果实色泽是苹果重要的外观品质,极大程度地决定了果实的商品价值。苹果果皮色泽与果皮花青苷含量密切相关,同时花青苷也是果实重要的功能营养成分,具有预防心脑血管疾病、抗氧化、延缓衰老等重要的保健功能[1-3]。因此,研究苹果果皮色泽的遗传特性及调控机制,改善果实色泽和提高果皮花青苷含量是苹果育种及相关领域研究关注的热点。
花青苷通过苯丙氨酸代谢途径,在多种结构基因及转录因子类调控基因的参与下合成。结构基因主要包括PAL、CHS、CHI、F3H、DFR、LDOX、ANS 和UFGT[4-5]。其中,CHS、CHI 以及F3H 为早期结构基因,而DFR、LDOX、ANS 和UFGT 为晚期结构基因,都在花青苷合成途径中起正向调控作用[6-7]。花青苷的生物合成还受到MYB、bHLH 和WD40 蛋白复合体(MYB-bHLH-WD40)的调控,其中MYB 转录因子发挥至关重要的作用[8-10]。MdMYB1、MdMYB10被认为是苹果花青苷生物合成的正向调控因子,直接激活MdDFR和MdUF3GT基因的表达,促进花青苷的生物合成[11-13]。报道还发现,茉莉酸甲酯处理嘎拉苹果幼苗后,MdMYB11 基因表达量提高,Md-MYB11基因转入苹果愈伤组织后,通过结合MdANS等结构基因启动子调控花青苷的合成,积累大量的花青苷与原花青苷[14]。除了MYB之外,其他家族转录因子也参与了花青苷的代谢。MdbZIP44 在激素ABA诱导下,与MdMYB1相互作用,增强了MdMYB1与下游靶基因的互作从而促进了花青苷的合成[15]。另有研究发现,在拟南芥及苹果叶片中转入MdERF3基因后,花青苷及原花青苷含量显著提高[16]。
2001富士品种自1993年由日本引入中国以来,以其丰产、质优的显著特点受到果农和消费者的普遍欢迎和认可[17]。珍富是2001富士的红色芽变,发现于山东省栖霞市,并于2021年8月24日获得品种登记证书。珍富与2001 富士果皮颜色都为条红类型,但珍富果皮表现出了更明显的红色,这种果皮色泽上的明显优势受到苹果种植户的重视,并在一定区域内得到了快速发展。然而珍富与2001 富士相比,除了明显的果皮颜色差异外,在果实品质、花青苷类物质组分及含量、花青苷合成相关基因的表达方面是否存在差异仍需进一步明确,为珍富苹果的示范推广提供数据支撑,也为进一步解析其果皮着色机制提供基础。
1 材料和方法
1.1 材料
2001 富士和珍富芽变苹果果实试材均采自山东省栖霞市庄园街道谢家沟村,两品种的砧木(八棱海棠)、栽培管理措施、果袋类型、套袋及摘袋时间均保持一致,2001富士及珍富果实采收时间见表1。
表1 2001 富士及珍富果实采收时间
Table 1 Harvesting date of Fuji 2001 and Zhenfu apple
?
1.2 方法
1.2.1 果实采摘 选取长势良好、树龄一致的苹果树,在树冠外围和内膛不同方向均匀采收。每个品种选择大小一致、无病虫害及磕碰伤的果实,随机分为3 组,每组30 个果实。第1 组果实于采摘当天进行果实性状相关指标测定;第2组果实在(25±1)℃、相对湿度85%~90%环境条件下贮藏14 d 后再进行果实相关指标测定。第3组果实在田间将果皮用液氮速冻后装入液氮罐中,于-80 ℃超低温冰箱中保存,测定时液氮冷冻研磨成粉末,用于花青苷含量及基因表达分析。
1.2.2 果实性状测定 采用淀粉-碘染色法[18]测定苹果成熟度;采用syntek 电子数显游标卡尺测定每个果实的纵横径,计算为果形指数;日本岛津TXB222L型电子天平测定每个果实单果质量;使用TMS-PRO 质构仪(圆盘探头直径为75 mm)测定果实破裂力和硬度;使用PR-101α 折光仪(日本ATAGO)测定可溶性固形物含量,采用CR-400色差计测定果皮颜色,所用光源为D65,分别测定果皮的亮度(L*)和饱和度(C*);可滴定酸含量采用酸碱滴定法测定,所用仪器为瑞士万通808电位滴定仪;维生素C 含量采用2,6-二氯靛酚滴定法测定,所用仪器为瑞士万通808 电位滴定仪;采用PAL-1 数显折光仪测定果实汁液中的可溶性固形物含量;固酸比以可溶性固形物含量和总酸含量的比例表示。以上每个指标测定30个果实。
1.2.3 花青苷提取及含量测定 取果皮样品1.00 g,经液氮研磨成匀浆,溶解于5 mL 的HCl-甲醇(0.5∶99.5,φ)溶液中,4 ℃及黑暗条件下提取24 h,然后在4 ℃条件下,12 000 r·min-1离心10 min,再过0.22µm 有机相滤膜,将1.5 mL 上清液转移至自动进样瓶中,用美国Waters HPLC 高效液相色谱仪检测。检测波长530 nm,柱温35 ℃,流速1.0 mL·min-1,进样5µL,梯度洗脱,溶液A(甲醇)和溶液B(10%甲酸水溶液)。洗脱条件如下:0 min,溶液A 17%,溶液B 83%;1 min,溶液A 17%,溶液B 83%;8 min,溶液A 35%,溶液B 65%;25 min,溶液A 37%,溶液B 63%;30 min,溶液A 17%,溶液B 83%。
1.2.4 RNA 提取和cDNA 第一条链合成 采用Trizol 提取试剂盒提取试验苹果果皮的总RNA。以2 种试材的RNA 为模板,使用第一链cDNA 合成试剂盒(RevertAid Premium Reverse Transcriptase)(Thermo Scientific™EP0733)将RNA 反转录成cDNA,用于后续研究。
1.2.5 引物设计及qRT-PCR 采用Step-one型荧光定量PCR 仪(ABI),反应体系为SYBR Green qPCR Master Mix 10 µL,cDNA 模板2 µL,上下游引物各0.4µL,ddH2O 7.2µL;扩增程序为95 ℃预变性3 min;95 ℃变性7 s,57 ℃退火10 s,72 ℃延伸15 s,共40 个循环。苹果花青素合成相关基因MdPAL、MdC4H、Md4CL、MdCHS、MdCHI、MdF3H、MdDFR、MdLDOX、MdANS、MdUFGT、MdMYB1、MdMYB10、MdMYB11、MdERF3、MdbZIP44、MdActin 的定量PCR引物序列见表2。
表2 实时荧光定量PCR 引物
Table 2 Primer seqnence used in qRT-PCR

?
1.2.6 数据分析 采用Excel 2010进行数据处理,采用DPS 18.10进行差异显著性分析,各图表中不同小写字母分别表示各处理在5%水平上的差异显著性。
2 结果与分析
2.1 2001 富士和珍富芽变苹果果实成熟期及外观比较
2001富士和珍富芽变苹果均属于晚熟品种,在所有栽培措施一致的情况下,果实完全成熟时确定为采收日期,与2001 富士相比,珍富果实采收日期提前了6 d,这表明果实发育期缩短了6 d(表1)。2001富士和珍富芽变苹果均为“条红”型苹果,但珍富果皮呈现更加明显的红色(图1)。采收期,珍富果皮亮度L*值及果皮颜色饱和度C*值均显著高于2001 富士的果皮。贮藏14 d 后,两种苹果果皮L*值及C*值均呈上升趋势,证实果皮颜色能够在贮藏过程中发生变化,且珍富果皮亮度L*值及果皮颜色饱和度C*值仍显著高于2001富士,这表明贮藏期珍富的色泽特性优于2001富士(图2)。2001富士和珍富果实的单果质量及纵横径比方面无显著差异,二者均呈圆形或近圆形,纵横径比即果形指数分别为0.90、0.88(图3)。

图1 2001 富士及珍富果实外观及颜色对比
Fig.1 Fruit appearance and colour comparison of Fuji 2001 and Zhenfu
A.2001 富士果实外观;B.珍富果实外观;C.2001 富士田间结果状;D.珍富田间结果状。
A.Fruit appearance of Fuji 2001;B.Fruit appearance of Zhenfu;C.Fruit of Fuji 2001 in the field;D.Fruit of Zhenfu in the field.
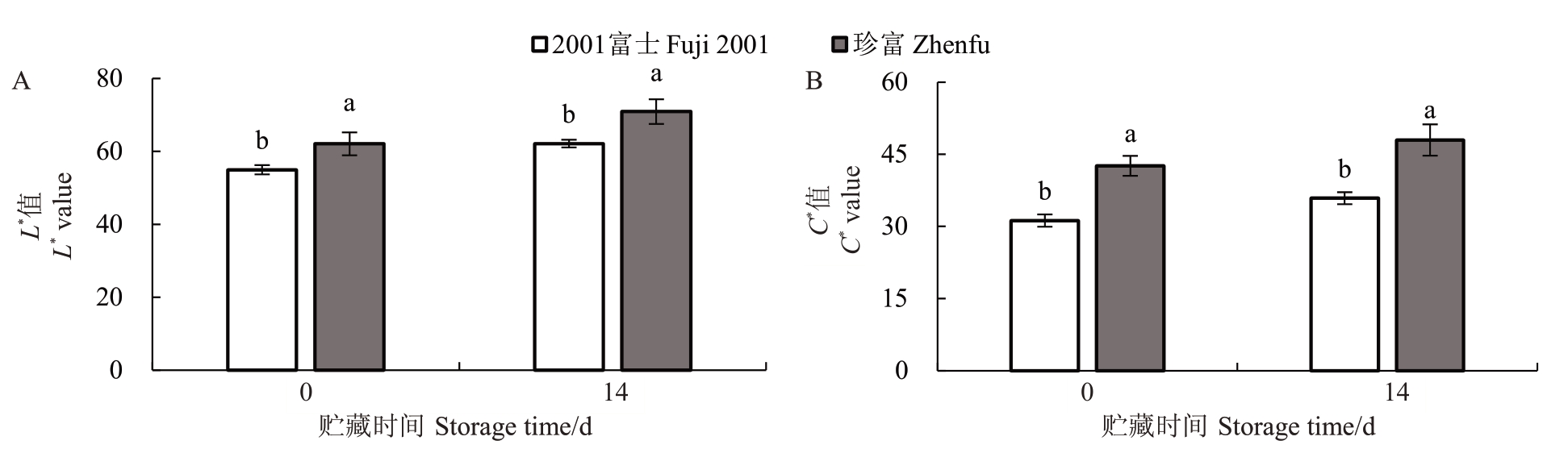
图2 2001 富士及珍富果皮亮度及饱和度比较
Fig.2 Peel brightness and saturation comparison of Fuji 2001 and Zhenfu
图中不同小写字母表示差异显著(p<0.05)。下同。
Different small letters represent significant difference at p<0.05.The same below.

图3 2001 富士及珍富果实单果质量及果形指数比较
Fig.3 Single fruit mass and fruit shape index of Fuji 2001 and Zhenfu
2.2 2001富士和珍富果皮花青苷类物质含量比较
苹果果皮色泽与果皮花青苷含量密切相关,进一步测定果皮花青素含量发现,采收期在2001富士和珍富果皮中都检测到了3 种花青苷类物质,分别为矢车菊素-3-半乳糖苷、矢车菊素-3-O-葡萄糖苷及花色素鼠李糖苷。其中,矢车菊素-3-半乳糖苷含量最高,是2001富士和珍富果皮中主要的花青苷组成成分。珍富果皮中矢车菊素-3-半乳糖苷、矢车菊素-3-O-葡萄糖苷及花色素鼠李糖苷含量分别是2001富士果皮中的2.21 倍、1.98 倍、1.60 倍,花青苷总量是2001富士果皮中的2.18倍。贮藏14 d后,2001富士和珍富果皮中花青苷含量均呈上升趋势。珍富果皮中3种花青苷含量及花青苷总量仍高于2001富士(表3)。
表3 2001 富士和珍富果皮花青苷类物质含量比较
Table 3 Comparison of anthocyanins contents in peel of Fuji 2001 and Zhenfu apple(mg·kg-1)
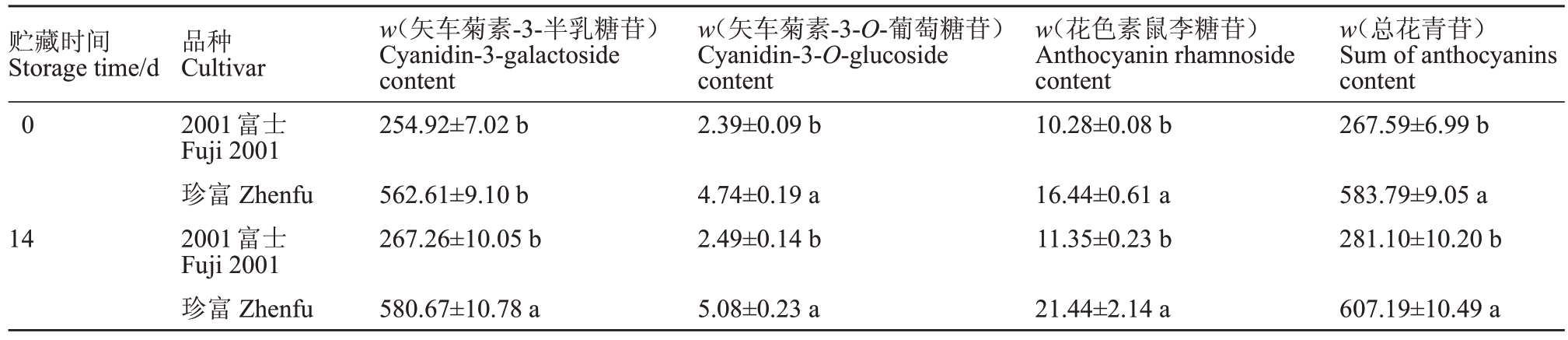
注:表中不同小写字母表示差异显著(p<0.05)。下同。
Note:Different small letters represent significant difference at p<0.05.The same below.
?
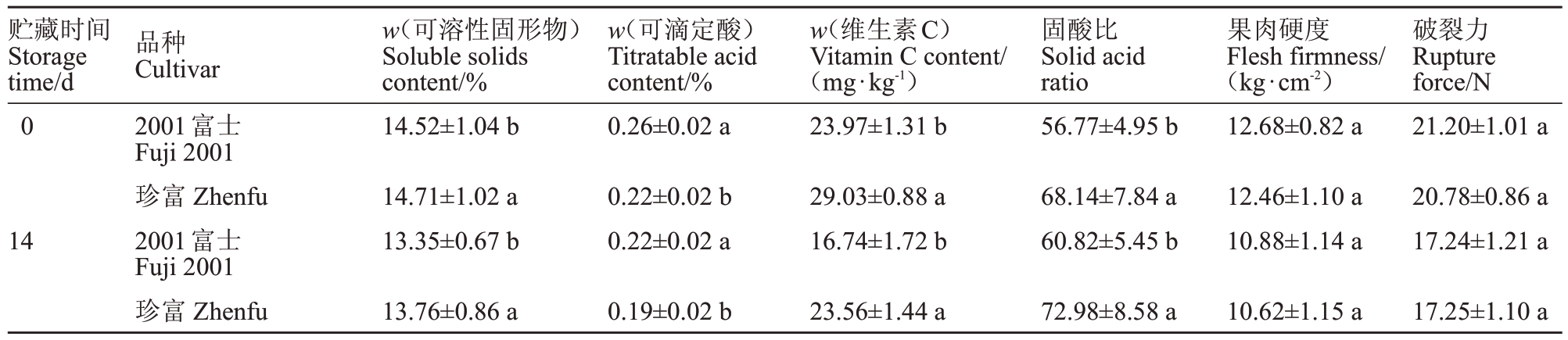
表4 2001 富士和珍富果实品质比较
Table 4 Quality comparison of Fuji 2001 and Zhenfu apple
?
2.3 2001富士和珍富果实内在品质比较
如图4 所示,采收期珍富果实的可溶性固形物含量在5%水平上显著高于2001 富士,而其可滴定酸含量也显著低于2001富士;珍富果实的维生素C含量及固酸比分别是2001富士的1.21、1.20倍;果肉硬度、破裂力方面二者无显著差异。贮藏14 d后,二者可溶性固形物含量分别降低8.06%、6.46%,但珍富果实的可溶性固形物含量显著高于2001富士,且其可滴定酸含量仍显著低于2001富士;二者维生素C含量都显著降低,但珍富仍是2001富士的1.41倍;二者固酸比分别提高了6.66%、6.63%,珍富是2001富士的1.20 倍;二者果肉硬度、破裂力均降低,且无显著差异。
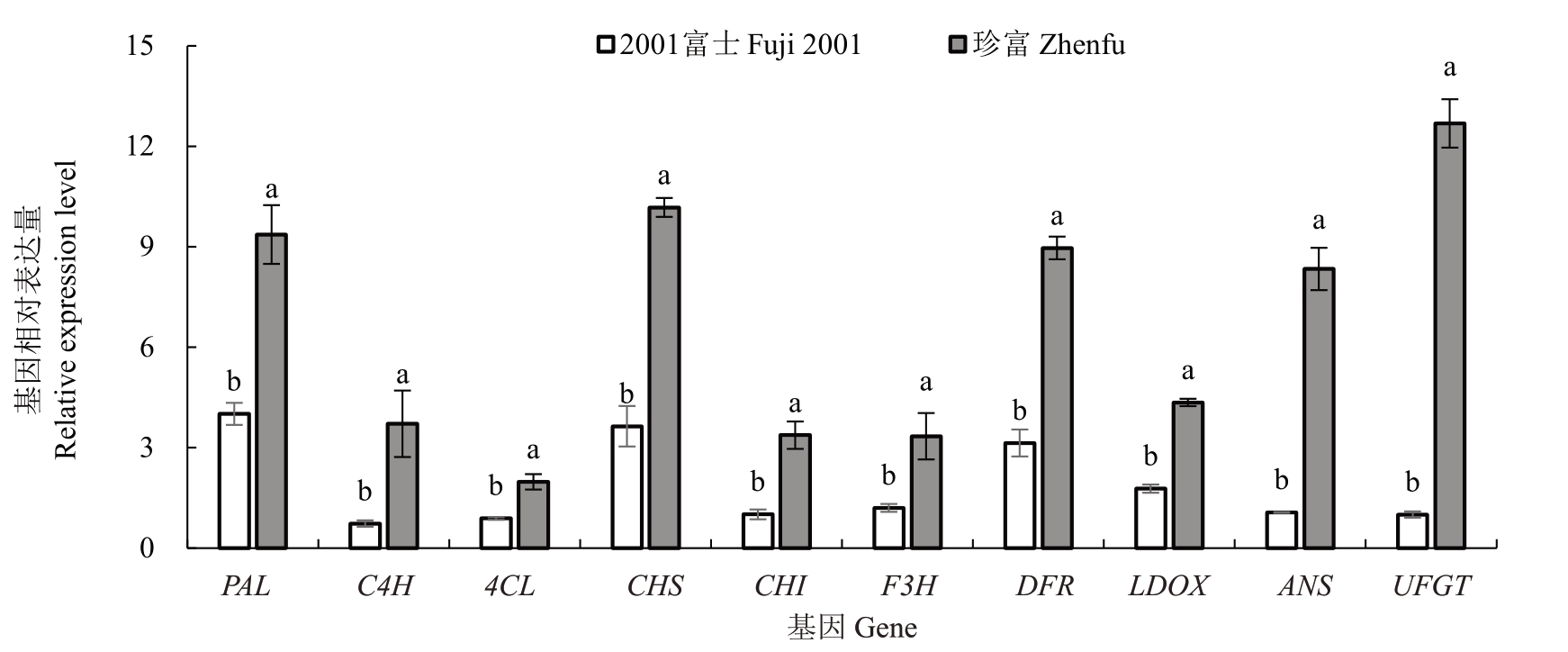
图4 花青苷合成途径中关键结构基因在2001 富士和珍富果皮中表达量比较
Fig.4 Comparison of expression levels of key structural genes in anthocyanin biosynthesis pathway in Fuji 2001 and Zhenfu
2.4 2001 富士和珍富果皮花青苷类物质合成途径相关基因表达量比较
花青苷合成途径中的关键结构基因在2001富士和珍富中出现了不同的表达模式。其中,珍富果皮中MdPAL、Md4CL、MdCHS、MdCHI、MdF3H、MdDFR、MdLDOX 表达量提高幅度较小,分别是2001 富士的2.34、2.24、2.80、3.35、2.78、2.85、2.45 倍,MdC4H、MdANS、MdUFGT表达量提高幅度更显著,分别是2001富士的5.07倍、7.83倍、12.65倍(图4)。
除了以上关键的结构基因外,转录因子也是影响花青苷合成的关键因素。因此,笔者挑选了部分已报道的正向调控花青苷合成的转录因子进行了表达量分析,发现MdMYB10、MdMYB1、MdMYB11、MdERF3、MdbZIP44 转录因子基因表达量在珍富果皮中均显著提高。其中,珍富果皮中MdMYB10、MdMYB11、MdERF3 表达量提高更显著,分别是2001富士的3.61倍、6.37倍、10.00倍(图5)。
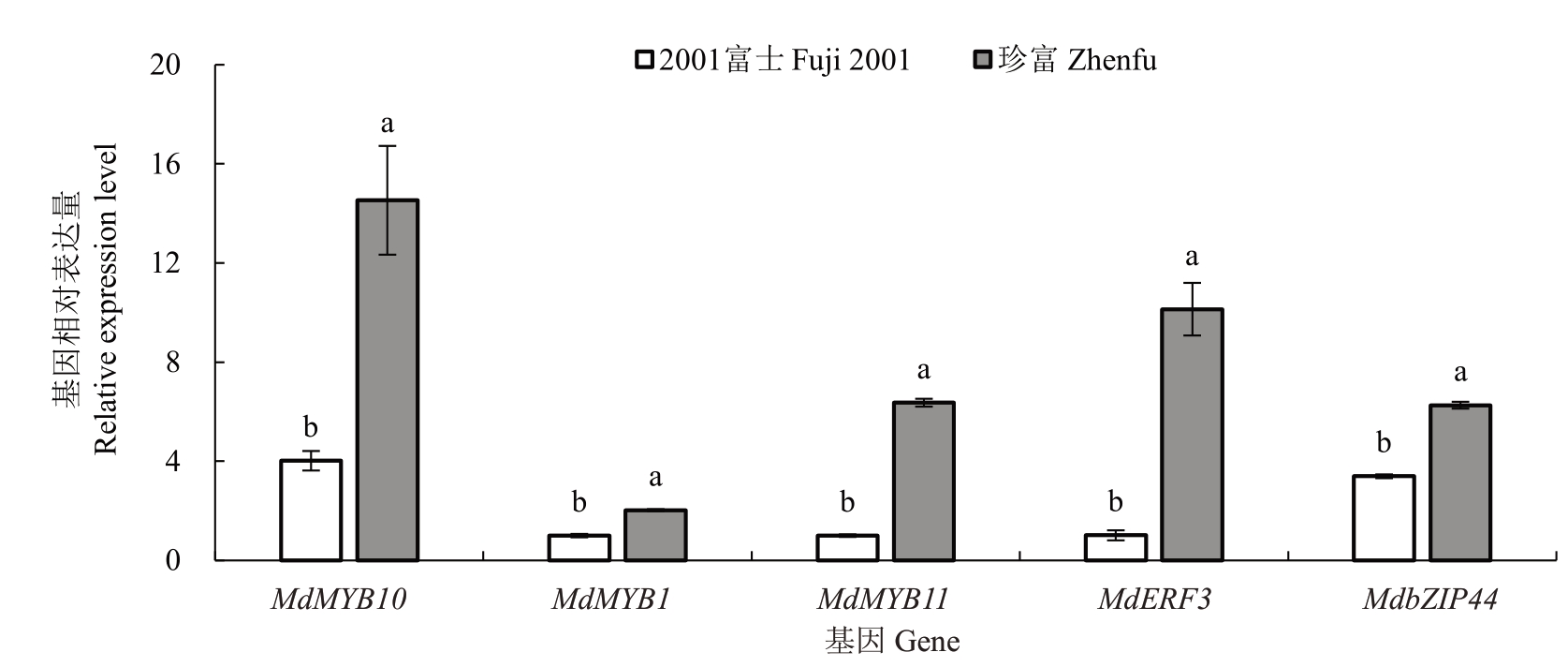
图5 花青苷合成调控转录因子基因在2001 富士和珍富中表达量比较
Fig.5 Comparison of expression levels of transcription factor genes regulating anthocyanin biosynthesis in Fuji 2001 and Zhenfu
3 讨 论
红富士在我国苹果产业独占鳌头(占比约70%),以红富士为主的品种结构使我国成为世界上最大的苹果生产国和消费国[20]。富士系苹果是经芽变选种的育种手段培育的系列品种,芽变选种的突出特点是优中选优,经此育种手段选育的长富2号、富士3号、富士8号、龙富等富士系苹果品种,占据苹果市场的很大比例,一代又一代新的红色优质芽变品种的出现有效推动了苹果产业的发展[21],探究芽变品种果实品质的形成机制对改善苹果品质、推动苹果产业发展具有重要意义。
2001富士属于红富士的红色和短枝芽变品种,其晚熟和耐贮这2 个性状没有改变,满足了我国的市场需求[22-23]。笔者在本研究中选择的芽变品种珍富的果实采收日期及盛花期至采收期时间较2001富士均明显缩短,证实珍富较2001 富士提早成熟,而这种提早成熟可能与乙烯正调控因子MdERF3有关,据报道ERF家族转录因子在植物生长中起着重要作用,参与调节植物对激素、胁迫、果实成熟的反应并调控花青素合成[24]。在本研究中,珍富果实中MdERF3 转录因子基因相较于2001 富士上调表达10 倍,因此笔者推测珍富的提早成熟可能与MdERF3基因的较高表达有关。
在内在品质方面,采收期珍富的可溶性固形物、维生素C含量及固酸比均显著高于2001富士,而可滴定酸含量显著低于2001富士,果肉硬度、破裂力均无显著差异;在外在品质方面,珍富果皮亮度L*值及果皮颜色饱和度C*值均显著高于2001富士的果皮,二者的单果质量、果形指数均无显著差异。以上结果证实,采收期不论是内在还是外在品质珍富都要优于2001富士;且两种苹果分别贮藏14 d后,上述差异仍存在,但二者除了固酸比升高外,可溶性固形物含量、硬度等其他指标都有所降低,这也证实苹果在采收后仍进行着一系列的生命活动,即苹果的后熟软化,苹果作为典型的呼吸跃变型果实,其在后熟过程中呼吸作用强度骤然升高,生成大量乙烯,同时可溶性糖等营养物质消耗增多[25];贮藏中构成果实细胞壁的果胶质、纤维素等物质降解加速了细胞壁结构的松弛,也是贮藏期果实硬度下降的原因之一[26]。
果实色泽也是苹果重要的外在商品性状,它直接影响苹果的市场竞争力[27-29]。苹果果皮红色主要由花青苷决定,花青苷在人类健康方面也扮演着重要的角色,具有抗氧化、提高记忆力、增进视力、预防肺部疾病和抗肿瘤等多种生理功能[3]。在本研究中,采收期及贮藏14 d 时,2001 富士和珍富果皮中都检测到了3 种花青苷类物质。其中,含量最高的为矢车菊素-3-半乳糖苷,该结果与其他报道中发现的苹果果皮中含量最多的花青苷种类是矢车菊-3-半乳糖苷的结果一致[19,30]。
目前关于苹果果皮花青苷合成基因在不同苹果果实、不同环境中的表达模式的研究已见诸多报道[29]。笔者在本研究中选取的10 个已报道与花青苷合成相关的结构基因,均在珍富果皮上调表达,这也证实上述结构基因的上调表达促进了其花青苷的合成积累,而这些结构基因的上调表达与MYB1、MYB10、MYB11 等MYB 类转录因子的调控相关[12-14]。另有研究证实,ERF家族转录因子也可以直接激活结构基因的启动子调控花青素生物合成,例如MdERF109 通过直接结合花青素结构基因MdCHS、MdUFGT 以及调节基因MdbHLH3 启动子并激活其转录,促进花青素生物合成[31]。ERF 家族转录因子不仅能够直接激活结构基因的启动子,还能够与MYB 类转录因子结合,通过MdERF3 依赖的途径促进乙烯的生物合成,从而增强乙烯途径对激活MdMYB1自身转录活性的促进作用,进而加速花青素生物合成[32]。值得注意的是,笔者在本研究中发现,珍富果皮中MdMYB10、MdMYB11表达量分别是2001富士的3.61、6.37倍,这也暗示珍富芽变后也可能存在类似的影响花青苷合成的调控模式,即MdERF3 上调表达后与MdMYB10、MdMYB11 结合相互作用,进而促进珍富果皮中花青苷合成结构基因的表达及含量的积累,但这种调控模式是否存在及其具体调控花青苷合成作用机制还需进一步解析验证。
4 结 论
采收期及贮藏14 d 后,红色芽变品种珍富果皮亮度L*值及果皮颜色饱和度C*值均显著高于2001富士;珍富可溶性固形物、维生素C含量及固酸比显著高于2001 富士,可滴定酸含量显著低于2001 富士,二者果肉硬度及破裂力无显著差异;珍富果皮中花青苷总量显著高于2001富士;珍富花青苷合成途径中的关键结构基因及MYB、EFR、bZIP44 等转录调控基因均显著上调表达。
[1] OLIVARES-SOTO H,BASTÍAS R M,CALDERÓN-ORELLANA A,LÓPEZ M D. Sunburn control by nets differentially affects the antioxidant properties of fruit peel in‘Gala’and‘Fuji’apples[J]. Horticulture,Environment,and Biotechnology,2020,61(2):241-254.
[2] 张媛雯,赵琳,谭伟.植物原花青素的应用研究进展[J].中国果菜,2023,43(7):24-28.ZHANG Yuanwen,ZHAO Lin,TAN Wei. Application progress of plant proanthocyanidins[J].China Fruit&Vegetable,2023,43(7):24-28.
[3] CÂMARA J S,LOCATELLI M,PEREIRA J A M,OLIVEIRA H,ARLORIO M,FERNANDES I,PERESTRELO R,FREITAS V,BORDIGA M. Behind the scenes of anthocyanins-from the health benefits to potential applications in food,pharmaceutical and cosmetic fields[J].Nutrients,2022,14(23):5133.
[4] FANG X,ZHANG L Z,SHANGGUAN L F,WANG L J. Md-MYB110a,directly and indirectly,activates the structural genes for the ALA-induced accumulation of anthocyanin in apple[J].Plant Science,2023,326:111511.
[5] SHI C Y,LIU L,WEI Z F,LIU J W,LI M,YAN Z L,GAO D T.Anthocyanin accumulation and molecular analysis of correlated genes by metabolomics and transcriptomics in sister line apple cultivars[J].Life,2022,12(8):1246.
[6] 王龙,王芳,汤蕾,童盼盼,张亚若,王江波.新疆红肉苹果不同时期果皮花青苷含量变化及其合成相关基因表达[J].江西农业学报,2021,33(8):6-10.WANG Long,WANG Fang,TANG Lei,TONG Panpan,ZHANG Yaruo,WANG Jiangbo.Changes of anthocyanin content and expression of synthesis-related genes in peel of Xinjiang red flesh apples in different periods[J].Acta Agriculturae Jiangxi,2021,33(8):6-10.
[7] ZHENG J,LIU L B,TAO H H,AN Y Y,WANG L J.Transcriptomic profiling of apple calli with a focus on the key genes for ALA-induced anthocyanin accumulation[J]. Frontiers in Plant Science,2021,12:640606.
[8] 许海峰.bHLH33 与MYB 抑制子参与苹果花青苷生物合成的分子机理[D].泰安:山东农业大学,2020.XU Haifeng. The molecular mechanism underlying anthocyanin biosynthesis in apple using bHLH33 and MYB repressors[D].Tai’an:Shandong Agricultural University,2020.
[9] WANG S,ZHANG Z,LI L X,WANG H B,ZHOU H,CHEN X S,FENG S Q. Apple MdMYB306-like inhibits anthocyanin synthesis by directly interacting with MdMYB17 and Mdb-HLH33[J].The Plant Journal,2022,110(4):1021-1034.
[10] LI W F,NING G X,ZUO C W,CHU M Y,YANG S J,MA Z H,ZHOU Q,MAO J,CHEN B H. MYB_SH [AL] QKY [RF]transcription factors MdLUX and MdPCL-like promote anthocyanin accumulation through DNA hypomethylation and MdF3H activation in apple[J].Tree Physiology,2021,41(5):836-848.
[11] 姜生辉.DNA 甲基化修饰MdMYB1 启动子调控苹果花青苷转运的机理[D].泰安:山东农业大学,2020.JIANG Shenghui.Mechanism of DNA methylation modifies Md-MYB1 promoter to regulate anthocyanin transport in apple[D].Tai’an:Shandong Agricultural University,2020.
[12] 马长青.苹果MdMYB1 启动子甲基化对套袋果实着色的调控研究[D].杨凌:西北农林科技大学,2019.MA Changqing. Regulation of the methylation of the MdMYB1 promoter on fruit coloration of Malus after bagging treatment[D].Yangling:Northwest A&F University,2019.
[13] 王美丽. MYB10 不同基因型对苹果果皮颜色的影响[D]. 拉萨:西藏大学,2020.WANG Meili.Effects of MYB10 different genotypes on the color of apple fruit skin[D].Lasa:Tibet University,2020.
[14] 安秀红.苹果MdTTG1、MdMYB9 与MdMYB11 基因调控花青苷合成的作用机制研究[D].泰安:山东农业大学,2013.AN Xiuhong. Studies on the mechanism by which MdTTG1,MdMYB9 and MdMYB11 Regulate anthocyanin biosythesis in apple[D].Tai’an:Shandong Agricultural University,2013.
[15] AN J P,YAO J F,XU R R,YOU C X,WANG X F,HAO Y J.Apple bZIP transcription factor MdbZIP44 regulates abscisic acid-promoted anthocyanin accumulation[J]. Plant,Cell & Environment,2018,41(11):2678-2692.
[16] 毕思琦,安建平,王小非,郝玉金,芮麟,李彤,韩月彭,由春香.苹果乙烯响应因子MdERF3 促进花青苷和原花青苷积累[J].园艺学报,2019,46(12):2277-2285.BI Siqi,AN Jianping,WANG Xiaofei,HAO Yujin,RUI Lin,LI Tong,HAN Yuepeng,YOU Chunxiang. Ethylene response factor MdERF3 promotes anthocyanin and proanthocyanidin accumulation in apple[J]. Acta Horticulturae Sinica,2019,46(12):2277-2285.
[17] 安佰娟,杨小奎,路超.2001 富士苹果的矮化密植栽培技术要点[J].落叶果树,2020,52(5):79-80.AN Baijuan,YANG Xiaokui,LU Chao. Key points of dwarfing and close planting techniques of Fuji 2001[J].Deciduous Fruits,2020,52(5):79-80.
[18] 卫世乾.不同成熟度苹果中多酚类物质的含量研究[J].许昌学院学报,2019,38(2):99-102.WEI Shiqian. Study on the contents of polyphenols in apples with different maturity[J].Journal of Xuchang University,2019,38(2):99-102.
[19] TAKOS A M,UBI B E,ROBINSON S P,WALKER A R. Condensed tannin biosynthesis genes are regulated separately from other flavonoid biosynthesis genes in apple fruit skin[J]. Plant Science,2006,170(3):487-499.
[20] 陈学森,李秀根,毛志泉,王楠,张宗营,马锋旺,丛佩华,张玉刚,郭黄萍,王志刚,姜召涛,徐月华.新种质创造支撑果品产业升级:红肉苹果和‘库尔勒香梨’种质资源利用以及‘红富士’芽变选种案例分析[J].果树学报,2021,38(1):128-141.CHEN Xuesen,LI Xiugen,MAO Zhiquan,WANG Nan,ZHANG Zongying,MA Fengwang,CONG Peihua,ZHANG Yugang,GUO Huangping,WANG Zhigang,JIANG Zhaotao,XU Yuehua. Fruit industry upgrading supported by new germplasm creation:Case study on the utilization of germplasm resources of red-fleshed apple and‘Kuerlexiangli’pear and the sports selection of‘Red Fuji’[J]. Journal of Fruit Science,2021,38(1):128-141.
[21] 陈学森,伊华林,王楠,张敏,姜生辉,徐娟,毛志泉,张宗营,王志刚,姜召涛,徐月华,李建明.芽变选种推动世界苹果和柑橘产业优质高效发展案例解读[J]. 中国农业科学,2022,55(4):755-768.CHEN Xuesen,YI Hualin,WANG Nan,ZHANG Min,JIANG Shenghui,XU Juan,MAO Zhiquan,ZHANG Zongying,WANG Zhigang,JIANG Zhaotao,XU Yuehua,LI Jianming. Interpretation of the case of bud sports selection to promote the high-quality and efficient development of the world’s apple and Citrus industry[J].Scientia Agricultura Sinica,2022,55(4):755-768.
[22] 陈学森,毛志泉,王志刚,王楠,张宗营,姜生辉,姜召涛,徐月华,东明学,李建明,隋秀奇.持续多代芽变选种及其芽变机理揭开‘红富士’在我国苹果产业独占鳌头的谜底[J].中国果树,2020(3):1-5.CHEN Xuesen,MAO Zhiquan,WANG Zhigang,WANG Nan,ZHANG Zongying,JIANG Shenghui,JIANG Zhaotao,XU Yuehua,DONG Mingxue,LI Jianming,SUI Xiuqi.Continuous multigenerational sports selection and its mechanism reveals the mystery of‘Red Fuji’in China’s apple industry[J]. China Fruits,2020(3):1-5.
[23] 宋来庆,赵玲玲,刘美英,唐岩,孙燕霞,李元军,姜中武.富士苹果芽变选种特点及影响因素分析[J]. 中国果菜,2016,36(2):54-56.SONG Laiqing,ZHAO Lingling,LIU Meiying,TANG Yan,SUN Yanxia,LI Yuanjun,JIANG Zhongwu. Analysis of main features and influencing factors of Fuji apple sport selection[J].China Fruit&Vegetable,2016,36(2):54-56.
[24] AN J P,ZHANG X W,BI S Q,YOU C X,WANG X F,HAO Y J. The ERF transcription factor MdERF38 promotes drought stress-induced anthocyanin biosynthesis in apple[J]. The Plant Journal,2020,101(3):573-589.
[25] LIU M C,PIRRELLO J,CHERVIN C,ROUSTAN J P,BOUZAYEN M. Ethylene control of fruit ripening:Revisiting the complex network of transcriptional regulation[J].Plant Physiology,2015,169(4):2380-2390.
[26] CORNUAULT V,POSÉ S,KNOX J P.Disentangling pectic homogalacturonan and rhamnogalacturonan-I polysaccharides:Evidence for sub-populations in fruit parenchyma systems[J].Food Chemistry,2018,246:275-285.
[27] 邓秀新,束怀瑞,郝玉金,徐强,韩明玉,张绍铃,段常青,姜全,易干军,陈厚彬.果树学科百年发展回顾[J].农学学报,2018,8(1):24-34.DENG Xiuxin,SHU Huairui,HAO Yujin,XU Qiang,HAN Mingyu,ZHANG Shaoling,DUAN Changqing,JIANG Quan,YI Ganjun,CHEN Houbin. Review on the centennial development of pomology in China[J]. Journal of Agriculture,2018,8(1):24-34.
[28] 束怀瑞,陈修德. 我国果树产业发展的时代任务[J]. 中国果树,2018(2):1-3.SHU Huairui,CHEN Xiude. The current task of the development of fruits industry in China[J].China Fruits,2018(2):1-3.
[29] 王金政,毛志泉,丛佩华,吕德国,马锋旺,任小林,束怀瑞,李保华,郭玉蓉,郝玉金,姜远茂,张新忠,杨欣,曹克强,赵政阳,韩振海,霍学喜,魏钦平.新中国果树科学研究70 年:苹果[J].果树学报,2019,36(10):1255-1263.WANG Jinzheng,MAO Zhiquan,CONG Peihua,LÜ Deguo,MA Fengwang,REN Xiaolin,SHU Huairui,LI Baohua,GUO Yurong,HAO Yujin,JIANG Yuanmao,ZHANG Xinzhong,YANG Xin,CAO Keqiang,ZHAO Zhengyang,HAN Zhenhai,HUO Xuexi,WEI Qinping. Fruit scientific research in New China in the past 70 years:Apple[J]. Journal of Fruit Science,2019,36(10):1255-1263.
[30] MAZZA G,VELIOGLU Y S. Anthocyanins and other phenolic compounds in fruits of red-flesh apples[J]. Food Chemistry,1992,43(2):113-117.
[31] MA H Y,YANG T,LI Y,ZHANG J,WU T,SONG T T,YAO Y C,TIAN J. The long noncoding RNA MdLNC499 bridges Md-WRKY1 and MdERF109 function to regulate early-stage lightinduced anthocyanin accumulation in apple fruit[J]. The Plant Cell,2021,33(10):3309-3330.
[32] AN J P,WANG X F,LI Y Y,SONG L Q,ZHAO L L,YOU C X,HAO Y J.EIN3-LIKE1,MYB1,and ETHYLENE RESPONSE FACTOR3 act in a regulatory loop that synergistically modulates ethylene biosynthesis and anthocyanin accumulation[J].Plant Physiology,2018,178(2):808-823.