硬度是果实品质分级的重要指标,直接影响果实机械采收、运输、贮藏加工等过程[1-2]。蓝莓(Vaccinium spp.)不同品种的果实硬度存在差异,硬肉品种在机械采收和长距离运输方面比软肉品种更有优势[3]。消费者对鲜食蓝莓的喜好程度与果实硬度呈显著正相关[4],高硬度果实有更高的脆度和弹性反馈力[5],更受消费者欢迎,培育高硬度果实品种对蓝莓产业健康可持续发展至关重要[3,6]。目前关于蓝莓果实硬度的报道集中在采后贮藏环节[4,7-8],关于发育时期果实硬度的报道较少,但越来越多研究表明发育期具有更高硬度的果实在成熟时倾向于保持其硬度特征[9]。
果实硬度下降与细胞壁结构、组分含量变化密切相关[10-12],植物细胞壁一般由纤维素、半纤维素和果胶组成,随着果实的发育、成熟或贮藏时间延长,细胞壁物质水解导致果实软化[12-13]。已有研究表明果胶甲酯酶(pectin methylesterase,PME)与果实软化无直接联系[14];多聚半乳糖醛酸酶(polygalacturonase,PG)酶活力在硬肉葡萄果实软化阶段几乎没有变化[15],沉默PG基因不会改变番茄果实可溶性果胶含量[16]。但在沉默PL基因的番茄果实中,果胶可溶性和解聚度都会受到抑制。多个物种中报道了果胶裂解酶(pectin lyase,PL)参与果实软化。香蕉(Musa acuminata)和草莓(Fragaria ananassa)果实成熟时期PL 酶活力显著增加[17-18],葡萄(Vitis vinifera)VvPL11 在果实发育期高表达,过表达VvPL11基因的番茄果实相比对照果实硬度降低[15]。沉默SlPL基因可抑制果肉细胞胞间层果胶降解,减缓番茄果实硬度下降[16]。沉默PL基因的草莓(F.ananassa)果实硬度显著高于对照果实[18]。果胶可溶性变化是蓝莓果实软化的主要原因之一,但其具体机制尚不完全清楚[19]。
笔者课题组前期采用优化的方法测定了36 个蓝莓品种的果实硬度,筛选得到硬肉品种Star 和软肉品种O’Neal,2 个品种遗传背景相似(O’Neal 为Star父本),果实硬度却存在显著差异[3],果胶裂解酶基因在果实硬度差异中发挥的作用尚不清晰。笔者在本研究中测定2 个蓝莓品种果实不同时期纤维素、半纤维素和果胶含量,比较果实不同发育时期的果肉细胞,分析细胞壁组分与果实硬度的相关性,基于果胶裂解酶基因全基因组鉴定和不同发育时期果实转录组数据,笔者课题组筛选到在蓝莓不同硬度果实中存在表达丰度差异的2 个VcPL 基因,克隆VcPLs 基因并进行番茄转基因功能验证,以期探明VcPL 基因在蓝莓硬肉品种和软肉品种果实硬度差异中的作用,丰富果实硬度变化研究机制,为选育高硬度蓝莓品种提供参考。
1 材料和方法
1.1 试验材料
蓝莓果实采自浙江师范大学蓝莓种质资源圃。随机选取同一区组的Star和O’Neal蓝莓树各20株,参考沈朱俐等[3]对蓝莓果实发育时期的划分,依据大小和着色程度筛选6个发育时期(图1)的足量(≥150个)果实,室温条件下放置1 h去除田间热,去除伤果和病果,选择果实大小和发育状态相似的100个果实。两个品种分别随机取60 个果实进行硬度测定,取6个果实用于果肉细胞解剖结构观察,剩余果实使用液氮速冻后放置在-80 ℃备用。
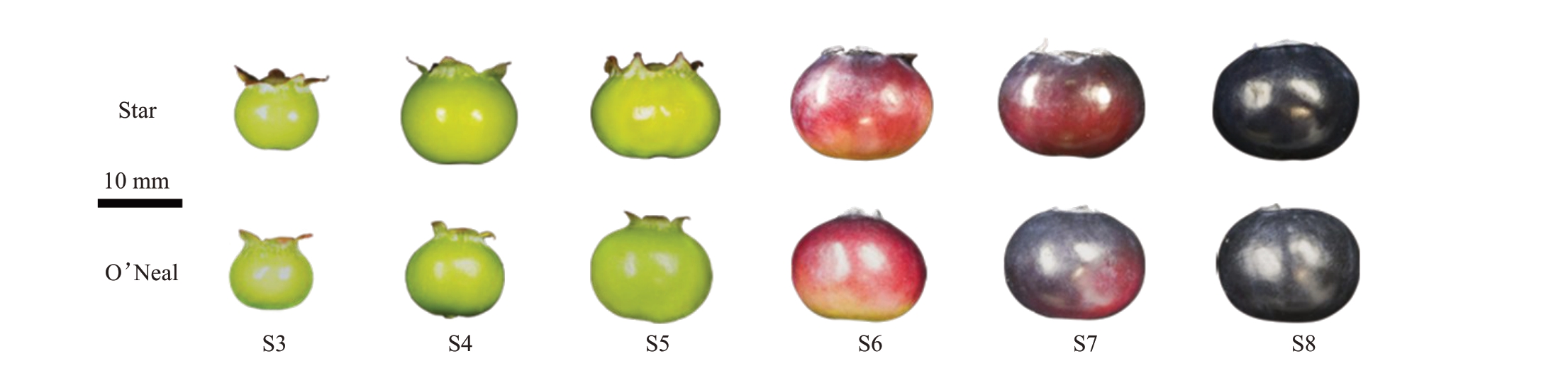
图1 O’Neal 和Star 不同发育时期的果实
Fig.1 O’Neal and Star fruits at different developmental stages
S3.坐果期;S4.膨大期;S5.白绿期;S6.红果期;S7.紫果期;S8.成熟期。下同。标尺为10 mm。
S3.Fruit setting;S4.Expansion;S5.White green;S6.Red;S7.Purple;S8.Dark blue.The same below.The bar is 10 mm.
1.2 果实硬度测定
使用Firmtech FT7 果实硬度计(UP GmbH Firmensitz,德国),参照沈朱俐等[3]的方法测定蓝莓不同发育时期果实的硬度。测量模式选择形变阈值模式(Deflection threshold),形变阈值根据果实发育时期设置为1.0(S3,坐果期;S4,膨大期)和2.0 mm(S5,白绿期;S6,红绿期;S7,紫果期;S8,成熟期)。将硬度计探头垂直对准果实赤道面进行测量,使用FT7 Control 软件(UP GmbH Firmensitz,德国)记录探头挤压的反馈质量并计算果实硬度。
1.3 果实解剖结构观察
将新鲜蓝莓果实纵向切开,切取边长为5 mm左右的带皮果肉小块,快速置于FAA固定液中。充分固定后分别用梯度乙醇(75%、85%、95%、两次100%乙醇)、透明剂、二甲苯浸泡,挥发二甲苯后使用液体石蜡浸泡两次。用石蜡包埋果肉样品,设置厚度6~11 µm 在切片机(Leica Biosystems RM2245)上切片,粘片和脱蜡后进行番红固绿对染,中性树胶封片置于显微镜下观察。
1.4 果实细胞壁物质含量和果胶裂解酶活力测定
参考海龙飞等[20]的方法测定纤维素、半纤维素和可溶性果胶含量,使用光谱法试剂盒(苏州科铭生物公司)测定果胶裂解酶活力,均设置3次生物学重复,使用Microsoft Excel 2021和SPSS 19.0软件进行图表制作和数据分析,采用Duncan新复极差法分析不同品种和发育时期之间的差异显著性。
1.5 VcPL基因克隆和表达模式分析
采用改良CTAB法分别提取Star和O’Neal蓝莓不同发育时期的果实RNA,利用试剂盒(TaKaRa,大连)反转录成cDNA。以Draper 蓝莓转录本序列为模板设计基因克隆引物(表1),采用Oligo Calc检查引物稳定性,PCR 扩增产物经割胶回收后连接到pMD-19T载体上,转化DH5α大肠杆菌,菌液PCR检测后送擎科生物技术有限公司(杭州,浙江)测序。使用Primer-BLAST设计Quantitative PCR(qPCR)引物(表1),由生工(上海)生物工程有限公司合成。以蓝莓VcGAPDH为内参基因进行qPCR,反应体系10 μL包括:2×SYBR Green qPCR premix(TaKaRa,大连)5 μL,cDNA 模板1 μL,上、下游引物(10 μmol∙L-1)各1 μL,双蒸水7 μL。反应程序为:94 ℃预变性3 min;94 ℃变性30 s,58 ℃退火30 s,72 ℃延伸30 s,30 个循环;循环结束后72 ℃10 min。每个样品进行3次生物学重复,使用2-ΔΔCt法计算相对表达量。
表1 VcPL 基因编码区克隆和qPCR 引物序列
Table.1 Primer sequences for coding region cloning and qPCR of VcPL genes
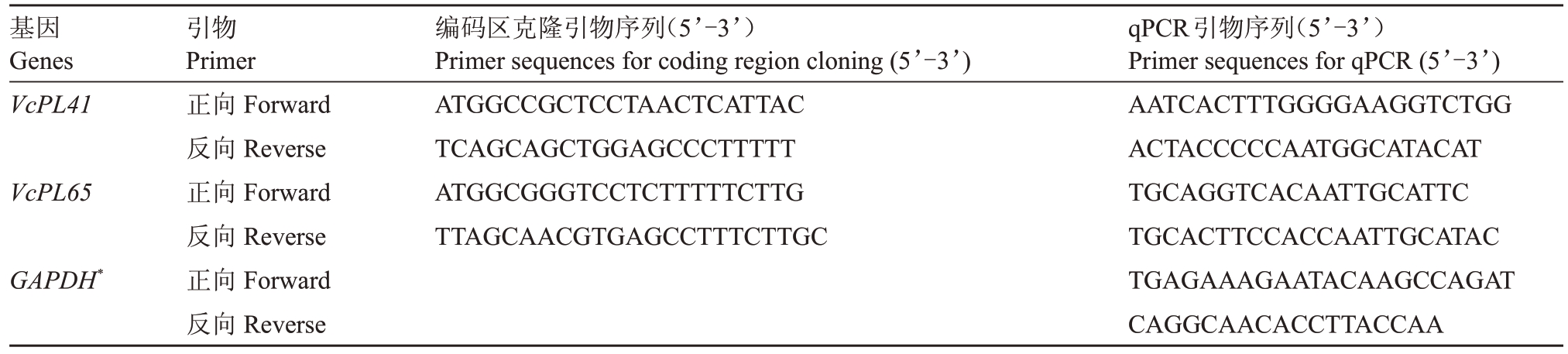
基因Genes VcPL41 VcPL65编码区克隆引物序列(5’-3’)Primer sequences for coding region cloning(5’-3’)ATGGCCGCTCCTAACTCATTAC TCAGCAGCTGGAGCCCTTTTT ATGGCGGGTCCTCTTTTTCTTG TTAGCAACGTGAGCCTTTCTTGC GAPDH*引物Primer正向Forward反向Reverse正向Forward反向Reverse正向Forward反向Reverse qPCR引物序列(5’-3’)Primer sequences for qPCR(5’-3’)AATCACTTTGGGGAAGGTCTGG ACTACCCCCAATGGCATACAT TGCAGGTCACAATTGCATTC TGCACTTCCACCAATTGCATAC TGAGAAAGAATACAAGCCAGAT CAGGCAACACCTTACCAA
1.6 VcPLs诱导表达载体构建
将克隆得到的基因连接到pMD19-T载体,用限制性核酸内切酶XhoⅠ和SpeⅠ(TaKaRa,大连)酶切VcPL-pMD19-T和pER8质粒,获得相同的双酶切位点。将目的片段连接到诱导表达载体pER8 中,转化大肠杆菌DH5α,进行菌液PCR和双酶切验证,将构建成功的载体转化农杆菌GV3101。
1.7 VcPL转基因功能验证
将Micro-Tom 种子进行消毒处理,在培养皿中暗培养3 d后移至光下,光照/黑暗周期为16 h/8 h培养4 d,黑暗和光照条件下的温度均为24 ℃。将萌发的幼苗移栽到土壤中,待子叶完全展开后切除,保留2~3 cm下胚轴用于菌液侵染,将1 mL于1.6构建成功的载体转化农杆菌GV3101菌液分多次滴加在切口平面处。暗培养1 d 后进行光照(光照/黑暗为16 h/8 h)培养,温度均为24 ℃;同时以pER8空载为对照进行转化。切口萌生新植株后提取基因组DNA,利用PCR鉴定转基因阳性植株用于后续试验。
待转基因阳性植株进入花后20 d,选择长势一致、果实大小相似的绿果期番茄,在其果面均匀喷施雌二醇,48 h后观察表型。使用Firmtech FT7测定番茄果实硬度,参考曹建康等[21]的方法对番茄果实中可溶性果胶进行提取和测定。利用CTAB法提取果实RNA,使用HiScript® Reverse Transcriptase Kit(Vazyme)反转录cDNA,以VcPLs基因引物进行qPCR,反应程序、反应体系、相对表达量计算方法同1.5。
2 结果与分析
2.1 不同发育时期果实硬度变化
2 个蓝莓品种的果实硬度总体表现为下降趋势,但S3 到S4 时期有小幅度上升(图2)。S4 到S6时期果实硬度快速下降,之后降幅趋于平缓。Star和O’Neal 都在S4 时期达到果实硬度最大值,果实硬度分别为1 061.5 g∙mm-1和1 207.7 g∙mm-1。Star和O’Neal在S6时期的果实硬度已经下降到273.4 g∙mm-1和227.1 g∙mm-1,O’Neal降幅远大于Star。2个品种果实硬度最小值出现在S8 时期,分别为217.0 g∙mm-1和165.9 g∙mm-1。
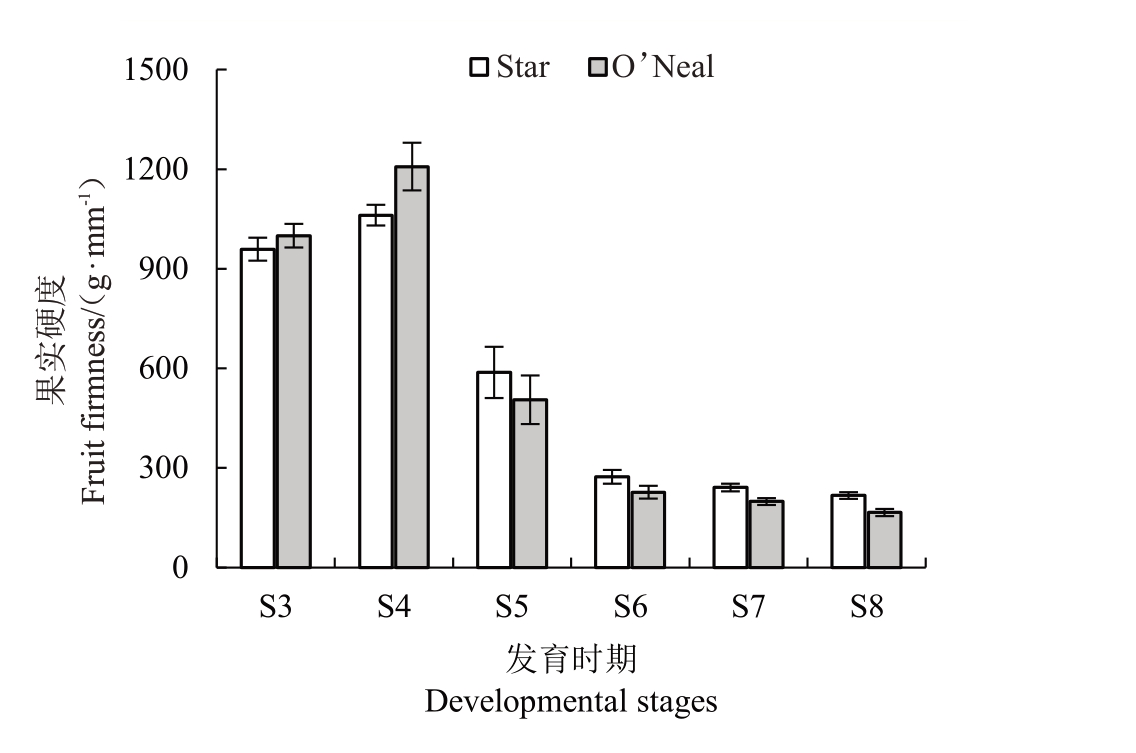
图2 Star 和O’Neal 不同发育时期的果实硬度
Fig.2 Fruit firmness of Star and O’Neal at different developmental stages
2.2 不同发育时期果实解剖结构观察
对蓝莓果实不同发育时期的果皮和果肉细胞结构进行观察,结果表明软肉品种O’Neal和硬肉品种Star 果肉细胞结构逐渐失序,O’Neal 中早于Star(图3)。2个蓝莓品种的果实在发育初期(S3和S4)近果皮处果肉细胞结构完整,排列紧密,细胞壁轮廓清晰。从S5时期开始O’Neal果实近果皮处果肉细胞出现结构破损,而Star果实中的细胞结构仍较为完整。在S6到S8时期,大量果肉细胞结构逐渐趋于不完整,失序状态严重,O’Neal比Star更加突出。

图3 Star 和O’Neal 不同发育时期果实解剖结构
Fig.3 Fruit dissection of Star and O’Neal at different developmental stages
O-S3 到O-S8 分别表示O’Neal S3 到S8 时期的果实,S-S3 到S-S8 分别表示Star S3 到S8 时期的果实。标尺为100 μm。O-S3 to O-S8 represented O’Neal fruits from stage S3 to S8.S-S3 to S-S8 represented Star fruits from stage S3 to S8.The bar is 100 μm.
2.3 果实细胞壁结构物质含量和果胶裂解酶活力
对果实纤维素、半纤维素、可溶性果胶等细胞壁结构物质含量和果胶裂解酶活力进行了测定,结果表明纤维素和半纤维素含量在果实硬度降低过程中总体均呈现下降趋势(图4-A、B)。除S4 时期外,2个品种间的纤维素含量存在显著差异,O’Neal纤维素含量(w,后同)最大值在S3 时期,为10.0 mg∙g-1,Star 出现在S4 时期,为8.20 mg∙g-1;2 个品种纤维素含量最小值均出现在S8 时期。果实发育期半纤维素含量变化较小,品种间仅在S4 和S5 两个时期存在显著差异(图4-B)。可溶性果胶含量随着果实软化呈上升趋势,从S5 时期开始快速增加,O’Neal 涨幅大于Star,最大值分别出现在S7 和S8 两个时期,O’Neal 果实中可溶性果胶含量显著高于Star(图4-C)。果胶裂解酶活力在S3 和S4 两个时期变化较小,与可溶性果胶含量增加趋势相似,酶活力从S5时期开始升高,O’Neal中趋势比Star更明显。果胶裂解酶在O’Neal S5 到S8 时期均保持在较高活力,且显著大于Star。Star中酶活力从S6时期才开始保持在高水平(图4-D)。
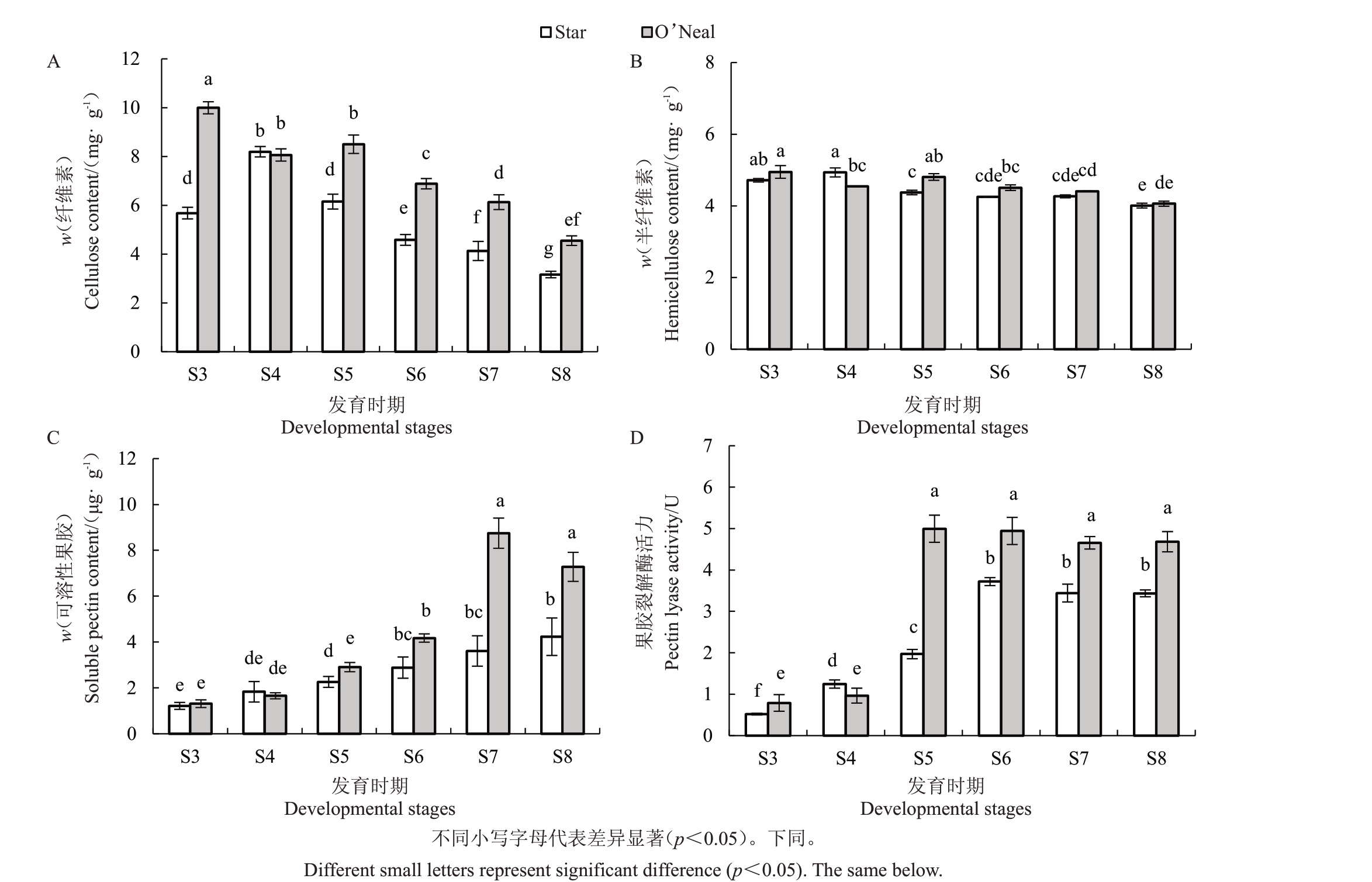
图4 不同发育时期果实细胞壁结构物质含量和果胶裂解酶活力
Fig.4 The contents of cell wall components and pectin lyase activity at fruit developmental stages
2.4 果实硬度与细胞壁结构物质含量、果胶裂解酶活力相关性分析
对细胞壁结构物质含量和果胶裂解酶活力与果实硬度进行相关性分析(表2),结果表明,纤维素和半纤维素含量与果实硬度呈显著或极显著正相关,相关系数分别为0.688和0.741。与可溶性果胶含量和果胶裂解酶活力均呈极显著负相关,其相关系数分别为-0.742 和-0.823,果胶裂解酶活力与果实硬度相关性最显著。可溶性果胶含量与果胶裂解酶活力呈极显著正相关,相关系数为0.727。
表2 蓝莓果实硬度和生理指标测定数据相关性分析
Table 2 Correlation analysis of the determination data of blueberry fruit hardness and physiological indicators
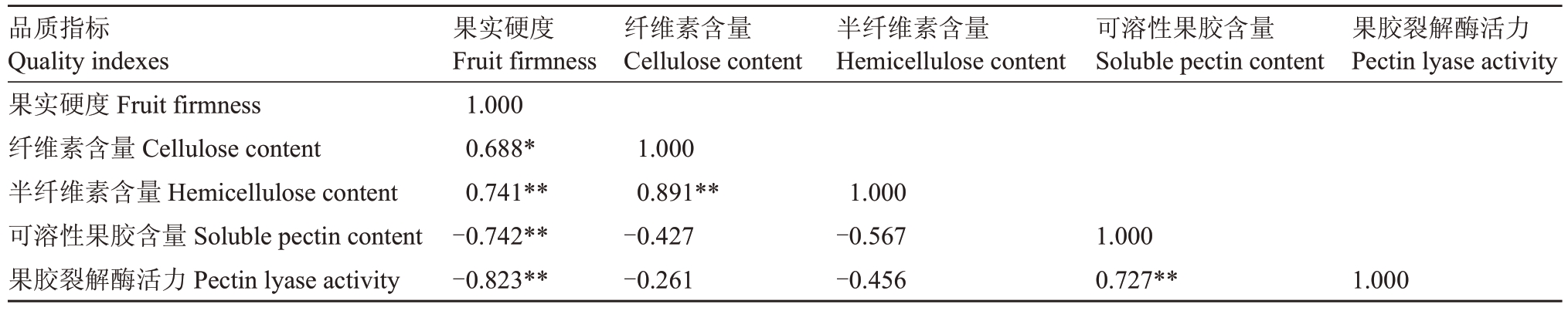
注:*和**分别表示0.05 和0.01 水平上显著相关。
Note:*and**indicate significant correlation at the 0.05 and 0.01 levels,respectively.
品质指标Quality indexes果实硬度Fruit firmness纤维素含量Cellulose content半纤维素含量Hemicellulose content可溶性果胶含量Soluble pectin content果胶裂解酶活力Pectin lyase activity果实硬度Fruit firmness 1.000 0.688*0.741**-0.742**-0.823**纤维素含量Cellulose content半纤维素含量Hemicellulose content可溶性果胶含量Soluble pectin content果胶裂解酶活力Pectin lyase activity 1.000 0.891**-0.427-0.261 1.000-0.567-0.456 1.000 0.727**1.000
2.5 VcPL基因克隆和表达模式分析
克隆得到VcPL41 和VcPL65 的编码区全长序列,长度为1230 bp 和1209 bp(图5),分别编码409个和402个氨基酸,Star和O’Neal中VcPL65氨基酸序列完全相同,VcPL41 的序列相似度大于99%。VcPL41 的氨基酸序列中包含果胶裂解酶保守结构域Motif 1(WⅠDH)、Motif 2(DGLⅠDAⅠMGSSAⅠTⅠSNNYM)和Motif 3(LVQRMPRCRHGYFHVVNN),在品种间有4 个氨基酸差异,分别为第3、92、181 和221位的氨基酸,其中第221位氨基酸变化发生在保守结构域Motif 1 中。O’Neal 中氨基酸分别是苏氨酸、缬氨酸、天冬氨酸和缬氨酸,在Star 对应位置是丙氨酸、异亮氨酸、组氨酸和甲硫氨酸(图6)。
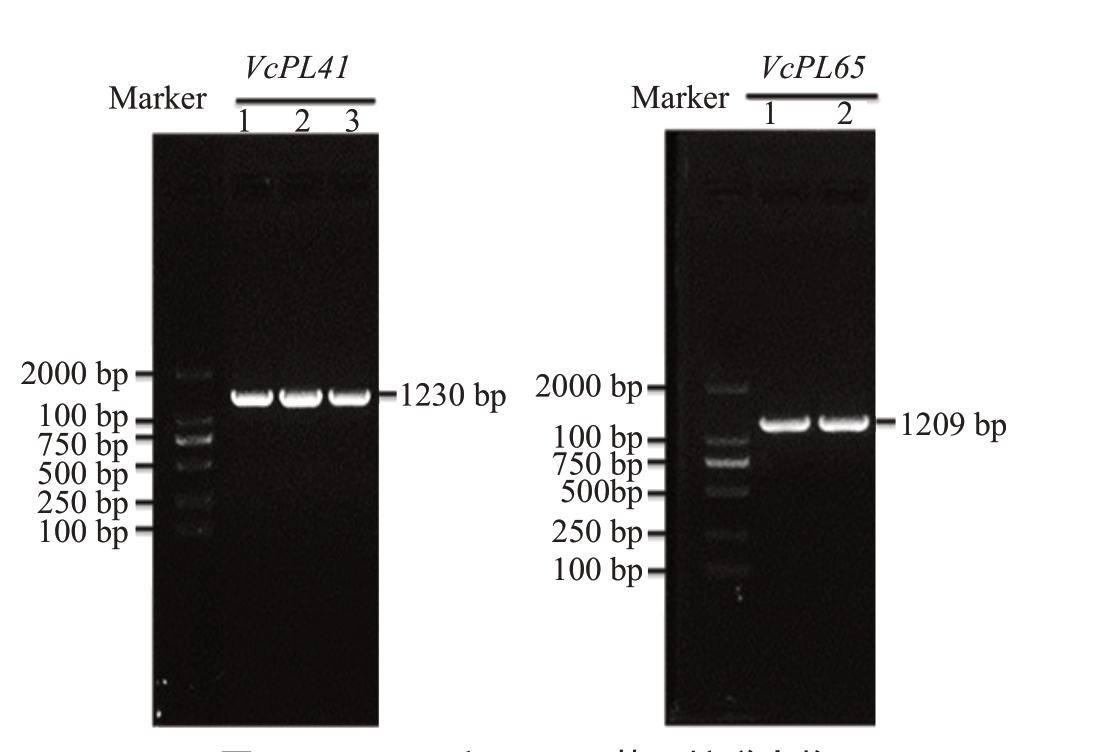
图5 VcPL41 和VcP65 基因扩增产物
Fig.5 Gel electrophoresis of amplified products of VcPL41 and VcPL65

图6 Star 和O’Neal 中VcPL41 氨基酸序列比对
Fig.6 Amino acid sequences alignment of VcPL41 in Star and O’Neal
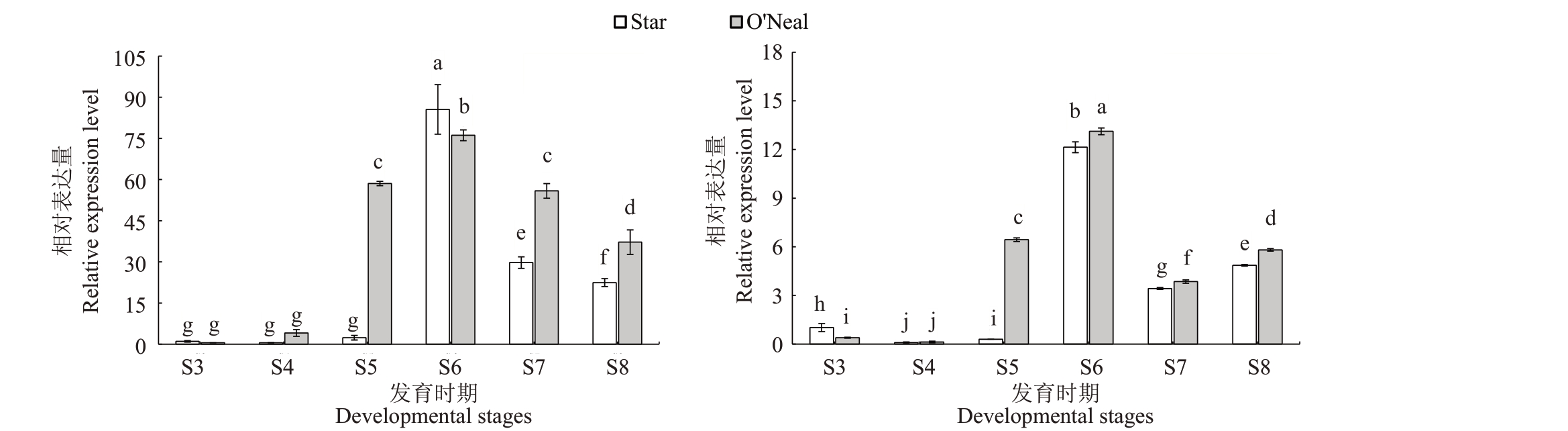
图7 蓝莓不同发育时期果实中VcPL 基因相对表达量
Fig.7 The relative expression levels of VcPL gene at different developmental stages in blueberry fruits
基因VcPL41 和VcPL65 相对表达量在Star 和O’Neal果实发育期表现为先升高后降低的趋势(图7)。2个基因在S3和S4时期表达水平很低,其相对表达量在O’Neal 中从S5 期开始快速增加,在S6 期达到最大值,分别为76.1和13.1。Star中2个基因的相对表达量从S6 时期快速上升,最大值也在S6 时期,分别为85.6 和12.1。VcPL41 和VcPL65 相对表达量在S6时期后下降,VcPL41降幅远大于VcPL65,但VcPL65的相对表达量在S8时期出现了小幅度增加。
2.6 番茄转基因功能验证
构建了VcPL41 和VcPL65 的过表达载体,遗传转化番茄后分别得到10 和14 株转基因植株,花后20 d 利用雌二醇在番茄绿果中诱导VcPLs 基因表达,结果表明VcPL41 和VcPL65 转基因番茄果实转色早于对照组(图8)。转基因番茄果实萼片边缘干枯,且VcPL65转基因番茄组更明显(图8-A)。番茄果实中VcPL41 和VcPL65 基因显著上调表达,表明诱导表达效果良好(图8-B),测定了对照组和转基因株系番茄果实硬度、可溶性果胶含量,超表达Vc-PLs 基因的番茄果实硬度均显著小于对照组,可溶性果胶含量均显著高于对照组,VcPL65表现更突出(图8-C、D)。
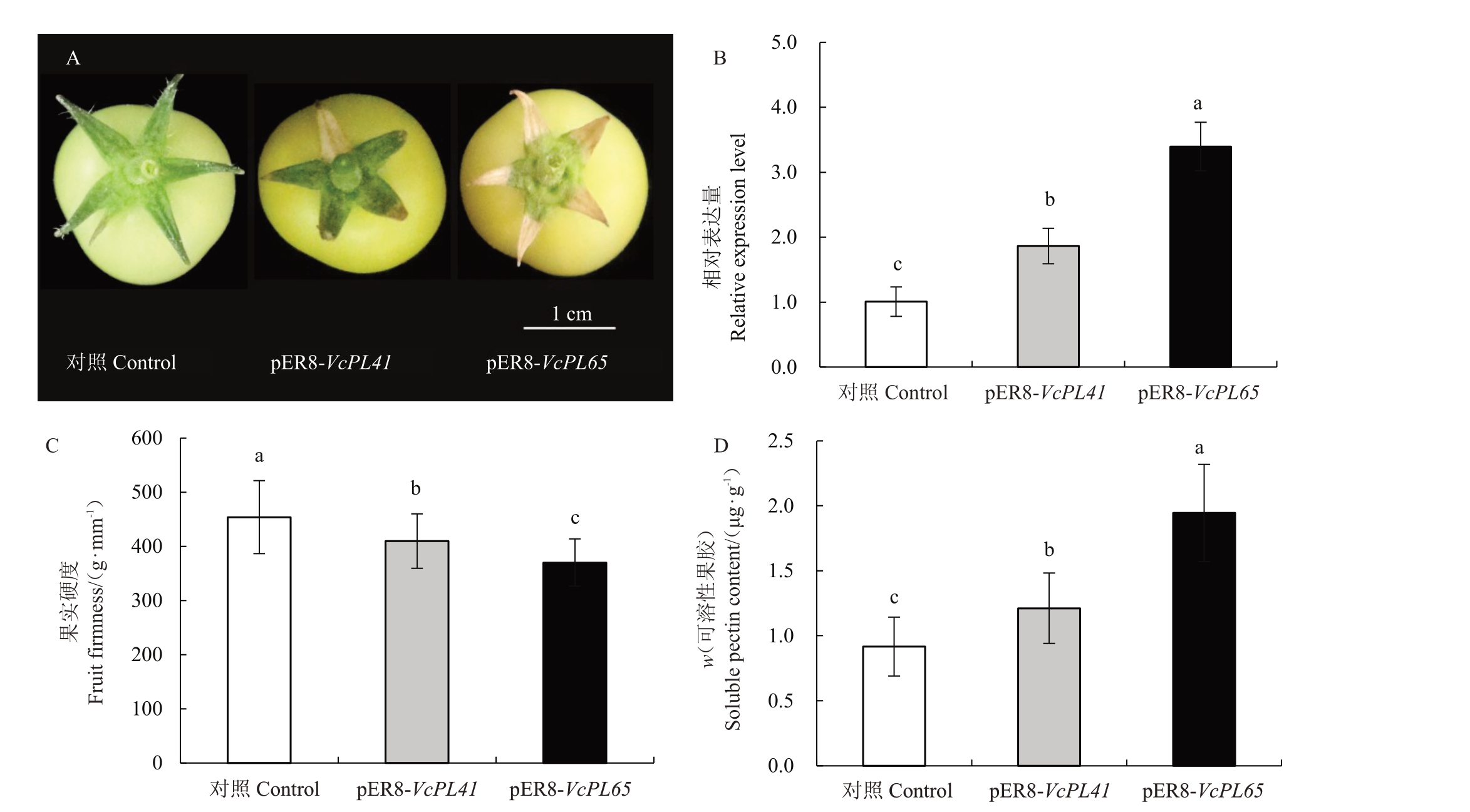
图8 超表达VcPLs 基因的番茄果实表型、相对表达量、果实硬度和可溶性果胶含量
Fig.8 Phenotype,soluble pectin content,fruit firmness and relative expression of VcPL gene in transgenic tomato
A.番茄果实表型;B.番茄中VcPL 基因相对表达量;C.番茄果实硬度;D.番茄果实可溶性果胶含量。
A.phenotype of tomato fruit;B.Relative expression of VcPL genes in tomato;C.Fruit firmness of tomato fruit;D.Soluble pectin content of tomato fruit.
3 讨 论
3.1 细胞壁物质对果实硬度的影响
果实细胞壁是由纤维素、半纤维素和果胶为主形成的交联结构,多种物质共同维持细胞壁稳定[10-13]。葡萄果实开始软化和着色时,细胞壁边界逐渐模糊,伴随着成熟加剧,大量出现细胞壁降解现象[15]。这与本研究结果相似,2 个蓝莓品种从S5 时期开始出现细胞结构的差别,软肉品种O’Neal细胞排列和大小相比硬肉品种Star更加杂乱。果实硬度下降过程中细胞壁物质纤维素和半纤维素逐渐降解,果胶可溶性增强,影响果实硬度的细胞壁物质在不同果实或同类果实不同品种之间存在差异[20,22-23]。共价结合果胶和纤维素是影响苹果品种秦冠果实硬度的主要组分,而半纤维素和水溶性果胶含量与富士果实硬度密切关联[24]。随着果实硬度的降低,一些苹果品种水溶性果胶、离子结合型果胶和共价结合型果胶均呈现下降趋势[25]。原果胶降解和纤维素水解是樱桃果实软化的关键因素[26],也有观点认为半纤维素和果胶含量变化是导致硬肉樱桃和软肉樱桃果实硬度差异的主要因素,硬肉樱桃在全红期的半纤维素和共价结合型果胶多于软肉品种,水溶性果胶和离子结合型果胶少于软肉品种[27]。笔者在本研究中发现,蓝莓果实软化过程中,纤维素含量降低,同时期的2个品种呈现显著差异;半纤维素含量也表现出逐渐降低的趋势,但降幅较小,2 个品种在S3、S6 到S8 共4 个时期没有显著差异。蓝莓硬肉品种Star可溶性果胶含量显著低于软肉品种O’Neal,特别是S5到S8时期,同时期果胶裂解酶活力在品种之间也存在显著差异,这表明果胶是影响蓝莓果实硬度的主要细胞壁物质,与苹果、樱桃等果实中的研究结果不完全相同,暗示蓝莓果实软化和硬度差异形成机制可能有其特殊性。
3.2 超表达蓝莓VcPL41 和VcPL65 促进番茄果实软化
果胶裂解酶通过β-反式消除作用催化降解去甲酯化多聚半乳糖醛酸α-1,4-糖苷键,在非还原性末端产生含有不饱和半乳糖醛酸残基的寡聚糖[28]。已有研究表明,PL 主要参与植物花器官发育、果实成熟软化、与病原微生物互作等过程[28]。抑制PL基因表达可以提高成熟期草莓果实硬度;在番茄中沉默SlPL基因可以降低可溶性果胶含量,使得采后果实表现出更强的抗病原体和抗腐烂能力[29];MiPel1参与杧果(Mangifera indica)果实成熟过程中的果胶降解从而促进果实软化[30]。本研究中VcPL41和Vc-PL65 两个果胶裂解酶基因在果实发育后期上调表达,软肉品种O’Neal 中上调表达早于硬肉品种Star,且VcPL41相对表达量变化大于VcPL65(图7),VcPL41和VcPL65的差异化表达模式可能是引起蓝莓果实硬度差别的原因之一,番茄[29]和欧洲甜樱桃[27]中果胶裂解酶基因研究结果与本研究结果相似。超表达VcPLs基因的番茄果实比对照果实转黄更早,还出现了萼片干枯表型,超表达番茄果实中可溶性果胶含量显著高于对照果实,果实硬度显著下降。这进一步表明VcPL41和VcPL65基因具有促进果实软化的功能。
值得注意的是,2 个品种VcPL41 有4 个氨基酸不同,而VcPL65氨基酸序列相同,这暗示VcPL41和VcPL65 基因在品种间表达模式的差异很可能是因为受不同转录因子调控。果胶裂解酶基因可以响应生长素[28,31]、脱落酸[27]、乙烯[32-33]等植物激素,硬肉品种Star 和O’Neal 果实同一时期的激素含量可能存在差别,导致PL 对植物激素不同程度的响应,造成表达模式不同。对于蓝莓是否属于呼吸跃变型尚存在争议,广为接受的观点是蓝莓乙烯释放量有品种特异性,目前已知大部分蓝莓品种无乙烯释放高峰。笔者课题组对Star 和O’Neal 果实乙烯前体物质1-氨基环丙基-1-羧酸(ACC)的测定初步表明Star和O’Neal均为非呼吸跃变型,脱落酸变化趋势一致且同时期无显著差异,吲哚乙酸(ⅠAA)在品种间表现为显著性差异(数据未发表)。将来可以使用外源ⅠAA 处理蓝莓不同发育时期的果实,揭示生长素及其响应转录因子对果胶裂解酶基因的调控作用,进一步阐明蓝莓硬肉品种和软肉品种果实硬度差异的形成机制。
4 结 论
蓝莓硬肉品种Star 和软肉品种O’Neal 果实硬度差异主要发生在S4 到S6 时期,近果皮处细胞层松散、细胞间隙增大、果胶裂解酶活力增强、可溶性果胶含量上升等与果实硬度下降紧密关联的变化在2个品种间呈显著差异。O’Neal果实中各项变化一般早于Star且更为显著。可溶性果胶含量与果胶裂解酶活力呈显著正相关,果实硬度与果胶裂解酶活力和可溶性果胶含量呈显著负相关,VcPL41 和Vc-PL65能够加速果实成熟软化进程。
[1] HU Y N,HAN Z Y,SUN Y Q,WANG S,WANG T,WANG Y,XU K N,ZHANG X Z,XU X F,HAN Z H,WU T.ERF4 affects fruit firmness through TPL4 by reducing ethylene production[J].The Plant Journal,2020,103(3):937-950.
[2] LⅠR,SUN S,WANG H J,WANG K T,YU H,ZHOU Z,XⅠN P Y,CHU J F,ZHAO T M,WANG H Z,LⅠJ Y,CUⅠX.FIS1 encodes a GA2-oxidase that regulates fruit firmness in tomato[J].Nature Communications,2020,11:5844.
[3] 沈朱俐,顾莉莉,李晓谊,李永强,宗宇,徐丽珊,郭卫东.基于形变距离和受压质量混合模式测定蓝莓果实硬度的方法建立和优化[J].果树学报,2023,40(1):169-179.SHEN Zhuli,GU Lili,LⅠXiaoyi,LⅠYongqiang,ZONG Yu,XU Lishan,GUO Weidong.Foundation and optimization of protocol for blueberry fruit firmness measurement under mix mode of deflection distance and pressure weight[J].Journal of Fruit Science,2023,40(1):169-179.
[4] GⅠONGO L,PONCETTA P,LORETTⅠP,COSTA F.Texture profiling of blueberries (Vaccinium spp.) during fruit development,ripening and storage[J].Postharvest Biology and Technology,2013,76:34-39.
[5] CAPPAⅠF,BENEVENUTO J,FERRÃO L,MUNOZ P.Molecular and genetic bases of fruit firmness variation in blueberry:A review[J].Agronomy,2018,8(9):174.
[6] 刘丙花,孙锐,王开芳,舒秀阁,孙蕾.不同蓝莓品种果实品质比较与综合评价[J].食品科学,2019,40(1):70-76.LⅠU Binghua,SUN Rui,WANG Kaifang,SHU Xiuge,SUN Lei.Comparison and comprehensive evaluation of fruit quality of different blueberry (Vaccinium spp.) varieties[J].Food Science,2019,40(1):70-76.
[7] LOBOS G A,BRAVO C,VALDÉS M,GRAELL J,LARA A Ⅰ,BEAUDRY R M,MOGGⅠA C.Within-plant variability in blueberry (Vaccinium corymbosum L.):Maturity at harvest and position within the canopy influence fruit firmness at harvest and postharvest[J].Postharvest Biology and Technology,2018,146:26-35.
[8] MONTECCHⅠARⅠNⅠM L,SⅠLVA-SANZANA C,VALDERRAMO L,ALEMANO S,GOLLÁN A,RⅠVADENEⅠRA M F,BELLO F,VÁZQUEZ D,BLANCO-HERRERA F,PODESTÁ F E,TRⅠPODⅠK E J.Biochemical differences in the skin of two blueberries (Vaccinium corymbosum) varieties with contrasting firmness:Ⅰmplication of ions,metabolites and cell wall related proteins in two developmental stages[J].Plant Physiology and Biochemistry,2021,162:483-495.
[9] HUANG B W,HU G J,WANG K K,FRASSE P,MAZA E,DJARⅠA,DENG W,PⅠRRELLO J,BURLAT V,PONS C,GRANELL A,LⅠZ G,VAN DER REST B,BOUZAYEN M.Ⅰnteraction of two MADS-box genes leads to growth phenotype divergence of all-flesh type of tomatoes[J].Nature Communications,2021,12:6892.
[10] ZEPEDA B,OLMEDO P,EJSMENTEWⅠCZ T,SEPÚLVEDA P,BALⅠC Ⅰ,BALLADARES C,DELGADO- RⅠOSECO J,FUENTEALBA C,MORENO A A,DEFⅠLⅠPPⅠB G,MENESES C,PEDRESCHⅠR,CAMPOS-VARGAS R.Cell wall and metabolite composition of berries of Vitis vinifera (L.) cv.Thompson Seedless with different firmness[J].Food Chemistry,2018,268:492-497.
[11] CHEN Y Z,ZHANG S,LⅠN H T,LU W J,WANG H,CHEN Y H,LⅠN Y F,FAN Z Q.The role of cell wall polysaccharides disassembly in Lasiodiplodia theobromae-induced disease occurrence and softening of fresh Longan fruit[J].Food Chemistry,2021,351:129294.
[12] BRUMMELL D A.Cell wall disassembly in ripening fruit[J].Functional Plant Biology,2006,33(2):103-119.
[13] FORLANⅠS,MASⅠERO S,MⅠZZOTTⅠC.Fruit ripening:The role of hormones,cell wall modifications,and their relationship with pathogens[J].Journal of Experimental Botany,2019,70(11):2993-3006.
[14] HUANG W J,CHEN M Y,ZHAO T T,HAN F,ZHANG Q,LⅠU X L,JⅠANG C Y,ZHONG C H.Genome-wide identification and expression analysis of polygalacturonase gene family in kiwifruit (Actinidia chinensis) during fruit softening[J].Plants,2020,9(3):327.
[15] LⅠW X,HE C,WEⅠH L,QⅠAN J K,XⅠE J N,LⅠZ Q,ZHENG X B,TAN B,LⅠJ D,CHENG J,WANG W,YE X,FENG J C.VvPL11 is a key member of the pectin lyase gene family involved in grape softening[J].Horticulturae,2023,9(2):182.
[16] ULUⅠSⅠK S,CHAPMAN N H,SMⅠTH R,POOLE M,ADAMS G,GⅠLLⅠS R B,BESONG T M D,SHELDON J,STⅠEGELMEYER S,PEREZ L,SAMSULRⅠZAL N,WANG D D,FⅠSK ⅠD,YANG N,BAXTER C,RⅠCKETT D,FRAY R,BLANCOULATE B,POWELL A L T,HARDⅠNG S E,CRAⅠGON J,ROSE J K C,FⅠCH E A,SUN L,DOMOZYCH D S,FRASER P D,TUCKER G A,GRⅠERSON D,SEYMOUR G B.Genetic improvement of tomato by targeted control of fruit softening[J].Nature Biotechnology,2016,34(9):950-952.
[17] MARÍN-RODRÍGUEZ M C,SMⅠTH D L,MANNⅠNG K,ORCHARD J,SEYMOUR G B.Pectate lyase gene expression and enzyme activity in ripening banana fruit[J].Plant Molecular Biology,2003,51(6):851-857.
[18] BALDⅠP,ORSUCCⅠS,MOSER M,BRⅠLLⅠM,GⅠONGO L,SⅠ-AMMOUR A.Gene expression and metabolite accumulation during strawberry (Fragaria × ananassa) fruit development and ripening[J].Planta,2018,248(5):1143-1157.
[19] CAPPAⅠF,AMADEU R R,BENEVENUTO J,CULLEN R,GARCⅠA A,GROSSMAN A,FERRÃO L F V,MUNOZ P.High-resolution linkage map and QTL analyses of fruit firmness in autotetraploid blueberry[J].Frontiers in Plant Science,2020,11:562171.
[20] 海龙飞,栗温新,李志谦,李猛,陈超阳,魏红丽,邹东方,何畅,冯建灿,叶霞.软/硬肉葡萄果实细胞壁结构、组分及降解酶活性的变化[J].果树学报,2023,40(4):690-698.HAⅠLongfei,LⅠWenxin,LⅠZhiqian,LⅠMeng,CHEN Chaoyang,WEⅠHongli,ZOU Dongfang,HE Chang,FENG Jiancan,YE Xia.Variations in cell wall microstructure and components and activities of their degradation enzymes in grapes with soft or hard textures[J].Journal of Fruit Science,2023,40(4):690-698.
[21] 曹建康,姜微波,赵玉梅.果蔬采后生理生化实验指导[M].北京:中国轻工业出版社,2007.CAO Jiankang,JⅠANG Weibo,ZHAO Yumei.Guidance of postharvest physiological and biochemical experiment of fruits and vegetables[M].Beijing:China Light Ⅰndustry Press,2007.
[22] 陈凯莉,许轲,张贤聪,王亚楠,汪志辉,王迅.果实中果胶代谢相关酶基因的研究进展[J].园艺学报,2017,44(10):2008-2014.CHEN Kaili,XU Ke,ZHANG Xiancong,WANG Yanan,WANG Zhihui,WANG Xun.Advances in genes information involved in pectin metabolism in fruit[J].Acta Horticulturae Sinica,2017,44(10):2008-2014.
[23] 周鹤莹,张玮,张卿,沈元月,秦岭,邢宇.森林草莓‘Ruegen’果胶裂解酶基因的克隆及荧光定量表达分析[J].园艺学报,2015,42(3):455-461.ZHOU Heying,ZHANG Wei,ZHANG Qing,SHEN Yuanyue,QⅠN Ling,XⅠNG Yu.The cloning and quantitative expression analysis of pectate lyase gene in Fragaria vesca[J].Acta Horticulturae Sinica,2015,42(3):455-461.
[24] 雷琴,任小林.秦冠和富士苹果果实成熟过程中的质地变化特性[J].西北农业学报,2007,16(1):213-216.LEⅠQin,REN Xiaolin.Characteristics of texture change with Qinguan and fuji apples during ripening[J].Acta Agriculturae Boreali-Occidentalis Sinica,2007,16(1):213-216.
[25] 高滋艺,范献光,杨惠娟,蒋小兵,杨亚州,赵政阳,党智宏.苹果发育过程中细胞壁代谢及果肉质地的变化[J].食品科学,2016,37(19):70-75.GAO Ziyi,FAN Xianguang,YANG Huijuan,JⅠANG Xiaobing,YANG Yazhou,ZHAO Zhengyang,DANG Zhihong.Correlation among cell wall components,related enzyme activities and texture of developing fruits of different apple (Malus×domestica)cultivars[J].Food Science,2016,37(19):70-75.
[26] 沈颖,李芳东,王玉霞,张序,李延菊,赵慧,张福兴.甜樱桃果实发育过程中细胞壁组分及其降解酶活性的变化[J].果树学报,2020,37(5):677-686.SHEN Ying,LⅠFangdong,WANG Yuxia,ZHANG Xu,LⅠYanju,ZHAO Hui,ZHANG Fuxing.A study on the variation of cell wall components and activities of their degradation enzymes in sweet cherry during fruit development[J].Journal of Fruit Science,2020,37(5):677-686.
[27] ZHAⅠZ F,XⅠAO Y Q,WANG Y Y,SUN Y T,PENG X,FENG C,ZHANG X,DU B Y,ZHOU X,WANG C,LⅠU Y,LⅠT H.Abscisic acid-responsive transcription factors PavDof2/6/15 mediate fruit softening in sweet cherry[J].Plant Physiology,2022,190(4):2501-2518.
[28] 陈乐天,王慧婷,韩靖鸾,栾莹.植物果胶裂解酶的研究现状及展望[J].华南农业大学学报,2019,40(5):71-77.CHEN Letian,WANG Huiting,HAN Jingluan,LUAN Ying.Research progress and perspective of plant pectin lysase[J].Journal of South China Agricultural University,2019,40(5):71-77.
[29] YANG L,HUANG W,XⅠONG F J,XⅠAN Z Q,SU D D,REN M Z,LⅠZ G.Silencing of SlPL,which encodes a pectate lyase in tomato,confers enhanced fruit firmness,prolonged shelf-life and reduced susceptibility to grey mould[J].Plant Biotechnology Journal,2017,15(12):1544-1555.
[30] CHOURASⅠA A,SANE V A,NATH P.Differential expression of pectate lyase during ethylene-induced postharvest softening of mango (Mangifera indica var.Dashehari) [J].Physiologia Plantarum,2006,128(3):546-555.
[31] DOMⅠNGO C,ROBERTS K,STACEY N J,CONNERTON Ⅰ,RUÍZ-TERAN F,MCCANN M C.A pectate lyase from Zinnia elegans is auxin inducible[J].The Plant Journal,1998,13(1):17-28.
[32] 田嘉,曾斌,罗淑萍,李秀根,李疆.‘库尔勒香梨’PsPL 基因的克隆与表达分析[J].果树学报,2015,32(6):1012-1019.TⅠAN Jia,ZENG Bin,LUO Shuping,LⅠXiugen,LⅠJiang.Cloning and expression analysis of PsPL gene in‘Korla fragrant pear’(Pyrus sinkiangensis Yu)[J].Journal of Fruit Science,2015,32(6):1012-1019.
[33] 温波,王亚兰,何丽丽,张峰,BOON-EK Y,柳士勇.桃果胶裂解酶编码基因PpPL1 的鉴定及其在果实成熟软化过程中的表达[J].核农学报,2020,34(8):1681-1689.WEN Bo,WANG Yalan,HE Lili,ZHANG Feng,BOON-EK Y,LⅠU Shiyong.Ⅰdentification of peach pectate lyase coding gene PpPL1 and its expression during fruit ripening and softening[J].Journal of Nuclear Agricultural Sciences,2020,34(8):1681-1689.