鹰嘴桃又名鹰嘴蜜桃,是蔷薇科(Rosaceae)李属(Prunus)桃(Prunus persica L.)下的一个品种。连平鹰嘴桃是广东省河源市连平县特产,该县鹰嘴桃于2015年被评为中国国家地理标志产品[1]。经过30多年的发展,当地相关种植技术已形成一套较成熟的体系。然而,近年来不少果园常常受到果肉组织海绵化病害的危害。据笔者前期研究发现,这是发生危害较重的一种生理性病害,果实成熟前10 d左右开始出现病害,病变果肉颜色变褐,呈海绵状,表皮甚至出现开裂症状[2]。果实海绵组织病害不仅降低了鹰嘴桃的营养价值,而且外部难以鉴别病害症状,导致果实分级困难,严重影响鹰嘴桃食用价值和商品价值。尽管对鹰嘴桃海绵果实组织病害的认识已经取得一些进展,但是鹰嘴桃海绵果实组织病害的发生原因及机制尚不明确。曾有调查者发现鹰嘴桃海绵组织病害的发生与太阳直射存在相关性,阳面果的发病率明显高于阴面果[2]。
细胞壁的分解、修饰等代谢作用会影响果肉的力学性能,有研究者发现果实发生海绵组织、裂果均与细胞壁代谢有关[3-6]。细胞壁代谢主要由植物细胞降解酶参与进行,包括多聚半乳糖醛酸酶(polygalacturonase,PG)、果胶甲基酯酶(pectin methylesterase,PME)、β-半乳糖苷酶(β-galactosidase,β-Gal)、木聚糖内切糖苷酶(xyloglucan endotransglucosylase,XET)、β-D-木糖苷酶(β-D-xylosidase,BXL)和膨胀素(expansin,EXP)[7]。先前研究报道杧果海绵组织中PG、PME 的表达水平显著上调,PG 和PME的过表达导致果胶降解,从而减少细胞黏附,可能是杧果海绵组织发生的主要原因之一[3-4]。另外,有研究者发现多果实开裂与PG、PME、β-Gal、BXL、XET、EXP 等细胞壁代谢酶的过量表达密切相关[5-6,8-9]。
此外,许多研究者发现果肉分解常常是一种或多种矿物质营养缺乏导致的,其中,缺钙是导致水果生理障碍相关的最常见因素之一[10]。钙是植物生长发育过程中的重要营养元素,在构建细胞壁、保持细胞膜完整性、信号转导和维持细胞离子平衡等过程中发挥关键作用[11]。果树缺钙能引起水心、苦果或内部崩溃等症状[4,12-14],其中,缺钙便是引发杧果海绵化的关键因素[4,15]。植物体内钙离子含量主要受钙离子运转蛋白和钙传感器调控[16]。植物调节钙离子跨膜运转的基因包括钙运转ATP 酶(calcium-transporting ATPase,ACA)、钙 通 道(calcium channel,TPC)、钙离子/阳离子交换蛋白(cation/calcium exchanger,CAX)和植物V 型ATP 酶(V-type ATPase,AVP)基因[17]。另外,钙离子传感器主要分为4 类,包括钙调蛋白(calmodulin protein,CaM)、类钙调蛋白(calmodulin-like protein,CML)、类钙调磷酸酶B蛋白(calcineurin-B-like protein,CBL)和钙依赖蛋白激酶(calmodulin-dependent protein kinase,CDPK)基因[16]。钙离子运转蛋白和钙传感器对维持植物的正常生长发育有重要作用[17],钙离子运转蛋白和钙传感器表达异常会导致钙离子代谢紊乱,诱发植物生理病害[14,18]。Ma 等[4]研究发现钙运转ATP 酶、钙离子/阳离子交换蛋白促进钙离子流向液泡,从而破坏细胞钙离子稳态,是杧果海绵组织发生的重要原因。
随着高通量测序技术的发展,转录组测序已成为探讨果树病害致病机制的有效途径[4,14,19]。转录组是指特定细胞或组织在某一阶段转录出的所有信使RNA(mRNA)的总和,能够揭示特定生物学过程中的分子机制。笔者在本研究中拟对鹰嘴桃海绵果实组织、非病害组织和健康果实组织进行转录组测序,比较分析差异表达基因,为进一步了解鹰嘴桃果肉海绵组织发生的分子机制以及采取相应的防治措施提供参考。
1 材料和方法
1.1 试验材料
2021年7月16日在广东省河源市连平县内,选取树龄相同的鹰嘴蜜桃树,分别采摘已成熟健康果实和发病果实,带回室内去皮后分别取健康果实组织(JKG)、病害果海绵组织(BGHM)和病害果非病变组织(BGFB),每种处理取3组重复(图1)。
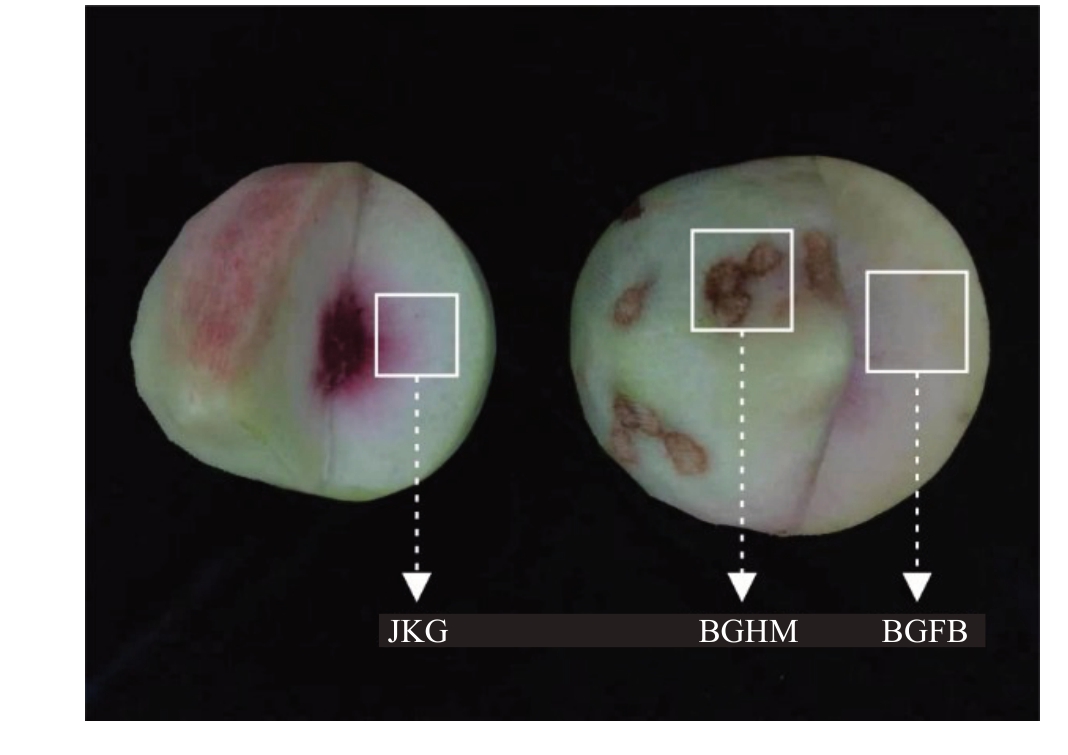
图1 鹰嘴桃健康果实(JKG)、海绵组织病害果实病害
组织(BGHM)与非病害组织(BGFB)
Fig.1 Healthy flesh(JKG),flesh with spongy tissue(BGHM),non-damage flesh with spongy tissue(BGFB)
of olecranon peach and healthy flesh
1.2 鹰嘴桃果实总RNA提取
将鹰嘴桃样本加液氮进行研磨,采用Trizol法提取总RNA[20]。利用1.5%琼脂糖凝胶电泳以及超微量分光光度计分别检测总RNA 的完整性、质量与浓度。测序文库的构建以及转录组测序委托北京贝瑞和康生物技术有限公司完成。利用HiSeq 6000测序仪以配对末端模式(PE150)对构建的文库进行测序。
1.3 转录组数据分析
使用FastQC v0.12.1(http://www.bioinformatics.babraham.ac.uk/projects/fastqc/)对下机原始数据进行评估,且使用Trimmomatic v0.39[21]去除接头,以及对低质量序列(序列质量值低于25 或序列长度小于50 bp)进行过滤。使用Hisat2 v2.2.1[22]将过滤后的序列与桃参考基因组(GenBank 登录号:GCF_000346465.2)进行比对。利用FADU v1.8.3[23]对鹰嘴桃转录本进行定量,计算TPM(Transcript per million),使用DESeq v1.34.0[24]进行差异基因表达分析,差异表达基因(differentially expressed genes,DEG)的筛选条件为p<0.01以及|log2FC|>1.0。利用egg-NOG-mapper v2.1.10[25]筛选到的差异基因进行GO功能注释和KEGG通路注释。使用TBtools v.1.112[26]进行GO功能富集分析和KEGG通路富集分析。
2 结果与分析
2.1 鹰嘴桃转录组数据比对
对下机数据进行过滤后,鹰嘴桃病害果海绵组织、病害果非病变组织和健康果实组织所获得的转录组序列在18 425 897~30 543 093条之间。转录组序列与桃参考基因组进行比对,结果显示比对率均在95%以上(表1)。
表1 鹰嘴桃样品转录组数据过滤与比对结果统计
Table 1 Summary of trimming and mapping results of the
transcription data
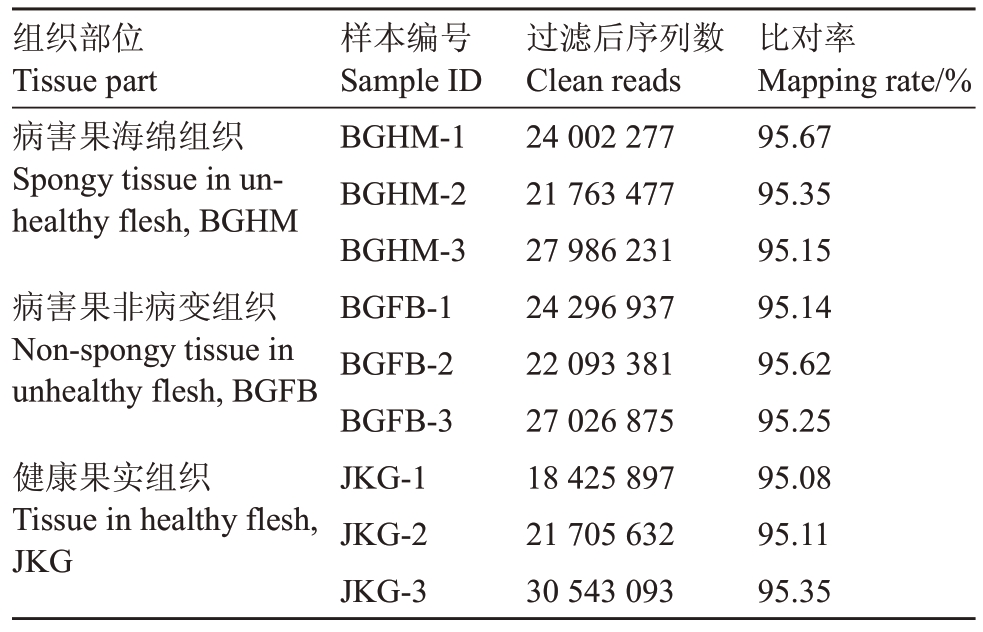
组织部位Tissue part病害果海绵组织Spongy tissue in unhealthy flesh,BGHM病害果非病变组织Non-spongy tissue in unhealthy flesh,BGFB健康果实组织Tissue in healthy flesh,JKG样本编号Sample ⅠD BGHM-1 BGHM-2 BGHM-3 BGFB-1 BGFB-2 BGFB-3 JKG-1 JKG-2 JKG-3过滤后序列数Clean reads 24 002 277 21 763 477 27 986 231 24 296 937 22 093 381 27 026 875 18 425 897 21 705 632 30 543 093比对率Mapping rate/%95.67 95.35 95.15 95.14 95.62 95.25 95.08 95.11 95.35
2.2 差异表达分析
根据差异倍数筛选,BGHM vs JKG 共筛选到4537个基因差异表达显著(图2-A~B),其中2127个基因表达下调,2410 个基因表达上调(图2-C)。BGFB vs JKG共筛选到4446个基因差异表达显著,其中2323 个基因表达下调,2123 个基因表达上调(图2-D)。根据差异倍数筛选,BGHM vs BGFB 共筛选到672个基因差异表达显著,其中133个基因表达下调,539个基因表达上调(图2-E)。
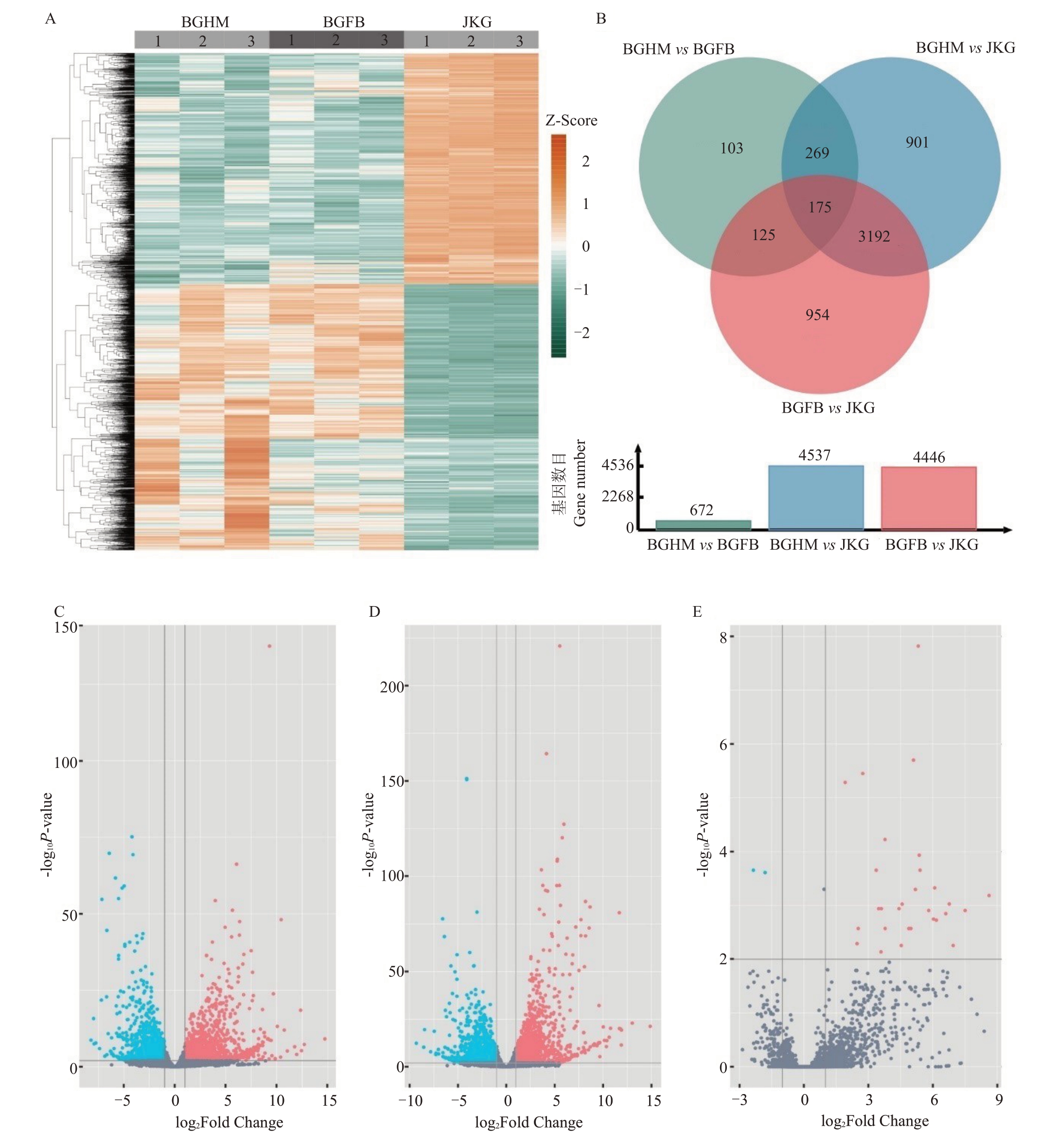
图2 鹰嘴桃样品差异基因表达分析
Fig.2 Differentially expressed genes in P.persica
A.鹰嘴桃样品差异表达基因;B.不同果实组织之间差异表达基因的韦恩图;C.病害果海绵组织(BGHM)与健康果实(JKG)差异表达基因火山图;D.病害果非病变组织(BGFB)与健康果实(JKG)差异表达基因火山图;E.病害果海绵组织(BGHM)与病害果非病变组织(BGFB)差异表达基因火山图。
A.Genes expressed in different fruit tissue sample; B.Venn diagram of differentially expressed genes in different fruit tissue sample; C.Volcano plots of differentially expressed genes between spongy tissue (BGHM) and healthy tissue (JKG); D.Volcano plots of differentially expressed genes between non-spongy tissue(BGFB)and healthy tissue(JKG);E.Volcano plots of differentially expressed genes between spongy tissue(BGHM)and non-spongy tissue(BGFB).
2.3 差异表达基因功能分析
经过GO 功能分析,BGHM vs JKG 差异表达基因注释到35 条分子功能术语、50 条细胞组分术语以及105 条生物过程的术语(图3)。其中,差异表达基因执行的分子功能前3 位是离子结合、氧化还原酶活性、无极分子实体跨膜转运蛋白活性,所处细胞组分前3 位是细胞质、陈旧的细胞质部分、膜,参与的生物学过程前3 位是对刺激的应答、对化学物质的应答、对有机物的应答(图3-A)。BGFB vs JKG 差异表达基因注释到34 条分子功能术语、44条细胞组分术语以及161 条生物过程的术语,其GO富集结果与BGHM vs JKG差异表达基因GO富集结果相似(图3-B)。BGHM vs BGFB差异表达基因注释到9 条分子功能术语、4 条细胞组分术语以及8 条生物过程的术语。其中,差异表达基因执行的分子功能前3 位是糖基转移酶活性、已糖基转移酶活性、葡糖转移酶,所处细胞组分前3位是细胞外围、内质网、外部封装结构,参与的生物学过程前3位是对刺激的应答、对有机物的应答、对含氧化合物的应答(图3-C)。

图3 鹰嘴桃海绵组织与健康果实组织差异表基因GO 功能富集分析
Fig.3 GO enrichment of differentially expressed genes between spongy tissue and healthy flesh
A.病害果海绵组织vs 健康果实组织;B.病害果非病变组织vs 健康果实组织;C.病害果海绵组织vs 病害果非病变组织。
A.BGHM vs JKG;B.BGFB vs JKG;C.BGHM vs BGFB.
KEGG 通路富集分析结果显示,BGHM vs JKG差异表达基因共注释到11条通路,其中主要富集在新陈代谢、碳水化合物代谢、能量代谢、光合作用相关通路(图4-A)。BGFB vs JKG 差异表达基因的KEGG 通路富集结果与BGHM vs JKG 差异表达基因KEGG通路相似(图4-B)。BGHM vs BGFB差异表达基因注释到9条通路,主要富集于新陈代谢、次生产物合成、次生产物代谢等通路(图4-C)。

图4 鹰嘴桃海绵组织与健康果实组织差异表基因KEGG 通路富集分析
Fig.4 KEGG enrichment of differentially expressed genes between spongy tissue and healthy flesh
A.病害果海绵组织vs 健康果实组织;B.病害果非病变组织vs 健康果实组织;C.病害果海绵组织vs 病害果非病变组织。
A.BGHM vs JKG;B.BGFB vs JKG;C.BGHM vs BGFB.
2.4 细胞壁代谢相关差异表达基因
本研究中,选择了细胞壁代谢相关的PG、PME、β-Gal、XET、BXL和EXP基因。BGHM vs JKG中发现33 个与细胞壁代谢相关的差异表达基因,其中8个基因下调,25 个基因上调;BGHM vs BGFB 中共鉴定出17个与细胞壁代谢相关的差异表达基因,其中1个基因下调,16个基因上调(图5)。多聚半乳糖醛酸酶(XP_007217288、XP_007215246、XP_007213880)、β-D-木糖苷酶7(XP_007225247)、木聚糖内切糖苷酶2(XP_007215773、XP_007215791、XP_07215793、XP_007217276、XP_007217342、XP_020409478)、膨胀素A4(XP_007205762、XP_007218834)在BGHM中表达均高于JKG和BGFB。其中,4个木聚糖内切糖苷酶2基 因 (XP_007215773、 XP_007215793、 XP_007217276、XP_007217342)在BGHM vs JKG、BGFB vs JKG、BGHM vs BGFB中均显著上调。

图5 细胞壁代谢过程相关的差异表达基因
Fig.5 The DEGs involved in cell wall metabolism
2.5 钙离子运转和钙传感相关差异表达基因
植物调节钙离子跨膜运转的基因包括钙运转ATP酶、钙通道、钙离子/阳离子交换蛋白、植物V型ATP 酶基因。BGHM vs JKG 中发现钙离子运转相关差异表达基因有11 个,其中5 个上调,6 个下调;BGFB vs JKG中钙离子中运转相关差异表达基因有4 个,皆为上调基因;BGHM vs BGFB 中,钙离子相关差异表达基因有3个,皆为上调基因(图6)。这些差异表达基因主要是钙运转ATP 酶与钙离子/阳离子交换蛋白基因。3个质膜型钙运转ATP酶13基因(XP_007225391、XP_007225392、XP_007225393),在BGHM 中表达均高于JKG 和BGFB。其中XP_007225391 在BGHM vs JKG、BGFB vs JKG、BGHM vs BGFB中均显著上调。
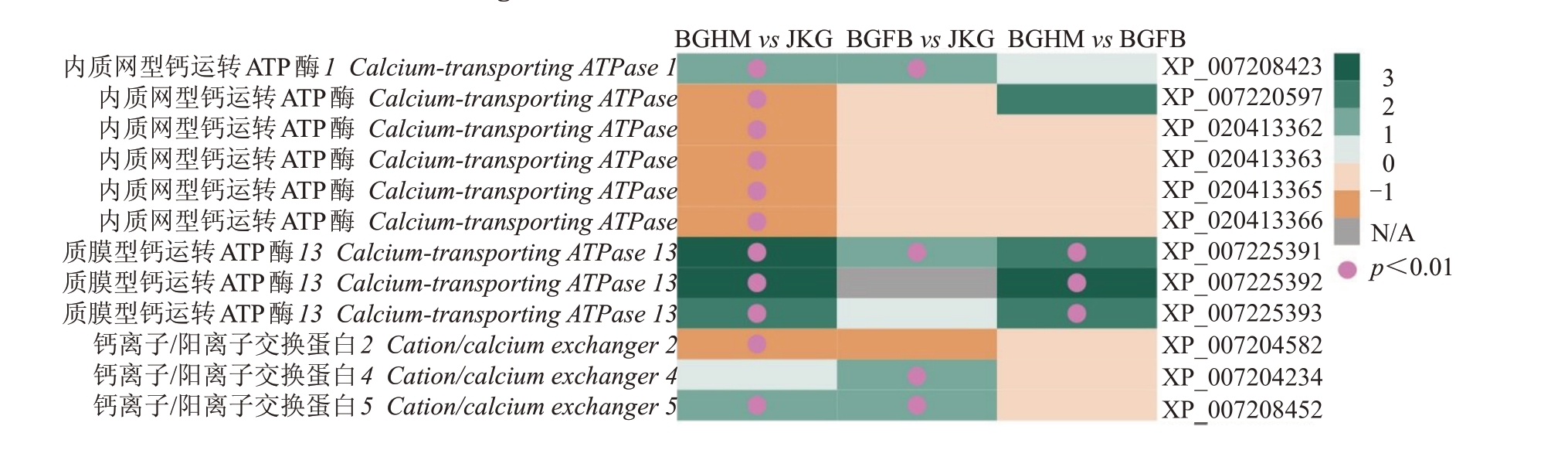
图6 钙离子传递相关的差异表达基因
Fig.6 The DEGs involved in calcium transport
钙离子传感器主要分为4 类,包括钙调蛋白、类钙调蛋白、类钙调磷酸酶B 蛋白和钙依赖蛋白激酶基因。转录组比较分析结果显示,BGHM vs JKG钙传感相关差异表达基因有28 个,其中15 个基因上调,13 个基因下调;BGFB vs JKG 钙传感相关差异表达基因有26个,其中13个基因上调,13个基因下调;BGHM vs BGFB 钙传感相关差异表达基因仅有6 个(图7)。这些差异表达基因包括钙调蛋白、类钙调蛋白和类钙调磷酸酶B 蛋白基因。笔者在本研究中发现钙调蛋白11(XP_007200520)、钙调蛋白18(XP_020419982)在BGHM 中表达量显著高于JKG、BGFB。其他钙传感器蛋白在BGHM 和BGFB 中出现不同程度的上调与下调:2 个类钙调磷酸B 蛋白1、3 个钙调蛋白3、钙调蛋白24、钙调蛋白25、钙调蛋白27、钙调蛋白31、钙调蛋白45、钙调蛋白48 基因在BGHM 和BGFB 均表现上调表达,而8个类钙调磷酸B蛋白7、2个钙调蛋白1、钙调蛋白23、钙调蛋白29 在BGHM 和BGFB 中均表现下调表达。

图7 钙感器相关的差异表达基因
Fig.7 The DEGs involved in calcium sensors
3 讨 论
果肉海绵组织的形成是一个复杂的过程,而该过程受到内部发育和外界环境因素共同影响。鹰嘴桃果肉海绵组织是发生危害较重的一种生理性病害,然而其发生机制尚不明确[2]。笔者在本研究中通过对病变鹰嘴桃病害组织、非病害组织,以及健康鹰嘴桃进行转录组测序和比较分析,初步探讨了不同果实组织之间基因表达差异。
鹰嘴桃果实组织海绵化,甚至出现开裂,可能是细胞壁的降解、修饰影响果肉的机械性能所导致。细胞壁降解涉及一系列细胞壁修饰酶、水解酶的调控作用,包括PG、PME、β-Gal、XET 和EXP[7]。笔者在本研究中通过比较转录组,发现多聚半乳糖醛酸酶At1g4810、多聚半乳糖醛酸酶QRT3、木聚糖内切糖苷酶2、β-D-木糖苷酶7、膨胀素A4 在病害果海绵组织中的表达量高于健康果和病害果实非病变组织中的表达量。PG是植物细胞壁降解的关键酶,主要促进果胶的水解[27],譬如猕猴桃软化过程中多聚半乳糖醛酸酶At1g48100呈上调表达[28]。此外,XET主要参与细胞壁降解和重塑的过程,水解木聚糖并重新连接至其他多糖[29-30]。研究表明木聚糖内切糖苷酶2、木聚糖内切糖苷酶5的高表达水平会导致果肉快速软化[31-32]。BXL 是细胞壁修饰酶,与XET 功能类似,主要参与分解细胞壁中木聚糖和阿拉伯木聚糖残基[33-34]。EXP 是一种引起植物细胞壁松弛的蛋白,是细胞壁的关键调节剂[35],而膨胀素A4 表达量上升被证实与木瓜软化有关[36]。因此,笔者推测鹰嘴桃果实组织海绵化可能与PG、XET、β-Gal、BXT、EXP 等一系列细胞壁水解酶基因的上调表达有关。
钙是调节水果质量的重要矿物质元素,特别是维持水果的硬度,减少腐烂和生理紊乱的发生[16]。钙代谢失衡是导致水果生理紊乱最常见因素之一,其中,杧果果实海绵组织便是缺钙引起的[4]。植物体内的钙离子主要存在于细胞壁,含量高,为60%~75%[37],钙离子可以与细胞壁成分结合、交联果胶残基增强细胞壁结构和通过降低细胞壁降解酶对其底物的可及性来稳定细胞膜[38-39]。调控细胞内和细胞间钙离子运转分布,对植物细胞的生长和代谢至关重要,水果钙代谢失衡可能是细胞水平上钙离子的异常分布导致局部缺乏所引起的[4,40-41]。植物可以通过一系列的钙离子跨膜蛋白酶以及钙离子感受器来调节细胞内钙离子含量,包括钙运转ATP酶、钙通道、钙离子/阳离子交换蛋白等钙转运跨膜蛋白,以及钙调蛋白、类钙调蛋白、类钙调磷酸酶B蛋白和钙依赖蛋白激酶等钙离子传感器[19,40,42]。钙转运ATP酶主要催化ATP水解,并且将钙从胞质溶胶流出到液泡、内质网、质体和细胞外部分[43]。笔者在本研究中发现,与健康果以及病害果非病变组织相比,3 个细胞质膜型钙运转ATP 酶13 基因在病害果海绵组织均表现上调表达。运转ATP 酶13 主要表达于质膜上,上调时调控钙离子流出,与植物缺钙紧密相关[44]。此外,笔者在本研究中发现类钙调磷酸酶B 蛋白11、类钙调蛋白18 表达量在病害果海绵组织中显著高于健康果和病害果非病变组织。其他类钙调磷酸酶B 蛋白、钙调蛋白、类钙调蛋白等钙传感器基因在病害果中出现不同程度的上调和下调。钙调蛋白、类钙调蛋白和类钙调磷酸酶B 蛋白是真核细胞中主要的钙离子传感器,将钙离子信号转化为转录反应、蛋白磷酸化和代谢变化等,在调节植物生长发育和非生物胁迫抗性方面发挥重要作用[45-47]。因此,笔者推测钙运转与钙传感器基因的上调或者下调致使钙代谢紊乱,从而降低鹰嘴桃的抗逆性,最终导致果实组织海绵化的发生。
4 结 论
笔者在本研究中对鹰嘴桃病害果海绵组织、非病害组织和健康果实组织进行转录组测序,在病害果海绵组织与非病害组织,以及与健康果实组织的比较中,鉴定出12个与细胞壁代谢、3个钙转运和23个钙传感器相关的差异表达基因,推测钙代谢以及细胞壁代谢异常在果实组织海绵化过程中发挥关键作用。
[1] 质检总局关于批准对西山焦枣等产品实施地理标志产品保护的公告(2015 年第96 号)[EB].2015-8-10[2023-9-17].http://cpgi.org.cn/?c=i&a=detail&cataid=3&id=973.Announcement of the General Administration of Quality Supervision,Ⅰnspection and Quarantine on approving the protection of geographical indication products for Xishan jujube and other products (2015 No.96).2015-8-10 [2023-9-17].http://cpgi.org.cn/?c=i&a=detail&cataid=3&id=973.
[2] 廖永林,王龙江,章玉苹,黄少华,李传瑛,刘伟玲.鹰嘴蜜桃果肉海绵组织病害调查[J].安徽农业科学,2016,44(33):123-124.LⅠAO Yonglin,WANG Longjiang,ZHANG Yuping,HUANG Shaohua,LⅠChuanying,LⅠU Weiling.Ⅰnvestigation on disease condition of spongy tissue in Yingzui peach[J].Journal of Anhui Agricultural Sciences,2016,44(33):123-124.
[3] OAK P,JHA V,DESHPANDE A,TANPURE R,DAWKAR V,MUNDHE S,GHUGE S,PRABHUDESAⅠS,KRⅠSHANPAL A,JERE A,GⅠRⅠA,GUPTA V.Transcriptional and translational perturbation in abiotic stress induced physiological activities and metabolic pathway networks in spongy tissue disorder of mango fruit[J].Postharvest Biology and Technology,2022,188:111880.
[4] MA X W,LⅠU B,ZHANG Y H,SU M Q,ZHENG B,WANG S B,WU H X.Unraveling correlations between calcium deficiency and spongy tissue in mango fruit flesh[J].Scientia Horticulturae,2023,309:111694.
[5] WANG Y Y,GUO L H,ZHAO X Q,ZHAO Y J,HAO Z X,LUO H,YUAN Z H.Advances in mechanisms and omics pertaining to fruit cracking in horticultural plants[J].Agronomy,2021,11(6):1045.
[6] YU J,ZHU M T,BAⅠM,XU Y S,FAN S G,YANG G S.Effect of calcium on relieving berry cracking in grape (Vitis vinifera L.)‘Xiangfei’[J].PeerJ,2020,8:e9896.
[7] SHⅠY N,LⅠB J,GRⅠERSON D,CHEN K S.Ⅰnsights into cell wall changes during fruit softening from transgenic and naturally occurring mutants[J].Plant Physiology,2023,192(3):1671-1683.
[8] CHEN J J,DUAN Y J,HU Y L,LⅠW M,SUN D Q,HU H G,XⅠE J H.Transcriptome analysis of atemoya pericarp elucidates the role of polysaccharide metabolism in fruit ripening and cracking after harvest[J].BMC Plant Biology,2019,19(1):219.
[9] FAN J,DU W,YANG X P,ZHANG J G,CHEN Q L,HU H J.Changes in calcium content and expression of calcium sensor-related genes during sand pear (Pyrus prifolia) fruit cracking[J].Scientia Horticulturae,2023,313:111911.
[10] DE BANG T C,HUSTED S,LAURSEN K H,PERSSON D P,SCHJOERRⅠNG J K.The molecular-physiological functions of mineral macronutrients and their consequences for deficiency symptoms in plants[J].New Phytologist,2021,229(5):2446-2469.
[11] THOR K.Calcium-nutrient and messenger[J].Frontiers in Plant Science,2019,10:440.
[12] GRⅠFFⅠTH C,EⅠNHORN T C.The effect of plant growth regulators on xylem differentiation,water and nutrient transport,and bitter pit susceptibility of apple[J].Scientia Horticulturae,2023,310:111709.
[13] SⅠNGH A,SHUKLA A K,MEGHWAL P R.Fruit cracking in pomegranate:Extent,cause,and management:A review[J].Ⅰnternational Journal of Fruit Science,2020,20(Suppl.3):S1234-S1253.
[14] YAO Y L,LⅠM W,LⅠN W Q,LⅠU S H,WU Q S,FU Q,ZHU Z Y,GAO Y Y,ZHANG X M.Transcriptome analysis of watercore in pineapple[J].Horticulturae,2022,8(12):1175.
[15] SHARMA R R,SⅠNGH R.The fruit pitting disorder:A physiological anomaly in mango(Mangifera indica L.)due to deficiency of calcium and boron[J].Scientia Horticulturae,2009,119(4):388-391.
[16] GAO Q Y,XⅠONG T T,LⅠX P,CHEN W X,ZHU X Y.Calcium and calcium sensors in fruit development and ripening[J].Scientia Horticulturae,2019,253:412-421.
[17] DE FREⅠTAS S T,JⅠANG C Z,MⅠTCHAM E J.Mechanisms involved in calcium deficiency development in tomato fruit in response to gibberellins[J].Journal of Plant Growth Regulation,2012,31(2):221-234.
[18] KURONUMA T,WATANABE H.Ⅰdentification of the causative genes of calcium deficiency disorders in horticulture crops:A systematic review[J].Agriculture,2021,11(10):906.
[19] 余贤美,王金政,薛晓敏,陈汝,聂佩显,王贵平,韩雪平.基于全转录组分析的苹果苦痘病发生机制初步研究[J].植物病理学报,2020,50(4):405-419.YU Xianmei,WANG Jinzheng,XUE Xiaomin,CHEN Ru,NⅠE Peixian,WANG Guiping,HAN Xueping.Preliminary studies on the mechanism of bitter pit in apple based on whole-transcriptomic sequencing analysis[J].Acta Phytopathologica Sinica,2020,50(4):405-419.
[20] SⅠMMS D,CⅠZDZⅠEL P,CHOMCZYŃSKⅠP.TRⅠzol:A new reagent for optimal single-step isolation of RNA[J].Focus,1993,15(4):532-535.
[21] BOLGER A M,LOHSE M,USADEL B.Trimmomatic:A flexible trimmer for Ⅰllumina sequence data[J].Bioinformatics,2014,30(15):2114-2120.
[22] KⅠM D,PAGGⅠJ M,PARK C,BENNETT C,SALZBERG S L.Graph-based genome alignment and genotyping with HⅠSAT2 and HⅠSAT-genotype[J].Nature Biotechnology,2019,37(8):907-915.
[23] CHUNG M,ADKⅠNS R S,MATTⅠCK J S A,BRADWELL K R,SHETTY A C,SADZEWⅠCZ L,TALLON L J,FRASER C M,RASKO D A,MAHURKAR A,DUNNⅠNG HOTOPP J C.FADU:A quantification tool for prokaryotic transcriptomic analyses[J].mSystems,2021,6(1):e00917-e00920.
[24] LOVE M Ⅰ,HUBER W,ANDERS S.Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2[J].Genome Biology,2014,15(12):550.
[25] CANTALAPⅠEDRA C P,HERNÁNDEZ-PLAZA A,LETUNⅠCⅠ,BORK P,HUERTA-CEPAS J.eggNOG-mapper v2:functional annotation,orthology assignments,and domain prediction at the metagenomic scale[J].Molecular Biology and Evolution,2021,38(12):5825-5829.
[26] CHEN C J,CHEN H,ZHANG Y,THOMAS H R,FRANK M H,HE Y H,XⅠA R.TBtools:An integrative toolkit developed for interactive analyses of big biological data[J].Molecular Plant,2020,13(8):1194-1202.
[27] ZHAⅠZ F,FENG C,WANG Y Y,SUN Y T,PENG X,XⅠAO Y Q,ZHANG X,ZHOU X,JⅠAO J L,WANG W L,DU B Y,WANG C,LⅠU Y,LⅠT H.Genome-wide identification of the Xyloglucan endotransglucosylase/Hydrolase (XTH) and Polygalacturonase(PG)genes and characterization of their role in fruit softening of sweet cherry[J].Ⅰnternational Journal of Molecular Sciences,2021,22(22):12331.
[28] CHOⅠH R,BAEK M W,JEONG C S,TⅠLAHUN S.Comparative transcriptome analysis of softening and ripening-related genes in kiwifruit cultivars treated with ethylene[J].Current Ⅰssues in Molecular Biology,2022,44(6):2593-2613.
[29] STRATⅠLOVÁ B,KOZMON S,STRATⅠLOVÁ E,HRMOVA M.Plant xyloglucan xyloglucosyl transferases and the cell wall structure:Subtle but significant[J].Molecules,2020,25(23):5619.
[30] LⅠX L,SU Q F,FENG Y C,GAO X H,WANG B C,TAHⅠR M M,YANG H J,ZHAO Z Y.Ⅰdentification and analysis of the xyloglucan endotransferase/hydrolase (XTH) family genes in apple[J].Scientia Horticulturae,2023,315:111990.
[31] WANG Y W,NAMBEESAN S U.Full-length fruit transcriptomes of southern highbush (Vaccinium sp.) and rabbiteye (V.virgatum Ait)blueberry[J].BMC Genomics,2022,23(1):733.
[32] SANTOS M,EGEA-CORTⅠNES M,GONÇALVES B,MATOS M.Molecular mechanisms involved in fruit cracking:A review[J].Frontiers in Plant Science,2023,14:1130857.
[33] 苏菁,庄军平,陈维信.香蕉果实成熟软化过程中β-D-木聚糖苷酶活性变化[J].西北植物学报,2007,27(7):1394-1398.SU Jing,ZHUANG Junping,CHEN Weixin.β-D-xylosidase activity in banana(Musa spp.)fruits during ripening and softening[J].Acta Botanica Boreali-Occidentalia Sinica,2007,27(7):1394-1398.
[34] CHEN Y H,XⅠE B,AN X H,MA R P,ZHAO D Y,CHENG C G,LⅠE M,ZHOU J T,KANG G D,ZHANG Y Z.Overexpression of the apple expansin-like gene MdEXLB1 accelerates the softening of fruit texture in tomato[J].Journal of Ⅰntegrative Agriculture,2022,21(12):3578-3588.
[35] ZHANG T,TANG H S,VAVYLONⅠS D,COSGROVE D J.Disentangling loosening from softening:Ⅰnsights into primary cell wall structure[J].The Plant Journal,2019,100(6):1101-1117.
[36] ZHU Q N,ZHANG K Y,CHEN W X,LⅠX P,ZHU X Y.Transcriptomic and metabolomic analyses reveal key factors regulating chilling stress-induced softening disorder in papaya fruit[J].Postharvest Biology and Technology,2023,205:112534.
[37] AGHDAM M S,HASSANPOURAGHDAM M B,PALⅠYATH G,FARMANⅠB.The language of calcium in postharvest life of fruits,vegetables and flowers[J].Scientia Horticulturae,2012,144:102-115.
[38] LU K S,YAN L,RⅠAZ M,BABAR S,HOU J Y,ZHANG Y L,JⅠANG C C.Exogenous boron alleviates salt stress in cotton by maintaining cell wall structure and ion homeostasis[J].Plant Physiology and Biochemistry,2023,201:107858.
[39] HUANG W N,SHⅠY N,YAN H,WANG H,WU D,GRⅠERSON D,CHEN K S.The calcium-mediated homogalacturonan pectin complexation in cell walls contributes the firmness increase in loquat fruit during postharvest storage[J].Journal of Advanced Research,2023,49:47-62.
[40] LⅠU J,JⅠANG Z T,QⅠY W,LⅠU Y F,DⅠNG Y D,TⅠAN X N,REN X L.MdCAX affects the development of the‘Honeycrisp’bitter pit by influencing abnormal Ca distribution[J].Postharvest Biology and Technology,2021,171:111341.
[41] DE FREⅠTAS S T,MCELRONE A J,SHACKEL K A,MⅠTCHAM E J.Calcium partitioning and allocation and blossomend rot development in tomato plants in response to whole-plant and fruit-specific abscisic acid treatments[J].Journal of Experimental Botany,2014,65(1):235-247.
[42] TⅠAN W,WANG C,GAO Q F,LⅠL G,LUAN S.Calcium spikes,waves and oscillations in plant development and biotic interactions[J].Nature Plants,2020,6(7):750-759.
[43] SEⅠFⅠKALHOR M,ALⅠNⅠAEⅠFARD S,SHOMALⅠA,AZAD N,HASSANⅠB,LASTOCHKⅠNA O,LⅠT.Calcium signaling and salt tolerance are diversely entwined in plants[J].Plant Signaling&Behavior,2019,14(11):1665455.
[44] CHEN H,YANG Q,FU H W,CHEN K,ZHAO S S,ZHANG C,CAⅠT C,WANG L H,LU W Z,DANG H,GAO M J,LⅠH Q,YUAN X Y,VARSHNEY R K,ZHUANG W J.Ⅰdentification of key gene networks and deciphering transcriptional regulators associated with peanut embryo abortion mediated by calcium deficiency[J].Frontiers in Plant Science,2022,13:814015.
[45] KLEⅠST T J,SPENCLEY A L,LUAN S.Comparative phylogenomics of the CBL-CⅠPK calcium-decoding network in the moss Physcomitrella,Arabidopsis,and other green lineages[J].Frontiers in Plant Science,2014,5:187.
[46] ZHU X Y,DUNAND C,SNEDDEN W,GALAUD J P.CaM and CML emergence in the green lineage[J].Trends in Plant Science,2015,20(8):483-489.
[47] TANG M F,XU C,CAO H H,SHⅠY,CHEN J,CHAⅠY,LⅠZ G.Tomato calmodulin-like protein SlCML37 is a calcium(Ca2+)sensor that interacts with proteasome maturation factor SlUMP1 and plays a role in tomato fruit chilling stress tolerance[J].Journal of Plant Physiology,2021,258/259:153373.