软枣猕猴桃[Actinidia arguta(Sieb.et Zucc.)Planch.ex Miq],为猕猴桃科(Actinidiaceae)猕猴桃属(Actinidia)大型落叶木质藤本植物[1]。果实表皮光滑无毛,品质风味独特[2]。其果实营养丰富,可鲜食,亦可加工成果酱、果酒等,同时具有抗氧化、降血糖和通便润肠等功效[3]。虽然中国软枣猕猴桃人工栽培发展较晚[4],但软枣猕猴桃是很有开发利用前景的野生浆果类果树[5],中国软枣猕猴桃人工栽培以露地为主,温室栽培起步相对较晚[6]。温室栽培作为保护地栽培的一种,可以较好地控制病虫害的发生及传播,避免因雨水不均导致涝害或果实糖度降低。研究表明,近年来,温室栽培适用于多种果树[7-9],猕猴桃属植物研究较晚,但设施栽培对猕猴桃的意义重大,可以有效控制猕猴桃溃疡病的发病率[10]。温室栽培需提前打破休眠,对果树本身需冷量有一定要求,由于软枣猕猴桃自身的需冷量要求不高,大部分品种在1000 h以内,其中魁绿与佳绿只需840 h、672 h左右即可[11],较低的需冷量可使软枣猕猴桃在温室栽培时提前升温不影响其结果性能,故进行温室栽培具有一定的可行性。在温室栽培条件下,整体物候期会提前2~3个月,在吉林长春地区约在当年6月份果实成熟,较露天栽培提早上市3个月左右,能够填补软枣猕猴桃鲜果市场的空窗期,提高经济效益。
目前软枣猕猴桃叶片的光合特性研究多在露地栽培条件下,温室条件下少见报道[12],因此深入研究温室栽培对软枣猕猴桃叶片光合特性及果实品质的影响,对建立科学、规范的温室栽培模式具有重要的理论指导意义。
1 材料和方法
1.1 试验地概况
试验于2021 年12 月至2022 年9 月在吉林农业大学软枣猕猴桃资源圃与设施农业基地内进行。试验采用日光温室栽培模式,传统的露地栽培为对照。日光温室跨度9.51 m,脊高4.50 m,长65.30 m。供水方式为滴灌,通过内、外遮阴帘调控温度,排风扇与湿帘控制温、湿度。种植土壤均为园土、沙子、草炭土体积比为3∶1∶1 配置而成。长春地区年平均日照时数2259~3016 h,年平均降水量568.5 mm,无霜期140~150 d,霜期156 d[13]。长春地区2020 年每月气温详见表1。
表1 长春地区2022 年每月气温
Table 1 Monthly temperature in Changchun in 2022℃
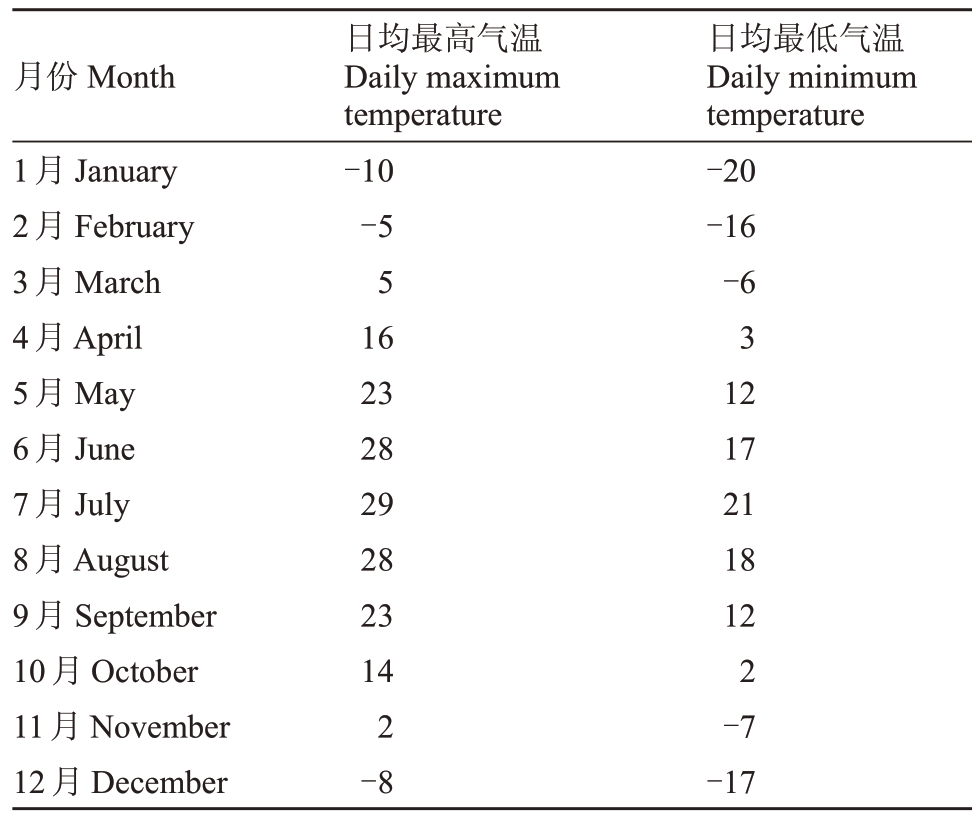
月份Month 1月January 2月February 3月March 4月April 5月May 6月June 7月July 8月August 9月September 10月October 11月November 12月December日均最高气温Daily maximum temperature-10-5 5 16 23 28 29 28 23 14 2-8日均最低气温Daily minimum temperature-20-16-6 3 12 17 21 18 12 2-7-17
1.2 试验材料
供试软枣猕猴桃品种为4 年生佳绿和魁绿,以雄株品种绿王为授粉树。温室及露地栽培均为单龙干整形。温室栽培于2021年12月20日进行升温处理(揭被升温),露地栽培随气候变化自然进行,试验期间两种栽培模式的整形修剪、肥水管理与病虫害管理等措施保持一致。
1.3 试验方法
试验共设两种栽培环境,标记结果枝基部第一花序前第3枚叶片,于盛花期(温室为4月1日,露地为6月5日)分别在两种栽培环境下进行光合指标及快速叶绿素荧光的测定,每个品种设20 个重复;选取功能叶片,通过石蜡切片法观察叶片结构[14];监测温室内两个品种果实的可溶性固形物含量(TSS),当其达到7%时进行采收,测量果形指数及单果质量,每个品种设30 个重复;待果实充分后熟后进行可溶性固形物含量、可溶性糖含量、可滴定酸含量以及维生素C 含量的测定,每个品种设3 次重复。露地栽培佳绿、温室栽培佳绿、露地栽培魁绿以及温室栽培魁绿分别用J1、J2、K1、K2代表。
1.3.1 光响应曲线的测定 光响应曲线测定参考王振兴等[15]的方法。于晴天上午使用CⅠRAS-3便携式光合测定系统(PP-SYSTEM,美国)对选取的植株叶片进行测定。分别以净光合速率(Pn)、胞间二氧化碳浓度(Ci)、气孔导度(Gs)、蒸腾速率(Tr)为纵轴,绘制响应曲线。利用双曲线修正模型进行计算,得出光合作用的最大净光合速率(Pnmax)、光饱和点(LSP)、光补偿点(LCP)、暗呼吸速率(Rd)以及表观量子效率(AQY)。
1.3.2 二氧化碳响应曲线的测定 CO2响应参考王振兴等[15]的方法测定,光合有效辐射(PAR)设1400 μmol ∙m-2 ∙s-1,CO2 浓 度 设 为0、100、150、200 μmol∙mol-1。Pn-CO2曲线初始斜率即为羧化效率(CE)。
1.3.3 快速叶绿素荧光诱导动力学曲线的测定 该试验参考郭建辉等[16]的方法,将叶片在黑暗条件下处理1 h,使用Pocket-PEA非调制式荧光仪(Hansatech,英国)测定软枣猕猴桃叶片快速叶绿素荧光诱导动力学曲线(O-J-Ⅰ-P曲线),参考Strasser等[17]的方法进行分析,并根据公式(Ft-Fo)/(Fm-Fo)进行标准化处理,每个品种均测20枚叶片。
1.3.4 叶片结构及气孔的观察 在盛花期(温室为4月1日,露地为6月5日)选取叶幕上层照光均匀一致的功能叶片进行石蜡切片的制作以及观察气孔形态,石蜡切片制作参考王振兴等[18]的方法,气孔形态观察参考徐清华[19]的方法。
1.3.5 果形指数的测定 在果实采收后,使用游标卡尺对软枣猕猴桃果实的纵径、横径、侧径进行测量,由于软枣猕猴桃为二歧聚伞花序,同一花序上着生3 个果实的选择位于中间的果实进行测量,以此保证数据的一致性。
1.3.6 果实品质的测定 在果实成熟期,每份资源采收30 个典型果实,放入4 ℃冰箱中进行果实后熟,待7 d后果实完全成熟时进行果实品质的测定。TSS 含量采用电子糖量仪(PAL-101,杭州齐威仪器有限公司,中国)测定;果实的可溶性糖含量采用试剂盒法进行检测(BC0030,Solarbio,中国);可滴定酸含量参考张治安等[20]的方法采用酸碱滴定法测定;维生素C 含量参考师恺丰[21]的方法采取钼蓝比色法测定。
1.4 数据分析
利用SPSS 20统计软件进行方差分析,使用Microsoft Office 2021进行数据处理与作图。
2 结果与分析
2.1 软枣猕猴桃温室栽培对叶片光响应的影响
由图1-A 可知,不同条件下各品种的Pn与PAR均呈正相关,在露地栽培条件下品种的Pn值显著高于温室栽培。PAR 在200~1600 μmol∙m-2∙s-1内露地栽培条件的Pn值与温室栽培间的差异逐渐增大,各栽培环境下的Pn排序为:佳绿露地栽培>魁绿露地栽培>佳绿温室栽培>魁绿温室栽培。温室栽培条件下佳绿Pnmax下降28.16%、魁绿Pnmax下降24.28%;如图1-B 所示,温室与露地条件下各处理Ci无显著差异;从图1-C可以看出,露地栽培软枣猕猴桃Gs随PAR的增加呈波动性变化,温室内趋于平缓;在温室栽培条件下的两个品种,其Gs的光响应曲线与Tr的光响应曲线变化趋于一致,Gs是影响Tr变化的主要因子之一(图1-C、D)。
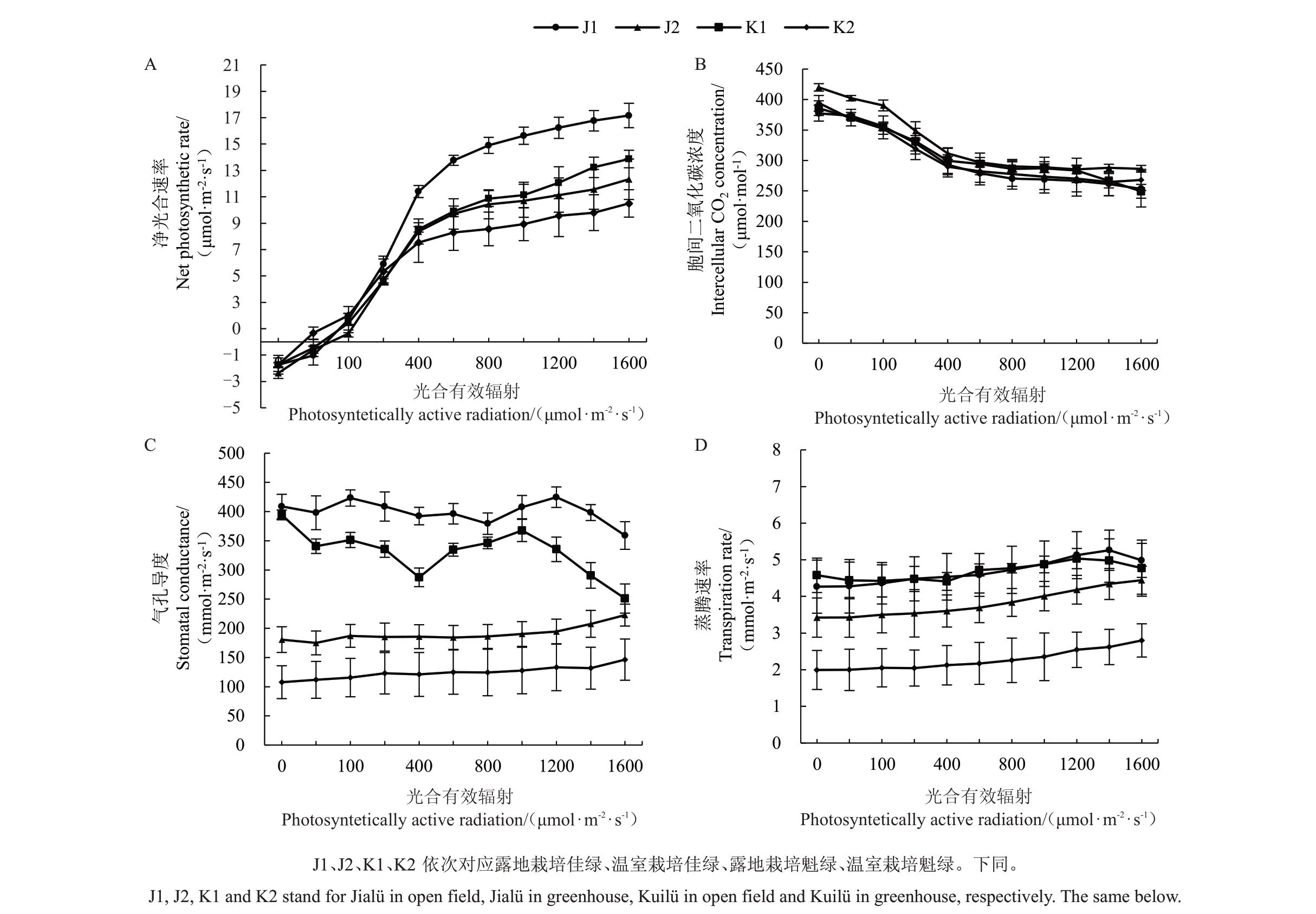
图1 不同栽培环境对软枣猕猴桃净光合速率(A)、胞间二氧化碳浓度(B)、气孔导度(C)、蒸腾速率(D)的影响
Fig.1 Effects of different cultivation environments on net photosynthetic rate(A),intercellular carbon dioxide concentration(B),stomatal conductance(C),and transpiration rate(D)of A.arguta
不同栽培条件下的软枣猕猴桃品种光响应特征参数存在差异(表2),温室栽培条件下两个品种Pnmax、LSP显著低于露地栽培,温室栽培佳绿LCP高于露地15.13%,而魁绿则表现相反;温室栽培下的佳绿Rd值与露地相比上升4.64%,魁绿下降12.47%;温室中佳绿的AQY 相对下降5.32%,魁绿上升24.7%。
表2 光响应曲线特征参数统计
Table 2 Statistics of characteristic parameters of photoresponse curve
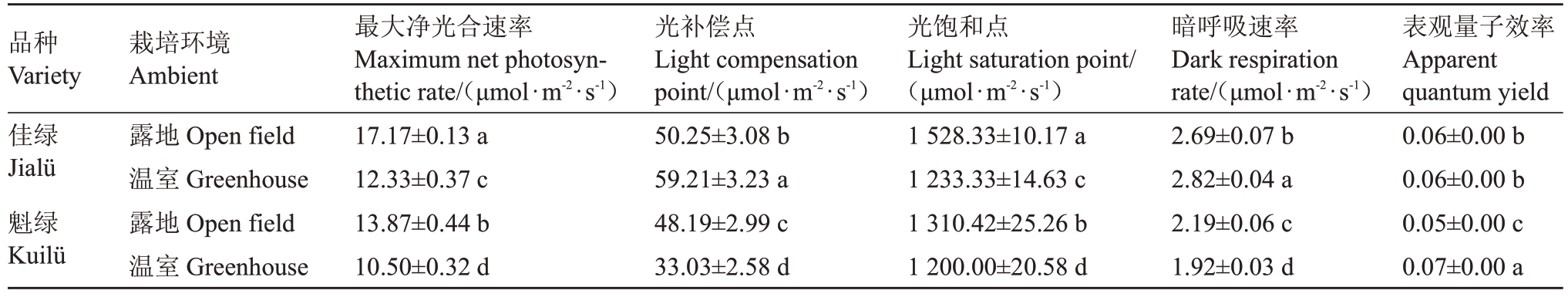
注:不同小写字母表示在p<0.05 水平显著差异。下同。
Note:Different small letters indicate significant differences at p<0.05.The same below.
品种Variety佳绿Jialü魁绿Kuilü表观量子效率Apparent quantum yield 0.06±0.00 b 0.06±0.00 b 0.05±0.00 c 0.07±0.00 a栽培环境Ambient露地Open field温室Greenhouse露地Open field温室Greenhouse最大净光合速率Maximum net photosynthetic rate/(μmol∙m-2∙s-1)17.17±0.13 a 12.33±0.37 c 13.87±0.44 b 10.50±0.32 d光补偿点Light compensation point/(μmol∙m-2∙s-1)50.25±3.08 b 59.21±3.23 a 48.19±2.99 c 33.03±2.58 d光饱和点Light saturation point/(μmol∙m-2∙s-1)1 528.33±10.17 a 1 233.33±14.63 c 1 310.42±25.26 b 1 200.00±20.58 d暗呼吸速率Dark respiration rate/(μmol∙m-2∙s-1)2.69±0.07 b 2.82±0.04 a 2.19±0.06 c 1.92±0.03 d
2.2 软枣猕猴桃温室栽培对叶片CO2响应的影响
将CO2浓度<200 μmol∙mol-1的Pn值进行线性回归后计算CE 值,如图2-A、B 所示,两个品种在不同栽培环境下CE值具显著性差异,但在相同栽培环境下两个品种之间并无显著性差异,回归曲线趋于一致。在温室栽培中两个品种CE值显著下降,表明在温室环境下两个软枣猕猴桃品种利用低浓度CO2的能力降低,并且温室内部CO2浓度与露地相比较低,是Pnmax低于露地栽培的原因之一。
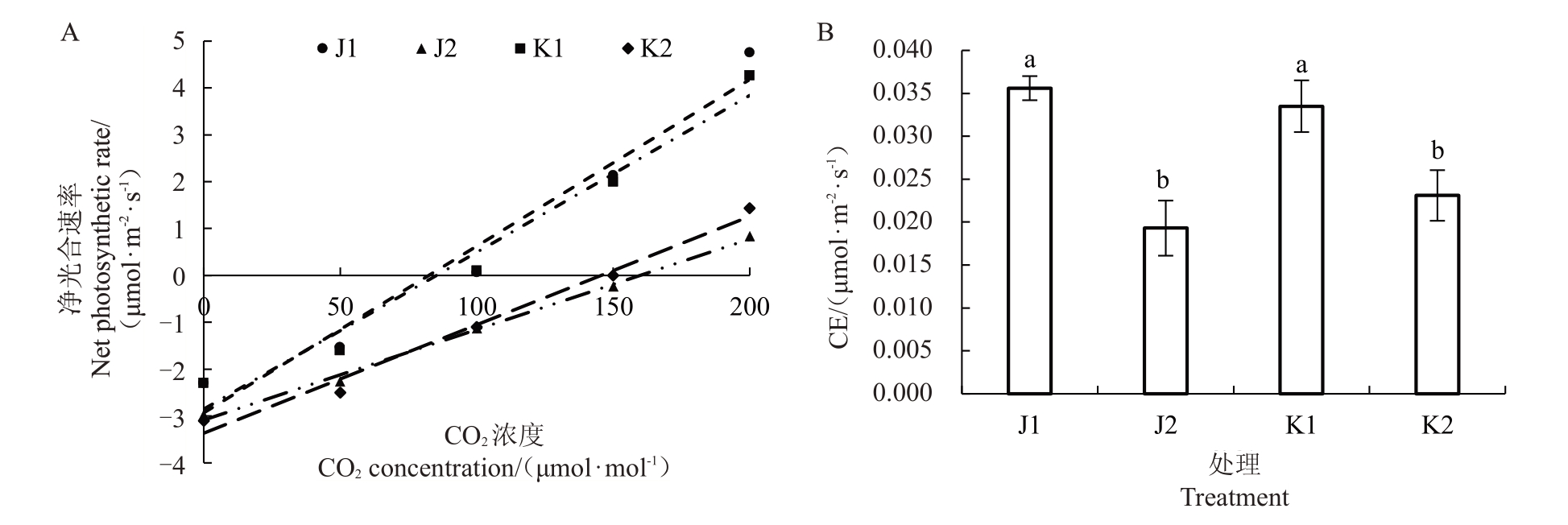
图2 不同栽培环境软枣猕猴桃Pn-CO2拟合曲线及CE 值差异分析
Fig.2 Pn-CO2 fitting curve and CE value difference analysis of A.arguta under different cultivation environments
不同小写字母表示在p<0.05 水平显著差异。下同。
Different small letters indicate significant differences at p<0.05.The same below.
2.3 软枣猕猴桃温室栽培对叶片叶绿素荧光特性的影响
如图3和图4所示,温室栽培增加了软枣猕猴桃两个品种叶片叶绿素荧光诱导动力学曲线中j点和i点的相对可变荧光,其Vk、Vj、Vi值与露地相比显著升高。以上结果表明,在温室环境下软枣猕猴桃叶片的放氧复合体及电子传递链的活性发生了一定的变化,但对叶绿素荧光诱导动力学曲线进行分析,温室栽培仍与露地栽培趋于一致。
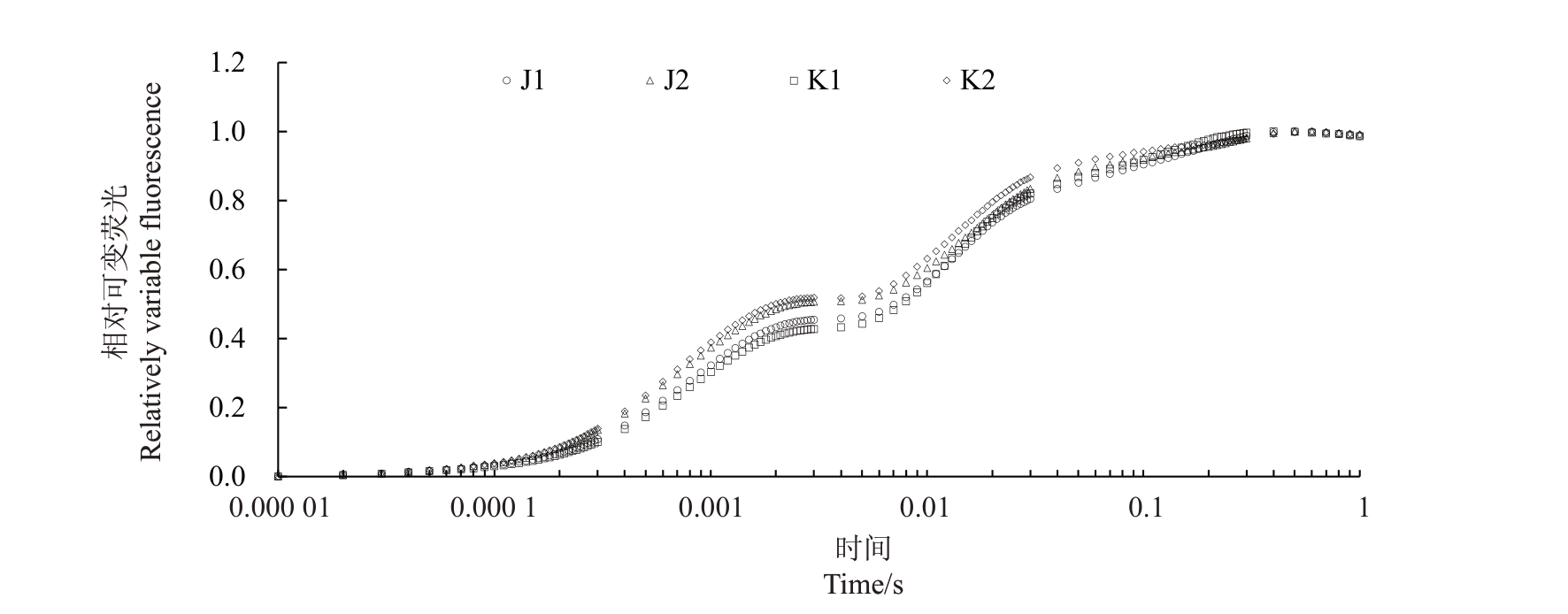
图3 不同栽培环境对软枣猕猴桃叶片快速叶绿素荧光特性的影响
Fig.3 Effect of different cultivation environments on the rapid chlorophyll fluorescence characteristics of A.arguta leaves
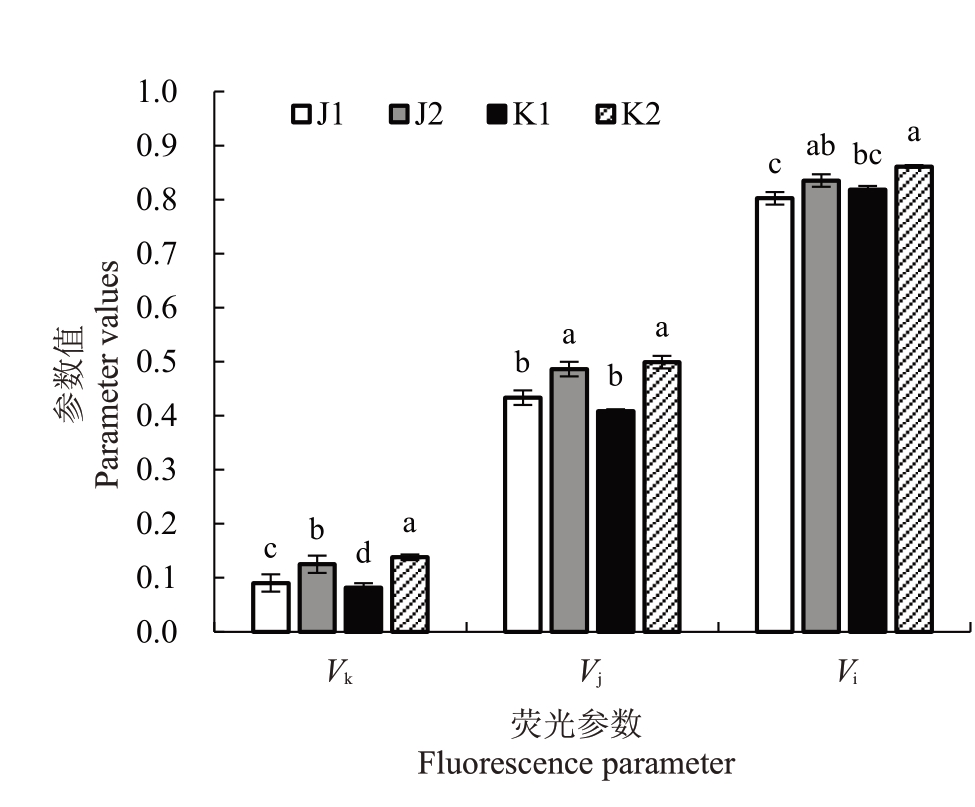
图4 不同栽培环境对软枣猕猴桃k、j、i 点相对可变荧光值的影响
Fig.4 Effect of different cultivation environments on the relative variable fluorescence values of k,j and i points of A.arguta
如图5 所示,对于单位活性的PSⅡ反应中心而言,不同栽培环境对软枣猕猴桃叶片的ETo/RC无影响,但温室栽培两个品种的ABS/RC、TRo/RC 值显著高于露地;温室栽培使两个品种反应中心吸收、耗散、捕获的光能增加,但用于电子传递的能量无显著变化。在温室内两个品种的DIo/CSm均显著升高,说明温室内的高温使软枣猕猴桃叶片单位面积的热耗散增加。
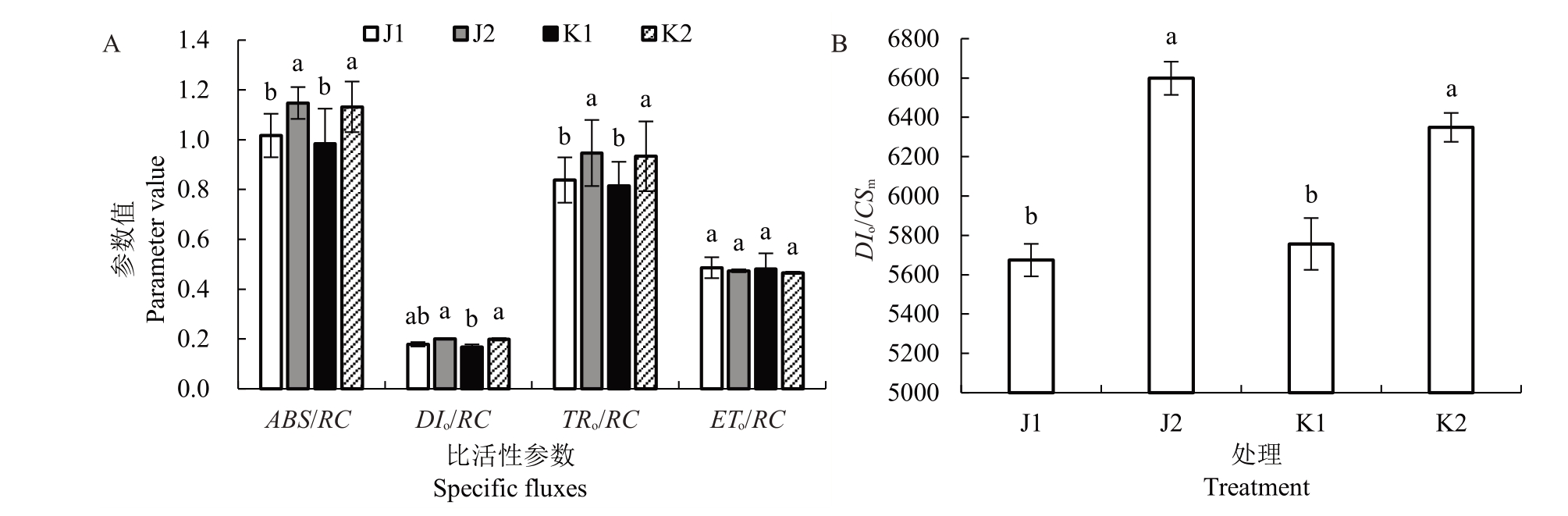
图5 不同栽培环境对软枣猕猴桃JIP-test 比活性参数与单位叶面积热耗散的影响
Fig.5 Effect of different cultivation environments on JIP-test specific fluxes and heat dissipation of excited leaf cross-section of A.arguta
A.比活性参数;B.单位叶面积热耗散。
A.Specific fluxes;B.Dissipated energy flux per cross-section.
2.4 栽培环境对软枣猕猴桃叶片结构的影响
如图6 所示,通过对不同环境下的叶片结构进行观察,两个品种在温室和露地栽培条件下均可见明显的栅栏组织与海绵组织,上下表皮为不规则单层细胞,栅栏组织多为长柱状,海绵组织多为不规则卵圆形,结构上无较大差异。
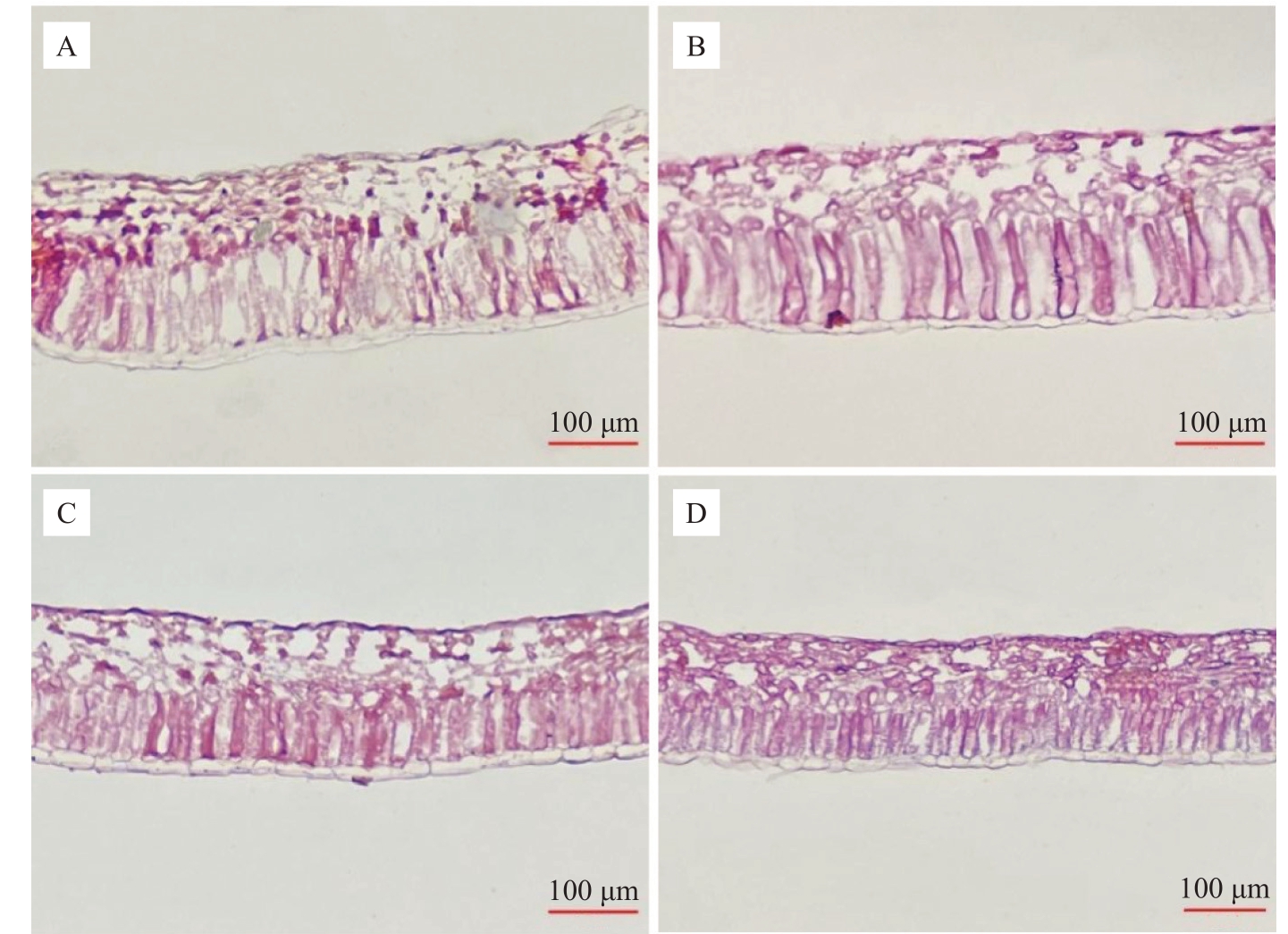
图6 不同栽培环境对软枣猕猴桃叶片结构的影响
Fig.6 Effects of different cultivation environments on stomatal structure of A.arguta
A、B、C、D 依次对应露地栽培佳绿、温室栽培佳绿、露地栽培魁绿、温室栽培魁绿。下同。
A,B,C and D stand for Jialü in open field,Jialü in greenhouse,Kuilü in open field,Kuilü in greenhouse,respectively.The same below.
由表3 可知,两个品种在温室中叶片的叶肉组织均受影响,表现在叶片厚度、栅栏组织、海绵组织及上下表皮厚度显著低于露地,叶片变薄,呈阴生植物特性。在温室中,佳绿的栅栏组织、海绵组织较魁绿发达,同时栅海比显著高于魁绿,表明佳绿的叶片较魁绿更为适应温室环境。
表3 不同栽培环境下软枣猕猴桃叶片叶肉组织差异分析
Table 3 Analysis of differences in mesophyll tissue of A.arguta leaves under different cultivation environments
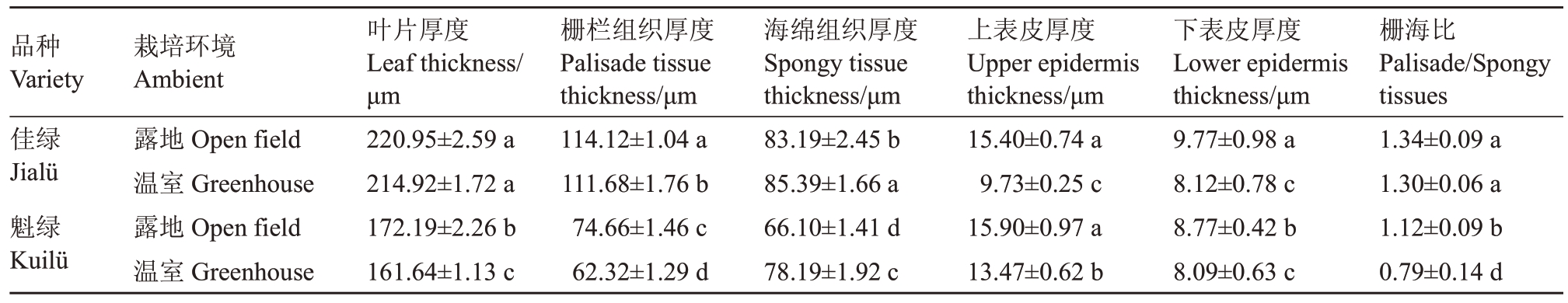
品种Variety佳绿Jialü魁绿Kuilü栽培环境Ambient露地Open field温室Greenhouse露地Open field温室Greenhouse叶片厚度Leaf thickness/μm 220.95±2.59 a 214.92±1.72 a 172.19±2.26 b 161.64±1.13 c栅栏组织厚度Palisade tissue thickness/μm 114.12±1.04 a 111.68±1.76 b 74.66±1.46 c 62.32±1.29 d海绵组织厚度Spongy tissue thickness/μm 83.19±2.45 b 85.39±1.66 a 66.10±1.41 d 78.19±1.92 c上表皮厚度Upper epidermis thickness/μm 15.40±0.74 a 9.73±0.25 c 15.90±0.97 a 13.47±0.62 b下表皮厚度Lower epidermis thickness/μm 9.77±0.98 a 8.12±0.78 c 8.77±0.42 b 8.09±0.63 c栅海比Palisade/Spongy tissues 1.34±0.09 a 1.30±0.06 a 1.12±0.09 b 0.79±0.14 d
如图7 所示,2 个品种在不同的栽培环境下,表皮气孔的大小、形状及开闭状态存在差异,露地环境下气孔明显较温室下密集。由表4 可知,温室条件下的2 个品种的气孔密度、气孔开张比、气孔开度、气孔面积、气孔长、气孔宽及气孔指数均呈显著低于露地栽培。
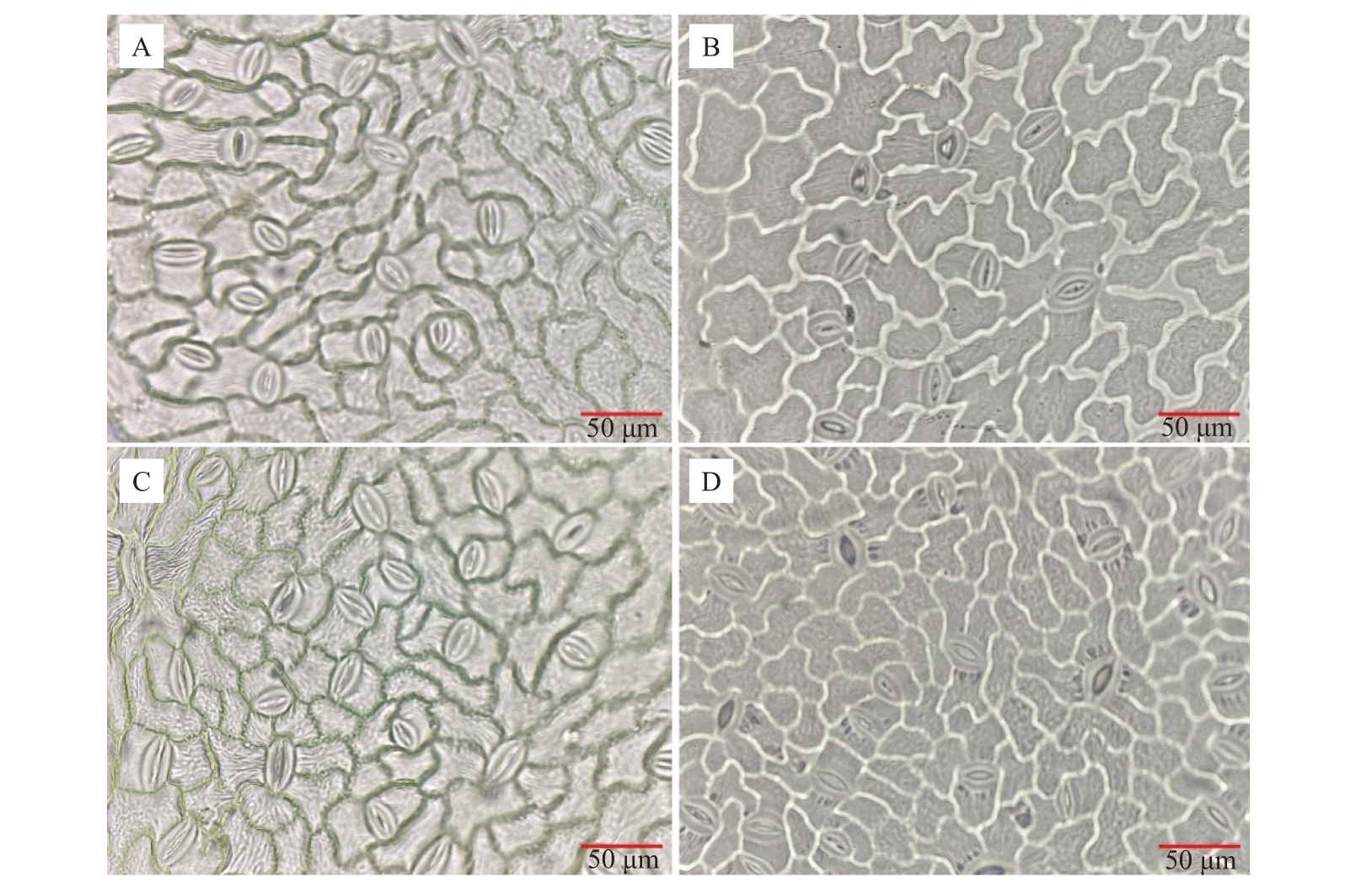
图7 不同栽培环境对软枣猕猴桃气孔结构的影响
Fig.7 Effects of different cultivation environments on stomatal structure of A.arguta
表4 不同栽培环境下软枣猕猴桃叶片气孔相关参数差异分析
Table 4 Analysis of differences in stomatal related parameters of A.arguta leaves under different cultivation environments
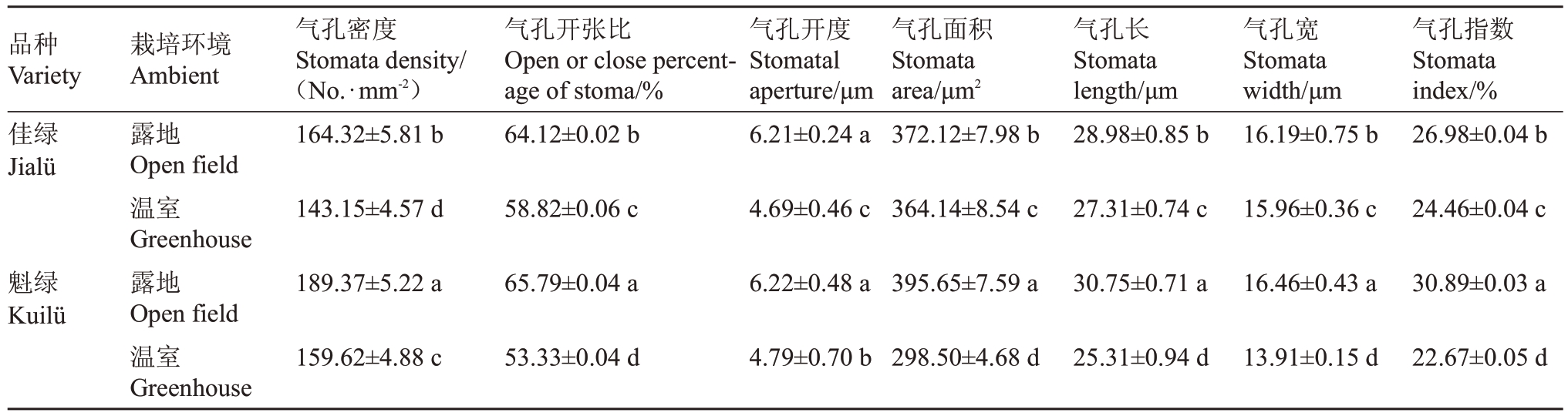
品种Variety佳绿Jialü气孔密度Stomata density/(No.∙mm-2)164.32±5.81 b气孔开张比Open or close percentage of stoma/%64.12±0.02 b栽培环境Ambient露地Open field温室Greenhouse露地Open field温室Greenhouse气孔开度Stomatal aperture/μm 6.21±0.24 a气孔面积Stomata area/μm2 372.12±7.98 b气孔长Stomata length/μm 28.98±0.85 b气孔宽Stomata width/μm 16.19±0.75 b气孔指数Stomata index/%26.98±0.04 b 143.15±4.57 d 58.82±0.06 c 4.69±0.46 c 364.14±8.54 c 27.31±0.74 c 15.96±0.36 c 24.46±0.04 c魁绿Kuilü 189.37±5.22 a 65.79±0.04 a 6.22±0.48 a 395.65±7.59 a 30.75±0.71 a 16.46±0.43 a 30.89±0.03 a 159.62±4.88 c 53.33±0.04 d 4.79±0.70 b 298.50±4.68 d 25.31±0.94 d 13.91±0.15 d 22.67±0.05 d
2.5 软枣猕猴桃温室栽培对果实品质的影响
对两个品种的果实进行单果质量与果形指数的调查(表5),发现在温室环境下,两个品种除魁绿果实横径外,其余指标均显著低于露地。温室栽培条件下佳绿果实的横、纵径分别比露地栽培低4.29%、8.79%;魁绿的纵径、侧径、单果质量分别比露地栽培低8.36%、2.41%、14.07%。综上所述,温室栽培对果实大小及单果质量影响较大。
表5 不同栽培环境对软枣猕猴桃果实表型特征的影响
Table 5 Effect of different cultivation environments on phenotypic characteristics of A.arguta
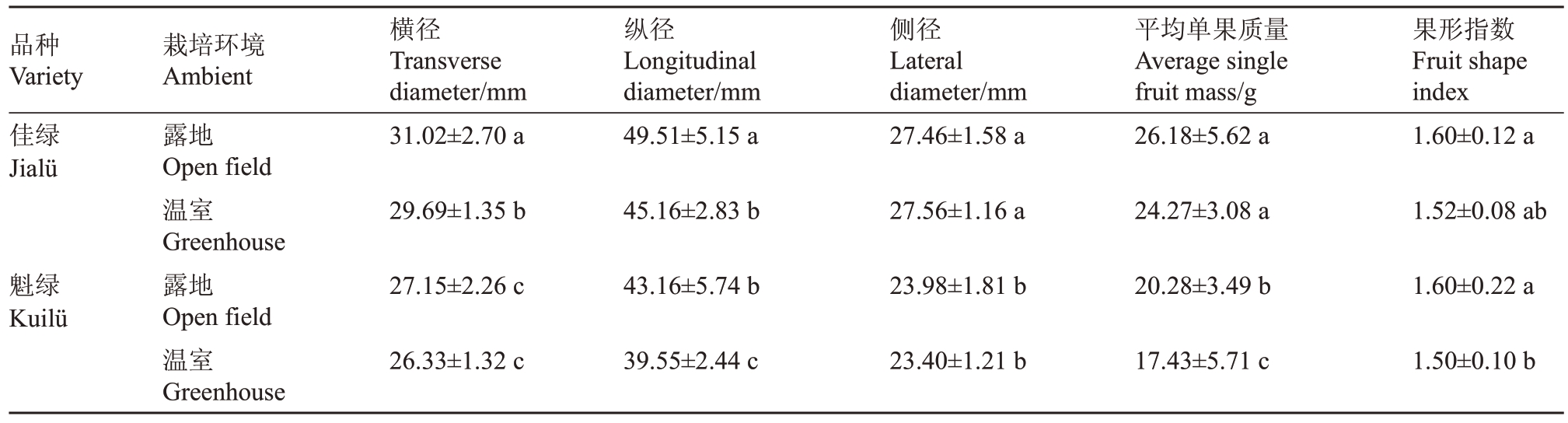
品种Variety佳绿Jialü横径Transverse diameter/mm 31.02±2.70 a纵径Longitudinal diameter/mm 49.51±5.15 a栽培环境Ambient露地Open field温室Greenhouse露地Open field温室Greenhouse侧径Lateral diameter/mm 27.46±1.58 a平均单果质量Average single fruit mass/g 26.18±5.62 a果形指数Fruit shape index 1.60±0.12 a 29.69±1.35 b 45.16±2.83 b 27.56±1.16 a 24.27±3.08 a 1.52±0.08 ab魁绿Kuilü 27.15±2.26 c 43.16±5.74 b 23.98±1.81 b 20.28±3.49 b 1.60±0.22 a 26.33±1.32 c 39.55±2.44 c 23.40±1.21 b 17.43±5.71 c 1.50±0.10 b
对果实进行生理指标的测定,结果见表6,温室栽培使两个品种成熟期提前70 d,佳绿与魁绿在温室栽培中,除可滴定酸含量显著上升外,可溶性固形物含量、可溶性糖含量以及维生素C 含量均无显著性差异,温室栽培对果实内在品质的影响较小。
表6 不同栽培环境对软枣猕猴桃果实内在品质的影响
Table 6 Effect of different cultivation environments on the internal quality of A.arguta
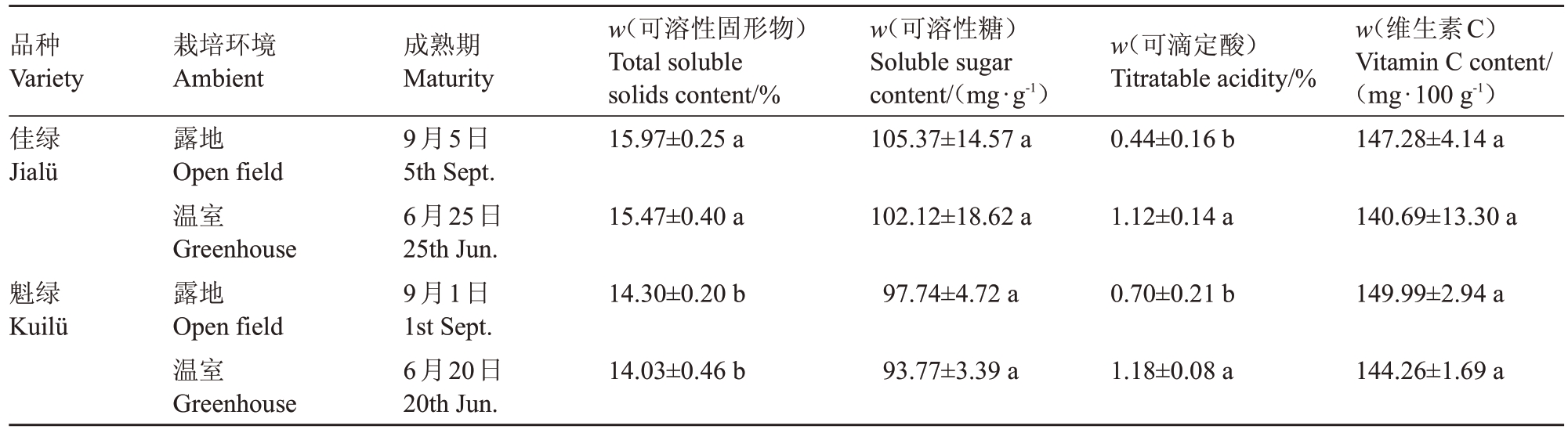
品种Variety佳绿Jialü w(可溶性固形物)Total soluble solids content/%15.97±0.25 a w(可溶性糖)Soluble sugar content/(mg∙g-1)105.37±14.57 a栽培环境Ambient露地Open field温室Greenhouse露地Open field温室Greenhouse成熟期Maturity 9月5日5th Sept.6月25日25th Jun.9月1日1st Sept.6月20日20th Jun.w(可滴定酸)Titratable acidity/%0.44±0.16 b w(维生素C)Vitamin C content/(mg∙100 g-1)147.28±4.14 a 15.47±0.40 a 102.12±18.62 a 1.12±0.14 a 140.69±13.30 a魁绿Kuilü 14.30±0.20 b 97.74±4.72 a 0.70±0.21 b 149.99±2.94 a 14.03±0.46 b 93.77±3.39 a 1.18±0.08 a 144.26±1.69 a
3 讨 论
温室栽培区别于露地栽培,其中光质差、日照时长短、高温高湿、CO2浓度低是温室环境的特点。果树在温室中栽培势必会受到环境因子的影响,首先改变叶片的形态建成,进而影响光合特性,最后导致果实品质发生变化。
不同植物对光照的需求有差异,研究表明,葡萄等其他树种对设施及弱光环境的适应性较为一致[22-25],导致叶片Pnmax与LSP 显著下降,与本研究中温室栽培的软枣猕猴桃叶片对环境适应的光响应模式相同。叶片的光适应性变化与叶片形态建成密不可分,其中温室内较低的Gs与Tr直接影响着气体交换,马微[26]对温室栽培葡萄的研究也表明其叶片的光合特性与之相似。
利用叶绿素荧光动力学方法可以快速、灵敏、无损伤地研究和探测各种逆境对植物光合生理的影响[27]。植物光合电子传递链中包括两个连续作用的光系统,光系统Ⅰ与光系统Ⅱ[28]。过高的温度与较弱的光照度通常会影响植物PSⅡ供体侧与受体侧的电子传递体活性,使其受到可逆或不可逆的损伤[29],吴久赟等[30]发现高温影响了PSⅡ中心捕获电子和电子传递,这与本研究中佳绿、魁绿在温室内的表现一致。由于本研究中的温室为玻璃温室,在冬春季时,于上午8:00—9:00 逐步打开顶、侧通风,维持温度在30 ℃以内,在夏季时,顶、侧通风则保持全天开放(阴雨天另作调整),温度过高时打开排风扇,但仍时有不可控因素导致30 ℃以上的温度,影响叶片,较高的Vj值证明在温室内软枣猕猴桃佳绿、魁绿叶片的PSⅡ的确受到了较高温度的影响,可能导致叶绿素类囊体膜的活性发生改变[31]。王振兴等[32]研究指出PSⅡ供体侧放氧复合体(OEC)的活性与受体侧电子传递链均易受到高温影响,明显的k 点随之出现,本研究表明温室中软枣猕猴桃叶片的Vk、Vj等值显著高于露地,这与多种热处理影响OEC敏感性的研究结果一致[33-35]。随着叶龄的增加,过剩光能和活性氧的不断积累可能会导致叶片出现日灼、早衰等现象[36-37]。
叶片的形态建成主要影响因素之一是光照,而形态建成后又会影响光合作用,所以叶片的形态建成对适应环境有重要作用[38]。由于温室中光照较差、温度和湿度较高等,两个品种叶片结构均为叶片大而薄、栅栏组织不发达,并且栅海比较低,气孔数较少,呈现出阴生叶的性质[39],且上下表皮细胞变薄,对逆境的适应性较差,符合温室中的植物特点。Chen等[40]研究指出,补光的马铃薯叶片的厚度显著高于对照,光照对叶片的形态建成具有决定作用。故有必要定期对温室表面玻璃进行清洗,以保证光照充足。此外,应根据物候期变化,通过排风或灌水方式调控湿度。适度补光可以促进植物生长[41-43],在光形态建成时期,夜间对软枣猕猴桃采用补光灯补光可以有效缓解短日照时长的影响。
由于温室栽培的软枣猕猴桃叶片光合能力低于露地,积累的同化物较少,因此果个小于露地。范盼盼[44]研究发现设施栽培的冬枣单果质量及果实纵横径显著低于露地栽培,与本研究结果一致。但陈海豹等[45]对杨梅的研究结果相反,产生这种差异的原因可能是不同种果树对温室栽培的适应性有差异。与露地栽培相比,温室中两个品种的可溶性固形物含量、可溶性糖含量及维生素C 含量与露地相比无显著差异,说明温室栽培对软枣猕猴桃的糖分及维生素C 积累无明显影响,但可滴定酸含量在温室条件下显著高于露地栽培,表明软枣猕猴桃的酸含量极易受温室条件影响,最终导致糖酸比下降。一方面由于温室内日照时长短于露地,有机物的积累较少,酸的分解能力降低;另一方面温室内的昼夜温差较小,夜间呼吸作用强于露地,营养消耗加剧,导致积累的有机物进一步流失。最终使糖含量下降,酸含量上升,糖酸比下降。
温室环境下两个品种间叶片结构及光能利用范围具有差异,具有较低LCP及较高栅海比等特征的软枣猕猴桃品种更适宜进行温室栽培,佳绿与魁绿在温室环境下的表现较为一致,但佳绿具有更高的栅海比,而魁绿叶片的LCP 较低,品种间的栽培特性差异应作为选择温室栽培品种的理论依据。后续计划对资源圃中其余品种进行温室栽培试验,比较需冷量、成熟期与温室栽培适应性的关系。对现有结果推测可知,大部分软枣猕猴桃品种进行温室栽培具有一定的可行性。
4 结 论
在温室栽培条件下,两个软枣猕猴桃品种对环境的适应性较为一致,首先体现为叶片形态建成趋向阴生叶,其次PSⅡ反应中心供体侧及受体侧活性较露地栽培降低,导致温室内叶片利用强光和低浓度CO2的能力低于露地,最终单果质量出现一定程度的下降,但内在品质无显著影响。
温室栽培中,不同品种对环境的适应性具有一定差异,在后续工作中,应根据品种的栽培特性,筛选适宜进行温室栽培的品种,这对建立科学、规范的软枣猕猴桃温室栽培具有重要的理论指导意义。
[1] 艾军.中国软枣猕猴桃种质资源[M].北京:中国农业出版社,2023.AⅠJun.Chinese Actinidia arguta germplasm resources[M].Beijing:China Agriculture Press,2023.
[2] 艾军.软枣猕猴桃栽培与加工技术[M].北京:中国农业出版社,2014.AⅠJun.Cultivation and processing technology of Actinidia arguta[M].Beijing:China Agriculture Press,2014.
[3] 许丽文,秦红艳,王衍莉,李嘉琪,张宝香,刘方圆.软枣猕猴桃成分分析及润肠通便功效的研究[J].特产研究,2023,45(3):18-23.XU Liwen,QⅠN Hongyan,WANG Yanli,LⅠJiaqi,ZHANG Bao xiang,LⅠU Fangyuan.Component analysis of Actinidia arguta and study on its effect of moistening intestines and defecating[J].Special Wild Economic Animal and Plant Research,2023,45(3):18-23.
[4] 齐秀娟,郭丹丹,王然,钟云鹏,方金豹.我国猕猴桃产业发展现状及对策建议[J].果树学报,2020,37(5):754-763.QⅠXiujuan,GUO Dandan,WANG Ran,ZHONG Yunpeng,FANG Jinbao.Development status and suggestions on Chinese kiwifruit industry[J].Journal of Fruit Science,2020,37(5):754-763.
[5] 方金豹,钟彩虹.新中国果树科学研究70 年:猕猴桃[J].果树学报,2019,36(10):1352-1359.FANG Jinbao,ZHONG Caihong.Fruit scientific research in New China in the past 70 years:Kiwifruit[J].Journal of Fruit Science,2019,36(10):1352-1359.
[6] 赵凤军.软枣猕猴桃温室栽培技术[J].北方果树,2020(6):24-26.ZHAO Fengjun.Cultivation techniques of Actinidia arguta in greenhouse[J].Northern Fruits,2020(6):24-26.
[7] 李玉婷,任利慧,王媛,周爱英,杨维,黄建.设施栽培模式下‘冬枣’光合效率限制因子研究[J].园艺学报,2023,50(3):647-656.LⅠYuting,REN Lihui,WANG Yuan,ZHOU Aiying,YANG Wei,HUANG Jian.Photosynthetic characteristics of Ziziphus jujuba‘Dongzao’under protected cultivation[J].Acta Horticulturae Sinica,2023,50(3):647-656.
[8] 戚行江,梁森苗,陈海豹,俞浙萍,孙鹂,郑锡良,张淑文.促早栽培对杨梅叶片形态及果实成熟与品质的影响[J].果树学报,2023,40(11):2403-2412.QⅠXingjiang,LⅠANG Senmiao,CHEN Haibao,YU Zheping,SUN Li,ZHENG Xiliang,ZHANG Shuwen.Effects of forcing cultivation on the leaf morphology,fruit ripening and quality of Myrica rubra[J].Journal of Fruit Science,2023,40(11):2403-2412.
[9] 孙晓华,叶丽红,刘杰才,李晓静,张之为,崔世茂,宋阳.日光温室不同结果枝类型对柑橘果实有机酸含量的影响[J].果树学报,2018,35(5):565-573.SUN Xiaohua,YE Lihong,LⅠU Jiecai,LⅠXiaojing,ZHANG Zhiwei,CUⅠShimao,SONG Yang.Effect of different types of fruiting shoots on organic acid content in citrus fruit grown in a solar greenhouse[J].Journal of Fruit Science,2018,35(5):565-573.
[10] 饶菁.避雨栽培下微环境对猕猴桃溃疡病防控和品质的影响研究[D].重庆:重庆三峡学院,2023.RAO Qing.Study on the effect of microenvironment on the prevention and control of Actinidia chinensis Planch.canker and quality under rain-shelter cultivation[D].Chongqing:Chongqing three gorges university,2023.
[11] 石广丽,艾军,秦红艳,赵滢,刘海双.不同软枣猕猴桃资源的需冷量[J].北方园艺,2018(16):81-84.SHⅠGuangli,AⅠJun,QⅠN Hongyan,ZHAO Ying,LⅠU Haishuang.Chilling requirement of different Actinidia argute[J].Northern Horticulture,2018(16):81-84.
[12] PⅠNTO D,SUT S,DALL'ACQUA S,DELERUE-MATOS C,RODRⅠGUES F.Actinidia arguta pulp:Phytochemical composition,radical scavenging activity,and in vitro cells effects[J].Chemistry&Biodiversity,2021,18(3):e2000925.
[13] 庄艳婷.长春地区10 种牡丹的引种栽培试验[D].长春:长春大学,2022.ZHUANG Yanting.Ⅰntroduction and cultivation of 10 resistant peonies in Changchun[D].Changchun:Changchun University,2022.
[14] 艾军,王英平,李昌禹,沈育杰.五味子花芽分化的形态学研究[J].特产研究,2009,31(4):22-24.AⅠJun,WANG Yingping,LⅠChangyu,SHEN Yujie.A study on morphogenesis of flower bud differentiation in Schisandra chinensis (Tnrcz) Baill.[J].Special Wild Economic Animal and Plant Research,2009,31(4):22-24.
[15] 王振兴,吕海燕,秦红艳,赵滢,刘迎雪,艾军,曹建冉,杨义明,沈育杰.盐碱胁迫对山葡萄光合特性及生长发育的影响[J].西北植物学报,2017,37(2):339-345.WANG Zhenxing,LÜ Haiyan,QⅠN Hongyan,ZHAO Ying,LⅠU Yingxue,AⅠJun,CAO Jianran,YANG Yiming,SHEN Yujie.Photosynthetic characteristics and growth development of Amur grape (Vitis amurensis Rupr.) under saline-alkali stress[J].Acta Botanica Boreali-Occidentalia Sinica,2017,37(2):339-345.
[16] 郭建辉,王振兴,孙丹,石广丽,张苏苏,艾军,于淼,刘雨萌,吴姝逸.不同光照强度对两份五味子资源PSⅡ活性的影响[J].西南农业学报,2022,35(12):2788-2793.GUO Jianhui,WANG Zhenxing,SUN Dan,SHⅠ Guangli,ZHANG Susu,AⅠJun,YU Miao,LⅠU Yumeng,WU Shuyi.Effect of different light intensity on PSⅡactivity of two resources of Schisandra chinensis[J].Southwest China Journal of Agricultural Sciences,2022,35(12):2788-2793.
[17] STRASSER R J,TSⅠMⅠLLⅠ-MⅠCHAEL M,SRⅠVASTAVA A.Analysis of the chlorophyll a fluorescence transient[M]//PAPAGEORGⅠOU G C,GOVⅠNDJEE.Chlorophyll a fluorescence,advances in photosynthesis and respiration.Dordrecht:Springer,2004,19:321-362.
[18] 王振兴,秦红艳,李昌禹,艾军,张庆田.刺五加不同叶位叶片光合特性及显微结构研究[J].特产研究,2011,33(3):30-33.WANG Zhenxing,QⅠN Hongyan,LⅠChangyu,AⅠJun,ZHANG Qingtian.Studies on the photosynthetic characteristics and microstructure of Acanthopanax senticosus (Rupr.et Maxim.)Harms.leaves at different position[J].Special Wild Economic Animal and Plant Research,2011,33(3):30-33.
[19] 徐清华.两种不同类型植物叶片形态解剖学指标观测方法的研究[D].哈尔滨:东北农业大学,2016.XU Qinghua.Research about observation methods of morphological and anatomical indicators in two different kinds of qlant leaves[D].Harbin:Northeast Agricultural University,2016.
[20] 张治安,陈展宇.植物生理学实验技术[M].长春:吉林大学出版社,2008.ZHANG Zhi’an,CHEN Zhanyu.Experimental techniques of plant physiology[M].Changchun:Jilin University Press,2008.
[21] 师恺丰.软枣猕猴桃种质资源在长春地区的生物学特性评价研究[D].长春:吉林农业大学,2022.SHⅠKaifeng.Study of the evaluation for biological characteristics of the Actinidia arguta germplasm resources in Changchun area[D].Changchun:Jilin Agricultural University,2022.
[22] 张银凤,蔡洪月,彭金根,刘学军,谢利娟,张华,王艳梅.深圳城市公园不同栽植环境对毛棉杜鹃生长的影响[J].南京林业大学学报(自然科学版),2023,47(2):197-204.ZHANG Yinfeng,CAⅠHongyue,PENG Jingen,LⅠU Xuejun,XⅠE Lijuan,ZHANG Hua,WANG Yanmei.Effects of different planting environments on the growth of Rhododendron moulmainense in Shenzhen urban parks[J].Journal of Nanjing Forestry University(Natural Sciences Edition),2023,47(2):197-204.
[23] 王海波,王孝娣,史祥宾,王宝亮,郑晓翠,刘凤之.葡萄不同品种对设施环境的适应性[J].中国农业科学,2013,46(6):1213-1220.WANG Haibo,WANG Xiaodi,SHⅠXiangbin,WANG Baoliang,ZHENG Xiaocui,LⅠU Fengzhi.Environmental adaptability of different grape cultivars in greenhouse[J].Scientia Agricultura Sinica,2013,46(6):1213-1220.
[24] 唐银,杨培蓉,吕宁宁,刘子晗,钟淑芳,黄金华,沈子宇,郑雪燕,许珊珊,曹光球,叶义全.遮阴对杉木幼苗生长及光合特性的影响[J].应用与环境生物学报,2023,29(5):1084-1092.TANG Yin,YANG Peirong,LÜ Ningning,LⅠU Zihan,ZHONG Shufang,HUANG Jinhua,SHEN Ziyu,ZHENG Xueyan,XU Shanshan,CAO Guangqiu,YE Yiquan.Effects of shading on growth and photosynthetic characteristics of Cunninghamia lanceolata seedlings[J].Chinese Journal of Applied and Environmental Biology,2023,29(5):1084-1092.
[25] 王志强,何方,牛良,刘淑娥.设施栽培油桃光合特性研究[J].园艺学报,2000,27(4):245-250.WANG Zhiqiang,HE Fang,NⅠU Liang,LⅠU Shue.A comparative research on photosynthesis of nectarine grown inside and outside greenhouses[J].Acta Horticulturae Sinica,2000,27(4):245-250.
[26] 马微.吐鲁番设施与露地栽培葡萄的生长发育差异分析[D].乌鲁木齐:新疆农业大学,2016.MA Wei.Analysis on difference of growth and development of grape in protected and open field cultivation in Turpan[D].Urumqi:Xinjiang Agricultural University,2016.
[27] 陈建明,俞晓平,程家安.叶绿素荧光动力学及其在植物抗逆生理研究中的应用[J].浙江农业学报,2006,18(1):51-55.CHEN Jianming,YU Xiaoping,CHENG Jia’an.The application of chlorophyll fluorescence kinetics in the study of physiological responses of plants to environmental stresses[J].Acta Agriculturae Zhejiangensis,2006,18(1):51-55.
[28] APPENROTH K J,STÖCKEL J,SRⅠVASTAVA A,STRASSER R J.Multiple effects of chromate on the photosynthetic apparatus of Spirodela polyrhiza as probed by OJⅠP chlorophyll a fluorescence measurements[J].Environmental Pollution,2001,115(1):49-64.
[29] 王振兴,艾军,李晓红,陈丽,沈育杰.不同胁迫对软枣猕猴桃叶片光系统活性的影响[J].华北农学报,2011,26(S1):69-73.WANG Zhenxing,AⅠJun,LⅠXiaohong,CHEN Li,SHEN Yujie.Effects of different stress on activity of photosystems in leaves of Actinidia arguta (Seib.et Zucc.) Plangch.ex Miq.[J].Acta Agriculturae Boreali-Sinica,2011,26(S1):69-73.
[30] 吴久赟,徐桂香,李海峰,曾晓燕,姜建福,刘勇翔,魏亦农,任红松.高温胁迫对葡萄叶绿素荧光和光合特性参数的影响[J].新疆农业科学,2021,58(12):2274-2281.WU Jiuyun,XU Guixiang,LⅠHaifeng,ZENG Xiaoyan,JⅠANG Jianfu,LⅠU Yongxiang,WEⅠYinong,REN Hongsong.Effects of heat stress on chlorophyll fluorescence and photosynthetic characteristic parameters in grape (Vitis vinifera L.‘Manicure finger’)[J].Xinjiang Agricultural Sciences,2021,58(12):2274-2281.
[31] 陈贻竹,李晓萍,夏丽,郭俊彦.叶绿素荧光技术在植物环境胁迫研究中的应用[J].热带亚热带植物学报,1995,3(4):79-86.CHEN Yizhu,LⅠXiaoping,XⅠA Li,GUO Junyan.The application of chlorophyll fluorescence technique in the study of responses of plants to environmental stresses[J].Journal of Tropical and Subtropical Botany,1995,3(4):79-86.
[32] 王振兴,艾军,陈丽,范书田,何伟,秦红艳,赵滢.软枣猕猴桃叶片光系统Ⅱ活性对不同温度的响应[J].西北植物学报,2015,35(2):329-334.WANG Zhenxing,AⅠJun,CHEN Li,FAN Shutian,HE Wei,QⅠN Hongyan,ZHAO Ying.Activity of photosystems Ⅱin leaves of Actinidia arguta under different temperature treatments[J].Acta Botanica Boreali-Occidentalia Sinica,2015,35(2):329-334.
[33] ZHANG C,ZHANG W C,YAN H F,NⅠY X,AKHLAQ M,ZHOU J N,XUE R.Effect of micro-spray on plant growth and chlorophyll fluorescence parameter of tomato under high temperature condition in a greenhouse[J].Scientia Horticulturae,2022,306:111441.
[34] ZHANG L X,CHANG Q S,HOU X G,WANG J Z,CHEN S D,ZHANG Q M,WANG Z,YⅠN Y,LⅠU J K.The effect of high-temperature stress on the physiological indexes,chloroplast ultrastructure,and photosystems of two herbaceous peony cultivars[J].Journal of Plant Growth Regulation,2023,42(3):1631-1646.
[35] SHEN J S,CHENG H F,LⅠX Q,PAN X D,HU Y E,JⅠN S H.Beneficial effect of exogenously applied calcium chloride on the anatomy and fast chlorophyll fluorescence in Rhododendron ×pulchrum leaves following short-term heat stress treatment[J].Agronomy,2022,12(12):3226.
[36] 李化龙,王景红,张维敏,柏秦凤,张焘,潘宇鹰,权文婷.高温胁迫对猕猴桃叶片叶绿素荧光特性的影响[J].应用气象学报,2021,32(4):468-478.LⅠHualong,WANG Jinghong,ZHANG Weimin,BAⅠQinfeng,ZHANG Tao,PAN Yuying,QUAN Wenting.Effects of high temperature stress on leaf chlorophyll fluorescence characteristics of kiwifruit[J].Journal of Applied Meteorological Science,2021,32(4):468-478.
[37] STRASSERF R J,SRⅠVASTAVA A,GOVⅠNDJEE.Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria[J].Photochemistry and Photobiology,1995,61(1):32-42.
[38] 鲁菁.异质性光环境对不同品种大豆叶片形态结构及光合特性的影响[D].雅安:四川农业大学,2019.LU Jing.Effects of heterogeneous light on morphological structure and photosynthetic characteristics of soybean leaves[D].Ya’an:Sichuan Agricultural University,2019.
[39] XⅠE F C,SHⅠZ J,ZHANG G Y,ZHANG C T,SUN X Y,YAN Y,ZHAO W,GUO Z X,ZHANG L,FAHAD S,SAUD S,CHEN Y J.Quantitative leaf anatomy and photophysiology systems of C3 and C4 turfgrasses in response to shading[J].Scientia Horticulturae,2020,274:109674.
[40] CHEN L L,ZHANG K,GONG X C,WANG H Y,GAO Y H,WANG X Q,ZENG Z H,HU Y G.Effects of different LEDs light spectrum on the growth,leaf anatomy,and chloroplast ultrastructure of potato plantlets in vitro and minituber production after transplanting in the greenhouse[J].Journal of Ⅰntegrative Agriculture,2020,19(1):108-119.
[41] 母德锦,吴美珍,余琼芬,胡小龙.LED 红光对蚕豆幼苗生长和生理生化特性的影响[J].中国瓜菜,2023,36(2):35-41.MU Dejin,WU Meizhen,YU Qiongfen,HU Xiaolong.Effects of LED red light on growth,physiology and biochemistry features of Vicia faba seedlings[J].China Cucurbits and Vegetables,2023,36(2):35-41.
[42] 刘志强,朱新红,刘勇鹏,王清,李春,张婵,姜俊.夜间不同LED 补光时段对番茄幼苗生长生理指标的影响[J].中国瓜菜,2022,35(8):79-85.LⅠU Zhiqiang,ZHU Xinhong,LⅠU Yongpeng,WANG Qing,LⅠChun,ZHANG Chan,JⅠANG Jun.LED lighting periods at night affects the growth and development of tomato seedlings[J].China Cucurbits and Vegetables,2022,35(8):79-85.
[43] 王舒亚,徐威,唐中祺,王鹏,景涛,刘琪,马正宇,吕剑,郁继华.不同补光时长对日光温室西葫芦生长、品质及产量的影响[J].中国瓜菜,2020,33(4):23-27.WANG Shuya,XU Wei,TANG Zhongqi,WANG Peng,JⅠNG Tao,LⅠU Qi,MA Zhengyu,LÜ Jian,YU Jihua.Effects of different duration of light supplementation on growth,quality and yield of Cucurbita pepo in greenhouse[J].China Cucurbits and Vegetables,2020,33(4):23-27.
[44] 范盼盼.不同栽培环境及其对冬枣生长结果和效益的影响[D].阿拉尔:塔里木大学,2021.FAN Panpan.Different cultivation environments and their effects on the growth,fruits and benefits of winter jujube[D].Alar:Tarim University,2021.
[45] 陈海豹,戚行江,张淑文,俞浙萍,孙鹂,郑锡良,梁森苗.不同促成设施栽培模式对杨梅果实品质形成的影响[J].浙江农业科学,2023,64(8):1885-1891.CHEN Haibao,QⅠXingjiang,ZHANG Shuwen,YU Zheping,SUN Li,ZHENG Xiliang,LⅠANG Senmiao.Effects of different cultivation models in facilitation facilities on fruit quality formation of bayberry[J].Journal of Zhejiang Agricultural Sciences,2023,64(8):1885-1891.