酿酒葡萄的品质决定了所酿葡萄酒的质量[1],在西北干旱地区,少雨和春秋的冷凉气候使葡萄生育期变短,中晚熟品种存在成熟度不够、含糖量不足以及产量低等问题,这对酿酒葡萄产业的可持续发展造成了严重影响[2]。前人研究表明,通过外源性喷施天然或合成诱导物,如脱落酸[3]、赤霉素[4]、茉莉酸甲酯[5]等能够有效保障作物营养供应,提高抗逆性,增强光合作用,最终达到优质丰产的生产目的。
调环酸钙(Pro-Ca)是一种在工业上合成的低毒、无污染的植物生长调节剂[6],且被认为能够促进植物生长和改善果实品质。研究发现,外源Pro-Ca可保护水稻幼苗细胞结构完整性,提高水稻幼苗的叶片光能吸收和利用效率,从而增强光合作用[7],并通过调节抗氧化酶活性和AsA-GSH循环系统、增加渗透溶质积累、降低活性氧(ROS)损伤来提高大豆幼苗抗氧化能力[8]。前人研究已表明,Pro-Ca 可通过提高烤烟叶绿素、游离脯氨酸、可溶性糖和可溶性蛋白含量,增强超氧化物歧化酶(superoxide dismutase,SOD)活性并降低丙二醛(malonaldehyde,MDA)含量以提升烤烟的抗逆能力[9]。赵肖琼等[10]研究发现Pro-Ca 以减少玉米叶片中超氧阴离子自由基、过氧化氢、MDA含量和降低相对电导率(relative electric conductivity,Rec),增强叶片中SOD等4种抗氧化酶的活性以及促进脯氨酸、可溶性蛋白等4 种渗透调节物质的累积,提升植株抗氧化能力。此外,刘丽等[11]研究发现,在正常条件下叶面喷施Pro-Ca 可以增加富士苹果叶片叶绿素含量,提高果实品质。Medjdoub 等[12]通过对苹果树喷施Pro-Ca,提高了植株的净光合速率和蒸腾速率,增加了植株体内的叶绿素含量,有效调节苹果树营养生长与生殖生长之间的关系,解决了植株营养生长过剩的问题。但外源Pro-Ca 在霞多丽葡萄生长发育过程中对叶片生理特性及果实品质的影响鲜有报道,笔者采用Pro-Ca 泡腾粒剂,对霞多丽葡萄全树喷施,以探明Pro-Ca对葡萄生理特性和果实品质的影响,并筛选出适宜的应用浓度,为提升酿酒葡萄品质提供理论依据。
1 材料和方法
1.1 试验材料和试验地概况
植物材料:试验于2022 年4—10 月在甘肃农业大学葡萄园进行,以11 年生酿酒葡萄霞多丽为试材,选择长势一致、无病虫害的植株,株行距0.75 m×1.5 m,单臂篱架整形,南北走向。
供试药剂:为安阳全丰生物科技有限公司生产的施必达牌Pro-Ca泡腾粒剂。
试验地概况:试验地(N 36°5′~37°10′,E 103°34′~103°47′)海拔约1517 m,属于中温带气候区,四季分明,水热同季,光照充足,降水少,蒸发大,气候干燥,易干旱,年降水量349.90 mm,年蒸发量1 664.00 mm,年日照时数2 476.40 h。
1.2 试验设计
试验共设4 个Pro-Ca 质量浓度处理:200(T1)、400(T2)、600(T3)、800 mg∙L-1(T4),以蒸馏水处理作为对照(CK)。分别于初花后22、42、62、82 d进行整株喷施,以叶片开始滴液为准,每个处理设3次重复,每个重复5株,且在坐果后对长势一致的果穗挂牌标记。
叶片采样时间为处理后第3天上午08:00,即初花后25、45、65、85 d分别采样,初花后105 d果实达到生产成熟时再采样1次,共采样5次;果实采样时间分别为初花后45、65、85、105 d,共采样4次,样品均在液氮充分冷冻之后存放于-80 ℃超低温冰箱备用。
1.3 测定项目与方法
1.3.1 光合作用相关指标测定 光合色素测定:参照高俊凤[13]的方法测定叶绿素a(Chl a)、叶绿素b(Chl b)和类胡萝卜素(Car)含量,采用80%丙酮提取新鲜叶片叶绿素,使用紫外分光光度计测定。
光合气体交换参数测定:选取葡萄新梢从基部数第3~5 节位长势良好的功能叶片,于晴朗天气上午09:00—11:00,采用LⅠ-6400XT光合作用测量系统软件测定各处理葡萄同一功能叶片光合气体交换参数,即净光合速率(Pn)、气孔导度(Gs)、蒸腾速率(Tr)、胞间CO2浓度(Ci)[14],3 次重复,对首次测定的叶片进行编号,并挂牌标记用于下次测定。
参照胡琳莉[15]的方法测定叶绿素荧光参数,利用调制式叶绿素荧光成像仪(Walz,Effeltrich,Germany)进行测定。
1.3.2 抗氧化系统相关指标测定 利用陈刚等[16]
的方法测定叶片相对电导率。采用硫代巴比妥酸法[17]测定丙二醛含量:称量0.5 g 干净葡萄叶片,加入5 mL 10%TCA 溶液进行充分研磨,4000 r∙min-1离心10 min 提取,使用移液枪吸取2 mL 上清液后,加入2 mL 0.6%TBA 沸水浴15 min,待试管冷却后进行离心,分别测定450、532、600 nm处吸光值并计算MDA 含量。参照曾钰[18]的方法测定游离脯氨酸(Pro)含量。
利用分光光度法测定过氧化氢(H2O2)含量[19],并稍作修改。称量0.5 g干净葡萄叶片,加入1.5 mL预冷的丙酮充分研磨成匀浆后,3000 r ∙min-1离心10 min,弃残渣。吸取1 mL 上清液加入0.1 mL 50 g∙L-1硫酸钛,再加入0.2 mL浓氨水,待沉淀形成后,3000 r∙min-1离心10 min,弃去上清液,留下沉淀。采用预冷的丙酮将沉淀洗涤至白色后,加入2 mol∙L-1硫酸5 mL进行溶解,然后在415 nm处测定吸光值并计算过氧化氢含量。利用羟胺法测定[20]超氧阴离子(O2-)释放速率,并稍作修改。称量0.2 g干净叶片加入2 mL磷酸钾缓冲液(50 mmol∙L-1,pH 7.8)充分研磨,4 ℃下10 000 r∙min-1离心10 min。吸取0.2 mL上清液,加入0.2 mL 磷酸钾缓冲液(50 mmol∙L-1,pH 7.8)和0.2 mL 盐酸羟胺(10 mmol ∙L-1)充分混匀。在25 ℃孵育1 h 后,再加入0.2 mL 对氨基苯磺酸(17 mmol∙L-1)和0.2 mL α-萘胺(7 mmol∙L-1)充分混合20 min,测定530 nm吸光度。用NaNO2在530 nm处吸光度做标准曲线,然后计算超氧阴离子释放速率。
酶活性测定:称取0.2 g干净葡萄叶片,加入2 mL含1%PVP 的50 mmol∙L-1 PBS 缓冲液(pH=7)研磨为匀浆后,4 ℃下16 000 r∙min-1离心20 min 后取上清液测定SOD、过氧化物酶(peroxidase ,POD)、过氧化氢酶(catalase ,CAT)活性。采用氮蓝四唑法测定[21]SOD活性;利用愈创木酚法测定470 nm处吸光度的增加来计算POD活性[22];通过检测过氧化氢在240 nm 下变化量来计算CAT 活性[23]。参考Ramzi等[24]的方法测定抗坏血酸过氧化物酶(ascorbate peroxidase,APX)活性。
1.3.3 果实品质相关指标测定 利用手持PAL-1数显折射仪(ATAGO CO,LTD,日本)测定果实可溶性固形物含量;采用氢氧化钠滴定法[25]测定可滴定酸含量;固酸比用可溶性固形物含量与可滴定酸含量之比表示。参照李艺[26]的方法测定葡萄果皮总酚含量;参照汤丽华等[27]的方法测定单宁含量;参照刘政海等[28]的方法测定类黄酮含量。
1.4 数据分析
采用Excel 2019 进行所有数据整理和作图,采用SPSS 23.0 对所有数据进行单因素方差分析(Duncan 法)和差异显著性分析。
2 结果与分析
2.1 Pro-Ca对霞多丽葡萄叶片光合作用的影响
2.1.1 Pro-Ca对霞多丽葡萄叶片光合色素含量的影响 初花后随着叶片的生长和发育,各处理Chl a、Chl b 和Chl t 含量均呈先升后降的变化趋势(图1-A~C),并在初花后65 d 时达到峰值,各物候期随着Pro-Ca 处理浓度的增加叶片Chl a、Chl b 和Chl t 含量呈先升后降的变化趋势。其中,在初花后45、65、85和105 d时,T3处理的Chl a含量较对照分别显著增加了21.58%、21.92%、15.17%和26.47%;在初花后65 d 时T3 处理的Chl b 含量高于其他处理,较对照显著提高了14.43%;在初花后25、45、65、85 和105 d时,T2、T3处理的总叶绿素含量均显著高于对照,且T3处理优于T2处理,分别较对照显著增加了14.45%、20.75%、20.03%、24.39%和24.82%。由图1-D 可知,各处理Car含量均随叶片的生长和发育,呈先升后降的变化趋势,且随着Pro-Ca处理浓度的上升叶片Car含量也呈先升后降的变化趋势,其中T1、T2、T3 处理在初花后45 至105 d 均较对照有所提高,T3处理在初花后25、45、65、85和105 d时Car含量最高,分别较对照显著提高了10.06%、6.34%、19.21%、24.04%、25.34%。
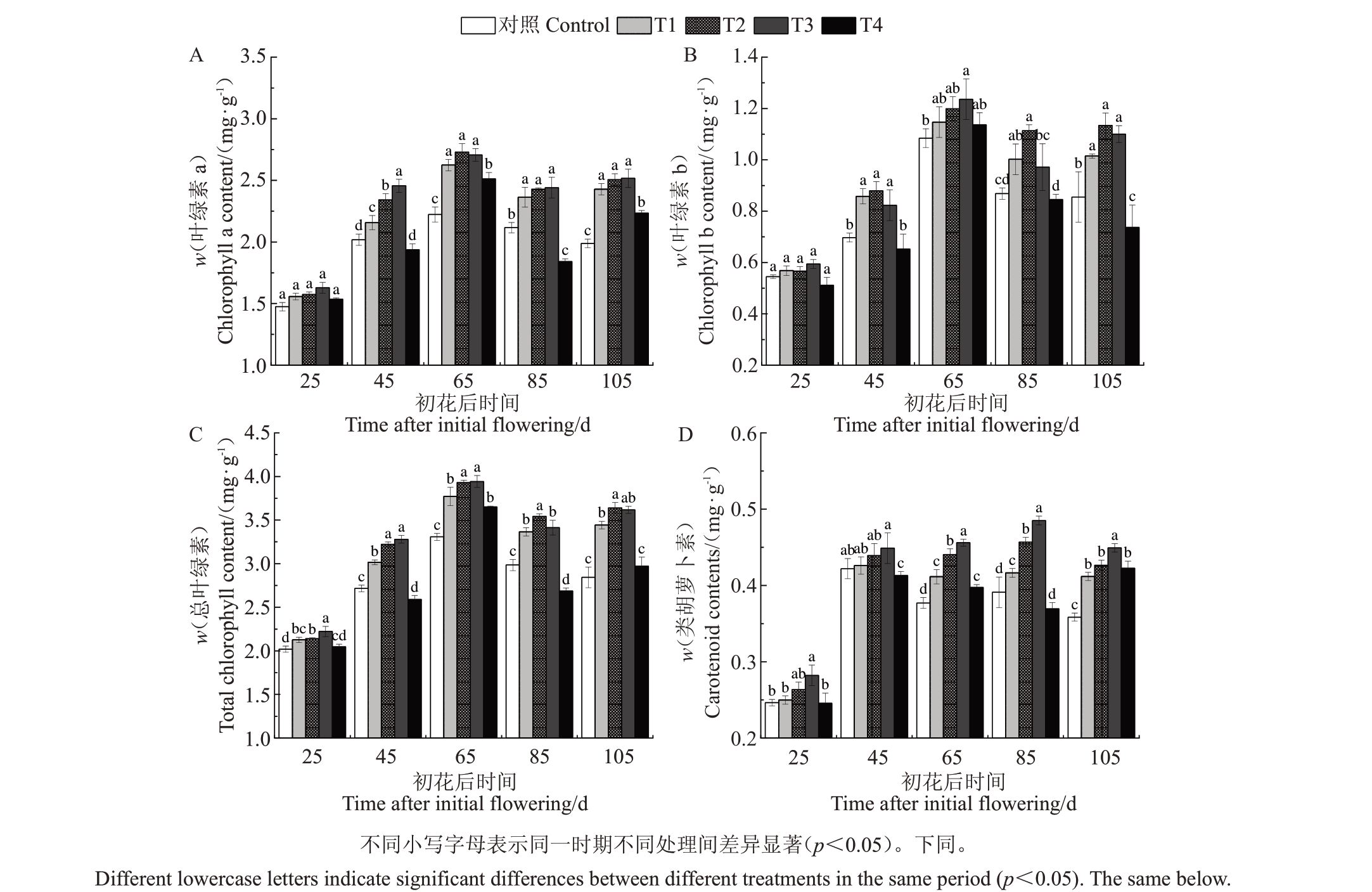
图1 Pro-Ca 对叶片叶绿素、类胡萝卜素含量的影响
Fig.1 Effects of calcium cycla mate on chlorophyll and carotenoid contents in leaves
2.1.2 Pro-Ca 对霞多丽葡萄叶片光合特性的影响由图2 所示,随着叶片的生长和发育,各处理叶片Pn、Tr、Gs和Ci均呈先升后降的变化趋势。叶片Pn在初花后45 d 时迅速升高(图2-A),且各时期随Pro-Ca 处理浓度的增加,均呈先升后降的变化趋势,与对照相比,除初花后105 d 的T1处理外,其余Pro-Ca处理均提高了叶片Pn,其中,T3处理效果最优,各时期平均较对照增加了18.69%;与对照相比,除初花后25和45 d外,其他各时期不同浓度的Pro-Ca处理均提升了叶片Tr(图2-B),且随着Pro-Ca处理浓度的增加,叶片Tr呈先升后降的变化趋势,其中,在初花后65、85和105 d时,T3处理对叶片Tr的提升效果较为突出,较对照分别显著提升10.50%、28.68%和8.64%;在初花后25~45 d叶片Gs增长迅速(图2-C),且与对照相比,除初花后45 d 的T1 处理外,各时期不同浓度的Pro-Ca 处理提升了叶片Gs,并随Pro-Ca处理浓度的增加,均呈先升后降的变化趋势,其中,在初花后45、65和85 d时T3处理的叶片Gs,分别较对照显著提升了24.69%、15.26%、7.24%;叶片Ci除初花后65和85 d,其他时期各处理之间均没有显著差异(图2-D),其中,在初花后65 和85 d 时T3 处理的Ci显著高于对照和其他处理,较对照分别显著增加了3.13%和5.55%。

图2 Pro-Ca 对葡萄叶片光合参数的影响
Fig.2 Effects of calcium cyclamate on photosynthetic parameters of grape leaves
2.1.3 Pro-Ca对霞多丽葡萄叶片叶绿素荧光参数的影响 随着叶片的生长和发育,各处理叶片Fv/Fm和Y(Ⅱ)均呈先升后降的变化趋势(图3-A~B),除初花后105 d 外,Fv/Fm和Y(Ⅱ)随Pro-Ca 处理浓度的增加也呈先升后降的变化趋势,在初花后105 d 时Pro-Ca处理对Fv/Fm有明显提升。其中,T2、T3处理的Fv/Fm在初花后25、45、85和105 d显著高于对照,且T3 处理优于T2 处理,分别较对照显著提升11.01%、13.93%、6.60%、7.55%。在初花后25、45、65、85 和105 d时,T3处理的Y(Ⅱ)在整个生育期分别较对照显著提高4.42%、7.35%、8.16%、3.69%、6.32%。由图3-C 可以看出,随着叶片的生长和发育,对照处理的qP先逐渐降低,然后在105 d时有所升高,且在初花后65~85 d 迅速降低,Pro-Ca 各处理的qP变化趋势并不一致。叶片qP随Pro-Ca处理浓度上升呈先升高后降低的变化趋势。其中,T3处理的qP在初花后45、65、85和105 d分别较对照显著增加6.67%、3.12%、10.00%、6.13%。
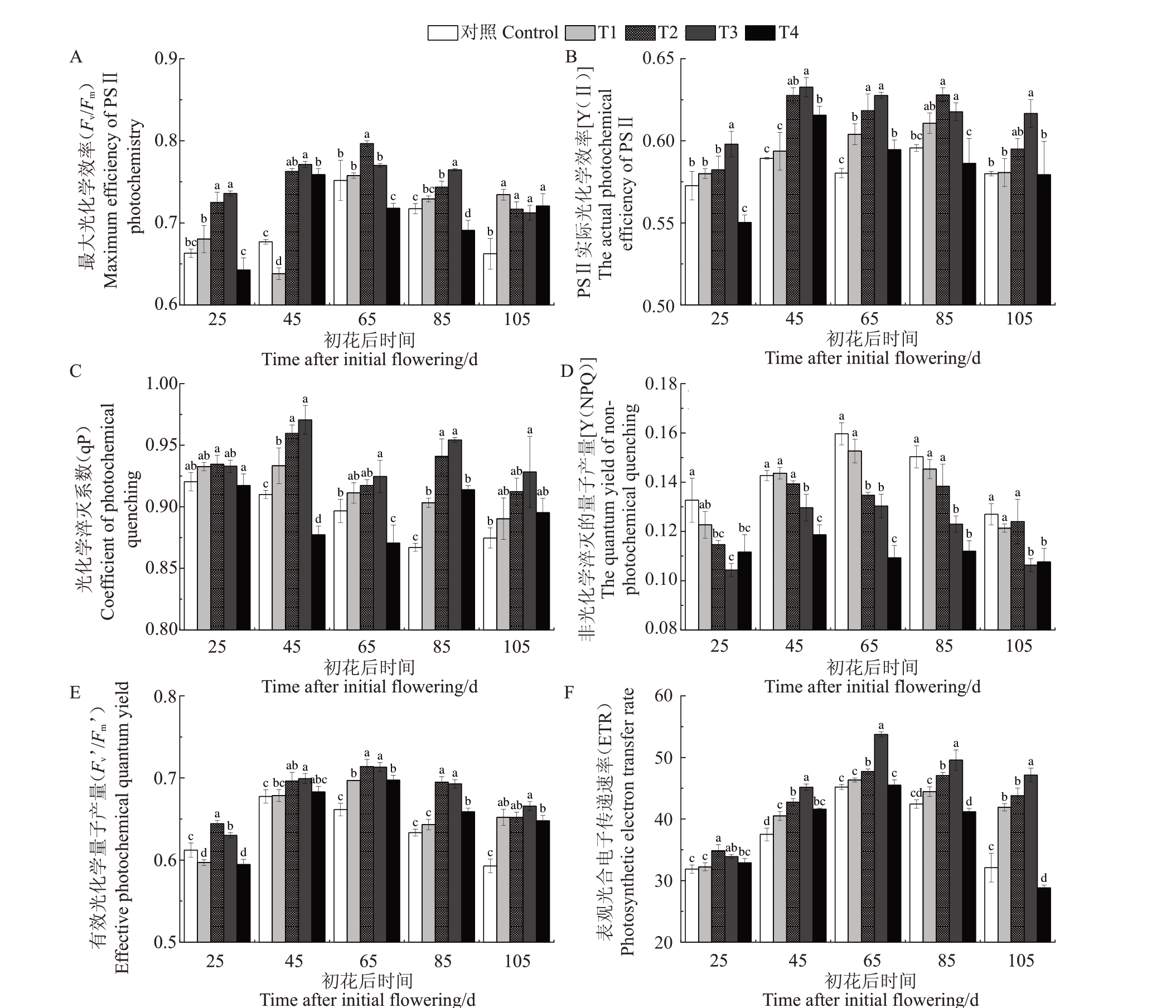
图3 Pro-Ca 对葡萄叶片叶绿素荧光参数的影响
Fig.3 Effects of prohexadione calcium on chlorophyll fluorescence parameters of grape leaves
由图3-D 可知,随着叶片的生长和发育,除T2和T4 处理外,各处理的Y(NPQ)呈先升后降的变化趋势,在初花后65 d 达到最大值;除初花后45 d的T1 处理外,各时期不同浓度Pro-Ca 处理的叶片Y(NPQ)较对照均有下降,且T3和T4处理显著降低了Y(NPQ);由图3-E~F可知,Fv’/Fm’和表观光合电子传递速率(ETR)随Pro-Ca 处理浓度的增加,均呈先升后降的变化趋势。其中,初花后25、45、65、85和105 d 时T3处理显著提升了叶片Fv’/Fm’和ETR,各时期分别平均较对照增加了7.05%和21.36%。
2.2 Pro-Ca对霞多丽葡萄叶片抗氧化特性的影响
2.2.1 Pro-Ca对霞多丽葡萄叶片膜透性及活性氧积累的影响 与对照相比,除T1 处理外,Pro-Ca 各处理于初花后25、45、65、85 和105 d 均降低了叶片相对电导率(Rec)(图4-A)。其中,初花后45 d 时T2、T3、T4 处理的叶片Rec 分别较对照显著降低了2.70%、2.73%、0.98%;在初花后45 d 时,T3、T4处理的MDA 含量较对照分别显著增加了9.64%、11.30%;在初花后65、85 和105 d 时O2-产生速率随Pro-Ca 处理浓度的增加呈先降后升的趋势(图4-C),其中,T1、T2、T3处理在初花后65和85 d显著降低了O2-产生速率,分别较对照降低20.24%、29.86%、34.42%和11.94%、13.78%、24.98%;与对照相比,T3处理的H2O2含量在初花后45 d较对照显著降低12.96%(图4-D);除初花后65 d 的T1 处理外,Pro-Ca处理在初花后45、65和85 d时均显著增加叶片Pro含量,且随Pro-Ca处理浓度的增加叶片Pro含量整体呈先升后降的变化趋势(图4-E),其中,T3处理的叶片Pro 含量在初花后45、65 和85 d 分别较对照显著提高42.02%、15.51%和24.60%。

图4 Pro-Ca 对葡萄叶片膜透性及活性氧积累的影响
Fig.4 Effects of Pro-Ca on membrane permeability and active oxygen accumulation in grape leaves
2.2.2 Pro-Ca 对霞多丽葡萄叶片保护酶活性的影响 如图5所示,随着葡萄植株的生长发育,Pro-Ca处理和对照处理的叶片APX、CAT、POD 和SOD 活性均呈先升后降的变化趋势。在初花后25、45、85和105 d时,T2处理的叶片APX活性最高,分别较对照提高了5.18%、9.70%、15.26%、26.53%(图5-A);Pro-Ca 处理和对照的CAT 活性在初花后25 和45 d差异均不显著(图5-B),T3处理的CAT活性在初花后65、85 和105 d 分别较对照显著提高16.83%、13.58%和31.70%;T3处理的POD活性在初花后45、65、85 和105 d 分别较对照显著提高25.62%、34.81%、12.00%、27.26%(图5-C);T3处理的SOD活性在初花后45、65 和105 d 分别较对照显著提高6.86%、13.15%、14.43%(图5-D)。

图5 Pro-Ca 对葡萄叶片抗氧化酶活性的影响
Fig.5 Effect of Pro-Ca on antioxidant enzyme activity in grape leaves
2.3 Pro-Ca对霞多丽葡萄成熟期果实品质的影响
不同浓度Pro-Ca对葡萄果实初花后105 d(果实达到成熟)的可溶性固形物、可滴定酸含量和固酸比,以及果皮总酚、单宁、总类黄酮含量的影响如表1 所示。与对照相比,除T4 处理的固酸比外,Pro-Ca 处理增加了果实可溶性固形物、可滴定酸含量和固酸比。其中,T1、T2、T3、T4处理均显著提高了果实可溶性固形物含量,分别较对照显著提升3.41%、7.18%、12.12%、4.17%;T2、T3、T4 处理显著增加可滴定酸含量,较对照显著增长7.02%、8.77%、7.02%。Pro-Ca 处理的果皮总酚含量与对照相比差异不显著。T3、T4处理使果皮类黄酮含量较对照显著增加21.9%、26.56%。Pro-Ca 处理降低了果皮单宁含量,其中,T1、T2、T3 处理较对照显著减少14.97%、16.24%、3.82%。综上所述,T3 处理可以显著提高葡萄成熟果实中可溶性固形物和果皮总类黄酮含量,增加果实固酸比和果皮总酚含量,且显著降低了果皮单宁含量。
表1 Pro-Ca 对葡萄成熟期果实品质的影响
Table 1 Effects of calcium cyclamate on fruit quality of grape at mature stage
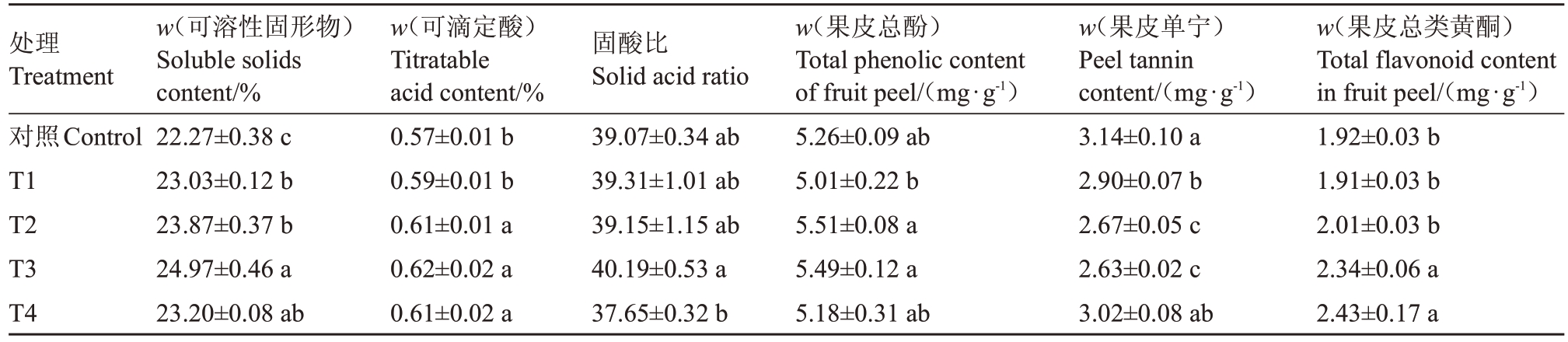
注:表中数据为平均值±标准差,不同小写字母表示同一时期不同处理间差异显著(p<0.05)。
Note:The data in the table are mean ± standard deviation.Different lowercase letters indicate significant differences between different treatments in the same period(p<0.05).
w(果皮总类黄酮)Total flavonoid content in fruit peel/(mg∙g-1)1.92±0.03 b 1.91±0.03 b 2.01±0.03 b 2.34±0.06 a 2.43±0.17 a处理Treatment对照Control T1 T2 T3 T4 w(可溶性固形物)Soluble solids content/%22.27±0.38 c 23.03±0.12 b 23.87±0.37 b 24.97±0.46 a 23.20±0.08 ab w(可滴定酸)Titratable acid content/%0.57±0.01 b 0.59±0.01 b 0.61±0.01 a 0.62±0.02 a 0.61±0.02 a固酸比Solid acid ratio 39.07±0.34 ab 39.31±1.01 ab 39.15±1.15 ab 40.19±0.53 a 37.65±0.32 b w(果皮总酚)Total phenolic content of fruit peel/(mg∙g-1)5.26±0.09 ab 5.01±0.22 b 5.51±0.08 a 5.49±0.12 a 5.18±0.31 ab w(果皮单宁)Peel tannin content/(mg∙g-1)3.14±0.10 a 2.90±0.07 b 2.67±0.05 c 2.63±0.02 c 3.02±0.08 ab
2.4 霞多丽葡萄叶片生理特性及果实品质的主成分分析
通过对霞多丽葡萄叶片及果实共29项指标进行主成分分析,结果如表2所示。霞多丽葡萄提取出3个主成分,各个主成分的特征值均大于1,且这3个主成分的累计方差贡献率为95.03%,说明酿酒葡萄霞多丽的3个主成分总体上可以反映出各指标的所有信息。对不同浓度Pro-Ca 处理霞多丽葡萄进行综合评价,综合得分排名由高到低依次为T3>T2>T4>T1>对照,根据得分高,处理效果好的原则,T3处理效果最佳,T2 次之,说明叶面喷施Pro-Ca 浓度为600 mg∙L-1(T3)效果最佳。(综合得分=方差贡献率1×FAC1+方差贡献率2×FAC2+方差贡献率3×FAC3)。
表2 Pro-Ca 处理对霞多丽葡萄的主成分得分表
Table 2 The principal component score table of Pro-Ca treatment on Chardonnay grapes
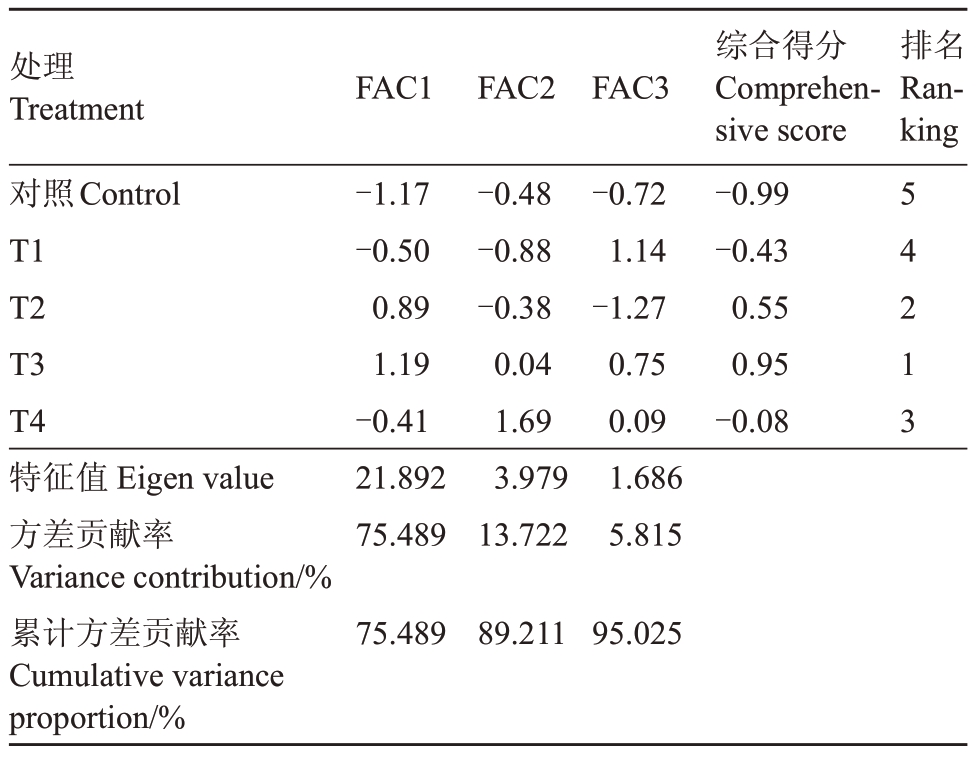
注:主成分分析中葡萄叶片相关指标均为各个物候期的平均值,果实品质指标为成熟期测定值。
Note: Ⅰn the principal component analysis, the related indexes of grape leaves were the average value of each phenological period, and the fruit quality index was the measured value of mature period.
处理Treatment FAC1 FAC2 FAC3排名Ranking对照Control T1 T2 T3 T4特征值Eigen value方差贡献率Variance contribution/%累计方差贡献率Cumulative variance proportion/%-1.17-0.50 0.89 1.19-0.41 21.892 75.489-0.48-0.88-0.38 0.04 1.69 3.979 13.722-0.72 1.14-1.27 0.75 0.09 1.686 5.815综合得分Comprehensive score-0.99-0.43 0.55 0.95-0.08 54213 75.489 89.211 95.025
3 讨 论
3.1 叶面喷施Pro-Ca 对霞多丽葡萄叶片光合作用的影响
植物90%的干物质积累是由光合作用产生的[29],且叶绿素在光合作用中起着非常关键的作用,其含量能够反映出植株对外部光照的适应性和光合作用的强度,高的叶绿素含量有助于维持高的光合速率,从而提高植株的光合速率[30-31],叶绿素主要参与了光能吸收、传递和转化。余明龙等[8]研究表明,施用Pro-Ca可提高大豆叶片叶绿素含量,从而提高植物叶片净光合速率,进而提高产量。试验研究发现,各时期叶面喷施适宜质量浓度(600 mg∙L-1)Pro-Ca 均显著增加葡萄叶片Chl a、Car 和Chl b 含量及Pn,这与Pro-Ca抑制植物体内生长素的合成有关,生长素在植物中参与调控叶绿素的合成和分解过程,Pro-Ca 通过抑制生长素的合成,可以促进叶绿素的积累[32]。Reekie 等[33]在草莓上的研究也发现,叶面喷施Pro-Ca 可以增加叶绿素含量,从而提高了Pn。光合作用不仅受叶片叶绿素含量的影响,也受气孔因素的限制,通过增加葡萄叶片的气孔密度和开度,减少叶片的气孔阻力,从而提高了叶片的气孔导度和净光合速率,而且蒸腾速率的改变与气孔的改变存在着一定的联系[34]。笔者的试验结果表明Pro-Ca处理提高了葡萄叶片Tr、Gs和Pn,与李瑶等[7]的研究结果一致,即外源Pro-Ca可以提高水稻幼苗的Gs和Tr,从而提高了叶片Pn。
作为光合作用的内部探针,植物体内的叶绿素荧光参数可以用来指示植物体内光合产物的吸收、转化和生理状态的变化,它不但会对碳循环的动态平衡产生影响,还会对植物的生长发育起重要作用[35-37]。植物叶片Fv/Fm(PSⅡ最大光能转换效率)值越大,PSⅡ光能转化效率越高,其PSⅡ活性越强[38];在叶绿素荧光参数中,Y(Ⅱ)指在叶片吸收的光能中用于光合电子转移的能量占比高低,高Y(Ⅱ)通常意味着高光合效率,具体包含了高效的光子吸收和电子转移[39],用于暗反应中碳同化的能量,PSⅡ功能降低,Y(Ⅱ)也随之下降。光合电子传递效率(ETR)主要反映了实际光强条件下的表观电子传递效率。qP是光合作用导致的光化学淬灭系数,它反映了PSⅡ天线色素在光合电子转移过程中所吸收的光能所占的比例[40]。NPQ 指PSⅡ天然色素吸收的不能用于光合电子转移,而以热能的形式耗散掉的光能部分,它反映了光系统对过剩光能的耗散能力[41-42]。笔者的试验中,不同时期叶面喷施不同浓度的Pro-Ca 均可不同程度提高叶片Fv/Fm、Y(Ⅱ)、qP、qN 和ETR 值,降低叶片NPQ 值,说明叶面喷施Pro-Ca 可增强酿酒葡萄的光合活性,提高其叶片PSⅡ光能转化效率和光能利用率,降低通过非光化学途径的能量耗散,最终导致光合产物的积累。
3.2 叶面喷施Pro-Ca对霞多丽葡萄叶片抗氧化特性的影响
植物体内抗氧化酶活性和活性氧、脯氨酸、MDA 含量以及叶片Rec 的变化反映了植物对生长环境的适应程度。其中,叶片Rec 可以体现出植物细胞膜被损伤的程度[43],MDA是一种植物细胞膜脂过氧化作用的结果产物,其易与细胞膜上的酶、蛋白等物质相结合从而破坏膜结构,所以MDA 可作为细胞膜被损害的标志之一[44]。试验结果表明,除T1处理外,Pro-Ca处理在初花后25、45、65、85和105 d均降低了叶片Rec;Pro-Ca对葡萄叶片MDA含量的影响并不明显,在初花后105 d 降低叶片MDA 含量,在初花后45 d 较高浓度的T3、T4 处理反而显著增加了其含量,表明较高浓度Pro-Ca可能会对葡萄造成药害,与李瑶[7]的研究结论相似,添加外源Pro-Ca可降低水稻幼苗Rec和MDA含量。活性氧是氧在植物体内正常代谢的副产物,其中,超氧阴离子、过氧化氢是植物体内自然存在的活性氧,在平衡细胞内部环境和信号传导中有重要意义,但过高水平的活性氧会对细胞结构造成损害[45]。研究发现叶面喷施Pro-Ca 可降低玉米幼苗超氧阴离子和过氧化氢含量[10]。试验研究结果表明,Pro-Ca 处理在初花后65 和85 d 较低浓度Pro-Ca 处理显著降低了叶片超氧阴离子产生速率,在初花后45和85 d较高质量浓度的T4处理(800 mg∙L-1)显著提高了超氧阴离子产生速率;Pro-Ca 处理对葡萄叶片过氧化氢含量在大部分时期无明显影响,在最后一次喷施Pro-Ca即初花后85 d,T4 处理显著提高了叶片过氧化氢含量,可能是高浓度的Pro-Ca溶液对葡萄叶片造成了损伤。脯氨酸是广泛存在于植物体内的氨基酸,参与了细胞渗透压调节,细胞内脯氨酸含量的提升可增强细胞的保水能力[46-48]。试验研究结果表明,除初花后65 d的T1处理外,Pro-Ca处理在初花后45、65、85 d显著提高了叶片脯氨酸含量,与前人研究相似,Pro-Ca提升了烤烟叶片游离脯氨酸含量[9]。
当植物体内活性氧浓度超过正常值时,植株本身的抗氧化系统就会将过量活性氧清除掉,其中SOD、POD、CAT、APX 在消除过剩活性氧中起关键作用。SOD 使植物细胞超氧阴离子歧化为过氧化氢和氧气,APX、CAT、和POD 进一步清除过氧化氢防止其对细胞产生毒害[49-50]。试验结果表明,随着葡萄叶片的生长发育,APX、CAT、POD、SOD活性均呈先增后降的变化趋势,这可能与叶片由幼嫩发育成熟直至衰老的过程有关,且在叶片生长发育前期Pro-Ca 处理对CAT、APX 活性影响并不明显,直至初花后105 d Pro-Ca 处理增强了CAT 和APX 活性。而Pro-Ca 处理在大部分时期显著提升POD 和SOD活性,与前人研究相似,Pro-Ca 增强了大豆CAT、POD、SOD等的活性[51]。
3.3 叶面喷施Pro-Ca 对霞多丽葡萄成熟期果实品质的影响
前人研究表明,Pro-Ca 处理可增加苹果可溶性固形物含量[11],提高轮台白杏光合效率、增加果实可溶性固形物含量[52]。笔者的试验研究结果也表明Pro-Ca 可以显著提高葡萄果实可溶性固形物含量,同时增大除T4处理外的果实固酸比,提升可滴定酸含量,与果实草酸、酒石酸、苹果酸含量提升相契合。Pro-Ca 处理显著降低除T4 处理外的果皮单宁含量,这可能与不同处理下果实成熟度有关。刘旭等[53]对葡萄不同成熟度的研究表明,随果实成熟度的加深果皮单宁逐渐降低,与笔者研究结果相一致。前人研究发现叶面喷施Pro-Ca可诱导玫瑰花类黄酮的形成[54]。Pro-Ca处理对果皮总酚含量无明显影响,而果皮总类黄酮含量在较高质量浓度Pro-Ca处理(600 mg∙L-1和800 mg∙L-1)后显著上升。因此,Pro-Ca处理可改善成熟期果实品质。
4 结 论
通过对不同浓度Pro-Ca处理下酿酒葡萄叶片生理特性及果实品质等29个指标进行主成分分析,综合评价了Pro-Ca处理对酿酒葡萄的影响,最后得出叶面喷施600 mg L-1Pro-Ca 为最适质量浓度,能够显著增强霞多丽葡萄叶片光合能力、降低膜脂过氧化水平并改善果实品质,对酿酒葡萄的生产具有指导意义。
[1] RUSTⅠONⅠL,ALTOMARE A,SHANSHⅠASHVⅠLⅠG,GRECO F,BUCCOLⅠERⅠR,BLANCO Ⅰ,COLA G,FRACASSETTⅠD.Microclimate of grape bunch and sunburn of white grape berries:Effect on wine quality[J].Foods,2023,12(3):621.
[2] JAHNKE G,SZŐKE B Á,STECKL S,SZÖVÉNYⅠÁ P,KNOLMAJERNÉ S G,NÉMETH C,JENEⅠB G,NYⅠTRAⅠNÉ S D Á.Delay in the ripening of wine grapes:Effects of specific phytotechnical methods on harvest parameters[J].Agronomy,2023,13(8):1963.
[3] 刘浩然,汪俏梅.脱落酸对番茄部分果实性状和营养品质的影响[J].核农学报,2020,34(12):2858-2864.LⅠU Haoran,WANG Qiaomei.Effect of abscisic acid on partial traits and nutritional quality in tomato fruit[J].Journal of Nuclear Agricultural Sciences,2020,34(12):2858-2864.
[4] 郭淑萍,杨顺林,杨玉皎,张永辉,孟富宣,何建军,张俊松,金杰.GA3 和CPPU 对无核翠宝葡萄果实品质的影响[J].果树学报,2022,39(10):1834-1844.GUO Shuping,YANG Shunlin,YANG Yujiao,ZHANG Yonghui,MENG Fuxuan,HE Jianjun,ZHANG Junsong,JⅠN Jie.Effect of GA3 and CPPU treatments on fruit quality of Wuhe Cuibao grape[J].Journal of Fruit Science,2022,39(10):1834-1844.
[5] 孙嘉茂,崔全石,王语晴,司雅静,时瑀繁,卜海东,袁晖,王爱德.苹果采前喷施EBR 与MeJA 对采后品质的影响[J].园艺学报,2022,49(10):2236-2248.SUN Jiamao,CUⅠQuanshi,WANG Yuqing,SⅠYajing,SHⅠYufan,BU Haidong,YUAN Hui,WANG Aide.Effects of brassinolides and methyl jasmonate spraying on the postharvest quality of apple fruit[J].Acta Horticulturae Sinica,2022,49(10):2236-2248.
[6] OWENS C L,STOVER E.Vegetative growth and flowering of young apple trees in response to prohexadione-calcium[J].Hort-Science,1999,34(7):1194-1196.
[7] 李瑶,郑殿峰,冯乃杰,冯胜杰,余明龙,黄露,张容郡,孟枫岩.调环酸钙对盐胁迫下水稻幼苗生长及抗性生理的影响[J].植物生理学报,2021,57(10):1897-1906.LⅠYao,ZHENG Dianfeng,FENG Naijie,FENG Shengjie,YU Minglong,HUANG Lu,ZHANG Rongjun,MENG Fengyan.Effects of prohexadione-calcium on growth and resistance physiology of rice seedlings under salt stress[J].Plant Physiology Journal,2021,57(10):1897-1906.
[8] 余明龙,黄露,郑殿峰,冯乃杰,牟保民,刘美玲.外源调环酸钙对盐碱胁迫下大豆幼苗生长及生理特性的影响[J].生态学杂志,2022,41(4):683-692.YU Minglong,HUANG Lu,ZHENG Dianfeng,FENG Naijie,MOU Baomin,LⅠU Meiling.Effects of exogenous prohexadione-calcium on growth and physiological characteristics of soybean seedlings under saline-alkali stress[J].Chinese Journal of Ecology,2022,41(4):683-692.
[9] 彭友,舒义鹏,叶征宇,徐益明,方平,杨宇.调环酸钙对盐胁迫下烤烟生理特性的影响[J].天津农业科学,2022,28(11):20-24.PENG You,SHU Yipeng,YE Zhengyu,XU Yiming,FANG Ping,YANG Yu.Effects of prohexadione calciumon physiological characteristicsof flue-cured tobaccounder salt stress[J].Tianjin Agricultural Sciences,2022,28(11):20-24.
[10] 赵肖琼,梁泰帅,李琪,张恒慧.调环酸钙对旱盐交叉胁迫下玉米幼苗生长及生理特性的影响[J].江苏农业科学,2023,51(1):99-103.ZHAO Xiaoqiong,LⅠANG Taishuai,LⅠQi,ZHANG Henghui.Ⅰmpacts of prohexadione-calcium on growth and physiological characteristics of maize seedlings under drought and salt cross stress[J].Jiangsu Agricultural Sciences,2023,51(1):99-103.
[11] 刘丽,高登涛,魏志峰,石彩云,徐玉西.调环酸钙对富士苹果生长及果实品质的影响[J].果树学报,2021,38(7):1084-1091.LⅠU Li,GAO Dengtao,WEⅠZhifeng,SHⅠCaiyun,XU Yuxi.Effects of prohexadione calcium on growth and fruit quality of Fuji apple[J].Journal of Fruit Science,2021,38(7):1084-1091.
[12] MEDJDOUB R,VAL J,BLANCO A.Ⅰnhibition of vegetative growth in red apple cultivars using prohexadione-calcium[J].The Journal of Horticultural Science and Biotechnology,2005,80(2):263-271.
[13] 高俊凤.植物生理学实验指导[M].北京:高等教育出版社,2006.GAO Junfeng.Experimental guidance for plant physiology[M].Beijing:Higher Education Press,2006.
[14] 张振兴,孙锦,郭世荣,童辉.钙对盐胁迫下西瓜光合特性和果实品质的影响[J].园艺学报,2011,38(10):1929-1938.ZHANG Zhenxing,SUN Jin,GUO Shirong,TONG Hui.Effects of supplemental calcium on the photosynthetic characteristics and fruit quality of watermelon under salt stress[J].Acta Horticulturae Sinica,2011,38(10):1929-1938.
[15] 胡琳莉.铵硝营养缓解小型大白菜幼苗弱光胁迫的生理和分子机制[D].兰州:甘肃农业大学,2016.HU Linli.Physiological and molecular mechanism of the alleviation role of ammonium:Nitrate in mini Chinese cabbage under low light intensity[D].Lanzhou:Gansu Agricultural University,2016.
[16] 陈刚,李胜.植物生理学实验[M].北京:高等教育出版社,2016.CHEN Gang,LⅠSheng.Plant physiology experiment[M].Beijing:Higher Education Press,2016.
[17] DⅠAO M,MA L,WANG J W,CUⅠJ X,FU A F,LⅠU H Y.Selenium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing chloroplast antioxidant defense system[J].Journal of Plant Growth Regulation,2014,33(3):671-682.
[18] 曾钰.外源脯氨酸对硼胁迫下枳砧生长及脯氨酸代谢的影响[D].武汉:华中农业大学,2021.ZENG Yu.Effects of exogenous proline on growth and proline metabolism of trifoliate orange rootstock under boron stress[D].Wuhan:Huazhong Agricultural University,2021.
[19] LⅠJ,YANG P,GAN Y T,YU J H,XⅠE J M.Brassinosteroid alleviates chilling-induced oxidative stress in pepper by enhancing antioxidation systems and maintenance of photosystem Ⅱ[J].Acta Physiologiae Plantarum,2015,37(11):222.
[20] VASQUEZ-VⅠVAR J,THⅠRUGNANAM K.Quantitative relationship between NADPH depletion and inhibition of NOX2-induced superoxide radical anion[J].Free Radical Biology and Medicine,2016,100:S30-S31.
[21] GⅠANNOPOLⅠTⅠS C N,RⅠES S K.Superoxide dismutases:Ⅰ.Occurrence in higher plants[J].Plant Physiology,1977,59(2):309-314.
[22] SHANNON L M,KAY E,LEW J Y.Peroxidase isozymes from horseradish roots.Ⅰ.Ⅰsolation and physical properties[J].Journal of Biological Chemistry,1966,241(9):2166-2172.
[23] HASANUZZAMAN M,HOSSAⅠN M A,FUJⅠTA M.Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings[J].Plant Biotechnology Reports,2011,5(4):353-365.
[24] MURSHED R,LOPEZ-LAURⅠF,SALLANON H.Microplate quantification of enzymes of the plant ascorbate-glutathione cycle[J].Analytical Biochemistry,2008,383(2):320-322.
[25] 陈屏昭,杜红波,秦燕芬,杨宏贵,李成树,莫正芬.果蔬品含酸量测定方法的改进及其应用[J].浙江农业科学,2013,54(4):451-453.CHEN Pingzhao,DU Hongbo,QⅠN Yanfen,YANG Honggui,LⅠChengshu,MO Zhengfen.Ⅰmprovement and application of the method for determining acid content in fruit and vegetable products[J].Journal of Zhejiang Agricultural Sciences,2013,54(4):451-453.
[26] 李艺.不同预处理方式对四种常见蔬菜营养品质的影响研究[D].杨凌:西北农林科技大学,2015.LⅠYi.Effects of different preprocessing methods on nutrition qualities of four common vegetables[D].Yangling:Northwest A&F University,2015.
[27] 汤丽华,张亮亮,张禾.紫外法和皮粉法测定塔拉单宁含量的对比研究[J].生物质化学工程,2022,56(6):31-35.TANG Lihua,ZHANG Liangliang,ZHANG He.Comparison on the determination of Tara tannin content with UV spectrophotometry and hide powder method[J].Biomass Chemical Engineering,2022,56(6):31-35.
[28] 刘政海,李六林,董志刚,谭伟,李晓梅,唐晓萍.梅鹿辄葡萄4个营养系果实酚类物质变化研究[J].中国酿造,2017,36(7):114-118.LⅠU Zhenghai,LⅠLiulin,DONG Zhigang,TAN Wei,LⅠXiaomei,TANG Xiaoping.Changes of phenolic compounds in four clones of Merlot grape[J].China Brewing,2017,36(7):114-118.
[29] TANG H,HU Y Y,YU W W,SONG L L,WU J S.Growth,photosynthetic and physiological responses of Torreya grandis seedlings to varied light environments[J].Trees,2015,29(4):1011-1022.
[30] 刘兆新,刘妍,刘婷如,何美娟,姚远,杨坚群,甄晓宇,栗鑫鑫,杨东清,李向东.控释复合肥对麦套花生光系统Ⅱ性能及产量和品质的调控效应[J].作物学报,2017,43(11):1667-1676.LⅠU Zhaoxin,LⅠU Yan,LⅠU Tingru,HE Meijuan,YAO Yuan,YANG Jianqun,ZHEN Xiaoyu,LⅠXinxin,YANG Dongqing,LⅠXiangdong.Effect of controlled-release compound fertilized on photosystem Ⅱperformance,yield and quality of intercropped peanut with wheat[J].Acta Agronomica Sinica,2017,43(11):1667-1676.
[31] 陆志峰,鲁剑巍,潘勇辉,鲁飘飘,李小坤,丛日环,任涛.钾素调控植物光合作用的生理机制[J].植物生理学报,2016,52(12):1773-1784.LU Zhifeng,LU Jianwei,PAN Yonghui,LU Piaopiao,LⅠXiaokun,CONG Rihuan,REN Tao.Physiological mechanisms in potassium regulation of plant photosynthesis[J].Plant Physiology Journal,2016,52(12):1773-1784.
[32] LⅠY,ZHOU H,FENG N J,ZHENG D F,MA G H,FENG S J,LⅠU M L,YU M L,HUANG X X,HUANG A Q.Physiological and transcriptome analysis reveals that prohexadione-calcium promotes rice seedling’s development under salt stress by regulating antioxidant processes and photosynthesis[J].PLoS One,2023,18(6):e0286505.
[33] REEKⅠE J Y,HⅠCKLENTON P R,STRUⅠK P C.Prohexadionecalcium modifies growth and increases photosynthesis in strawberry nursery plants[J].Canadian Journal of Plant Science,2005,85(3):671-677.
[34] 田钰君,许云飞,郭延平.叶面喷施钾肥对无花果光合生理和叶绿素荧光特性的影响[J].西北农林科技大学学报(自然科学版),2023,51(7):126-132.TⅠAN Yujun,XU Yunfei,GUO Yanping.Effects of foliar potassium application on photosynthetic physiology and chlorophyll fluorescence characteristics of Ficus carica L.[J].Journal of Northwest A&F University(Natural Science Edition),2023,51(7):126-132.
[35] 徐兴利,金则新,何维明,王兴龙,车秀霞.不同增温处理对夏蜡梅光合特性和叶绿素荧光参数的影响[J].生态学报,2012,32(20):6343-6353.XU Xingli,JⅠN Zexin,HE Weiming,WANG Xinglong,CHE Xiuxia.Effects of different day/night warming on the photosynthetic characteristics and chlorophyll fluorescence parameters of Sinocalycanthus chinensis seedlings[J].Acta Ecologica Sinica,2012,32(20):6343-6353.
[36] 王义婧,李岩,徐胜,何兴元,陈玮,吴娴.高浓度臭氧对美国薄荷(Monarda didyma L.)叶片光合及抗性生理特征的影响[J].生态学杂志,2019,38(3):696-703.WANG Yijing,LⅠYan,XU Sheng,HE Xingyuan,CHEN Wei,WU Xian.Effects of elevated ozone concentrations on photosynthetic and resistant physiological characteristics of Monarda didyma L.leaves[J].Chinese Journal of Ecology,2019,38(3):696-703.
[37] 齐晓媛,王文莉,胡少卿,刘梦雨,郑成淑,孙宪芝.外源褪黑素对高温胁迫下菊花光合和生理特性的影响[J].应用生态学报,2021,32(7):2496-2504.QⅠ Xiaoyuan,WANG Wenli,HU Shaoqing,LⅠU Mengyu,ZHENG Chengshu,SUN Xianzhi.Effects of exogenous melatonin on photosynthesis and physiological characteristics of chrysanthemum seedlings under high temperature stress[J].Chinese Journal of Applied Ecology,2021,32(7):2496-2504.
[38] 王晓芳,相昆,孙岩,崔冬冬,亓雪龙,王来平,翟衡,李勃.叶面肥对‘巨峰’葡萄光氧化胁迫的缓解效应[J].果树学报,2017,34(3):312-320.WANG X F,XⅠANG K,SUN Y,CUⅠD D,QⅠX L,WANG L P,ZHAⅠH,LⅠB.Protective effects of foliar fertilizer on‘Kyoho’grape under photooxidation[J].Journal of Fruit Science,2017,34(3):312-320.
[39] 蔡倩,白一光.果粮间作对仁用杏生长及叶绿素荧光参数的影响[J].农业科技与装备,2020(6):1-4.CAⅠQian,BAⅠYiguang.Effect of fruit-grain intercropping on growth and chlorophyll fluorescence parameters of kernel-apricot[J].Agricultural Science&Technology and Equipment,2020(6):1-4.
[40] 赵昕,宋瑞清,阎秀峰.接种AM 真菌对喜树幼苗生长及光合特性的影响[J].植物生态学报,2009,33(4):783-790.ZHAO Xin,SONG Ruiqing,YAN Xiufeng.Effects of arbuscular mycorrhizal fungal inoculation on growth and photosynthesis of Camptotheca acuminata seedlings[J].Chinese Journal of Plant Ecology,2009,33(4):783-790.
[41] 王建华,任士福,史宝胜,刘炳响,周玉丽.遮阴对连翘光合特性和叶绿素荧光参数的影响[J].生态学报,2011,31(7):1811-1817.WANG Jianhua,REN Shifu,SHⅠBaosheng,LⅠU Bingxiang,ZHOU Yuli.Effects of shades on the photosynthetic characteristics and chlorophyll fluorescence parameters of Forsythia suspensa[J].Acta Ecologica Sinica,2011,31(7):1811-1817.
[42] 裴斌,张光灿,张淑勇,吴芹,徐志强,徐萍.土壤干旱胁迫对沙棘叶片光合作用和抗氧化酶活性的影响[J].生态学报,2013,33(5):1386-1396.PEⅠBin,ZHANG Guangcan,ZHANG Shuyong,WU Qin,XU Zhiqiang,XU Ping.Effects of soil drought stress on photosynthetic characteristics and antioxidant enzyme activities in Hippophae rhamnoides Linn.seedings[J].Acta Ecologica Sinica,2013,33(5):1386-1396.
[43] 何凤,刘攀峰,王璐,杜兰英,庆军,杜庆鑫,杜红岩.干旱胁迫及复水对杜仲苗生理特性的影响[J].植物生理学报,2021,57(3):661-671.HE Feng,LⅠU Panfeng,WANG Lu,DU Lanying,QⅠNG Jun,DU Qingxin,DU Hongyan.Effect of drought stress and rewatering on physiological characteristics of Eucommia ulmoides seedling[J].Plant Physiology Journal,2021,57(3):661-671.
[44] 张露婷,吴江,梅丽,吴家胜.喜树种源耐盐能力评价及耐盐指标筛选[J].林业科学,2011,47(11):66-72.ZHANG Luting,WU Jiang,MEⅠLi,WU Jiasheng.Saline tolerance of Camptotheca acuminata provenances and the index selection for saline tolerance[J].Scientia Silvae Sinicae,2011,47(11):66-72.
[45] 马丹颖,季东超,徐勇,陈彤,田世平.活性氧调控植物细胞自噬的研究进展[J].植物学报,2019,54(1):81-92.MA Danying,JⅠDongchao,XU Yong,CHEN Tong,TⅠAN Shiping.Advances in the regulation on autophagy by reactive oxygen species in plant cells[J].Chinese Bulletin of Botany,2019,54(1):81-92.
[46] 王宁,袁美丽,陈浩,李真真,张铭鑫.干旱胁迫及复水对入侵植物节节麦幼苗生长及生理特性的影响[J].草业学报,2019,28(1):70-78.WANG Ning,YUAN Meili,CHEN Hao,LⅠZhenzhen,ZHANG Mingxin.Effects of drought stress and rewatering on growth and physiological characteristics of invasive Aegilops tauschii seedlings[J].Acta Prataculturae Sinica,2019,28(1):70-78.
[47] 张海娜,鲁向晖,金志农,李阳,王瑞峰,李宗勋,刘利昆.高温条件下稀土尾砂干旱对4 种植物生理特性的影响[J].生态学报,2019,39(7):2426-2434.ZHANG Haina,LU Xianghui,JⅠN Zhinong,LⅠYang,WANG Ruifeng,LⅠZongxun,LⅠU Likun.Effects of drought on physiological characteristics of seedlings of four species grown on rare earth mill tailings at high temperatures[J].Acta Ecologica Sinica,2019,39(7):2426-2434.
[48] 项洪涛,齐德强,李琬,郑殿峰,王月溪,王彤彤,王立志,曾宪楠,杨纯杰,周行,赵海东.低温胁迫下外源ABA 对开花期水稻叶鞘激素含量及抗寒生理的影响[J].草业学报,2019,28(4):81-94.XⅠANG Hongtao,QⅠDeqiang,LⅠWan,ZHENG Dianfeng,WANG Yuexi,WANG Tongtong,WANG Lizhi,ZENG Xiannan,YANG Chunjie,ZHOU Hang,ZHAO Haidong.Effect of exogenous ABA on the endogenous hormone levels and physiology of chilling resistance in the leaf sheath of rice at the flowering stage under low temperature stress[J].Acta Prataculturae Sinica,2019,28(4):81-94.
[49] 张俊霞,刘晓鹏,向极钎.植物抗氧化系统对逆境胁迫的动态响应[J].湖北民族学院学报(自然科学版),2015,33(4):435-439.ZHANG Junxia,LⅠU Xiaopeng,XⅠANG Jiqian.Dynamic response of antioxidant systems to adversity stress in plants[J].Journal of Hubei University for Nationalities (Natural Science Edition),2015,33(4):435-439.
[50] 曾元元,娄玉霞,曹同.匍枝青藓(Brachythecium procumbens)对铅胁迫的生理响应[J].上海师范大学学报(自然科学版),2010,39(5):544-550.ZENG Yuanyuan,LOU Yuxia,CAO Tong.The physiological response to lead stress of the moss Brachythecium procumbens[J].Journal of Shanghai Normal University (Natural Sciences),2010,39(5):544-550.
[51] FENG N J,YU M L,LⅠY,JⅠN D,ZHENG D F.Prohexadionecalcium alleviates saline-alkali stress in soybean seedlings by improving the photosynthesis and up-regulating antioxidant defense[J].Ecotoxicology and Environmental Safety,2021,220:112369.
[52] 章世奎,罗晓琴,王亚铜,侯忠友,吾买尔江·巴拉提,樊国全.调环酸钙对轮台白杏营养生长和果实品质的影响[J].新疆农业科学,2021,58(5):846-853.ZHANG Shikui,LUO Xiaoqin,WANG Yatong,HOU Zhong you,Wumaierjiang Balati,FAN Guoquan.Effects of prohexadione calcium on the growth and fruit quality of Luntaibaixing apricot[J].Xinjiang Agricultural Sciences,2021,58(5):846-853.
[53] 刘旭,陈敏,武轩,金小朵,张振文.赤霞珠葡萄转色后不同成熟度指标的变化[J].食品科学,2016,37(22):230-236.LⅠU Xu,CHEN Min,WU Xuan,JⅠN Xiaoduo,ZHANG Zhenwen.Changes in different maturity indices of cabernet Sauvignon (Vitis vinifera L.) grape after veraison[J].Food Science,2016,37(22):230-236.
[54] SCHMⅠTZER V,VEBERⅠC R,STAMPAR F.Prohexadione-Ca application modifies flavonoid composition and color characteristics of rose (Rosa hybrida L.) flowers[J].Scientia Horticulturae,2012,146:14-20.