由解淀粉欧文氏菌(Erwinia amylovora)侵染引起的梨火疫病(pear fire blight)是蔷薇科仁果类果树生产中最具毁灭性的细菌病害[1]。2016年在中国新疆首次发现该病害,2020 年被列入中国《一类农作物病虫害名录》[2],2022 年被列入《重点管理外来入侵物种名录》[3]。该病害危害梨、苹果、山楂、榅桲等果树,尤其是在香梨上传播极为迅速。梨火疫病菌可侵染果树的花,并作为侵染源向叶片、嫩梢及幼果传染,同时修剪的枝干伤口也是其侵染的主要途径。病原菌一旦入侵植物体内,便可终身定殖,进而扩散蔓延至整个植株,同时迅速流行,难以控制和根除[4]。梨火疫病病情发生、蔓延态势严峻,目前已经在中国新疆和甘肃2 个省(自治区)70 个县、市(区)发生并造成严重危害[5]。如果不能采取有效防控措施遏制该病害的传播扩散,将对新疆乃至全国林果产业带来重大威胁。
生产上对梨火疫病的防治包括检疫、修剪和铲除病株、药剂防治、生物防治及选育抗病品种等措施[6-7]。但目前生产上缺乏抗梨火疫病的果树品种,通过移除发病枝干可以有效地阻止火疫病的传播,但严重影响果树产量;化学农药的大量使用引起的病菌抗药性、环境污染以及果实农药残留等问题日益突出。研究和实践表明,利用具有抗菌作用的有益微生物及其活性物质防治植物病害,能达到化学农药的良好防效,并且具有选择性强、不易产生抗性、安全高效等突出优势。近年来国外在梨火疫病生物防治的相关研究和应用中取得了一定进展。其中,美国研发的荧光假单胞菌(Pseudomonas fluorescens)A506、草生欧文氏菌(E. herbicola)C9-1等商品菌剂已得到了实际应用,防治效果接近于农用链霉素[8-9]。Zeller 等[10]分离获得的内生细菌菌株E.herbicola 89 对花腐的控制率达到70%。Ait 等[11]研究表明,在田间喷施Bacillus subtilis QST713,花腐率可降低66%。目前国内在针对梨火疫病生物防治方面的研究尚属起步阶段,徐琳赟等[12]从分离获得的梨树内生菌中筛选梨火疫病菌拮抗菌株Klebsiella sp.TN50、Paenibacillus sp.HN89 和Pseudomonas sp.SN37,通过喷施接种盆栽杜梨苗能够显著降低嫩枝的枝枯率和病情指数。鲁晏宏等[13]以杜梨苗为接种材料,通过土壤接种法测定了拮抗菌株对梨火疫病菌的防病效果,其中,Bacillus velezensis JE4 防效最佳,可超过73%。近期,吕天宇等[14]在植物酵素液分离细菌中筛选出的一株对梨火疫病有拮抗作用的菌株B.velezensis FX1,经优化后的发酵液能显著降低花腐率。
黏细菌是一类具有多细胞群体行为和复杂生活史的高等原核生物,能够以活的微生物细胞或其他高分子作为食物获取营养[15],同时产生丰富的具有抗菌活性的酶类和次生代谢产物[16],此外,黏细菌能够形成抗逆性强的子实体和黏孢子,从而使黏细菌具有良好的环境适应性和稳定的定殖能力[17],还有研究发现,黏细菌具有调控微生物群落结构的功能[18]。黏细菌的这些特性使其被视为一类具有重要生防潜力的新型生防微生物资源。近年来一些温室和田间试验表明,施用黏细菌能显著减轻苗木立枯病[19]、黄瓜枯萎病[18]、辣椒炭疽病[20]和稻瘟病[21]等病害的危害。目前关于黏细菌生防潜力的研究主要集中在植物病原真菌方面,相比之下只有少数的研究报道了黏细菌在植物细菌性病害生物防治中的应用潜力,李周坤课题组筛选出一株对胡萝卜软腐果胶杆菌(Pectobacterium carotovorum subsp. carotovorum)具有良好捕食特性的黏细菌菌株BS,能够显著降低马蹄莲软腐病的发病率[22];Dong 等[23]筛选出一株对茄科罗尔斯通氏菌(Ralstonia solanacearum)具有较强捕食能力的黄色黏球菌(Myxococcus xanthus)R31,盆栽试验结果表明,菌株R31对番茄青枯病的防效达到81.9%。然而这些研究目前也仅限于细菌性土传病害,而关于黏细菌对如梨火疫病等主要在植株地上部传播和危害的细菌性病害的生防潜力评估还鲜见报道。
笔者基于前期建立的黏细菌小型菌种资源库,针对梨火疫病菌通过平板捕食试验筛选出具有较强捕食能力的黏细菌菌株,进一步通过离体花序和盆栽杜梨苗测定黏细菌菌株对梨火疫病的生防效果。研究结果将为发掘黏细菌生防资源、探索梨火疫病生物防治新途径奠定科学基础。
1 材料和方法
1.1 材料
1.1.1 黏细菌菌株 笔者课题组在前期研究中从新疆喀什地区、阿克苏地区、巴音郭楞蒙古自治州、昌吉回族自治州等地采集农田土壤样品,采用兔粪诱导法、大肠杆菌诱导法和梨火疫病菌诱导法,从中分离、纯化出46株黏细菌纯培养物[24]。
1.1.2 供试病原菌 梨火疫病菌(E.amylovora)菌株Ea.017,来源于新疆库尔勒市的香梨分离物,由新疆农业大学农学院微生物实验室分离并保存。
1.1.3 培养基 NA 培养基、NB 培养基、LBS 培养基、VY/4 培养基、CYE 培养基、TPM 培养基参照王婷[25]的研究。
1.2 黏细菌捕食梨火疫病菌能力测定
1.2.1 黏细菌与病原菌的准备 挑取活化好的梨火疫病菌Ea.017 单菌落接入NB 培养液中,在30 ℃、160 r∙min-1恒温摇床中震荡培养24 h,12 000 r∙min-1,离心1 min,收集菌体,用无菌水漂洗3次后,重悬至109 cfu∙mL-1,备用。将黏细菌纯培养物在VY/4 平板上活化后,刮取适量黏细菌菌落转接至LBS培养液中,30 ℃、160 r∙min-1摇培3~4 d。得到的黏细菌菌悬液于12 000 r∙min-1离心1 min,去除上清液,收集菌体之后用无菌水漂洗3 次,将成团的黏细菌菌体充分打散,最后用无菌水重悬至OD600=1.0,备用。
1.2.2 菌苔捕食试验 参考Li 等[22]的方法在TPM无营养固体培养基上垂直接种100 μL 梨火疫病菌菌悬液,自然风干后将3 μL黏细菌悬液接种在梨火疫病菌菌苔中央,自然风干。以梨火疫病菌菌苔中央接种3 μL无菌水为对照,30 ℃恒温培养5 d,每株黏细菌3次重复。定时观察黏细菌的扩展情况。5 d后用无菌接种环刮下菌苔并用无菌水混匀,采用稀释涂布的方法,每个梯度取100 μL 于NA 平板上涂布均匀,平板放置于30 ℃恒温培养箱培养至单菌落长出,统计梨火疫病菌菌落数量,计算残留活菌数量,评估捕食能力。
1.2.3 对峙培养试验 在TPM 无营养固体培养基上垂直悬空接种100 μL梨火疫病菌菌悬液,自然风干,在梨火疫病菌菌苔边缘邻近位置接种3 μL黏细菌菌悬液,对照组接种3 μL 无菌水,待风干后置于30 ℃恒温培养箱培养5 d,3次重复。其间定时观察黏细菌运动方向及运动距离,5 d后用无菌接种环刮下菌苔并用无菌水混匀,采用稀释涂布的方法,每个梯度取100 μL 于NA 平板上涂布均匀,平板放置30 ℃恒温培养箱培养至单菌落长出,统计梨火疫病菌菌落数量,计算残留活菌数量,评估捕食能力。
1.3 黏细菌除菌发酵滤液的抑菌作用测定
将捕食梨火疫病菌能力较强的黏细菌菌株接种于LBS 液体培养基中,在30 ℃、160 r ∙min-1
摇床中震荡培养至稳定期。取菌液在4 ℃条件下,12 000 r ∙min-1 离心20 min,收集上清液,用0.22 μm微孔滤膜过滤除菌,获得除菌发酵滤液。取1 mL除菌发酵滤液与0.1 mL梨火疫病菌菌悬液(109 cfu∙mL-1)在30 ℃条件下静置共培养24 h后,在NA固体平板上稀释涂布,计算梨火疫病菌活菌数量。以LBS培养液与梨火疫病菌共培养为对照。
1.4 黏细菌对梨火疫病防效的生物测定
1.4.1 黏细菌和病原菌接种液的制备 将待测黏细菌菌株活化后接种至3 mL LBS 液体培养基中,30 ℃、160 r∙min-1摇培2~3 d后,接种至200 mL VY/4培养液中,30 ℃、160 r∙min-1摇培3~4 d,用移液枪将瓶底菌球充分打散后得到黏细菌接种液,备用。将梨火疫病菌Ea.017 活化,挑取单菌落接入NB 液体培养基中,在28 ℃、160 r∙min-1恒温摇床中震荡培养24 h至菌液OD600=1.0,用无菌水稀释至107 cfu∙mL-1作为接种液。
1.4.2 香梨离体花序接种 将梨园采集的花枝插入0.05% NaCl 溶液中保湿防腐。用手持压力喷雾器将待测黏细菌菌液喷雾接种梨花序,每个黏细菌菌株接种50 朵花序,3 次重复。在28 ℃、70%空气湿度的人工气候箱中培养24 h后喷雾接种梨火疫病菌菌悬液。将接种后的花序置于人工气候箱中28 ℃、70%空气湿度继续培养,于3、5和7 d后定时观察记录发病情况,统计花腐率、计算防效。同时设喷施农用链霉素(华北制药厂生产,有效成分72%)4000倍液对照,以无菌水代替黏细菌菌液作为对照。试验结束后将发病植株材料干热灭菌后销毁。花腐率/%=(病花数/总花数)×100;花腐防效/%=(对照花腐率-处理花腐率)/对照花腐率×100。
1.4.3 盆栽杜梨苗接种(1)黏细菌对梨火疫病的保护性防效:试验在温室中进行,选用2 年生盆栽杜梨苗为接种材料。用手持式压力喷雾器将待测黏细菌菌液喷雾至叶片及枝条完全湿润,24 h 后喷雾接种病原菌菌悬液,每个黏细菌菌株喷雾3 盆(每盆约20 个枝条),3 次重复。同时设农用链霉素(华北制药厂,有效成分72%)4000倍液对照和无菌水对照(即先喷施无菌水24 h 后再接种梨火疫病菌)。接种后的杜梨苗置于28~30 ℃、相对湿度70%的日光温室中培养。其间每天观察发病情况,记录发病枝条数、测定枝枯长度、枝枯长度占接种枝条长度的比例及发病级别,计算发病率和病情指数,统计防效。试验结束后将发病植株材料干热灭菌后销毁。
(2)黏细菌对梨火疫病的治疗性防效:在治疗性试验中黏细菌和病原菌的接种顺序与保护性试验相反,即先在杜梨苗上喷施接种梨火疫病菌菌液24 h后再喷施黏细菌菌液,其他试验材料、培养条件及防效调查方法等均与保护性试验一致。试验结束后将植株材料干热灭菌后销毁。参照李燕等[26]的方法,制定梨火疫病原菌接种盆栽杜梨苗的病情分级标准:0级,枝条无病斑;Ⅰ级,枝条病斑长度占接种枝条长度的1/3;Ⅲ级,枝条病斑长度占接种枝条长度的>1/3~2/3;Ⅴ级,枝条病斑长度占接种枝条长度的2/3以上。发病率/%=(发病枝条数/接种总枝条数)×100;病情指数/%=∑(各级发病枝条数×病级代表值)/(接种总枝条数×最高级值)×100;枝枯防效/%=(对照病情指数-处理病情指数)/对照病情指数×100。
1.5 黏细菌菌株的鉴定
1.5.1 形态和培养特征 根据Bergey’s Manual of Systematic Bacteriology[27]和《原核生物学》(第2版)[28]中的黏细菌分类标准,观察记录黏细菌子实体的颜色及形态、菌落特征,革兰氏染色后观察营养细胞和黏孢子形态,并据此对黏细菌进行初步鉴定。
1.5.2 16S rRNA 和lepA 基因序列测定及分析 采用细菌基因组提取试剂盒(TⅠANamp Bacteria DNA Kit,TⅠANGEN)提取黏细菌总DNA。以细菌通用引物27F(5’-AGAGTTTGATCCTGGCTCAG-3’)和1492R(5’- TACGGCTACCTTGTTACGACTT-3’)扩增16S rRNA 基因[29]。参照Stackebrandt 等[30]报道的 引 物 对BAUP1(5’-CATCGCCCACATCGAYCAYGGNAA-3’)和BⅠDN1:(5’-CATGTGCAGCAGGCCNARRAANCC-3’)扩增lepA 基因。PCR扩增体系(25 μL):2×SanTaq PCR Mix 12.5 μL,模板DNA 1 μL,引物对(10 μmol ∙L-1)各0.5 μL,ddH2O 10.5 μL。PCR 反应程序:94 ℃预变性5 min;94 ℃变性30 s,55 ℃复性30 s,72 ℃延伸60 s,35个循环;72 ℃延伸10 min。取PCR产物在10 g∙L-1的琼脂糖1×TAE缓冲系统电泳,检测合格后将PCR扩增产物送至生工生物工程(上海)股份有限公司测序。将所得序列在NCBⅠ数据库(https://www.ncbi.nlm.nih.gov/)中进行BLAST比对分析,选取与其相似度最高的模式菌株基因序列,利用MEGA7.0软件以邻接法(Neighbor-Joining)构建多基因系统发育树。
1.6 数据分析
利用SPSS Statistics 19.0 软件进行数据统计分析,使用Origin 2021 和Microsoft Excel 2019 绘制数据统计图。
2 结果与分析
2.1 菌苔捕食试验结果
以梨火疫病菌为靶标病原菌,对46株黏细菌的菌苔捕食能力进行测定,经过5 d后大部分黏细菌都能够完全扩散并覆盖整个病原细菌菌苔,并且在跨过的区域形成子实体。此时将整个菌落刮取,通过稀释涂布法于NA固体平板中统计梨火疫病菌的残留活菌数,发现所有供试黏细菌菌株均对梨火疫病菌具有捕食性,但不同黏细菌菌株捕食梨火疫病菌的能力存在明显差异,残留活菌数较对照组(9.7×108 cfu∙mL-1)下降至(3.7×103)~(2.4×107)cfu∙mL-1。其中黏细菌菌株WCH05 捕食梨火疫病菌的能力最强,残留活菌数下降至3.7×103 cfu∙mL-1,其次是菌株FB02 和WCH03,残留活菌数分别下降至2.0×104 cfu∙mL-1和3.8×104 cfu∙mL-1(表1)。
表1 不同黏细菌菌株捕食梨火疫病菌后的残留活菌数
Table 1 Survival of E.amylovora after preying by different myxobacteria
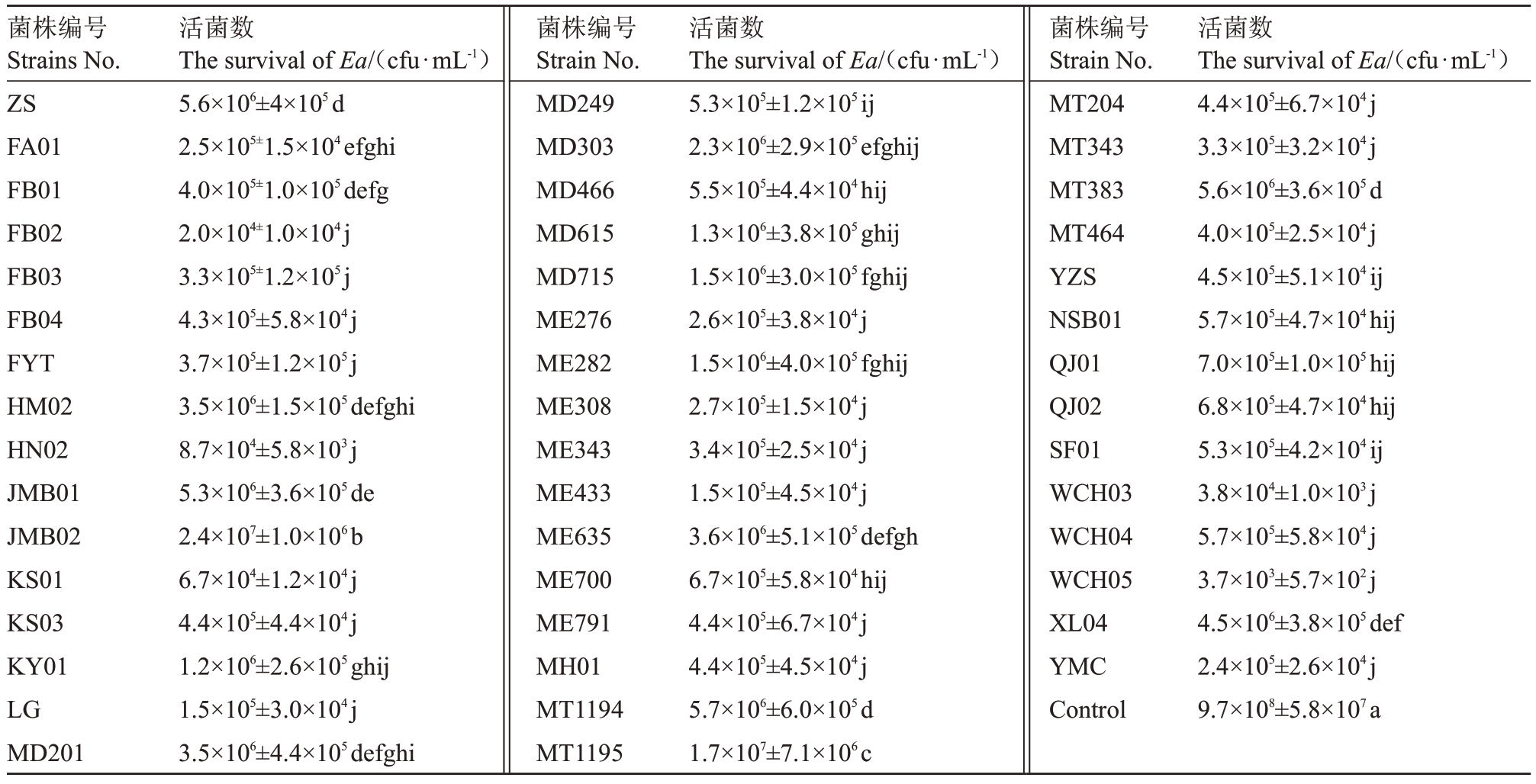
注:数据为平均值±标准误。不同小写字母表示在0.05 水平上差异显著。下同。
Note:Data were presented as mean±sx.Data with different small letters indicated significant difference at 0.05 level.The same below.
菌株编号Strains No.ZS FA01 FB01 FB02 FB03 FB04 FYT HM02 HN02 JMB01 JMB02 KS01 KS03 KY01 LG MD201活菌数The survival of Ea/(cfu∙mL-1)5.6×106±4×105d 2.5×105±1.5×104efghi 4.0×105±1.0×105defg 2.0×104±1.0×104j 3.3×105±1.2×105j 4.3×105±5.8×104j 3.7×105±1.2×105j 3.5×106±1.5×105defghi 8.7×104±5.8×103j 5.3×106±3.6×105de 2.4×107±1.0×106b 6.7×104±1.2×104j 4.4×105±4.4×104j 1.2×106±2.6×105ghij 1.5×105±3.0×104j 3.5×106±4.4×105defghi菌株编号Strain No.MD249 MD303 MD466 MD615 MD715 ME276 ME282 ME308 ME343 ME433 ME635 ME700 ME791 MH01 MT1194 MT1195活菌数The survival of Ea/(cfu∙mL-1)5.3×105±1.2×105ij 2.3×106±2.9×105efghij 5.5×105±4.4×104hij 1.3×106±3.8×105ghij 1.5×106±3.0×105fghij 2.6×105±3.8×104j 1.5×106±4.0×105fghij 2.7×105±1.5×104j 3.4×105±2.5×104j 1.5×105±4.5×104j 3.6×106±5.1×105defgh 6.7×105±5.8×104hij 4.4×105±6.7×104j 4.4×105±4.5×104j 5.7×106±6.0×105d 1.7×107±7.1×106c菌株编号Strain No.MT204 MT343 MT383 MT464 YZS NSB01 QJ01 QJ02 SF01 WCH03 WCH04 WCH05 XL04 YMC Control活菌数The survival of Ea/(cfu∙mL-1)4.4×105±6.7×104j 3.3×105±3.2×104j 5.6×106±3.6×105d 4.0×105±2.5×104j 4.5×105±5.1×104ij 5.7×105±4.7×104hij 7.0×105±1.0×105hij 6.8×105±4.7×104hij 5.3×105±4.2×104ij 3.8×104±1.0×103j 5.7×105±5.8×104j 3.7×103±5.7×102j 4.5×106±3.8×105def 2.4×105±2.6×104j 9.7×108±5.8×107a
2.2 对峙培养试验结果
为了进一步统计和评估菌苔捕食试验中捕食能力较强的黏细菌菌株的运动捕食能力,将菌苔捕食试验中捕食能力较强的黏细菌菌株WCH05、FB02和WCH03 与梨火疫病菌对峙培养,定时观察黏细菌的运动方向和扩展速度,发现3 株黏细菌均能向梨火疫病菌运动并且捕食梨火疫病菌,具有明显的趋向性。培养5 d后刮取菌苔并稀释涂布,计算梨火疫病菌残留活细胞数,结果表明,菌株WCH05 向外扩展捕食梨火疫病菌的能力最强,梨火疫病菌的残留活菌数较对照组(2.8×108 cfu∙mL-1)下降到2.6×103 cfu∙mL-1。其次是菌株WCH03 和FB02,梨火疫病菌残留活菌数分别下降至1.3×104 cfu∙mL-1和3.0×104 cfu∙mL-1(图1)。
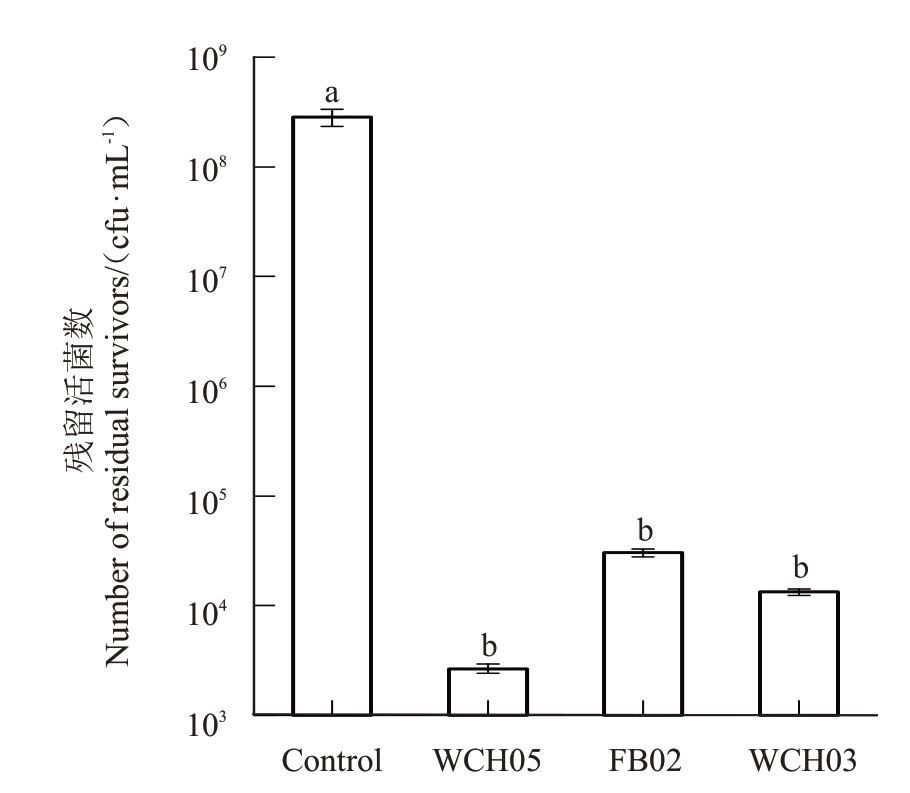
图1 在对峙培养中黏细菌对梨火疫病菌菌苔的捕食作用
Fig.1 Myxobacteria strains prey on Ea lawn in confront culture
2.3 黏细菌除菌发酵滤液对梨火疫病菌的抑菌作用
将黏细菌菌株WCH05、FB02和WCH03的除菌发酵滤液与梨火疫病菌共培养24 h,稀释涂布后测定梨火疫病菌残留活菌数。结果(图2)表明,菌株WCH05、FB02、WCH03的除菌发酵滤液对梨火疫病菌并无抑菌作用,残留活菌数与对照并无显著差异。说明菌株WCH05、FB02、WCH03 主要通过直接接触的方式捕食梨火疫病菌。
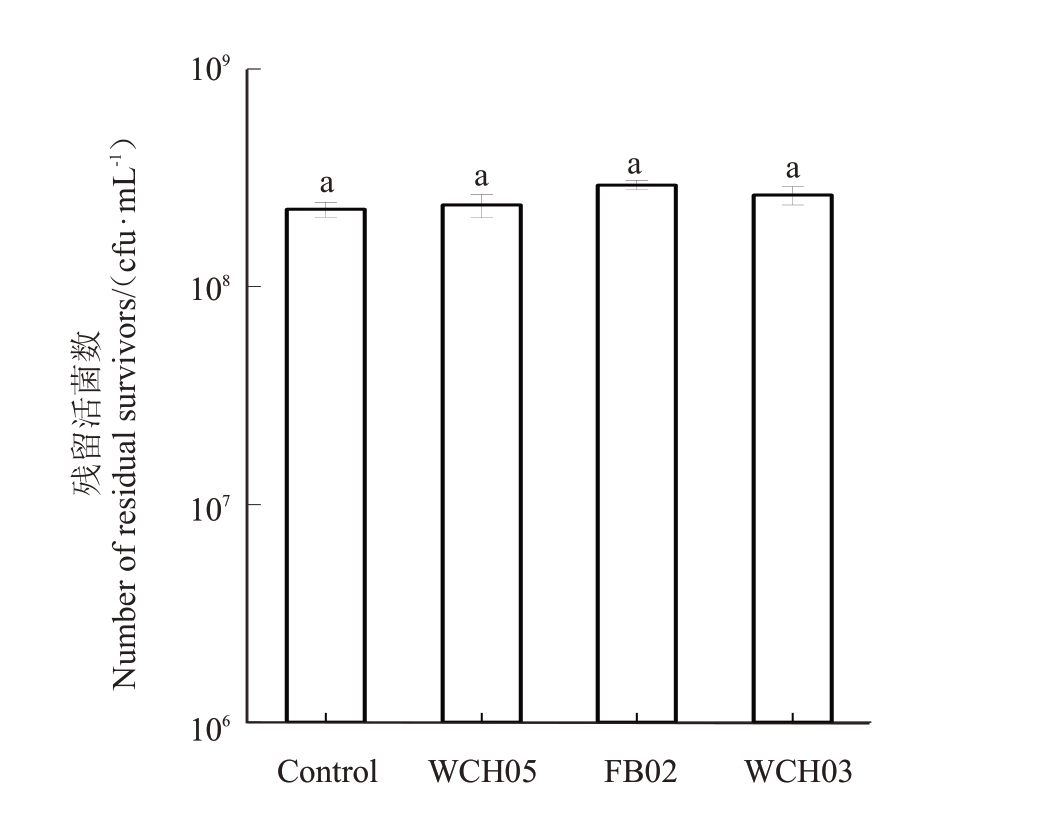
图2 黏细菌发酵滤液对梨火疫病菌的影响
Fig.2 Effect of the myxobacteria ferment filtrate on Ea growth
2.4 黏细菌对梨火疫病的防效
将初步筛选出的对梨火疫病菌捕食能力较强的3 株黏细菌菌株(WCH05、FB02 和WCH03),通过离体花序、杜梨苗接种,测定其对该病害的生防效果。
2.4.1 香梨离体花序的保护性防效 在离体香梨花序上喷施黏细菌菌液,再接种病原菌后,观察发现(表2),在病原菌接种后第3 天,未喷施黏细菌菌液的对照的大量香梨花序开始出现花腐症状,而喷施黏细菌的处理能在一定程度上减轻花腐症状,降低花腐率。其中菌株WCH05的防效最高,第3天的保护性防效达到92.12%,7 d时平均防效达68.35%,与农用链霉素的防效(68.20%)接近;其次是FB02(63.24%),而菌株WCH03 的平均防效最低,为50.36%。
表2 黏细菌接种对梨花腐预防作用的室内生测结果
Table 2 Bioassay results of the preventive control effect of myxobacteria strains to pear blossom rot
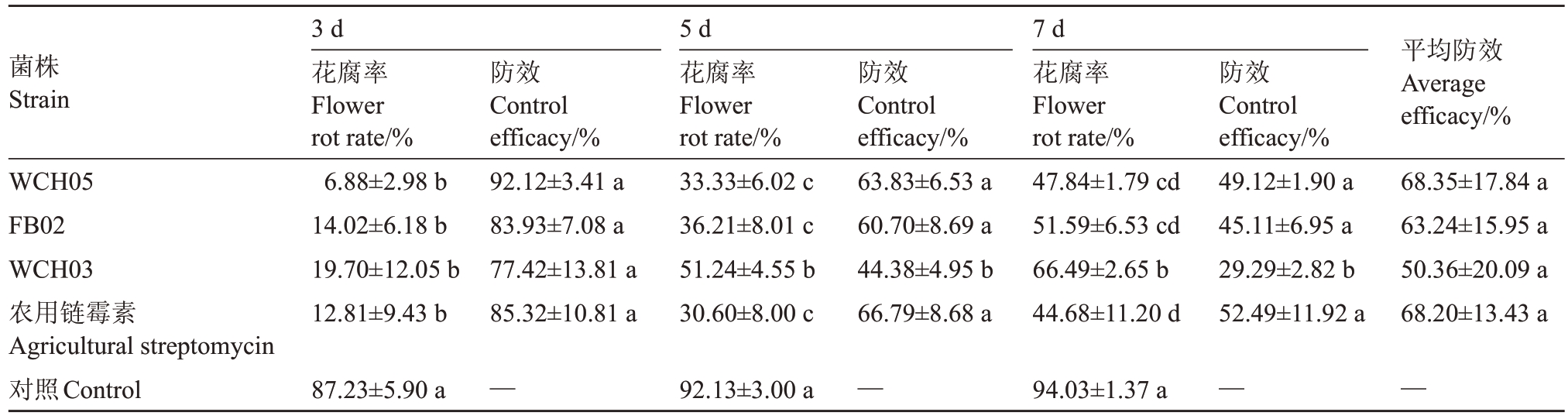
菌株Strain平均防效Average efficacy/%WCH05 FB02 WCH03农用链霉素Agricultural streptomycin对照Control 3 d花腐率Flower rot rate/%6.88±2.98 b 14.02±6.18 b 19.70±12.05 b 12.81±9.43 b防效Control efficacy/%92.12±3.41 a 83.93±7.08 a 77.42±13.81 a 85.32±10.81 a 5 d花腐率Flower rot rate/%33.33±6.02 c 36.21±8.01 c 51.24±4.55 b 30.60±8.00 c防效Control efficacy/%63.83±6.53 a 60.70±8.69 a 44.38±4.95 b 66.79±8.68 a 7 d花腐率Flower rot rate/%47.84±1.79 cd 51.59±6.53 cd 66.49±2.65 b 44.68±11.20 d防效Control efficacy/%49.12±1.90 a 45.11±6.95 a 29.29±2.82 b 52.49±11.92 a 68.35±17.84 a 63.24±15.95 a 50.36±20.09 a 68.20±13.43 a 87.23±5.90 a —92.13±3.00 a —94.03±1.37 a ——
2.4.2 黏细菌对杜梨苗梨火疫病的保护性及治疗性防效 选择对香梨花腐具有较好预防效果的黏细菌菌株WCH05、FB02和WCH03在盆栽杜梨苗上进行梨火疫病的保护性和治疗性的防治试验。
(1)保护性试验结果(表3,图3)表明,在杜梨苗上事先喷施黏细菌菌液能显著降低杜梨苗嫩枝的枝枯率和病情指数。其中防效最好的WCH05 第7 天的防效最高(84.58%),到21 d 仍能保持在80.19%,平均防效达81.53%,略低于农用链霉素(87.50%);其次是FB02(平均防效76.38%)和WCH03(平均防效71.44%)。
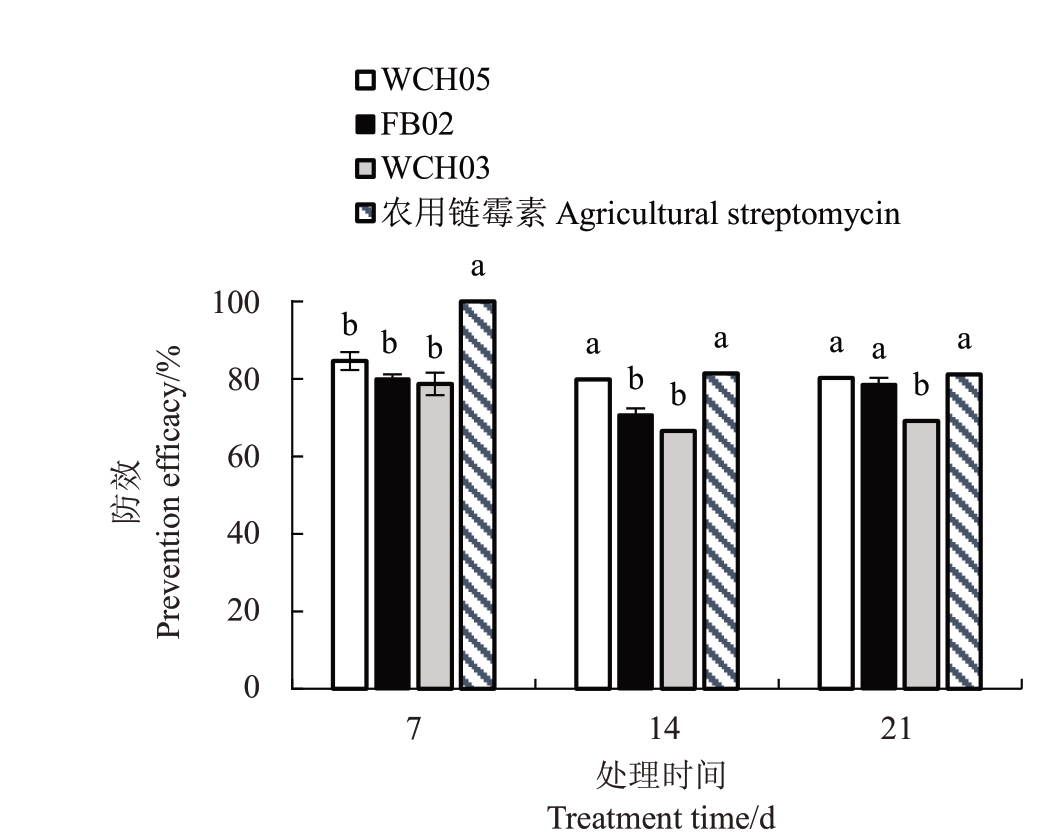
图3 黏细菌菌株预处理(喷施)对杜梨苗梨火疫病的保护性防效
Fig.3 Protective control efficacy of the myxobacteria strains to the fire blight of P.betuleafolia
表3 黏细菌对杜梨苗梨火疫病的保护性防效
Table 3 Protective control effect of the myxobacteria strains to fire blight of Pyrus betuleafolia
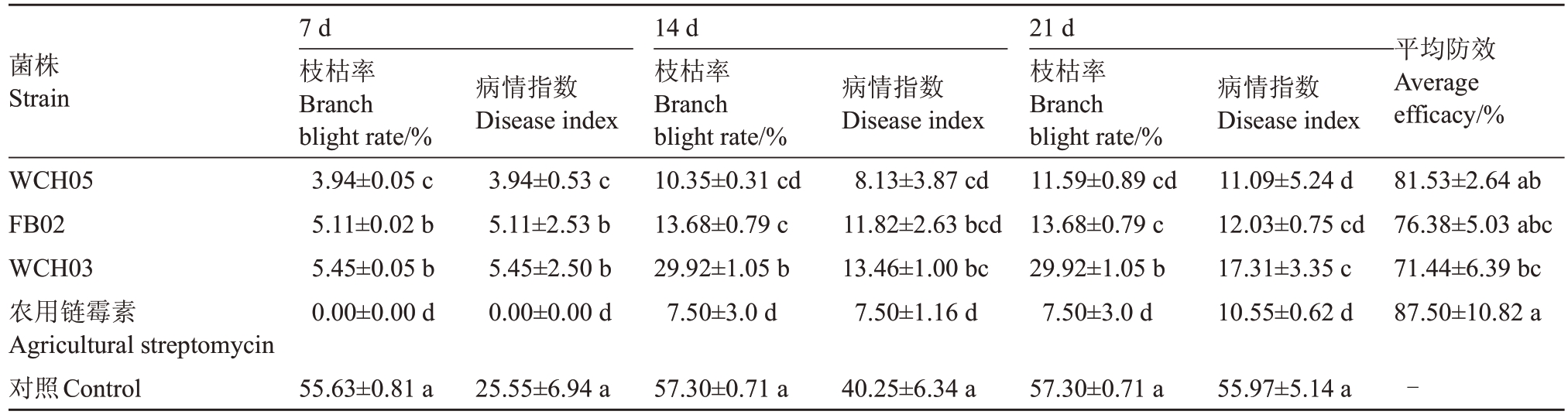
菌株Strain平均防效Average efficacy/%WCH05 FB02 WCH03农用链霉素Agricultural streptomycin对照Control 7 d枝枯率Branch blight rate/%3.94±0.05 c 5.11±0.02 b 5.45±0.05 b 0.00±0.00 d病情指数Disease index 3.94±0.53 c 5.11±2.53 b 5.45±2.50 b 0.00±0.00 d 14 d枝枯率Branch blight rate/%10.35±0.31 cd 13.68±0.79 c 29.92±1.05 b 7.50±3.0 d病情指数Disease index 8.13±3.87 cd 11.82±2.63 bcd 13.46±1.00 bc 7.50±1.16 d 21 d枝枯率Branch blight rate/%11.59±0.89 cd 13.68±0.79 c 29.92±1.05 b 7.50±3.0 d病情指数Disease index 11.09±5.24 d 12.03±0.75 cd 17.31±3.35 c 10.55±0.62 d 81.53±2.64 ab 76.38±5.03 abc 71.44±6.39 bc 87.50±10.82 a 55.63±0.81 a 25.55±6.94 a 57.30±0.71 a 40.25±6.34 a 57.30±0.71 a 55.97±5.14 a -
(2)治疗性试验结果(表4,图4)表明,喷施WCH05、FB02 和WCH03 菌液具有明显的治疗效果,枝枯率和病情指数显著降低。防效最好的菌株WCH05 第7 天的防效达到76.44%,14~21 d 的防效有所下降,平均防效达到63.84%,略低于农用链霉素(67.32%);其次是WCH03(平均防效54.88%)和FB02(平均防效51.13%)。

图4 黏细菌菌株预处理(喷施)对杜梨苗梨火疫病的治疗性防效
Fig.4 Therapeutic control efficacy of the myxobacteria strains to the fire blight of P.betuleafolia
表4 黏细菌对杜梨苗梨火疫病的治疗性防效
Table 4 Therapeutic control effect of myxobacteria strains to fire blight of P.betuleafolia
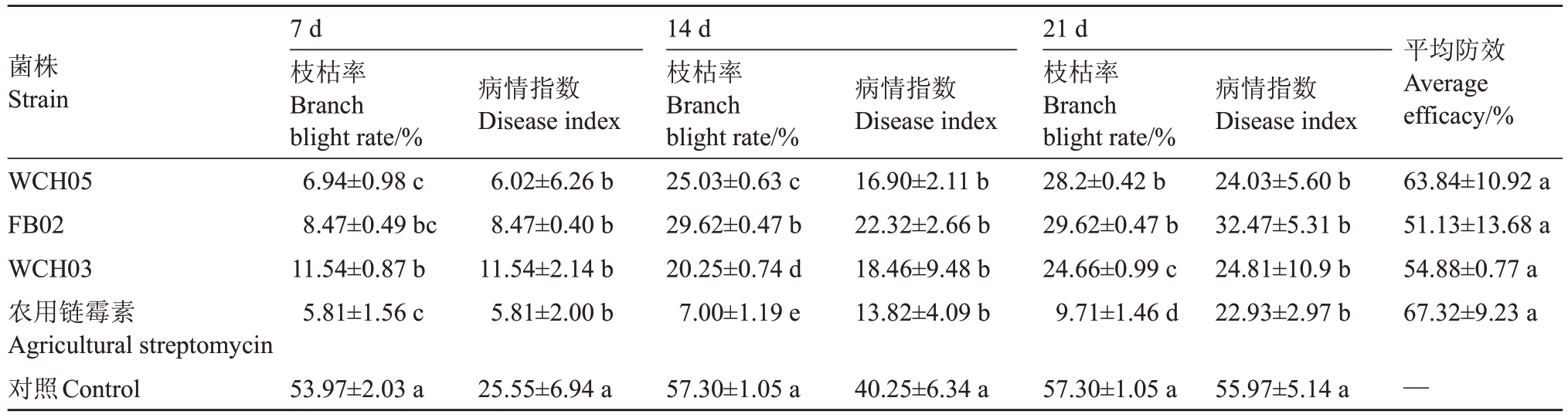
a a a a b b 80%a Preventionefficac b b菌株Strain平均防效Average efficacy/%b效40%WCH05 FB02 WCH03农用链霉素Agricultural streptomycin对照Control 20%60%防0%7 d枝枯率Branch blight rate/%6.94±0.98 c 8.47±0.49 bc 11.54±0.87 b 5.81±1.56 c病情指数Disease index 6.02±6.26 b 8.47±0.40 b 11.54±2.14 b 5.81±2.00 b 7d 14d 21d 14 d枝枯率Branch blight rate/%25.03±0.63 c 29.62±0.47 b 20.25±0.74 d 7.00±1.19 e病情指数Disease index 16.90±2.11 b 22.32±2.66 b 18.46±9.48 b 13.82±4.09 b 21 d枝枯率Branch blight rate/%28.2±0.42 b 29.62±0.47 b 24.66±0.99 c 9.71±1.46 d病情指数Disease index 24.03±5.60 b 32.47±5.31 b 24.81±10.9 b 22.93±2.97 b 63.84±10.92 a 51.13±13.68 a 54.88±0.77 a 67.32±9.23 a处理时间Treatment time/d 53.97±2.03 a 25.55±6.94 a 57.30±1.05 a 40.25±6.34 a 57.30±1.05 a 55.97±5.14 a —
2.5 黏细菌菌株WCH05、WCH03、FB02的鉴定
2.5.1 形态学鉴定 通过观察记录黏细菌子实体的颜色及形态、菌落特征以及细胞形态,根据《伯杰氏细菌鉴定手册》中黏细菌分类标准,初步判断WCH05、WCH03、FB02为黏球菌属菌株。其中菌株WCH05和FB02形态相似,在VY/4平板上呈薄膜状扩展,子实体多为球形,单生,粉红色,营养细胞细杆状,黏孢子球形。菌株WCH03 在VY/4 平板上菌膜上有整齐排列的子实体,子实体卵球形,单生,黄色,营养细胞细杆状,黏孢子球形(图5)。
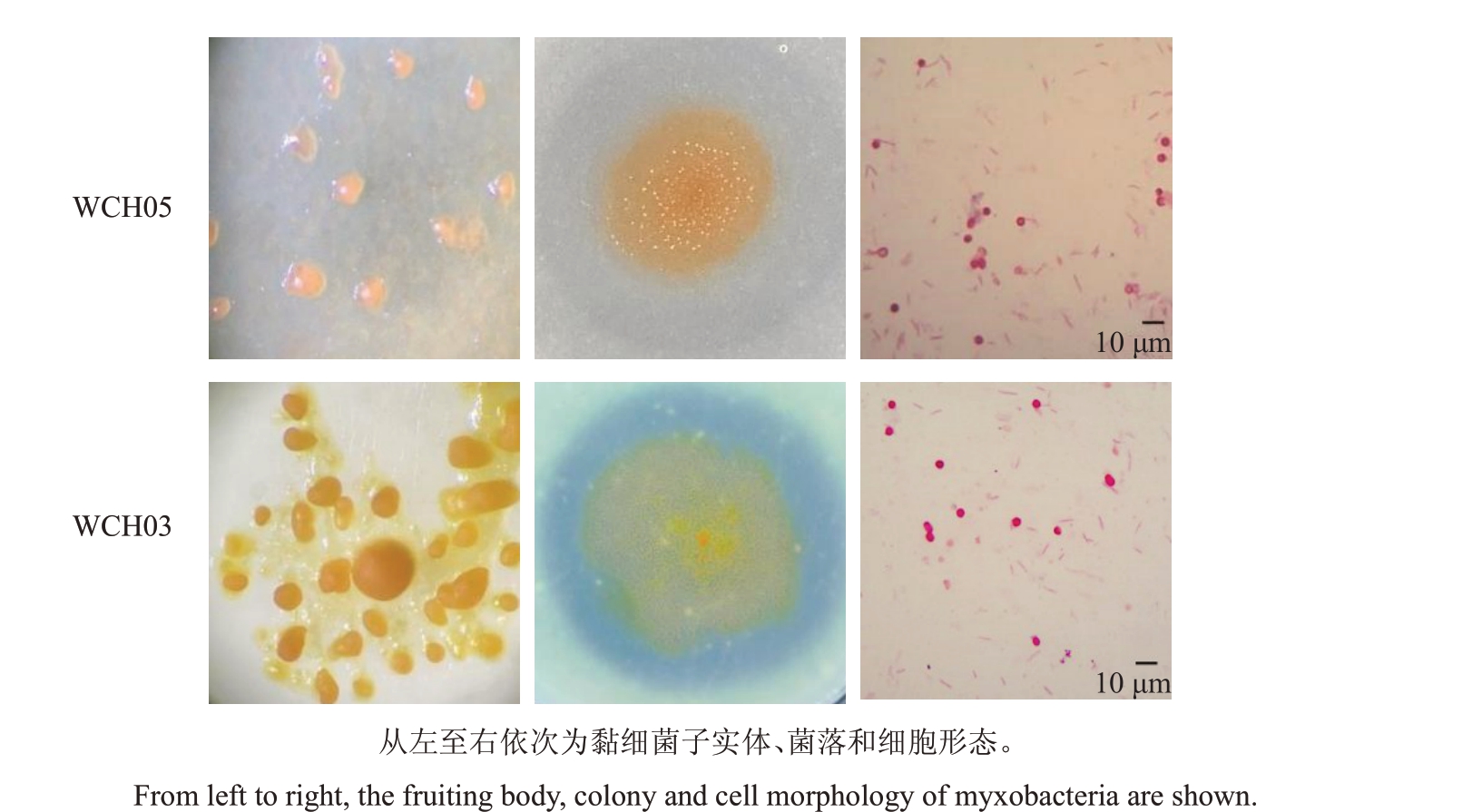
图5 黏细菌菌株WCH05 和WCH03 的形态特征
Fig.5 Morphological characteristics of myxobacteria strains WCH05 and WCH03
2.5.2 16S rRNA 和lepA 基因序列分析 以提取的WCH05、FB02 和WCH03 菌株的基因组DNA 为模板进行16S rRNA的PCR以及持家基因lepA PCR扩增、测序。将获得的序列提交至GeneBank,获得16S rRNA GenBank 登录号分别为ON406568、ON024012、ON024053;lepA 登 录 号 分 别 为ON313804、ON313766、ON313803。将测序结果在NCBⅠ数据库中进行在线Blast,使用DNAStar 软件进行序列相似性比对。选取与其相似度最高的模式菌株的基因序列,利用MEGA7.0软件邻接法构建多基因联合系统进化树。结果(图6)表明,黏细菌菌株WCH05、FB02 与橙色黏球菌模式菌株Myxococcus fulvus 124B02(CP006003)聚为一簇,WCH03与黄色黏球菌M.xanthus strain R31(CP068048)、M.xanthus strain GH3.5.6c2(CP017169)模式菌株聚为一支。综合培养性状、形态特征,将WCH05 和FB02鉴定为橙色黏球菌,WCH03鉴定为黄色黏球菌。
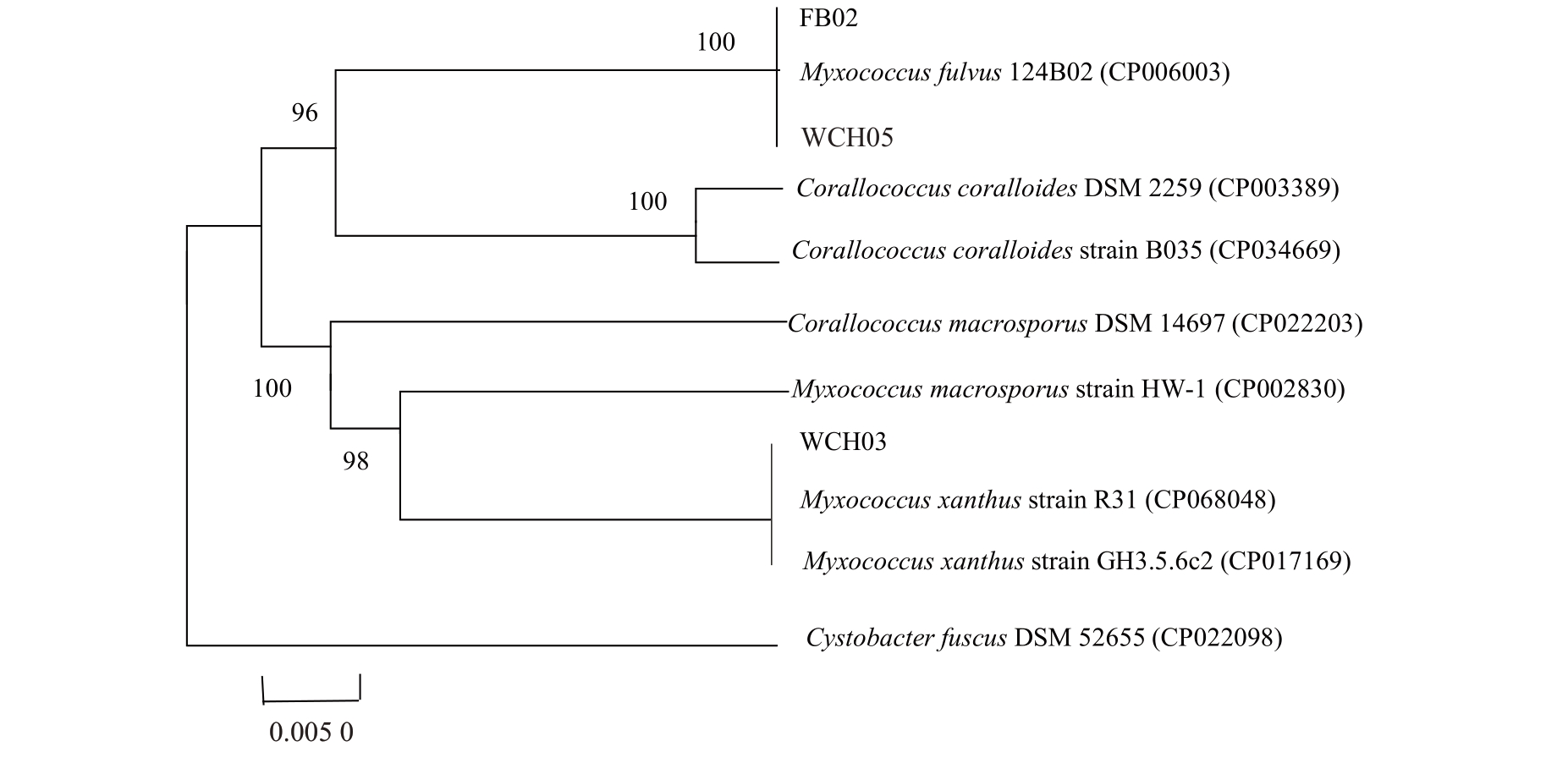
图6 基于16S rRNA、持家基因lepA 基因序列构建的黏细菌菌株的多基因系统发育树
Fig.6 Polygenetic phylogenetic tree tree of myxobacteria strains based on 16S rRNA gene sequences and lepA gene sequences
3 讨 论
梨火疫病的入侵给中国林果产业带来严重威胁,特别对中国新疆地区香梨产业带来巨大风险。近年来基于有益微生物的生防菌剂在梨火疫病生物防治中作用的研究受到了学者的重视,也取得了一定的成果。有许多微生物已被用于梨火疫病的生物防治,但关于黏细菌在梨火疫病生物防治中的研究和应用目前尚属空白。近年来大量研究表明,黏细菌在植物病害的生物防治方面具有重要的应用潜力。在抗植物病原真菌方面,珊瑚球菌(Corallococcus)[22,18]、黏球菌(Myxococcus)[31]、Sorangiym cellulosum[32]、Nannocystis exedens[33]以及其他一些捕食性黏细菌[34-35]对多种植物病原真菌表现出良好的生物防治效果。事实上,黏细菌对细菌的捕食和拮抗效果更佳,在植物细菌性病害的生物防治方面具有广阔的应用前景[24]。笔者基于前期建立的黏细菌小型菌种资源库,通过菌苔捕食和平板对峙筛选出3 株对梨火疫病菌具有较强捕食能力的黏细菌菌株WCH05、FB02 和WCH03,并通过离体花序和盆栽杜梨苗接种试验,首次证实了黏细菌在梨火疫病生物防治中的应用潜力。研究结果不仅为梨火疫病的生物防治提供了新的微生物资源,也为进一步研究和开发黏细菌生防菌剂在梨火疫病生物防治中的应用奠定了基础。
迄今为止,针对植物病原菌,分离和应用拮抗菌来进行生物防治依然是最为活跃的研究领域。研究者已筛选到大量具有抑制植物病原菌效果的拮抗菌株,包括芽孢杆菌(Bacillus)、假单胞菌(Pseudomonas)、链霉菌(streptomyces)、溶杆菌(Lysobacter)和木霉(Trichoderma)等[36-40]。这些菌株的生防机制主要是在生长代谢过程中产生多种拮抗病原菌的抗生素类物质、毒素、细菌素、蛋白质类抗菌物质等,达到抑制或杀灭病原菌的效果。而黏细菌能通过独特的狼群式群体行为和滑行运动主动捕食细菌、真菌和酵母菌等微生物活体,其产生的次级代谢产物被认为在黏细菌捕食过程中发挥着重要作用[41-42]。其中M.xanthus DK1622 产生的抗生素TA 和Corallococcus coralloides 产生的corallopyronin 已被证明可以抑制Escherichia coli MG1655 和Staphylococcus aureus的生长,在捕食过程中扮演重要的角色[43-44]。在本研究中,黏细菌菌株WCH05、FB02 和WCH03 在固体平板表面对梨火疫病菌均表现出高的捕食能力,但将其无菌发酵滤液与梨火疫病菌共培养后,发现这3株菌株的无菌发酵滤液对梨火疫病菌的生长并无影响,结合菌苔捕食试验结果,推测这3 株黏细菌对梨火疫病菌的有效杀伤依赖于菌体间的直接接触,而黏细菌菌株产生的胞外次级产物可能在捕食作用中并未起到主要作用。与本研究结果相似,Pan 等[45]的研究表明在液体条件下,M. xanthus对大肠杆菌的捕食作用依赖于胞外多糖的物理接触;李周坤等[46]研究发现Myxococcus sp.BS 所分泌的次级代谢物或者酶类具有一定抑菌作用,但是与直接捕食病原细菌相比较,该作用是次要的。
黏细菌作为一类广泛分布于土壤中的“土著菌”[47],对土壤环境的适应性更强,更易在土壤中定殖,因此近些年来关于黏细菌在植物病害生物防治方面的研究和应用主要集中在土传病害方面。与土壤环境相比,果树的叶际生存环境条件更加严苛,其可被利用的营养成分较少,温湿度变化及紫外线辐射对微生物的生存也有很大影响[48]。笔者通过本研究虽然已证实黏细菌在温室条件下对梨火疫病具有显著的生防效果,但在田间自然环境下其抗逆性能、定殖性能及防治效果还需在后续的工作中进一步证实。此外一些问题也制约了黏细菌在梨火疫病生物防治中的实际应用。如黏细菌的自溶特性直接限制了黏细菌菌剂的规模化制备和货架期。黏细菌在液体培养基中生长聚集成团,严重影响了喷雾施用。因此如何强化黏细菌在生长过程中的细胞分散性,建立和优化黏细菌发酵工艺,这些问题都需要在后续的工作中深入研究。
4 结 论
本研究中,基于前期分离、纯化获得的黏细菌菌种资源,从中筛选出3 株对梨火疫病菌具有高效捕食能力的黏细菌菌株M.fulvus WCH05、M.xanthus WCH03 和M. fulvus FB02,进一步通过离体花序和盆栽杜梨苗防效测定,发现这3 株黏细菌菌株均表现出良好的生防效果,其中黏细菌菌株WCH05 的防效最佳,表明黏细菌在梨火疫病的生物防治中具有潜在的应用价值。
[1] HEⅠTEFUSS R.Fire blight,history,biology,and management[J].Journal of Phytopathology,2012,160(7/8):440.
[2] 中华人民共和国农业农村部.中华人民共和国农业农村部公告第333 号:一类农作物病虫害名录[EB/OL].(2020-09-15)[2022-12-21].http://www.moa.gov.cn/nybgb/2020/202010/202011/t20201130_6357326.htm.Ministry of Agriculture and Rural Affairs of the People’s Republic of China.Announcement No.333 of the Ministry of Agriculture and Rural Affairs of the People’s Republic of China:CategoryⅠlist of crop diseases and pests[EB/OL].(2020-09-15)[2022-12-21].http://www.moa.gov.cn/nybgb/2020/202010/202011/t20201130_6357326.htm.
[3] 中华人民共和国农业农村部.中华人民共和国农业农村部公告第567 号:重点管理外来入侵物种名录[EB/OL].(2022-12-20)[2022-12-21].http://www.moa.gov.cn/govpublic/KJJYS/202211/t20221109_6415160.htm.Ministry of Agriculture and Rural Affairs of the People’s Republic of China.Announcement No.567 of the Ministry of Agriculture and Rural Affairs of the People’s Republic of China:List of alien invasive species under key management[EB/OL].(2022-12-20)[2022-12-21].http://www.moa.gov.cn/govpublic/KJJYS/202211/t20221109_6415160.htm.
[4] 黄伟,盛强,罗明,马德英,张春竹.新疆库尔勒香梨火疫病的发生特点及防控建议[J].植物保护,2022,48(6):207-213.HUANG Wei,SHENG Qiang,LUO Ming,MA Deying,ZHANG Chunzhu.Occurrence status of fire blight on Korla fragrant pear in Xinjiang and the control proposals[J].Plant Protection,2022,48(6):207-213.
[5] 中华人民共和国农业农村部.农业农村部办公厅关于印发《全国农业植物检疫性有害生物分布行政区名录》的通知[EB/OL].(2019-05-16)[2022-12-21].http://www.moa.gov.cn/govpublic/ZZYGLS/202207/t20220707_6404284.htm.Ministry of Agriculture and Rural Affairs of the People’s Republic of China.Notice of the General Office of the Ministry of Agriculture and Rural Affairs on Printing and Distributing the National List of Administrative Regions Where Agricultural Plant Quarantine Pests Are Distributed[EB/OL].(2019-05-16)[2022-12-21].http://www.moa.gov.cn/govpublic/ZZYGLS/202207/t20220707_6404284.htm.
[6] ZHAO Y Q,TⅠAN Y L,WANG L M,GENG G M,ZHAO W J,HU B S,ZHAO Y F.Fire blight disease,a fast-approaching threat to apple and pear production in China[J].Journal of Ⅰntegrative Agriculture,2019,18(4):815-820.
[7] DOOLOTKELDⅠEVA T,BOBUSHOVA S,SCHUSTER C,KONURBAEVA M,LECLERQUE A.Ⅰsolation and genetic characterization of Erwinia amylovora bacteria from Kyrgyzstan[J].European Journal of Plant Pathology,2019,155(2):677-686.
[8] VANNESTE J L,YU J.Biological control of fire blight using Erwinia herbicola EH252 and Pseudomonas fluorescens A506 separately or in combination[J].Acta Horticulturae,1996,411:351-354.
[9] LⅠNDOW S E,MCGOURTY G,ELKⅠNS R.Ⅰnteractions of antibiotics with Pseudomonas fluorescens strain A506 in the control of fire blight and frost injury to pear[J].Phytopathology,1996,86(8):841.
[10] ZELLER W,WOLF B.Studies on biological control of fire blight[J].Acta Horticulturae,1996,411:341-346.
[11] AⅠT BAHADOU S,OUⅠJJA A,BOUKHARⅠM A,TAHⅠRⅠA.Development of field strategies for fire blight control integrating biocontrol agents and plant defense activators in Morocco[J].Journal of Plant Pathology,2017,99:51-58.
[12] 徐琳赟,古丽孜热·曼合木提,韩剑,蒋萍,黄伟,罗明.香梨内生拮抗细菌的筛选及对梨火疫病的生防潜力[J].西北植物学报,2021,41(1):132-141.XU Linyun,Gulizzier ∙Manhemuti,HAN Jian,JⅠANG Ping,HUANG Wei,LUO Ming.Screening of endophytic antagonistic bacteria from‘Kuerlexiangli’pear and their biocontrol potential against fire blight disease[J].Acta Botanica Boreali-Occidentalia Sinica,2021,41(1):132-141.
[13] 鲁晏宏,郝金辉,罗明,黄伟,盛强,王宁,詹发强,龙宣杞,包慧芳.梨火疫病拮抗菌筛选及温室防效测定[J].微生物学通报,2021,48(10):3690-3699.LU Yanhong,HAO Jinhui,LUO Ming,HUANG Wei,SHENG Qiang,WANG Ning,ZHAN Faqiang,LONG Xuanqi,BAO Huifang.Screening of antagonistic bacteria against Erwinia amylovora and its control effect in greenhouse[J].Microbiology China,2021,48(10):3690-3699.
[14] 吕天宇,贺旭,罗明,韩剑,包慧芳,黄伟.梨火疫病菌拮抗细菌FX1 培养基及摇瓶发酵条件优化[J].中国生物防治学报,2022,38(6):1553-1565.LÜ Tianyu,HE Xu,LUO Ming,HAN Jian,BAO Huifang,HUANG Wei.Optimization of culture medium and flask fermentation conditions for Bacillus velezensis FX1 against Erwinia amylovora[J].Chinese Journal of Biological Control,2022,38(6):1553-1565.
[15] MUÑOZ- DORADO J,MARCOS- TORRES F J,GARCÍABRAVO E,MORALEDA-MUÑOZ A,PÉREZ J.Myxobacteria:Moving,killing,feeding,and surviving together[J].Frontiers in Microbiology,2016,7:781.
[16] THⅠERY S,KAⅠMER C.The predation strategy of Myxococcus xanthus[J].Frontiers in Microbiology,2020,11:2.
[17] DAWⅠD W.Biology and global distribution of myxobacteria in soils[J].FEMS Microbiology Reviews,2000,24(4):403-427.
[18] YE X F,LⅠZ K,LUO X,WANG W H,LⅠY K,LⅠR,ZHANG B,QⅠAO Y,ZHOU J,FAN J Q,WANG H,HUANG Y,CAO H,CUⅠZ L,ZHANG R F.A predatory myxobacterium controls cucumber Fusarium wilt by regulating the soil microbial community[J].Microbiome,2020,8(1):49.
[19] DAHM H,BRZEZⅠŃSKA J,WRÓTNⅠAK-DRZEWⅠECKA W,GOLⅠŃSKA P,RÓŻYCKⅠH,RAⅠM.Myxobacteria as a potential biocontrol agent effective against pathogenic fungi of economically important forest trees[J].Dendrobiology,2015,74:13-24.
[20] RAZA W,LⅠNG N,ZHANG R F,HUANG Q W,XU Y C,SHEN Q R.Success evaluation of the biological control of Fusarium wilts of cucumber,banana,and tomato since 2000 and future research strategies[J].Critical Reviews in Biotechnology,2017,37(2):202-212.
[21] LⅠZ K,YE X F,CHEN P L,JⅠK,ZHOU J,WANG F,DONG W L,HUANG Y,ZHANG Z G,CUⅠZ L.Antifungal potential of Corallococcus sp.strain EGB against plant pathogenic fungi[J].Biological Control,2017,110:10-17.
[22] LⅠZ K,WANG T,LUO X,LⅠX M,XⅠA C Y,ZHAO Y Q,YE X F,HUANG Y,GU X Y,CAO H,CUⅠZ L,FAN J Q.Biocontrol potential of Myxococcus sp.strain BS against bacterial soft rot of calla lily caused by Pectobacterium carotovorum[J].Biological Control,2018,126:36-44.
[23] DONG H H,XU X,GAO R X,LⅠY Q,LⅠA Z,YAO Q,ZHU H H. Myxococcus xanthus R31 suppresses tomato bacterial wilt by inhibiting the pathogen Ralstonia solanacearum with secreted proteins[J].Frontiers in Microbiology,2022,12:801091.
[24] 白欣禾,韩剑,窦新玉,罗明,崔中利,李周坤,吕文.新疆农田土壤中粘细菌的分离鉴定及其捕食特性[J].新疆农业大学学报,2022,45(6):462-473.BAⅠXinhe,HAN Jian,DOU Xinyu,LUO Ming,CUⅠZhongli,LⅠZhoukun,LÜ Wen.Ⅰsolation,identification and predation characteristics of myxobacteria from farmland soils in Xinjiang[J].Journal of Xinjiang Agricultural University,2022,45(6):462-473.
[25] 王婷.新型生防粘细菌Myxococcus sp.BS 的分离及粘细菌对细菌性软腐病菌的捕食机理研究[D].南京:南京农业大学,2018.WANG Ting.Ⅰsolation of a novel biocontrol Myxococcus sp.BS and the myxobacterial predation mechanism against bacterial soft rot[D].Nanjing:Nanjing Agricultural University,2018.
[26] 李燕,李洪涛,叶良超,周俊祥,罗明.苹果细菌性枯枝病菌的生物学特性及抑菌药剂筛选[J].中国农学通报,2021,37(4):112-119.LⅠYan,LⅠHongtao,YE Liangchao,ZHOU Junxiang,LUO Ming.Biological characteristics of Pseudomonas syringae pv.syringae causing shoot dieback disease on apple and screening of bactericides[J].Chinese Agricultural Science Bulletin,2021,37(4):112-119.
[27] BOONE D R,CASTENHOLZ R W,GARRⅠTY G M.Bergey’s manual of systematic bacteriology[M].Berlin:Springer Science&Business Media,2001.
[28] BALOUS A,TRUPER H G,DWORKⅠN M,HARDER W,SCHLEⅠFER K H.The prokaryotes[M].Berllin:Springer,1992:3418-3487.
[29] ZHANG X J,YAO Q,CAⅠZ P,XⅠE X L,ZHU H H.Ⅰsolation and identification of myxobacteria from saline-alkaline soils in Xinjiang,China[J].PLoS One,2013,8(8):e70466.
[30] STACKEBRANDT E,PÄUKER O,ERHARD M.Grouping myxococci (Corallococcus) strains by matrix-assisted laser desorption ionization time-of-flight(MALDⅠTOF)mass spectrometry:Comparison with gene sequence phylogenies[J].Current Microbiology,2005,50(2):71-77.
[31] KⅠM S T,YUN S C.Biocontrol with Myxococcus sp.KYC 1126 against anthracnose in hot pepper[J].The Plant Pathology Journal,2011,27(2):156-163.
[32] HOCKⅠNG D,COOK F D.Myxobacteria exert partial control of damping-off and root disease in container-grown tree seedlings[J].Canadian Journal of Microbiology,1972,18(10):1557-1560.
[33] TAYLOR W J,DRAUGHON F A. Nannocystis exedens:A potential biocompetitive agent against Aspergillus flavus and Aspergillus parasiticus[J].Journal of Food Protection,2001,64(7):1030-1034.
[34] HOMMA Y.Perforation and Lysis of hyphae of Rhizoctonia solaniand conidia of Cochliobolus miyabeanus by soil myxobacteria[J].Phytopathology,1984,74(10):1234.
[35] MELⅠAH S,KUSUMAWATⅠD Ⅰ,ⅠLYAS M.Preliminary study of myxobacteria as biocontrol agents for Panama disease pathogen,tropical race 4 Fusarium odoratissimum[J].ⅠOP Conference Series:Earth and Environmental Science,2020,457(1):012060.
[36] FⅠRA D,DⅠMKⅠĆ Ⅰ,BERⅠĆ T,LOZO J,STANKOVⅠĆ S.Biological control of plant pathogens by Bacillus species[J].Journal of Biotechnology,2018,285:44-55.
[37] OLORUNLEKE F E,KⅠEU N P,HÖFTE M.Recent advances in Pseudomonas biocontrol[M]//MURⅠLLO J,VⅠNATZER B A,JACKSON R W,ARNOLD D L.Bacteria-plant interactions:advanced research and future trends.UK:Caister Academic Press,2015:167-198
[38] LAW J W F,SER H L,KHAN T M,CHUAH L H,PUSPARAJAH P,CHAN K G,GOH B H,LEE L H.The potential of Streptomyces as biocontrol agents against the rice blast fungus,Magnaporthe oryzae(Pyricularia oryzae)[J].Frontiers in Microbiology,2017,8:3.
[39] LⅠN L,XU K W,SHEN D Y,CHOU S H,GOMELSKY M,QⅠAN G L.Antifungal weapons of Lysobacter,a mighty biocontrol agent[J].Environmental Microbiology,2021,23(10):5704-5715.
[40] MARTÍNEZ B,ⅠNFANTE D,REYES Y.Trichoderma spp.y su función en el control de plagas en los cultivos[J].Revista De Protección Vegetal,2013,28(1):1-11.
[41] PÉREZ J,CONTRERAS-MORENO F J,MARCOS-TORRES F J,MORALEDA-MUÑOZ A,MUÑOZ-DORADO J.The antibiotic crisis:How bacterial predators can help[J].Computational and Structural Biotechnology Journal,2020,18:2547-2555.
[42] AREND K Ⅰ,SCHMⅠDT J J,BENTLER T,LÜCHTEFELD C,EGGERⅠCHS D,HEXAMER H M,KAⅠMER C. Myxococcus xanthus predation of Gram-positive or Gram-negative bacteria is mediated by different bacteriolytic mechanisms[J].Applied and Environmental Microbiology,2021,87(5):e02382-e02420.
[43] XⅠAO Y,WEⅠX M,EBRⅠGHT R,WALL D.Antibiotic production by myxobacteria plays a role in predation[J].Journal of Bacteriology,2011,193(18):4626-4633.
[44] ⅠRSCHⅠK H,JANSEN R,HÖFLE G,GERTH K,REⅠCHENBACH H.The corallopyronins,new inhibitors of bacterial RNA synthesis from myxobacteria[J].The Journal of Antibiotics,1985,38(2):145-152.
[45] PAN H W,HE X S,LUX R,LUAN J,SHⅠW Y.Killing of Escherichia coli by Myxococcus xanthus in aqueous environments requires exopolysaccharide-dependent physical contact[J].Microbial Ecology,2013,66(3):630-638.
[46] 李周坤,叶现丰,杨凡,黄彦,范加勤,王辉,崔中利.黏细菌捕食生物学研究进展及其在农业领域的应用潜力[J].南京农业大学学报,2021,44(2):208-216.LⅠZhoukun,YE Xianfeng,YANG Fan,HUANG Yan,FAN Jiaqin,WANG Hui,CUⅠZhongli.The predation biology of myxobacteria and its application in agricultural field[J].Journal of Nanjing Agricultural University,2021,44(2):208-216.
[47] ZHOU X W,LⅠS G,LⅠW,JⅠANG D M,HAN K,WU Z H,LⅠY Z.Myxobacterial community is a predominant and highly diverse bacterial group in soil niches[J].Environmental Microbiology Reports,2014,6(1):45-56.
[48] 潘建刚,呼庆,齐鸿雁,张洪勋,庄国强,白志辉.叶际微生物研究进展[J].生态学报,2011,31(2):583-592.PAN Jiangang,HU Qing,QⅠHongyan,ZHANG Hongxun,ZHUANG Guoqiang,BAⅠZhihui.Advance in the research of phyllospheric microorganism[J].Acta Ecologica Sinica,2011,31(2):583-592.