马家柚[Citrus grandis(L.)Osbeck]是江西省上饶市广丰区的地方特色品种,因其果大、营养价值高、独特的香味和耐贮性而广受消费者欢迎,它也是中国首个通过临床验证治疗糖尿病的保健食品[1]。有机酸是决定柑橘类水果内在品质的重要成分。其含量过高或过低都会影响水果的正常风味。柠檬酸是柑橘类水果中的主要有机酸,不同种类的柑橘类水果的柠檬酸含量及其积累模式在贮藏期间存在很大差异[2]。杨岚琪等[3]研究发现湖南新引进的4 个宽皮柑橘品种(春见、早蜜椪柑、金秋砂糖橘、春香)和4 个甜橙品种(锦红、橘湘珑、锦秀、橘湘元)果实的有机酸含量在整个贮藏过程中均呈下降趋势;李永杰等[4]也发现红美人杂柑果实的有机酸含量在贮藏过程中下降;但汪妮娜等[5]发现三红蜜柚、红肉蜜柚和桂红柚1 号在贮藏过程中有机酸含量逐渐上升。
柑橘中的柠檬酸首先在线粒体中由磷酸烯醇式丙酮酸(PEP)与CO2结合,在磷酸烯醇式丙酮酸羧化酶(PEPC)催化下生成草酰乙酸(OAA),然后在柠檬酸合成酶(CS)的作用下由OAA和乙酰-CoA合成,随后柠檬酸通过跨膜转运方式进入液泡中积累,并依赖转运载体和离子通道进入细胞质中被降解利用。赵淼等[6]发现在不同早、晚熟柑橘品种果实发育过程中PEPC 和CS 活性都呈现降低-升高-降低的表达模式,与柠檬酸含量变化的趋势呈显著正相关;而在靖安椪柑果实发育过程中CitCS2 和Cit-PEPC1相对表达先增加后减少,柠檬酸含量呈现相同变化趋势[7];柠檬酸合成后多余部分在液泡膜V型质子泵和P 型质子泵的参与下,通过特异转运蛋白运输到液泡中进行储存;Li 等[8]通过对高橙(高酸)和温州蜜柑(低酸)两个柑橘品种研究,发现VHA-c4 通过与转录因子ERF13 发生蛋白互作促进柠檬酸的积累;而温州蜜柑果实中V-PPase 和VATPase被报道促进柠檬酸在液泡中的积累[9]。
当果实中的有机酸含量达到一定水平时,柠檬酸开始在细胞质中降解和被利用。柠檬酸的分解主要有三种途径:GABA、谷氨酰胺和ACL 途径。其中,GABA 和谷氨酰胺途径是果实中最重要的柠檬酸降解途径。在GABA途径中,细胞质中的柠檬酸被顺乌头酸酶(Cyt-Aco)分解成异柠檬酸,异柠檬酸被异柠檬酸脱氢酶(NADP-IDH)分解成α-酮戊二酸(α-KG),随后在谷氨酸脱氢酶、大冬氨酸转氨酶或丙氨酸转氨酶的作用下生成谷氨酸。谷氨酸一方面被谷氨酸脱羧酶(GAD)催化,生成γ-氨基丁酸(GABA)进入GABA途径,随后GABA在GABA转氨酶(GABA-T)的作用下生成琥珀酸半醛,最后在琥珀酸半醛脱氢酶的作用下生成琥珀酸;另一方面在谷氨酰胺合成酶(GS)的催化下形成谷氨酰胺。张规富等[10]发现水分胁迫处理下的椪柑果实中有机酸含量显著增加,而柠檬酸降解基因CitAco3、CitIDH3和CitGAD5表达量显著下调,说明这些基因可能与柠檬酸的降解有关;Chen 等[11]研究发现早熟椪柑果实中柠檬酸降解相关基因CitAco3、CitIDH1/3、CitGAD4/5、CitGS2的表达水平均显著高于普通椪柑,推测可能是早熟椪柑果实中柠檬酸含量显著低于普通椪柑的重要原因。在ACL 途径中,柠檬酸由ATP-柠檬酸裂解酶(ACL)催化生成草酰乙酸和乙酰辅酶A,纽荷尔、温州蜜柑果实汁胞中CitACL 基因表达随着果实成熟显著升高,柠檬酸含量则显著降低[12];纽荷尔幼果经过热处理后,主要通过ACL 途径降解柠檬酸[13]。
目前关于马家柚果实中有机酸的研究主要集中在果实发育过程中,而针对马家柚果实在采后贮藏过程中有机酸含量的变化规律以及分子机制研究较少[1,14-15]。笔者在本研究中以马家柚为试材,研究果实有机酸组分在贮藏期间含量及相关基因表达的变化,探明贮藏早期柠檬酸含量显著变化的调控机制,为提高马家柚贮藏品质提供理论依据。
1 材料和方法
1.1 试验材料
马家柚果实采自江西省上饶市广丰区芦林镇果园。选取6 株生长势相当、树龄为12 年生的枳砧成年结果树,2 株为1 个生物重复。果实采收时,选择成熟度和大小一致、无病虫害、表面无机械损伤的果实在室温(20±2 ℃)下贮藏,每10 d每个重复取3个果实,果肉用液氮速冻后贮藏于-80 ℃的超低温冰箱中进行后续研究。
1.2 测定指标及方法
1.2.1 有机酸的提取与含量测定 果实中的有机酸的提取参考赵淼等[6]的方法,略作改动。称取4 g左右的果肉样品,加入5.0 mL 的80%乙醇,35 ℃水浴20 min,室温下10 000 r·min-1离心15 min,取上清液至25 mL 容量瓶,重复提取3 次后定容。取1 mL 提取液,旋转蒸干后加入1 mL 抽滤排气的超纯水溶解,使用一次性注射器吸取后用直径13 mm、孔径0.44µm的水系滤头过滤,滤液用于HPLC测定酸组分及含量。
色谱条件为Shim-pack VP-ODS 反相色谱柱(4.6 mm×250 mm,5 μm),柱温为30 ℃,流动相为0.01 mol·L-1 H2SO4(0.543 mL 浓硫酸定容至1 L,用氢氧化钾调节pH=2.6,抽滤排气后使用),流速为0.5 mL·min-1;进样体积为20 μL。用二极管阵列检器检测PDA(岛津SPD-M20A)(只需氘灯,需要把钨灯关闭);检测波长为210 nm。
1.2.2 RNA 的提取和逆转录 采用华越洋试剂盒提取马家柚果肉的RNA,采用琼脂糖凝胶电泳法检测RNA 完整性;采用TaKaRa 公司的反转录试剂盒(Cat.#RR047A)合成cDNA 第一链用于荧光定量分析。反转录体系为5 × PrimeScript Buffer 4 µL,PrimeScript RT Enzyme MIX Ⅰ1µL,Oligo dT Primer(50µmol·L-1)1µL,Random 6 mers(100µmol·L-1)2µL,Total RNA(1µg·µL-1)1µL,RNase Free ddH2O补齐至20µL。cDNA储存于-20 ℃冰箱用于后续试验。
1.2.3 实时荧光定量PCR 相关基因表达水平分析在Bio-Rad CFX 96-PCR 仪上进行,试剂来自TaKa-Ra公司的SYBR®Preminx Ex TaqTM试剂盒,体系为:ddH2O(灭菌蒸馏水)8.0 µL,SYBR®Preminx Ex Taq 10.0µL,PCR Forward Primer(10µmol·L-1)0.5µL,PCR Reverse Primer(10 µmol·L-1)0.5 µL,cDNA 1.0 µL,总体积20µL。内参基因与柠檬酸合成、转运和降解相关基因序列来自马家柚基因组数据库(http://citrus.hzau.edu.cn)[16],引物用Primer 5 设计,引物序列见表1。每个样品设置3次生物学重复,利用2-∆∆Ct方法进行数据分析。
表1 荧光定量PCR 引物序列
Table 1 Primer sequences of real-time fluorescence quantitative PCR

?
1.3 数据统计与分析
使用Microsoft Excel 2010 和SPSS Statistic 17.0软件对数据进行整理,显著性分析采用ANOVA 式方差分析和Duncan 差异显著性检验(p<0.05),使用Origin2018进行图表绘制。
2 结果与分析
2.1 马家柚果实贮藏期间有机酸含量的变化
马家柚果实中有机酸包括柠檬酸、苹果酸、酒石酸和奎宁酸等,其中柠檬酸为最主要的有机酸组分,采收时占总有机酸的73.4%。总有机酸含量(w,后同)随贮藏时间的增加呈现先上升后下降,最后趋于平稳的变化趋势(图1),在贮藏0~40 d(贮藏初期)逐渐上升至峰值(7.0 mg·g-1),随后在贮藏40~70 d 时呈明显下降趋势,并在贮藏70 d 时为最低值(3.8 mg·g-1),在贮藏80 d时回升至平稳;柠檬酸含量变化趋势与总有机酸含量变化基本保持一致,在贮藏70 d时下降至最低值2.7 mg·g-1,贮藏70~80 d短暂上升后直至贮藏结束(150 d)没有明显变化;而苹果酸、奎宁酸、酒石酸的含量在整个贮藏期变化较为平稳。
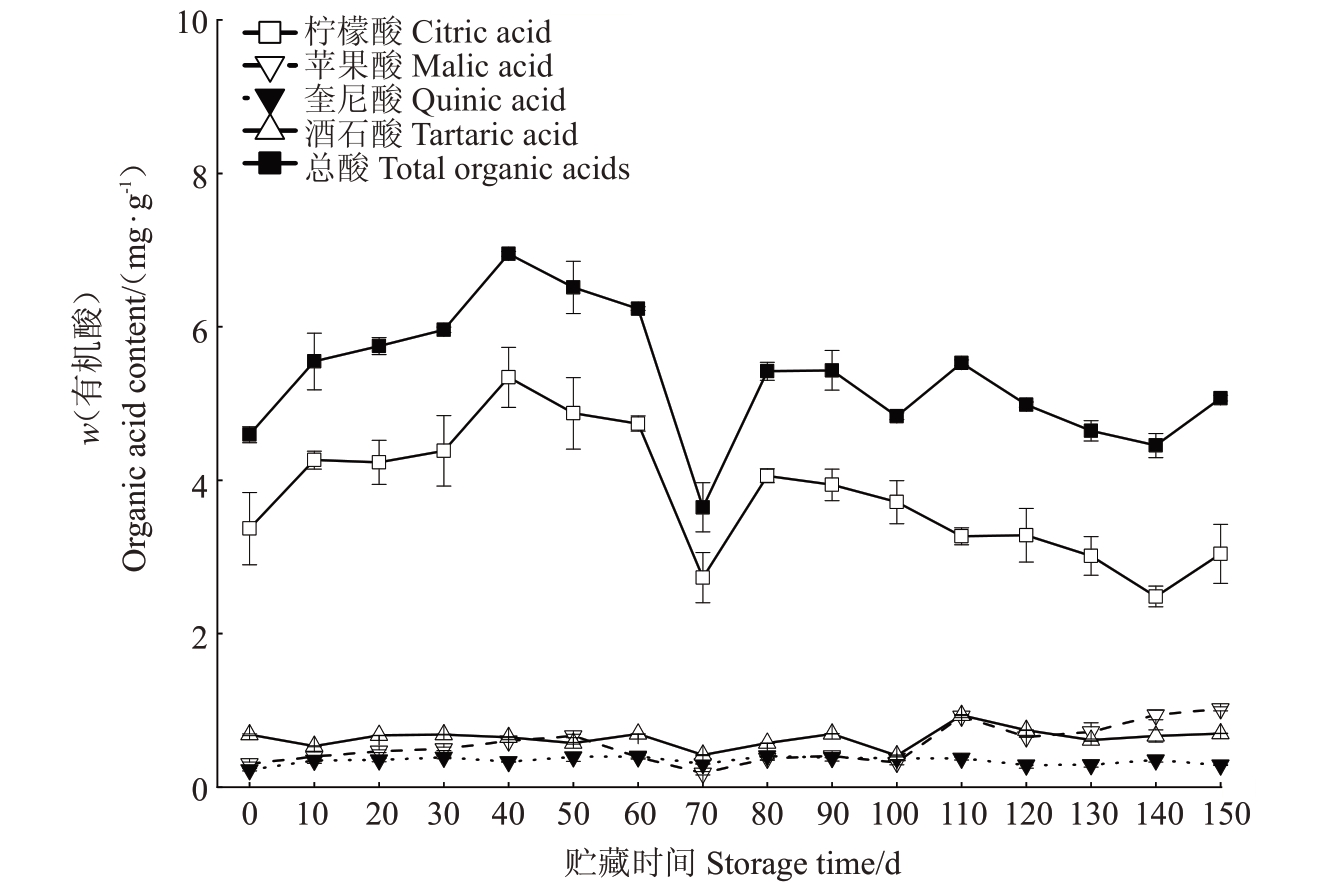
图1 马家柚果实贮藏期间有机酸含量的变化
Fig.1 Changes in organic acid content during storage of Majiayou pomelo fruit
2.2 马家柚果实贮藏期柠檬酸合成相关基因表达分析
选择柠檬酸含量显著变化的贮藏0、20、40、70 d马家柚果实进行柠檬酸代谢相关基因的表达分析(图2)。在马家柚果实贮藏过程中,CmPEPC1基因相对表达量在贮藏0~40 d 基本呈上升趋势,在贮藏40~70 d 时显著降低;CmPEPC2 基因相对表达量在贮藏0~40 d 时变化不明显,但在贮藏40~70 d 时显著降低;CmCS1 基因相对表达量在贮藏开始20 d基本保持不变,在贮藏40 d 时显著上升,贮藏40~70 d 时没有显著变化;CmCS1 在整个贮藏过程中呈现先上升后下降趋势:CmCS2在贮藏第40天时上升至峰值,在贮藏40~70 d时显著下降。以上结果可推测,CmPEPC1和CmCS1/2与贮藏0~40 d柠檬酸含量增加有关,而CmPEPC1/2 和CmCS2 可能调控了贮藏40~70 d期间柠檬酸和含量的减少。
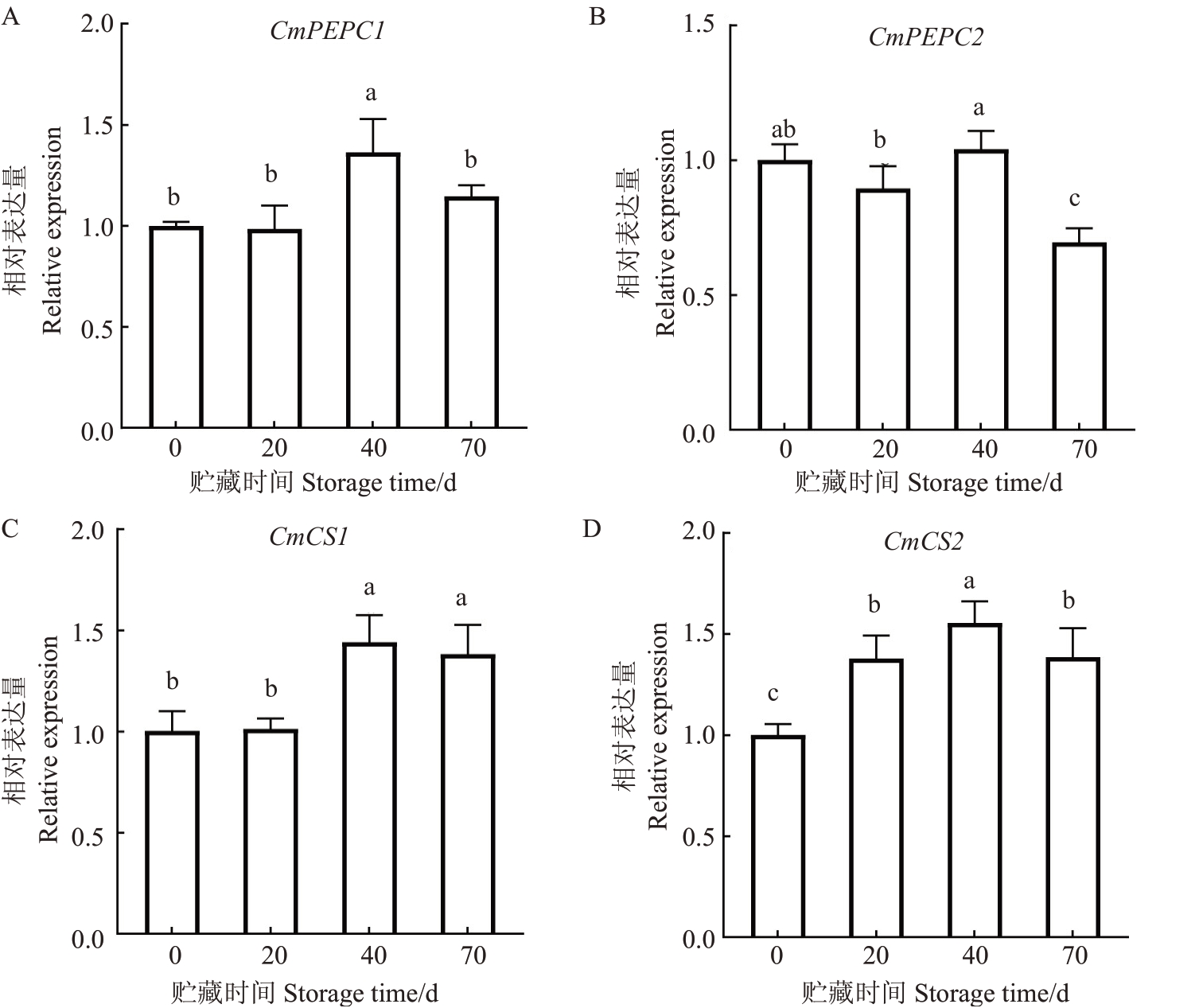
图2 马家柚果实贮藏期间柠檬酸合成相关基因的表达量
Fig.2 Expression of genes related to citric acid synthesis during storage of Majiayou pomelo fruit
不同小写字母表示在p<0.05 水平差异显著。下同。
Different small letters indicate significant differences at the level of p<0.05.The same below.
2.3 马家柚果实贮藏期柠檬酸转运相关基因表达分析
柠檬酸通过多种转运体和质子泵协同作用向液泡中转运。对贮藏0、20、40、70 d马家柚果实进行柠檬酸转运相关基因的表达分析(图3),阳离子质子转运体基因CmCHX的相对表达量在贮藏0~40 d时显著上升,并在贮藏第40 天时显著上升至最大值,贮藏40~70 d 没有显著差异;线粒体二羧酸载体基因CmDIC相对表达量在贮藏0~40 d逐渐下降,贮藏40~70 d没有显著差异;质子泵基因CmVHA-c4相对表达量呈先上升(贮藏0~40 d)后下降(贮藏40~70 d)的趋势;而CmVHP2 基因相对表达量在贮藏0~40 d没有显著差异,贮藏40~70 d显著下降。由以上结果可知,CmCHX、CmVHA-c4 和CmVHP2 基因表达量与此期间柠檬酸含量变化趋势相似,而CmDIC基因表达量则与之相反。
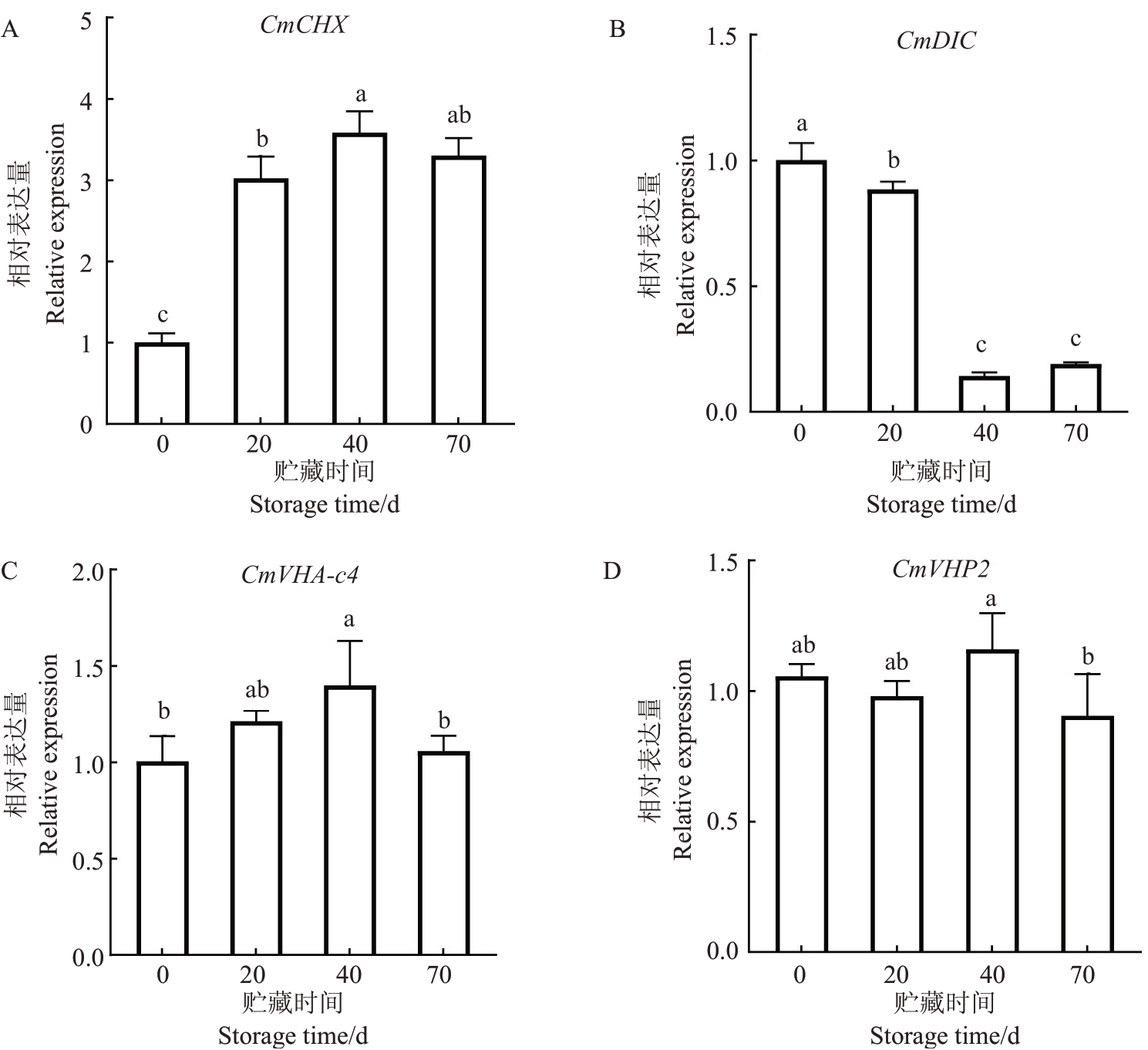
图3 马家柚果实贮藏期间柠檬酸转运相关基因的表达量
Fig.3 Expression of genes related to organic acid transport during the storage of Majiayou pomelo fruit
2.4 马家柚果实贮藏期间柠檬酸降解相关基因表达分析
对贮藏0、20、40、70 d马家柚果实进行柠檬酸降解相关基因的表达分析(图4)。在马家柚贮藏前期,CmACL1 基因相对表达量持续显著上升,CmACL3基因在贮藏40 d时显著上升至最大值,贮藏40~70 d时显著下降;CmAco3基因表达量在贮藏20 d时达到最大值,随后趋于平稳;CmNAD-IDH2 基因在贮藏0~40 d 时没有显著差异,贮藏40~70 d 时显著下降;CmNAD-IDH3 基因呈先下降后上升再下降的波动趋势;CmGS2和CmGAD5基因相对表达量在贮藏40 d之前均呈现下降趋势,贮藏40~70 d时差异不显著。γ-氨基丁酸转氨酶基因CmGABA-T 的相对表达量在贮藏0~40 d的时候没有显著的变化,在贮藏40~70 d 显著上升,贮藏70 d 时柠檬酸含量最低。由以上结果可知,只有柠檬酸降解基因CmGS2和CmGAD5 在贮藏0~40 d 期间与柠檬酸含量呈现相反情况,推测与此期间果实柠檬酸含量上升有关。

图4 马家柚果实贮藏期间柠檬酸降解相关基因的表达量
Fig.4 Expression of genes related to citric acid degradation during storage of Majiayou pomelo fruit
3 讨 论
柠檬酸是柑橘类水果中的主要有机酸,占有机酸总量的63.7%~96.7%。不同柑橘品种在贮藏期间果实中有机酸的组成和含量变化趋势各不相同。一般来说,贮藏期间柑橘类果实中的有机酸含量总体呈下降趋势[17-19];但也有部分品种呈现先上升,再下降,所谓“返酸”的现象,如泰国柚在贮藏过程中总酸及柠檬酸含量逐渐增加[20],六月早柚果实在贮藏20 d出现低程度的“返酸”现象[21];水晶蜜柚以及琯溪蜜柚果实在贮藏过程中有机酸含量在贮藏45~60 d 时出现高峰之后逐渐减少[22];李宏祥等[23]研究发现三个不同采收期的桃溪蜜柚果实在贮藏0~60 d 有机酸含量都出现了增加;徐世荣等[24]研究发现,琯溪蜜柚果实在贮藏过程中柠檬酸含量逐渐增加。笔者在本研究中发现马家柚果实在贮藏40 d 前也会出现“返酸”现象,柠檬酸含量在贮藏初期(0~40 d)显著增加,且在贮藏40 d时达到最大值,在贮藏70 d下降至最低值。因此,为了解马家柚果实贮藏前期柠檬酸显著积累的原因,笔者在本研究中对此期间柠檬酸代谢相关基因的相对表达量进行了分析。
柑橘果实内柠檬酸的含量是其合成、转运和降解相关基因协同调控的结果。柠檬酸的合成主要与PEPC和CS基因相关,有大量研究表明,PEPC和CS基因表达量或者酶活性的增加与琯溪蜜柚[25]、脐橙[26]、砂梨[27]、菠萝[28]、蜂糖李[29]、柠檬[30]等果实发育前期或贮藏期柠檬酸或苹果酸积累增加呈显著正相关。笔者在本研究中通过对马家柚果实贮藏早期柠檬酸合成相关基因表达分析,发现PEPC1/2和CS1/2基因相对表达量变化趋势与柠檬酸含量基本动态一致,均在贮藏后40 d时达到最大值,与前人研究结果相符,因此PEPC1/2 和CS1/2 可能参与马家柚果实内柠檬酸在贮藏前期的合成。
在植物细胞中,有机酸在线粒体中合成,主要储存在液泡中,并在细胞质中进行降解,其在细胞内膜系统中的运输主要通过转运蛋白或通道实现,而质子泵蛋白为有机酸相关次级转运蛋白提供必需的电化学势梯度和能量,因此,质子泵蛋白在有机酸的转运过程中发挥着重要作用。液泡膜上的质子泵有3种类型:V-ATPase,V-PPase 和P-type ATPase。拟南芥中AtVHA-a2 和AtVHA-a3 基因突变,会导致植物中有机酸含量降低[31];而柠檬果实VHA蛋白C亚基的激活会导致柠檬酸的积累增加[32];同样VHP的活性与葡萄[33]、番茄[34]、柠檬[35-36]果实中有机酸含量呈正相关。笔者在本研究中发现,CmVHA-c4和CmVHP2基因的表达量变化趋势与马家柚果实内柠檬酸含量变化的趋势一致,由此推测CmVHA-c4 和CmVHP2在调控果实中柠檬酸在液泡中的积累方面可能发挥了重要作用,后期可对二者的生物功能进行进一步的研究。线粒体二羧酸转运蛋白DIC能促进线粒体与细胞质中三羧酸盐阴离子与二羧酸盐阴离子的交换,并负责拟南芥叶片液泡内柠檬酸以及苹果酸选择性地转运[37-38];在柑橘上,CHX和DIC转运蛋白可能参与果实液泡中的柠檬酸向细胞质的转运[39]。笔者在本研究中发现马家柚果实在贮藏前期,即柠檬酸含量持续增加时,CmDIC 基因表达显著下调,推测可能是CmDIC的下调表达,抑制了有机酸向线粒体的转运和代谢,从而增加了液泡和细胞质中柠檬酸的含量。
柠檬酸运输到细胞质后,主要通过GABA 和谷氨酰胺途径降解。在谷氨酰胺途径中,谷氨酰胺合成酶(GS)起主导作用。有研究表明在果实发育后期,CsGS1 基因的高表达,导致奉节72-1[40]、红橘砧脐橙果实[41]、赣州脐橙果实[42]柠檬酸含量降低。在GABA 途径中,谷氨酸在GAD 的催化下生成GABA。易明亮等[14]、Liu 等[43]和宋江涛等[44]的研究表明,CsGAD1、CsGAD2 和GAD5 基因表达上调会导致果实柠檬酸含量显著下降;Sheng等[45]通过外源喷施GABA抑制果实中GAD基因表达,从而导致果实中柠檬酸含量显著增加;笔者在本研究中发现,CmGS2 和CmGAD5 基因表达都是在贮藏40 d 前显著下调,延缓了柠檬酸的降解速率,从而使得该贮藏阶段马家柚果实柠檬酸含量上升。因此推测,马家柚果实中柠檬酸可能主要通过谷氨酰胺和GABA途径进行降解。
4 结 论
笔者在本研究中通过对马家柚贮藏过程中的有机酸含量变化以及贮藏早期柠檬酸积累调控相关基因表达分析,明确了在马家柚整个贮藏阶段有机酸的组成以及含量变化规律,并得出马家柚果实在贮藏期间的主要有机酸为柠檬酸,在贮藏40 d 时出现明显返酸现象;这种变化可能主要由柠檬酸合成基因PEPC1/2和CS1/2、有机酸转运相关基因CmDIC、VHA-c4 和VHP2 以及降解基因GS2、GAD5 表达调控;为进一步研究柑橘果实贮藏期间柠檬酸积累调控机制奠定了理论基础。
[1] NIE Z P,WAN C P,CHEN C Y,CHEN J Y. Comprehensive evaluation of the postharvest antioxidant capacity of majiayou pomelo harvested at different maturities based on PCA[J].Antioxidants,2019,8(5):136.
[2] 卢晓鹏,李菲菲,谢深喜.柑橘果实柠檬酸积累调控基因研究进展[J].果树学报,2018,35(1):118-127.LU Xiaopeng,LI Feifei,XIE Shenxi.Citrate accumulation in citrus fruit:A molecular perspective[J]. Journal of Fruit Science,2018,35(1):118-127.
[3] 杨岚琪,郭静,金燕,龙桂友,龙晴柔,盛玲.湖南省8 个品种柑橘品质和贮藏性的评价[J].保鲜与加工,2022,22(4):34-45.YANG Lanqi,GUO Jing,JIN Yan,LONG Guiyou,LONG Qingrou,SHENG Ling. Evaluation on fruit quality and storage trait of eight citrus cultivars in Hunan Province[J]. Storage and Process,2022,22(4):34-45.
[4] 李永杰,金国强,邱晓莹,何碧波.“红美人”杂柑果实品质特性及低温贮藏对品质的影响[J].中国南方果树,2021,50(4):28-32.LI Yongjie,JIN Guoqiang,QIU Xiaoying,HE Bibo.Characteristics of fruit quality of hongmeiren mandarin and effect of low storage temperature on fruit quality[J]. South China Fruits,2021,50(4):28-32.
[5] 汪妮娜,黄宏明,陈东奎,陈香玲,刘福平,王茜,黄其椿,张兰,张尧良,廖惠红.几个柚品种果实贮藏期的生理生化及粒化指数差异[J].中国南方果树,2021,50(2):40-43.WANG Nina,HUANG Hongming,CHEN Dongkui,CHEN Xiangling,LIU Fuping,WANG Qian,HUANG Qichun,ZHANG Lan,ZHANG Yaoliang,LIAO Huihong. Differences of physiological,biochemical and granulation index of fruits during storage in several pumelo varieties[J]. South China Fruits,2021,50(2):40-43.
[6] 赵淼,吴延军,蒋桂华,谢鸣,林毅,蔡永萍.柑橘果实有机酸代谢研究进展[J].果树学报,2008,25(2):225-230.ZHAO Miao,WU Yanjun,JIANG Guihua,XIE Ming,LIN Yi,CAI Yongping. Advances in research on the metabolism of organic acids in citrus fruits[J]. Journal of Fruit Science,2008,25(2):225-230.
[7] 高阳,阚超楠,陈楚英,陈明,陈金印.‘靖安椪柑’果实发育阶段柠檬酸含量变化及其代谢相关基因的表达分析[J].果树学报,2018,35(8):936-946.GAO Yang,KAN Chaonan,CHEN Chuying,CHEN Ming,CHEN Jinyin. Changes of citric acid content and the related gene expression at different developmental stages of fruits in‘Jing’an Ponkan’[J].Journal of Fruit Science,2018,35(8):936-946.
[8] LI S J,YIN X R,XIE X L,ALLAN A C,GE H,SHEN S L,CHEN K S.The Citrus transcription factor,CitERF13,regulates citric acid accumulation via a protein-protein interaction with the vacuolar proton pump,CitVHA-c4[J]. Scientific Reports,2016,6:20151.
[9] HUSSAIN B S.柑橘液胞型焦磷酸质子泵基因鉴定及其在蔗糖和柠檬酸积累调控中的功能验证[D].武汉:华中农业大学,2020.HUSSAIN B S. Identification of citrus vacuolar H+-pyrophosphatase genes and their function in the regulation of sucrose and citrate accumulation[D].Wuhan:Huazhong Agricultural University,2020.
[10] 张规富,卢晓鹏,谢深喜.不同时期水分胁迫对椪柑果实柠檬酸代谢相关基因表达的影响[J]. 果树学报,2015,32(4):525-535.ZHANG Guifu,LU Xiaopeng,XIE Shenxi. Influence of water stress in different development stage on the citric acid metabolism-related genes expression in the Ponkan fruits[J]. Journal of Fruit Science,2015,32(4):525-535.
[11] CHEN M,JIANG Q,YIN X R,LIN Q,CHEN J Y,ALLAN A C,XU C J,CHEN K S.Effect of hot air treatment on organic acid- and sugar-metabolism in Ponkan (Citrus reticulata) fruit[J].Scientia Horticulturae,2012,147:118-125.
[12] HU X M,SHI C Y,LIU X,JIN L F,LIU Y Z,PENG S. Genome-wide identification of citrus ATP-citrate lyase genes and their transcript analysis in fruits reveals their possible role in citrate utilization[J].Molecular Genetics and Genomics,2015,290(1):29-38.
[13] 杨滢滢,高阳,向妙莲,陈明,陈金印.‘纽荷尔’脐橙果实发育过程中几种柠檬酸代谢相关基因的表达特征分析[J].果树学报,2016,33(4):400-408.YANG Yingying,GAO Yang,XIANG Miaolian,CHEN Ming,CHEN Jinyin. Expression profile analysis of several citric acid metabolism related genes in the‘Newhall’navel orange during fruit development[J]. Journal of Fruit Science,2016,33(4):400-408.
[14] 易明亮,张王妮,杨莉,匡柳青,刘德春,刘勇,胡威.‘马家柚’果实发育期有机酸含量变化及柠檬酸代谢相关基因的表达分析[J].江西农业大学学报,2022,44(4):841-851.YI Mingliang,ZHANG Wangni,YANG Li,KUANG Liuqing,LIU Dechun,LIU Yong,HU Wei.Analysis of organic acid content and expression of citric acid metabolism related genes during fruit development of‘Majia’pomelo[J]. Acta Agriculturae Universitatis Jiangxiensis,2022,44(4):841-851.
[15] 张念,彭怡霖,陈细羽,焦必宁,张耀海.不同成熟期琯溪蜜柚果实功能成分的差异分析[J]. 果树学报,2022,39(6):1042-1053.ZHANG Nian,PENG Yilin,CHEN Xiyu,JIAO Bining,ZHANG Yaohai.Analysis on the difference of founctional components of Guanxi pomelo fruits during maturity[J]. Journal of Fruit Science,2022,39(6):1042-1053.
[16] LU Z H,HUANG Y,MAO S Y,WU F F,LIU Y,MAO X Q,ADHIKARI P B,XU Y T,WANG L,ZUO H,RAO M J,XU Q.The high-quality genome of pummelo provides insights into the tissue-specific regulation of citric acid and anthocyanin during domestication[J].Horticulture Research,2022,9:uhac175.
[17] 匡晓东,向敏,王聪田,杨文学.安农无核蜜香柚采后生理及保鲜技术研究[J].湖南农业科学,2008(5):119-120.KUANG Xiaodong,XIANG Min,WANG Congtian,YANG Wenxue.Research on postharvest physiology and freshness preservation technology of Annong nuclear-free honey pomelo[J].Hunan Agricultural Sciences,2008(5):119-120.
[18] 施堂红,刘晓政,严晓丽,占元毅.常山胡柚果品品质及贮藏过程中变化规律的研究[J].食品工程,2013(4):34-38.SHI Tanghong,LIU Xiaozheng,YAN Xiaoli,ZHAN Yuanyi.Research on the quality of citrus Chang Shan-huyou and change rule during storage[J].Food Engineering,2013(4):34-38.
[19] 纪颖,黄华明.建阳橘柚采后贮藏期间生理和营养成分变化的研究[J].食品工程,2015(3):37-39.JI Ying,HUANG Huaming. Study on‘Jianyang’tangelo nutrition and physical changes during the period of postharvest storage[J].Food Engineering,2015(3):37-39.
[20] 刘娟,阮音音,曾丽萍,李新国,LONG Chunrui.泰国柚常温贮藏的采后品质变化[J].热带生物学报,2018,9(4):418-422.LIU Juan,RUAN Yinyin,ZENG Liping,LI Xinguo,LONG Chunrui. The changes of postharvest quality in Thai pomelo fruits stored at room temperature[J]. Journal of Tropical Biology,2018,9(4):418-422.
[21] 徐成妍,王乃玉,蔡净蓉,赵俊跃,佘文琴.六月早柚果实常温贮藏期间糖酸含量变化[J]. 福建农业学报,2022,37(6):765-773.XU Chengyan,WANG Naiyu,CAI Jingrong,ZHAO Junyue,SHE Wenqin.Flavor of Liuyuezao pummelos as affected by sugar and acid contents in storage at ambient temperature[J].Fujian Journal of Agricultural Sciences,2022,37(6):765-773.
[22] 周先艳,朱春华,周东果,高俊燕,李进学,赖新朴,杨帆,岳建强.水晶蜜柚和琯溪蜜柚贮藏期间果实不同部位品质变化[J].食品工业科技,2018,39(19):291-295.ZHOU Xianyan,ZHU Chunhua,ZHOU Dongguo,GAO Junyan,LI Jinxue,LAI Xinpu,YANG Fan,YUE Jianqiang.Changes of quality of different parts of Crystal and Guanxi honey pomelo during storage[J].Science and Technology of Food Industry,2018,39(19):291-295.
[23] 李宏祥,马巧利,林雄,廖小娜,曾平章,陈金印.采收成熟度对桃溪蜜柚贮藏品质及抗氧化性的影响[J].食品与发酵工业,2019,45(13):191-198.LI Hongxiang,MA Qiaoli,LIN Xiong,LIAO Xiaona,ZENG Pingzhang,CHEN Jinyin. Effects of harvest maturity on storage quality and antioxidative capacity of Taoxi pomelo[J]. Food and Fermentation Industries,2019,45(13):191-198.
[24] 徐世荣,杨华丽,李小婷,张智伟,潘东明.采后‘琯溪蜜柚’果实柠檬酸合成酶基因的克隆与表达分析[J].中国果树,2017(3):10-15.XU Shirong,YANG Huali,LI Xiaoting,ZHANG Zhiwei,PAN Dongming. Cloning and expression analysis of citrate synthase gene in postharvest‘Guanximiyou’fruits[J].China Fruits,2017(3):10-15.
[25] 张小红,赵依杰,潘东明,林航.琯溪蜜柚果实采后有机酸代谢[J].果树学报,2010,27(2):193-197.ZHANG Xiaohong,ZHAO Yijie,PAN Dongming,LIN Hang.Study on the post-harvest metabolism of organic acids in fruit of Guanxi-miyou pomelo cultivar[J]. Journal of Fruit Science,2010,27(2):193-197.
[26] 文涛,熊庆娥,曾伟光,刘远鹏.脐橙果实发育过程中有机酸合成代谢酶活性的变化[J].园艺学报,2001,28(2):161-163.WEN Tao,XIONG Qing’e,ZENG Weiguang,LIU Yuanpeng.Changes of organic acid synthetase activity during fruit development of Navel orange (Citrus sinesis Osbeck)[J].Acta Horticulturae Sinica,2001,28(2):161-163.
[27] 蒋爽,岳晓燕,滕元文,王晓庆,施春晖,徐芳杰,张学英,白松龄,骆军.不同砂梨果实中糖酸含量及代谢相关基因表达分析[J].果树学报,2016,33(增刊1):65-70.JIANG Shuang,YUE Xiaoyan,TENG Yuanwen,WANG Xiaoqing,SHI Chunhui,XU Fangjie,ZHANG Xueying,BAI Songling,LUO Jun. The contents of sugars and acids,and the expression analysis of metabolism-associated genes in fruit of Pyrus pyrifolia[J]. Journal of Fruit Science,2016,33(Suppl.1):65-70.
[28] 张秀梅,杜丽清,孙光明,弓德强,陈佳瑛,李伟才,谢江辉.菠萝果实发育过程中有机酸含量及相关代谢酶活性的变化[J].果树学报,2007,24(3):381-384.ZHANG Xiumei,DU Liqing,SUN Guangming,GONG Deqiang,CHEN Jiaying,LI Weicai,XIE Jianghui.Changes in organic acid concentrations and the relative enzyme activities during the development of Cayenne pineapple fruit[J]. Journal of Fruit Science,2007,24(3):381-384.
[29] 王小红,陈红,董晓庆.‘蜂糖李’果实发育过程中有机酸含量变化及其与苹果酸代谢相关酶的关系[J].果树学报,2018,35(3):293-300.WANG Xiaohong,CHEN Hong,DONG Xiaoqing. Changes in organic acids content during‘Fengtang’plum (Prunus salicina)fruit development in relation to malic acid metabolism related enzymes[J].Journal of Fruit Science,2018,35(3):293-300.
[30] SADKA A,DAHAN E,OR E,COHEN L.NADP+-isocitrate dehydrogenase gene expression and isozyme activity during citrus fruit development[J].Plant Science,2000,158(1/2):173-181.
[31] KREBS M,BEYHL D,GÖRLICH E,AL-RASHEID K A S,MARTEN I,STIERHOF Y D,HEDRICH R,SCHUMACHER K.Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation[J].Proceedings of the National Academy of Sciences of the United States of America,2010,107(7):3251-3256.
[32] BRUNE A,MÜLLER M,TAIZ L,GONZALEZ P,ETXEBERRIA E. Vacuolar acidification in Citrus fruit:Comparison between acid lime(Citrus aurantifolia)and sweet lime(Citrus limmetioides) juice cells[J]. Journal of the American Society for Horticultural Science,2002,127(2):171-177.
[33] VENTER M,GROENEWALD J H,BOTHA F C. Sequence analysis and transcriptional profiling of two vacuolar H+-pyrophosphatase isoforms in Vitis vinifera[J]. Journal of Plant Research,2006,119(5):469-478.
[34] MILNER I D,HO L C,HALL J L.Properties of proton and sugar transport at the tonoplast of tomato (Lycopersicon esculen-tum)fruit[J].Physiologia Plantarum,1995,94(3):399-410.
[35] GUO L X,SHI C Y,LIU X,NING D Y,JING L F,YANG H,LIU Y Z. Citrate accumulation-related gene expression and/or enzyme activity analysis combined with metabolomics provide a novel insight for an orange mutant[J]. Scientific Reports,2016,6:29343.
[36] TAN F Q,ZHANG M,XIE K D,FAN Y J,SONG X,WANG R,WU X M,ZHANG H Y,GUO W W. Polyploidy remodels fruit metabolism by modifying carbon source utilization and metabolic flux in Ponkan mandarin (Citrus reticulata Blanco)[J]. Plant Science,2019,289:110276.
[37] LALOI M. Plant mitochondrial carriers:An overview[J]. Cellular and Molecular Life Sciences,1999,56(11/12):918-944.
[38] LEE C P,ELSÄSSER M,FUCHS P,FENSKE R,SCHWARZLÄNDER M,MILLAR A H. The versatility of plant organic acid metabolism in leaves is underpinned by mitochondrial malate-citrate exchange[J]. The Plant Cell,2021,33(12):3700-3720.
[39] LIN Q,LI S J,DONG W C,FENG C,YIN X R,XU C J,SUN C D,CHEN K S. Involvement of CitCHX and CitDIC in developmental-related and postharvest-hot-air driven citrate degradation in citrus fruits[J].PLoS One,2015,10(3):e0119410.
[40] 付莉莉,吴巨勋,伊华林.脐橙晚熟突变体及其野生型果实柠檬酸代谢基因表达分析[J].园艺学报,2016,43(1):38-46.FU Lili,WU Juxun,YI Hualin. Expression analysis of citrate metabolic related genes in late-ripening mutant of navel orange and its wild type[J].Acta Horticulturae Sinica,2016,43(1):38-46.
[41] 苏媚,伊华林.砧木对脐橙果实品质及糖酸代谢相关基因表达的影响[J].中国南方果树,2016,45(5):34-37.SU Mei,YI Hualin. Influence of rootstocks on navel orange fruit quality and expression of genes related to sugar-acid metabolism[J].South China Fruits,2016,45(5):34-37.
[42] 马雨尘.柑橘果实柠檬酸降解GS 途径转录调控研究[D].杭州:浙江大学,2020.MA Yuchen.Transcriptional regulation of citric acid degradation GS pathway in citrus fruits[D]. Hangzhou:Zhejiang University,2020.
[43] LIU X,HU X M,JIN L F,SHI C Y,LIU Y Z,PENG S A.Identification and transcript analysis of two glutamate decarboxylase genes,CsGAD1 and CsGAD2,reveal the strong relationship between CsGAD1 and citrate utilization in citrus fruit[J]. Molecular Biology Reports,2014,41(9):6253-6262.
[44] 宋江涛,谌丹丹,公旭晨,商祥明,李春龙,蔡永喜,岳建平,王帅玲,张卜芬,谢宗周,刘继红.人工疏果对‘爱媛28’橘橙果实糖酸含量及代谢基因表达的影响[J].中国农业科学,2022,55(23):4688-4701.SONG Jiangtao,CHEN Dandan,GONG Xuchen,SHANG Xiangming,LI Chunlong,CAI Yongxi,YUE Jianping,WANG Shuailing,ZHANG Bufen,XIE Zongzhou,LIU Jihong. Effects of artificial fruit thinning on sugar and acid content and expression of metabolism-related genes in fruit of Beni-Madonna tangor[J].Scientia Agricultura Sinica,2022,55(23):4688-4701.
[45] SHENG L,SHEN D D,LUO Y,SUN X H,WANG J Q,LUO T,ZENG Y L,XU J,DENG X X,CHENG Y J. Exogenous γ-aminobutyric acid treatment affects citrate and amino acid accumulation to improve fruit quality and storage performance of postharvest citrus fruit[J].Food Chemistry,2017,216:138-145.