桑树是桑科(Moraceae)桑属(Morus)多年生木本植物,广泛分布于我国及世界各地[1-2]。桑葚为桑树的聚合果,具有较高的营养价值。近年来,越来越多的研究报道了桑葚的营养价值,并证实了桑葚中富含多种生物活性化合物,如维生素C、酚酸(绿原酸、咖啡酸)、类黄酮化合物(芦丁、槲皮素和山烯酚)、花青素(花青素-3-O-葡萄糖苷)等,在抗氧化、抗炎和治疗糖尿病等方面具有重要的生理功能[3-7]。目前我国桑葚的品种,从外观上主要分为白色、红色和黑色3类,而这3类桑葚营养物质及抗氧化活性存在显著差异[8]。鉴于此,客观、合理、科学地评价不同类别桑葚的品质,研究其品质形成机制具有重要意义。
我国桑树品种资源丰富,不同品种桑葚外观特性与内在成分存在显著差异[9]。对河北试种的9 个果桑品种(安椹、节曲、蒙桑、8632、物45、鲁诱7 号、桂花、白玉王、东光大白)的营养分析表明,不同品种表现出不同的特性,如桂花出汁率和含水率最高,总酸含量最低,安葚的总黄酮含量、总多酚含量、DPPH 清除能力和ABTS 清除能力显著高于其他品种[10]。对中桑5801、大十和台湾果桑等5 种桑葚进行研究发现,黑色和红色桑葚中可检测到大量的花青素,而在白色桑葚中均未发现花青素[11]。对桑葚不同生长阶段黄酮类化合物含量变化的研究发现,桑葚在成熟后黄酮类化合物含量较高[12]。基于拟靶向代谢组学技术对紫色(大十)、白色(白玉王)和红色(红果72C001)桑葚不同生长期进行代谢物品质分析,发现不同品种桑葚中氨基酸和糖醇类物质随桑葚生育期而逐渐增加;红色和紫色桑葚中类黄酮和酚酸类物质随生育期而逐渐累积,而白色桑葚累积不明显;不同生长阶段桑葚的总糖含量随之上升;紫色桑葚总酚含量和抗氧化活性随桑葚成熟不断提高,而白色桑葚随桑葚成熟下降,红色桑葚则无明显变化。此外,马来酸、苹果酸、奎尼酸、泛酸等有机酸含量在桑葚发育过程中不断降低[13]。
水果的品质分外观品质和内在品质两个方面,其中单果质量、果形指数、色泽等为果实外观品质,决定着果实的商品价值;而可溶性糖、有机酸、多酚、黄酮等为果实的内在品质,决定着果实的营养及口感,且不同品种和不同发育时期对桑葚的理化品质和营养物质有显著影响。笔者对河北省主栽的2个不同颜色桑葚品种(安葚和桂花)的单果质量、果实硬度2项外观品质和可溶性固形物、可滴定酸、总黄酮、总酚、葡萄糖、蔗糖、果糖、苹果酸、琥珀酸和酒石酸等10 项内在营养物质进行测定和评价,研究2 个品种间桑葚有效成分的含量差异,并对差异较大的蔗糖合成与代谢关键酶基因进行表达分析,以期为桑葚资源的营养价值评价及桑葚产品的开发和深加工奠定基础。
1 材料和方法
1.1 试验材料
选择河北省承德市承德医学院蚕业研究所桑园为试验区,选取生长势基本一致、栽培管理措施基本相同的7 年生稳定结果的安葚(As)和桂花(Gh)品种为试材,安葚属白桑(Morus alba L.),由承德医学院蚕业研究所培育,果实颜色呈紫黑色;桂花属白桑(M.alba L.),为河北省遵化市农家品种,果实颜色呈白色。对2 个品种分别进行套袋授粉,在授粉后(DAP)10、20、30、40、50 d 依据果实颜色进行取样,每个品种采集500 g,鲜样用于测定单果质量、果实硬度、可溶性固形物与可滴定酸含量;其他样品用液氮冷冻后分别于-80 ℃超低温冰箱储存及真空冷冻干燥后备用。
1.2 单果质量、果实硬度、可溶性固形物与可滴定酸含量的测定
采用千分之一天平进行果实质量测量,每时期测定20 个单果。采用配有5 mm 柱塞的渗透计(SMTT50,东京,日本)测量果实硬度。采用便携式糖度计测定可溶性固形物(TSS)含量,每时期测定20 个单果。采用NaOH 滴定法测定可滴定酸(TA)含量,取10.0 g 桑葚冷冻干燥样品研磨,匀浆,过滤后用0.1 mol·L-1 NaOH标准溶液滴定。
1.3 总黄酮和总酚含量的测定
采用NaNO2-Al(NO3)3 比色法测定总黄酮含量[14],将桑葚样本冻干粉碎后过60 目筛,称取干样50 mg,加入1.5 mL 60%的乙醇,震荡均匀,6000 r·min-1 离心10 min,取上清液,测定样品提取液的总黄酮浓度。取1 mg 芦丁,溶于10 mL 60%乙醇中,即为0.1 mg·mL-1的标准液。采用福林-酚比色法测定总酚含量[15],称取0.1 g 上述粉碎的样品,加入1.0 mL 60%的乙醇溶液,震荡均匀,在超声波提取器中75 ℃提取30 min。提取液以4000 r·min-1离心10 min,取上清液,测定样品提取液的总多酚浓度。称取没食子酸5 mg,溶于10 mL 蒸馏水中,即为0.5 mg·mL-1的标准液(50 ℃加热溶解)。测定分别设定3个生物学重复。
1.4 可溶性糖含量的测定
称取20 mg的冻干样品粉末,加入500 μL甲醇、异丙醇、水体积比为3∶3∶2的提取液,涡旋3 min,将混合物在冰水中超声30 min,在4 ℃、14 000 r·min-1下离心5 min 后收集上清液。上清液通过0.45 µm醋酸纤维素过滤器过滤,使用安捷伦1260 仪器(安捷伦科技有限公司,美国)进行高效液相色谱分析。在Agilent DB-5MS(30 m×0.25 mm×0.25 μm)上,以1.0 mL·min-1的流速在35 ℃分离样品,使用每种糖(葡萄糖,果糖和蔗糖)的标准曲线进行分析,测定设定3个生物学重复。
1.5 有机酸含量的测定
称量50 mg 冻干样品粉末,立即加入500 μL 的-20 ℃预冷的70%甲醇水提取液,涡旋3 min,在4 ℃、12 000 r·min-1下离心10 min后收集上清液。上清液通过0.45µm醋酸纤维素过滤器过滤,然后使用安捷伦1260仪器(安捷伦科技有限公司,美国)进行高效液相色谱分析。洗脱体系由40 mmol·L-1 KH2PO4-H3PO4缓冲液(pH 2.4)组成,流速为0.35 mL·min-1。在ACQUⅠTY HSS T3 柱(1.8 µm,100 mm×2.1 mm)上分离有机酸,使用每种有机酸(苹果酸,琥珀酸和酒石酸)的标准曲线进行分析,测定设定3个生物学重复。
1.6 糖合成相关基因荧光定量PCR分析
使用大连宝生物工程有限公司生产的TaKaRa MiniBEST Universal RNA Extraction Kit试剂盒提取桑树10、20、30、40、50 DAP 果实总RNA,反转录使用大连宝生物工程有限公司生产的PrimeScript™RT reagent Kit 试剂盒合成cDNA,实时荧光定量PCR(qRT-PCR)使用大连宝生物工程有限公司生产的SYBR Premix Ex TaqTM Ⅱ。基因序列在川桑基因组数据库中获取(https://morus.biodb.org/),以桑树Ribosomal protein L15 为内参基因(表1)。qRTPCR反应体系组成:SYBR Premix Ex TaqTM Ⅱ5 μL,cDNA 0.5 μL,正向引物0.4 μL,反向引物0.4 μL,加水至10 μL。反应程序:95 ℃预变性30 s;95 ℃变性5 s,60 ℃退火20 s,72 ℃延伸40 s,共40 个循环。PCR 扩增反应在CF×96 TM Real-Time PCR Detection System(Applied Biosystems,Forter City,CA,美国)仪器上进行,每个样品3 次生物学重复,3 次技术重复,反应结束后应用2-△△CT算法[16]进行分析。
表1 基因的qRT-PCR 引物
Table 1 List of qRT-PCR primer sequences used for genes
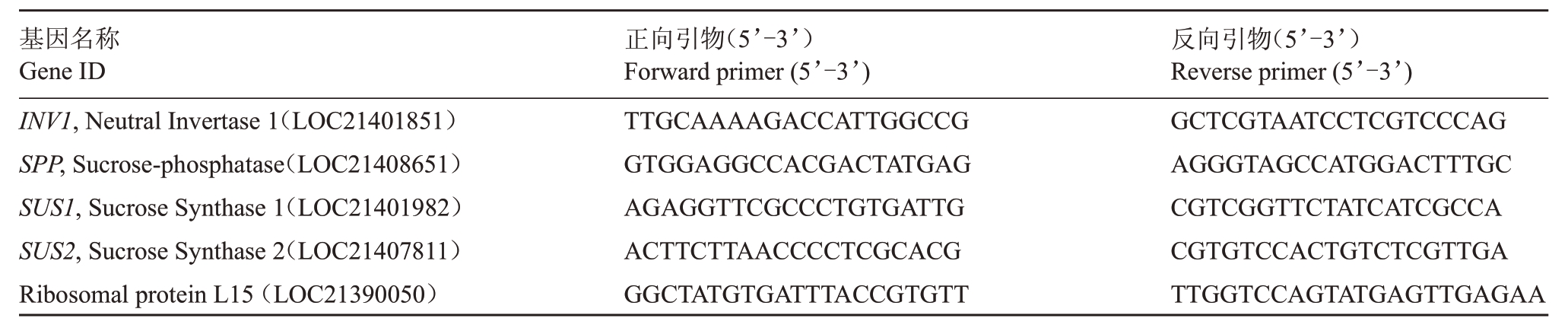
基因名称Gene ⅠD INV1,Neutral Ⅰnvertase 1(LOC21401851)SPP,Sucrose-phosphatase(LOC21408651)SUS1,Sucrose Synthase 1(LOC21401982)SUS2,Sucrose Synthase 2(LOC21407811)Ribosomal protein L15(LOC21390050)正向引物(5’-3’)Forward primer(5’-3’)TTGCAAAAGACCATTGGCCG GTGGAGGCCACGACTATGAG AGAGGTTCGCCCTGTGATTG ACTTCTTAACCCCTCGCACG GGCTATGTGATTTACCGTGTT反向引物(5’-3’)Reverse primer(5’-3’)GCTCGTAATCCTCGTCCCAG AGGGTAGCCATGGACTTTGC CGTCGGTTCTATCATCGCCA CGTGTCCACTGTCTCGTTGA TTGGTCCAGTATGAGTTGAGAA
1.7 数据分析
使用SPSS 27.0软件进行统计分析,使用单因素方差分析计算样品之间的差异性,在0.05 水平进行Duncan’s 检验(p≤0.05),数据表示为平均值± SD(标准差),每个样本3个独立重复。
2 结果与分析
2.1 果实发育时期形态特征变化
图1-A 为安葚(As)和桂花(Gh)不同发育时期果实形态特征。通过果实质量测定发现,在整个发育时期桂花单果质量较安葚大,在成熟期(50 DAP)桂花单果质量为8.13 g,安葚单果质量为6.89 g(图1-B);桂花单果质量从青果期到转色期(20~30 DAP)增幅较大,其中在青果期单果质量为5.67 g,转色期达到7.32 g(图1-B)。硬度测定结果表明,安葚在整个发育时期(10 DAP 除外)硬度显著高于桂花;安葚的果实硬度在20 DAP 时下降,而桂花的果实硬度在10 DAP时开始下降,且从青果期到转色期(20~30 DAP)降低幅度较大(图1-C)。结果表明,桂花果实快速生长期在20~30 DPA,在此期间果实质量和硬度发生显著变化(图1-B~C)。

图1 安葚(As)和桂花(Gh)果实的形态特征
Fig.1 Morphological characteristics of the Anshen(As)and Guihua(Gh)fruits
A.果实发育期间表型;B.果实质量变化;C.果实硬度变化。数据以平均值±标准差表示。不同字母表示在p≤0.05 水平上差异显著。下同。
A.Phenotypes of fruit changes;B.Weight of fruit changes;C.Firmness of fruit changes.Data were represented by the mean values±standard deviation.Different letters indicated signifificant difference at the p≤0.05 level.The same below.
2.2 果实发育过程中的物质成分分析
安葚和桂花中可溶性固形物含量随着果实发育和成熟呈现增加趋势,在果实发育初始阶段二者无显著差异,在成熟期,桂花的可溶性固形物含量显著高于安葚(图2-A)。可滴定酸含量随着果实发育呈现下降趋势,在成熟期,安葚的可滴定酸含量显著高于桂花(图2-B)。测定总黄酮和总酚结果表明,安葚和桂花的总黄酮和总酚含量在果实发育初期差异不显著(图2-C~D)。在发育20 d 以后,桂花的总黄酮和总酚含量逐渐降低,安葚的总黄酮和总酚含量显著增加,且均显著高于桂花。直至果实成熟,桂花和安葚总黄酮含量(w,后同)分别是0.03 mg·g-1、9.68 mg·g-1,总酚含量分别是20.13 mg·g-1、84.89 mg·g-1(图2-C~D)。

图2 安葚(As)和桂花(Gh)果实发育过程中物质含量的变化
Fig.2 Changes of nutrient contents in Anshen(As)and Guihua(Gh)fruits during fruit development
2.3 果实发育过程中可溶性糖与有机酸含量分析
在桂花果实中,蔗糖含量随着果实发育持续上升,在50 DPA 时含量达到156.32 mg·g-1,安葚在果实发育初期蔗糖含量下降,在30 DPA 以后呈现增加趋势,在50 DAP 时含量达到37.56 mg·g-1(图3-A)。葡萄糖和果糖含量在整个果实发育和成熟过程中逐渐增加,且桂花中葡萄糖和果糖含量显著高于安葚(图3-B~C);但在50 DAP 时,安葚果实中果糖含量显著高于桂花(图3-C)。在安葚果实中,与葡萄糖和果糖含量相比,蔗糖的积累水平最低(图3-A~C)。

图3 安葚(As)和桂花(Gh)果实发育过程中可溶性糖与有机酸含量的变化
Fig.3 Changes in sugar and organic acid content of Anshen(As)and Guihua(Gh)during fruit development
苹果酸含量在2 个品种中呈现缓慢增加的模式,但在30 DAP 以后,桂花中苹果酸积累呈现下降的趋势(图3-D)。琥珀酸含量在果实发育过程中呈现先升高后降低的趋势,在30 DAP 时含量最高,安葚为58.15 mg·g-1,桂花为63.12 mg·g-1(图3-E)。而酒石酸含量在2 个品种中的变化趋势相反,安葚在20 DAP 时酒石酸含量上升,桂花则在40 DAP 时呈现上升的趋势(图3-F)。琥珀酸和酒石酸在安葚和桂花果实中积累量差异不大,而苹果酸含量在桂花中较高。
2.4 果实发育过程中可溶性总糖、有机酸含量和糖酸比分析
随着果实发育,2个品种桑葚糖酸比逐渐增大,糖酸比在7.99~81.06 之间,到成熟期(50 DAP)时,达到最大值,安葚为81.06,桂花为57.17,且在各个不同发育时期,除10 DAP 外,安葚糖酸比显著大于桂花(图4)。
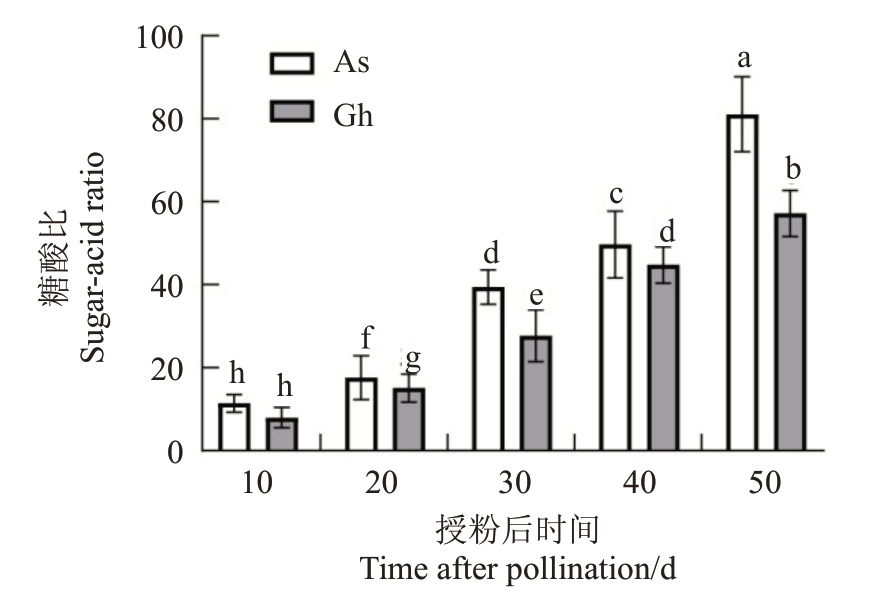
图4 安葚(As)和桂花(Gh)果实发育过程中糖酸比
Fig.4 Sugar-acid ratio of Anshen(As)and Guihua(Gh)during fruit development
2.5 果实发育过程中糖代谢相关基因的表达分析
为探讨2个品种果实发育过程中差异最大物质蔗糖代谢的分子机制,笔者采用qRT-PCR 检测了相关关键基因的表达水平。结果表明,安葚中的中性转化酶1(Neutral Ⅰnvertase 1,INV1)基因表达量随着果实发育呈现上升趋势,而桂花中,在30 DPA 时达到最高水平,而后在40 DPA 时表达量出现下降(图5-A)。蔗糖磷酸酶(Sucrose-phosphatase,SPP)基因在桂花整个发育时期表达量均显著高于安葚(图5-B)。蔗糖合酶1(Sucrose Synthase 1,SUS1)基因在2个品种中均呈现上升的表达趋势(图5-C),而安葚中的蔗糖合酶2(SUS2)基因表达量在30 DPA 和50 DPA 时出现下降。相反,桂花中该基因表达量在这2 个时间点呈现升高趋势(图5-D)。以上结果表明2 个品种中关键基因的表达模式存在差异,这些基因与蔗糖的代谢相关。

图5 安葚(As)和桂花(Gh)果实发育过程中糖代谢相关基因的表达
Fig.5 Expression profiling of genes related to sugar metabolism during fruit development in Anshen(As)and Guihua(Gh)
相对表达在10 DAP 条件下与安葚果实中的值进行归一化。数据以平均值±标准差表示。不同字母表示在p≤0.05 水平上差异显著。
Relative expression was normalized against the value in the Anshen fruit at 10 DPA. Data were represented by the mean values ± standard deviation.Different letters indicated signifificant difference at the p≤0.05 level.
3 讨 论
桑葚是中国最具价值的天然产物之一,富含丰富的次生代谢产物,如黄酮类、多糖类化合物[3-4]。笔者研究的安葚和桂花2个桑葚品种在河北承德栽培较为广泛,目前为止,关于他们在发育时期的物理及营养物质变化的信息很少。果长、单果质量、硬度、可溶性固形物和可滴定酸含量是果实的外观形态和物理化学特征,可以用来指示果实成熟度、采收时间和保质期[17]。果实成熟表现为硬度降低、可滴定酸含量减少和可溶性固形物含量增加[18]。笔者研究发现,从20 DPA开始,桂花的果实大小显著增加,果实硬度显著下降,而安葚果实的这些变化从30 DPA开始,表明桂花发育速度较安葚更快。此外,可溶性固形物和可滴定酸含量可以反映水果的口感,2 个品种的可溶性固形物含量增加幅度相似,而安葚的可滴定酸含量在40 DPA时出现上升。
总黄酮和总酚是抗氧化剂中最重要的抗氧化化合物。不同桑葚基因型成熟期的总黄酮和总酚含量存在显著差异[19]。本研究结果表明,安葚果实的总黄酮和总酚含量显著高于桂花,表明安葚果实的抗氧化能力高于桂花。前期研究表明,相对于深色品种,白色品种的总黄酮和总酚含量较低,且抗氧化活性呈现一致的趋势[10]。糖和有机酸之间的平衡影响着果实的口感[19],前期研究表明,桑葚中主要可溶性糖是葡萄糖和果糖,蔗糖含量较少[20]。在成熟期,桂花和安葚的葡萄糖和果糖含量均存在较高水平,而2个品种在果实发育过程中蔗糖含量的变化差异较大。对于桂花,蔗糖积累发生在整个发育时期,而安葚在30~50 DPA 之间出现蔗糖积累(图3-A),这可能是2个品种口感差异的主要原因。苹果酸是桑葚中重要的有机酸之一,有机酸中苹果酸含量的高低对桑葚的口感发挥重要作用[21]。研究表明,安葚和桂花中的优势酸是苹果酸,其次是琥珀酸和酒石酸。在整个果实发育和成熟期间,桂花中苹果酸含量显著高于安葚。2 个桑葚品种的糖酸比在7.99~81.06之间,其中在成熟期桂花的糖酸比为57.17,安葚的糖酸比显著大于桂花,为81.06,风味最佳。
甜度是桑树栽培育种最重要的品质性状之一。蔗糖、果糖和葡萄糖是植物中的重要成分,影响果实品质与口感[22]。本研究对2个桑葚品种可溶性糖测定,发现二者蔗糖含量差异显著。本研究对蔗糖代谢关键酶基因进行表达分析,结果表明,中性转化酶1 在桂花中10~30 DAP 表达量显著高于安葚,中性转化酶主要功能为催化蔗糖不可逆地降解为葡萄糖和果糖,是蔗糖代谢的关键酶[23]。笔者在本研究中共分析两个蔗糖合成酶,表达分析表明,蔗糖合成酶1 基因在2 个桑葚品种中表达量随着果实发育呈上升趋势,而蔗糖合酶2 基因在安葚40~50 DAP 间表达量较高且显著高于桂花。蔗糖合酶在尿苷二磷酸(UDP)存在下,可催化蔗糖裂解为尿苷二磷酸葡萄糖(UDPG)和果糖[24],这与安葚在成熟期(50 DAP)果糖含量高于桂花的结果一致。植物的蔗糖是由蔗糖磷酸合酶(sucrose phosphate synthase)催化尿苷二磷酸葡萄糖和6-磷酸果糖形成蔗糖-6-磷酸,再由磷酸蔗糖磷酸酶进一步水解蔗糖-6-磷酸形成的[25]。本研究表明,桂花中磷酸蔗糖磷酸酶基因表达量显著高于安葚,这可能是桂花中蔗糖含量显著高于安葚的原因。
4 结 论
综上,桂花果实的蔗糖、苹果酸和可溶性固形物含量高于安葚,安葚果实的总黄酮、总酚和可滴定酸含量高于桂花,而桂花果实的发育速度比安葚快。此外,中性转化酶1、蔗糖磷酸酶与蔗糖合酶2 基因可以作为调控桑葚糖含量的候选基因。研究结果有助于更好地了解桑葚果实发育过程中营养成分的变化动态,为桑葚果实品质形成研究奠定基础。
[1] KⅠM Ⅰ,LEE J.Variations in anthocyanin profiles and antioxidant activity of 12 genotypes of mulberry (Morus spp.) fruits and their changes during processing[J]. Antioxidants,2020,9(3):242.
[2] SHREELAKSHMⅠS V,NAZARETH M S,KUMAR S S,GⅠRⅠDHAR P,PRASHANTH K V H,SHETTY N P. Physicochemical composition and characterization of bioactive compounds of mulberry(Morus indica L.)fruit during ontogeny[J]. Plant Foods for Human Nutrition,2021,76(3):304-310.
[3] 杨婉媛,陈晓维,罗文珊,余元善,陈树鹏,郭冬玲,卜智斌.桑葚的功效成分及加工利用研究进展[J]. 中国果菜,2022,42(12):48-53.YANG Wanyuan,CHEN Xiaowei,LUO Wenshan,YU Yuanshan,CHEN Shupeng,GUO Dongling,BU Zhibin. Research progress of functional components and processing and utilization of mulberry[J]. China Fruit & Vegetable,2022,42(12):48-53.
[4] LⅠN C Y,LAY H L. Characteristics of fruit growth,component analysis and antioxidant activity of mulberry (Morus spp.)[J].Scientia Horticulturae,2013,162:285-292.
[5] WANG R S,DONG P H,SHUAⅠX X,CHEN M S. Evaluation of different black mulberry fruits(Morus nigra L.)based on phenolic compounds and antioxidant activity[J]. Foods,2022,11(9):1252.
[6] CHUMROENPHAT T, SOMBOONWATTHANAKUL Ⅰ,SAENSOUK S,SⅠRⅠAMORNPUN S. The diversity of biologically active compounds in the rhizomes of recently discovered Zingiberaceae plants native to north eastern Thailand[J]. Pharmacognosy Journal,2019,11(5):1014-1022.
[7] 窦子微,杨璐,程平,张志刚,李宏.不同品种桑葚营养品质分析及综合评价[J].新疆农业科学,2023,60(1):127-139.DOU Ziwei,YANG Lu,CHENG Ping,ZHANG Zhigang,LⅠHong. Analysis and comprehensive evaluation of nutritional quality of different mulberry varieties[J]. Xinjiang Agricultural Sciences,2023,60(1):127-139.
[8] ⅠQBAL S,YOUNAS U,UDDⅠN S,CHAN K W,SARFRAZ R A,UDDⅠN M K. Proximate composition and antioxidant potential of leaves from three varieties of mulberry (Morus sp.):A comparative study[J].Ⅰnternational Journal of Molecular Sciences,2012,13(6):6651-6664.
[9] LEE Y,HWANG K T. Changes in physicochemical properties of mulberry fruits (Morus alba L.) during ripening[J]. Scientia Horticulturae,2017,217:189-196.
[10] 贾漫丽,李娜,王彬彬,范伟,夏爱华,李季生.9 个品种桑果营养、香气成分与抗氧化活性评价[J]. 果树学报,2022,39(2):221-231.JⅠA Manli,LⅠNa,WANG Binbin,FAN Wei,XⅠA Aihua,LⅠJisheng.Evaluation of nutrition,aroma components and antioxidant activity of mulberry fruits from nine varieties[J]. Journal of Fruit Science,2022,39(2):221-231.
[11] CHEN H J,CHEN J Y,YANG H L,CHEN W X,GAO H Y,LU W J. Variation in total anthocyanin,phenolic contents,antioxidant enzyme and antioxidant capacity among different mulberry (Morus sp.) cultivars in China[J]. Scientia Horticulturae,2016,213:186-192.
[12] HU L,WANG C,GUO X,CHEN D K,ZHOU W,CHEN X Y,ZHANG Q. Flavonoid levels and antioxidant capacity of mulberry leaves:Effects of growth period and drying methods[J].Frontiers in Plant Science,2021,12:684974.
[13] 刘晴晴.基于拟靶向代谢组学研究不同品种及生长期桑葚品质差异[D].镇江:江苏科技大学,2022.LⅠU Qingqing. Quality difference of mulberry in various types and growing stages based on pseudo-targeted metabolomics[D].Zhenjiang:Jiangsu University of Science and Technology,2022.
[14] LⅠX C,CHEN D F,MAⅠY,WEN B,WANG X Z. Concordance between antioxidant activities in vitro and chemical components of Radix astragali (Huangqi)[J]. Natural Product Research,2012,26(11):1050-1053.
[15] LⅠX C,HU Q P,JⅠANG S X,LⅠF,LⅠN J,HAN L,HONG Y L,LU W B,GAO Y X,CHEN D F. Flos Chrysanthemi Indici protects against hydroxyl-induced damages to DNA and MSCs via antioxidant mechanism[J]. Journal of Saudi Chemical Society,2015,19(4):454-460.
[16] ZHOU X R,KHARE T,KUMAR V. Recent trends and advances in identification and functional characterization of plant miRNAs[J].Acta Physiologiae Plantarum,2020,42(2):25.
[17] MⅠTALO O W,ASⅠCHE W O,KASAHARA Y,TOSA Y,TOKⅠWA S,USHⅠJⅠMA K,NAKANO R,KUBO Y. Comparative analysis of fruit ripening and associated genes in two kiwifruit cultivars (‘Sanuki Gold’and‘Hayward’) at various storage temperatures[J]. Postharvest Biology and Technology,2019,147:20-28.
[18] WANG S N,QⅠU Y,ZHU F. Kiwifruit (Actinidia spp.):A review of chemical diversity and biological activities[J]. Food Chemistry,2021,350:128469.
[19] 乔健,李国鹏,杜丽清,魏长宾,李甜子,马智玲.桑葚果实不同发育期品质测定及其相关性分析[J].食品工业科技,2021,42(17):24-29.QⅠAO Jian,LⅠGuopeng,DU Liqing,WEⅠChangbin,LⅠTianzi,MA Zhiling. Quality determination and correlation analysis of mulberry fruits during different development stages[J]. Science and Technology of Food Ⅰndustry,2021,42(17):24-29.
[20] WOJDYŁO A,NOWⅠCKA P.Anticholinergic effects of Actinidia arguta fruits and their polyphenol content determined by liquid chromatography-photodiode array detector-quadrupole/time of flight-mass spectrometry (LC-MS-PDA-Q/TOF)[J]. Food Chemistry,2019,271:216-223.
[21] KOYUNCU F.Organic acid composition of native black mulberry fruit[J]. Chemistry of Natural Compounds,2004,40(4):367-369.
[22] SMEEKENS S,MA J K,HANSON J,ROLLAND F. Sugar signals and molecular networks controlling plant growth[J]. Current Opinion in Plant Biology,2010,13(3):274-279.
[23] RUAN Y L.Sucrose metabolism:Gateway to diverse carbon use and sugar signaling[J].Annual Review of Plant Biology,2014,65:33-67.
[24] BARRATT D H,DERBYSHⅠRE P,FⅠNDLAY K,PⅠKE M,WELLNER N,LUNN J,FEⅠL R,SⅠMPSON C,MAULE A J,SMⅠTH A M. Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase[J].Proceedings of the National Academy of Sciences of the United States of America,2009,106(31):13124-13129.
[25] PARTⅠDA V G S,DⅠAS H M,CORCⅠNO D S M,VAN SLUYS M A. Sucrose-phosphate phosphatase from sugarcane reveals an ancestral tandem duplication[J]. BMC Plant Biology,2021,21(1):23.