梨小食心虫(Grapholita molesta)为世界和中国重大果树害虫,因其钻蛀性、隐蔽性、世代重叠严重等特点,其防治较为困难[1-3]。目前梨小食心虫多采用化学药剂防控,药剂主要包括阿维菌素、氯虫苯甲酰胺、高效氯氟氰菊酯、毒死蜱等,化学药剂长期频繁不规范使用已导致害虫产生了不同程度的抗性[4],其化学防治效果逐年下降[5-6],阿维菌素为生物源类杀虫剂,生产上主要用于防治多种害虫[7-9]。因其杀虫活性强、杀虫谱广,在梨园中常用于防治梨小食心虫、梨木虱、黄粉蚜和山楂叶螨等多种害虫[10-12],年使用次数5~7次。因其用药不规范,20 a(年)间,阿维菌素在梨园中的施药浓度由0.5%制剂稀释4000倍增至5%制剂稀释1000倍,增加了40倍。笔者课题组测定了山西省不同区域的田间种群,部分种群已具中等抗性水平(待发表)。作为梨园主要防治药剂,阿维菌素在当前及今后较长时间内仍频繁使用,因而,如何合理科学使用该药剂成为生产上迫切需要解决的问题。目前阿维菌素田间使用次数主要从农药残留和果品安全性等角度来确定,未从其抗性角度研究其使用技术;阿维菌素对梨小食心虫防控作用也多集中于研究其毒力和田间防效等,对其抗性发展规律、抗性现实遗传力及交互抗性等研究较少。笔者在本研究中以室内敏感种群为参照,以田间种群为基础,测定阿维菌素致死中浓度处理的梨小食心虫抗性发展和无药剂处理的敏感性变化趋势,获得其抗性变化趋势、发展速度、抗性遗传力及其与梨园常用药剂交互抗性,从抗性发展角度为该药剂合理使用、延缓抗性发展等提供理论依据。
1 材料和方法
1.1 供试虫源
供试梨小食心虫敏感种群(SS)为室内继代饲养100代以上;田间种群(F)为山西省运城市盐湖区酥梨园采集的种群。饲养条件为:温度(25±1)℃、相对湿度70%~80%,光照度3000~4000 lx,光周期为L/D=15 h/9 h,下同。
1.2 供试药剂
阿维菌素(avermectin)原药,纯度92.0%,山东齐发药业有限公司;高效氯氟氰菊酯(lambda-cyhalothrin)原药,纯度96.0%,山东潍坊润丰化工股份有限公司;吡虫啉(imidacloprid)原药,纯度96.0%,辽宁宏峰科技有限公司;氯虫苯甲酰胺(chlorantraniliprole)原药,纯度96.0%,辽宁省沈阳丰收农药有限公司。
1.3 毒力测定
采用浸果法。具体参考庾琴等[13-14]的方法,略有改动。挑选同一品种、大小一致、性状良好的苹果幼果,洗净晾干后,放入药液中浸泡10 s,取出后用滤纸吸掉多余药液,平稳放于底部铺有湿滤纸、具盖的塑料容器(直径为10 cm、高4 cm)中,将附有50粒待孵化卵的卵纸轻放于幼果上,有卵面接触果面,盖上盖,器皿中保持相对湿度90%以上;以0.02%的吐温-80 水溶液为对照。每个处理设置3 次重复。接卵后78 h调查蛀果数,依据蛀果孔洞处有无新鲜虫粪判定为幼虫是否死亡[14],记录死亡虫数。
抗性倍数(resistance ratio,RR)=待测梨小食心虫种群LC50/相对敏感种群LC50。
参考黄彦娜[15]抗性倍数划分标准:RR≤3 为敏感、3<RR≤5为敏感性降低、5<RR≤10为低水平抗性、10<RR≤40为中等水平抗性、40<RR≤160为高水平抗性、RR>160为极高水平抗性。
1.4 梨小食心虫田间抗性品系汰选和敏感性恢复
在室内条件下,根据1.3获得的LC50浓度阿维菌素对梨小食心虫田间种群(F)进行汰选,每代处理一次,处理时以药液完全湿润幼果且不流失为度。此后每代均使用上一代LC50浓度进行汰选,连续施药6 次后种群为田间抗性品系(FR);连续6 代未施药种群为田间对照品系(FS),以实验室饲养的敏感品系(SS)为试验参考品系。
1.5 抗性风险评估
1.5.1 抗性现实遗传力估算 抗性现实遗传力(h2)采用Tabashnik等[16]的阈性状分析法计算,其中,R为选择反应,S 为选择差异,i 为选择强度,δP为表性标准差。计算公式分别为:
R=[log(终LC50)-log(始LC50)]/n,其中,n 为选择代数,终LC50为选择n 代后的LC50,始LC50为选择前亲代的LC50。
S=i×δP。
i=1.583-0.019 333 6P+0.000 042 8P2+3.651 941/P(10<P<80),P=100-平均校正死亡率,平均校正死亡率为抗性选育过程中各代死亡率用Abbott公式校正后的平均值。
δP=[1/2(初斜率+终斜率)]-1,初斜率为选择前亲本毒力回归方程的斜率,终斜率为选择n 代后的毒力回归方程斜率。
1.5.2 抗性发展速率预测 根据现实遗传力h2,可预测抗性上升x 倍所需代数[Gx=lgx/(h2S)],以及不同选择压力(50%~90%)下抗性上升10倍所需代数:G=R-1=(h2S)-1[17]。
1.6 交互抗性测定
测定梨小食心虫3 个品系对梨园中常用的3 种药剂高效氯氟氰菊酯、吡虫啉和氯虫苯甲酰胺的毒力,毒力测定方法同1.3。参照SS 品系LC50,计算3个品系抗性倍数,评估其是否存在交互抗性。
1.7 数据处理
利用Excel 软件整理数据,利用SPSS 24.0 进行单因素方差分析,求出斜率值、LC50、卡方值、自由度和标准偏差等。
RR<1 表示负交互抗性,1≤RR<5 表示无交互抗性,RR>5表示有交互抗性[18]。
2 结果与分析
2.1 阿维菌素对梨小食心虫不同品系毒力和抗性变化趋势
如表1 所示,阿维菌素对梨小食心虫田间种群LC50为1.092 mg·L-1,抗性倍数4.608倍,为敏感性降低。该种群经LC50阿维菌素汰选,随着汰选代数增加,其LC50不断增加,汰选至第2 代时由低敏感升至低抗性水平;汰选至第4 代和6 代时,其抗性倍数分别为11.966 倍和20.304 倍,增至中等抗性水平。未接触阿维菌素的田间种群敏感性逐渐上升,未施药4 代后,田间种群抗性倍数降至2.802 倍,为敏感;未接触农药6 代时,梨小食心虫对阿维菌素的抗性为2.001 倍,敏感性进一步提高。结果说明,已对阿维菌素敏感性降低的田间种群连续使用阿维菌素处理使其对阿维菌素抗性水平快速升高;而连续未接触阿维菌素的品系其敏感性逐渐提高。
表1 阿维菌素对梨小食心虫的初孵幼虫的毒力
Table 1 Toxicity of avermectin to the G.molesta neonate larvae
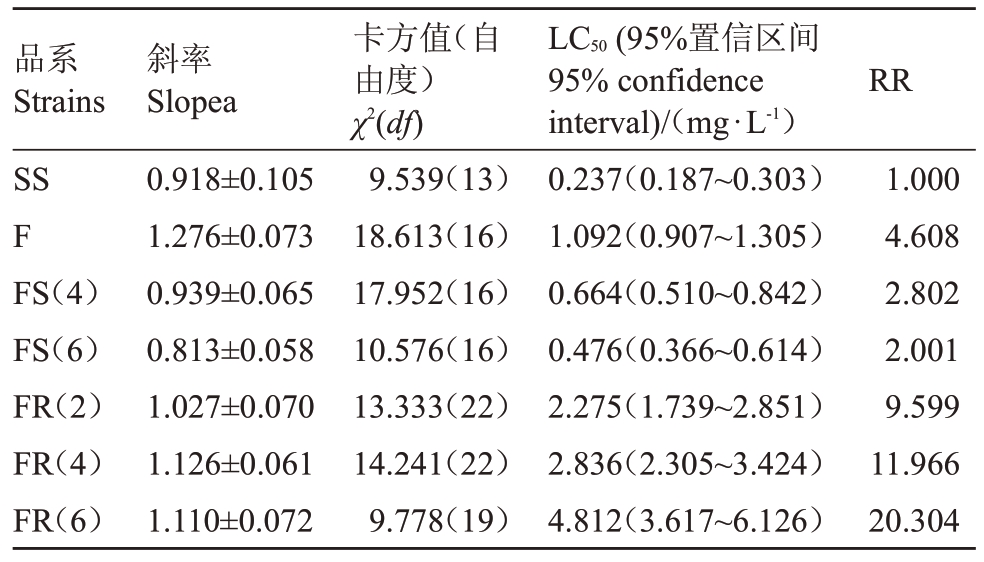
注:SS.敏感品;F.田间种群;FS.F 种群连续6 代未接触阿维菌素的田间对照品系;FR.F 种群连续6 代接触LC50 阿维菌素后的田间抗性品系;RR.抗性倍数;表中数据为平均数±标准差。下同。
Note: SS. Sensitive strain; F. Field population; FS. Field control strain from F population without exposure to avermectin for six consecutive generations; FR. Field resistant strain from F population after exposure to LC50 of avermectin for six consecutive generations;RR.Resistance ratio.Data in the table are mean±SD.The same below.
?
2.2 梨小食心虫对阿维菌素抗性现实遗传力及抗性发展速率
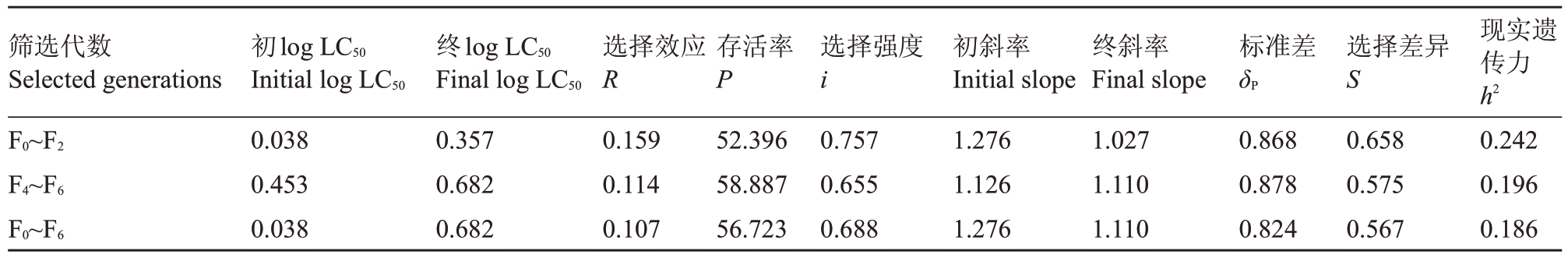
结果(表2)表明,用阿维菌素汰选田间种群梨小食心虫6代,抗性现实遗传力h2=0.186,其中F0~F2汰选阶段的现实遗传力为0.242,F4~F6汰选阶段的现实遗传力为0.196。结果说明,梨小食心虫对阿维菌素存在快速产生抗性的风险。
表2 梨小食心虫田间种群汰选品系的抗性现实遗传力
Table 2 Realized heritability of resistance strains of G.molesta in field population
?
通过阿维菌素汰选田间抗性种群所得到的抗性现实遗传力h2和选择差异S,分别计算在选择压力为50%~90%的情况下,梨小食心虫田间种群对阿维菌素抗性增加10 倍所需要的代数。结果(图1)表明,在抗性遗传力为0.186的情况下,阿维菌素对梨小食心虫抗性上升10 倍需代数4~9 代。结果说明,梨小食心虫抗性增加较快,较高浓度阿维菌素处理时,其产生中等抗性风险较大。
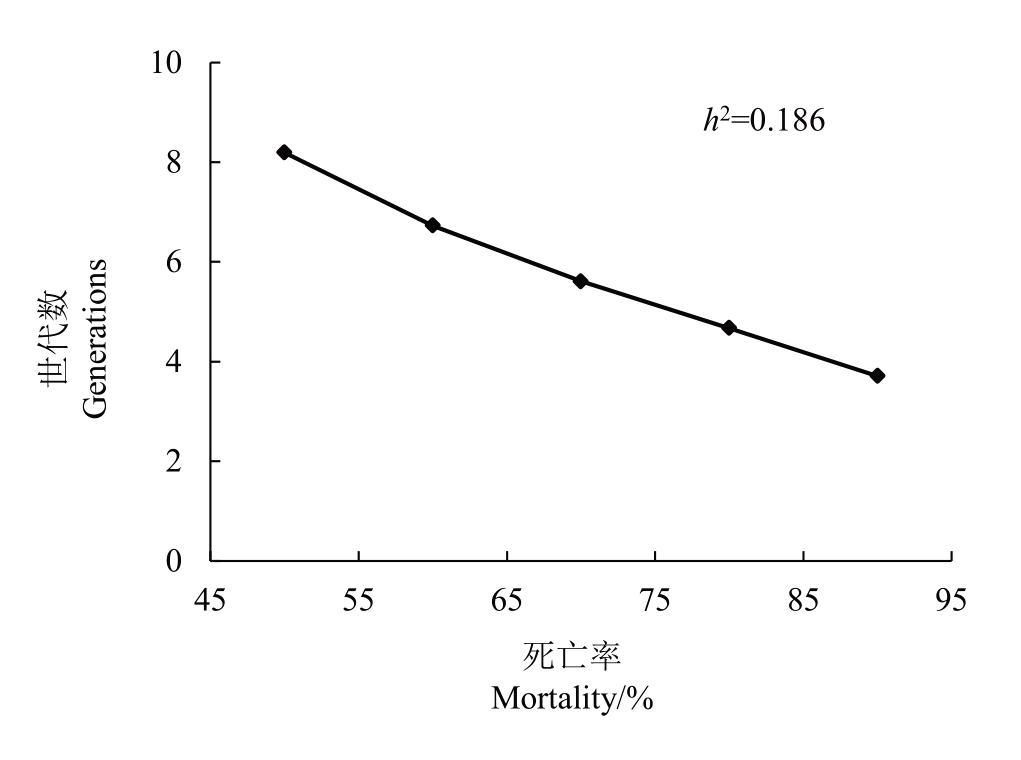
图1 不同选择压力下梨小食心虫对阿维菌素的抗性上升10 倍所需世代数
Fig.1 Generations required for a 10-fold increase in LC50 of avermectin to G.molesta under different selection pressures
2.3 梨小食心虫不同品系对3种药剂的交互抗性
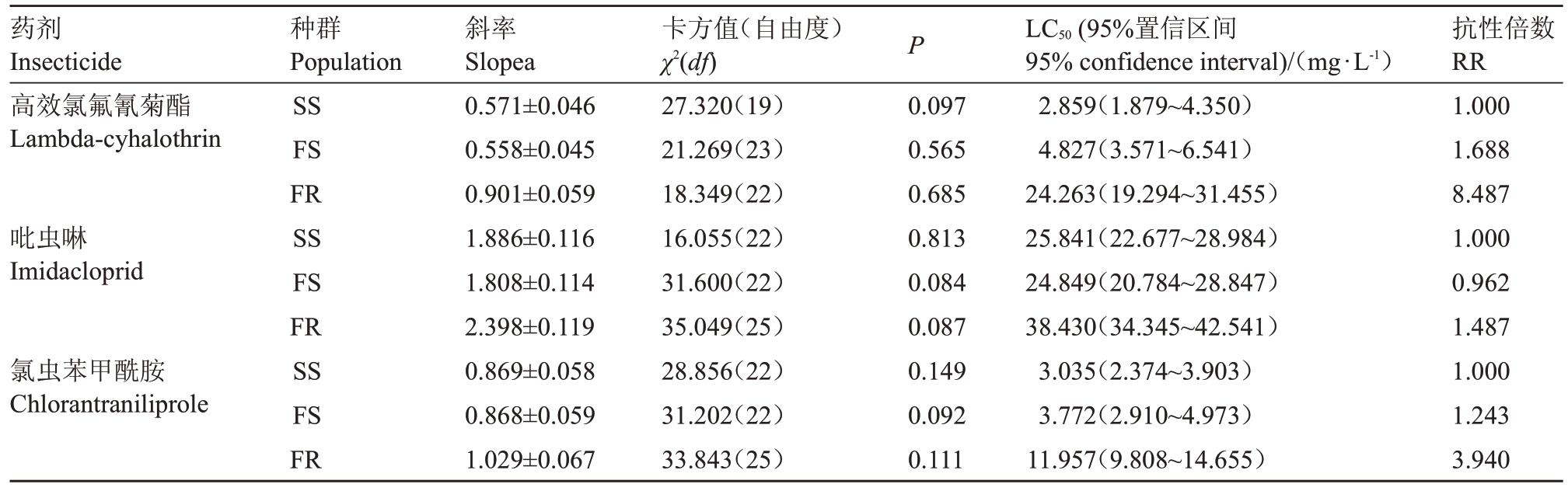
结果(表3)表明,田间种群阿维菌素汰选6代的FR 品系对高效氯氟氰菊酯的抗性倍数为8.487,存在交互抗性;对氯虫苯甲酰胺抗性倍数为3.940,抗性倍数增加;对吡虫啉抗性倍数为1.487,无交互抗性。田间种群连续6 代未接触阿维菌素的FS 品系对高效氯氟氰菊酯、吡虫啉和氯虫苯甲酰胺的抗性倍数分别为1.688、0.962 和1.243,均无交互抗性。结果说明,对阿维菌素敏感品系与试验选择的3 种药剂均无交互抗性;随着其抗性增至中等抗性水平,其与高效氯氟氰菊酯有交互抗性。
表3 梨小食心虫不同品系对3 种杀虫剂的交互抗性
Table 3 Cross-resistance of three insecticides to different strains of G.molesta
?
3 讨 论
阿维菌素可有效防控多种害虫[7-9],如使用不规范,害虫易对其产生抗药性或敏感性下降[19]。笔者在本研究中发现,梨园现有使用技术条件下(阿维菌素用药5~7次),试验采集的梨小食心虫田间种群已对阿维菌素敏感性降低;对该种群连续使用阿维菌素半致死剂量处理2 代后,梨小食心虫由低敏感性升至低等抗性水平,当进一步用药至第4代时,其抗性水平升至中等抗性水平;而未接触阿维菌素4 代后其抗性从4.608 倍降至2.802 倍,由低敏感性提高至敏感性。因而,在田间使用时,阿维菌素不仅从农药残留和食品安全间隔期来考虑其使用次数,也要从抗性变化角度来考虑其间隔使用技术,保证其处于敏感性或低敏感性水平,来保障其较好防治效果。
药剂抗性现实遗传力是其抗性风险评估的重要参数。阿维菌素对不同害虫抗性现实遗传力不同,对易产生抗性的小菜蛾和不易产生抗性的橘全爪螨,其抗性现实遗传力分别为0.130 和0.047 5,在50%~99%选择压力下两种害虫对其抗性上升10 倍分别需要3~16 代[20]和12~26 代[21];笔者在本研究中发现,阿维菌素对梨小食心虫抗性现实遗传力h2=0.186,在50%~90%选择压力下,梨小食心虫对阿维菌素抗性上升10 倍需4~9 代。说明阿维菌素对梨小食心虫抗性上升快,风险较大。这可能与梨小食心虫化学防控技术要求有关,梨小食心虫化学防治时,要求在成虫盛发期后3~5 d时施药,此时初孵幼虫接触农药的浓度较高,这可能是其抗性增加快速原因之一。同时,阿维菌素汰选6代,F0~F2汰选阶段的h2为0.242,F4~F6汰选阶段h2为0.196,表现出汰选前期现实遗传力大于汰选后期的现实遗传力,这与甲氨基阿维菌素苯甲酸盐、虱满脲对草地贪夜蛾[22]、Bt[17]和溴氰虫酰胺[23]对小菜蛾抗性的筛选结果相似,说明梨小食心虫初始种群中可能存在抗性基因,子代表现型可能由遗传变异来实现,抗性发展较快;随着抗性筛选持续,敏感基因可能被淘汰,抗性发展减慢。
现有研究结果表明,高等抗性水平鳞翅目害虫易对其他杀虫剂产生交互抗性,在小菜蛾[24-25]、二化螟[26]、黏虫[27]、草地贪夜蛾[28]、斜纹夜蛾[29]等害虫中均有发现。笔者在本研究中发现,田间种群未接触阿维菌素的对照品系对高效氯氟氰菊酯、吡虫啉和氯虫苯甲酰胺均无交互抗性;而汰选6 代的抗性品系对氯虫苯甲酰胺的抗性倍数明显增加,对高效氯氟氰菊酯存在交互抗性;随着梨小食心虫对阿维菌素抗性增加,其对梨园常用药剂可能存在交互抗性风险。因而,在田间梨小食心虫化学防治中,在使用阿维菌素时不仅要考虑使用次数,也要科学安排使用间隔时间,减少或限制高效氯氟氰菊酯和氯虫苯甲酰胺使用次数,避免或延缓其抗药性产生和发展,保证药剂防治效果。
4 结 论
连续使用阿维菌素半致死剂量处理2 代后,梨小食心虫由低敏感性升至低等抗性水平,进一步用药至第4代时,其抗性水平升至中等抗性水平;而未接触阿维菌素的4 代后其由低敏感性提高至敏感性。阿维菌素对梨小食心虫抗性现实遗传力h2=0.186,在50%~90%选择压力下,梨小食心虫对阿维菌素抗性上升10倍需4~9代。田间种群未接触阿维菌素的对照品系对高效氯氟氰菊酯、吡虫啉和氯虫苯甲酰胺均无交互抗性;而汰选6 代的抗性品系对氯虫苯甲酰胺的抗性倍数增加,对高效氯氟氰菊酯存在交互抗性。田间使用阿维菌素时可通过间隔用药或与无交互抗性药剂轮换使用来减缓抗性发展。
[1] 王雪婷.陕西几个地区梨小食心虫及李小食心虫种群动态监测[D].杨凌:西北农林科技大学,2019.WANG Xueting. Population dynamics monitoring of Grapholita molesta and Grapholita funebrana in several areas of Shaanxi[D].Yangling:Northwest A&F University,2019.
[2] 柴晓晗.桃园环境和品种差异对梨小食心虫两性引诱剂防控效果的影响[D].太谷:山西农业大学,2021.CHAI Xiaohan. Effects of cultivation characteristics and varieties of peach orchards on the control efficiency of the dual-sex attractant for Grapholita molesta[D]. Taigu:Shanxi Agricultural University,2021.
[3] 韩慧,毛瑞卿,赵志国,张利军,高玲玲,郭艳琼.3 种不同类型杀虫剂对梨小食心虫的室内毒力测定[J]. 山西农业科学,2019,47(8):1470-1473.HAN Hui,MAO Ruiqing,ZHAO Zhiguo,ZHANG Lijun,GAO Lingling,GUO Yanqiong.Indoor toxicity of three types of insecticides against Grapholita molesta Busck[J]. Journal of Shanxi Agricultural Sciences,2019,47(8):1470-1473.
[4] 刘月英,周昭旭,张美娇,曹利军.浸卵法测定甘肃省梨小食心虫田间种群对8 种杀虫剂的敏感性[J].农药学学报,2022,24(4):819-824.LIU Yueying,ZHOU Zhaoxu,ZHANG Meijiao,CAO Lijun.Sensitivity of field populations of Grapholita molesta in Gansu Province to eight insecticides using the egg dipping method[J].Chinese Journal of Pesticide Science,2022,24(4):819-824.
[5] GUO Y Q,CHAI Y P,ZHANG L J,ZHAO Z G,GAO L L,MA R Y.Transcriptome analysis and identification of major detoxification gene families and insecticide targets in Grapholita molesta (Busck) (Lepidoptera:Tortricidae)[J]. Journal of Insect Science,2017,17(2):43.
[6] MONTEIRO L B,WITT L G,GUILOSKI I C,DOS SANTOS R S S,SILVA DE ASSIS H C.Evaluation of resistance management for the oriental fruit moth (Lepidoptera:Tortricidae) to insecticides in Brazilian apple orchards[J]. Journal of Economic Entomology,2020,113(3):1411-1418.
[7] 蔡明飞,沈健,仵均祥.不同杀虫剂对梨小食心虫卵和成虫的室内防效[J].果树学报,2010,27(4):636-640.CAI Mingfei,SHEN Jian,WU Junxiang.Indoor control efficiency of six pesticides on eggs and adults of oriental fruit moth,(Grapholitha molesta)[J].Journal of Fruit Science,2010,27(4):636-640.
[8] 王芳.不同药剂对梨小食心虫不同虫态的药效及亚致死效应的研究[D].太谷:山西农业大学,2019.WANG Fang. Control effciency of different pesticides on different stages of G. molesta and research of sublethal effects[D].Taigu:Shanxi Agricultural University,2019.
[9] 张治科,尚小霞,马荣.棉蚜及异色瓢虫对不同药剂的敏感性测定[J].中国瓜菜,2022,35(9):91-94.ZHANG Zhike,SHANG Xiaoxia,MA Rong.Sensitivity of different insecticides against Aphis gossypii and Harmonia axyridis[J].China Cucurbits and Vegetables,2022,35(9):91-94.
[10] 朱红艳,伍涛,李先明,涂俊凡,杨夫臣,刘政,秦仲麒.棚架梨园主要虫害危害特点及绿色防治技术应用[J].现代园艺,2018(6):43-44.ZHU Hongyan,WU Tao,LI Xianming,TU Junfan,YANG Fuchen,LIU Zheng,QIN Zhongqi. Characteristics of main pests in shed pear orchard and application of green control technology[J].Contemporary Horticulture,2018(6):43-44.
[11] 金炜.安徽省几种特色水果主要虫害发生调查及防治研究[D].合肥:安徽农业大学,2016.JIN Wei. Research of the occurrence characteristic and control effect on main fruit pest in Anhui province[D].Hefei:Anhui Agricultural University,2016.
[12] 潘升水.南方采摘梨园主要病虫害综合防治技术[J].林业与生态,2020(12):36-37.PAN Shengshui. Integrated control technology of main diseases and insect pests in picking pear orchards in southern China[J].Forestry and Ecology,2020(12):36-37.
[13] 庾琴,杜恩强,郭晓君,封云涛,张润祥.不同杀虫剂及其组合对梨小食心虫初孵幼虫的毒力及田间防效[J]. 植物保护,2020,46(5):265-269.YU Qin,DU Enqiang,GUO Xiaojun,FENG Yuntao,ZHANG Runxiang. Toxicity of six insecticides and their mixtures to the neonate larvae of Grapholita molesta (Busck) and their field control effects[J].Plant Protection,2020,46(5):265-269.
[14] 庾琴,封云涛,郭晓君,杜恩强,郭贵明,张润祥.梨小食心虫初孵幼虫药效测定方法研究[J]. 果树学报,2017,34(11):1483-1489.YU Qin,FENG Yuntao,GUO Xiaojun,DU Enqiang,GUO Guiming,ZHANG Runxiang. Testing method of pesticide on Grapholitha molesta (Busck) neonate larvae[J]. Journal of Fruit Science,2017,34(11):1483-1489.
[15] 黄彦娜.陕西关中地区禾谷缢管蚜抗药性监测及共生菌检测[D].杨凌:西北农林科技大学,2018.HUANG Yanna. Insecticide resistance monitoring and symbionts detection in Rhopalosiphum padi field populations from Guanzhong area of Shaanxi Province[D].Yangling:Northwest A&F University,2018.
[16] TABASHNIK B E,MCGAUGHEY W H.Resistance risk assessment for single and multiple insecticides:responses of indianmeal moth (Lepidoptera:Pyralidae) to Bacillus thuringiensis[J].Journal of Economic Entomology,1994,87(4):834-841.
[17] TABASHNIK B E. Resistance risk assessment:Realized heritability of resistance to Bacillus thuringiensis in diamondback moth(Lepidoptera:Plutellidae),tobacco budworm(Lepidoptera:Noctuidae),and Colorado potato beetle (Coleoptera:Chrysomelidae)[J]. Journal of Economic Entomology,1992,85(5):1551-1559.
[18] 沈晋良,谭建国,肖斌,谭福杰,尤子平.我国棉铃虫对拟除虫菊酯类农药的抗性监测及预报[J].昆虫知识,1991,28(6):337-341.SHEN Jinliang,TAN Jianguo,XIAO Bin,TAN Fujie,YOU Ziping. Monitoring and forecasting of resistance of cotton bollworm to pyrethroid pesticides in China[J].Entomological Knowledge,1991,28(6):337-341.
[19] 周宇航,李凤良,金剑雪,李文红,程英.贵州菜区南美斑潜蝇对杀虫剂的敏感性监测[J].中国瓜菜,2022,35(5):42-50.ZHOU Yuhang,LI Fengliang,JIN Jianxue,LI Wenhong,CHENG Ying. Susceptibility monitoring of Liriomyza huidobrensis to insecticides in vegetable production area in Guizhou[J].China Cucurbits and Vegetables,2022,35(5):42-50.
[20] 梁延坡,吴青君,张友军,徐宝云,谢圣华,吉训聪.小菜蛾对阿维菌素的抗性风险评估及交互抗性的室内测定[J].热带生物学报,2010,1(3):228-232.LIANG Yanpo,WU Qingjun,ZHANG Youjun,XU Baoyun,XIE Shenghua,JI Xuncong. Resistance risk assessment and cross-resistance of Plutella xylostella to abamectin[J]. Journal of Tropical Biology,2010,1(3):228-232.
[21] 何恒果,赵志模,闫香慧,王进军.橘全爪螨对阿维菌素和甲氰菊酯的抗性现实遗传力及风险评估[J]. 应用生态学报,2011,22(8):2147-2152.HE Hengguo,ZHAO Zhimo,YAN Xianghui,WANG Jinjun.Resistance Realized heritability and risk assessment of Panonychus citri to avermectin and fenpropathrin[J]. Chinese Journal of Applied Ecology,2011,22(8):2147-2152.
[22] 赵金凤,邱良妙,丁雪玲,姚凤銮,卢学松,郑宇,翁启勇.草地贪夜蛾对甲氨基阿维菌素苯甲酸盐和虱螨脲的抗性风险评估[J].植物保护,2022,48(4):88-93.ZHAO Jinfeng,QIU Liangmiao,DING Xueling,YAO Fengluan,LU Xuesong,ZHENG Yu,WENG Qiyong. Risk assessment of the resistance to emamectin benzoate and lufenuron in Spodoptera frugiperda[J].Plant Protection,2022,48(4):88-93.
[23] 徐巨龙.小菜蛾对十种杀虫剂的抗性检测及对溴氰虫酰胺的抗性风险评估[D].泰安:山东农业大学,2020.XU Julong.Resistance detection of diamondback moth to ten insecticides and resistant risk assessment of cyantraniliprole[D].Tai’an:Shandong Agricultural University,2020.
[24] 李腾武,高希武,郑炳宗.小菜蛾对阿维菌素的抗性遗传分析及交互抗性研究[J].植物保护,1999,25(6):12-14.LI Tengwu,GAO Xiwu,ZHENG Bingzong. Genetic analysis and cross resistance of Plutella xylostella to avermectins[J].Plant Protection,1999,25(6):12-14.
[25] 梁沛,高希武,郑炳宗,戴洪波.小菜蛾对阿维菌素的抗性机制及交互抗性研究[J].农药学学报,2001,3(1):41-45.LIANG Pei,GAO Xiwu,ZHENG Bingzong,DAI Hongbo.Study on resistance machanisms and cross-resistance of abamectin in diamondback moth Plutella oxylostella (L.)[J]. Chinese Journal of Pesticide Science,2001,3(1):41-45.
[26] 许小龙,顾中言,韩丽娟,周建高,娄金贵.阿维菌素系列化合物对4 种重要鳞翅目害虫的毒力研究[J]. 华东昆虫学报,2005,14(2):184-187.XU Xiaolong,GU Zhongyan,HAN Lijuan,ZHOU Jiangao,LOU Jingui.Toxicity of abamectin series compounds to four important lepidopteral pests[J]. Entomological Journal of East China,2005,14(2):184-187.
[27] 宋月芹,王海涛,陈玉国,王圣印,孙会忠.黏虫抗甲氨基阿维菌素苯甲酸盐种群的交互抗性与生化抗性机制[J].农药学学报,2017,19(1):18-24.SONG Yueqin,WANG Haitao,CHEN Yuguo,WANG Shengyin,SUN Huizhong. Cross- resistance and biochemical resistance mechanisms of emamectin benzoate resistant population of Mythimna separata[J]. Chinese Journal of Pesticide Science,2017,19(1):18-24.
[28] 蒋兴川,沈怿丹,孙劲超,李秀霞,黄勇,董永成,操海群.氯虫苯甲酰胺和甲维盐对草地贪夜蛾幼虫的毒力及解毒酶活性的影响[J].环境昆虫学报,2019,41(5):961-967.JIANG Xingchuan,SHEN Yidan,SUN Jinchao,LI Xiuxia,HUANG Yong,DONG Yongcheng,CAO Haiqun. Effect of chlorantraniliprole and emamectin benzoate on toxicity and detoxification enzymes activity in Spodoptera frugiperda larva[J].Journal of Environmental Entomology,2019,41(5):961-967.
[29] 贺金.斜纹夜蛾对阿维菌素抗性风险分析及其抗性生化机理[D].泰安:山东农业大学,2009.HE Jin. Resistance risk analysis and biochemical mechanism of Spodoptera litura to avermectin[D]. Tai’an:Shandong Agricultural University,2009.