COP9 信号复合体(constitutively photomorphogennic signalosome,CSN)最初从拟南芥中被鉴定为光形态发生的重要调节体[1]。CSN 是泛素-蛋白酶体途径中一种进化上高度保守的多蛋白复合物,其特异性地将泛素化的蛋白质引导至26S 蛋白酶体,从而促进植物降解[2]。在高等真核生物中,CSN 亚基命名法已经统一,各个CSN 亚基现在被称为CSN1-CSN8[3]。CSN 亚基可以独立地行使功能,也可以结合成CSN 复合体发挥作用,其中CSN5 的作用最受关注[4]。在拟南芥中发现AtCSN5a 和AtCSN5b基因,均为编码CSN5的基因[5]。在草莓、葡萄、水稻的基因组中,则只含有一个CSN5基因[6-7]。
CSN5 包含MPN(Mpr1p-Pad1p-N-terminal)结构域,主要识别信号因子,激活下游信号,从而调控一系列生物学功能,MPN 域蛋白具有生化活性,完整的CSN5是COP9信号复合体稳定所必需的[8]。目前CSN5 已经在多个物种中被克隆并分析,各种功能也陆续被发现,如双子病毒C2蛋白与CSN5相互作用并改变拟南芥中基于CUL1 的SCF 泛素E3 连接酶,最终涉及激素信号调控[9]。在番茄中CSN4和CSN5 通过JA 信号通路调节COⅠ1 和响应根结线虫感染[10]。CSN5a通过热胁迫来恢复AUX/ⅠAA水平,从而调节拟南芥中生长素含量[11]。因此,CSN5在生物胁迫和非生物胁迫中起着重要的调控作用。然而,CSN5在草莓上的分子基础目前还不清楚。
草莓是研究非呼吸跃变型果实发育成熟机制的模式植物[12-13]。基于笔者实验室草莓果实中的5个发育时期的转录组分析,筛选到一个随着果实发育表达量增加的COP9亚基,编码MPN保守结构域,将其命名为FaCSN5,并推测其可能涉及草莓果实发育调控。笔者在本研究中首先从八倍体草莓红颜果实中克隆到FaCSN5的CDS序列,并对其理化性质、蛋白结构、蛋白纯化、亚细胞定位、组织表达和农杆菌瞬时侵染草莓果实等进行预测和研究,为深入研究FaCSN5在草莓果实发育中的分子机制和功能奠定基础。
1 材料和方法
1.1 材料
试验材料为北京农学院科技园区日光温室基质栽培的八倍体草莓红颜(Fragaria × ananassa‘Benihoppe’)。温室内昼夜气温范围为10~30 ℃、空气相对湿度为60%~80%、日照时间为8 h,肥水按当地常规管理。在2023 年2 月第二茬果时,于上午10:00 对根、茎、叶、花、果实和种子各组织部位取样。根据前人的研究[14],采集小绿期、大绿期、褪绿期、白果期、始红期、片红期和全红期等不同发育时期的草莓果实,并用液氮速冻,-80 ℃保存。
1.2 总RNA提取及反转录
采用难提植物总RNA小提试剂盒(美基生物科技有限公司)提取样品总RNA,利用Hi fair®Ⅲ1st Strand cDNA Synthesis Super Mix for qPCR(翌圣生物公司)试剂盒反转录合成cDNA,放置-80 ℃保存备用。
1.3 草莓CSN5生物学分析
FaCSN5保守结构域通过NCBⅠ(https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi)预 测;FaCSN5蛋白的跨膜结构域使用TMHMM 2.0(https://services.healthtech.dtu.dk/services/TMHMM-2.0/)分析;FaCSN5 分子质量、等电点、蛋白质分子式和不稳定系数利用Prot Param(https://web.expasy.org/protparam/)在线预测;FaCSN5 蛋白质二级和三级结构利用NPS(https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_gor4.html)和Swiss Model(http://swissmodel.expasy.org/)在线分析;根据得到的FaCSN5 氨基酸序列,利用MEGA 软件进行氨基酸序列的同源性比对和系统进化树构建;通过在线软件Plant CARE(http://bioinformatics.psb.ugent.be/webtools/plantcare/html/)分析FaCSN5 启动子的顺式作用元件。
1.4 圆片温育与激素处理
取长势一致的白果期果实,使用打孔器和刀片将果实制备成圆片,放于温育平衡液中平衡30 min,其圆片直径和厚度分别为0.75 cm 和0.1 cm,温育平衡液包含10 mmol·L-1 MgCl2、5 mmol·L-1 CaCl2、10 mmol·L-1 EDTA、5 mmol·L-1维生素C、200 mmol·L-1甘露醇和50 mmol·L-1 MES(pH = 5.5)。将平衡好的果实圆片随机取5 g分别放入到含有100 μmol·L-1茉莉酸甲酯(methyl jasmonate,MeJA)、100 μmol·L-1赤霉素(gibberellin,GA)和100 μmol·L-1脱落酸(abscisic acid,ABA)的平衡液中,25 ℃、140 r·min-1孵育。于1、2、3、4、5 h分别取1 g处理好的圆片,液氮速冻,-80 ℃保存。
1.5 实时荧光定量PCR
利用Primer 5 软件设计实时荧光定量引物(表1),以红颜草莓根、茎、叶、花、种子、各发育时期的果实和圆片温育样品的cDNA为模板,草莓的Actin基因为内参基因,采用Bio-Rad CFX96 荧光定量PCR仪检测FaCSN5在不同部位和不同处理下的表达情况。扩增程序使用三步法反应程序,实时荧光定量加样体系和反应程序均参考Trans Start Top Green qPCR Super Mix(全式金生物技术有限公司)说明书。试验采取3次重复,相对表达量采用2-ΔΔCt计算。
表1 本研究所用引物
Table 1 Primers used in this study

引物名称Primer name FaCSN5-F FaCSN5-R FaCSN5Pro-F FaCSN5Pro-R RT-qPCR-FaCSN5-F RT-qPCR-FaCSN5-R RT-qPCR-FaActin-F RT-qPCR-FaActin-R FaCSN5-His-F FaCSN5-His-R FaCSN5-GFP-F FaCSN5-GFP-R attB FaCSN5-F attB FaCSN5-R引物序列(5’-3’)Primer sequence(5’-3’)ATGGATGCGGAAATAGCGCAG ACTTTCAATCATTGGCTCAGGGC GTCTATTGTTCTTTTCTTTCT ATCACTGCGGGAGAG TATTCCCAGACTAACAAACAGGC GAGTGGGGTCAATAACAACAGCC GCCAACCGTGAGAAGATG TCCAGAGTCAAGAACAATACCAG ATGGGTCGCGGATCCGAATTCATGGATGCGGAAATAGCGC TGCGGCCGCAAGCTTGTCGACACTTTCAATCATTGGCTCAGGG GGTCGACATTTAAATACTAGTATGGATGCGGAAATAGCGC GCCCTTGCTCACCATGGTACCACTTTCAATCATTGGCTCAGGG GGGGACAAGTTTGTACAAAAAAGCAGGCTTC CCATCTTCTACTACGACGAGGC GGGGACCACTTTGTACAAGAAAGCTGGGTC TGCCTGTTTGTTAGTCTGGGAA
1.6 草莓FaCSN5原核表达载体构建及蛋白纯化
基于二倍体森林草莓同源基因FvCSN5(XM_004291211),采用Snap Gene 软件设计引物(表1),以红颜草莓的cDNA 为模板,选用2 × Phanta MaxMaster Mix(诺唯赞生物科技股份有限公司)克隆携有pET30a 载体同源臂的目的片段,PCR 产物经1%琼脂糖凝胶电泳检测后,用琼脂糖凝胶DNA回收试剂盒(美基生物科技有限公司)回收目的条带。使用内切酶EcoRⅠ和SalⅠ对pET30a 表达载体进行双酶切,通过1%的琼脂糖凝胶电泳进行分离,将pET30a 载体片段切胶回收。选用同源重组酶Clon Express ⅡOne Step Cloning Kit(诺唯赞生物科技有限公司)连接携有载体同源臂的FaCSN5 目的片段和pET30a 载体片段。将连接产物转化到大肠杆菌DH5α(唯地生物技术有限公司),菌落PCR 验证后将阳性菌送金唯智生物科技有限公司进行测序。
提取重组质粒,命名为pET30a-FaCSN5,转化大肠杆菌感受态BL21(DE3),涂板于含有卡那霉素(100 mg·L-1)LB固体培养基上37 ℃过夜培养,菌落PCR 验证。挑取阳性单菌落,接种于20 mL 含有卡那霉素(100 mg·L-1)LB 液体培养基上,在37 ℃、220 r·min-1活化4~6 h,将菌液加至300 mL含有卡那霉素(100 mg·L-1)的LB液体培养基中,调OD600值为0.1,在摇床上继续摇动,直到OD600值为0.6~0.8,加入终浓度为0.5 mmol·L-1的ⅠPTG,在16 ℃、140 r·min-1诱导过夜。收集诱导后菌液300 mL,4 ℃离心机8000 r·min-1离心4 min,向沉淀中加入30 mL Binding Buffer(PBS 缓冲液,5 mmol·L-1咪唑,调pH 至7.4)和0.3 mL 100µmol·L-1 PMSF,超声20 min(350 W,超声开3 s,超声关2 s),4 ℃离心机8000 r·min-1离心15 min,取上清液。利用Ni-NTA 纯化目标蛋白,具体操作参照其说明书,采用含有500 mmol·L-1咪唑的PBS 洗脱蛋白,参考杨洁[15]的方法进行SDSPAGE和Western Blot检测。
1.7 亚细胞定位
选定酶切位点SpeⅠ和KpnⅠ对pCAMBⅠA-Super1300-GFP 表达载体进行双酶切,利用Snap Gene软件设计引物(表1),使用携有载体同源臂的引物克隆目的片段,采用同源重组法连接载体片段和目的片段。经菌落PCR验证的阳性菌,送金唯智生物科技有限公司测序验证,提取重组质粒35S::FaCSN5-GFP,过表达载体也用该重组质粒,将其转化农杆菌GV3101,对烟草下表皮进行侵染,3 d后在激光共聚焦下观察pCAMBⅠA-Super1300-GFP 和35S::FaCSN5-GFP的绿色荧光在烟草细胞的位置。
1.8 FaCSN5 RNAi载体构建
Gateway 的方法构建FaCSN5 RNAi 载体,设计引物(表1),BP 反应具体操作见赛默飞Gateway™BP Clonase™Ⅱ酶混合物说明书,LR 反应具体操作见赛默飞Gateway™LR Clonase™Ⅱ酶混合物说明书。
1.9 FaCSN5瞬时侵染草莓果实
对FaCSN5 RNAi、转化的空载体(对照)和OE阳性农杆菌,挑取阳性单菌落于5 mL含有50µg·mL-1壮观霉素或50µg·mL-1卡那霉素和50µg·mL-1利福平的YEB 液体培养基中,28 ℃,200 r·min-1水平震荡培养过夜。取适量菌液加入到10 mL含有50µg·mL-1卡那霉素和50 µg·mL-1利福平的YEB 液体培养基中,调OD600值为0.1,28 ℃,200 r·min-1震荡培养至OD600值为1.0~1.2。6000 r·min-1离心5 min,收集菌体,用果实侵染液重悬菌体,调OD600值为0.4~0.6,避光、28 ℃静置2 h,果实侵染液包含10 mmol·L-1 MES、10 mmol·L-1 MgCl2和200µmol·L-1 AS。挑选发育健康的褪绿期果实做上标记,每种农杆菌各注射6个,每天拍照记录表型,注射6 d后取样,去掉种子切取注射部位,液氮速冻,-80 ℃保存。试验设置3次重复。
2 结果与分析
2.1 草莓FaCSN5基因的克隆
红颜草莓cDNA为模板的PCR扩增条带大小约1080 bp(图1-A),在小绿期(SG)、大绿期(LG)、白果期(Wt)、始红期(ⅠR)、片红期(PR)5 个草莓果实发育时期,COP9 亚基的表达量从大绿期开始随着果实发育而增加(图1-B)。采用MEGA11 邻接法(Neighbor-Joining method)构建的系统发育树显示该基因的编码区(coding sequence,CDS)也为1080 bp,与二倍体草莓FvCSN5(XM_004291211)和月季CSN5b(XM_024310633.2)同源性非常高,相似率分别达100%和94.24%(图2),因此将该基因命名为FaCSN5。此外,栽培品种艳丽草莓从GDR(https://www.rosaceae.org/)Blast 获得了8 条基因序列[16],克隆的FaCSN5 与艳丽草莓8 条基因序列的碱基和氨基酸序列相似率分别达98.97%和99.35%。
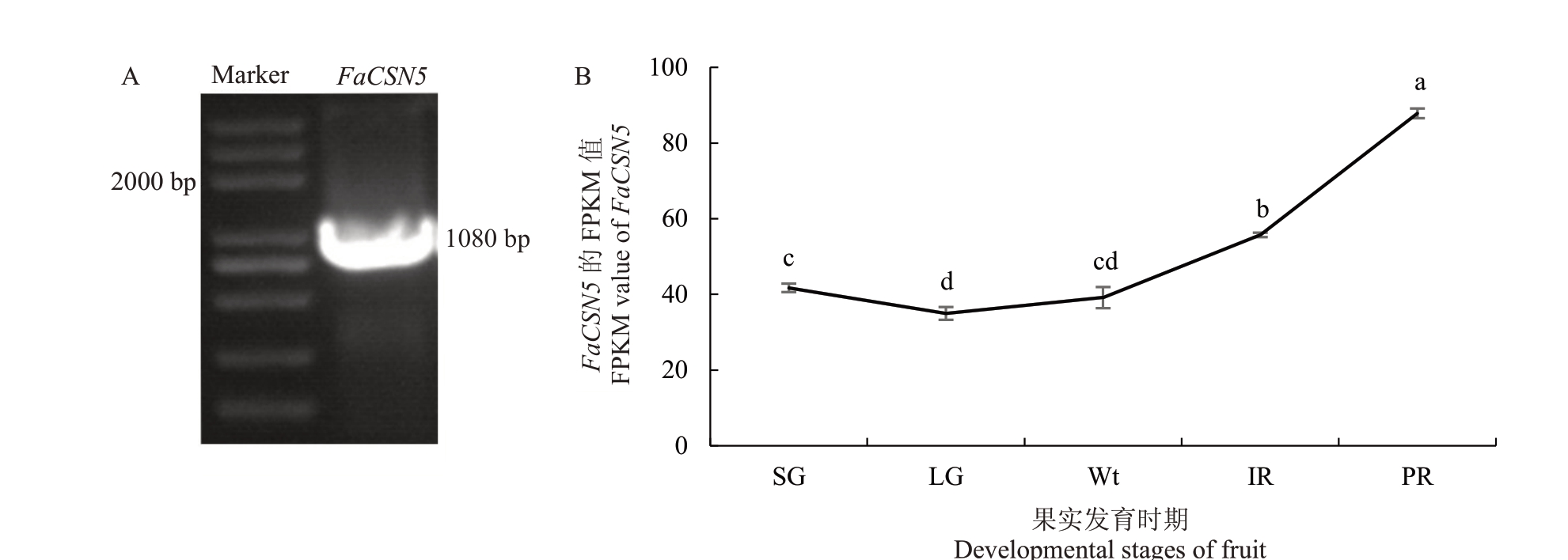
图1 FaCSN5 基因克隆及果实不同发育时期的FPKM 值
Fig.1 Cloning of FaCSN5 gene and FPKM values of fruits at different developmental stages
A.FaCSN5 CDS 区PCR 扩增;Marker.5000 bp Marker;B.果实不同发育时期的FPKM 值;SG.小绿期;LG.大绿期;Wt.白果期;ⅠR.始红期;PR.片红期。不同小写字母表示在不同条件下基因相对表达量的差异显著(p<0.05)。下同。
A.PCR amplification of the FaCSN5 CDS region;Marker.5000 bp Marker;B.FPKM values of fruits at different developmental stages.SG.Small green stage;LG.Large green stage;Wt.White stage;ⅠR.Ⅰnitial red stage;PR.Partial red stage.Different small letters indicate that the relative expression of genes under different conditions is significantly different(p<0.05).The same below.
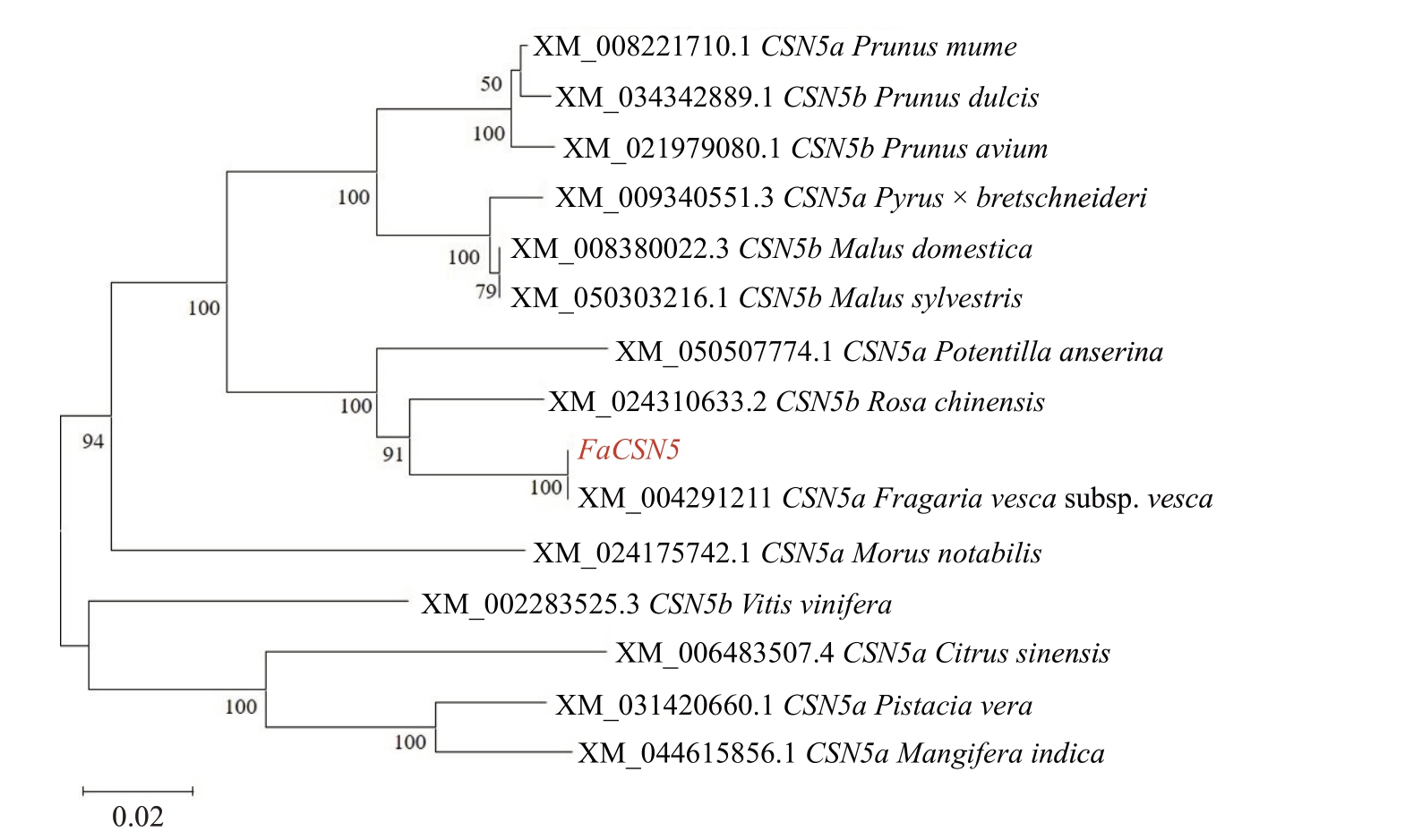
图2 FaCSN5 的系统进化分析
Fig.2 Phylogenetic analysis of FaCSN5
2.2 草莓FaCSN5基因的生物信息学分析
FaCSN5 基因的分子式为C1797H2773N471O558S14,编码359 个氨基酸,蛋白质分子质量为40.35 ku,等电点(pⅠ)为4.93,为酸性蛋白,含有31个正电荷氨基酸残基(Arg + Lys),48 个负电荷氨基酸残基(Asp +Glu),不稳定系数是41.41,亲水性平均值为-0.421,因此,FaCSN5蛋白可能为不稳定的疏水性蛋白。
利用NCBⅠ网站以及Blast 工具分析FaCSN5 蛋白的保守结构,结果显示FaCSN5 在51~323 位氨基酸之间含有一个MPN 保守结构域(图3)。采用TMHMM 2.0 对蛋白的跨膜结构域进行分析,发现FaCSN5无跨膜结构(图4)。

图3 FaCSN5 蛋白的保守结构域
Fig.3 The conserved domain of FaCSN5 protein
图4 FaCSN5 的跨膜域分析
Fig.4 Analysis of the transmembrane domain of FaCSN5
利用NPS 和Swiss Model 在线分析蛋白质二级和三级结构(图5)。FaCSN5蛋白的二级结构由4个结构组成,分别为α螺旋(α-helix)、β转角(Beta turn)、无规则卷曲(random coil)和延伸链(extended strand)(图5-A),Alpha helix 包含144 个氨基酸,占比为40.11%;Beta turn 包含11 个氨基酸,占比为3.06%;Random coil 包含152个氨基酸,占比为42.34%;Extended strand包含52个氨基酸,占比为14.48%。
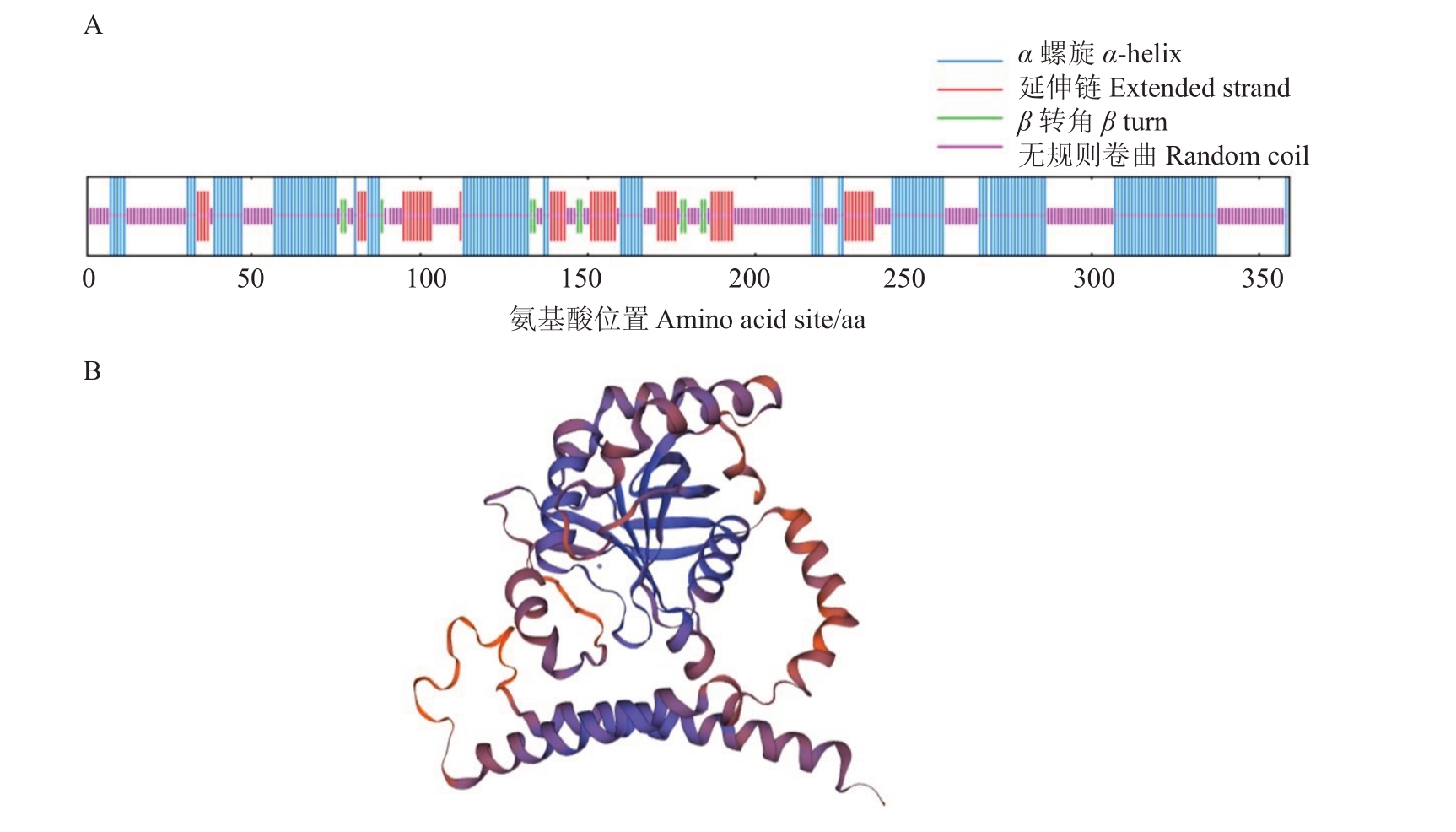
图5 FaCSN5 二级结构和三级结构
Fig.5 The secondary structure and tertiary structure of FaCSN5
A.FaCSN5 二级结构预测;B.FaCSN5 三级结构预测。
A.Prediction of the secondary structure of FaCSN5;B.Prediction of the tertiary structure of FaCSN5.
2.3 草莓FaCSN5的原核表达分析
FaCSN5-His 原核表达分析如图6 所示,对包括SDS-PAGE(图6-A)及Anti-His 抗体进行Western Blot 检测(图6-B),FaCSN5-His 目的蛋白条带大小在55~70 ku之间,约66 ku,其中含His标签0.84 ku。
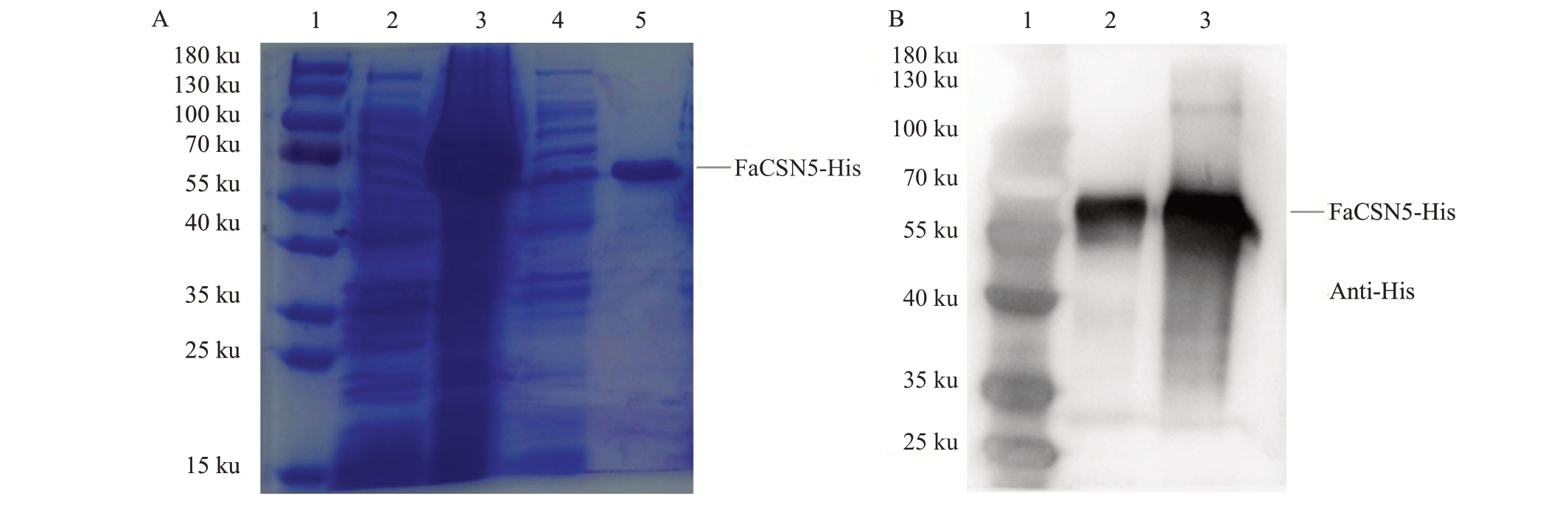
图6 FaCSN5-His 原核表达分析
Fig.6 FaCSN5-His prokaryotic expression analysis
A.SDS-PAGE 电泳胶图。1.180 ku Marker;2.诱导前菌体;3.诱导后菌体;4.超声破碎后的上清液;5.纯化的蛋白;2~5.上样20µL。B.免疫印迹显影。1.180 ku Marker;2.超声破碎后的上清液;3.纯化的蛋白;2 与3.上样3µL。
A.SDS-PAGE gel plots.1.180 ku Marker;2.Pre-induction organism;3.Post-induction organism;4.Supernatant after sonication;5.Purified protein;2-5.20µL on the sample.B.Western Blot plots.1.180 ku Marker;2.Supernatant after sonication;3.Purified protein;2 and 3.3µL on the sample.
2.4 草莓FaCSN5的亚细胞定位
草莓FaCSN5-GFP融合蛋白的亚细胞定位分析如图7所示,细胞核和细胞质有绿色荧光,表明FaC-SN5-GFP融合蛋白定位于细胞核和细胞质。
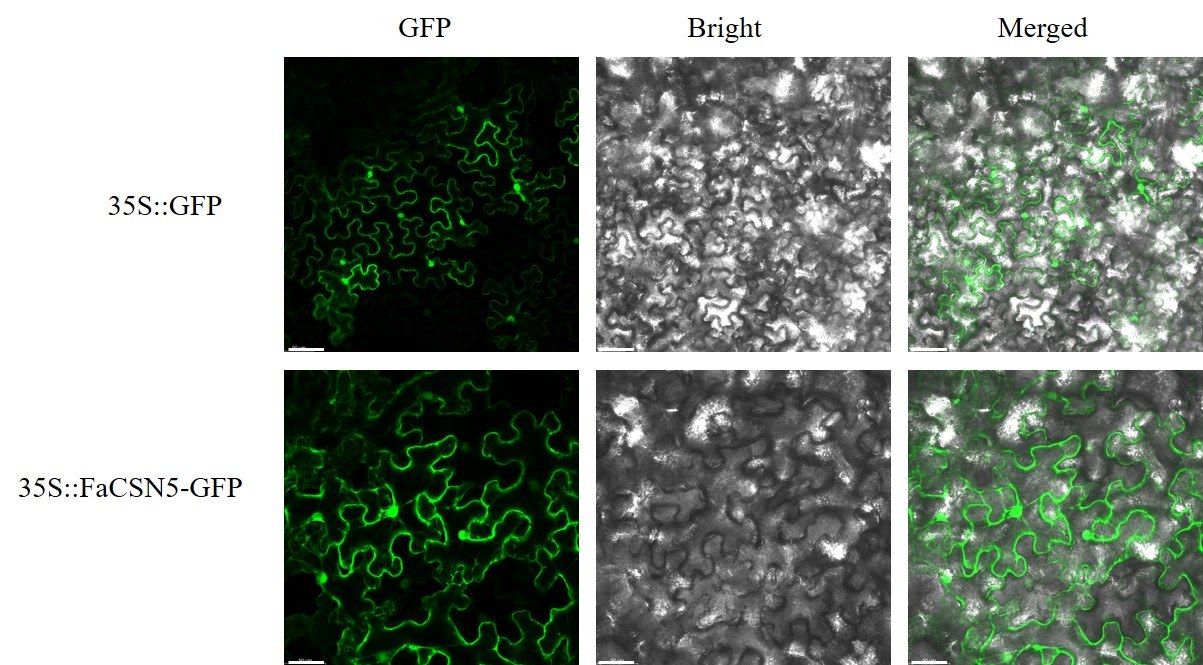
图7 FaCSN5-GFP 亚细胞定位
Fig.7 FaCSN5-GFP subcellular localization
GFP.绿色荧光蛋白通道;Bright.明场;Merge.合并图片。Bars=50 μm。GFP.Signal(green);Bright.Bright field;Merge.Overlay signals.Bars=50 μm.
2.5 草莓FaCSN5的时空表达
草莓根、茎、叶、花、种子和全红期果实的FaCSN5相对表达量如图8-A所示,根的FaCSN5相对表达量最高,其次是全红期果实、茎、叶和花,种子的表达量最低。其中,根的FaCSN5表达量可达种子的8倍,表明FaCSN5具有明显的组织特异性。如图8-B所示,在草莓果实发育的不同阶段,FaCSN5 相对表达量从褪绿期开始就一直呈现上升的趋势,在全红时期达到最高,表明FaCSN5 参与了草莓果实的发育。
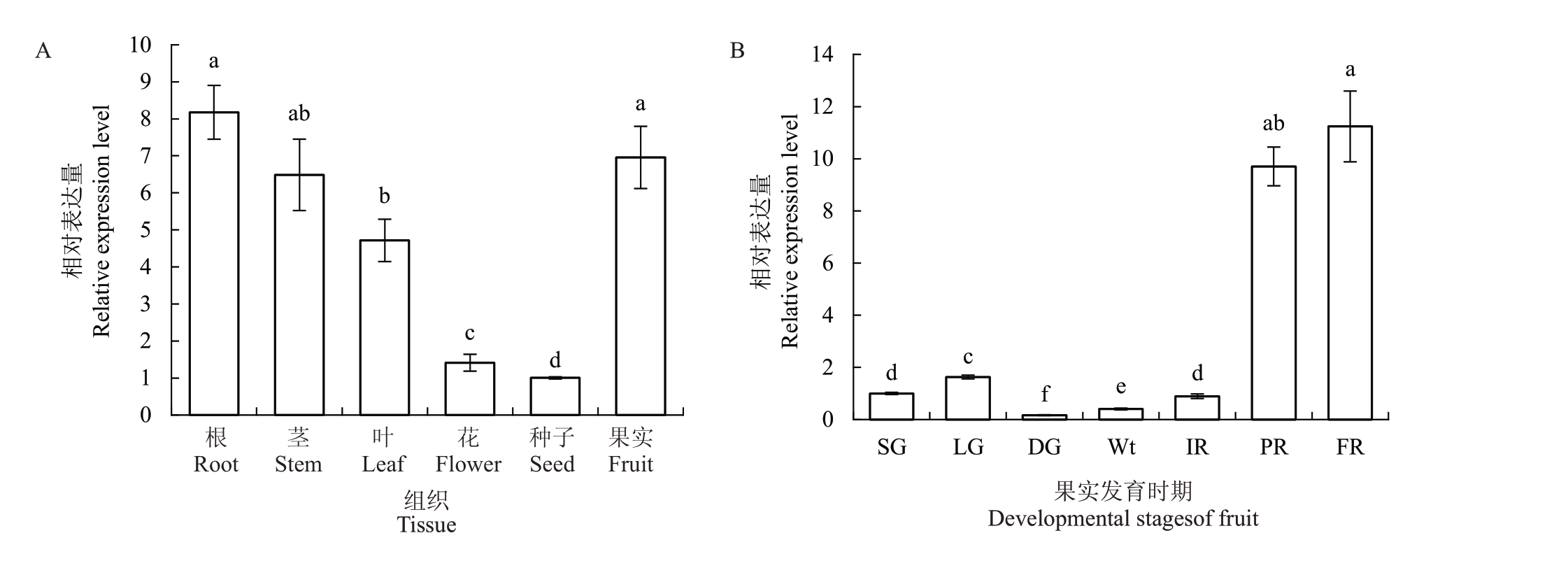
图8 FaCSN5 在不同组织部位和果实发育时期的相对表达量
Fig.8 Relative expression of FaCSN5 in different tissues of strawberry and at development stages of fruit
A.草莓不同组织部位FaCSN5 的相对表达量;B.草莓果实不同发育时期FaCSN5 的相对表达量。DG.褪绿期;FR.全红期。
A.The relative expression of the FaCSN5 in various tissue parts of strawberry; B.The relative expression of FaCSN5 at different developmental stages of strawberry fruit.DG.De-greening stage;FR.Full red stage.
2.6 草莓FaCSN5促进草莓果实成熟
为验证FaCSN5 在草莓果实发育中的功能,分别构建FaCSN5 RNAi 和FaCSN5 OE 载体,转化GV3101 农杆菌,通过农杆菌介导瞬时侵染草莓果实,每天拍照记录表型(图9-A),通过RT-qPCR检测FaCSN5 表达水平(图9-B)。结果表明,FaCSN5 过表达能够促进草莓果实成熟;沉默FaCSN5 表达则会抑制草莓果实成熟。以上结果初步证明FaCSN5在草莓果实成熟过程中起着重要作用。
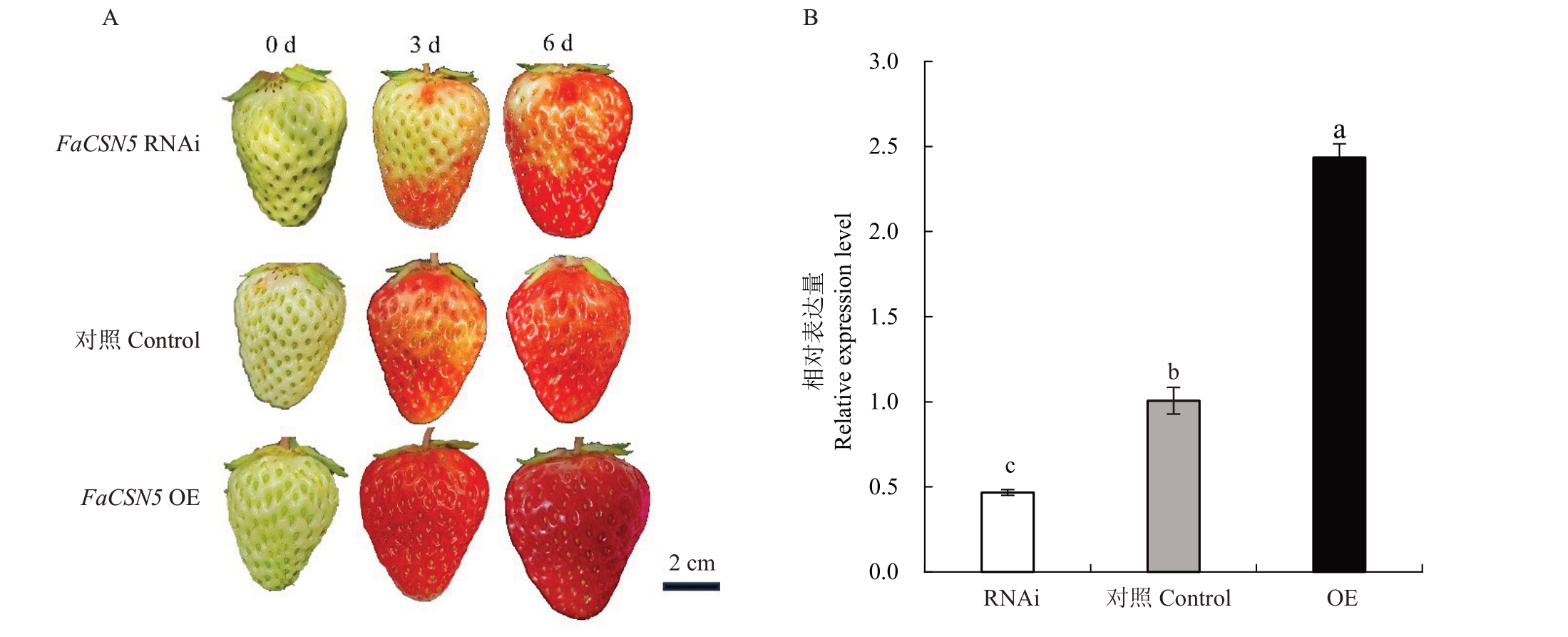
图9 FaCSN5 正调控草莓果实成熟
Fig.9 FaCSN5 positively regulated strawberry fruit ripening
A. FaCSN5 RNAi、对照和FaCSN5 OE 农杆菌注射草莓果实的表型观察;B.RT-qPCR 分析FaCSN5 RNAi、对照和FaCSN5 OE 果实中FaCSN5 基因的相对表达量。
A.Phenotypic observations of strawberry fruit injected with FaCSN5 RNAi, control, and FaCSN5 OE of agrobacterium; B.RT-qPCR analysis of the relative expression of the FaCSN5 gene in fruits of the FaCSN5 RNAi,control and FaCSN5 OE.
2.7 草莓FaCSN5在激素诱导下的表达分析
为了进一步分析FaCSN5 的功能,以红颜草莓基因组DNA为模板,扩增FaCSN5起始密码子至上游约2000 bp 的碱基序列(图10),PCR 产物长度1726 bp,与NCBⅠ查询到的二倍体草莓启动子序列进行比对,其相似率为100%。将FaCSN5启动子序列与八倍体草莓艳丽的启动子序列[16]进行比较,发现2条启动子序列符合条件,并且与FaCSN5启动子序列的相似率达88.07%。表2显示了FaCSN5基因启动子的顺式作用元件及其功能,FaCSN5 启动子有不同类型的响应元件,它们的功能也各不相同,其中,茉莉酸甲酯响应元件、赤霉素响应元件为激素响应元件。
图10 FaCSN5 启动子克隆
Fig.10 Cloning of FaCSN5 promoter
表2 FaCSN5 启动子顺式作用元件分析
Table 2 Analysis of cis-acting elements of FaCSN5 promoter
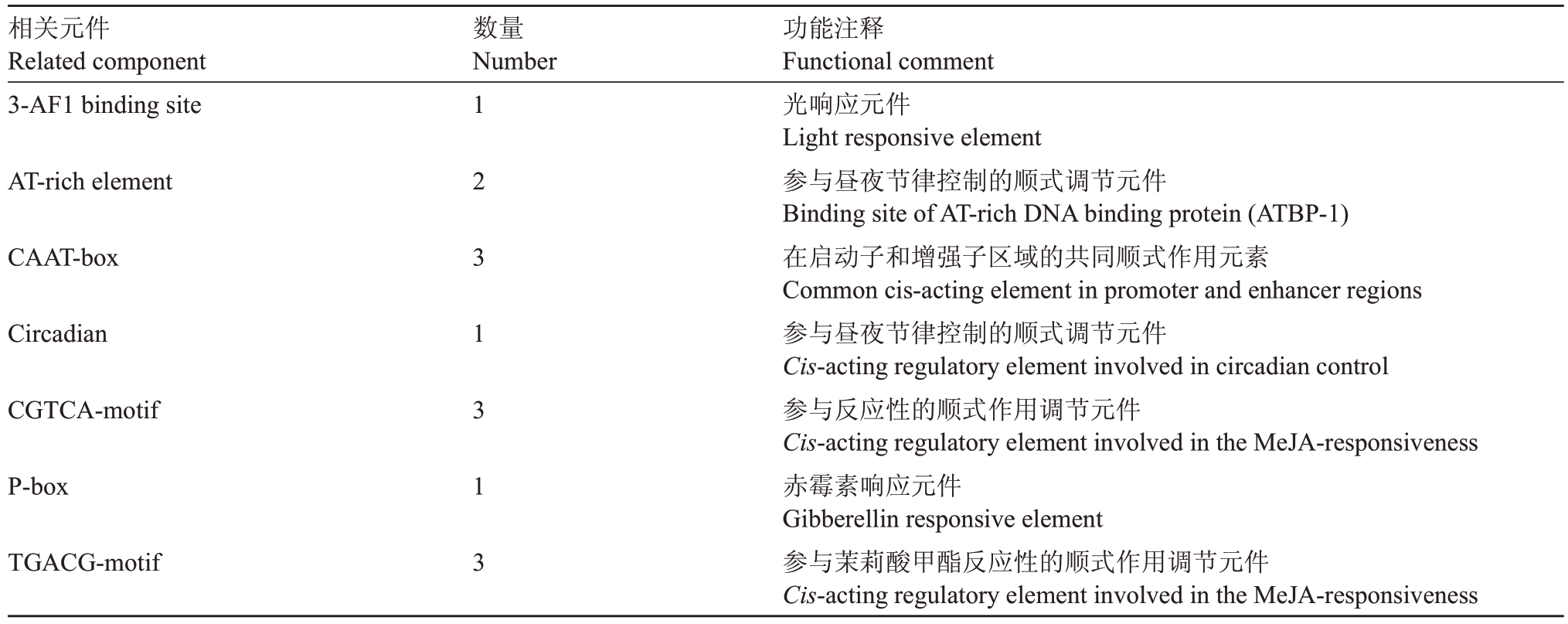
相关元件Related component 3-AF1 binding site数量Number AT-rich element CAAT-box Circadian CGTCA-motif P-box TGACG-motif 1 2 3 1 3 1 3功能注释Functional comment光响应元件Light responsive element参与昼夜节律控制的顺式调节元件Binding site of AT-rich DNA binding protein(ATBP-1)在启动子和增强子区域的共同顺式作用元素Common cis-acting element in promoter and enhancer regions参与昼夜节律控制的顺式调节元件Cis-acting regulatory element involved in circadian control参与反应性的顺式作用调节元件Cis-acting regulatory element involved in the MeJA-responsiveness赤霉素响应元件Gibberellin responsive element参与茉莉酸甲酯反应性的顺式作用调节元件Cis-acting regulatory element involved in the MeJA-responsiveness
FaCSN5 基因表达水平会受到MeJA、GA 和ABA不同程度的诱导,图11表示不同外源激素处理草莓果实后FaCSN5 基因表达水平。外施MeJA 处理1 h 后,FaCSN5 基因表达水平略低于对照组,处理后的2~5 h,FaCSN5基因表达水平均高于对照组;外施GA 处理1~5 h 后,FaCSN5 基因表达水平均高于对照组,在处理1 h 后表达量最高。外施ABA 处理后FaCSN5 基因表达水平均高于对照组,且在处理3 h后表达量最高。
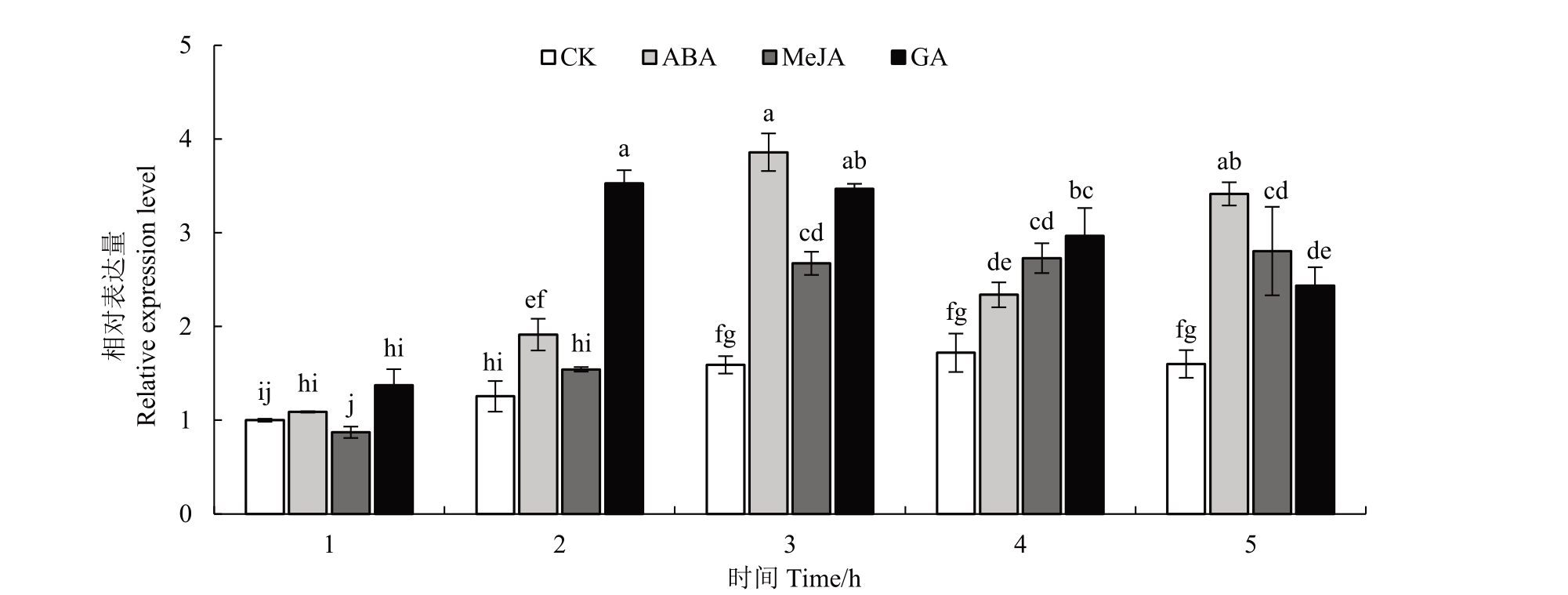
图11 不同外源激素处理草莓果实后FaCSN5 基因表达水平
Fig.11 Expression levels of FaCSN5 gene after different exogenous hormone treatments in strawberry fruit
3 讨 论
草莓是蔷薇科多年生草本植物,味道甘甜,香气浓郁,含有丰富的营养物质。草莓是研究非呼吸跃变型果实发育成熟机制的模式植物[12-13]。草莓果实发育和成熟受到诸多因素的影响,如温度、光照等因素的影响,其中植物激素的协同作用扮演了重要的角色[17]。
CSN参与协调植物的生长发育[18]。笔者在本研究中以草莓果实不同发育时期转录组数据为基础,克隆出草莓CSN5 基因。FaCSN5 只有一个MPN 功能域,系统发育分析表明,其与二倍体草莓和月季等蔷薇科植物的进化关系较近,说明CSN5 在进化过程中结构和功能较为保守。原核表达FaCSN5-His后,通过SDS-PAGE 和Western Blot 检测,发现FaCSN5-His目的蛋白条带约66 ku。通过亚细胞定位分析,FaCSN5-GFP融合蛋白定位于细胞核和细胞质。
关于CSN 的表达模式已有一些研究报道,如GmCSN5s 在大豆的根和根瘤中的表达量高于其他组织[19];HbCSN5 在橡胶树的胶乳中表达量最高,在雄花中表达量最低[20];OsCSN5基因在水稻的茎尖分生组织中表达量较高[21]。笔者在本研究中利用RTqPCR 检测了草莓根、茎、叶、花、种子和全红期果实的相对表达量,发现FaCSN5 在草莓根中表达量最高,其次是全红期果实、茎、叶和花,种子的表达量最低,表明FaCSN5具有组织特异性。FaCSN5在全红期表达量最高,在褪绿期表达量最低,从褪绿期到全红期,FaCSN5表达量呈现上升的趋势,表明FaCSN5可能参与草莓果实的发育。为了验证FaCSN5在果实发育过程中的功能,笔者通过农杆菌介导瞬时侵染草莓果实记录表型,检测FaCSN5的表达水平,结果表明,FaCSN5过表达能够促进草莓果实成熟;沉默FaCSN5表达则会抑制草莓果实成熟。以上研究表明FaCSN5 在草莓果实成熟过程中起着重要作用。
CSN参与包括乙烯、脱落酸、生长素、水杨酸等多种植物激素的调控过程[22-25]。OsCSN1通过CUL1的E4连接酶降解SLR3,最终影响内源激素GA的合成[26]。笔者在本研究中发现FaCSN5 启动子区域含有茉莉酸甲酯和赤霉素激素响应元件,推测FaCSN5基因表达可能受到这两种激素诱导。因此,通过圆片温育和外源激素处理试验,发现茉莉酸甲酯和赤霉素处理后,FaCSN5基因表达明显受到诱导,且显著响应赤霉素。草莓是非呼吸跃变型果实,脱落酸在草莓果实发育过程中起着重要的作用,通过脱落酸处理后,发现FaCSN5基因表达显著受到脱落酸的调控。
综上所述,FaCSN5 基因可能通过多种激素信号转导途径促进草莓果实的成熟。
4 结 论
FaCSN5 蛋白条带约66 ku,在细胞核和细胞质存在定位。FaCSN5 表达受到脱落酸、茉莉酸甲酯和赤霉素的诱导调控,推测FaCSN5 基因可能通过多种激素信号转导途径促进草莓果实的成熟。总之,本研究结果为深入研究FaCSN5 在草莓果实中的分子机制和功能奠定了基础。
[1] WEⅠN,CHAMOVⅠTZ D A,DENG X W.Arabidopsis COP9 is a component of a novel signaling complex mediating light control of development[J].Cell,1994,78(1):117-124.
[2] SⅠNHA A,ⅠSRAELⅠR,CⅠRⅠGLⅠANO A,GⅠHAZ S,TRABELCY B,BRAUS G H,GERCHMAN Y,FⅠSHMAN A,NEGRⅠR,RⅠNALDⅠT,PⅠCK E.The COP9 signalosome mediates the Spt23 regulated fatty acid desaturation and ergosterol biosynthesis[J].The FASEB Journal,2020,34(4):4870-4889.
[3] QⅠN N X,XU D Q,LⅠJ G,DENG X W.COP9 signalosome:Discovery,conservation,activity,and function[J].Journal of Ⅰntegrative Plant Biology,2020,62(1):90-103.
[4] DUBⅠEL D,ROCKEL B,NAUMANN M,DUBⅠEL W.Diversity of COP9 signalosome structures and functional consequences[J].FEBS Letters,2015,589(19):2507-2513.
[5] KWOK S F,SOLANO R,TSUGE T,CHAMOVⅠTZ D A,ECKER J R,MATSUⅠM,DENG X W.Arabidopsis homologs of a c-Jun coactivator are present both in monomeric form and in the COP9 complex,and their abundance is differentially affected by the pleiotropic cop/det/fus mutations[J].The Plant Cell,1998,10(11):1779-1790.
[6] JⅠN D,LⅠB S,DENG X W,WEⅠN.Plant COP9 signalosome subunit 5,CSN5[J].Plant Science,2014,224:54-61.
[7] CUⅠK C,LⅠU M,KE G H,ZHANG X Y,MU B,ZHOU M,HU Y,WEN Y Q.Transient silencing of VvCSN5 enhances powdery mildew resistance in grapevine(Vitis vinifera)[J].Plant Cell,Tissue and Organ Culture,2021,146(3):621-633.
[8] DENTⅠS,FERNANDEZ-SANCHEZ M E,ROGGE L,BⅠANCHⅠE.The COP9 signalosome regulates Skp2 levels and proliferation of human cells[J].Journal of Biological Chemistry,2006,281(43):32188-32196.
[9] LOZANO-DURÁN R,ROSAS-DÍAZ T,GUSMAROLⅠG,LUNA A P,TACONNAT L,DENG X W,BEJARANO E R.Geminiviruses subvert ubiquitination by altering CSN-mediated derubylation of SCF E3 ligase complexes and inhibit jasmonate signaling in Arabidopsis thaliana[J].The Plant Cell,2011,23(3):1014-1032.
[10] SHANG Y F,WANG K X,SUN S C,ZHOU J,YU J Q.COP9 signalosome CSN4 and CSN5 subunits are involved in jasmonatedependent defense against root- knot nematode in tomato[J].Frontiers in Plant Science,2019,10:1223.
[11] SⅠNGH A K,YADAV B S,DHANAPAL S,BERLⅠNER M,FⅠNKELSHTEⅠN A,CHAMOVⅠTZ D A.CSN5A subunit of COP9 signalosome temporally buffers response to heat in Arabidopsis[J].Biomolecules,2019,9(12):805.
[12] LⅠAO X,LⅠM S,LⅠU B,YAN M L,YU X M,ZⅠH L,LⅠU R Y,YAMAMURO C.Ⅰnterlinked regulatory loops of ABA catabolism and biosynthesis coordinate fruit growth and ripening in woodland strawberry[J].Proceedings of the National Academy of Sciences of the United States of America,2018,115(49):E11542-E11550.
[13] MOYA-LEÓN M A,STAPPUNG Y,MATTUS-ARAYA E,HERRERA R.Ⅰnsights into the genes involved in ABA biosynthesis and perception during development and ripening of the Chilean strawberry fruit[J].Ⅰnternational Journal of Molecular Sciences,2023,24(10):8531.
[14] 郑珍珍,陈雪雪,沈元月,黄芸.转录因子FabHLH148 参与草莓果实的颜色发育[J].果树学报,2022,39(8):1358-1367.ZHENG Zhenzhen,CHEN Xuexue,SHEN Yuanyue,HUANG Yun.Transcription factor FabHLH148 is involved in the color development of strawberry fruit[J].Journal of Fruit Science,2022,39(8):1358-1367.
[15] 杨洁.丹参SmMYB36 与SmCSN5 蛋白的互作关系鉴定及其作用分析[D].杨凌:西北农林科技大学,2021.YANG Jie.Ⅰdentification and analysis of interaction between SmMYB36 and SmCSN5 protein in Salvia miltiorrhiza[D].Yangling:Northwest A&F University,2021.
[16] MAO J X,WANG Y,WANG B T,LⅠJ Q,ZHANG C,ZHANG W S,LⅠX,LⅠJ,ZHANG J X,LⅠH,ZHANG Z H.High-quality haplotype-resolved genome assembly of cultivated octoploid strawberry[J].Horticulture Research,2023,10(1):uhad002.
[17] GU T T,JⅠA S F,HUANG X R,WANG L,FU W M,HUO G T,GAN L J,DⅠNG J,LⅠY.Transcriptome and hormone analyses provide insights into hormonal regulation in strawberry ripening[J].Planta,2019,250(1):145-162.
[18] MUSAZADE E,LⅠU Y X,REN Y X,WU M,ZENG H,HAN S N,GAO X W,CHEN S H,GUO L Q.OsCSN1 regulates the growth and development of rice seedlings through the degradation of SLR1 in the GA signaling pathway[J].Agronomy,2022,12(12):2946.
[19] 张梦柯.GmCSN5s 的克隆及其调控大豆适应低磷胁迫的机制初探[D].广州:华南农业大学,2019.ZHANG Mengke.Clonging and charaterizatino of GmCSN5s functions in soybean adaptation to phosphorus deficiency[D].Guangzhou:South China Agricultural University,2019.
[20] 吴绍华,张世鑫,邓小敏,陈月异,田维敏.橡胶树COP9 家族基因的克隆及表达分析[J].热带作物学报,2019,40(2):281-288.WU Shaohua,ZHANG Shixin,DENG Xiaomin,CHEN Yueyi,TⅠAN Weimin.Gene cloning and expression analysis of the COP9 signalosome members in laticifer cells of rubber tree[J].Chinese Journal of Tropical Crops,2019,40(2):281-288.
[21] 龚茵茵.CSN5 通过调节AsA 的稳态介导水稻盐胁迫响应的分子机制[D].长沙:湖南大学,2020.GONG Yinyin.The molecular mechanism of CSN5 mediates salt stress response in rice by regulating AsA homeostasis[D].Changsha:Hunan University,2020.
[22] SHⅠB Z,HOU J Q,YANG J,HAN ⅠJ,TU D Y,YE S Q,YU J F,LⅠL J.Genome-wide analysis of the CSN genes in land plants and their expression under various abiotic stress and phytohormone conditions in rice[J].Gene,2023,850:146905.
[23] ZHENG Q Z,ZHANG L,ZHANG Q,PANG Z Y,SUN Y,YⅠN Z,LOU Z Y.Discovery of interacting proteins of ABA receptor PYL5 via covalent chemical capture[J].ACS Chemical Biology,2019,14(12):2557-2563.
[24] JⅠN D,WU M,LⅠB S,BÜCKER B,KEⅠL P,ZHANG S M,LⅠJ G,KANG D M,LⅠU J,DONG J,DENG X W,ⅠRⅠSH V,WEⅠN.The COP9 signalosome regulates seed germination by facilitating protein degradation of RGL2 and ABⅠ5[J].PLoS Genetics,2018,14(2):e1007237.
[25] BAⅠX X,HUANG X L,TⅠAN S X,PENG H,ZHAN G M,GOHER F,GUO J,KANG Z S,GUO J.RNAi-mediated stable silencing of TaCSN5 confers broad-spectrum resistance to Puccinia striiformis f.sp.tritici[J].Molecular Plant Pathology,2021,22(4):410-421.
[26] HAN S N,LⅠU Y X,BAO A,ZENG H,HUANG G H,GENG M,ZHANG C Y,ZHANG Q,LU J M,WU M,GUO L Q.OsCSN1 regulates the growth of rice seedlings through the GA signaling pathway in blue light[J].Journal of Plant Physiology,2023,280:153904.