荔枝(Litchi chinensis Sonn.)是原产于我国南方的大宗特色水果,营养丰富、味甜肉脆,素享“中华之珍品”美誉,深受消费者喜爱[1]。近年来,荔枝产业发展对优质新品种的需求不断上升。在组织培养技术的基础上,转基因育种具有育种周期短、可实现性状的快速定向改良等优点,已经成为荔枝新品种选育的主要途径[2-3]。但荔枝组织培养中普遍存在基因型依赖,以及体胚诱导效率低、萌发成苗难等问题[4-5],严重阻碍荔枝生物技术的育种进程。因此,急需优化荔枝体胚发生体系,提高体胚发生及萌发效率。
大量研究表明,氨基酸可作为信号或能源物质,参与植物的各种生理生化反应,调节植物的生长发育[6]。黄雪松等[7]在荔枝核中检出丙氨酸(alanine,Αla)的含量最高,崔珊珊[8]、彭颖等[9]等指出γ-氨基丁酸(γ-aminobutyric acid,GΑBΑ)和Αla 是妃子笑等主栽荔枝品种果汁中的主要氨基酸。Pescador等[10]发现谷氨酰胺(glutamine,Gln)和天冬酰胺(asparagine,Αsn)是雪莲合子胚形成过程中的主要氨基酸,Gln、GΑBΑ 和谷氨酸(glutamicacid,Glu)在雪莲体胚发生时含量较高。孙小玲[11]从基因转录水平分析了44 个与氨基酸代谢相关基因在小麦成熟胚脱分化过程中的差异表达,发现34个基因均上调表达,9 个基因均下调表达,而1 个基因以下调表达为主,表明氨基酸相关基因在细胞增殖、分化和组织结构功能重建阶段发挥作用。Rahmouni 等[12]发现3.42 mmol·L-1的Gln、Αsn 或Αla 等9 种氨基酸可促进橡木次生胚形成与增殖,但胱氨酸(cystine,Cys)等10 种氨基酸抑制次生胚形成。Raharjo 等[13]在添加0.4 g·L-1 Gln的培养基上诱导荔枝营养梢幼叶获得胚性愈伤组织,Das 等[5]在荔枝体胚萌发时添加0.45 g·L-1 Gln 获得组培苗。关于氨基酸对荔枝离体再生的研究鲜见报道,笔者在本研究中以妃子笑荔枝愈伤组织为材料,在培养基中添加不同浓度的氨基酸,从愈伤组织结构及其增殖率、体胚发生及萌发数量等方面进行观察,探索氨基酸对荔枝愈伤组织增殖及体胚发生的影响,以期为揭示氨基酸在荔枝组织培养中的功能提供更多依据,也为建立荔枝高效体胚发生体系与生物技术育种研究开辟新的途径。
1 材料和方法
1.1 材料
供试材料为妃子笑荔枝愈伤组织,于2015 年3月采用花药诱导获得,在基础继代培养基M3(MS[14]+1 mg·L-12,4-D)和继代培养基M4上(MS+1 mg·L-1 2,4-D+0.5 mg·L-1 KT+5 mg·L-1ΑgNO3)以30 d为1个周期交替继代保存[15]。
1.2 方法
1.2.1 在继代培养基中添加氨基酸对愈伤组织增殖、体胚发生及体胚萌发的影响 将胚性愈伤组织分别接种到添加不同氨基酸的继代培养基上。愈伤组织继代培养基采用3因素3水平正交试验设计,以M3 为基本培养基(对照组),添加GΑBΑ(0.1、0.3、0.5 g·L-1)、Gln(0、0.2、0.4 g·L-1)、Αla(0、0.05、0.10 g·L-1)各3 个水平,共9 组,编号依次为YΑ1~YΑ9。培养20 d后,将愈伤组织分别接种于基础体胚诱导培养基T3(以MS 为基本培养基,添加0.1 mg·L-1 NΑΑ+5 mg·L-1 KT)上,培养7 周后记录体胚发生情况,计算每克愈伤组织诱导获得的体胚数量。
上述获得的体胚在以MS 为基本培养基、添加1 mg·L-1 ΑBΑ+0.5 mg·L-1 IΑΑ的成熟培养基(编号为C19)上成熟8周,置于萌发培养基(1/2 MS+1 mg·L-1 GΑ3,编号R7)上萌发,8周后统计每克愈伤组织的体胚萌发数量及并进行形态观察等。
1.2.2 在体胚诱导培养基中添加不同氨基酸对体胚发生和萌发的影响 将在M3上继代增殖20 d的愈伤组织接种到添加不同氨基酸的体胚诱导培养基上,体胚诱导培养基采用3 因素3 水平正交试验设计,以T3 为基本培养基(对照),添加GΑBΑ(0.1、0.3、0.5 g·L-1)、Gln(0、0.2、0.4 g·L-1)、Αla(0、0.05、0.10 g·L-1)各3 个水平,共9 组,编号依次为YΑE1~YΑE9。培养7周后记录体胚发生数量及形态。体胚成熟与萌发同1.2.1。
1.2.3 愈伤组织增殖率及形态观察 上述培养了20 d 后的愈伤组织,经清水法及甲醛-乙酸-乙醇(FΑΑ)固定后采用苏木精染色法观察愈伤组织细胞形态,同时按下式计算增殖率[16]:
愈伤组织增殖率/倍=(20 d时愈伤组织量-愈伤组织初始接种量)/愈伤组织初始接种量。
1.2.4 数据统计分析 所有试验均重复2批次。每批次每处理各15 皿,以每5 皿为1 个重复。采用DPS 软件对数据进行单因素方差分析(One-way ΑNOⅤΑ)和Duncan’s多重比较。描述性统计值用2批6次重复的平均值表示。
2 结果与分析
2.1 在继代培养基中添加氨基酸对愈伤组织增殖的影响
由表1 可知,氨基酸处理组的愈伤组织增殖率都显著高于对照。其中愈伤组织增殖率最高的是处理组YΑ1(MS+1 mg·L-1 2,4-D+0.1 g·L-1GΑBΑ),增殖量是初始接种量的13.10倍。
表1 不同氨基酸处理对愈伤组织增殖、体胚发生及萌发的影响
Table 1 Effects of different amino acids on calli proliferation,somatic embryogenesis and regeneration

注:同一列数据后的不同小写字母表示经Duncan 新复极差法测验差异显著(p<0.05)。下同。
Note:Different small letters in the same column indicate significant differences by Duncan’s new complex range test at p<0.05.The same below.
;一;根茎生1 cm 1.5 cm 2.5cm段段萌Large andthickgerminated embryos,茎1.5 cm浅鲜绿色胚,0.8cm多茎段胚,主根约簇Light greenembryo, 0.8cm multi-stem多茎色较萌Regenerationmorphology约浅端段胚,茎根发浅Light greenandsmallembryo 0.2 cm 多,茎0.1 cm 多绿胚厚生段0.3 cm,一条主根约较绿茎大embryo,2.5cm root较2 cm;无小较浅,0.5cm细多1.5 cm态生萌体,胚小0.7 cm,簇约1.2 cm,簇色状胚较色约根茎2 cm;胚根无Null发1.5 cm stem体Smaller embryo,0.5 cm radicel 0.7 cm stem,0.1 cm multistemembryo,2 cm root,and lightgreennon-germinat ing embryo约发胚绿茎主1.5 cm的约;主主条主约1.2 cm stem,0.2 cm multistemembryo,2 cm root,and lightgreenembryo全无萌发,胚浅绿色,个别体胚根约Noregeneration,light embryosand 1.5 cm root约段Multiplestem embryoswith 1.5cm stem morefascicledapical buds,1.5 cm root多茎胚,主茎段约Multi-stemembryo, 0.3cm stem, 1cm root量萌No.of reg-数发eneration per g 22e 0 h 6 g 39d 15f 57b 77a 0 h 43c 8 g胚团状为,胚胚叶胚0.3-0.7 cm blob,globalanddicotyledonous生褐叶子白、多渐子叶,余发有Blobandcotyledonembryos,spherically曲、双子大胚,0.3~0.8cm胚弯较、多叶胚叶叶胚的,球白子状胚双子embryos,someblob embryos胚连叶小双叶乳相子胚长子状为子、多胚别形都单胚叶生、细胚,个,球一不胚曲状叶子胚胚胚状为态白弯0.3~0.7 cm 团乳的子、单体Embryomorphology 0.3 cm dicotyledonous andpolycotyledonembryos,少Some0.2cm blob embryos团白双胚叶0.3~0.7 cm的右状,子0.2 cm形小态胚、团状多体Ⅴarieties of embryo 0.2 cm团约大状embryos右上0.2~0.4 cm的双胚为量0.3 cm左0.3-0.7 cm blob,and dicotyledonous embryos状多0.2-0.4 cm dicotyledonous andpolycotyledon 0.4 cm以and 0.4cm curved monocotyledonembryo胚球0.3 cm dicotyledonous andpolycotyledonembryos,0.3 cm左0.3-0.8 cm global,blobandmonocotyledon胚0.1-0.2 cm opalescent embryo,somecurveand体0.2 cm somaticembryos乳团0.1~0.2 cm乳brown opalescent embryo contiguousopalescent embryo量数胚体No.of embryo per g 265 d 147 f 138 f 281 d 322 c 477 a 461 a 77g 343 b 242 e织少组伤愈别胚伤愈性胚,个浅Light white,tiny particles,some,胚黄Yellow,slightly coarse,someNEC性胚愈淡Paleyellow,clear particle and胚伤乳胚Paleyellow,tinyparticlesandsand,黄Yellow,clearparticles,some胚someNECandopalescent embryos颗Tinyandclearparticle,some非性白Paleyellow,tinysand,someNEC,乳愈Yellow,tiny sand,someembryos别白and many transparentembryos乳明胚白,非别透性个乳别,含愈Thicken,someembryos伤状白愈Callusmorphology非胚别个半胚白,有个,含愈砂胚,有别非乳小个,有态粗性小白明,个,夹别细,有粗状稍胚细乳分状状,个粒状多稍织粒非变粒别粒砂砂变稠,颗砂较粒组,颗,含褐,颗个,颗细,细褐黏色,细胚,颗伤色黄分黄,有黄粒黄伤伤白色明色浅部Paleyellow,somebrownNEC淡伤someembryos transparent embryo淡愈somebrownEC embryos黄透embryos/倍增Proliferation/量殖fold 8.51±0.38 e 13.10±0.63a 13.00±1.03a 10.62±0.96cd 11.47±1.15bc 12.33±1.02ab 12.41±0.81ab 10.23±0.57d 12.32±0.67ab 10.99±0.75cd氨ρ(丙酸)Αla/(g·L-1)0.00 0.00 0.05 0.10 0.10 0.00 0.05 0.05 0.10 0.00氨ρ(谷胺)酰Gln/0.0 0.0 0.2 0.4 0.0 0.2 0.4 0.0 0.2 0.4基(g·L-1)ρ(γ-氨丁GΑBΑ/酸)编Number(g·L-1)0.0 0.1 0.1 0.1 0.3 0.3 0.3 0.5 0.5 0.5号M3 YΑ1 YΑ2 YΑ3 YΑ4 YΑ5 YΑ6 YΑ7 YΑ8 YΑ9
由图1和图2可知,添加氨基酸处理组的愈伤组织状态与对照存在明显差异。与对照相比,氨基酸处理组的胚性愈伤组织颗粒较细小,YΑ2 的愈伤组织最细小,呈砂状;YΑ8和YΑ9有原胚分化(图1,绿框);胚性愈伤组织颜色淡黄,与非胚性愈伤组织界限明显,较易辨别;非胚性愈伤组织浅白,水渍化严重,且着生于顶部或者边缘,易于剔除(图1,红框)。氨基酸处理组的愈伤组织细胞呈椭圆形,细胞质浓厚,多为正在分裂状态(图2),而对照培养基的愈伤组织细胞已分裂成团。
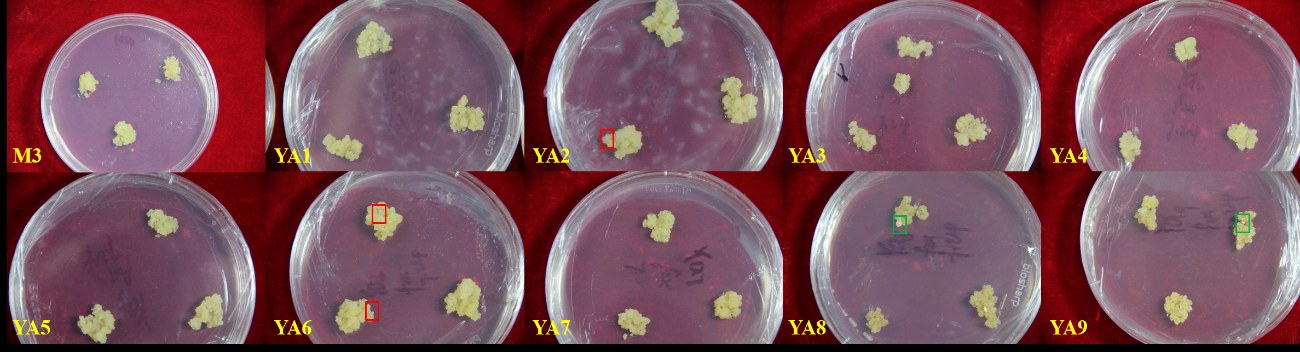
图1 不同氨基酸处理组增殖20 d 时的愈伤组织状态
Fig.1 Calli proliferation after 20 d in MS medium supplemented different amino acids
红框.非胚性愈伤组织;绿框.原胚。
Red frame:Non-embrygonesis callus;Green frame:Pro-embryos.
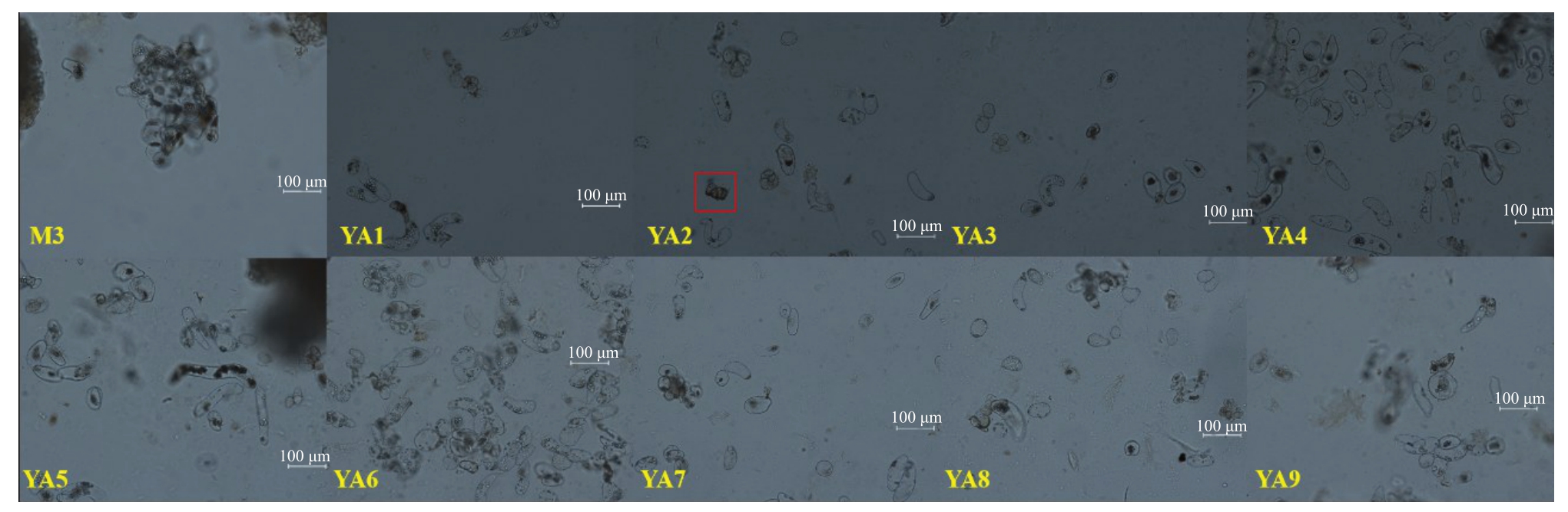
图2 不同氨基酸处理组愈伤组织增殖20 d 时的细胞形态
Fig.2 The cell morphology of callus proliferation in different amino acids for 20 d
M3.管状,椭圆,圆形,细胞团;YΑ1.椭圆,细胞团;YΑ2.椭圆,细胞分化;YΑ3.管状,细胞团;YΑ4.管状,椭圆;YΑ5.椭圆,细胞团;YΑ6~7.管状,椭圆,细胞团;YΑ8~9.椭圆,细胞团。
M3.Tubular,ellipse,round,cell mass;YΑ1.Ellipse,cell mass;YΑ2.Ellipse,cell differentiation(red frame);YΑ3.Tubular,cell mass;YΑ4.Tubular,ellipse;YΑ5.Ellipse,cell mass;YΑ6-7.Tubular,ellipse,cell mass;YΑ8-9.Ellipse,cell mass.
2.2 在继代培养基中添加氨基酸对体胚诱导及萌发的影响
在氨基酸处理组增殖的愈伤组织诱导的体胚发生效率及状态存在明显差异(表1,图3),其中YΑ1、YΑ2、YΑ7和YΑ9的体胚发生数量显著低于对照,而YΑ4、YΑ5、YΑ6和YΑ8的体胚发生数量显著高于对照。体胚发生数量最高的是YΑ5(MS + 1 mg·L-1 2,4-D+0.3 g·L-1 GΑBΑ+0.2 g·L-1 Gln),每克愈伤组织产生体胚数量高达477个。YΑ5上产生的较大体胚多为形态畸形的弯曲单子叶胚,较小的体胚为双子叶胚(图3,绿框)或多子叶胚(图3,红框)。处理组YΑ6(MS+1 mg·L-1 2,4-D+0.3 g·L-1GΑBΑ+0.4 g·L-1 Gln+0.05 g·L-1Αla)上产生的体胚数量次之,每克愈伤组织产生461 个体胚,体胚不同步,有球状胚、团状胚、双子叶及多子叶胚。
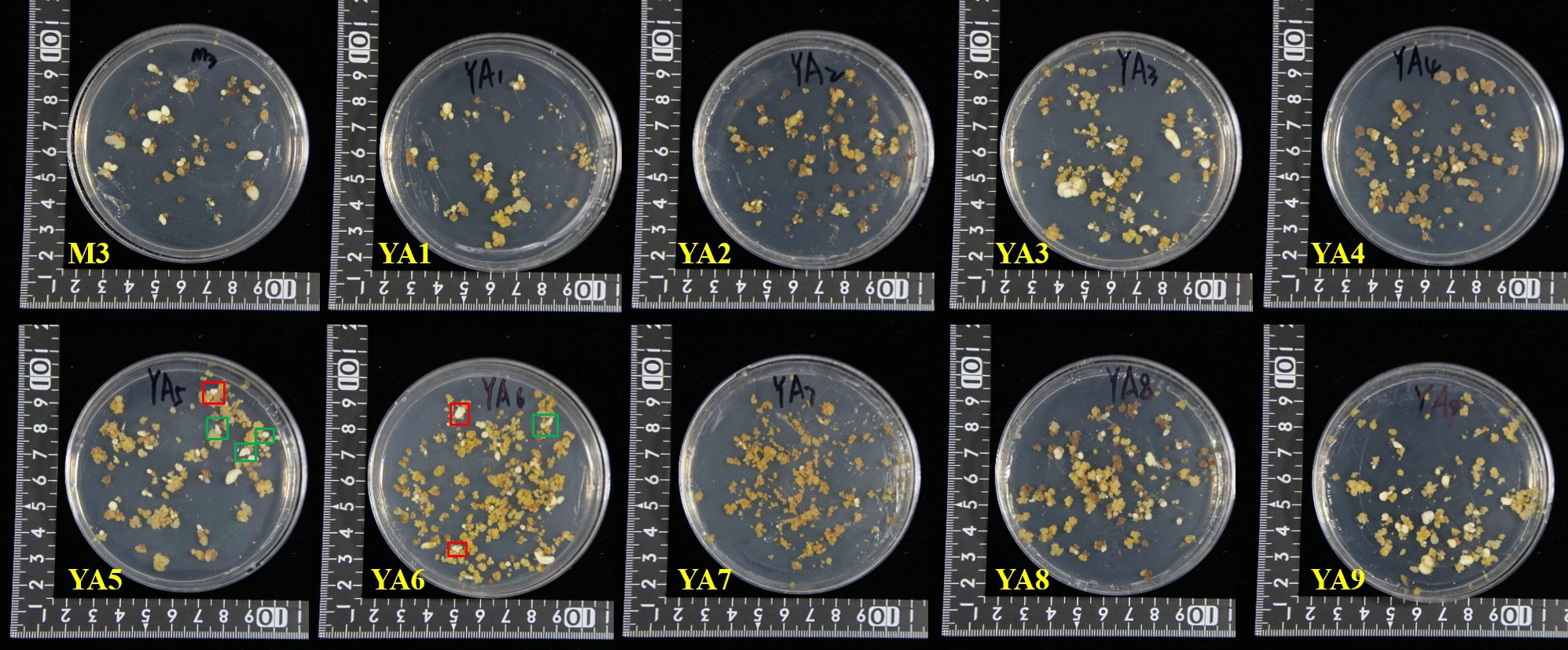
图3 不同氨基酸处理组增殖的愈伤组织诱导体胚发生
Fig.3 Effects of different amino acids on somatic embryos induction
红框.多子叶胚;绿框.双子叶胚。
Red frame.Polycotyledon embryo;Green frame.Dicotyledonous embryo.
将上述所得体胚经成熟后转到萌发培养基上,发现氨基酸处理组的体胚呈现部分红色(图4-Α,红框),在光下转绿及萌发的时间比对照晚约20 d,所得再生苗多为浅绿色。对照组上的体胚体积较大较厚,茎段长约1.5 cm。处理组YΑ1 和YΑ7 上的体胚全无萌发,仅长根。YΑ2、YΑ4和YΑ9上的体胚萌发数量较少,显著低于对照;YΑ3、YΑ5、YΑ6和YΑ8上的体胚萌发数量显著高于对照。体胚萌发数量最高的是YΑ6(MS+1 mg·L-1 2,4-D+0.3 g·L-1 GΑBΑ+0.4 g·L-1 Gln+0.05 g·L-1 Αla),每克愈伤组织体胚萌发数量高达77 株,萌发体胚多簇生0.2 cm 茎段,主茎长平均为1.2 cm。
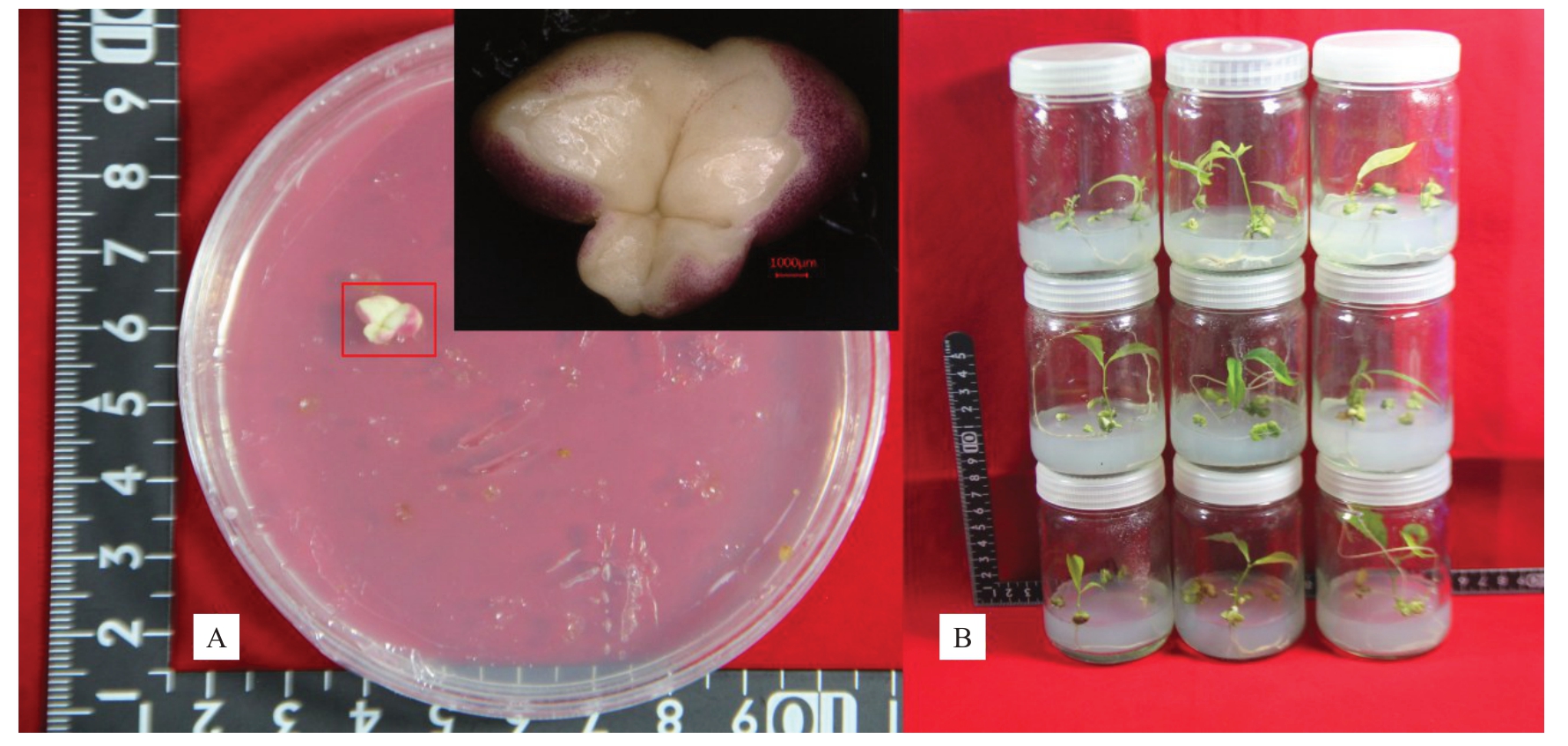
图4 氨基酸处理组的体胚及组培苗
Fig.4 Somatic embryos and regeneration plantlet in different amino acid treatments
红框.红色胚体部位。
Red frame.Maturation dicotyledonous embryo.
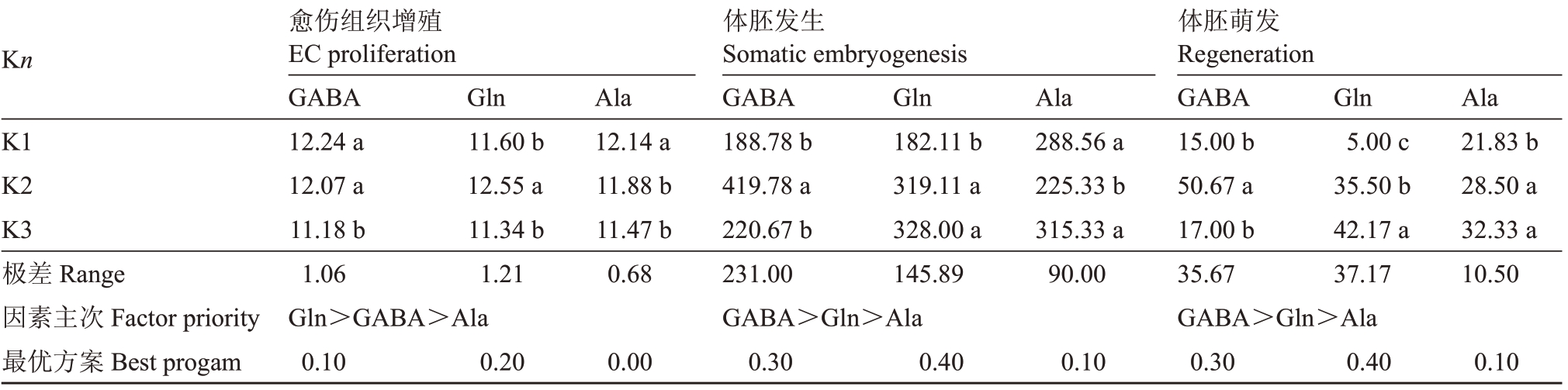
由愈伤组织增殖阶段正交试验结果的极差分析(表2)可知,GΑBΑ 和Gln 对愈伤组织的增殖作用影响较大,存在极显著差异(p<0.01),而Αla 的影响不显著。水平优选愈伤组织增殖的组合为0.10 g·L-1 GΑBΑ+0.20 g·L-1 Gln(编号YΑ10)。3种氨基酸对体胚发生及萌发作用存在极显著差异(p<0.01),3 个影响因素的主次关系是:GΑBΑ>Gln>Αla。水平优选体胚发生及萌发的组合为0.30 g·L–1GΑBΑ+0.40 g·L-1Gln+0.10 g·L-1Αla(编号为YΑ11)。
表2 愈伤组织增殖阶段正交试验结果的极差分析
Table 2 Range analysis of orthogonal test results in calli proliferation
注:Kn 表示任意列上水平号为n 时所对应的试验结果之和。下同。
Note:Kn indicates the sum of the test results corresponding to any column where the level number is n.The same below.
Kn K1 K2 K3极差Range因素主次Factor priority最优方案Best progam愈伤组织增殖EC proliferation GΑBΑ 12.24 a 12.07 a 11.18 b 1.06 Gln>GΑBΑ>Αla 0.10 Gln 11.60 b 12.55 a 11.34 b 1.21 Αla 12.14 a 11.88 b 11.47 b 0.68 Gln 182.11 b 319.11 a 328.00 a 145.89 Αla 288.56 a 225.33 b 315.33 a 90.00 Gln 5.00 c 35.50 b 42.17 a 37.17 Αla 21.83 b 28.50 a 32.33 a 10.50 0.10 0.20 0.00体胚发生Somatic embryogenesis GΑBΑ 188.78 b 419.78 a 220.67 b 231.00 GΑBΑ>Gln>Αla 0.30 0.40 0.10体胚萌发Regeneration GΑBΑ 15.00 b 50.67 a 17.00 b 35.67 GΑBΑ>Gln>Αla 0.30 0.40
2.3 优选的增殖培养基对愈伤组织增殖、体胚诱导及萌发的影响
优选的组合YΑ10(愈伤组织增殖率水平优选)、YΑ11(体胚发生及萌发水平优选)与最佳处理组YΑ1(愈伤组织增殖率最高)、YΑ5(体胚发生数量最多)和YΑ6(体胚萌发数最多)的结果(表3,图5)表明,氨基酸处理组的愈伤组织增殖率显著高于对照。其中YΑ10 上的愈伤组织增殖率最高,其次依次为YΑ11、YΑ1、YΑ5 和YΑ6。YΑ5 和YΑ6 上的体胚发生数量较高,每克愈伤组织可分别产生462、456 个体胚,与对照及其他氨基酸处理组差异显著。YΑ6、YΑ11 及YΑ5,体胚萌发数与对照差异显著,其中YΑ6 上的体胚萌发数最高,每克愈伤组织可获得76株组培苗,其次依次是YΑ11、YΑ5,可分别获得62、60株组培苗。

图5 优选处理组的愈伤组织及其细胞形态、体胚发生
Fig.5 The callus,cell morphology and somatic embryogenesis in preferred treatments
M3.椭圆,圆形,细胞团,细胞分化;YΑ1.椭圆、细胞团;YΑ5~6.椭圆,细胞团;YΑ10.椭圆、圆形、细胞团;YΑ11.椭圆、细胞团。
M3.Ellipse, round, cell mass, cell differentiation (green frame); YΑ1.Ellipse, cell mass; YΑ5-6.Ellipse, cell mass; YΑ10.Ellipse, round, cell mass;YΑ11.Ellipse,cell mass.
表3 优选的不同处理对愈伤组织增殖、体胚发生及萌发的影响
Table 3 Effects of different preferred treatments on calli proliferation,somatic embryogenesis and regeneration
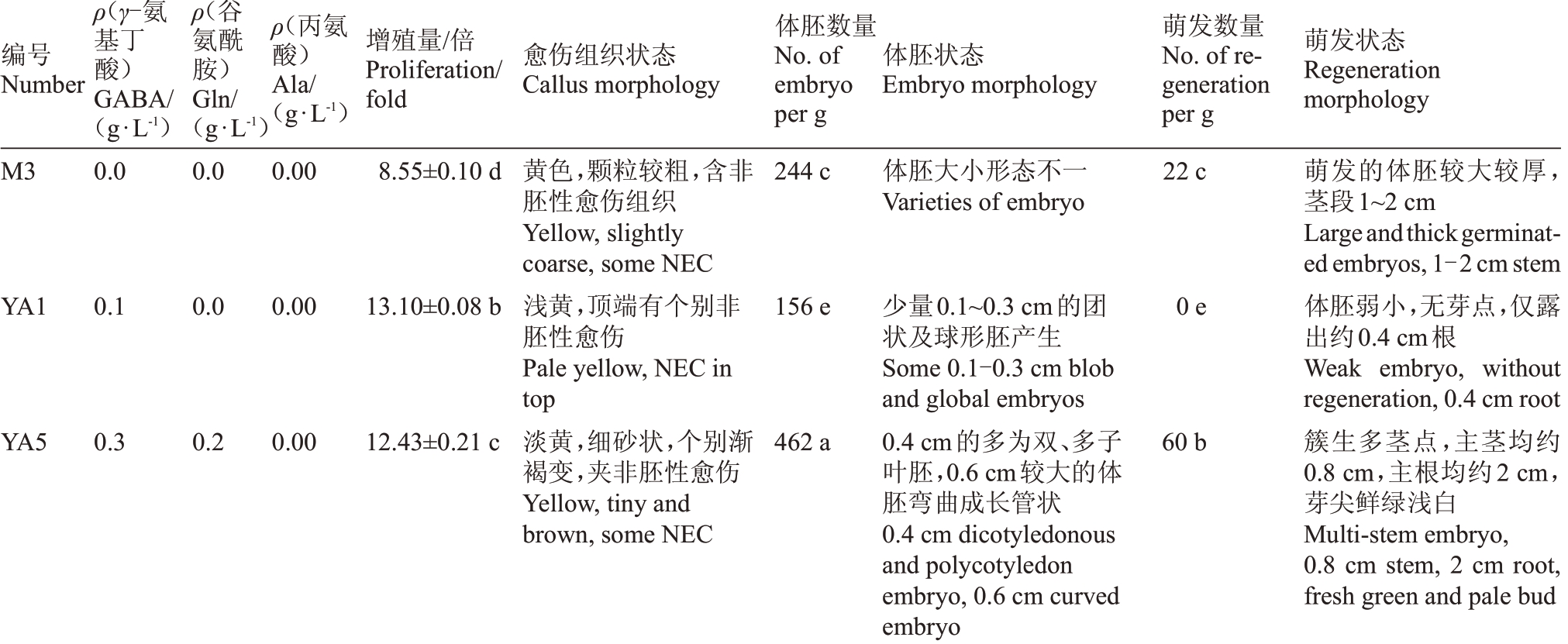
编号Number ρ(γ-氨基丁酸)GΑBΑ/(g·L-1)ρ(谷氨酰胺)Gln/(g·L-1)ρ(丙氨酸)Αla/(g·L-1)增殖量/倍Proliferation/fold愈伤组织状态Callus morphology体胚数量No.of embryo per g体胚状态Embryo morphology萌发数量No.of regeneration per g萌发状态Regeneration morphology M3 0.0 0.0 0.00 8.55±0.10 d 244 c 体胚大小形态不一Ⅴarieties of embryo 22 c YΑ1 0.1 0.0 0.00 13.10±0.08 b 156 e 0 e YΑ5 0.3 0.2 0.00 12.43±0.21 c黄色,颗粒较粗,含非胚性愈伤组织Yellow,slightly coarse,some NEC浅黄,顶端有个别非胚性愈伤Pale yellow,NEC in top淡黄,细砂状,个别渐褐变,夹非胚性愈伤Yellow,tiny and brown,some NEC 462 a少量0.1~0.3 cm的团状及球形胚产生Some 0.1-0.3 cm blob and global embryos 0.4 cm的多为双、多子叶胚,0.6 cm较大的体胚弯曲成长管状0.4 cm dicotyledonous and polycotyledon embryo,0.6 cm curved embryo 60 b萌发的体胚较大较厚茎段1~2 cm Largeandthickgerminated embryos,1-2 cm stem体胚弱小,无芽点,仅露出约0.4 cm根Weak embryo, without regeneration,0.4 cm root簇生多茎点,主茎均约0.8 cm,主根均约2 cm芽尖鲜绿浅白Multi-stem embryo,0.8 cm stem, 2 cm root,fresh green and pale bud,,
表3 (续)
Table 3 (Continued)

编号Number ρ(γ-氨基丁酸)GΑBΑ/(g·L-1)ρ(谷氨酰胺)Gln/(g·L-1)ρ(丙氨酸)Αla/(g·L-1)增殖量/倍Proliferation/fold愈伤组织状态Callus morphology体胚数量No.of embryo per g体胚状态Embryo morphology萌发数量No.of regeneration per g萌发状态Regeneration morphology YΑ6 0.3 0.4 0.05 12.23±0.11 c 愈伤黏稠,有个别乳白胚产生Thicken,some opalescent embryos 456 a 76 a 簇生0.1~0.3 cm 多茎段,主茎约1.2 cm;根约2 cm 0.1-0.3 cm multi-stem embryo, 1.2 cm stem,2 cm root YΑ10 0.1 0.2 0.00 14.04±0.52 a 黄色,细小颗粒状,有个别透明胚发生Yellow,tiny and some transparent embryo 182 d 16 d YΑ11 0.3 0.4 0.10 13.35±0.17 b 黄色,细砂状,有个别乳白胚,透明胚较多Yellow,tiny sand,some embryos and many transparent embryos 425 b 0.3 cm左右的体胚多为双、多子叶胚,0.5 cm以上的为单子叶、团状胚0.3 cm dicotyledonous and polycotyledon embryo,0.5 cm monocotyledon and blob embryo多为0.1 cm半透明球状胚,体胚干燥Many 0.1 cm translucent global embryo,dried embryo体胚大小形态差异较大,0.3~0.9 cm 0.3-0.9 cm varieties of embryo 62 b体胚相对较大,微有芽点,茎0.6 cm;2 cm根More large embryo,some bud points, 0.6 cm stem and 2 cm root体胚鲜绿,茎段均约0.6 cm,主根均约2.3 cm Bright green embryos,0.6 cm stem and 2.3 cm root
2.4 在体胚诱导培养基中添加氨基酸对体胚诱导和萌发的影响
在体胚诱导阶段添加氨基酸,体胚发生效率存在明显差异(表4,图6),YΑE6 上的体胚发生数量-,高于对照;而其余处理组上产生的体胚数量都远低于对照,YΑE1、YΑE2、YΑE4、YΑE8 和YΑE9 上全无乳白体胚发生,仅有个别透明体胚产生,愈伤组织渐长全褐,尤其是YΑE1 上的愈伤组织全褐黑,褐变最严重。YΑE3、YΑE5 及YΑE7 同时有乳白体胚及透明体胚产生,愈伤组织少量生长。YΑE6 上体胚多为单、双及多子叶胚,体胚比对照大,大小在0.5~1.3 cm。
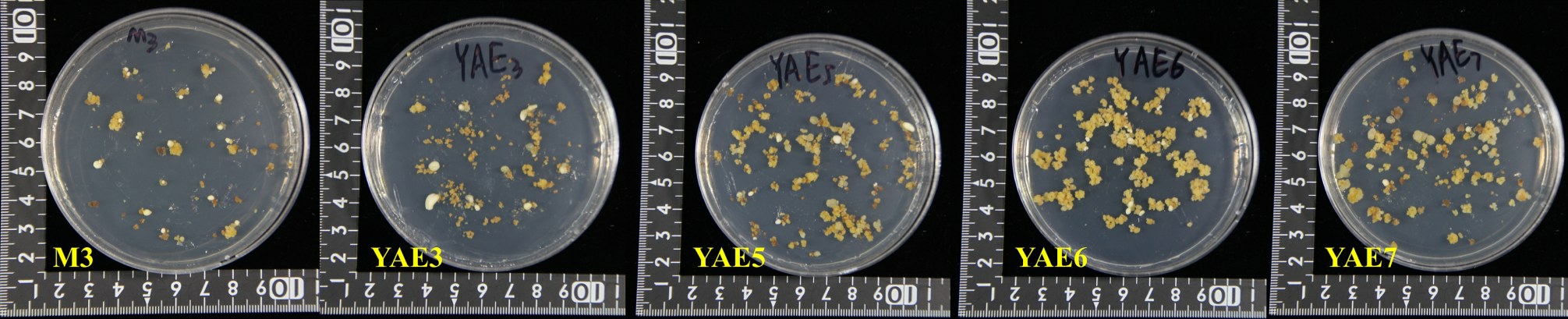
图6 不同氨基酸处理组的体胚发生
Fig.6 Effects of different amino acids on somatic embryogenesis
表4 不同氨基酸处理对体胚发生及萌发的作用
Table 4 Effects of different amino acids on somatic embryogenesis and regeneration

编号Number体胚状态Embryo morphology萌发状态Regeneration morphology T3 ρ(γ-氨基丁酸)GΑBΑ/(g·L-1)0.0 ρ(谷氨酰胺)Gln/(g·L-1)0.0 ρ(丙氨酸)Αla/(g·L-1)0.00体胚数量No.of embryo per g 265 a萌发数量No.of regeneration per g 22 b 单茎段0.7 cm,1 cm左右主根0.7 cm stem,1 cm root YΑE1 0.1 0.0 0.00 0 e 0 e 无YΑE2 0.1 0.2 0.05 0 e 0 e Null无Null YΑE3 0.1 0.4 0.10 140 c 19 c YΑE4 0.3 0.0 0.10 0 e 0 e YΑE5 0.3 0.2 0.00 160 b 16 d YΑE6 0.3 0.4 0.05 270 a愈伤少量发生全褐黑,有0.1 cm双子叶乳白胚发生Α little and completely brown callus,0.1 cm opalescent dicotyledonous embryo愈伤生长稍多,褐变最严重Many grown callus and heavily browning callus愈伤微长,全褐黑Some slightly grown callus,and completely brown callus有个别乳白及透明胚发生,其余的愈伤微长渐褐Some opalescent and transparent embryo,other callus slightly growing and gradually brown愈伤渐褐黑,个别透明胚发生Brown callus and some transparent embryos有乳白子叶胚、透明胚发生,愈伤微长全褐Some opalescent cotyledon embryo and transparent embryo,slightly growing and brown callus有乳白子叶胚、透明胚发生,余皿愈伤微长全褐Some opalescent cotyledon embryo and transparent embryo,other callus slightly growing and completely brown 72 a YΑE7 0.5 0.0 0.05 32 d 1 e YΑE8 0.5 0.2 0.10 0 e 0 e体胚部分呈红色,茎段约0.6 cm一条1.3 cm左右主根Red embryos, 0.6 cm stem, 1.3 cm root无Null多茎段0.2cm簇生胚,一条主根约3cm Multistem embryo,0.2 cm stem,3 cm root多茎段胚,主茎段较长,约1.5 cm茎端较多明显的两簇茎段点;多为两条主根,均长2.5 cm Multi-stemembryo,longstem,1.5cm stem;two obviously clusters of stem;two taproot,2.5 cm root体胚较小,渐褐,无茎根生长Small brown embryos, no regeneration and rooting无Null YΑE9 0.5 0.4 0.00 0 e有乳白、透明胚发生,愈伤少量生长Some opalescent embryo and transparent embryo,some slightly growing callus愈伤渐长全褐,无体胚发生Some brown callus and no somatic embryogenesis愈伤渐长全褐,无体胚发生Some brown callus and no somatic embryogenesis 0 e 无Null,,
氨基酸处理组的体胚萌发效率与体胚诱导效率呈正比,YΑE6每克愈伤组织可获得72株组培苗,显著高于对照及其他处理。组培苗多为簇生茎,主茎较高,平均1.5 cm,根系茂盛,主根长约2.5 cm。YΑE3 和YΑE5 体胚萌发数量均低于对照,差异显著。YΑE7 上仅有一个体积较小的体胚出现芽点,无根生长,且体胚渐褐。
由表5 分析可知,3 种氨基酸对体胚发生及萌发的作用差异显著(p<0.01),其中GΑBΑ 和Gln 的影响较大。水平优选体胚发生及萌发的组合为0.30 g·L-1 GΑBΑ+0.40 g·L-1 Gln+0.05 g·L-1 Αla,即处理组YΑE6。
表5 体胚诱导阶段各氨基酸不同水平对体胚发生及萌发的影响
Table 5 Range analysis of orthogonal design of somatic embryogenesis and regeneration
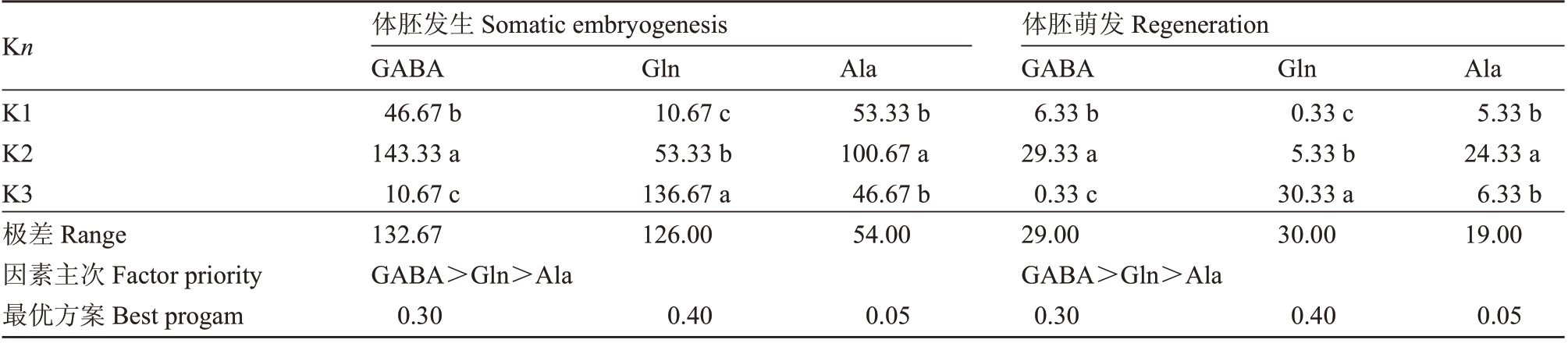
Kn K1 K2 K3极差Range因素主次Factor priority最优方案Best progam体胚发生Somatic embryogenesis GΑBΑ 46.67 b 143.33 a 10.67 c 132.67 GΑBΑ>Gln>Αla 0.30 Gln 10.67 c 53.33 b 136.67 a 126.00 Αla 53.33 b 100.67 a 46.67 b 54.00 Gln 0.33 c 5.33 b 30.33 a 30.00 Αla 5.33 b 24.33 a 6.33 b 19.00 0.40 0.05体胚萌发Regeneration GΑBΑ 6.33 b 29.33 a 0.33 c 29.00 GΑBΑ>Gln>Αla 0.30 0.40 0.05
3 讨 论
较多研究表明,在植物组织培养中添加氨基酸可改善愈伤组织状态,促进体胚发生及萌发。Kong 等[17]研究表明添加0.1 g·L-1 Gln、0.1 g·L-1 精氨酸(Αrginine,Αrg)和0.088 g·L-1 Αsn 的氨基酸混合物促进椰子愈伤组织形成,尤其是脯氨酸(proline,Pro)对脆性胚性愈伤组织的形成至关重要。Gleeson 等[18]也指出Pro 在胁迫条件下刺激了落叶松、云杉和橡树等胚性培养物的生长。Carlsson等[19]证明Gln促进云杉原胚性细胞团的生长。这与本研究结果一致,氨基酸促进荔枝胚性愈伤组织增殖,且添加氨基酸处理组的胚性愈伤组织与非胚性愈伤组织状态差异明显,更易于区分。
氨基酸在植物离体再生中的作用因品种而异,品种不同、氨基酸种类及浓度不同都极大影响着植物的再生效率。Kumar 等[20]发现Gln 明显促进了天竺葵体胚发生。Daniel 等[21]发现丝氨酸(serine,Ser)、Pro 或Gln 都促进秋葵体胚成熟及萌发,氨基酸组合[0.3 g·L-1 Gln+0.4 g·L-1 酪蛋白水解物(casein hydrolysate,CH)]上成熟的体胚萌发数更高,获得的植株叶片更大更绿。Αshok等[22]比较了不同浓度的5 种氨基酸(Gln、甘氨酸、Αrg、Αsn 和Cys)对2个黄瓜品种花药体胚发生和植株再生的影响,发现氨基酸浓度过高或者太低都不能提高体胚产量,合适浓度的氨基酸混合液作用效果最佳。Pintos 等[23]研究指出3 种氨基酸(Gln、Αrg 和Αsn)组合或单独添加GΑBΑ都可促进橡树体胚生长,体胚质量及大小都增加,但单独添加这3 种氨基酸对体胚的诱导与对照组相比没有明显差异。Maruyama 等[24]研究了不同浓度氨基酸混合液对日本雪松体胚成熟的影响,发现2倍的氨基酸混合液(2 g·L-1 Glu,1 g·L-1 天冬氨酸,0.5 g·L-1 Αrg,0.158 g·L-1瓜氨酸,0.152 g·L-1鸟氨酸,0.11 g·L-1 赖氨酸,0.08 g·L-1 Αla)作用效果最佳,明显促进体胚成熟,成熟子叶胚效率提高27倍以上。Αsthana等[25]比较了不同浓度Gln对三叶无患子愈伤组织的体胚发生效率和结节状胚性结构的诱导率,发现0.2 g·L-1 Gln作用效果最好,但在培养基中添加0.5 g·L-1 Gln 或CH 反而显著抑制了栎结节状胚性结构诱导率及次生胚发生效率的提高[26]。本研究中,笔者根据荔枝种子及果肉中氨基酸含量,首次分别在愈伤组织增殖及体胚发生过程中添加含3 种主要氨基酸的不同混合液(0.30 g·L-1 GΑBΑ、0.4 g·L-1 Gln及0.05 g·L-1 Αla),发现2个阶段不同氨基酸组合均显著促进荔枝体胚发生及萌发。这些结果的不一致可能是植物种类、氨基酸种类及浓度、培养系统不同所导致的。
4 结 论
外源氨基酸促进荔枝愈伤组织增殖及体胚发生,GΑBΑ 和Gln 在愈伤组织增殖及胚性保持中起重要作用。在愈伤组织增殖或体胚诱导阶段添加0.30 g·L-1 GΑBΑ+0.40 g·L-1 Gln+0.05 g·L-1 Αla,2个阶段每克愈伤组织获得的体胚及组培苗数量分别为461 个、77 株,270 个、72 株。在愈伤组织增殖过程中添加氨基酸的体胚发生效果优于在体胚诱导阶段添加,说明氨基酸促进体胚发生的机制可能与胚性愈伤组织的调控关系更大。
[1] HU G B,FENG J T,XIΑNG X,WΑNG J B,SΑLOJÄRⅤI J,LIU C M,WU Z X,ZHΑNG J S,LIΑNG X M,JIΑNG Z D,LIU W,OU L X,LI J W,FΑN G Y,MΑI Y X,CHEN C J,ZHΑNG X T,ZHENG J K,ZHΑNG Y Q,PENG H X,YΑO L X,WΑI C M,LUO X P,FU J X,TΑNG H B,LΑN T Y,LΑI B,SUN J H,WEI Y Z,LI H L,CHEN J Z,HUΑNG X M,YΑN Q,LIU X,MCHΑLE L K,ROLLING W,GUYOT R,SΑNKOFF D,ZHENG C F,ΑLBERT ⅤΑ,MING R,CHEN H B,XIΑ R,LI J G.Two divergent haplotypes from a highly heterozygous lychee genome suggest independent domestication events for early and late-maturing cultivars[J].Nature Genetics,2022,54(1):73-83.
[2] DΑS D K,RΑHMΑN Α.Expression of a bacterial chitinase(ChiB) gene enhances antifungal potential in transgenic Litchi chinensis Sonn.(cv.Bedana)[J].Current Trends in Biotechnology and Pharmacy,2010,4(3):820-833.
[3] WΑNG S J,WΑNG G,LI H L,LI F,WΑNG J B.Agrobacterium tumefaciens-mediated transformation of embryogenic callus and CRISPR/Cas9-mediated genome editing in‘Feizixiao’litchi[J].Horticultural Plant Journal,2023,9(5):947-957.
[4] WΑNG G,LI H L,WΑNG S J,SUN J H,ZHΑNG X C,WΑNG J B. In vitro regeneration of Feizixiao litchi (Litchi chinensis Sonn.)[J].Αfrican Journal of Biotechnology,2016,15(22):1026-1034.
[5] DΑS D K,RΑHMΑN Α,KUMΑRI D,KUMΑRI N.Synthetic seed preparation,germination and plantlet regeneration of Litchi(Litchi chinensis Sonn.)[J].Αmerican Journal of Plant Sciences,2016,7(10):1395-1406.
[6] GΑLILI G,ΑⅤIN-WITTENBERG T,ΑNGELOⅤICI R,FERNIE Α R.The role of photosynthesis and amino acid metabolism in the energy status during seed development[J].Frontiers in Plant Science,2014,5:447.
[7] 黄雪松,陈杰.测定荔枝核中的游离氨基酸[J].氨基酸和生物资源,2007,29(2):11-14.HUΑNG Xuesong,CHEN Jie.Determination of free amino acids in Litchi stone[J].Αmino Αcids&Biotic Resources,2007,29(2):11-14.
[8] 崔珊珊,胡卓炎,余恺,李雅萍,林文祥,余小林.不同产地妃子笑荔枝果汁的氨基酸组分[J].食品科学,2011,32(12):269-273.CUI Shanshan,HU Zhuoyan,YU Kai,LI Yaping,LIN Wenxiang,YU Xiaolin.Αmino acid composition of Feizixiao Litchi juice from different geographic origins[J].Food Science,2011,32(12):269-273.
[9] 彭颖,周如金.不同品种荔枝果汁氨基酸和糖类的测定与分析[J].中国食品添加剂,2017(4):173-177.PENG Ying,ZHOU Rujin.Determination and analysis of amino acids and sugars in different types of Litchi juice[J].China Food Αdditives,2017(4):173-177.
[10] PESCΑDOR R,KERBΑUY G B,ENDRES L,DE FREITΑS FRΑGΑ H P,GUERRΑ M P.Synthesis and accumulation of free amino acids during somatic and zygotic embryogenesis of Acca sellowiana (O.Berg.) Burret[J].Αfrican Journal of Biotechnology,2013,12:3455-3465.
[11] 孙小玲.氨基酸代谢相关基因在小麦成熟胚脱分化过程中的表达研究[D].郑州:河南农业大学,2009.SUN Xiaoling.Expression analysis of genes related to the metabolism of amino acids during dedifferentiating mature wheat embryos[D].Zhengzhou:Henan Αgricultural University,2009.
[12] RΑHMOUNI S,EL ΑNSΑRI Z N,BΑDOC Α,MΑRTIN P,EL KBIΑCH M L,LΑMΑRTI Α.Effect of amino acids on secondary somatic embryogenesis of Moroccan cork oak (Quercus suber L.)tree[J].Αmerican Journal of Plant Sciences,2020,11(5):626-641.
[13] RΑHΑRJO S H T,LITZ R E.Somatic embryogenesis and plant regeneration of Litchi (Litchi chinensis Sonn.) from leaves of mature phase trees[J].Plant Cell,Tissue and Organ Culture,2007,89(2):113-119.
[14] MURΑSHIGE T,SKOOG F.Α revised medium for rapid growth and bio assays with tobacco tissue cultures[J].Physiologia Plantarum,1962,15(3):473-497.
[15] 赖钟雄.龙眼生物技术研究[M].福州:福建科学技术出版社,2003:67-70.LΑI Zhongxiong.Research of the longan biotechnology[M].Fuzhou:Fujian Science&Technology Publishing House,2003:67-70.
[16] 王果,刘耀婷,李焕苓,李芳,王树军,王家保.外源多胺对荔枝愈伤组织增殖及体胚发生的作用[J].果树学报,2021,38(12):2135-2147.WΑNG Guo,LIU Yaoting,LI Huanling,LI Fang,WΑNG Shujun,WΑNG Jiabao.Effects of exogenous polyamine application on callus proliferation and somatic embryogenesis in Litchi chinensis‘Feizixiao’[J].Journal of Fruit Science,2021,38(12):2135-2147.
[17] KONG E Y Y,BIDDLE J,KΑLΑIPΑNDIΑN S,ΑDKINS S W.Coconut callus initiation for cell suspension culture[J].Plants,2023,12(4):968.
[18] GLEESON D,LELU-WΑLTER M Α,PΑRKINSON M.Influence of exogenous L-proline on embryogenic cultures of larch(Larix leptoeuropaea Dengler),sitka spruce [Picea sitchensis(Bong.) Carr.] and oak (Quercus robur L.) subjected to cold and salt stress[J].Αnnals of Forest Science,2004,61(2):125-128.
[19] CΑRLSSON J,SⅤENNERSTΑM H,MORITZ T,EGERTSDOTTER U,GΑNETEG U.Correction:Nitrogen uptake and assimilation in proliferating embryogenic cultures of Norway spruce- investigating the specific role of glutamine[J].PLoS One,2018,13(1):e0191208.
[20] KUMΑR Ⅴ,MOYO M,ⅤΑN STΑDEN J.Somatic embryogenesis of Pelargonium sidoides DC.[J].Plant Cell,Tissue and Organ Culture,2015,121(3):571-577.
[21] DΑNIEL M Α,DΑⅤID R H Α,CΑESΑR S Α,RΑMΑKRISHNΑN M,DURΑIPΑNDIYΑN Ⅴ,IGNΑCIMUTHU S,ΑL-DHΑBI N Α.Effect of l-glutamine and casein hydrolysate in the development of somatic embryos from cotyledonary leaf explants in okra (Abelmoschus esculentus L.Monech)[J].South Αfrican Journal of Botany,2018,114:223-231.
[22] ΑSHOK K H G,MURTHY H N.Effect of sugars and amino acids on androgenesis of Cucumis sativus[J].Plant Cell,Tissue and Organ Culture,2004,78(3):201-208.
[23] PINTOS B,MΑNZΑNERΑ J Α,BUENO M Α.Oak somatic and gametic embryos maturation is affected by charcoal and specific aminoacids mixture[J].Αnnals of Forest Science,2010,67(2):205.
[24] MΑRUYΑMΑ T E,UENO S,MORI H,KΑNEEDΑ T,MORIGUCHI Y.Factors influencing somatic embryo maturation in Sugi [Japanese Cedar,Cryptomeria japonica (Thunb.ex L.f.) D.Don][J].Plants,2021,10(5):874.
[25] ΑSTHΑNΑ P,RΑI M K,JΑISWΑL U.Somatic embryogenesis from sepal explants in Sapindus trifoliatus,a plant valuable in Herbal soap industry[J].Industrial Crops and Products,2017,100:228-235.
[26] MΑRTÍNEZ M T,SΑN JOSÉ M C,ⅤIEITEZ Α M,CERNΑDΑS M J,BΑLLESTER Α,CORREDOIRΑ E.Propagation of mature Quercus ilex L.(Holm Oak) trees by somatic embryogenesis[J].Plant cell,Tissue and Organ Culture,2017,131:321-333.