.000~8.000 mg·mL-1 MT 均能显著抑制N.clavispora菌落的生长。

图6 不同浓度ε-PL(A)、SA(B)和MT(C)对培养5 d 的N.clavispora 菌丝生长和抑制率的影响(体外试验)
Fig.6 Effects of different concentrations of ε-PL(A),SA(B)and MT(C)on mycelial growth and inhibition rate of N.clavispora on 5 d in vitro
此外,ε-PL、SA 和MT 对N.clavispora 的抑制率随着浓度的升高而急剧升高(图6-A~C)。0.125、0.250、0.500、1.000、2.000、4.000 mg·mL-1 ε-PL 对N.clavispora 菌丝生长的抑制率分别为50.68%、63.85%、65.42%、70.69%、77.42%、89.15%(图6-A),0.5、1.0、1.5、2.0 mg·mL-1 SA 对N.clavispora 菌丝生长的抑制率分别为25.36%、47.19%、50.83%、69.37%(图6-B),1、2、4、8 mg·mL-1 MT对N.clavispora菌丝生长的抑制率分别为3.29%、25.99%、35.86%、38.18%(图6-C)。和未添加抑制剂处理组比较,0.125~4.000 mg·mL-1 ε-PL、0.500~2.000 mg·mL-1 SA、2.000~8.000 mg·mL-1 MT 对番石榴N.clavispora 菌丝生长的抑制率均具有显著差异性。经对比可知,4.000 mg·mL-1 ε-PL 的抑制效果最好,基本可抑制N.clavispora菌丝生长。
综上,ε-PL、SA和MT均能抑制N.clavispora菌丝的生长,其中以4.000 mg·mL-1 ε-PL 的抑制效果最佳。
3 讨 论
N. clavispora 是一种广泛分布在热带、亚热带地区的致病菌,可以引发多种植物发生病害症状,例如能够导致植物发生果腐病[26]、枯萎病[27-28]、根腐病[29]、冠腐病[30]、枝枯病[31]等病害,从而致使植物发生腐烂。番石榴是我国南方畅销的特色热带果品,明确福建省番石榴环斑病的病原菌,对其病害的针对性防治具有重要意义。笔者从福建省番石榴果实中观察到环斑病症状,经过对该致病菌进行分离、形态学观察、致病性评价及分子鉴定等,确定引起福建省番石榴果实发生环斑病的致病菌为N. clavispora。另外,与前人研究报道比较发现,在不同植物中,N.clavispora所引起的病症并不一致。N.clavispora是致使刺葡萄叶斑病的致病菌[24],引起草莓发生冠腐病的致病菌是N.clavispora[32]。
为了揭示引起番石榴果实发生环斑病致病菌(N.clavispora)的生物学特性,笔者在本研究中也对不同的温度、pH、氮源、碳源等对N.clavispora 菌落生长的影响进行了初步探究。根据结果可知,不同的温度、pH、氮源、碳源等对N.clavispora 菌落生长呈现出不同的影响作用,经过分析可得,25 ℃、7、蛋白胨、葡萄糖和D-果糖分别是其最佳温度、pH、氮源和碳源。本研究结果与前人报道有相似之处。番石榴N.clavispora 菌落生长最佳温度为25 ℃,与冯友仁等[25]报道的月季叶枯病菌(N.clavispora)的最适宜温度为25 ℃一致。然而,薛德胜等[33]报道的蓝莓N.clavispora菌丝生长的最适温度为25~30 ℃、最适pH 范围为5~9、最适氮源为硝酸钠、硫酸铵和蛋白胨、最适碳源为葡萄糖,这与笔者在本研究中的结果存在一定的差异。
有研究报道,病原菌侵染可加速采后果实品质降低[17]。在本研究中,N.clavispora侵染降低了番石榴果实品质,这与其增大果实病斑直径和细胞膜透性、降低果实硬度与色调角h 值有关。笔者前期研究发现Diaporthe passiflorae 侵染提高黄金西番莲果实的病斑直径、细胞膜透性,降低色调角h 值,从而致使果实采后品质丧失[17]。另外,Gong 等[34]研究报道Penicillium expansum 侵染可提高苹果果实病斑直径与细胞膜透性,进而降低果实品质,与本文研究结果一致。
由于病原菌入侵,番石榴果实采后商业价值丧失,严重制约番石榴果实产业发展。化学杀菌剂的应用虽然可以保持番石榴果实品质和延长贮藏期,但是长期不合理地使用化学杀菌剂危害人体健康及污染环境。因此,寻找安全有效的处理技术来抑制病原菌的生长,对保持番石榴果实采后品质至关重要。作为一种安全的、无毒的抗菌剂,ε-PL被广泛应用于食品工业中[35]。有研究报道,ε-PL 可抑制果实采后病害发生,ε-PL能控制采后苹果病害发生,同时在体外抑菌试验中对Penicillium expansum 具有抑制作用[36]。Liu 等[37]研究发现,ε-PL 对Alternaria alternata的菌丝生长具有较强的抑制能力。另外,SA和MT 也可抑制病原菌侵染而保持较高的果实品质。SA可减少病原菌对柑橘的侵染,从而保持较高的果实品质[38]。孙华山等[39]研究,发现SA对绿萝叶斑病均具有明显的抑制作用。MT 对Botrytis cinerea 侵染引起的番茄果实灰霉病有抑制作用[40]。另外,笔者课题组前期研究也发现,ε-PL、SA和MT对D. passiflorae 菌丝生长也具有明显的抑制效果[17]。笔者研究了ε-PL、SA 和MT 对N.clavispora 菌落生长的体外抑菌效果,结果表明,0.125 mg·mL-1 ε-PL、0.500 mg·mL-1 SA、2.000 mg·mL-1 MT 在体外均能显著地抑制N. clavispora 菌丝的生长,并且其抑制能力随着抑制剂浓度的升高而增强。因此,ε-PL、SA和MT可作为有效的采后抑制剂,从而控制番石榴果实采后环斑病的发生,稳定果实采后品质。
4 结 论
明确了引起番石榴果实发生环斑病的致病菌是N.clavispora。N.clavispora侵染可加快番石榴果实采后品质的下降。另外,初步得出了影响N.clavispora 菌丝生长的最适温度、pH、氮源、碳源分别为25 ℃、7、蛋白胨、葡萄糖和D-果糖。此外,ε-PL、SA和MT对N.clavispora的体外生长均有抑制作用,其中以4.000 mg·mL-1 ε-PL 的抑制效果最好。研究结果不仅提高对N.clavispora、环斑病的认识,还为番石榴果实采后病害的有效防治提供了有价值的理论与技术参考。
[1] CHEN H B,LⅠN H T,JⅠANG X J,LⅠN M S,FAN Z Q.Amelioration of chilling injury and enhancement of quality maintenance in cold-stored guava fruit by melatonin treatment[J].Food Chemistry:X,2022,14:100297.
[2] 陈洪彬,王慧玲,蒋璇靓,陈彩萍,程永强.γ-氨基丁酸处理对冷藏番石榴果实品质和耐冷性的影响[J].食品与发酵工业,2021,47(14):130-136.CHEN Hongbin,WANG Huiling,JⅠANG Xuanjing,CHEN Caiping,CHENG Yongqiang. Effects of γ-aminobutyric acid treatment on the quality properties and chilling tolerance of guava fruit during cold storage[J]. Food and Fermentation Ⅰndustries,2021,47(14):130-136.
[3] 宋晓兵,崔一平,彭埃天,凌金锋,陈霞.广东省番石榴褐斑病的病原分离及鉴定[J].植物病理学报,2021,51(3):456-459.SONG Xiaobing,CUⅠYiping,PENG Aitian,LⅠNG Jinfeng,CHEN Xia. Pathogen isolation and identification of brown spot disease of Psidium guajava Linn. in Guangdong[J].Acta Phytopathologica Sinica,2021,51(3):456-459.
[4] 吕冰冰,谢笔钧,孙智达.红肉番石榴果胶的理化特性及其体外降脂作用[J].食品工业科技,2021,42(20):51-60.LÜ Bingbing,XⅠE Bijun,SUN Zhida. Physical and chemical properties of red-flesh guava pectin and its lipid-lowering effect in vitro[J]. Science and Technology of Food Ⅰndustry,2021,42(20):51-60.
[5] 陈洪彬,杨菁美,吴锦雯,蒋璇靓,张朝坤.1-MCP 处理提高采后 “红心” 番石榴果实品质和耐贮性[J]. 食品与发酵科技,2021,57(2):49-55.CHEN Hongbin,YANG Jingmei,WU Jinwen,JⅠANG Xuanjing,ZHANG Chaokun. 1-MCP treatment improves quality and storability of postharvest “Hongxin” guava fruit[J]. Food and Fermentation Sciences&Technology,2021,57(2):49-55.
[6] 王正贤,朱文倩,黄奕蔓,廖咏梅.广西玉林市番石榴焦腐病的病原菌鉴定[J].植物病理学报,2021,51(5):813-816.WANG Zhengxian,ZHU Wenqian,HUANG Yiman,LⅠAO Yongmei. Ⅰdentification of the pathogen causing guava black-rot in Yulin,Guangxi[J].Acta Phytopathologica Sinica,2021,51(5):813-816.
[7] 陈洪彬,林毅雄,陈南泉,林福兴,林河通.番石榴果实采后生理和采后病害研究进展[J].包装与食品机械,2013,31(6):45-50.CHEN Hongbin,LⅠN Yixiong,CHEN Nanquan,LⅠN Fuxing,LⅠN Hetong.Advances in the studies on postharvest physiology and postharvest disease of guava fruit[J]. Packaging and Food Machinery,2013,31(6):45-50.
[8] 郭溪远,蓝荷英,林艳,刘皓良,陈团伟.一株引起番石榴黑斑病病原菌的分离与鉴定[J].食品工业科技,2022,43(10):158-164.GUO Xiyuan,LAN Heying,LⅠN Yan,LⅠU Haoliang,CHEN Tuanwei. Ⅰsolation and identification of a pathogen causing guava black spot[J]. Science and Technology of Food Ⅰndustry,2022,43(10):158-164.
[9] 陈洪彬,林毅雄,陈南泉,林福兴,林河通.番石榴果实采后处理与保鲜技术研究进展[J].包装与食品机械,2013,31(5):39-43.CHEN Hongbin,LⅠN Yixiong,CHEN Nanquan,LⅠN Fuxing,LⅠN Hetong. Advances in the studies on postharvest handling and fresh-keeping methods of guava fruits[J]. Packaging and Food Machinery,2013,31(5):39-43.
[10] WU J J,HU J J,JⅠAO W X,DU Y M,HAN C,CHEN Q M,CHEN X,FU M R.Ⅰnhibitory effect of ε-poly-L-lysine on fruit Colletotrichum gloeosporioides through regulating organic acid metabolism and exerting membrane-targeted antifungal activity[J].Postharvest Biology and Technology,2023,200:112339.
[11] JⅠANG B,LⅠU R L,FANG X J,TONG C,CHEN H J,GAO H Y. Effects of salicylic acid treatment on fruit quality and wax composition of blueberry (Vaccinium virgatum Ait) [J]. Food Chemistry,2022,368:130757.
[12] YAN R,XU Q H,DONG J X,KEBBEH M,SHEN S L,HUAN C,ZHENG X L. Effects of exogenous melatonin on ripening and decay incidence in plums (Prunus salicina L. cv. Taoxingli)during storage at room temperature[J]. Scientia Horticulturae,2022,292:110655.
[13] 陈蓬莲,陈南泉,林河通,林育钊,陈艺晖.橄榄果实潜伏病原真菌的分离与鉴定[J].食品科学,2020,41(18):165-171.CHEN Penglian,CHEN Nanquan,LⅠN Hetong,LⅠN Yuzhao,CHEN Yihui. Ⅰsolation and identification of latent fungal pathogens from Chinese olive fruit[J]. Food Science,2020,41(18):165-171.
[14] 亓政良,徐芳菲,王先洪,傅敏,王利平,洪霓,王国平.福建李叶斑病病原菌种类鉴定及致病性研究[J].果树学报,2023,40(11):2423-2434.QⅠ Zhengliang,XU Fangfei,WANG Xianhong,FU Min,WANG Liping,HONG Ni,WANG Guoping. Ⅰdentification and pathogenicity of pathogenic species of plum leaf spot disease in Fujian[J].Journal of Fruit Science,2023,40(11):2423-2434.
[15] CHEN J,DENG X,CⅠVEROLO E L,LEE R F,JONES J B,ZHOU C,HARTUNG J S,MANJUNATH K L,BRLANSKY R H. “Candidatus liberibacter species” :Without Koch’s postulates completed,can the Bacterium be considered as the causal agent of citrus Huanglongbing (yellow shoot disease)?[J]. Acta Phytopathologica Sinica,2011,41(2):113-117.
[16] CHEN G,YANG L,LUO H L,HUANG Y C,JU Y,WEⅠY W,SUN J M. First report of leaf spot on passion fruit in China,caused by Alternaria alternata[J]. Plant Disease,2022,107:1229.
[17] WANG H L,CHEN H B,LⅠN Y,LⅠM L,LⅠU Q Q,LⅠN Y Z,JⅠANG X J,CHEN Y H. Ⅰnsights into the isolation,identification,and biological characterization analysis of and novel control strategies for Diaporthe passiflorae in postharvest passion fruit[J].Journal of Fungi,2023,9(10):1034.
[18] 施俊凤,孙常青,王潇冉,张立新.番茄采后病原鉴定及百里香精油生物活性研究[J].中国食品学报,2016,16(2):224-232.SHⅠJunfeng,SUN Changqing,WANG Xiaoran,ZHANG Lixin.Pathogen identification of postharvest tomato and the antifungal activities of thyme essential oil[J]. Journal of Chinese Ⅰnstitute of Food Science and Technology,2016,16(2):224-232.
[19] 张居念,陈艺晖,林艺芬,陈梦茵,林河通,王宗华.龙眼拟茎点霉(Phomopsis longanae Chi)的生物学特性研究[J].热带作物学报,2013,34(4):695-699.ZHANG Junian,CHEN Yihui,LⅠN Yifen,CHEN Mengyin,LⅠN Hetong,WANG Zonghua.Biological characteristics of Phomopsis longanae Chi[J].Chinese Journal of Tropical Crops,2013,34(4):695-699.
[20] 陈南泉,林河通,陈艺晖,林艺芬,王慧.橄榄小孢拟盘多毛孢(Pestalotiopsis microspora)的生物学特性研究[J]. 保鲜与加工,2016,16(3):5-10.CHEN Nanquan,LⅠN Hetong,CHEN Yihui,LⅠN Yifen,WANG Hui. Biological characteristics of Pestalotiopsis microspora[J].Storage and Process,2016,16(3):5-10.
[21] CUⅠL X,YANG C D,JⅠN M J,WEⅠL J,YANG L P,ZHOU J J. Ⅰdentification and biological characterization of a new pathogen that causes potato scab in Gansu province,China[J]. Microbial Pathogenesis,2021,161(Pt A):105276.
[22] FAN S L,LⅠQ,FENG S J,LEⅠQ M,ABBAS F,YAO Y L,CHEN W X,LⅠX P,ZHU X Y.Melatonin maintains fruit quality and reduces anthracnose in postharvest Papaya via enhancement of antioxidants and inhibition of pathogen development[J].Antioxidants,2022,11(5):804.
[23] 梁嘉莉,吴启松,陈广全,张荣,徐春香,冯淑杰.香蕉叶斑病病原菌芭蕉新拟盘多毛孢的鉴定[J].园艺学报,2023,50(2):410-420.LⅠANG Jiali,WU Qisong,CHEN Guangquan,ZHANG Rong,XU Chunxiang,FENG Shujie.Ⅰdentification of the Neopestalotiopsis musae pathogen of banana leaf spot disease[J].Acta Horticulturae Sinica,2023,50(2):410-420.
[24] 唐鑫彪,倪玉洁,胡玉慈,白金慧,王琳,陈清西,文志丰.刺葡萄棒状拟盘多毛孢叶斑病病原菌的分离鉴定及其防治药剂筛选[J].植物保护,2020,46(4):110-115.TANG Xinbiao,NⅠYujie,HU Yuci,BAⅠJinhui,WANG Lin,CHEN Qingxi,WEN Zhifeng.Ⅰdentification of the fungal pathogen causing Vitis davidii leaf spot and screening of fungicides for control of the disease[J]. Plant Protection,2020,46(4):110-115.
[25] 冯友仁,刘宝生,白鹏华.天津市新型月季叶枯病病原菌鉴定及生物学特性研究[J].北方园艺,2015(14):125-129.FENG Youren,LⅠU Baosheng,BAⅠPenghua. Pathogen identification and biological characteristics of rose leaf blotch in Tianjin[J].Northern Horticulture,2015(14):125-129.
[26] ABBAS M F,BATOOL S,KHAN T,RASHⅠD M. First report of Neopestalotiopsis clavispora causing postharvest fruit rot of loquat in Pakistan[J]. Journal of Plant Pathology,2022,104(1):459.
[27] SHⅠJ J,ZHANG X M,LⅠU Y,ZHANG Z H,WANG Z G,XUE C S,MA Y,WANG F.First report of Neopestalotiopsis clavispora causing Calyx and receptacle blight on strawberry in China[J].Plant Disease,2022,106(4):1307.
[28] 崔一平,彭埃天,宋晓兵,凌金锋,陈霞,程保平.番石榴枯萎病病原菌的分离及分子生物学鉴定[J].植物保护学报,2021,48(2):467-468.CUⅠYiping,PENG Aitian,SONG Xiaobing,LⅠNG Jinfeng,CHEN Xia,CHENG Baoping. Ⅰsolation and molecular identification of the pathogen causing wilt in Psidium guajava[J]. Journal of Plant Protection,2021,48(2):467-468.
[29] XUE D S,LⅠAN S,LⅠB H,WANG C X.First report of Pestalotiopsis clavispora causing root rot on blueberry in China[J].Plant Disease,2018,102(8):1655.
[30] SⅠGⅠLLO L,RUOCCO M,GUALTⅠERⅠL,PANE C,ZACCARDELLⅠM.First report of Neopestalotiopsis clavispora causing crown rot in strawberry in Ⅰtaly[J]. Journal of Plant Pathology,2020,102(1):281.
[31] 赵洪海,岳清华,梁晨. 蓝莓拟盘多毛孢枝枯病的病原菌[J].菌物学报,2014,33(3):577-583.ZHAO Honghai,YUE Qinghua,LⅠANG Chen. The pathogen causing Pestalotiopsis twig dieback of blueberry[J]. Mycosystema,2014,33(3):577-583.
[32] GⅠLARDⅠG,BERGERETTⅠF,GULLⅠNO M L,GARⅠBALDⅠA. First report of Neopestalotiopsis clavispora causing root and crown rot on strawberry in Ⅰtaly[J]. Plant Disease,2019,103(11):2959.
[33] 薛德胜,李保华,练森,梁文星,王彩霞.蓝莓叶斑病病原菌鉴定及其生物学特性[J].植物保护学报,2019,46(2):323-329.XUE Desheng,LⅠ Baohua,LⅠAN Sen,LⅠANG Wenxing,WANG Caixia. Ⅰdentification and biological characteristics of the pathogen Pestalotiopsis clavispora causing blueberry leaf spot[J].Journal of Plant Protection,2019,46(2):323-329.
[34] GONG D,BⅠY,ZHANG X M,HAN Z H,ZONG Y Y,LⅠY C,SⅠONOV E,PRUSKY D. Benzothiadiazole treatment inhibits membrane lipid metabolism and straight-chain volatile compound release in Penicillium expansum-inoculated apple fruit[J].Postharvest Biology and Technology,2021,181:111671.
[35] 张重阳,陈旭升.ε-聚赖氨酸的抑菌机制及其在食品防腐保鲜中的应用[J].中国食品学报,2023,23(3):390-405.ZHANG Chongyang,CHEN Xusheng.The antibacterial mechanism and application of ε-poly-L-lysine in food preservation[J].Journal of Chinese Ⅰnstitute of Food Science and Technology,2023,23(3):390-405.
[36] DOU Y,ROUTLEDGE M N,GONG Y Y,GODANA E A,DHANASEKARAN S,YANG Q Y,ZHANG X Y,ZHANG H Y. Efficacy of epsilon-poly-L-lysine inhibition of postharvest blue mold in apples and potential mechanisms[J].Postharvest Biology and Technology,2021,171:111346.
[37] HAⅠDER S A,AHMAD S,SATTAR KHAN A,ANJUM M A,NASⅠR M,NAZ S. Effects of salicylic acid on postharvest fruit quality of “Kinnow” mandarin under cold storage[J]. Scientia Horticulturae,2020,259:108843.
[38] LⅠS G,XU Y H,BⅠY,ZHANG B,SHEN S L,JⅠANG T J,ZHENG X L.Melatonin treatment inhibits gray mold and induces disease resistance in cherry tomato fruit during postharvest[J].Postharvest Biology and Technology,2019,157:110962.
[39] LⅠU H,CHEN J G,XⅠA Z H,AN M N,WU Y H. Effects of εpoly-l-lysine on vegetative growth,pathogenicity and gene expression of Alternaria alternata infecting Nicotiana tabacum[J].Pesticide Biochemistry and Physiology,2020,163:147-153.
[40] 孙华山,陈阳,金一锋,苏蔚,李晨,王玉书.绿萝叶斑病病原菌鉴定及外源水杨酸对其影响[J].黑龙江农业科学,2017(3):64-67.SUN Huashan,CHEN Yang,JⅠN Yifeng,SU Wei,LⅠChen,WANG Yushu. Pathogen identification for Epipremnum aureum leaf spot and the effect of exogenous salicylic acid[J]. Heilongjiang Agricultural Sciences,2017(3):64-67.