干旱是制约农业生产的主要因素之一,会造成作物大面积减产[1]。黄土高原地区是中国最大的苹果优势产区[2],该地区适宜苹果的生长,生产出的苹果品质好、风味佳。但黄土高原地区常年干旱缺水,且大部分苹果种植地位于山区,缺乏灌溉条件。因此,干旱缺水是中国黄土高原地区苹果产业发展的限制因素之一。水对植物的生存至关重要,缺水会限制植物的生长[3]。干旱胁迫会对植物的各种生物活动产生影响,如种子萌发、繁殖和成熟。干旱胁迫会影响植物的形态、生理、生化和代谢途径,并最终导致植物生产力的降低[4-5]。植物也进化出相应的耐旱策略以应对水分胁迫,可通过对植物细胞、组织、器官及整个植株的调控维持生存。通过气孔的调控和更大更深的根系来增加水分运输,从而减少水分的损失。通过抗氧化活性系统清除活性氧(ROS),保持膜的完整性,与胁迫相关的蛋白质和水通道蛋白活性也有助于植物产生耐旱性。通过脯氨酸(PRO)等渗透物质的积累维持细胞膨胀压力。脱落酸(ABA)是植物适应环境胁迫的重要信号分子,在干旱胁迫下,ABA 可以调控植物的气孔开放,从而减缓植物体内水分的亏缺,增强植物抗旱性[6]。9-顺式-环氧类胡萝卜素双加氧酶(NCED)是干旱引发诱导的ABA生物合成的关键酶,NCED基因属于一个具有9个成员的多基因家族[7]。
在西北农林科技大学洛川苹果试验站种质资源圃,笔者发现P5、L51、L37、LC36、L7、LC54、ZN18和C31 的果实具有特异性,可作为品种资源选育抗逆优质的苹果新品种。为探究其抗旱性,笔者在本研究中以这8 份苹果种质资源为试验材料,以抗旱性较强的富平楸子和新疆野苹果为对照,对各苹果种质资源的抗旱性进行研究,测定各苹果种质资源的净光合速率(Pn)、叶绿素含量、叶片相对含水量、相对电导率、丙二醛(MDA)含量、过氧化氢(H2O2)含量、超氧阴离子(O2-)含量、抗氧化酶活性、PRO含量、ABA 含量及其合成相关基因的表达量,并利用隶属函数法对各苹果种质资源的抗旱性进行评价。
1 材料和方法
1.1 试验材料
以1年生P5(Malus asiatica)、L51(M.robusta)、L37(M. hybrid‘Dwarf Tree’)、LC36(M. hybrid‘Cranberry’)、L7(M.soulardii)、LC54(M.domestica‘Oekonomierat Echter-meyer’)、ZN18(M. domestica,Sciros×Scifresh)和C31(M.domestica‘Trail’)为试验材料,选取富平楸子(M.prunifolia)和新疆野苹果(M.sieversii)为对照,于2022 年春采用芽接法嫁接于平邑甜茶植株上,试验于2022 年6 月在西北农林科技大学园艺场的避雨棚内进行。植株定植于塑料盆(30 cm×18 cm)中,栽植基质为V 黄土∶V 沙∶V 有机质=5∶1∶1,放置于避雨棚中,定期进行浇水、除草等生长管理工作。
1.2 试验方法
待所有试验材料高度为70~80 cm时,挑选高度一致的植株分为对照组和处理组进行试验处理。将处理组于处理前1 d浇透水后停止浇水,直至处理第9天各苹果种质资源因极度缺水出现显著差异后复水,对照组每天正常浇水。于处理的第0天开始,每隔1 d进行Pn、叶绿素含量、叶片相对含水量、相对电导率的测定,并采集植株中部完全成熟的叶片,用锡箔纸包住后立即用液氮快速冷冻,并于-80 ℃下储存。
1.3 生理指标测定
叶片相对含水量测定,称取叶片鲜质量(FW)后,将叶片浸泡在蒸馏水中24 h,用吸水纸吸干表面水分后,测量叶片饱和质量(TW),烘干至恒质量后测量叶片干质量(DW),计算叶片相对含水量,每个种质资源5次重复。叶片相对含水量计算公式如下:
RWC/%=(FW-DW)/(TW-DW)×100。
相对电导率测定,利用打孔器在叶片上打20个圆片,避开叶脉,装入15 mL 离心管,加入10 mL 纯净水,浸泡4 h 后,混匀利用电导率仪测量电导率(S1),沸水浴20 min,冷却至室温后混匀再次测量电导率(S2),测量纯净水的电导率(S0),计算叶片相对电导率,每个种质资源5 次重复。相对电导率计算公式如下:
MDA、PRO含量测定,按照生产厂家说明书(苏州科铭生物技术有限公司,江苏苏州),利用相应试剂盒进行测定。
1.4 Pn及叶绿素含量测定
在晴朗天气的上午,利用CⅠRAS-3 便携式光合作用系统(CⅠRAS,Amesbury,MA,USA)测定各苹果种质资源的Pn。
将叶片剪碎成细条状称取0.1 g 置于15 mL 试管中,加入8 mL 80%丙酮,将叶片全部浸没,避光浸泡24 h,其间每隔一定时间对试管进行晃动,直至叶片上的绿色完全褪去。混匀吸取1 mL加入比色皿,利用UV-2600分光光度计(日本岛津)分别在663 nm、645 nm、470 nm 处测定吸光值,计算各苹果种质资源的总叶绿素含量,每个种质资源5次重复。
1.5 活性氧含量及抗氧化酶活性测定
按照生产厂家说明书(苏州科铭生物技术有限公司,江苏苏州),利用相应试剂盒测定H2O2含量、O2-含量、超氧化物歧化酶(SOD)活性和过氧化物酶(POD)活性。
1.6 ABA含量的测定
称取0.1 g 经研磨的冻样于2 mL 离心管中,加入1 mL 经-20 ℃预冷的提取液(V 异丙醇∶V 甲醇∶V 乙酸=79∶20∶1),涡旋震荡混匀,4 ℃提取12 h,4 ℃条件下12 000 r·min-1离心10 min,用一次性注射器吸取上清液经0.22 μm 有机过滤器过滤后加入棕色进样瓶,利用液质联用仪测定[8]。
1.7 RNA提取及qRT-PCR分析
使用植物RNA 分离试剂盒提取总RNA[Wolact,Vicband Life Sciences Company(HK)Limited],再利用PrimeScript 第一链cDNA 合成试剂盒(TaKaRa,日本)反转录合成cDNA。实时荧光定量PCR 采用SYBR Premix Ex Taq ⅡKit(TaKaRa,Tokyo,Japan),以MdMDH(MDP0000197620)作为内参基因,试验所用引物序列见表1,使用2-△△CT方法计算相对表达量[9]。
表1 试验所用引物
Table 1 The primers used in this study

引物用途Primer function定量PCR内参Reference for qPCR MdNCED1定量MdNCED1 qPCR MdNCED3定量MdNCED3 qPCR基因名称Gene name MDH引物序列(5’-3’)Primer sequence(5’-3’)F:CGTGATTGGGTACTTGGAAC R:TGGCAAGTGACTGGGAATGA MdNCED1 F:AAGCAGCGTTATGTGTACGGAACC R:GCCAGGTCCCAGGTCATAGAGG MdNCED3 F:AACCAGCCGTATCAGCCAAGAAC R:TCCACGAGCCCGAACATCCC
1.8 隶属函数的计算
考虑到试验材料遗传背景不同,各项生理指标存在较大差异,故利用短期干旱第0天和第9天各项指标的相对变化率进行隶属函数的计算,以评价各苹果种质资源的抗旱性。
若该指标与抗旱性呈正相关,该指标的隶属函数计算公式为:
若该指标与抗旱性呈负相关,该指标的隶属函数计算公式为:
式中,U(X)为隶属函数值,X 指某一指标的相对变化率[(S 第9 天-S 第0 天)/S 第0 天×100%],S为某一指标的测量数值;Xmax指某一指标相对变化率的最大值,Xmin指某一指标相对变化率的最小值。在测定的指标中,与抗旱性负相关的有相对电导率、MDA含量、H2O2含量和O2-含量,其余指标与抗旱性呈正相关。
1.9 数据分析
使用SPSS Statistics 26.0进行数据统计分析,并使用单因素分析和Tukey的多重比较(p<0.05)进行显著性分析。使用Origin 2022b绘图。
2 结果与分析
2.1 自然干旱胁迫下各苹果种质资源的表型及生理指标
在自然干旱胁迫后,各苹果种质资源的叶片均出现不同程度的失水萎蔫(图1),干旱处理第9天各苹果种质资源间的差异最显著,其中LC54叶片的萎蔫程度最为严重。在干旱处理后,各苹果种质资源的叶片相对含水量(w,后同)显著降低(图2-A),变化范围为0.72%~30.3%,其中LC54的叶片相对含水量降幅最大。在干旱处理后,各苹果种质资源叶片的相对电导率显著升高(图2-B),变化范围为14.16%~61.99%。在干旱胁迫下,各苹果种质资源叶片的MDA 含量也显著升高,变化范围为9.42%~65.82%,在干旱处理第9 天,LC54 的MDA 含量最高,LC36 的MDA 含量最低(图2-C)。在干旱胁迫第0 天,各苹果种质资源叶片的PRO 含量维持在较低水平,分布范围为9.81~17.11 μg·g-1,在干旱处理第9天,各苹果种质资源叶片的PRO含量显著增加,分布范围为67.8~152.66 μg·g-1,LC36的PRO含量在干旱处理后显著高于其他种质资源,LC54、L37、P5的PRO 含量在干旱处理后显著低于其他种质资源(图2-D)。
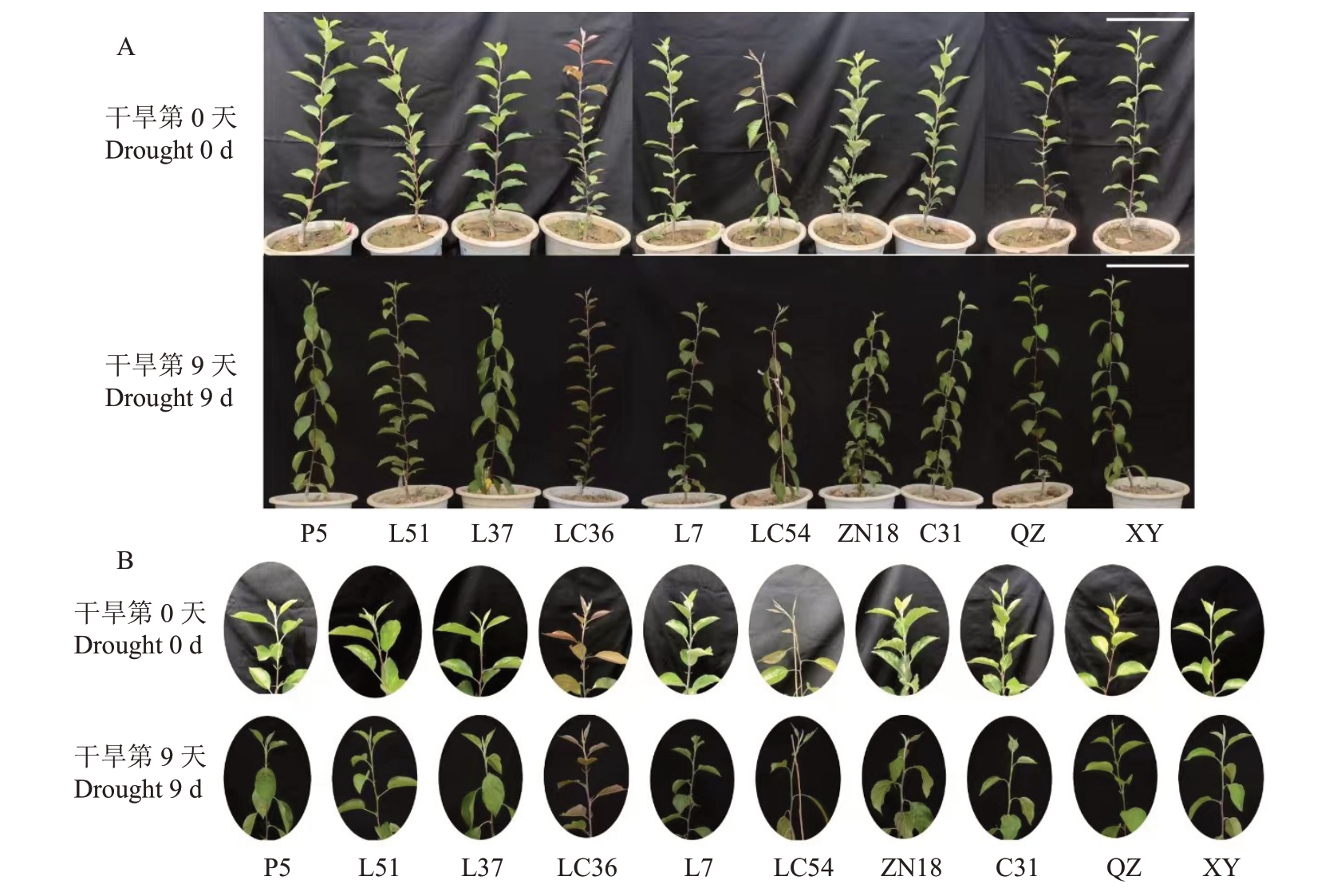
图1 自然干旱胁迫表型
Fig.1 Phenotypic of natural drought stress
A.自然干旱胁迫植株表型;B.自然干旱胁迫植株顶端表型。P5、L51、L37、LC36、L7、LC54、ZN18、C31 分别表示8 份苹果种质资源,QZ 表示富平楸子,XY 表示新疆野苹果。比标尺为30 cm。
A. Plant phenotype under natural drought stress; B.Top phenotype of plants under natural drought stress. P5, L51, L37, LC36, L7, LC54, ZN18,C31 represent eight resources respectively,QZ denotes M.prunifolia,XY denotes M.sieversii.Scale bars is 30 cm.
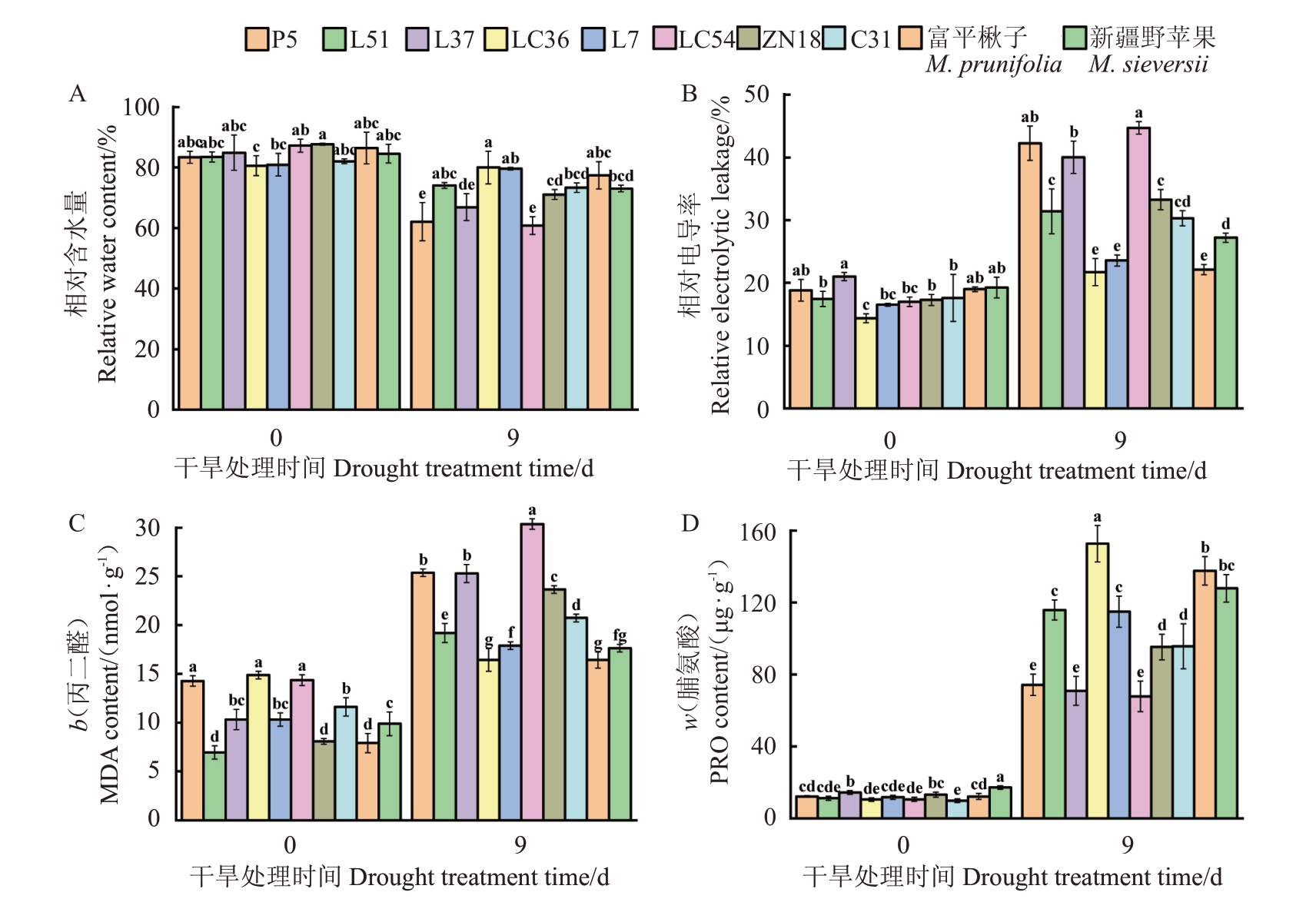
图2 干旱前后各苹果种质资源生理指标的变化
Fig.2 Changes of physiological indexes of apple germplasm resources before and after drought
不同小写字母表示各苹果种质资源间存在显著差异(p<0.05)。下同。
Different small letters indicate significant differences among apple germplasm resources(p<0.05).The same below.
2.2 自然干旱胁迫下各苹果种质资源的Pn和叶绿素含量
在干旱处理第0 天,各苹果种质资源叶片的Pn在12.3~19.06 μmol·m-2·s-1之间,在干旱处理第9天,各苹果种质资源叶片的Pn显著降低,变化范围为40.65%~68.03%,其中LC54的Pn显著低于其他种质资源,LC36、富平楸子和新疆野苹果的Pn较高(图3-A)。在干旱处理后,各苹果种质资源叶片的叶绿素含量也显著降低(图3-B),这些结果表明,在干旱处理后,各苹果种质资源均遭受到了不同程度的损伤,其中以LC54的损伤最严重,初步表明在各苹果种质资源中LC54的抗旱性最差。
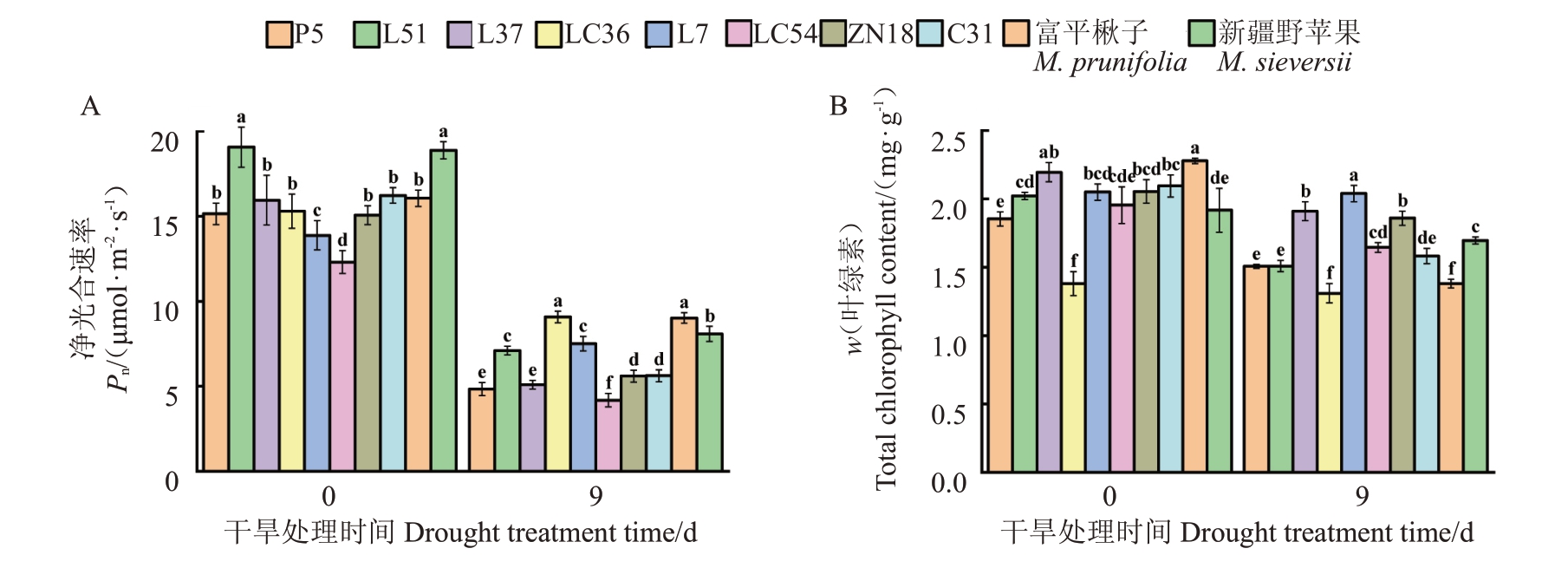
图3 干旱前后各苹果种质资源Pn和叶绿素含量的变化
Fig.3 Changes of net photosynthetic rate and chlorophyll content of apple germplasm resources before and after drought
2.3 自然干旱胁迫下各苹果种质资源的活性氧含量及抗氧化酶活性
植物在遭受到干旱胁迫时会产生大量的O2-等活性氧(ROS),以抵御外界环境的变化[10],然而过量的ROS 积累会导致植物氧化损伤[11]。在干旱处理第0天,各苹果种质资源叶片的H2O2含量较低,在干旱胁迫第9 天,各苹果种质资源的H2O2含量(b,后同)显著增加,分布范围为25.23~35.49 μmol·g-1,LC36的H2O2含量显著低于其他种质资源(图4-A)。如图4-B所示,在干旱处理第0天,各苹果种质资源叶片的O2-含量分布范围为29.31~42.69 nmol·g-1,在干旱处理第9天,各苹果种质资源叶片的O2-含量显著增加,LC54、P5和L37的O2-含量显著高于其他种质资源。为防止过量的ROS对植物的损伤,植物可通过相应抗氧化酶系统清除植物体内过量的ROS[12]。通过对各苹果种质资源SOD活性和POD活性测定可知,在干旱处理后,各苹果种质资源叶片内的SOD活性和POD活性显著升高。在干旱处理第9天,L7的POD活性最高,LC54的POD活性最低,LC36的SOD活性最高,LC54的SOD活性最低(图4-C~D)。

图4 干旱前后各苹果种质资源抗氧化酶系统的变化
Fig.4 Changes of malondialdehyde content and antioxidant system of apple germplasm resources before and after drought
2.4 自然干旱胁迫下各苹果种质资源的ABA含量
ABA 在植物应对干旱等胁迫时具有重要作用[13],因此测量了各苹果种质资源叶片的ABA含量(图5-A)。在干旱处理第0天,各苹果种质资源叶片的ABA 含量有显著差异,但均维持在相对较低水平,分布范围为14.60~45.97 ng·g-1,在干旱处理第9天,各苹果种质资源叶片的ABA 含量显著增高,分布范围为117.52~526.48 ng·g-1,LC54 的ABA 含量显著高于其他种质资源。通过测定各苹果种质资源与ABA 生物合成有关的2 个基因发现,MdNCED1和MdNCED3 基因相对表达量均在干旱处理第0 天维持在较低水平,在干旱处理第9天显著升高,与叶片内ABA含量的变化趋势相一致(图5-B、C)。

图5 干旱前后各苹果种质资源ABA 含量及其合成基因表达量
Fig.5 ABA content and synthetic gene expression of apple germplasm resources before and after drought
2.5 利用隶属函数法评价各苹果种质资源的抗旱性
以各苹果种质资源干旱胁迫第0 天和第9 天各指标的相对变化率计算隶属函数值,以各苹果种质资源隶属函数值的平均值为依据进行抗旱性的评价,平均隶属函数值越高说明对干旱的敏感度越低,则愈抗旱。综合Pn、叶绿素含量、叶片相对含水量、相对电导率、MDA含量、H2O2含量、O2-含量、SOD活性、POD活性、PRO含量、ABA含量共计11项指标,计算出各苹果种质资源的平均隶属函数值,结果(表2)表明,LC36 的平均隶属函数值最大,表明在干旱胁迫下LC36 各指标的相对变化程度最小,与其他种质资源相比抗旱性最强。平均隶属函数值最小的是LC54,表明其抗旱性最弱。由此笔者得出各苹果种质资源的抗旱性依次为:LC36>L7>富平楸子>新疆野苹果>L51>C31>P5>ZN18>L37>LC54。
表2 各苹果种质资源抗旱性隶属函数值
Table 2 Value of drought resistance membership function of apple germplasm resources
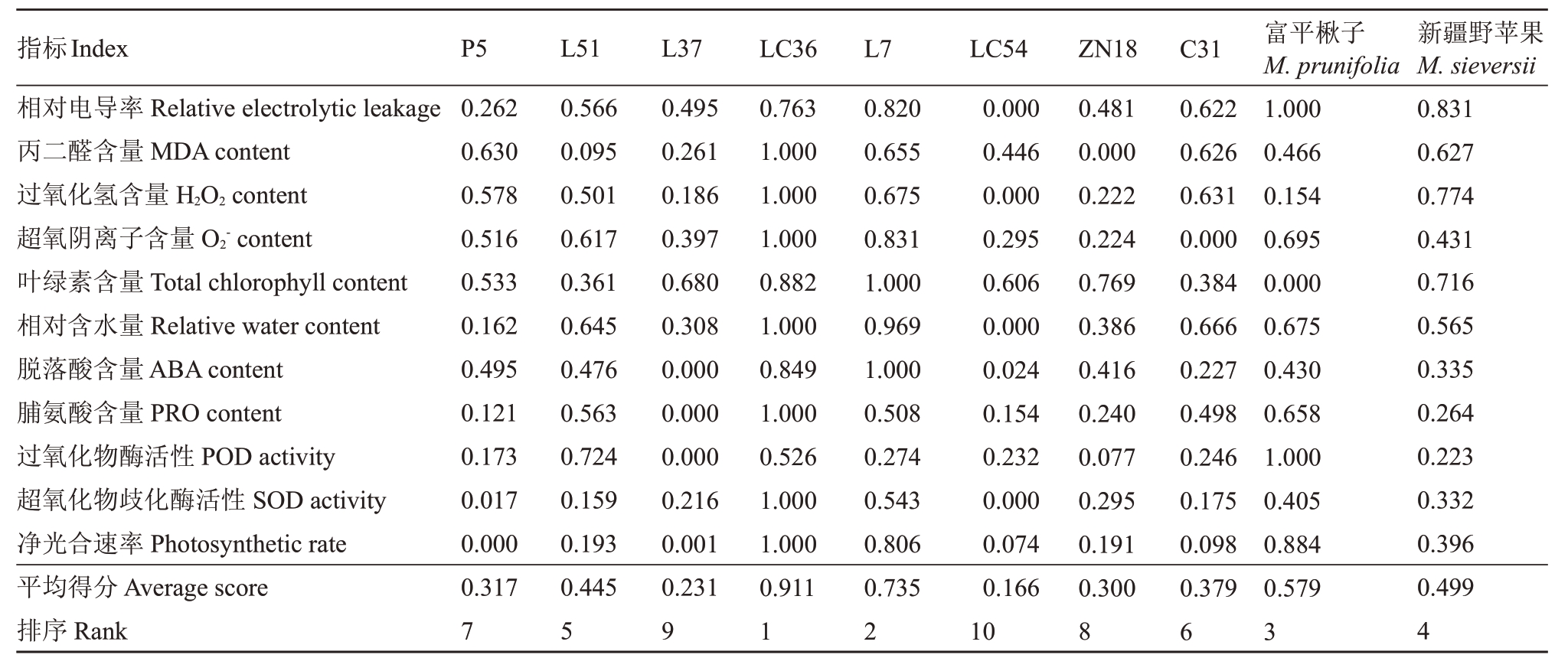
指标Ⅰndex相对电导率Relative electrolytic leakage丙二醛含量MDA content过氧化氢含量H2O2 content超氧阴离子含量O2-content叶绿素含量Total chlorophyll content相对含水量Relative water content脱落酸含量ABA content脯氨酸含量PRO content过氧化物酶活性POD activity超氧化物歧化酶活性SOD activity净光合速率Photosynthetic rate平均得分Average score排序Rank P5 0.262 0.630 0.578 0.516 0.533 0.162 0.495 0.121 0.173 0.017 0.000 0.317 7 L51 0.566 0.095 0.501 0.617 0.361 0.645 0.476 0.563 0.724 0.159 0.193 0.445 5 L37 0.495 0.261 0.186 0.397 0.680 0.308 0.000 0.000 0.000 0.216 0.001 0.231 9 LC36 0.763 1.000 1.000 1.000 0.882 1.000 0.849 1.000 0.526 1.000 1.000 0.911 1 L7 0.820 0.655 0.675 0.831 1.000 0.969 1.000 0.508 0.274 0.543 0.806 0.735 2 LC54 0.000 0.446 0.000 0.295 0.606 0.000 0.024 0.154 0.232 0.000 0.074 0.166 10 ZN18 0.481 0.000 0.222 0.224 0.769 0.386 0.416 0.240 0.077 0.295 0.191 0.300 8 C31 0.622 0.626 0.631 0.000 0.384 0.666 0.227 0.498 0.246 0.175 0.098 0.379 6富平楸子M.prunifolia 1.000 0.466 0.154 0.695 0.000 0.675 0.430 0.658 1.000 0.405 0.884 0.579 3新疆野苹果M.sieversii 0.831 0.627 0.774 0.431 0.716 0.565 0.335 0.264 0.223 0.332 0.396 0.499 4
3 讨 论
干旱严重制约了农业的发展,在生产上每年造成重大损失[14]。干旱胁迫会影响植物的生长发育,在植物中表现出不同的形态、生理、生化和分子变化[4-5]。光合作用是植物碳同化最重要的代谢过程[15],植物在遭受干旱胁迫后会对光合器官造成损伤,导致植物光合能力的下降[16]。在本研究中,干旱胁迫后各苹果种质资源叶片的Pn显著降低,这与前人研究结果一致[17-18]。叶绿素是植物主要的光合色素,在光合色素获取光方面起着重要作用[19]。Zhao等[20]研究发现,长期水分胁迫下植株的叶绿素含量均降低,梁博文[21]研究发现,自然干旱胁迫下植株的叶绿素浓度显著降低,这与笔者在本研究中的结果一致。本研究中,短期干旱处理后各苹果种质资源的叶绿素含量显著降低。研究表明,在干旱胁迫下,植物叶绿素的降解主要与活性氧(ROS)的过量产生有关[22-23]。这些研究结果表明,植物在干旱胁迫中Pn的降低可能与叶绿素含量的降低有关。
植物在进行光合作用时会产生大量活性氧(ROS)[24-25],活性氧(ROS)会引起膜脂质的过氧化和去酯化,并导致蛋白质变性,从而进一步损伤植物细胞[26]。前人研究表明,植物在干旱胁迫下,体内的活性氧(ROS)水平会显著增加[27-29]。在本研究中,干旱胁迫后各苹果种质资源的O2-含量显著增加,各苹果种质资源O2-含量的增长范围在84.31%~197.97%之间。H2O2含量在干旱胁迫第0 天的含量较低,在干旱胁迫第9 天显著增高,这与前人研究结果相同[14,30]。此外,笔者在本研究中测定了各苹果种质资源的POD、SOD 活性,结果表明,干旱胁迫后,各苹果种质资源的POD、SOD 活性显著增强,且抗旱性越强的种质资源抗氧化酶活性越强。这些研究结果表明,各苹果种质资源在干旱胁迫下,植株体内的活性氧(ROS)因胁迫显著增加,同时植株产生大量抗氧化酶抑制活性氧(ROS)的产生,但LC36等种质资源的抗氧化酶SOD、POD 活性较高,因此积累的活性氧(ROS)较少,表现出更强的抗旱能力。
植物在干旱胁迫下ABA 含量会显著增加[31],ABA可维持植物水分状态、增强光合作用从而减弱干旱的影响[32]。在本研究中,干旱胁迫后各苹果种质资源的ABA含量显著升高,且LC36的ABA含量增幅最大,这与前人研究结果相同。9-顺式-环氧类胡萝卜素双加氧酶(NCED)是ABA 合成的关键酶[33]。在本研究中,笔者检测了MdNCED1 和MdNCED3 的基因表达量,结果表明,MdNCED1 和MdNCED3 的基因表达量在干旱处理第0 天维持在较低水平,在干旱处理第9 天显著升高,与叶片内ABA含量的变化相一致。前人研究表明,在干旱胁迫下,PRO 等渗透保护剂可降低活性氧(ROS)对植物细胞膜的损伤[34],同时它们不会干扰细胞水平的正常代谢过程[35]。在本研究中,在干旱胁迫第9天,各苹果种质资源的PRO含量显著增加,这与前人研究结果相同[36-37]。
隶属函数值是从隶属度的角度出发,运用模糊数学的基本理论,采用隶属度函数法计算得到的综合评估值[38-39]。目前,隶属函数法在作物抗性评价方面广泛应用。冯琛等[40]利用隶属函数法对不同苹果矮化砧穗组合的抗旱性进行研究,发现宫藤富士/SH6组合的抗旱性比宫藤富士/G935、宫藤富士/M9-T337的强。王健强等[41]利用隶属函数法对7种矮化砧木进行了抗旱性评价,研究结果表明冀砧1号和SH40的抗旱性较其他砧木更强。在本研究中,利用11种测定指标进行了隶属函数分析,对各苹果种质资源的抗旱性进行评价,研究发现各苹果种质资源的抗旱性依次为:LC36>L7>富平楸子>新疆野苹果>L51>C31>P5>ZN18>L37>LC54。
4 结 论
在干旱胁迫下植物的Pn降低、膜完整性被破坏、ABA和PRO含量显著增加,但由于各苹果种质资源对干旱的抗性不同,干旱胁迫前后各指标的变化幅度也不同,根据隶属函数值得出各苹果种质资源的抗旱性依次为:LC36>L7>富平楸子>新疆野苹果>L51>C31>P5>ZN18>L37>LC54。LC36、L7 两份种质资源的抗旱性高于普遍认为抗旱性强的富平楸子和新疆野苹果,而其他资源的抗旱性则低于富平楸子和新疆野苹果,因此,LC36和L7是改善苹果抗旱性的重要资源。
[1] KHAN M A,ⅠQBAL M,AKRAM M,AHMAD M,HASSAN M W,JAMⅠL M. Recent advances in molecular tool development for drought tolerance breeding in cereal crops:A review[J].Zemdirbyste-Agriculture,2013,100(3):325-334.
[2] 刘璐,王景红,柏秦凤,张维敏,张焘.气候变化对黄土高原苹果主产地物候期的影响[J].果树学报,2020,37(3):330-338.LⅠU Lu,WANG Jinghong,BAⅠQinfeng,ZHANG Weimin,ZHANG Tao. Ⅰmpact of climate changes on apple’s phenophases in the main producing areas of the Loess Plateau in China[J].Journal of Fruit Science,2020,37(3):330-338.
[3] GUPTA A,RⅠCO-MEDⅠNA A,CAÑO-DELGADO A Ⅰ. The physiology of plant responses to drought[J]. Science,2020,368(6488):266-269.
[4] GONZÁLEZ- VⅠLLAGRA J,OMENA- GARCⅠA R P,RODRⅠGUES-SALVADOR A,NUNES-NESⅠA,COHEN J D,REYES-DÍAZ M M. Differential physiological and metabolic responses in young and fully expanded leaves of Aristotelia chilensis plants subjected to drought stress[J]. Environmental and Experimental Botany,2022,196:104814.
[5] ZANDALⅠNAS S Ⅰ,MⅠTTLER R,BALFAGÓN D,ARBONA V,GÓMEZ-CADENAS A.Plant adaptations to the combination of drought and high temperatures[J]. Physiologia Plantarum,2018,162(1):2-12.
[6] WANG X,ZHANG J,SONG J,HUANG M,CAⅠJ,ZHOU Q,DAⅠT,JⅠANG D. Abscisic acid and hydrogen peroxide are involved in drought priming-induced drought tolerance in wheat(Triticum aestivum L.)[J].Plant Biology,2020,22(6):1113-1122.
[7] SU Z J,LⅠX H,HAO Z F,XⅠE C X,LⅠM S,WENG J F,ZHANG D G,LⅠANG X L,WANG Z G,GAO J L,ZHANG S H.Association analysis of the nced and rab28 genes with phenotypic traits under water stress in maize[J].Plant Molecular Biology Reporter,2011,29(3):714-722.
[8] 敬媛媛. 苹果FERONⅠA 类受体激酶MdMRLK2 响应干旱、低温及腐烂病菌侵染的功能分析[D].杨凌:西北农林科技大学,2022.JⅠNG Yuanyuan. Characterization of FERONⅠA receptor-like kinase MdMRLK2 in response to drought,cold and Valsa mali infection in Malus[D]. Yangling:Northwest A & F University,2022.
[9] LⅠVAK K J,SCHMⅠTTGEN T D.Analysis of relative gene expression data using real-time quantitative PCR and the 2- ΔΔCT method[J].Methods,2001,25(4):402-408.
[10] KOCSY G,TARⅠⅠ,VANKOVÁ R,ZECHMANN B,GULYÁS Z,POÓR P,GALⅠBA G.Redox control of plant growth and development[J].Plant Science,2013,211:77-91.
[11] MUKARRAM M,CHOUDHARY S,KURJAK D,PETEK A,KHAN M M A. Drought:Sensing,signalling,effects and tolerance in higher plants[J]. Physiologia Plantarum,2021,172(2):1291-1300.
[12] CHAPMAN J M,MUHLEMANN J K,GAYOMBA S R,MUDAY G K. RBOH-dependent ROS synthesis and ROS scavenging by plant specialized metabolites to modulate plant development and stress responses[J].Chemical Research in Toxicology,2019,32(3):370-396.
[13] LEE S C,LⅠM C W,LAN W Z,HE K,LUAN S.ABA signaling in guard cells entails a dynamic protein-protein interaction relay from the PYL-RCAR family receptors to ion channels[J].Molecular Plant,2013,6(2):528-538.
[14] YANG J,WANG M,ZHOU S S,XU B Y,CHEN P H,MA F W,MAO K. The ABA receptor gene MdPYL9 confers tolerance to drought stress in transgenic apple (Malus domestica)[J]. Environmental and Experimental Botany,2022,194:104695.
[15] MA X S,XⅠA H,LⅠU Y H,WEⅠH B,ZHENG X G,SONG C Z,CHEN L,LⅠU H Y,LUO L J. Transcriptomic and metabolomic studies disclose key metabolism pathways contributing to wellmaintained photosynthesis under the drought and the consequent drought-tolerance in rice[J]. Frontiers in Plant Science,2016,7:1886.
[16] SONG X Y,ZHOU G S,HE Q J. Critical leaf water content for maize photosynthesis under drought stress and its response to rewatering[J].Sustainability,2021,13(13):7218.
[17] ZHAO S,GAO H B,JⅠA X M,WANG H B,KE M,MA F W.The HD-Zip Ⅰtranscription factor MdHB-7 regulates drought tolerance in transgenic apple (Malus domestica)[J]. Environmental and Experimental Botany,2020,180:104246.
[18] JⅠNG Y Y,LⅠU C H,LⅠU B B,PEⅠT T,ZHAN M H,LⅠC R,WANG D N,LⅠP M,MA F W. Overexpression of the FERONIA receptor kinase MdMRLK2 confers apple drought tolerance by regulating energy metabolism and free amino acids production[J].Tree Physiology,2023,43(1):154-168.
[19] SAGLAM A,TERZⅠR,DEMⅠRALAY M. Effect of polyethylene glycol induced drought stress on photosynthesis in two chickpea genotypes with different drought tolerance[J].Acta Biologica Hungarica,2014,65(2):178-188.
[20] ZHAO S,GAO H B,JⅠA X M,WEⅠJ T,MAO K,MA F W.Md-HB-7 regulates water use efficiency in transgenic apple (Malus domestica) under long-term moderate water deficit[J]. Frontiers in Plant Science,2021,12:740492.
[21] 梁博文.多巴胺和褪黑素对干旱和养分胁迫下苹果矿质养分吸收的调控研究[D].杨凌:西北农林科技大学,2018.LⅠANG Bowen.Regulatory function of dopamine and melatonin on mineral nutrient uptake in Malus under drought and nutrient stresses[D].Yangling:Northwest A&F University,2018.
[22] GHOBADⅠM,TAHERABADⅠS,GHOBADⅠM E,MOHAMMADⅠG R,JALALⅠ-HONARMAND S.Antioxidant capacity,photosynthetic characteristics and water relations of sunflower(Helianthus annuus L.)cultivars in response to drought stress[J].Ⅰndustrial Crops and Products,2013,50:29-38.
[23] JⅠANG Y W,HUANG B R.Drought and heat stress injury to two cool-season turfgrasses in relation to antioxidant metabolism and lipid peroxidation[J].Crop Science,2001,41(2):436-442.
[24] SAⅠBO N J M,LOURENÇO T,OLⅠVEⅠRA M M.Transcription factors and regulation of photosynthetic and related metabolism under environmental stresses[J]. Annals of Botany,2009,103(4):609-623.
[25] CHAVES M M,FLEXAS J,PⅠNHEⅠRO C. Photosynthesis under drought and salt stress:Regulation mechanisms from whole plant to cell[J].Annals of Botany,2009,103(4):551-560.
[26] BOWLER C,MONTAGU M V,ⅠNZE D.Superoxide dismutase and stress tolerance[J].Annual Review of Plant Physiology and Plant Molecular Biology,1992,43:83-116.
[27] JⅠA D F,JⅠANG Q,VAN NOCKER S,GONG X Q,MA F W.An apple (Malus domestica) NAC transcription factor enhances drought tolerance in transgenic apple plants[J].Plant Physiology and Biochemistry,2019,139:504-512.
[28] REN Y R,YANG Y Y,ZHANG R,YOU C X,ZHAO Q,HAO Y J.MdGRF11,an apple 14-3-3 protein,acts as a positive regulator of drought and salt tolerance[J]. Plant Science,2019,288:110219.
[29] WANG Y P,CHEN Q,ZHENG J Z,ZHANG Z J,GAO T T,LⅠC,MA F W. Overexpression of the tyrosine decarboxylase gene MdTyDC in apple enhances long-term moderate drought tolerance and WUE[J].Plant Science,2021,313:111064.
[30] 王允,张逸,刘灿玉,张志焕,曹逼力,徐坤.干旱胁迫下外源ABA 对姜叶片活性氧代谢的影响[J].园艺学报,2016,43(3):587-594.WANG Yun,ZHANG Yi,LⅠU Canyu,ZHANG Zhihuan,CAO Bili,XU Kun.Effects of exogenous abscisic acid on active oxygen metabolism in ginger leaves under drought stress[J]. Acta Horticulturae Sinica,2016,43(3):587-594.
[31] 赵东黎,鲍茹雪,李梦桃,陈新,王文泉.木薯ABA 受体Me-PYL4a 在拟南芥中过表达增强其耐旱性[J].农业生物技术学报,2022,30(12):2290-2300.ZHAO Dongli,BAO Ruxue,LⅠMengtao,CHEN Xin,WANG Wenquan. Overexpression of cassava (Manihot esculenta)ABA receptor MePYL4a in Arabidopsis thaliana enhances its drought tolerance[J]. Journal of Agricultural Biotechnology,2022,30(12):2290-2300.
[32] DE SOUZA T C,MAGALHAES P C,DE CASTRO E M,DE ALBUQUERQUE P E P,MARABESⅠM A. The influence of ABA on water relation,photosynthesis parameters,and chlorophyll fluorescence under drought conditions in two maize hybrids with contrasting drought resistance[J]. Acta Physiologiae Plantarum,2013,35(2):515-527.
[33] CHEN K,LⅠG J,BRESSAN R A,SONG C P,ZHU J K,ZHAO Y.Abscisic acid dynamics,signaling,and functions in plants[J].Journal of Ⅰntegrative Plant Biology,2020,62(1):25-54.
[34] BANDURSKA H. Does proline accumulated in leaves of water deficit stressed barley plants confine cell membrane injury? Ⅰ.Free proline accumulation and membrane injury index in drought and osmotically stressed plants[J].Acta Physiologiae Plantarum,2000,22(4):409-415.
[35] SⅠNGH M,KUMAR J,SⅠNGH S,SⅠNGH V P,PRASAD S M.Roles of osmoprotectants in improving salinity and drought tolerance in plants:A review[J]. Reviews in Environmental Science and Bio/Technology,2015,14(3):407-426.
[36] 高传彩,惠基运,魏玉兰,张蕊,刘建廷,肖伟,李玲.干旱及复水对‘红富士’苹果生长及果实品质和产量的影响[J].山东农业大学学报(自然科学版),2021,52(2):194-200.GAO Chuancai,HUⅠJiyun,WEⅠYulan,ZHANG Rui,LⅠU Jianting,XⅠAO Wei,LⅠLing. Effects of drought and rehydration on the growth,fruit quality and yield of‘Red Fuji’apple[J]. Journal of Shandong Agricultural University (Natural Science Edition),2021,52(2):194-200.
[37] 单皓,罗海婧,张松,张久刚,张虎,崔爱民,薛超,张永清.不同抗旱性小豆根系对干旱-复水的生理生态响应[J].干旱地区农业研究,2023,41(1):94-100.SHAN Hao,LUO Haijing,ZHANG Song,ZHANG Jiugang,ZHANG Hu,CUⅠAimin,XUE Chao,ZHANG Yongqing.Physiological and ecological response of different drought-tolerant adzuki beans root system to drought-rehydration[J]. Agricultural Research in the Arid Areas,2023,41(1):94-100.
[38] 孟雨.干旱胁迫对小麦生长的影响及品种抗旱性鉴定方法研究[D].郑州:河南农业大学,2022.MENG Yu.Effects of drought stress on wheat growth and identification methods of drought resistance of varieties[D]. Zhengzhou:Henan Agricultural University,2022.
[39] 武新娟,唐贵,隋冬华,张冬雪,孙晶,张静华,张鹍,宋鹏慧,吴雨蹊.20 个马铃薯品种抗旱性鉴定及评价指标筛选[J].中国瓜菜,2021,34(3):47-51.WU Xinjuan,TANG Gui,SUⅠDonghua,ZHANG Dongxue,SUN Jing,ZHANG Jinghua,ZHANG Kun,SONG Penghui,WU Yuxi. Evaluation index selection and drought resistance identification of 20 potato varieties[J].China Cucurbits and Vegetables,2021,34(3):47-51.
[40] 冯琛,黄学旺,李兴亮,周佳,李天红.不同苹果矮化砧穗组合的抗旱性比较研究[J].园艺学报,2022,49(5):945-957.FENG Chen,HUANG Xuewang,LⅠXingliang,ZHOU Jia,LⅠTianhong. Comparative study on drought resistance of different apple dwarfing rootstock and scion combinations[J].Acta Horticulturae Sinica,2022,49(5):945-957.
[41] 王健强,李佳,苏怡,郝奕樊,左家乐,石濛,李中勇,张学英,徐继忠. 7 种苹果矮化砧木的抗旱性评价[J]. 中国果树,2019(6):38-41.WANG Jianqiang,LⅠJia,SU Yi,HAO Yifan,ZUO Jiale,SHⅠMeng,LⅠZhongyong,ZHANG Xueying,XU Jizhong. Evaluation analysis of different apple dwarfing stocks on drought resistance[J].China Fruits,2019(6):38-41.