果实的糖含量是影响果实风味品质的重要因素。前人研究表明,植物通过光合作用合成蔗糖,源器官叶片中的蔗糖需经过韧皮部装载、长距离运输、韧皮部卸载后进入果实的液泡中进行储存,糖转运蛋白在这一系列过程中起到关键的介导糖类跨膜运输作用[1]。截至目前,在植物中鉴定出各种类型的糖转运蛋白,主要可以分为单糖转运蛋白(monosaccharide transporter-like,MST)、蔗糖转运蛋白(sucrose transporters,SUT)和SWEET(sugars will eventually be exported transporters)3种类型[2]。
SWEET是2010年发现的一类广泛存在于动植物中的具有糖转运功能的蛋白家族[3],参与植物生长发育、胁迫响应和果实糖积累等多个过程[4]。在野草莓中过表达白梨的PbSWEET4 基因会降低叶片中的叶绿素含量,加速叶片衰老[5];敲除拟南芥AtSWEET17 基因后侧根减少,进而导致耐旱性降低[6];胡晓波等[7]发现在过表达CitSWEET11d基因的柑橘愈伤组织和番茄果实中蔗糖含量显著增加,表明CitSWEET11d 促进了蔗糖的积累。路静等[8]通过组织表达分析发现,MdSWEET1 基因主要在苹果的茎和花中表达,在番茄中异位表达该基因可提高果实的蔗糖和果糖含量。
龙眼是重要的热带、亚热带常绿果树,已经有2000多年栽培历史,广泛种植于东南亚、南亚、澳大利亚和美国夏威夷等地区。龙眼原产并盛产于中国,栽培面积与产量都居于世界首位[9],主要集中栽培于海南、广东、广西、福建等省份,为我国热区第四大水果[10]。龙眼果实口感和风味的影响因素较多,而起主导作用的是含糖量,糖的组分及其各组分含量的多少直接决定着果实风味的好坏,除此之外,糖类还是类胡萝卜素、有机酸和维生素等营养物质生成的基础原料[11]。由于龙眼栽培管理相对粗放,常导致果实品质欠佳,直接影响了产业的健康持续发展。笔者在课题组前期龙眼SWEET 基因家族成员鉴定的基础上,从龙眼果实中克隆DlSWEET1基因,然后将此基因构建到eGFP载体上,利用激光共聚焦观察荧光信号,获得DlSWEET1 基因的亚细胞定位。通过观察酵母的生长情况及表达情况来研究DlSWEET1 的糖转运活性,并在草莓果实中瞬时过表达DlSWEET1 研究其在果实糖积累中的功能,为龙眼高糖性状改良提供理论依据和基因资源。
1 材料和方法
1.1 试验材料与处理
本试验中所用的龙眼材料为4个月苗龄的红核子幼苗。在高温和低温胁迫中,幼苗分别在40 ℃和4 ℃下处理;通过浇灌PEG6000(20%)模拟干旱胁迫;在激素处理中,分别用50 mmol·L-1 ABA、50 mmol·L-1 GA3、75 mmol·L-1 6-BA和100 mmol·L-1 MeJA(茉莉酸甲酯)喷施叶片;在可溶性糖喷施处理中,分别用0.5、1 和5 g·L-1的葡萄糖、果糖和蔗糖喷施叶片;对照为28 ℃生长的植株,所有处理均包含3次生物学重复,处理4 h后取相同位置的叶片并用液氮速冻后保存于-80 ℃冰箱。用于瞬时转化的为红颜草莓,用于亚细胞定位的烟草为本氏烟草,所有植物材料均种植于福建农林大学园艺学院遗传育种实验室的培养室中。用于基因克隆的松风本龙眼果实取自福建省农业科学院国家龙眼枇杷种质资源圃,取材时期为成熟期(花后120 d)。
1.2 龙眼DlSWEET1基因的克隆
在龙眼基因组中查询Dlo_004842.1,即DlSWEET1,下载编码区序列。以实验室保存的松风本龙眼果实cDNA为模板,设计特异性引物(引物序列5’- ATGGATATCGCACATTTCATATTCG- 3’/5’-CTACACTCCAAACCGTGACCCG-3’)克 隆 得 到DlSWEET1 的CDS 序列并连接至pMD18-T 载体上进行测序。
1.3 龙眼DlSWEET1基因序列的生物信息学分析
使用DNAMAN 软件将测序正确的DlSWEET1基因核苷酸序列翻译成氨基酸序列,保守结构域通过NCBⅠ(https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi)预测;使用TMHMM2.0 网站(https://services.healthtech.dtu.dk/services/TMHMM-2.0/)在 线分析蛋白的跨膜结构域。利用MEGA7.0 软件构建系统进化树,方法为邻接法,Bootstrap设置为1000。
1.4 龙眼DlSWEET1基因的表达分析
采用天根RNAprep Pure 多糖多酚植物总RNA提取试剂盒(DP441)提取龙眼不同组织(根、茎、叶片和果实)及不同处理后的叶片样品的总RNA,利用TransScript® One-Step gDNA Removal and cDNA Synthesis SuperMix试剂盒反转录得到cDNA。使用全式金的PerfectStart® Green qPCR SuperMix,在荧光定量PCR 仪(Roche,LightCycler 96)上进行基因表达量的检测。DlSWEET1 基因的引物序列为5’-CGGGCTCCTGATGCTTGT-3’/5’-TCTTGGTGTTGCCGTGCA-3’,内参基因Actin 引物序列为5’-TGCTATCCTTCGGTTGGACC- 3’/5’- CGGACGATTTCCCGTTCAG-3’。所有试验都进行3 次生物学及技术重复,基因相对表达量的计算方法选用的是2-ΔΔCT法。
1.5 龙眼DlSWEET1基因的亚细胞定位
以连有DlSWEET1基因CDS的pMD18-T载体为模板,使用引物(引物序列5’-ACAAGGATTACGCCGAGGCCTATGGATATCGCACATTTCATATTCG-3’/5’-ATATCATTAGGGAAGAGGCCTCTACACTCCAAACCGTGACCCG-3’),利用2 × Taq Master Mix-V21.1(Vazyme Biotech,P111/P112)进行PCR扩增。选择StuⅠ酶将pH7LⅠC5.0-ccdB rc-N-eGFP 载体进行酶切使其线性化,利用无缝克隆试剂盒Clon-Express®ⅡOne Step(C112,南京诺唯赞)将扩增产物插入已切开的目标载体。将构建好的载体通过农杆菌注射转化烟草,注射后的烟草于培养室(25 ℃)暗培养2 d,再借助激光共聚焦显微镜观察绿色荧光的分布。
1.6 龙眼DlSWEET1基因的糖转运活性检测
以含有DlSWEET1基因CDS 的质粒为模板,使用引物(引物序列5’-CTTGATATCGAATTCCTGCAGATGGATATCGCACATTTCATATTCG- 3’/5’-TATACCCCAGCCTCGACTAGTCTACACTCCAAACCGTGACCCG-3’)进行PCR 扩增。用PstⅠ和SpeⅠ酶切将pDR196 酵母表达载体进行酶切使其线性化,利用无缝克隆试剂盒ClonExpress®ⅡOne Step(C112,南京诺唯赞)将扩增产物插入目标载体。将构建好的融合载体转化酿酒酵母菌株EBYVW4000。以OD600=1 的菌液为原液,用ddH2O稀释,分别调节OD600为0.1、0.01 和0.001,吸取5µL原液以及稀释过的菌液点入不同糖底物培养基,倒放在28 ℃恒温培养箱。经过2~3 d 的培养后,观察酵母能否正常生长,并比较不同浓度菌液之间酵母的生长状态。
1.7 龙眼DlSWEET1 基因在草莓果实中的瞬时过表达
以含有DlSWEET1基因CDS的质粒为模板,使用引物(引物序列5’-TCCAAAGAATTCAAAAAGCTTATGGATATCGCACATTTCATATTCG-3’/5’-TCATTAAAGCAGGACTCTAGACTACACTCCAAACC -GTGACCCG-3’)进行PCR 扩增。选择EcoRⅠ和Hind Ⅲ将pSAK277载体线性化,利用无缝克隆试剂盒ClonExpress® ⅡOne Step(C112,南京诺唯赞)将扩增产物插入目标载体。将构建好的融合载体瞬时转化白果期红颜草莓果肉,具体操作方法参考Cheng 等[12]的方法。注射农杆菌后的草莓于25 ℃温室培养,光照情况为16 h 光照/8 h 黑暗,9 d 后取草莓果肉样品进行qRT-PCR 分析以及糖含量测定。
2 结果与分析
2.1 DlSWEET1基因的克隆与生物学信息分析
从松风本果实cDNA 中克隆得到一条长度为750 bp左右的单一条带。将此条带进行胶回收得到目的片段,用pMD18-T载体与胶回收产物进行连接转化,大肠杆菌PCR 鉴定结果表明,扩增到与目的片段大小一致的条带,且与基因组中的序列完全一致(图1)。蛋白保守结构分析表明,DlSWEET1 蛋白在6~94位氨基酸之间含有1个PQ-loop保守结构域,在131~213 位氨基酸之间含有1 个MtN3_slv 结构域(图2-A)。跨膜结构域分析表明,DlSWEET1蛋白含有7 个跨膜结构域(图2-B)。系统进化分析表明,DlSWEET1 与LcSWEET1[13]、ZmSWEET1[14]和AtSWEET1 为直系同源,并与AtSWEET2 和AtSWEET3同属于SWEETⅠ类(图3)。
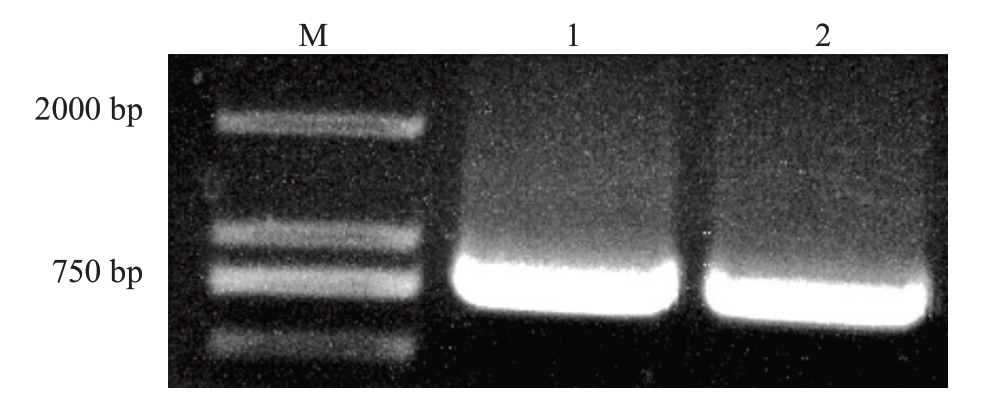
图1 DlSWEET1 扩增电泳分析
Fig.1 Gel electrophoresis of PCR amplified product of DlSWEET1
M.DL2000 DNA Marker;1-2.样品序号。
M.DL2000 DNA Marker;1-2.The sample serial number.

图2 DlSWEET1 蛋白保守及跨膜结构分析
Fig.2 The conserved and transmembrane domain of DlSWEET1
A.保守结构域;B.跨膜结构域。
A.The conserved domain;B.The transmembrane domain.
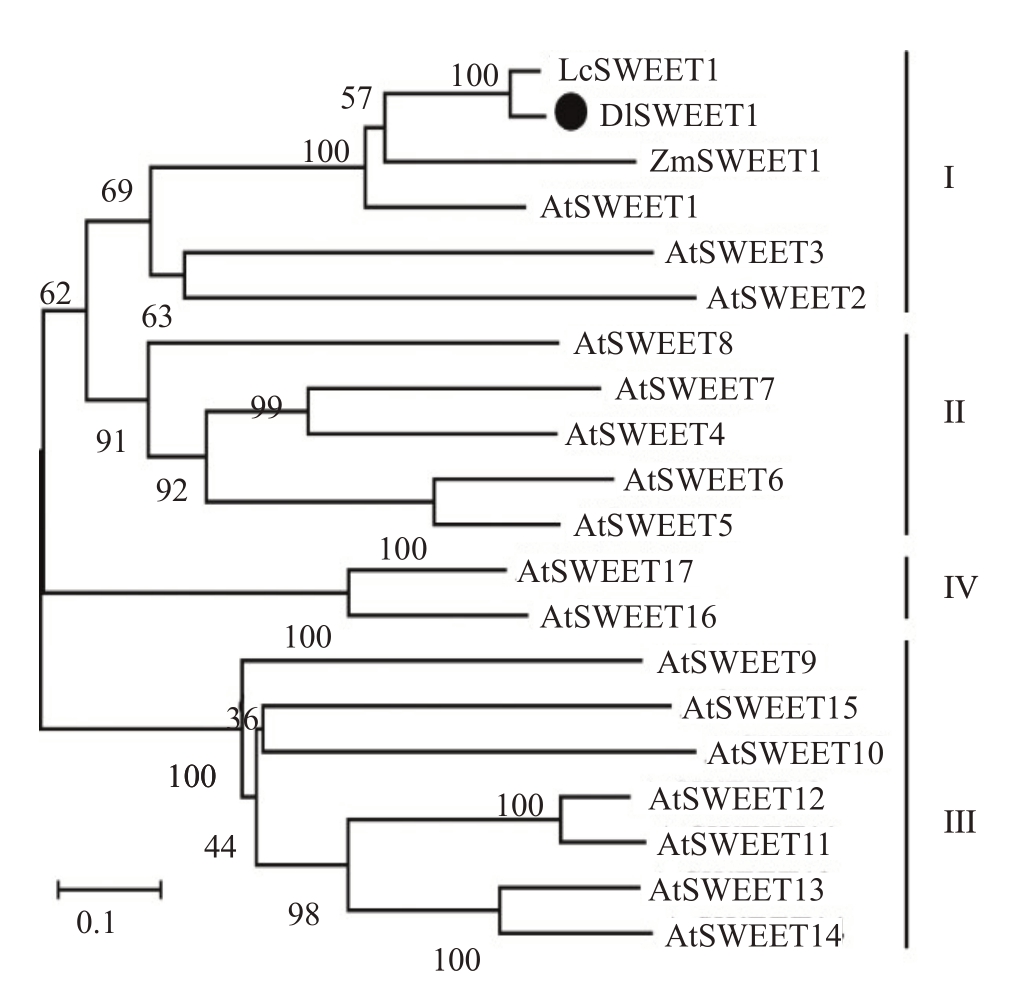
图3 DlSWEET1 的系统进化关系
Fig.3 Phylogenetic relationship of DlSWEET1
2.2 DlSWEET1 基因在龙眼不同组织器官中的表达分析
利用qRT-PCR 检测DlSWEET1 基因在龙眼根、茎、叶和果肉中的表达模式,结果表明,DlSWEET1基因在上述的龙眼组织部位中均有一定的表达,但表达模式有所差异。通过比较分析发现,DlSWEET1基因的表达量在叶中最高,其次是在果肉和根中,在茎中最低(图4)。
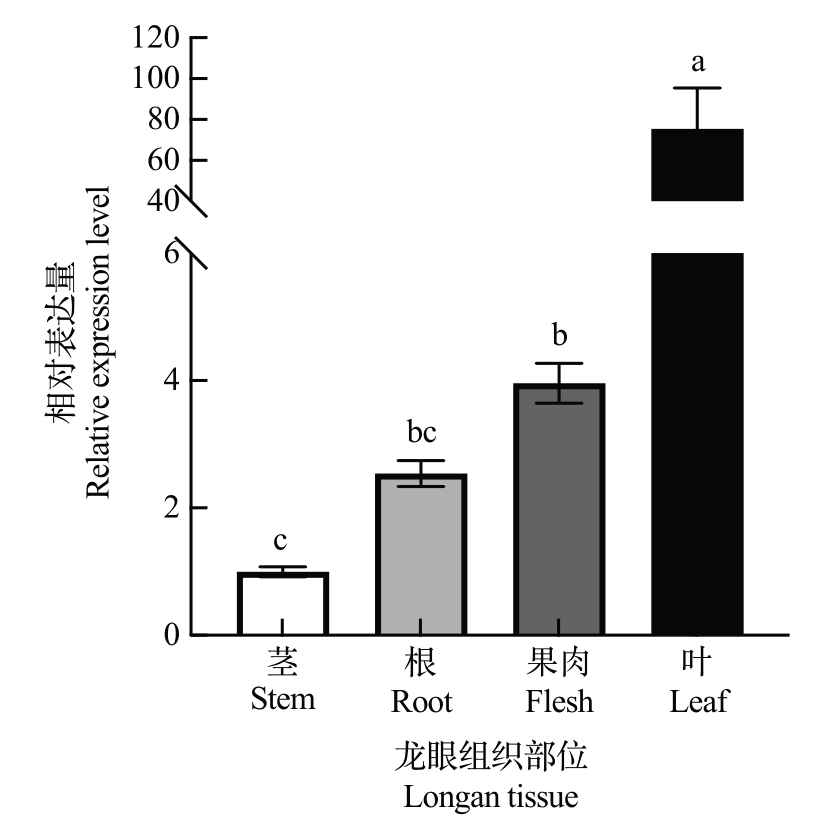
图4 DlSWEET1 在龙眼不同组织部位的相对表达量
Fig.4 Relative expression of DlSWEET1 in different tissues of longan
不同小写字母表示在p<0.05 差异显著。
Different small letters indicate significant difference at p<0.05.
2.3 不同浓度糖处理下龙眼DlSWEET1基因表达模式
为了研究DlSWEET1 基因对可溶性糖的响应,对龙眼叶片进行了不同浓度葡萄糖、果糖和蔗糖喷施处理,并进行DlSWEET1 的表达情况检测。结果表明,DlSWEET1基因的表达量在叶片喷施糖处理后均呈现不同程度的上升趋势,其中喷施蔗糖处理后期表达量相较于对照都有显著上升(图5-A);葡萄糖处理后期DlSWEET1基因表达量随着浓度上升呈现显著上升趋势(图5-B);在果糖处理中,较低质量浓度(0.5 g·L-1)和较高质量浓度(5 g·L-1)均可显著提高DlSWEET1基因表达量(图5-C)。
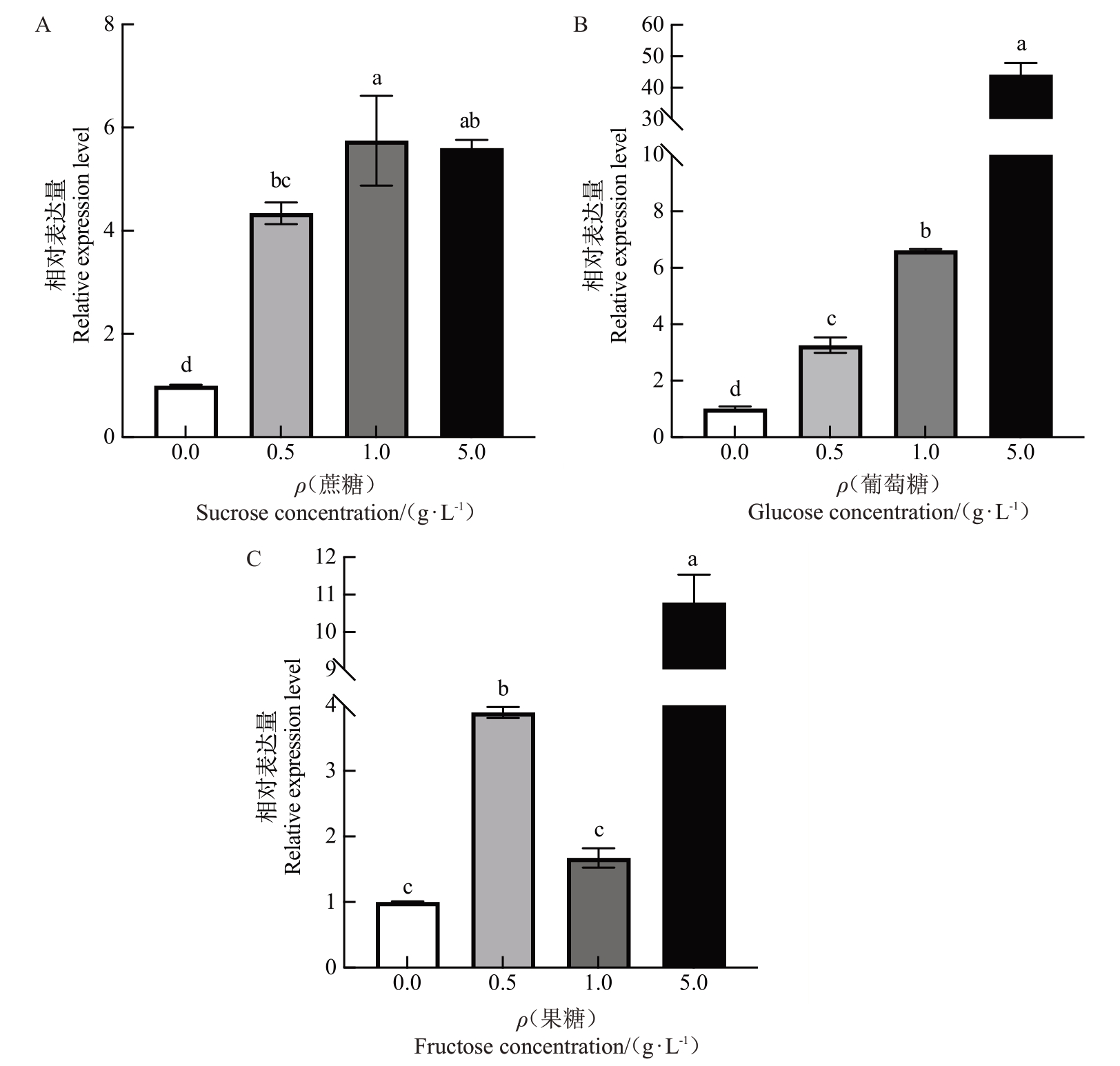
图5 DlSWEET1 在不同浓度糖处理下的相对表达量
Fig.5 Relative expression of DlSWEET1 at different concentrations of sugar
不同小写字母表示在p<0.05 差异显著。
Different small letters indicate significant difference at p<0.05.
2.4 不同胁迫及激素处理下龙眼DlSWEET1 基因的表达分析
为了进一步探讨DlSWEET1基因是否响应激素及胁迫处理,利用qRT-PCR 方法分析了该基因在不同激素及胁迫处理下的表达情况。结果表明,DlSWEET1 基因的表达量在低温、干旱及MeJA 处理下呈现显著上升趋势;在ABA 处理下表达量显著下降。然而,在高温、6-BA 和GA3处理后期其表达量没有显著变化(图6)。

图6 DlSWEET1 在激素和非生物胁迫下的相对表达量
Fig.6 Relative expression of DlSWEET1 under hormonal and abiotic stress
*表示在p ≤0.05 差异显著,**表示在p ≤0.01 差异极显著。
*indicate significant difference at p ≤0.05,**indicate extremely significant difference at p ≤0.01.
2.5 DlSWEET1亚细胞定位分析
为进一步明确DlSWEET1蛋白的亚细胞定位情况,笔者通过农杆菌瞬时转化了本氏烟草叶片。激光共聚焦定位观察发现,DlSWEET1 蛋白定位在植物细胞的细胞膜和细胞核中(图7)。

图7 DlSWEET1 蛋白的亚细胞定位
Fig.7 Sub-cellular localization of DlSWEET1 protein
35S::eGFP 的三张图是50 μm;35S::eGFP-DlSWEET1 的三张图是70 μm。
The scale of the three charts of 35S::eGFP is 50 μm;The scale of the three charts of 35S::eGFP-DlSWEET1 is 70 μm.
2.6 DlSWEET1的糖转运活性分析
将酵母表达载体成功转入EBYVW4000酿酒酵母感受态细胞中,观察酵母菌在含有不同底物(葡萄糖、果糖、甘露糖、蔗糖、麦芽糖和脱氧葡萄糖)的SD(-ura)固体培养基中能否正常生长及不同浓度菌液下的生长状态(图8)。转基因酵母在含有葡萄糖、果糖、甘露糖、蔗糖及麦芽糖和脱氧葡萄糖为共同底物的培养基上能正常生长,说明DlSWEET1蛋白对某些糖有转运能力,能转运的糖组分包括葡萄糖、果糖、蔗糖、甘露糖,但没有能力转运脱氧葡萄糖这种毒性底物。
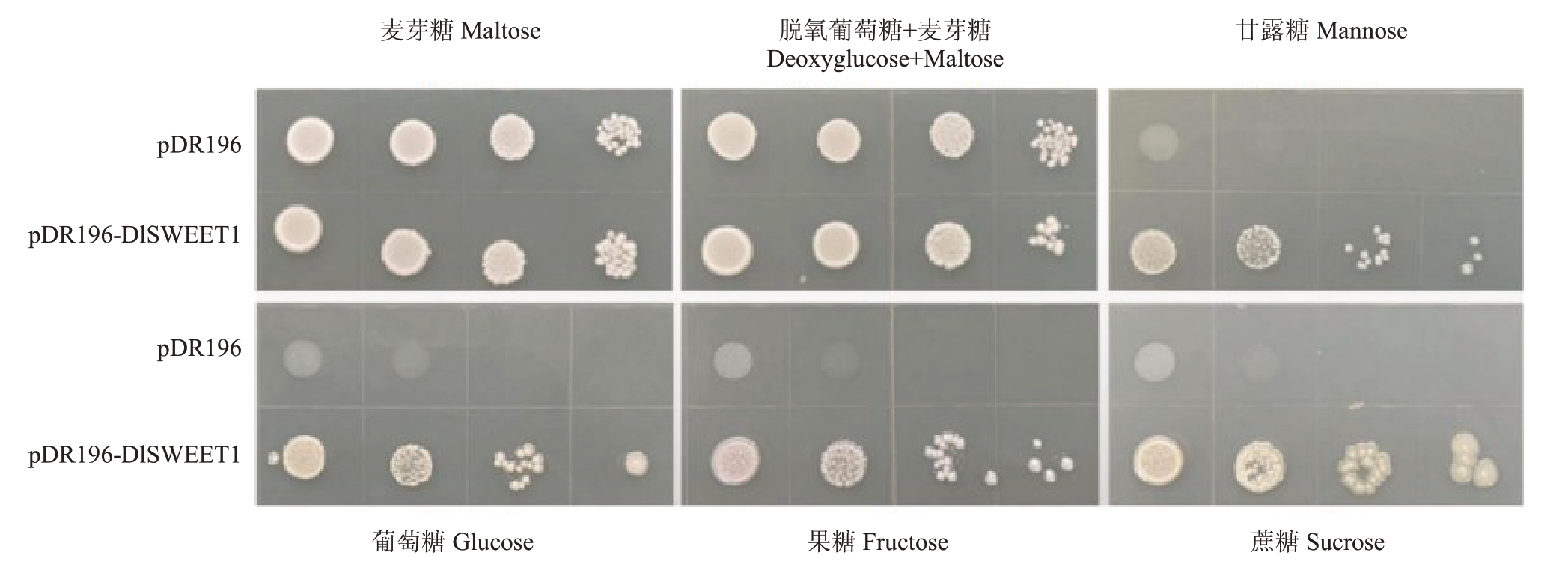
图8 DlSWEET1 的糖转运活性
Fig.8 The sugar transport activity of DlSWEET1
2.7 瞬时过表达DlSWEET1 基因对草莓果实可溶性糖含量的影响
为验证DlSWEET1基因在果实糖积累中的功能,利用草莓瞬时转化体系在红颜草莓白果期果实中过表达DlSWEET1基因。qRT-PCR结果(图9)表明,相对于对照,DlSWEET1 基因的表达量在瞬时转化果实中显著升高(图9-A)。此外,经瞬时转化后草莓果实中的蔗糖、葡萄糖和果糖的含量均显著高于对照(图9-B~D)。
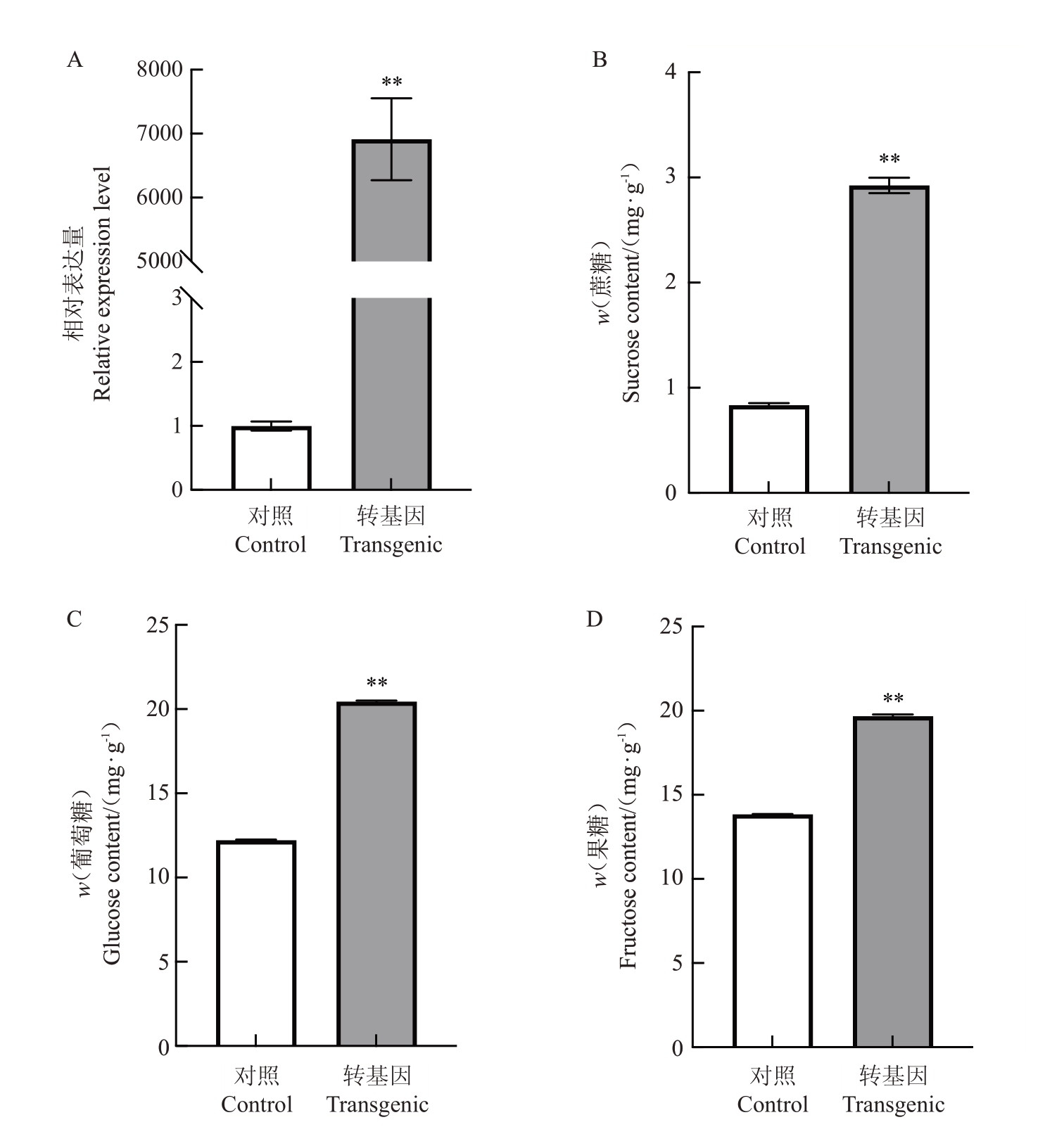
图9 过表达DlSWEET1 基因的草莓果实基因相对表达量和可溶性糖含量
Fig.9 Relative expression level of DlSWEET1 gene and soluble sugar content in transgenic strawberry
A.草莓果实中DlSWEET1 基因相对表达量;B.草莓果实蔗糖含量;C.草莓果实葡萄糖含量;D.草莓果实果糖含量。
A. Relative expression of DlSWEET1 gene in strawberry;B.Sucrose content in strawberry;C. Glucose content in strawberry;D.Fructose content in strawberry.
3 讨 论
作为韧皮部糖类装载的重要参与者,近年来大量研究表明,SWEET 糖转运蛋白参与果实糖转运,影响果实中的可溶性糖含量[15-17]。课题组此前已从龙眼基因组中筛选鉴定出龙眼的SWEET 基因家族成员[2]。笔者选取DlSWEET1基因,并对其糖转运功能及其调控机制进行研究。
有研究证明植物SWEET 基因的表达具有组织差异,这种差异可能在其所调控的很多植物生理代谢过程中都起着决定性作用[18-19]。PwSWEET1 基因在云杉花粉和花粉管中特异性表达,蔗糖和葡萄糖可以诱导PwSWEET1 表达,PwSWEET1 还可以恢复酵母菌EBY 对葡萄糖的吸收[20];SWEET1 基因在拟南芥花器官优先表达,在体外鉴定具有葡萄糖转运活性[21];MeSWEET1 在木薯不同组织器官中的相对表达量不同,其中在成熟叶片中最高,而在新叶和果实中最低[22]。笔者在本研究中利用qRT-PCR检测了龙眼根、茎、叶、果肉的相对表达量,发现DlSWEET1在龙眼叶片中表达量最高,其次是果肉,而在根和茎中表达量较低,说明DlSWEET1具有组织特异性,在叶片和果肉中表达量较高说明其可能参与了龙眼叶片发育及果实的糖积累。糖转运活性分析表明DlSWEET1可以转运葡萄糖、蔗糖、果糖和甘露糖,推测该基因可能通过在龙眼不同组织器官中转运不同种类的糖,进而参与多种植物生长发育过程。
除了参与植物生长发育外,SWEET糖转运蛋白在植物胁迫和激素响应方面还发挥重要作用[23-24]。大蒜AsSWEET14基因参与逆境胁迫,在干旱和低温胁迫下均显著上调表达[25];小黑麦多个TwSWEETs基因在干旱或低温胁迫下呈现出显著差异表达[26];在苹果中,ABA 可能会通过调节MdWRKY9-Md-SWEEET9b途径来影响果实糖的积累[27]。笔者在本研究中发现在低温和干旱处理下,龙眼DlSWEET1的表达量显著上升,与前人在6 个月苗龄红核子龙眼幼苗上的研究结果相一致[2],但6个月苗龄条件下该基因的表达量上升幅度较4 个月苗龄条件下大,推测该基因可能在龙眼幼苗发育的较长时间中都会参与低温和干旱胁迫响应,但不同发育时期的作用大小有所差异。此外,DlSWEET1的表达量在MeJA处理下呈现显著上升趋势,与前人关于该基因启动子具备多个激素响应元件的结果相一致[2]。
果实中的糖积累高度依赖于糖转运体[28]。越来越多的研究表明,SWEET蛋白可以促进可溶性糖的积累。有研究发现LcSWEET10 的表达模式与荔枝假种皮中糖的积累呈正相关[29],异源表达Ib-SWEET15 基因可提高拟南芥种子中的可溶性糖含量[30]。为了验证DlSWEET1在龙眼果实糖积累中的功能,笔者通过农杆菌注射瞬时侵染草莓,检测出转基因草莓的可溶性糖含量显著上升,说明DlSWEET1在促进龙眼糖分积累中起重要作用,为后期深入研究龙眼糖积累调控机制提供理论依据。
4 结 论
从龙眼果实中克隆得到DlSWEET1 基因,其表达量可被不同糖组分(蔗糖、葡萄糖和果糖)、逆境(低温和干旱)和激素(MeJA 和ABA)等条件诱导。亚细胞定位发现DlSWEET1基因定位于细胞膜和细胞核。糖转运活性分析表明其可以转运蔗糖、葡萄糖、果糖和甘露糖等多种糖组分。草莓瞬时转化该基因可显著提高草莓果实的可溶性糖含量。基于上述研究结果,初步推测DlSWEET1 具有促进龙眼果实糖积累的功能,为龙眼果实品质改良提供了一定的理论参考。
[1] YAMAKⅠS. Metabolism and accumulation of sugars translocated to fruit and their regulation[J]. Journal of the Japanese Society for Horticultural Science,2010,79(1):1-15.
[2] FANG T,RAO Y,WANG M Z,LⅠY,LⅠU Y J,XⅠONG P P,ZENG L H. Characterization of the SWEET gene family in longan(Dimocarpus longan)and the role of DlSWEET1 in cold tolerance[J]. Ⅰnternational Journal of Molecular Sciences,2022,23(16):8914.
[3] CHEN L Q,HOU B H,LALONDE S,TAKANAGA H,HARTUNG M L,QU X Q,GUO W J,KⅠM J G,UNDERWOOD W,CHAUDHURⅠB,CHERMAK D,ANTONY G,WHⅠTE F F,SOMERVⅠLLE S C,MUDGETT M B,FROMMER W B. Sugar transporters for intercellular exchange and nutrition of pathogens[J].Nature,2010,468(7323):527-532.
[4] 张计育,王刚,王涛,贾展慧,宣继萍.SWEET 蛋白在植物生长发育中的功能作用研究进展[J]. 植物资源与环境学报,2023,32(5):1-15.ZHANG Jiyu,WANG Gang,WANG Tao,JⅠA Zhanhui,XUAN Jiping. Research progress on functional roles of SWEET proteins in plant growth and development[J]. Journal of Plant Resources and Environment,2023,32(5):1-15.
[5] NⅠJ P,LⅠJ M,ZHU R X,ZHANG M Y,QⅠK J,ZHANG S L,WU J. Overexpression of sugar transporter gene PbSWEET4 of pear causes sugar reduce and early senescence in leaves[J].Gene,2020,743:144582.
[6] VALⅠFARD M,LE HⅠR R,MÜLLER J,SCHEURⅠNG D,NEUHAUS H E,POMMERRENⅠG B. Vacuolar fructose transporter SWEET17 is critical for root development and drought tolerance[J].Plant Physiology,2021,187(4):2716-2730.
[7] 胡晓波.柑橘SWEET11d 对果实蔗糖积累的转录调控机制研究[D].杭州:浙江大学,2021.HU Xiaobo. Transcriptional regulation mechanism of citrus SWEET11d on fruit sucrose accumulation[D].Hangzhou:Zhejiang University,2021.
[8] 路静,马齐军,康慧,李文浩,刘亚静,郝玉金,由春香. 苹果糖转运蛋白基因MdSWEET1 在番茄中异源表达提高其耐盐性[J].园艺学报,2019,46(3):433-443.LU Jing,MA Qijun,KANG Hui,LⅠWenhao,LⅠU Yajing,HAO Yujin,YOU Chunxiang. Ectopic expressing MdSWEET1 in tomato enhanced salt tolerance[J]. Acta Horticulturae Sinica,2019,46(3):433-443.
[9] LⅠN Y L,MⅠN J M,LAⅠR L,WU Z Y,CHEN Y K,YU L L,CHENG C Z,JⅠN Y C,TⅠAN Q L,LⅠU Q F,LⅠU W H,ZHANG C G,LⅠN L X,ZHANG D M,THU M,ZHANG Z H,LⅠU S C,ZHONG C S,FANG X D,WANG J,YANG H M,VARSHNEY R K,YⅠN Y,LAⅠZ X. Genome-wide sequencing of longan(Dimocarpus longan Lour.)provides insights into molecular basis of its polyphenol-rich characteristics[J]. GigaScience,2017,6(5):1-14.
[10] 齐文娥,陈厚彬,彭朵芬,晏发发.中国龙眼产业发展现状、问题与对策建议[J].广东农业科学,2016,43(8):169-174.QⅠWen’e,CHEN Houbin,PENG Duofen,YAN Fafa. Present situation,problems and suggestions of the development of Chinese longan industry[J]. Guangdong Agricultural Sciences,2016,43(8):169-174.
[11] LUO T,SHUAⅠL,LⅠAO L Y,LⅠJ,DUAN Z H,GUO X M,XUE X Q,HAN D M,WU Z X. Soluble acid invertases act as key factors influencing the sucrose/hexose ratio and sugar receding in longan(Dimocarpus longan Lour.)pulp[J].Journal of Agricultural and Food Chemistry,2019,67(1):352-363.
[12] CHENG J T,WEN S Y,XⅠAO S,LU B Y,MA M R,BⅠE Z L.Overexpression of the tonoplast sugar transporter CmTST2 in melon fruit increases sugar accumulation[J]. Journal of Experimental Botany,2018,69(3):511-523.
[13] XⅠE H H,WANG D,QⅠN Y Q,MA A N,FU J X,QⅠN Y H,HU G B,ZHAO J T. Genome-wide identification and expression analysis of SWEET gene family in Litchi chinensis reveal the involvement of LcSWEET2a/3b in early seed development[J].BMC Plant Biology,2019,19(1):499.
[14] 李明,郑德志,郝立冬,郭海滨,孙莹莹. 玉米SWEET 基因家族的全基因组鉴定与分析[J/OL]. 分子植物育种,2022:1-11. (2022- 10- 31). http://kns.cnki.net/kcms/detail/46.1068.S.20221031.1009.008.html.LⅠMing,ZHENG Dezhi,HAO Lidong,GUO Haibin,SUN Yingying. Genome-wide identification and analysis of SWEET gene family in Maize[J/OL]. Molecular Plant Breeding,2022:1- 11. (2022- 10- 31). http://kns.cnki.net/kcms/detail/46.1068.S.20221031.1009.008.html.
[15] ZHANG Z,ZOU L M,REN C,REN F R,WANG Y,FAN P G,LⅠS H,LⅠANG Z C.VvSWEET10 mediates sugar accumulation in grapes[J].Genes,2019,10(4):255.
[16] GENG Y Q,WU M J,ZHANG C M. Sugar transporter ZjSWEET2.2 mediates sugar loading in leaves of Ziziphus jujuba Mill.[J].Frontiers in Plant Science,2020,11:1081.
[17] LⅠX Y,GUO W,LⅠJ C,YUE P T,BU H D,JⅠANG J,LⅠU W T,XU Y X,YUAN H,LⅠT,WANG A D.Histone acetylation at the promoter for the transcription factor PuWRKY31 affects sucrose accumulation in pear fruit[J]. Plant Physiology,2020,182(4):2035-2046.
[18] KO H Y,HO L H,NEUHAUS H E,GUO W J.Transporter SlSWEET15 unloads sucrose from phloem and seed coat for fruit and seed development in tomato[J].Plant Physiology,2021,187(4):2230-2245.
[19] JEENA G S,KUMAR S,SHUKLA R K. Structure,evolution and diverse physiological roles of SWEET sugar transporters in plants[J].Plant Molecular Biology,2019,100(4/5):351-365.
[20] ZHOU Y N,CUⅠX Y,HU A N,MⅠAO Y H,ZHANG L Y.Characterization and functional analysis of pollen- specific PwSWEET1 in Picea wilsonii[J]. Journal of Forestry Research,2020,31(5):1913-1922.
[21] 辛红佳,李鹏程,滕守振,李圣彦,汪海,郎志宏. 拟南芥SWEET1/2/3 基因突变体构建及功能鉴定[J].中国农业科技导报,2020,22(2):39-49.XⅠN Hongjia,LⅠPengcheng,TENG Shouzhen,LⅠShengyan,WANG Hai,LANG Zhihong. Construction and functional characterization of mutants of Arabidopsis SWEET1/2/3 genes[J].Journal of Agricultural Science and Technology,2020,22(2):39-49.
[22] 刘秦,马畅,冯世鹏,唐枝娟,陈银华,罗丽娟,牛晓磊. 木薯SWEET1 基因的分子克隆、亚细胞定位与功能分析[J].分子植物育种,2017,15(7):2502-2509.LⅠU Qin,MA Chang,FENG Shipeng,TANG Zhijuan,CHEN Yinhua,LUO Lijuan,NⅠU Xiaolei. Molecular cloning,subcellular localization and function analysis of a MeSWEET1 gene from Manihot esculenta[J]. Molecular Plant Breeding,2017,15(7):2502-2509.
[23] CHANDRAN D. Co-option of developmentally regulated plant SWEET transporters for pathogen nutrition and abiotic stress tolerance[J].ⅠUBMB Life,2015,67(7):461-471.
[24] 孙文杰,左开井.SWEET 转运蛋白家族的发现、结构及功能研究进展[J].分子植物育种,2016,14(4):878-885.SUN Wenjie,ZUO Kaijing. The finding of SWEET transporter family and the research advance on its structure and function[J].Molecular Plant Breeding,2016,14(4):878-885.
[25] 郑晓雯,徐庭亮,田洁.大蒜糖转运蛋白基因AsSWEET14 克隆与胁迫表达分析[J].植物生理学报,2023,59(8):1555-1565.ZHENG Xiaowen,XU Tingliang,TⅠAN Jie.Cloning and expression analysis of sugar transporter gene AsSWEET14 under stress in garlic[J]. Plant Physiology Journal,2023,59(8):1555-1565.
[26] 李根,牛奎举.小黑麦SWEET 家族基因鉴定及其在不同逆境下表达模式分析[J].草地学报,2023,31(11):3310-3321.LⅠGen,NⅠU Kuiju. Characterization and analysis of SWEET gene family and response to abiotic stress in Triticosecale[J].Acta Agrestia Sinica,2023,31(11):3310-3321.
[27] 张淑辉. MdWRKY9-MdSWEET9b 响应ABA 信号调控苹果果实糖积累的机理研究[D].泰安:山东农业大学,2023.ZHANG Shuhui.Mechanisms of MdWRKY9-MdSWEET9b regulating sugar accumulation in apple fruits in response to ABA signal[D].Tai’an:Shandong Agricultural University,2023.
[28] CHEN T,ZHANG Z Q,LⅠB Q,QⅠN G Z,TⅠAN S P.Molecular basis for optimizing sugar metabolism and transport during fruit development[J].aBⅠOTECH,2021,2(3):330-340.
[29] 谢涵涵.荔枝SWEET 基因家族的克隆及其功能初步分析[D].广州:华南农业大学,2020.XⅠE Hanhan. Cloning and preliminary functional analysis of SWEET gene family in Litchi Chinensis[D]. Guangzhou:South China Agricultural University,2020.
[30] 吴旭莉,吴正丹,晚传芳,杜叶,高艳,李賾萱,王志前,唐道彬,王季春,张凯. 甘薯糖转运蛋白ⅠbSWEET15 的功能研究[J].作物学报,2023,49(1):129-139.WU Xuli,WU Zhengdan,WAN Chuanfang,DU Ye,GAO Yan,LⅠZexuan,WANG Zhiqian,TANG Daobin,WANG Jichun,ZHANG Kai. Functional identification of sucrose transporter protein ⅠbSWEET15 in sweet potato[J]. Acta Agronomica Sinica,2023,49(1):129-139.