蜡质作为植物与外界接触的第一道屏障,具有控制非气孔水分散失和气体交换,保护植物免受紫外线辐射、真菌或昆虫引起的机械损伤以及其他非生物和生物环境胁迫的作用[1-2]。角质层经过索氏提取器分馏后剩余不可溶的残留物称为聚合物基质,经酸水解得到角质,可溶性部分称为蜡质[3]。蜡质可以分为表皮外蜡和表皮内蜡。外蜡沉积在角质层外面,可以通过阿拉伯树胶从植物表面剥离[4],当外蜡完全去除后,可以用有机溶剂萃取镶嵌在角质层中的内蜡[5],完成内外蜡的分离。蜡质主要是由碳原子数在18~34碳之间的超长链脂肪酸及其衍生物组成,包括醛、烷烃、支链烷烃、烯烃、伯醇、仲醇、不饱和脂肪醇、酮和蜡酯等[6]。此外,还含有萜类和其他微量次级代谢物如固醇和类黄酮类物质。研究发现不同植物物种、同一物种的不同基因型和不同地理位置,以及不同的发育阶段和贮藏环境中表皮蜡质组分均存在差异[7-9]。
常用的植物表皮蜡质提取方法有两种:一种是表皮圆片提取法[10-11],其操作简便,提取溶剂用量少,提取效率高,但该方法会提取出部分果肉中的脂溶性物质,结果误差较大;另一种是整果浸提法[12-13],该方法通过将整果浸泡于溶剂中,需要大量的提取溶剂。两种方法都是利用有机溶剂的萃取性能对蜡质进行提取。因此,选择合适的有机溶剂对于植物表皮蜡质的研究具有重要意义。然而,常用的提取试剂,如三氯甲烷[12]、二氯甲烷[14]等,大多毒性较高,对实验人员的身心健康具有一定危害。因此,急需对蜡质的提取溶剂进行筛选、优化,寻找一种低毒甚至无毒的绿色有机溶剂作为蜡质提取试剂。目前,关于溶剂选取、体积配比、浸提时间、提取温度及不同料液比等对蜡质提取效果的影响已经有部分研究报道。如Yin等[15]的研究表明先用氯仿和甲醇混合液(体积比3∶1)萃取,再用氯仿提取,苹果梨果皮蜡质提取效果最好。李珍慈等[16]通过研究发现库尔勒香梨果皮蜡质的最佳条件为三氯甲烷与二氯甲烷混合液(体积比2∶1)作为提取溶剂,1∶2.5(g∶mL)料液比,75 s提取时间。张微等[17]明确了玉露香梨蜡质提取的最佳条件为三氯甲烷和二氯甲烷体积比2∶1、提取时间为60 s、提取温度为40 ℃、料液比为1∶2(g∶mL)。上述方法虽然较好地改善了梨果皮蜡质提取的效果,但忽视了三氯甲烷等溶剂毒性对人体健康的危害。笔者在本研究中以三氯甲烷试剂作为对照,另外选取了7 种常见的有机溶剂作为提取试剂,以翠冠梨果实为实验材料进行整果蜡质浸提,比较不同溶剂毒性与蜡质提取效果,筛选适宜的低毒高效提取溶剂,为后续蜡质的研究工作奠定基础。
1 材料和方法
1.1 试材及取样
在南京农业大学梨工程技术研究中心湖熟基地采集盛花期后90 d的翠冠梨果实样品。挑选大小一致、无机械损伤、无病虫害的果实,用网套包裹立即运回实验室。乙醚、丙酮购自南京农业大学实验材料供应中心;三氯甲烷(分析纯、色谱纯)、甲醇购自南京辉亚生物科技有限公司;碳酸二甲酯、乙酸丁酯、乙酸乙酯、正己烷购自南京晚晴化玻仪器有限公司。
1.2 蜡质的提取
首先通过先前报道的三维激光扫描测量方法对每个翠冠梨果实表面积精确测定[18],并做好记录。将翠冠果实置于烧杯中,加入300 mL三氯甲烷提取溶剂浸泡梨果实,用玻璃棒缓慢搅动,避免破坏梨果表皮,提取75 s;向提取液加入2 μL的0.01 g·mL-1二十四烷作为内标,使用氮吹仪吹干。接下来,分别使用碳酸二甲酯、乙醚、乙酸丁酯、丙酮、乙酸乙酯、甲醇、正己烷作为提取溶剂按照相同方法提取果实表皮蜡质。每个处理3个重复,每个重复6个梨果实。
1.3 蜡质的衍生与含量测定
取1 mg 吹干的蜡质粗提物,加入1 mL 氯仿重新溶解,加入40 μL 吡啶和40 μL N,O-双(三甲基硅烷基)三氟乙酰胺[N,O-bis(trimethylsilyl)trifluoroacetamide,BSTFA],70 ℃水浴1 h,再次利用氮吹仪吹干所有试剂,加入1.2 mL色谱级的氯仿溶解。
通过气相色谱-质谱联用仪(Bruker 450-GC,Bruker 320-MS)和色谱柱(BR-5MS,30 m× 0.25 mm×0.25 μm)对提取的样品进行检测。氦气用作载气,流速为1.2 mL·min-1。采用以下参数:进样量,1.0 μL;进样口温度,280 ℃;传输线温度,280 ℃;离子源温度,250 ℃;四级杆温度,150 ℃;电子能量(EⅠ),70 eV;扫描范围,50~650 m·z-1。升温程序如下:50 ℃持续2 min。然后,以40 ℃·min-1的速率将温度增加到200 ℃,保持2 min。最后,以3 ℃·min-1的速率升高至320 ℃,保持30 min。
蜡质成分经GC-MS检测后得到其离子峰,使用70-750-750的筛选条件,依据NⅠST 2013质谱库进行检索判定,确定物质种类,并对离子峰进行面积积分,通过物质峰面积,内标二十四烷峰面积以及梨果实表面积等数值计算物质含量。所有化合物含量相加记为该样品的总蜡质含量。
1.4 数据分析
实验数据采用Microsoft Excel 2016 与Graph-Pad Prism 9 软件进行统计和分析,使用方差分析2012软件进行方差分析,采用Origin 2022进行主成分分析(Principal component analysis,PCA)。所有图片通过Adobe Ⅰllustrator 2021软件进行组合。
2 结果与分析
2.1 不同溶剂提取蜡质组分数量差异比较
使用八种提取溶剂对翠冠果实进行整果蜡质提取,通过GC-MS检测梨表皮蜡质成分,共检测到54种化合物(表1)。如图1所示,乙醚和甲醇提取的蜡质化合物数量最少,分别只有28和24种,提取效果差;乙酸乙酯提取数量最多,为41种;碳酸二甲酯和三氯甲烷提取数量相同,均为40种。
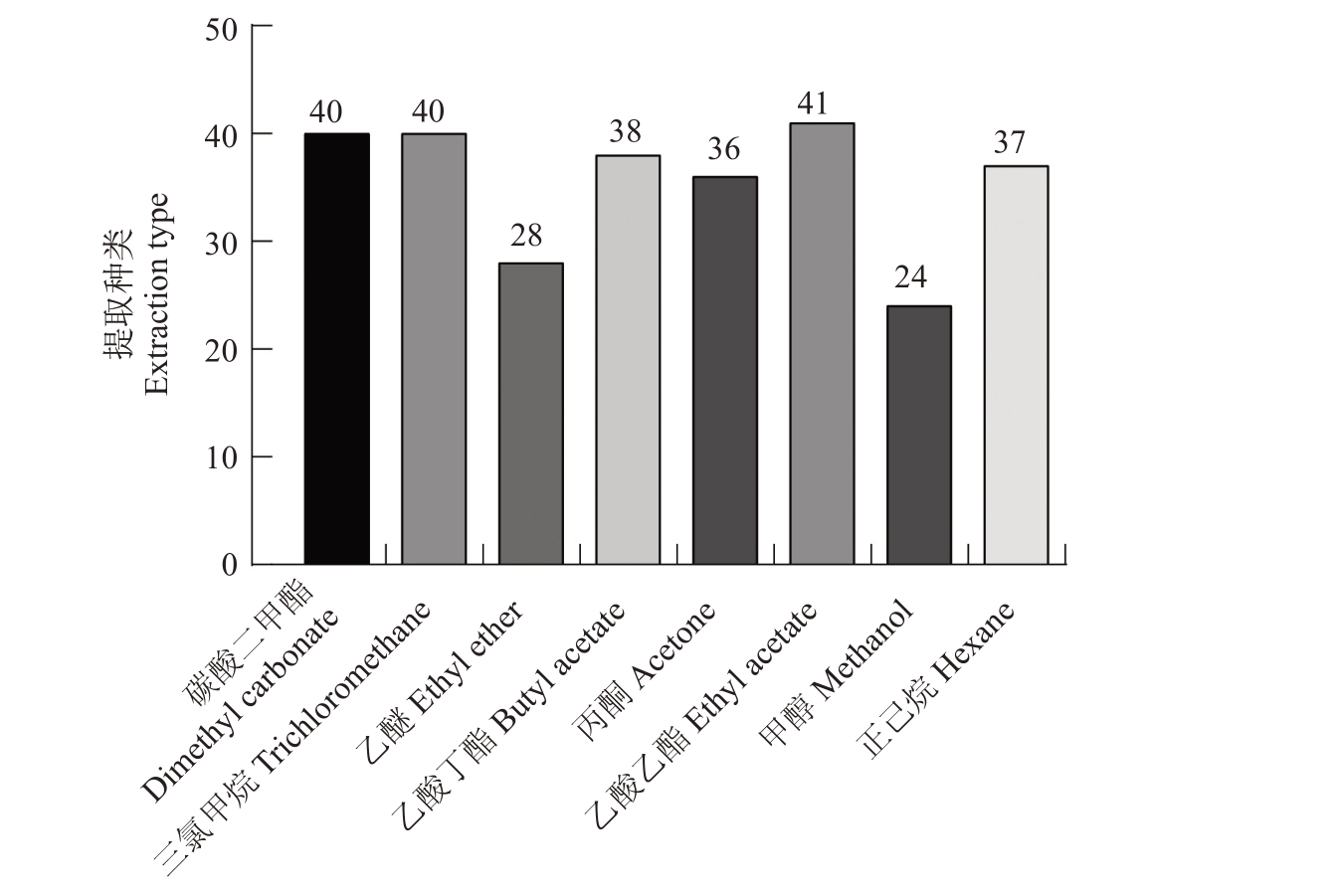
图1 不同溶剂提取出物质种类比较
Fig.1 Comparison of substances extracted with different solvents
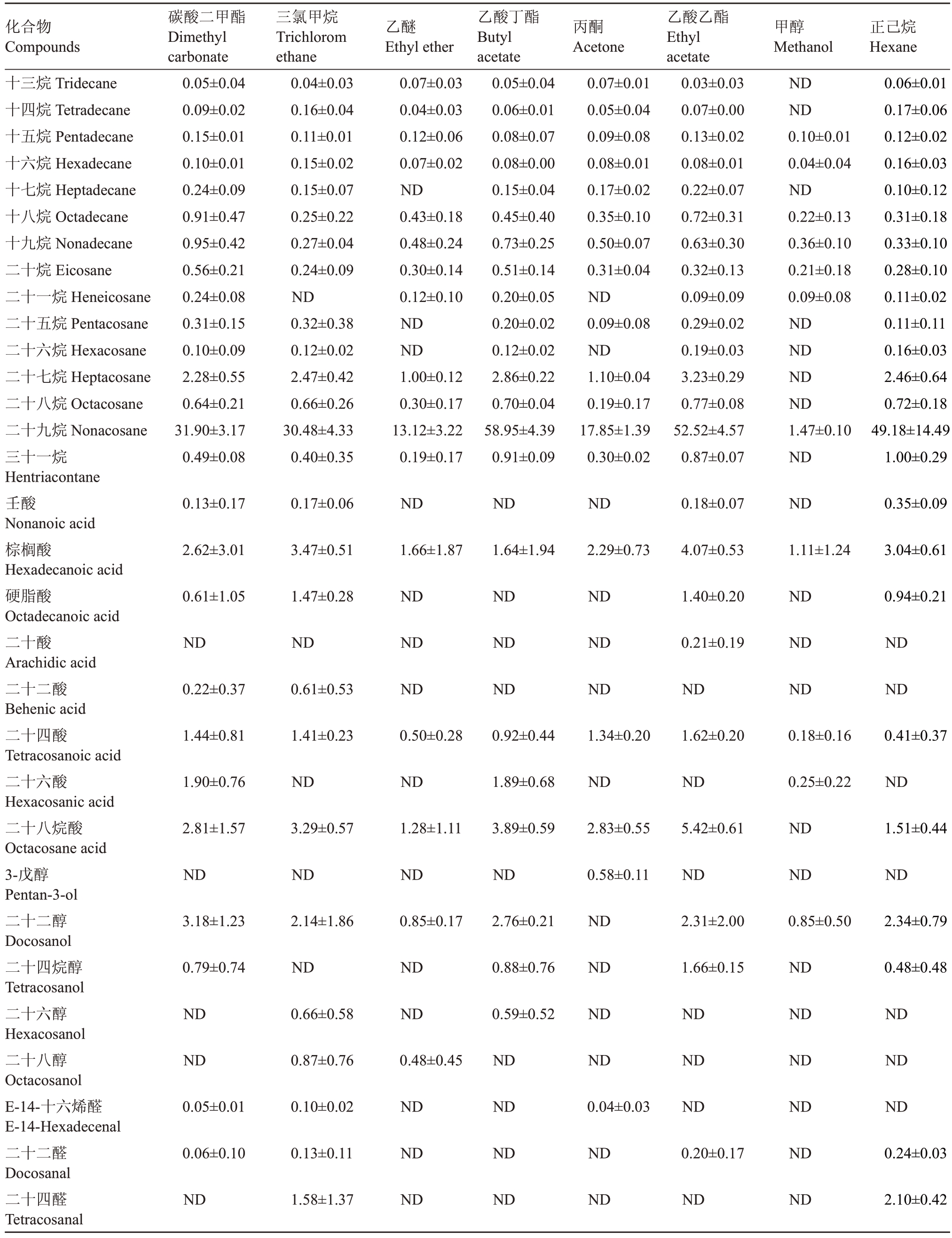
表1 不同溶剂提取梨果皮蜡质具体化合物含量
Table 1 Contents of chemical compositions extracted from pear peel wax with different solvents (μg·cm-2)
注:ND 为未检测到。下同。
Note:ND is not detected.The same below.
化合物Compounds碳酸二甲酯Dimethyl carbonate三氯甲烷Trichlorom ethane乙醚Ethyl ether乙酸丁酯Butyl acetate丙酮Acetone乙酸乙酯Ethyl acetate甲醇Methanol正己烷Hexane十三烷Tridecane十四烷Tetradecane十五烷Pentadecane十六烷Hexadecane十七烷Heptadecane十八烷Octadecane十九烷Nonadecane二十烷Eicosane二十一烷Heneicosane二十五烷Pentacosane二十六烷Hexacosane二十七烷Heptacosane二十八烷Octacosane二十九烷Nonacosane三十一烷Hentriacontane壬酸Nonanoic acid棕榈酸Hexadecanoic acid硬脂酸Octadecanoic acid二十酸Arachidic acid二十二酸Behenic acid二十四酸Tetracosanoic acid二十六酸Hexacosanic acid二十八烷酸Octacosane acid 3-戊醇Pentan-3-ol二十二醇Docosanol二十四烷醇Tetracosanol二十六醇Hexacosanol二十八醇Octacosanol E-14-十六烯醛E-14-Hexadecenal二十二醛Docosanal二十四醛Tetracosanal 0.05±0.04 0.09±0.02 0.15±0.01 0.10±0.01 0.24±0.09 0.91±0.47 0.95±0.42 0.56±0.21 0.24±0.08 0.31±0.15 0.10±0.09 2.28±0.55 0.64±0.21 31.90±3.17 0.49±0.08 0.04±0.03 0.16±0.04 0.11±0.01 0.15±0.02 0.15±0.07 0.25±0.22 0.27±0.04 0.24±0.09 ND 0.32±0.38 0.12±0.02 2.47±0.42 0.66±0.26 30.48±4.33 0.40±0.35 0.07±0.03 0.04±0.03 0.12±0.06 0.07±0.02 ND 0.43±0.18 0.48±0.24 0.30±0.14 0.12±0.10 ND ND 1.00±0.12 0.30±0.17 13.12±3.22 0.19±0.17 0.05±0.04 0.06±0.01 0.08±0.07 0.08±0.00 0.15±0.04 0.45±0.40 0.73±0.25 0.51±0.14 0.20±0.05 0.20±0.02 0.12±0.02 2.86±0.22 0.70±0.04 58.95±4.39 0.91±0.09 0.07±0.01 0.05±0.04 0.09±0.08 0.08±0.01 0.17±0.02 0.35±0.10 0.50±0.07 0.31±0.04 ND 0.09±0.08 ND 1.10±0.04 0.19±0.17 17.85±1.39 0.30±0.02 0.03±0.03 0.07±0.00 0.13±0.02 0.08±0.01 0.22±0.07 0.72±0.31 0.63±0.30 0.32±0.13 0.09±0.09 0.29±0.02 0.19±0.03 3.23±0.29 0.77±0.08 52.52±4.57 0.87±0.07 ND ND 0.10±0.01 0.04±0.04 ND 0.22±0.13 0.36±0.10 0.21±0.18 0.09±0.08 ND ND ND ND 1.47±0.10 ND 0.06±0.01 0.17±0.06 0.12±0.02 0.16±0.03 0.10±0.12 0.31±0.18 0.33±0.10 0.28±0.10 0.11±0.02 0.11±0.11 0.16±0.03 2.46±0.64 0.72±0.18 49.18±14.49 1.00±0.29 0.13±0.17 0.17±0.06 ND ND ND 0.18±0.07 ND 0.35±0.09 2.62±3.01 3.47±0.51 1.66±1.87 1.64±1.94 2.29±0.73 4.07±0.53 1.11±1.24 3.04±0.61 0.61±1.05 1.47±0.28 ND ND ND 1.40±0.20 ND 0.94±0.21 ND ND ND ND ND 0.21±0.19 ND ND 0.22±0.37 0.61±0.53 ND ND ND ND ND ND 1.44±0.81 1.41±0.23 0.50±0.28 0.92±0.44 1.34±0.20 1.62±0.20 0.18±0.16 0.41±0.37 1.90±0.76 ND ND 1.89±0.68 ND ND 0.25±0.22 ND 2.81±1.57 3.29±0.57 1.28±1.11 3.89±0.59 2.83±0.55 5.42±0.61 ND 1.51±0.44 ND ND ND ND 0.58±0.11 ND ND ND 3.18±1.23 2.14±1.86 0.85±0.17 2.76±0.21 ND 2.31±2.00 0.85±0.50 2.34±0.79 0.79±0.74 ND ND 0.88±0.76 ND 1.66±0.15 ND 0.48±0.48 ND 0.66±0.58 ND 0.59±0.52 ND ND ND ND ND 0.87±0.76 0.48±0.45 ND ND ND ND ND 0.05±0.01 0.10±0.02 ND ND 0.04±0.03 ND ND ND 0.06±0.10 0.13±0.11 ND ND ND 0.20±0.17 ND 0.24±0.03 ND 1.58±1.37 ND ND ND ND ND 2.10±0.42
表1 (续) Table 1 (Continued)

化合物Compounds碳酸二甲酯Dimethyl carbonate三氯甲烷Trichlorom ethane乙醚Ethyl ether乙酸丁酯Butyl acetate丙酮Acetone乙酸乙酯Ethyl acetate甲醇Methanol正己烷Hexane二十五醛Pentacosanal NDβ-谷甾醇beta-Sitosterol β-香树精β-amyrin α-香树精α-amyrin羽扇豆醇Lupeol三十烷酸乙酯Ethyl triacontanoate豆甾-3,5-二烯Stigmastan-3,5-diene高根二醇Erythrodiol乌发醇Uvaol白桦脂醛Betulinaldehyde齐墩果酸Oleanolic acid白桦脂酸Betulinic acid 1-十六烯1-Hexadecene 3,4-二羟基苯甲醛3,4- Dihydroxybenzaldehyde 2-羟基丙酸2-Hydroxypropionic acid甘油Glycerol对苯二酚Hydroquinone邻苯二甲酸二甲酯Dimethyl phthalate 2,6-二叔丁基对甲酚Butylated hydroxytoluene 1,6-二氧杂环十二烷-7,12-二酮1,6-Dioxacyclododecane-7,12-dione对苯二甲酸Terephthalic acid邻苯二甲酸环丁基异丁酯Phthalic acid,cyclobutyl isobutyl ester 2,2’-亚甲基双-(4-甲基-6-叔丁基苯酚)Phenol,2,2'-methylenebis[6-(1,1-dimethylethyl)-4-methyl-ND 0.77±0.11 ND ND ND 1.03±0.15 ND 0.84±0.16 ND ND ND ND 1.64±1.42 ND ND ND 3.32±0.37 3.43±0.58 4.98±1.15 3.92±0.29 6.82±0.42 5.41±0.58 2.3±0.32 0.48±0.47 4.00±0.43 5.59±0.97 4.73±1.23 6.11±0.53 8.66±0.40 6.16±0.52 3.00±0.36 0.63±0.70 9.79±0.99 9.95±1.50 27.44±6.65 11.60±1.32 31.99±1.68 17.99±1.47 7.77±1.21 1.65±0.42 1.22±1.06 ND ND ND ND ND ND ND 1.65±0.17 1.67±0.28 0.61±0.53 1.39±1.21 1.85±0.14 1.77±0.16 ND 0.58±0.51 1.82±1.59 2.88±0.45 2.90±0.52 3.25±0.36 4.60±0.41 3.99±0.30 1.18±1.02 ND 4.80±0.41 7.12±1.07 4.90±1.28 8.11±1.08 9.45±0.81 8.62±0.59 2.43±2.11 ND 17.67±0.94 18.09±2.56 36.05±9.67 25.35±5.67 61.76±9.18 39.21±3.38 15.07±1.76 ND 94.89±21.76 106.21±95.89 111.22±97.83 188.62±51.69 314.99±19.45 260.52±20.04 77.76±23.78 1.96±2.07 12.51±10.99 22.26±4.13 44.45±19.53 25.55±8.81 80.45±4.54 30.88±26.80 ND ND ND 0.10±0.05 ND ND ND ND ND ND ND ND ND ND 0.09±0.08 0.07±0.07 ND ND ND 0.76±0.30 ND ND ND 0.94±0.25 ND 1.48±0.28 2.05±1.50 0.20±0.17 3.16±0.63 1.92±0.25 8.08±0.56 2.75±1.15 15.09±6.94 0.30±0.11 ND ND ND 0.17±0.15 0.50±0.41 ND ND ND ND ND ND ND 0.54±0.09 ND ND 0.07±0.07 0.22±0.14 ND 4.74±2.32 0.06±0.05 1.00±0.04 ND 0.10±0.04 0.04±0.04 ND ND ND 0.12±0.10 ND ND 0.06±0.05 ND 0.63±0.80 0.88±0.65 ND 0.25±0.37 0.24±0.08 0.48±0.04 ND 0.44±0.10 0.12±0.12 ND ND 0.12±0.10 1.43±0.23 0.09±0.08 0.16±0.01 0.06±0.05 1.05±0.49 0.95±0.24 0.78±0.20 0.90±0.32 1.05±0.48 1.39±0.49 0.93±0.49 0.46±0.18
2.2 不同溶剂提取蜡质总量和组分含量差异比较
如图2 所示,不同溶剂提取梨果皮蜡质含量差异显著,从0.08 mg·cm-2(甲醇)到0.56 mg·cm-2(丙酮)不等。与对照三氯甲烷(0.24 mg·cm-2)相比,丙酮(0.56 mg·cm-2)、乙酸乙酯(0.48 mg·cm-2)、乙酸丁酯(0.36 mg·cm-2)和乙醚(0.32 mg·cm-2)提取的总蜡含量较高,且差异显著(p<0.05);碳酸二甲酯(0.22 mg·cm-2)提取的总蜡含量与对照相比无显著差异;甲醇(0.13 mg·cm-2)和正己烷(0.08 mg·cm-2)提取的总蜡含量显著低于对照。
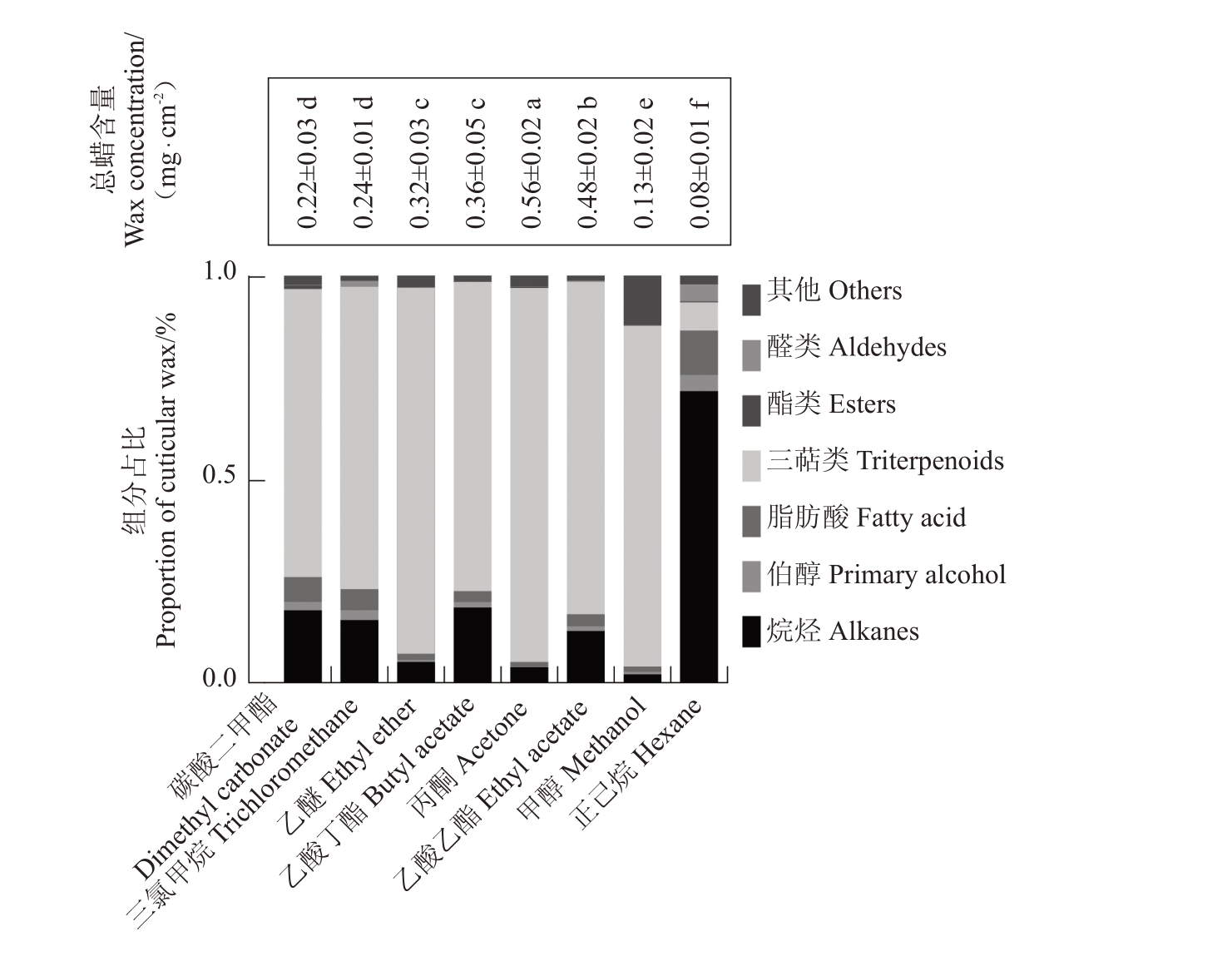
图2 不同溶剂提取梨果皮总蜡质含量及组分占比
Fig.2 Total wax content and component proportion of pear peel extracted with different solvents
显著性差异采用t 检验。结果为3 次生物学重复的平均值。不同小写字母表示p<0.05 水平显著差异。下同。
The significant difference was tested by t-test.The results were the average of three biological repetitions.Different small letters indicate significant differences at p<0.05.The same below.
对8 种溶剂提取的含量较高蜡质化合物分为7类,相对含量结果显示碳酸二甲酯、乙酸乙酯和乙酸丁酯提取的蜡质化合物组分与三氯甲烷提取效果相似。这些化合物主要包括烷烃、伯醇、脂肪酸、三萜类、酯、醛,以及其他未分类化合物。其中,碳酸二甲酯提取的蜡质化合物占比与三氯甲烷的提取效果无显著差异,二者提取的烷烃类占比分别为18.7%和15.1%,伯醇占比分别为1.9%和1.6%,脂肪酸占比分别为5.0%和5.2%,三萜类占比分别为71.3%和75.5%,表明两种溶剂提取蜡质组分具有极高相似性。此外,正己烷提取烷烃占比达73.7%,但对三萜类提取效果较差,仅为6.3%。相反,乙醚和丙酮对三萜类化合物的提取效果较好,分别达到88.7%和92.1%,而对烷烃的提取效果较差,仅为6.1%和3.8%。
7类化合物绝对含量表明不同溶剂提取果皮蜡质中不同化学组分效果差异显著(表2),乙酸丁酯提取烷烃效果最好,为66.13 μg·cm-2,而甲醇提取烷烃效果最差,为2.66 μg·cm-2;碳酸二甲酯和三氯甲烷提取的烷烃含量分别为39.10 μg·cm-2和36.31 μg·cm-2,差异不显著;三氯甲烷提取伯醇效果最好,为5.51 μg·cm-2,丙酮效果最差,仅为0.58μg·cm-2;对于脂肪酸的提取,乙酸乙酯效果最好,达到14.42 μg·cm-2,甲醇提取率最低,为1.75 μg·cm-2,碳酸二甲酯的提取量高于三氯甲烷,为13.70 μg·cm-2,但差异不显著。丙酮溶剂对于三萜类化合物的提取效率相对较高,达到518.72 μg·cm-2,其提取出的齐墩果酸含量达到314.99 μg·cm-2,正己烷提取三萜类物质的能力最差,仅5.29 μg·cm-2,差异显著,碳酸二甲酯(155.96 μg·cm-2)和三氯甲烷(175.53 μg·cm-2)的提取效果差异不显著。综上,乙酸丁酯和乙酸乙酯提取效果最好;碳酸二甲酯和三氯甲烷提取效果次之,且提取效果无显著差异;正己烷对烷烃、伯醇、脂肪酸提取效果好,但对三萜类化合物的提取效果极差;丙酮的提取效果与正己烷相反,其可高效提取三萜类物质;而甲醇对各类物质的提取效果均不理想。
表2 不同溶剂提取梨果皮蜡质各化学组分含量
Table 2 Contents of chemical components of pear peel wax extracted with different solvents (μg·cm-2)
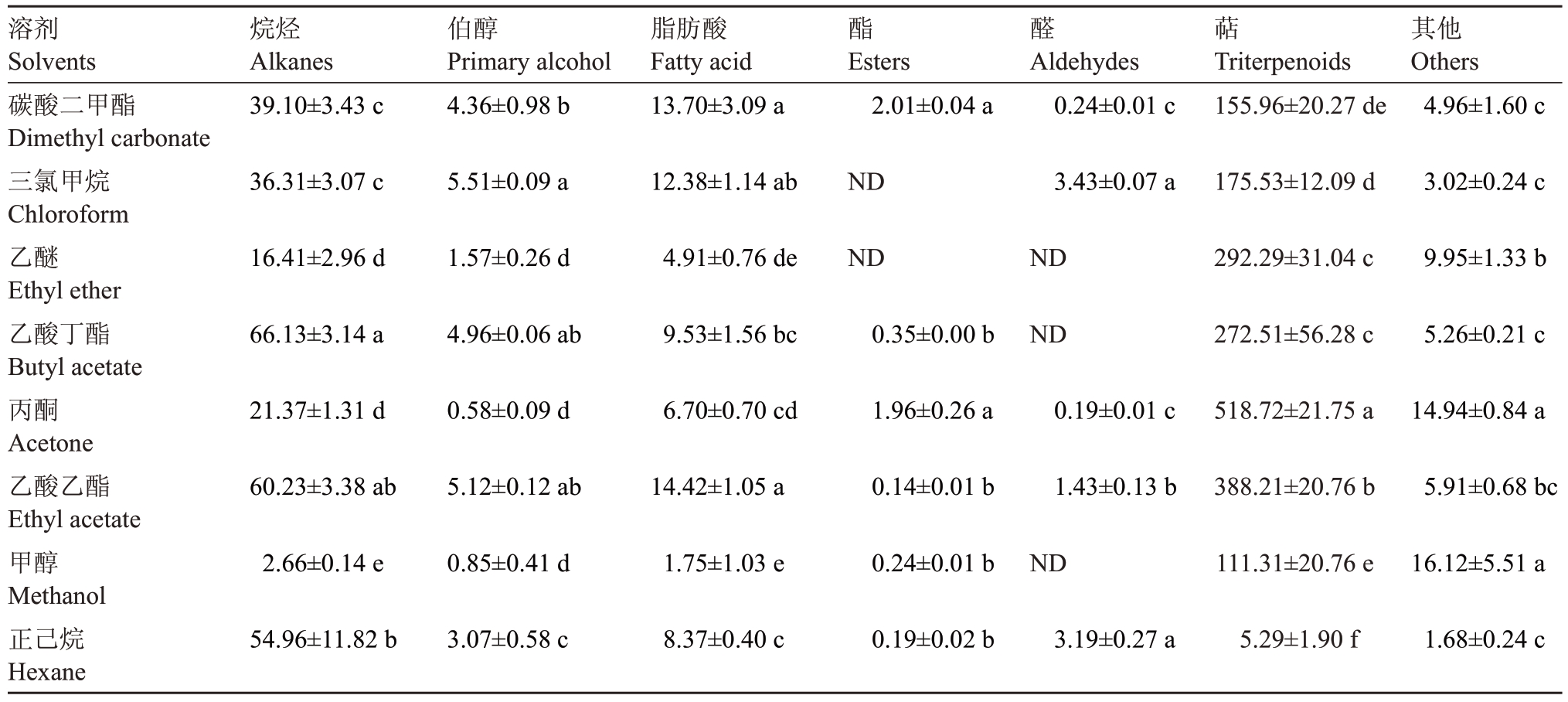
注:显著性差异采用单因素方差分析。结果为3 次生物学重复的平均值。
Note:One-way ANOVA was used for significant difference.The results were the average of three biological repetitions.
溶剂Solvents碳酸二甲酯Dimethyl carbonate三氯甲烷Chloroform乙醚Ethyl ether乙酸丁酯Butyl acetate丙酮Acetone乙酸乙酯Ethyl acetate甲醇Methanol正己烷Hexane烷烃Alkanes 39.10±3.43 c伯醇Primary alcohol 4.36±0.98 b脂肪酸Fatty acid 13.70±3.09 a酯醛萜Esters 2.01±0.04 a Aldehydes 0.24±0.01 c Triterpenoids 155.96±20.27 de其他Others 4.96±1.60 c 36.31±3.07 c 5.51±0.09 a 12.38±1.14 ab ND 3.43±0.07 a 175.53±12.09 d 3.02±0.24 c 16.41±2.96 d 1.57±0.26 d 4.91±0.76 de ND ND 292.29±31.04 c 9.95±1.33 b 66.13±3.14 a 4.96±0.06 ab 9.53±1.56 bc 0.35±0.00 b ND 272.51±56.28 c 5.26±0.21 c 21.37±1.31 d 0.58±0.09 d 6.70±0.70 cd 1.96±0.26 a 0.19±0.01 c 518.72±21.75 a 14.94±0.84 a 60.23±3.38 ab 5.12±0.12 ab 14.42±1.05 a 0.14±0.01 b 1.43±0.13 b 388.21±20.76 b 5.91±0.68 bc 2.66±0.14 e 0.85±0.41 d 1.75±1.03 e 0.24±0.01 b ND 111.31±20.76 e 16.12±5.51 a 54.96±11.82 b 3.07±0.58 c 8.37±0.40 c 0.19±0.02 b 3.19±0.27 a 5.29±1.90 f 1.68±0.24 c
2.3 不同溶剂提取梨果皮蜡质中不同链长化合物和萜类化合物的差异比较
对不同溶剂提取的梨果皮蜡质具体化合物进行检测分析,发现不同溶剂提取的烷烃C16~C31化合物含量范围为2.55 μg·cm-2(甲醇溶剂)~65.87 μg·cm-2(乙酸丁酯溶剂)(图3)。梨果实表皮蜡质中的烷烃主要是二十九烷(C29),乙醚、丙酮和甲醇对其提取效果较差,乙酸丁酯、乙酸乙酯和正己烷对C29提取效果较好;不同溶剂提取的伯醇化合物(C22-C28)含量差异较大,丙酮溶剂提取量为0 μg·cm-2,而三氯甲烷提取量达5.51 μg·cm-2,此外,乙醚和甲醇的提取量相对较少,而碳酸二甲酯、乙酸乙酯和乙酸丁酯的提取量较大,与三氯甲烷差异不显著(图4-A);碳酸二甲酯对十六烷酸(C16)和二十八烷酸(C28)等脂肪酸(C16-C28)的提取效果最好,达12.56 μg·cm-2,而甲醇的提取效果最差,仅为1.75 μg·cm-2(图4-B)。此外,笔者发现丙酮对齐墩果酸、羽扇豆醇、乌发醇和白桦脂酸等三萜类化合物提取效果最好,为518.72 μg·cm-2,而正己烷的提取效果最差,仅为5.29 μg·cm-2(图5)。
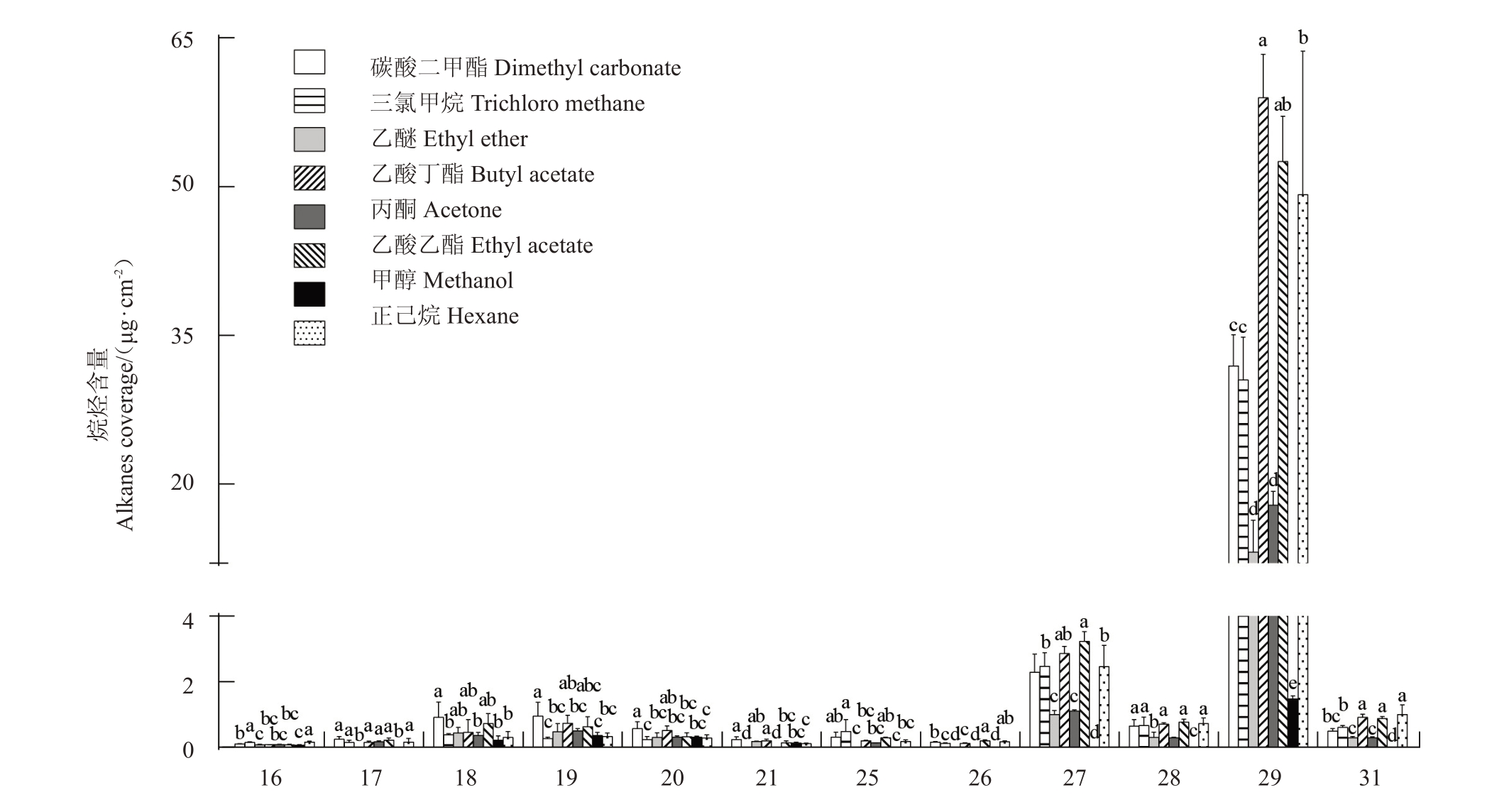
图3 不同溶剂提取蜡质烷烃链长分布比较
Fig.3 Comparison of chain length distribution of waxy alkanes extracted with different solvents
碳链长度Carbon chain length
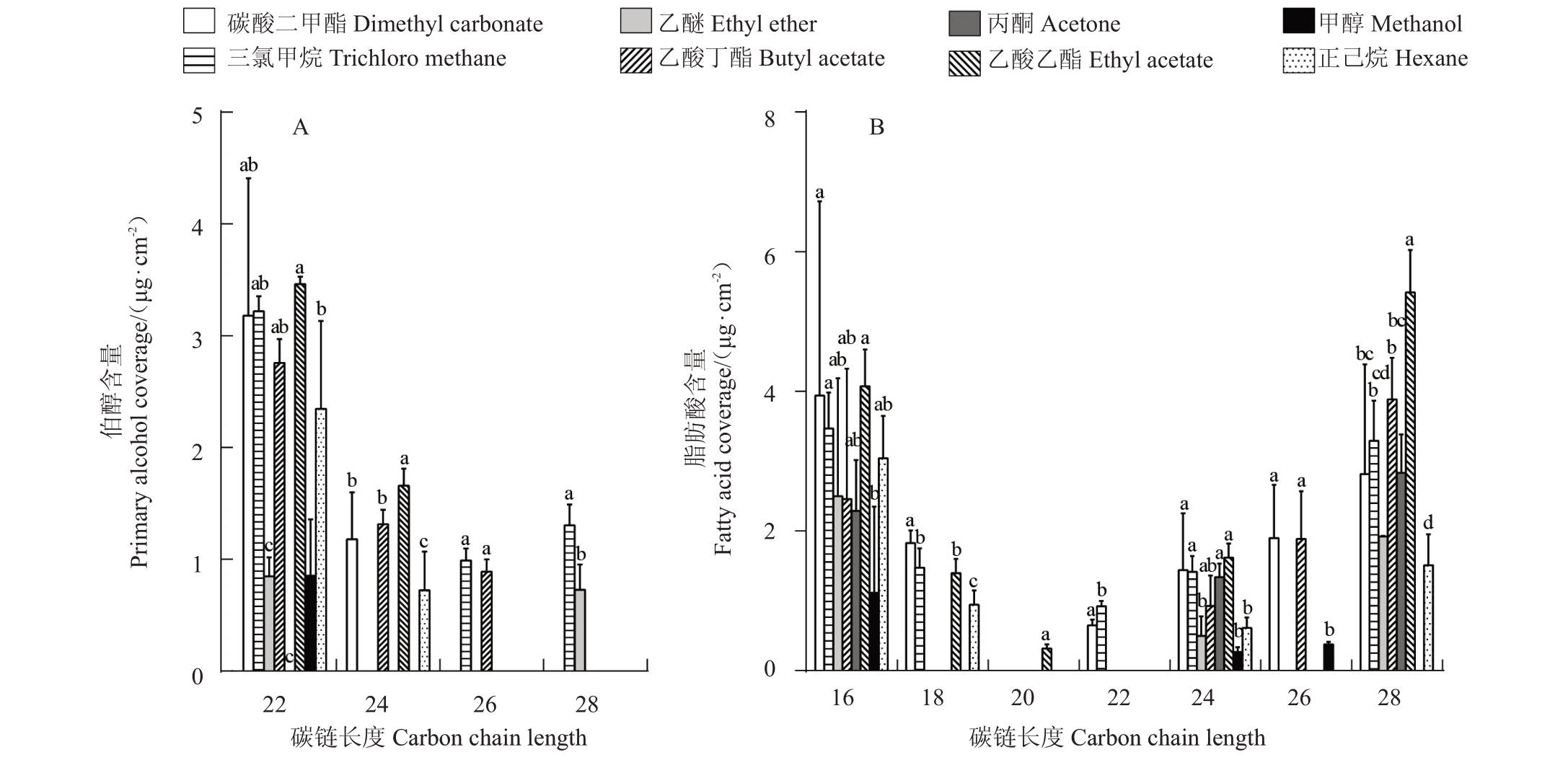
图4 不同溶剂提取蜡质伯醇(A)和脂肪酸(B)链长分布比较
Fig.4 Comparison of chain length distribution of waxy primary alcohols(A)and fatty acids(B)extracted with different solvents
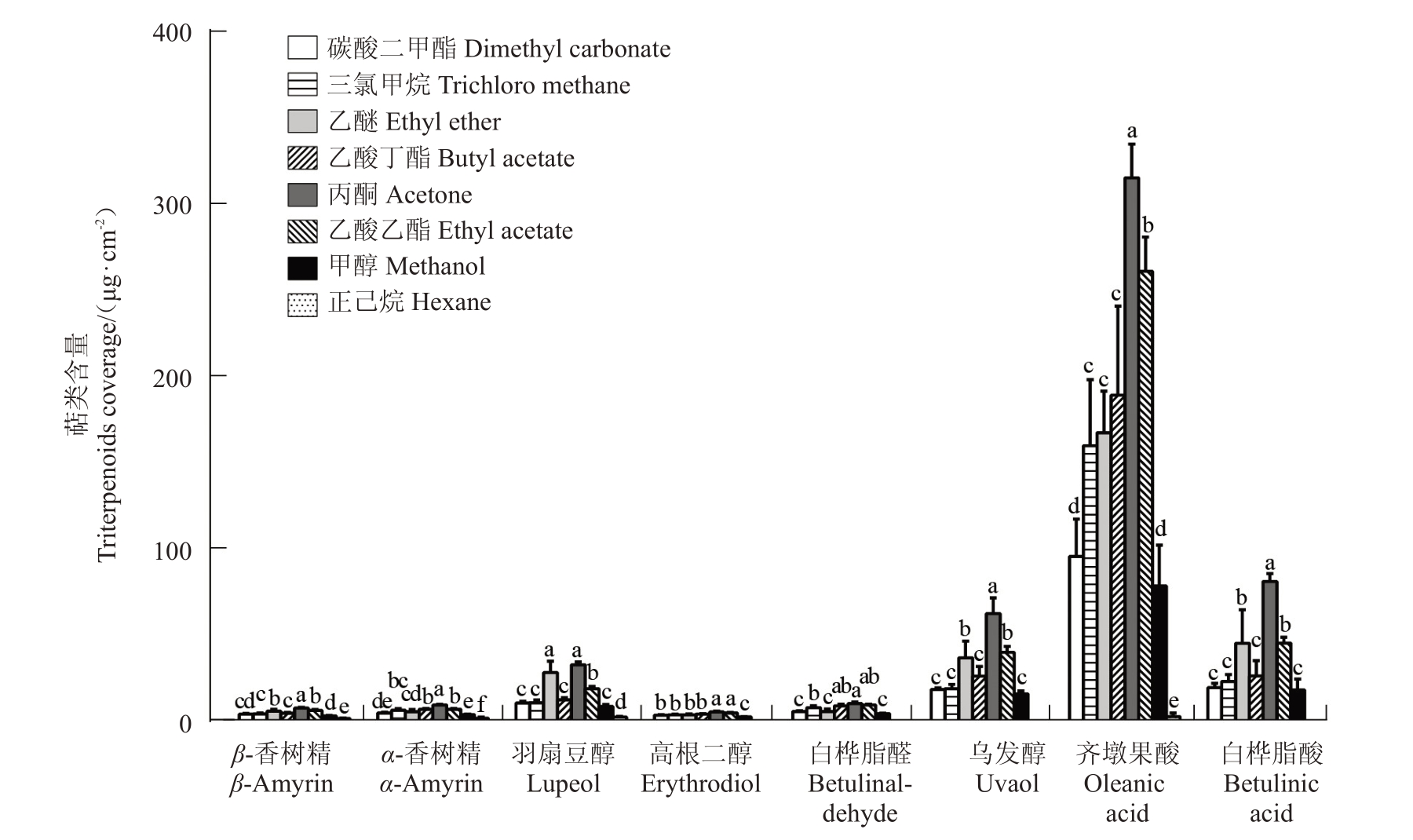
图5 不同溶剂提取蜡质萜类化合物比较
Fig.5 Comparison of waxy triterpenoids extracted with different solvents
2.4 不同溶剂提取蜡质含量主成分分析
为了进一步了解不同溶剂提取蜡质的效果,对提取的54 种蜡质化合物进行了主成分分析(图6)。PC1(94.2%)和PC2(4.0%)共描述了98.2%的数据差异性。根据54 种蜡质化合物主成分分析,将8 种溶剂处理分为5 类:乙酸丁酯(Ba1-3)和乙酸乙酯(Ea1-3)形成一组,其共同特征是两种溶剂提取的蜡质中二十九烷含量较高;丙酮(A1-3)和乙醚(Ee1-3)为第二组,其蜡质提取物中萜类物质含量较高,但烷烃含量较低;甲醇(M1-3)因提取的萜类和烷烃含量较低,单独为第三组;正己烷(H1-3)因提取蜡质中烷烃含量较高,萜类物质含量最低,单独构成第四组;而三氯甲烷(T1-3)和碳酸二甲酯(Dc1-3)提取的蜡质化合物无显著差异,共同组成第五组(图6)。该分类结果与前文各溶剂提取蜡质化合物组分和含量一致。
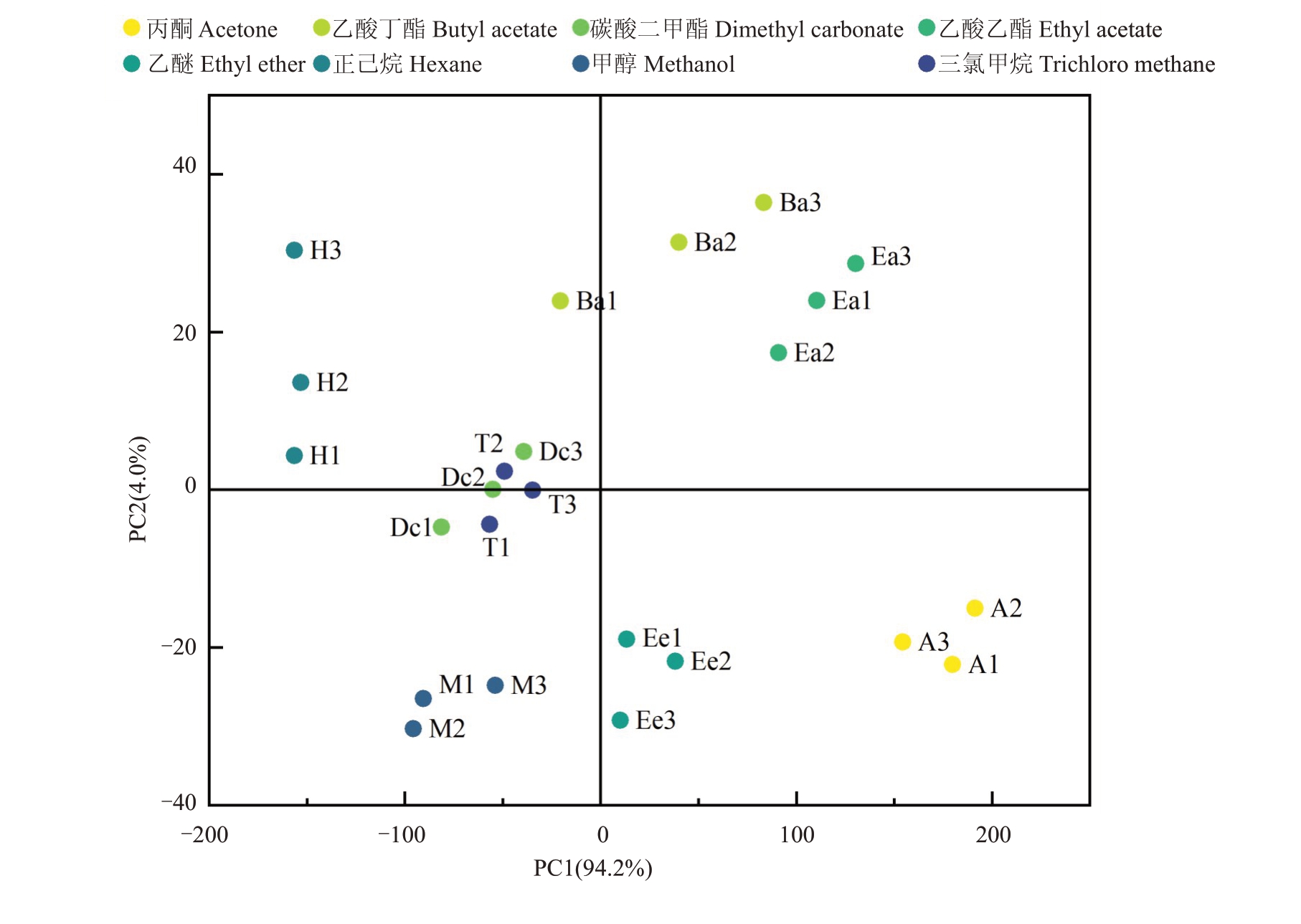
图6 不同溶剂提取蜡质含量主成分分析得分图
Fig.6 Principal component analysis score diagram of wax content extracted with different solvents
A1-3.丙酮;Ba1-3.乙酸丁酯;Dc1-3.碳酸二甲酯;Ea1-3.乙酸乙酯;Ee1-3.乙醚;H1-3.正己烷;M1-3.甲醇;T1-3.三氯甲烷。
A1-3.Acetone;Ba1-3.Butyl acetate;Dc1-3.Dimethyl carbonate;Ea1-3.Ethyl acetate;Ee1-3.Ethyl ether;H1-3.Hexane;M1-3.Methanol;T1-3.Chloroform.
3 讨 论
笔者在本研究中选取了8 种有机溶剂,以最常用的蜡质提取溶剂三氯甲烷为对照,采用相同条件方法分别提取翠冠梨果实表皮蜡质,比较不同溶剂的蜡质提取效果,以便筛选到与三氯甲烷提取效果相近但毒性较低的安全溶剂,进而在蜡质提取过程中替代三氯甲烷,保证实验人员在操作过程中的身心健康与安全。通过查阅国家卫生和计划生育委员会《食品安全国家标准急性经口毒性试验》(2015)数据可知,正己烷半数致死量(median lethal dose,LD50)数值最大,达到了28 710 mg·kg-1,其毒性最低,仅为三氯甲烷的1/32,乙酸丁酯和碳酸二甲酯次之,其毒性为三氯甲烷的1/14。尽管正己烷具有低毒特性,但由于正己烷闪点为-22 ℃,属于高度挥发的无色液体,极易燃,其蒸气与空气可形成爆炸性混合物,遇明火、高热极易燃烧爆炸,所以其用于实验存在较大安全隐患,并且其对于萜类物质的提取效果较差;乙酸丁酯的蜡质提取效果优于三氯甲烷,且其毒性仅为三氯甲烷的1/14,但由于其沸点为126.6 ℃,不易挥发,在蜡质提取过程中吹干溶剂较难。丙酮对萜类物质提取效果约是三氯甲烷的3 倍,但其对萜类以外物质的提取效果差,可作为蜡质萜类物质的优选提取溶剂;甲醇和乙醚的蜡质提取效果均较差。因此,上述5 种溶剂均不适合作为蜡质提取的最优改良溶剂。乙酸乙酯LD50为5620 mg·kg-1,属于2 级(实际无毒)毒性,且其蜡质提取效果优于三氯甲烷,适合作为最优替代溶剂;碳酸二甲酯LD50为13 000 mg·kg-1,其毒性为三氯甲烷的1/14,具有熔、沸点范围窄,溶解性能好,蒸发速度较快(沸点:90 ℃),闪点高(17 ℃)、蒸汽压低和空气中爆炸下限高(3.1%)等特点,且提取效果与三氯甲烷相当,可以作为替代溶剂。此外,研究表明使用中等极性的溶剂可以最大化萃取蜡质成分,包括极度疏水的碳氢化合物和含有(多个)官能团的极性更大的化合物[15,19]。笔者在本研究中筛选出乙酸乙酯和碳酸二甲酯均属于中间极性溶剂,与该结果保持一致。因此,乙酸乙酯和碳酸二甲酯这两种低毒高效的梨果皮蜡质提取溶剂将为安全有效地开展梨果皮蜡质研究及其他果树表皮蜡质研究工作提供新的选择。
植物表皮的蜡质含量、组分与结构极为复杂,并且容易受到外界环境的影响[20]。不同物种的不同品种间、同一品种的不同组织器官之间、同一器官的不同发育时期之间都存在差异。表皮蜡质的高效提取是开展植物蜡质相关研究的第一步,也是最重要的一步工作。因此,仅以三氯甲烷作为蜡质的提取溶剂并不能满足所有蜡质研究的需求,而根据研究对象和目标选择适宜的蜡质提取溶剂将使研究结论更可靠,更高效。针对不同物种蜡质提取方法的优化,研究者做出了大量工作,例如,王敏力等[21]通过EB酶解液提取柑橘果实蜡质,获得了与先前报道一致的蜡质成分。王雨菲等[22]通过二氯甲烷、正己烷、乙酸乙酯、甲醇4种溶剂提取葡萄果实蜡质,发现二氯甲烷与正己烷混合比3∶1(mL∶mL),液料比2∶1(mL∶g),浸泡7.5 min 蜡质提取最佳。郭焰等[23]发现通过按照料液比1∶15 加入乙酸乙酯提取玫瑰花蜡质效果最好,并且对具有多种生理功能的二十八烷醇提取效果较好,达到了23.16%。冯秀静等[24]采用亚临界丁烷提取甘蔗蜡发现料液比为1∶20(g∶mL),提取温度为70 ℃,提取3 次效果最好。此外,部分研究者关注某一类特定蜡质化合物的提取。例如,枸杞表皮蜡质主要成分为烷烃,杨爱梅等[25]选用正己烷提取枸杞表皮蜡质开展相关研究。因此,本研究筛选的蜡质萜类物质提取溶剂——丙酮,可作为梨果皮萜类物质研究的高效提取溶剂,为梨果皮等植物表皮蜡质的提取和研究提供更安全、高效的选择。
4 结 论
笔者在本研究中筛选出3 种蜡质优良提取试剂,乙酸乙酯蜡质提取效果最好,碳酸二甲酯蜡质提取效果与三氯甲烷相当,二者均毒性较低,挥发性较好,可作为三氯甲烷替代溶剂,丙酮是提取萜类化合物优势型溶剂。
[1] SAMUELS L,KUNST L,JETTER R.Sealing plant surfaces:Cuticular wax formation by epidermal cells[J].Annual Review of Plant Biology,2008,59:683-707.
[2] LARA Ⅰ,BELGE B,GOULAO L F.A focus on the biosynthesis and composition of cuticle in fruits[J].Journal of Agricultural and Food Chemistry,2015,63(16):4005-4019.
[3] BECKER M,KERSTⅠENS G,SCHÖNHERR J.Water permeability of plant cuticles:Permeance,diffusion and partition coefficients[J].Trees,1986,1(1):54-60.
[4] BUSCHHAUS C,JETTER R.Composition differences between epicuticular and intracuticular wax substructures:How do plants seal their epidermal surfaces?[J].Journal of Experimental Botany,2011,62(3):841-853.
[5] VAN MAARSEVEEN C,JETTER R.Composition of the epicuticular and intracuticular wax layers on Kalanchoe daigremontiana (Hamet et Perr.de la Bathie) leaves[J].Phytochemistry,2009,70(7):899-906.
[6] LEE S B,SUH M C.Advances in the understanding of cuticular waxes in Arabidopsis thaliana and crop species[J].Plant Cell Reports,2015,34(4):557-572.
[7] GUO N,GAO J H,HE Y J,GUO Y J.Compositae plants differed in leaf cuticular waxes between high and low altitudes[J].Chemistry&Biodiversity,2016,13(6):710-718.
[8] WANG M L,WU H Q,XU J,LⅠC L,WANG Y,WANG Z H.Five fatty acyl-coenzyme A reductases are involved in the biosynthesis of primary alcohols in Aegilops tauschii leaves[J].Frontiers in Plant Science,2017,8:1012.
[9] WU X,YⅠN H,CHEN Y Y,LⅠL,WANG Y Z,HAO P P,CAO P,QⅠK J,ZHANG S L.Chemical composition,crystal morphology and key gene expression of cuticular waxes of Asian pears at harvest and after storage[J].Postharvest Biology and Technology,2017,132:71-80.
[10] SALADⅠÉ M,MATAS A J,ⅠSAACSON T,JENKS M A,GOODWⅠN S M,NⅠKLAS K J,REN X L,LABAVⅠTCH J M,SHACKEL K A,FERNⅠE A R,LYTOVCHENKO A,O'NEⅠLL M A,WATKⅠNS C B,ROSE J K C.A reevaluation of the key factors that influence tomato fruit softening and integrity[J].Plant Physiology,2007,144(2):1012-1028.
[11] CURRY E.Effects of 1-MCP applied postharvest on epicuticular wax of apples (Malus domestica Borkh.) during storage[J].Journal of the Science of Food and Agriculture,2008,88(6):996-1006.
[12] WU X,YⅠN H,SHⅠZ B,CHEN Y Y,QⅠK J,QⅠAO X,WANG G M,CAO P,ZHANG S L.Chemical composition and crystal morphology of epicuticular wax in mature fruits of 35 pear (Pyrus spp.) cultivars[J].Frontiers in Plant Science,2018,9:679.
[13] CHU W J,GAO H Y,CAO S F,FANG X J,CHEN H J,XⅠAO S Y.Composition and morphology of cuticular wax in blueberry (Vaccinium spp.) fruits[J].Food Chemistry,2017,219:436-442.
[14] ARRENDALE R F,SEVERSON R F,CHORTYK O T,STEPHENSON M G.Ⅰsolation and identification of the wax esters from the cuticular waxes of green tobacco leaf[J].Beiträge Zur Tabakforschung Ⅰnternational,1988,14(2):67-84.
[15] YⅠN Y,BⅠY,CHEN S J,LⅠY C,WANG Y,GE Y H,DⅠNG B,LⅠY C,ZHANG Z.Chemical composition and antifungal activity of cuticular wax isolated from Asian pear fruit(cv.Pingguoli)[J].Scientia Horticulturae,2011,129(4):577-582.
[16] 李珍慈,江英,秦婕,陶海燕,王陈强.库尔勒香梨表皮蜡质提取条件研究及成分分析[J].中国酿造,2016,35(4):158-162.LⅠZhenci,JⅠANG Ying,QⅠN Jie,TAO Haiyan,WANG Chenqiang.Extraction conditions and component analysis of epicuticular wax of Korla fragrant pears[J].China Brewing,2016,35(4):158-162.
[17] 张微,赵迎丽,杨志国,王亮,陈会燕.‘玉露香’梨果皮蜡质含量提取方法及成分研究[J].食品科技,2022,47(1):34-40.ZHANG Wei,ZHAO Yingli,YANG Zhiguo,WANG Liang,CHEN Huiyan.Extraction method and analysis of waxy components from‘Yuluxiang’pear pericarp[J].Food Science and Technology,2022,47(1):34-40.
[18] 蒙小玉,穆悦,胡杨,吴潇,朱辰,王慧敏,陶书田,张绍铃,殷豪.几种果实表面积的三维激光扫描测定[J].园艺学报,2022,49(9):1998-2006.MENG Xiaoyu,MU Yue,HU Yang,WU Xiao,ZHU Chen,WANG Huimin,TAO Shutian,ZHANG Shaoling,YⅠN Hao.Determination of fruit surface area of several fruit by 3D laser scanning technology[J].Acta Horticulturae Sinica,2022,49(9):1998-2006.
[19] STAMMⅠTTⅠL,DERRⅠDJ S,GARREC J P.Leaf epicuticular lipids of Prunus laurocerasus:Ⅰmportance of extraction methods[J].Phytochemistry,1996,43(1):45-48.
[20] 龚成胜,刘文革.蔬菜作物果实和叶片表皮蜡质研究进展[J].中国瓜菜,2019,32(5):1-6.GONG Chengsheng,LⅠU Wenge.Research progress on epidermis wax in fruits and leaves of vegetable crops[J].China Cucurbits and Vegetables,2019,32(5):1-6.
[21] 王敏力,刘德春,杨莉,曾琼,王玥辰,吴启,刘山蓓,刘勇.不同种类柑橘的蜡质结构与成分比较[J].园艺学报,2014,41(8):1545-1553.WANG Minli,LⅠU Dechun,YANG Li,ZENG Qiong,WANG Yuechen,WU Qi,LⅠU Shanbei,LⅠU Yong.Comparative analysis of different Citrus wax morphological structure and composition[J].Acta Horticulturae Sinica,2014,41(8):1545-1553.
[22] 王雨菲,吕云皓,宋昕昕,江英.木纳格葡萄表皮蜡质提取工艺优化及其成分分析[J].食品工业科技,2023,44(3):221-229.WANG Yufei,LÜ Yunhao,SONG Xinxin,JⅠANG Ying.Optimization on extraction technology and component analysis of wax from munage grapes pericarp[J].Science and Technology of Food Ⅰndustry,2023,44(3):221-229.
[23] 郭焰,董成虎,白羽嘉,冯作山.玫瑰花蜡质提取工艺的研究[J].保鲜与加工,2012,12(3):34-37.GUO Yan,DONG Chenghu,BAⅠYujia,FENG Zuoshan.Study on the extraction technology of rose wax[J].Storage and Process,2012,12(3):34-37.
[24] 冯秀静,陆海勤,陈淋转,李凯.亚临界丁烷提取甘蔗滤泥中蔗蜡的工艺优化[J].中国调味品,2020,45(9):73-76.FENG Xiujing,LU Haiqin,CHEN Linzhuan,LⅠKai.Optimization of extraction process of sugarcane wax from sugarcane filter mud by subcritical butane[J].China Condiment,2020,45(9):73-76.
[25] 杨爱梅,吴古飞,杜静,李春雷,袁惠君,王丽丽.枸杞表皮蜡质层成分及显微结构的研究[J].食品工业科技,2011,32(12):112-114.YANG Aimei,WU Gufei,DU Jing,LⅠChunlei,YUAN Huijun,WANG Lili.Study on component and microscopic structure of wax of Lycium barbarum L.[J].Science and Technology of Food Ⅰndustry,2011,32(12):112-114.