近年在福建连城等地的李产区发生一种新的叶部病害,根据其症状表现称之为李叶斑病。该病初期在李叶上产生黄色至浅褐色病点,之后逐渐扩展成形状不规则的灰色至深褐色病斑,发生严重时叶片干枯。该病有扩展蔓延和逐年加重的趋势,已引起当地的高度关注。
据Olawole等[1]报道,在美国的李产区发生一种细菌病害,导致李叶枯焦,潮湿时病部产生菌脓,研究证实系由叶缘焦枯病菌(Xylella fastidiosa)所致。我国四川的李产区也发生有李叶枯病,叶片边缘或尖端形成不规则的褐色斑点,继而扩展为较大的灰褐色病斑。经病原鉴定证实是由两种镰刀菌(Fusarium pernambucanum 和F.sulawesiense)引起的[2]。2021年在我国广西李产区发现李黄斑病,该病田间症状表现为最初叶上形成小的黄色斑点,逐渐扩展为不规则深褐色凹陷病斑,其边缘显现黄色晕圈。研究表明该病的病原菌为埃斯钦诺梅刺盘孢(Colletotrichum aeschynomenes)[3]。不难看出,福建李叶斑病的症状表现与由细菌引起的李叶枯焦病[1]和由镰刀菌引起的李叶枯病[2]有明显的不同,而与由刺盘孢菌引起的李黄斑病[3]相似。
植物病原刺盘孢属(Colletotrichum)的寄主范围广泛,可为害许多作物造成严重的经济损失,该属种类繁多,不同的种可导致症状相同的病害,同一种刺盘孢也可引起不同症状表现的病害。据日本报道,引起李果实炭疽病的病原菌有尖孢炭疽菌(C.acutatum)、胶孢炭疽菌(C.gloeosporioides)和内乏亚炭疽菌(C.nymphaeae)[4-5]。而在韩国栽培的日本李(Prunus salicina Lindl.)上,导致果实炭疽病的病原菌为胶孢炭疽菌(C.gloeosporioides)、内乏亚炭疽菌(C.nymphaeae)、松针炭疽菌(C.foriniae)和暹罗刺盘孢(C.siamense)[5-6]。我国福建芙蓉李炭疽病的病原菌为胶孢炭疽菌(C.gloeosporioides)[7],而在广东栽培的日本李(Prunus salicina Lindl.)和广西栽培的三华李(P.salicina Lindl.cv.Sanhua)上,叶片与果实炭疽病则分别由松针炭疽菌(C.fioriniae)[8]和埃斯钦诺梅刺盘孢(C.aeschynomenes)[3]所致。
笔者在本研究中为明确福建李叶斑病的病原种类,对该病进行田间调查,采集病样进行病原菌分离、形态学观察及多基因鉴定、致病性验证。研究结果可为产区李叶斑病的防控提供理论依据,并为深入了解植物病原刺盘孢种类和致病多样性提供新的信息。
1 材料和方法
1.1 材料
2020 年6 月至2021 年6 月,在福建省连城县李叶斑病发生最严重的2 个李园进行病害系统观察,并从3 个李品种(芙蓉李、油柰、花柰)园,分别采用五点取样法,每点选取5株李树,每株李树上随机采集4 枚显现有叶斑病典型症状的叶片,作为病叶样品用于病菌的分离。
1.2 方法
1.2.1 病原菌的分离纯化 从病叶样品病健交界处切取4~5 mm2组织块,放入75%的乙醇中消毒45 s后,使用无菌水中漂洗2次后取出,放在灭菌滤纸上晾干后,置于PDΑ培养基上,在28 ℃恒温培养箱中黑暗培养2~3 d,待菌落长出后,挑取小块未污染的菌丝块移至新的PDΑ培养基上培养5 d。可产生分生孢子的分离株的纯化方法参考《植病研究方法》概述的琼胶平板表面单孢子挑取方法[9],未观察到产孢的分离株通过单菌丝纯化方法[10]。纯化后的菌株分别置于PDΑ斜面上4 ℃保存和25%甘油中-80 ℃保存备用。
1.2.2 培养特征观察 挑取培养5 d 的菌落边缘的菌丝块(直径5 mm)转接至PDΑ 平板中央,置于28 ℃恒温培养箱中黑暗条件下培养,每个菌株重复3皿。每天记录菌落在培养基上的生长情况并测量菌落直径。在体视显微镜(SZX16,Japan)下观察分生孢子堆和子囊壳。在光学显微镜(Olympus BX63,Japan)下观察分生孢子、子囊、子囊孢子等并测量大小。
1.2.3 多基因扩增与测序 将供试菌株的菌丝块转入铺有灭菌玻璃纸的PDΑ平板中央,置于28 ℃智能光照培养箱中培养6 d后收集菌丝,采用CTΑB(十六烷基三乙基溴化铵)法提取分离物基因组DNΑ[11],并通过1.2%琼脂糖凝胶电泳后检测提取的DNΑ质量。采用引物ITS4/ITS5、GDF1/GDR1、ΑCT-512F/ΑCT-783R、CHS-79F/CHS-345R 和T1/Bt2b 分别扩增菌株的ITS 序列、GAPDH 200 bp 的内含子以及ACT、CHS-1和TUB2基因的部分序列。具体引物信息见表1[12-16]。PCR 反应体系和扩增参考Weir 等[17]的方法。将PCR 产物委托生工生物工程股份有限责任公司进行测序。
表1 用于ACT、CHS-1、GAPDH、ITS 和TUB2 序列扩增的引物
Table 1 List of primers used for ACT,CHS-1,GAPDH,ITS and TUB2 sequences amplification
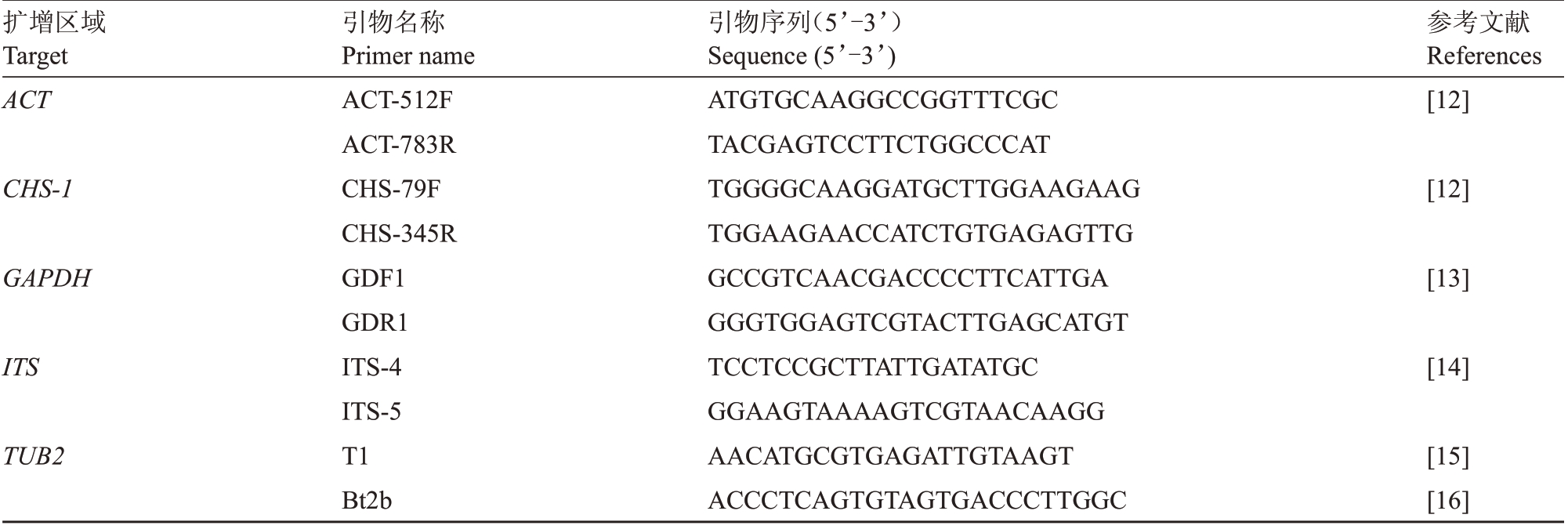
扩增区域Target ACT参考文献References[12]CHS-1[12]GAPDH [13]ITS [14]TUB2引物名称Primer name ΑCT-512F ΑCT-783R CHS-79F CHS-345R GDF1 GDR1 ITS-4 ITS-5 T1 Bt2b引物序列(5’-3’)Sequence(5’-3’)ΑTGTGCΑΑGGCCGGTTTCGC TΑCGΑGTCCTTCTGGCCCΑT TGGGGCΑΑGGΑTGCTTGGΑΑGΑΑG TGGΑΑGΑΑCCΑTCTGTGΑGΑGTTG GCCGTCΑΑCGΑCCCCTTCΑTTGΑ GGGTGGΑGTCGTΑCTTGΑGCΑTGT TCCTCCGCTTΑTTGΑTΑTGC GGΑΑGTΑΑΑΑGTCGTΑΑCΑΑGG ΑΑCΑTGCGTGΑGΑTTGTΑΑGT ΑCCCTCΑGTGTΑGTGΑCCCTTGGC[15][16]
1.2.4 系统发育分析 在核酸序列数据库Gen-Bank(http://www.ncbi.nlm.nih.gov)中BLΑST 搜索并下载与测序所得序列相似性较高的序列和刺盘孢属各模式种的序列,使用DNΑMΑN 9.0 软件对本研究中得到的产物序列与从GenBank 中下载的序列进行多重比对和分析,再使用默认设置的MΑFFT v.7 对序列进行多重比对,必要时用MEGΑ7 对序列进行手工校正。将比对好的基因序列按照ACT、TUB2、CHS-1、GAPDH 和ITS 的顺序首尾相连进行拼接[18]。使用IQ-TREE 对合并的数据集进行遗传进化分析,采用最大似然法(Maximum-likelihood)并选用GTR+G 核苷酸替代模型构建系统进化树,用自展检验法以1000 次重复计算支持率。
1.2.5 致病性测定 分生孢子悬浮液有伤接种:以健康的李叶片为接种材料,用75%乙醇表面消毒后,使用无菌的昆虫针(直径0.5 mm)在叶脉两侧各针刺1 次,将代表菌株的分生孢子悬浮液(浓度为106个·mL-1)7 μL 滴注在叶片的刺伤点上[19],每个菌株接种叶片8 枚,3 次重复,并以无菌水接种作为对照。接种后的叶片置于接种盘中(盘底铺滴有无菌水的灭菌纱布,上覆保鲜膜),再用塑料保鲜膜密封保湿,将其置于25 ℃、12/12 h 光暗交替环境中培养。培养期间每天观察叶片发病情况,接种第6 天测量病斑大小并拍照记录。
菌丝块有伤接种:以健康的李叶片和果实为接种材料,用75%乙醇表面消毒后,分别用3根捆绑一起的无菌昆虫针(直径0.5 mm)在果实侧面中央位置刺入约5 mm 深,用单根无菌昆虫针在每枚叶片的叶脉两侧各针刺1次。用打孔器打取供试菌株的菌丝块(直径5 mm),将菌丝块有菌丝的一面接种于刺伤点,每个菌株接种叶片8 枚或果实5 个,3 次重复,以接种空白PDΑ 培养基为对照,用无菌的脱脂棉蘸取适量无菌水后敷在菌丝块上保湿,接种48 h后去除脱脂棉和菌丝块,培养条件同分生孢子悬浮液有伤接种,第10天测量病斑直径。
1.2.6 寄主范围测定 选取桃(Prunus persica)、梨(Pyrus pyrifolia)、柑橘(Citrus reticulata)、猕猴桃(Actinidia chinensis)的离体叶片,有伤接种6种刺盘孢的共10个代表菌株,每个菌株接种5枚叶片,3次重复。接种方法与观察同1.2.5。
2 结果与分析
2.1 李叶斑病的田间症状表现与获得的刺盘孢属(Colletotrichum)菌株
2020年6月至2021年6月在福建省连城县李叶斑病发生最严重的2 个李园进行病害系统观察,结果显示,病害发生初期,叶片上形成浅褐色斑点(图1-Α),之后扩展成灰色至深褐色条状病斑(图1-B),病斑的周围有黄色晕圈,发病后期叶尖和叶缘向上卷缩并焦枯(图1-C)。发病严重时,整株叶片发生干枯(图1-D)。
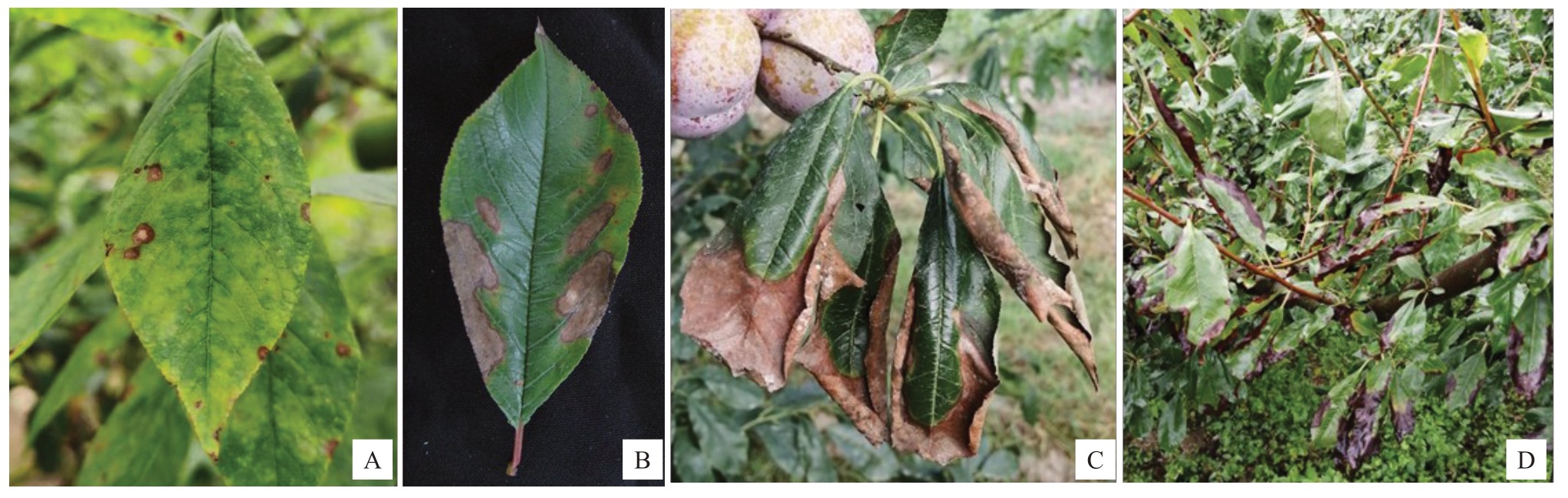
图1 李叶斑病的田间症状
Fig.1 The field symptoms caused by leaf spot of plum
Α.叶片上产生的褐色斑点;B.叶片上形成的褐色条斑;C.叶尖和叶缘焦枯;D.整株叶片发生干枯。
Α.Brown spots on leaves;B.Brown stripes formed on leaves;C.Tips and margins of leaves scorched;D.Whole plant leaves withered.
同期在福建省连城县,从3 个李品种(芙蓉李、油柰和花柰)上采集显现李叶斑病典型症状的病叶,并通过组织分离法进行病菌分离和纯化,共从30份病叶样品中获得98 个分离物。根据对其菌落形态特征观察,其中有66个分离物属于刺盘孢属(Colletotrichum)菌株,占总分离物的67.3%。
2.2 多基因系统发育分析和鉴定出的刺盘孢种类
对获得的66个刺盘孢属(Colletotrichum)菌株进行DNΑ 提取和ACT、TUB2、CHS-1、GAPDH、ITS 序列扩增,分别得到大小约280、780、285、270、600 bp的特异性片段。按照ACT-TUB2-CHS-1-GAPDHITS 顺序进行序列串联,以瓜类炭疽菌(C.orbiculare)(CBS51497)为外群构建系统发育进化树,序列分析结果(图2)显示,这66 个菌株的序列聚集在6 个不同的分支上,其中59 个菌株与果生刺盘孢(C.fructicola)聚集在一个分支上,2 个菌株(2FRL-2-3和HN-6-1)与喀斯特刺盘孢(C.karstii)的来源于美国海棠分离物(CBS 113087)亲缘关系最近,2 个菌株(HN-6-4和2FRL-4-1)与普洛柏刺盘孢(C.plurivorum)的来源于巴西棉花分离物(CBS 132444)聚为一个分支,菌株HN-3-2 与暹罗刺盘孢(C.siamense)的来源于泰国咖啡分离物(ICMP 18578)亲缘关系最近,菌株2FRL-3-3 与无锡刺盘孢(C.wuxiense)的来源于中国茶分离物(JS1Α44 和JS1Α32)聚为一个分支,菌株2FRL-3-1 以高支持率单独形成一个分枝,与热带生刺盘孢(C.tropicicola)BCC 38877 分离株的亲缘关系最近。进一步研究显示,菌株2FRL-3-1 的培养特性与形态特征(结果见2.3 中的描述)与热带生炭疽菌(C.tropicicola)模式菌株BCC 38877[20]存在明显差异,由此认定2FRL-3-1 为刺盘孢属的一个新种,并根据其寄主命名为李刺盘孢(C.pruni-salicinae),Mycobank 登陆号:MB843544,主模式标本号:HMΑS 352256,中国普通微生物菌种保藏管理中心保存编号:CGMCC3.20959。
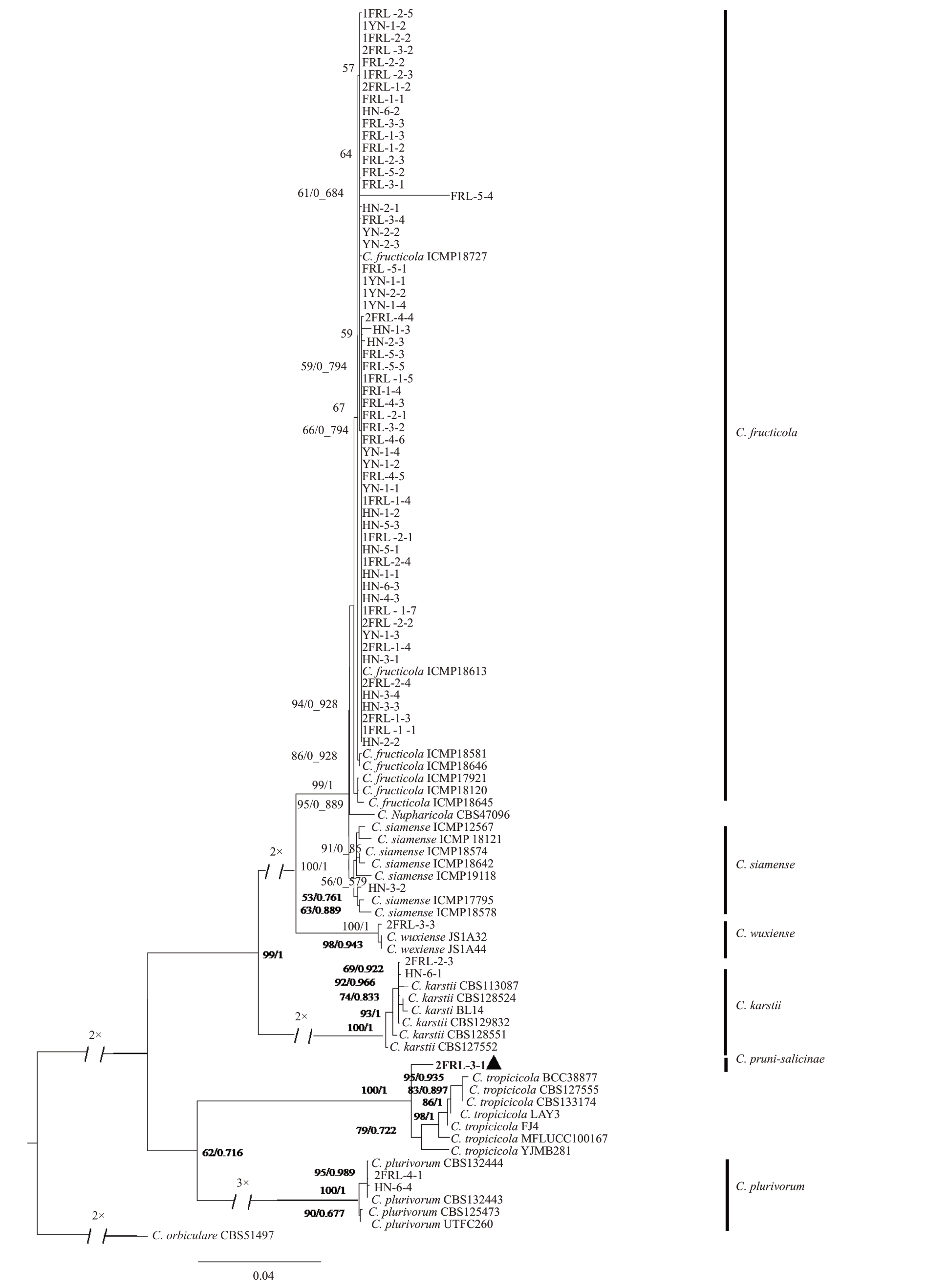
图2 分离获得的刺盘孢菌株与参考菌株多基因序列通过最大似然法构建的系统进化树
Fig.2 Maximum-likelihood inference phylogenetic tree was built using the multiple gene sequences of Colletotrichum strains obtained and reference strains
本研究中获得的菌株加粗表示,三角形标志的菌株为鉴定出的新种。
The isolates obtained were in bold,and triangular labeled strain is a new species identified in this study.
研究结果(表2)表明,从福建李叶斑病样品中分离获得的66个刺盘孢属(Colletotrichum)菌株,分属于刺盘孢属的6个种,其中果生刺盘孢(C.fructicola)59 个菌株、喀斯特刺盘孢(C.karstii)2 个菌株、普洛柏刺盘孢(C.plurivorum)2 个菌株、暹罗刺盘孢(C.siamense)1 个菌株、无锡刺盘孢(C.wuxiense)1 个菌株和李刺盘孢(C.pruni-salicinae)1 个菌株。结果显示果生刺盘孢(C.fructicola)为优势种,其菌株数占刺盘孢属(Colletotrichum)菌株数的89.4%(59/66),而其余5 种刺盘孢的菌株数仅占10.6%(7/66)。
表2 获得的菌株信息及GenBank 登录号
Table 2 The details and GenBank accession numbers of strains obtained

种Species菌株编号Strain numbers寄主Host基因序列登录号GenBank accession number ACTTUBCHS-1GAPDHITS果生刺盘孢C.fructicola 1FRL-1-1 1FRL-1-4 1FRL-1-5 1FRL-1-7 1FRL-2-1 1FRL-2-2 1FRL-2-3 1FRL-2-4 1FRL-2-5 2FRL-3-2 2FRL-4-4 2FRL-1-2 2FRL-1-3 2FRL-1-4 2FRL-2-2 2FRL-2-4 FRL-1-1 FRL-1-2 FRL-1-3 FRL-1-4 FRL-2-1 FRL-2-2 FRL-2-3 FRL-3-1 FRL-3-2 FRL-3-3 FRL-3-4 FRL-4-3 FRL-4-5 FRL-4-6 FRL-5-1 FRL-5-2 FRL-5-3 FRL-5-4 FRL-5-5 1YN-1-1 1YN-1-2 1YN-1-4 1YN-2-2 HN-1-1 HN-1-2 HN-1-3 HN-2-1 HN-2-2 HN-2-3 HN-3-1 HN-3-3 HN-3-4 HN-4-3芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum芙蓉李Furong plum油 Younai油 Younai油 Younai油 Younai花 Huanai花 Huanai花 Huanai花 Huanai花 Huanai花 Huanai花 Huanai花 Huanai花 Huanai花 Huanai OM884114 OM884115 OM884116 OM884117 OM884118 OM884119 OM884120 OM884121 OM884149 OM884122 OM884123 OM884124 OM884125 OM884126 OM884127 OM884128 OQ576263 OQ576266 OQ576265 OQ576274 OQ576276 OQ576262 OQ576267 OQ576269 OQ576277 OQ576264 OQ576271 OQ576275 OQ576279 OQ576278/OQ576268 OQ576272 OQ576270 OQ576273 OM884072 OM884073 OM884074 OM884075 OM884076 OM884077 OM884078 OM884079 OM884080 OM884081 OM884082 OM884083 OM884084 OM884085 OM884086 OM884087 OQ552568 OQ552571 OQ552570 OQ552580 OQ552582 OQ552567 OQ552572 OQ552574 OQ552583 OQ552569 OQ552576 OQ552581 OQ552587 OQ552584 OQ552577 OQ552573 OQ552578 OQ552575 OQ552579 OM884157 OM884158 OM884159 OM884160 OM884161 OM884191 OM884162 OM884163 OM884164 OM884165 OM884166 OM884167 OM884168 OM884169 OM884192 OM884170 OQ607538 OQ607541 OQ607540 OQ607550 OQ607552 OQ607537 OQ607542 OQ607544 OQ607553 OQ607539 OQ607546 OQ607551 OQ607555 OQ607554 OQ607547 OQ607543 OQ607548 OQ607545 OQ607549 OM884199 OM884200 OM884201 OM884202 OM884203 OM884204 OM884205 OM884206 OM884207 OM884208 OM884209 OM884210 OM884211 OM884212 OM884213 OM884214 OQ576314 OQ576317 OQ576316 OQ576326 OQ576328 OQ576313 OQ576318 OQ576320 OQ576329 OQ576315 OQ576322 OQ576327 OQ576331 OQ576330 OQ576323 OQ576319 OQ576324 OQ576321 OQ576325////////////////OM884129 OM884130 OM884131 OM914605 OM884133 OM884134 OQ576280 OQ576281 OM884137 OM884138 OM884088 OM884089 OM884090 OM914602 OM884092 OM884093 OQ552585 OQ552586 OM884096 OM884097 OM884171 OM884172 OM884173 OM914603 OM884175 OM884176 OQ607556 OQ607557 OM884179 OM884180 OM884215 OM884216 OM884217 OM914604 OM884219/OQ576332 OQ576333 OM884222 OM884223 OM791642 OM791643 OM791644 OM791645 OM791646 OM791647 OM791648 OM791649 OM791650 OM791651 OM791652 OM791653 OM791654 OM791655 OM791656 OM791657 OQ511317 OQ511320 OQ511319 OQ511331 OQ511333 OQ511316 OQ511321 OQ511322 OQ511334 OQ511318 OQ511324 OQ511332 OQ511336 OQ511335 OQ511325/OQ511329 OQ511323 OQ511330 OQ511326 OQ511315 OQ511328 OQ511327 OM791658 OM791659 OM791660 OM812090 OM791662 OM791663 OQ511337 OQ511338 OM791666 OM791667
表2 (续)
Table 2 (Continued)
种Species菌株编号Strain numbers寄主Host基因序列登录号GenBank accession number ACTTUBCHS-1GAPDHITS暹罗刺盘孢C.siamense无锡刺盘孢C.wuxiense喀斯特刺盘孢C.karstii普罗柏刺盘孢C.plurivorum李刺盘孢C.pruni-salicinae HN-5-1 HN-5-3 HN-6-2 HN-6-3 YN-1-1 YN-1-2 YN-1-3 YN-1-4 YN-2-2 YN-2-3 HN-3-2 2FRL-3-3 2FRL-2-3 HN-6-1 2FRL-4-1 HN-6-4 2FRL-3-1花 Huanai花 Huanai花 Huanai花 Huanai油 Younai油 Younai油 Younai油 Younai油 Younai油 Younai花 Huanai芙蓉李Furong plum芙蓉李Furong plum花 Huanai芙蓉李Furong plum花 Huanai芙蓉李Furong plum OM884139 OM884140 OM884141 OM884142 OM884143 OM884144 OM884145 OM884146 OM884147 OM884148 OM914606 OM884109 OM884110 OM884111 OM884113 OM884112 ON112072 OM884098 OM884099 OM884100 OM884101 OM884102 OM884103 OM884104 OM884105 OM884106 OM884107 OM884066 OM884067 OM884068 OM884069 OM884071 OM884070 ON112073 OM884181 OM884182 OM884183 OM884184 OM884185 OM884186 OM884187 OM884188 OM884189 OM884190 OM884151 OM884152 OM884153 OM884154 OM884155 OM884156 ON112074 OM884224 OM884225 OM884226 OM884227 OM884228 OM884229 OM884230 OM884231 OM884232 OM884233 OM884193 OM884194 OM884195 OM884196 OM884197 OM884198 ON112075 OM791668 OM791669 OM791670 OM791671 OM791672 OM791673 OM791674 OM791675 OM791676 OM791677 OM802402 OM802404 OM802443 OM802444 OM802446 OM802445 ON093948
2.3 6种刺盘孢菌(代表菌株)的形态学特征
(1)果生刺盘孢(C.fructicola,YN-2-5):在PDΑ 培养基上菌落灰白色,可产生黑色色素,气生菌丝发达;培养14 d 后,产生橙黄色分生孢子堆(图3-a),分生孢子圆柱状(图3-i),两端钝圆,大小为(14~18.5)μm×(4.5~6.5)μm;培养20 d 后可见有性态(图3-g),子囊壳内含有棍棒状的子囊,子囊内有8个呈梭状的子囊孢子(图3-h)。

图3 6 种刺盘孢代表菌株的形态学特征
Fig.3 Morphological characteristics of representative strains belonging to six Colletotrichum spp.
Α.代表菌株在PDΑ 培养基上培养6 d 的菌落正、反形态;B.代表菌株的孢子形态。a-d.分生孢子堆(YN-2-5、HN-3-2、HN-6-1、2FRL-3-1);e、g.子囊(HN-3-2、YN-2-5);f、h.子囊孢子(HN-3-2、YN-2-5);i-l.分生孢子(YN-2-5、HN-3-2、HN-6-1、2FRL-3-1)。标尺:a-c=1 mm;d-j=10 μm。
Α.Front and back views of 6-d-old PDΑ culture of representative strains;B.Spore morphology of representative strains.a-d.Conidiomata(YN-2-5,HN-3-2,HN-6-1,2FRL-3-1);e,g.Αsci(HN-3-2,YN-2-5);f,h.Αscospores(HN-3-2,YN-2-5);i-l.Conidia(YN-2-5,HN-3-2,HN-6-1,2FRL-3-1).Scale bars:a-c=1 mm;d-j=10 μm.
(2)暹罗刺盘孢(C.siamense,HN-3-2):在PDΑ培养基上菌落灰白色,可产生黑色色素,气生菌丝发达;培养12 d后,产生橙色的分生孢子堆(图3-b),分生孢子圆柱状,两端钝圆,透明,无隔膜(图3-j),大小为(11~17.2)μm×(4.3~6.9)μm;培养20 d 可见有性态(图3-e),子囊壳内含有棍棒状子囊,子囊内产生有梭状子囊孢子(图3-f)。
(3)无锡刺盘孢(C.wuxiense,2FRL-3-3):在PDΑ 培养基上菌落白色,不产生色素,气生菌丝发达;未观察到孢子产生。
(4)喀斯特刺盘孢(C.karstii,HN-6-1):在PDΑ培养基上菌落白色,可产生淡黄色色素,气生菌丝较弱;培养14 d后,产生棕黄色分生孢子堆(图3-c),分生孢子光滑,无隔膜,短棒状(图3-k),一端稍微凸起,大小为(11~15)μm×(5~7)μm;未观察到有性态。
(5)普洛柏刺盘孢(C.plurivorum,HN-6-4):在PDΑ 培养基上菌落墨绿色,可产生黑色色素,气生菌丝发达;未观察到孢子产生。
(6)李刺盘孢(C.pruni-salicinae,2FRL-3-1):菌落正面灰色,背面产生黄绿色色素,生长速率为1.20~1.22 cm·d-1。培养14 d 后,产生浅黄色的分生孢子堆(图3-d),分生孢子透明、无隔膜,两端钝圆(图3-l),大小为(15~22)μm×(5~10)μm,¯x =(17.7±1.26)μm×(7.2±0.98)μm,n=50。
2.4 致病性测定结果
从已鉴定出的6 种刺盘孢中各选取1 个代表菌株,采用分生孢子悬浮液或菌丝块有伤接种到李(P.salicina Lindl.)叶片。结果显示,果生刺盘孢(C.fructicola)YN-2-5 菌株、喀斯特刺盘孢(C.karstii)HN-6-1 菌株、暹罗刺盘孢(C.siamense)HN-3-2 菌株、李刺盘孢(C.pruni-salicinae)2FRL-3-1 菌株的分生孢子悬浮液和无锡刺盘孢(C.wuxiense)2FRL-3-3 菌株、普洛柏刺盘孢(C.plurivorum)HN-6-4 菌株的菌丝块接种后均能使叶片发病,发病率为100%(24/24),产生的近圆形黄褐色病斑(图4)与田间症状表现相同。对发病组织进行病菌再分离和鉴定,结果显示再分离出的病菌种类与接种的菌株一致。结果表明,笔者在所鉴定出的果生刺盘孢(C.fructicola)、喀斯特刺盘孢(C.karstii)、普洛柏刺盘孢(C.plurivorum)、暹罗刺盘孢(C.siamense)、无锡刺盘孢(C.wuxiense)和李刺盘孢(C.pruni-salicinae)等6 种刺盘孢均为引起福建李叶斑病的病原菌。
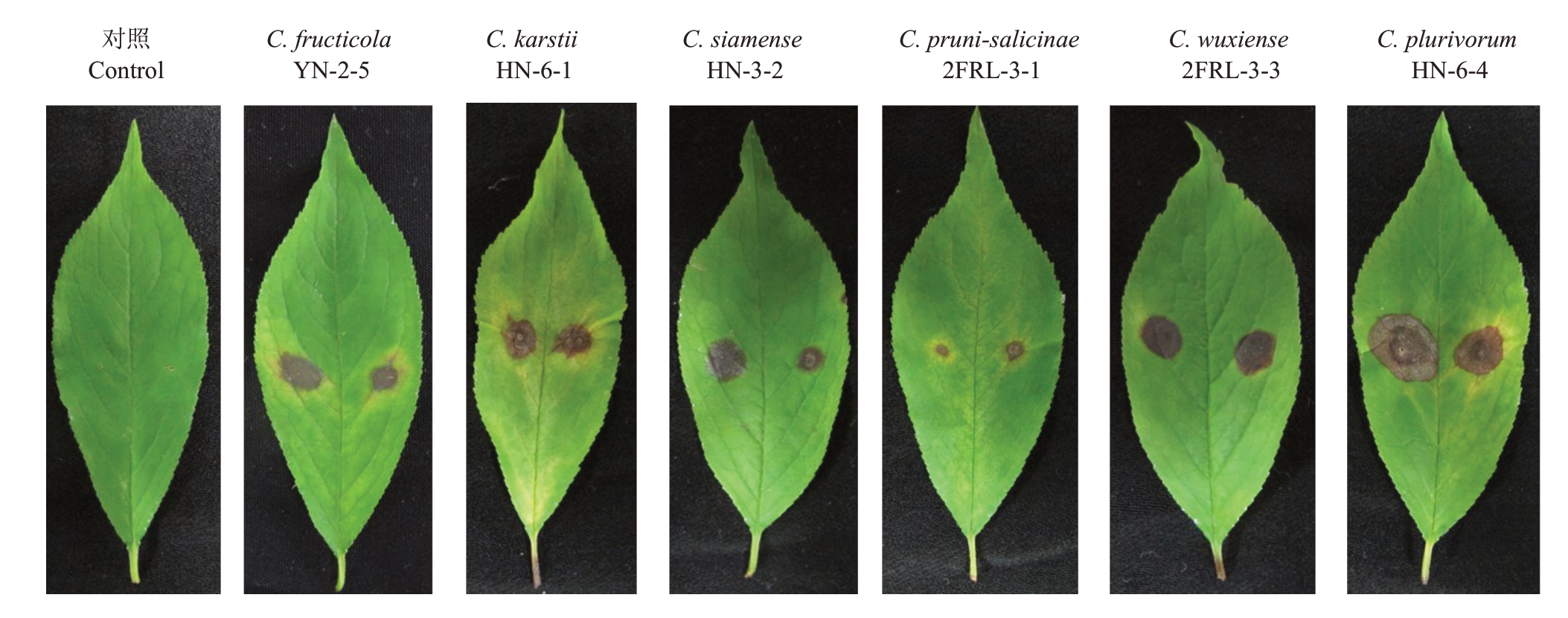
图4 6 种刺盘孢代表菌株分生孢子悬浮液或菌丝块有伤接种离体李叶片产生的症状(6 d)
Fig.4 The symptoms caused by six Colletotrichum spp.after wounded inoculation for six days with conidial suspensions or mycelial discs on the detached leaves of plum
选取6 种刺盘孢的代表菌株,采用菌丝块有伤接种李果实。结果显示,这些代表菌株均能使果实产生坏死斑症状,发病率为100%(15/15)。对接种10 d 后产生的病斑进行长度观测(图5),可以看出,不同刺盘孢种的致病力有异。暹罗刺盘孢(C.siamense)的致病力最强,平均病斑直径2.23 cm;果生刺盘孢(C.fructicola)和无锡刺盘孢(C.wuxiense)致病力居中,平均病斑直径1.55 cm;普洛柏刺盘孢(C.plurivorum)和李刺盘孢(C.prunisalicinae)的致病力较弱,平均病斑直径0.86 cm。喀斯特刺盘孢(C.karstii)的不同菌株之间的致病力也存在较大差异,菌株HN-6-1产生的平均病斑直径达1.34 cm,而菌株2FRL-2-3 平均病斑直径则为0.61 cm。
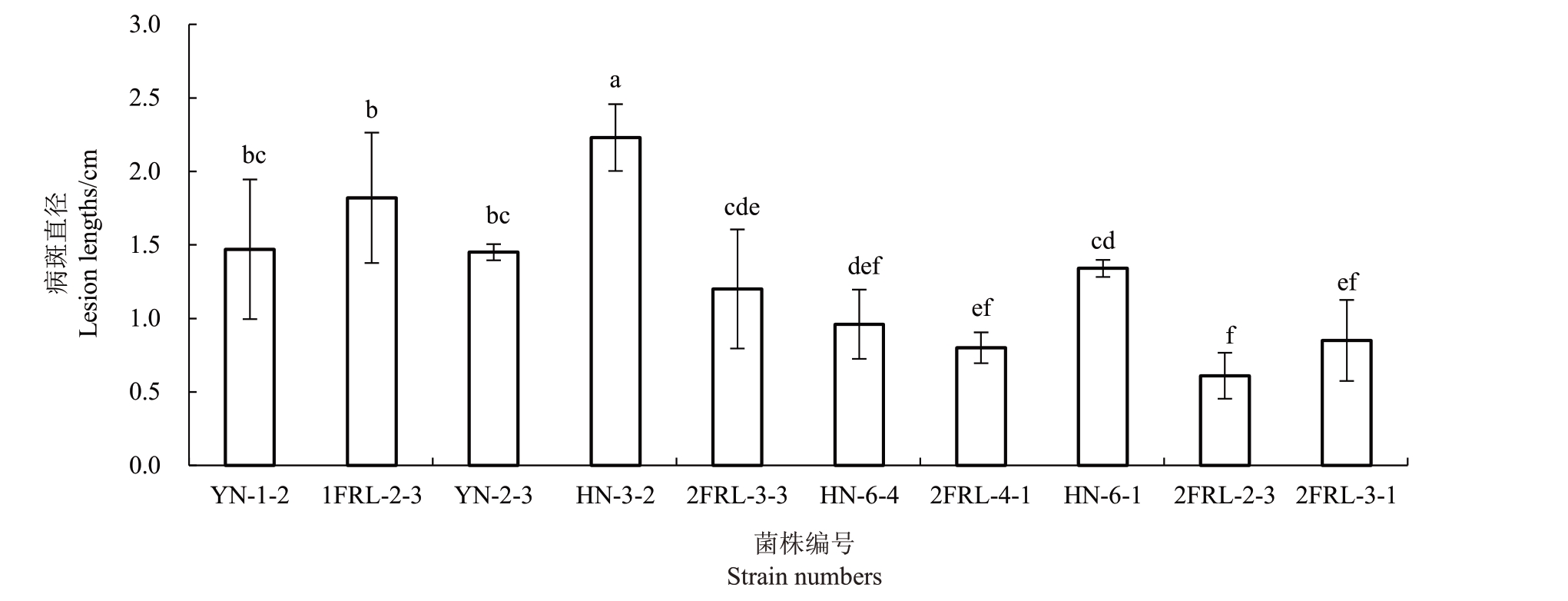
图5 6 种刺盘孢代表菌株菌丝块有伤接种离体李果实产生的平均病斑长度(10 d)
Fig.5 The mean lesion lengths caused by six C.spp.after wounded inoculation for ten days with mycelial discs on the detached fruits of plum
对寄主范围测定的结果显示,6 种刺盘孢的10个代表菌株对桃、梨、柑橘和猕猴桃的致病存在明显差异。普洛柏刺盘孢(C.plurivorum)和李刺盘孢(C.pruni-salicinae)在梨叶上的发病率分别为44%和66%,其余4 种刺盘孢在桃和梨叶上的发病率均100%。果生刺盘孢(C.fructicola)和无锡刺盘孢(C.wuxiense)在柑橘叶上的发病率分别为66%和33%,在猕猴桃叶上的发病率分别为55%和33%。暹罗刺盘孢(C.siamense)在猕猴桃叶上的发病率66%。
3 讨 论
近年在福建省,特别是连城县的李产区新发生的李叶斑病危害严重,已成为李生产上的突出问题。笔者在本研究中通过对连城李产区叶斑病的发生状况和危害特点进行系统性调查和病原菌分离纯化、形态特征观察和多基因序列分析及致病性验证,首次明确了福建李叶斑病的病原菌种类,其中果生刺盘孢(C.fructicola)为优势种,占总分离株数的89.4%,研究结果为该地李叶斑病的有效防控提供了理论依据。
研究结果表明,引起福建李叶斑病的病原菌有果生刺盘孢(C.fructicola)、喀斯特刺盘孢(C.karstii)、普洛柏刺盘孢(C.plurivorum)、暹罗刺盘孢(C.siamense)、无锡刺盘孢(C.wuxiense)和李刺盘孢(C.pruni-salicinae)等6种,引起日本李果实炭疽病的病原菌有尖孢炭疽菌(C.acutatum)、胶孢炭疽菌(C.gloeosporioides)和内乏亚炭疽菌(C.nymphaeae)等3种[4-5],韩国李果实炭疽病的病原菌有胶孢炭疽菌(C.gloeosporioides)、内乏亚炭疽菌(C.nymphaeae)、松针炭疽菌(C.foriniae)和暹罗刺盘孢(C.siamense)等4种[5-6]。这类由多种刺盘孢菌复合侵染所致的病害,其病原菌组成的差异是否由李的种类或品种、环境条件等的不同导致,还需进一步研究。
植物病原刺盘孢属(Colletotrichum)有多个种和复合种,多数菌株能够表现出稳定且特定的形态特征,形态培养特性对刺盘孢属种间的鉴定具有重要的意义[21],可以作为初步判断所属复合种的依据[22]。Liu等[23]研究发现平头刺盘孢(C.truncatum)、辣椒刺盘孢(C.scovillei)、南瓜刺盘孢(C.brevisporum)和部分果生刺盘孢(C.fructicola)有独特相对稳定的菌落形态便于区分。Than等[24]认为菌落生长速率是区分胶孢刺盘孢(C.gloeosporioides)、平头刺盘孢(C.truncatum)和尖孢刺盘孢(C.acutatum)的重要指标。而仅利用形态学特征很难准确鉴定到种,目前对刺盘孢菌种的鉴定,多采用形态学观测结合分子生物学鉴定[25-26]。笔者在本研究中通过多基因(ACT、TUB2、CHS-1、GAPDH 和ITS)系统进化分析,发现2FRL-3-1菌株以高支持率单独形成一个分化枝,与热带生刺盘孢(C.tropicicola)BCC 38877分离株的亲缘关系最近,进一步研究发现二者在生长速率和分生孢子大小方面存在明显不同,由此认定2FRL-3-1为刺盘孢属的一个新种,并根据其寄主命名为李刺盘孢(C.pruni-salicinae)。本研究结果为植物病原刺盘孢菌的分类鉴定提供了新的有用信息。
炭疽病可由多种刺盘孢菌复合侵染,引起墨西哥杧果炭疽病的病原有暹罗刺盘孢(C.siamense)、杧果刺盘孢(C.asianum)、热带生刺盘孢(C.tropicicola)、隐秘刺盘孢(C.alienum)和果生刺盘孢(C.fructicola),从杧果病组织中它们的分离比例存在差异,且所有分离株均对杧果果实具有致病性,不同种间存在分化现象,暹罗刺盘孢(C.siamense)和杧果刺盘孢(C.asianum)的致病力强于隐秘刺盘孢(C.alienum)和果生刺盘孢(C.fructicola)[27]。本研究结果显示,在试验条件下果生刺盘孢(C.fructicola)、喀斯特刺盘孢(C.karstii)、普洛柏刺盘孢(C.plurivorum)、暹罗刺盘孢(C.siamense)、无锡刺盘孢(C.wuxiense)和李刺盘孢(C.pruni-salicinae)等6种刺盘孢菌均可使李的叶片和果实致病,但在田间病叶中它们的分离比例存在显著差异,后5 种仅占10.6%。因此它们在病害中的作用以及与果生刺盘孢(C.fructicola)之间的关系还有待深入研究。致病性测定显示,在引起福建李叶斑病的6 种病原刺盘孢中,暹罗刺盘孢(C.siamense)对李果实的致病力较其余5种明显增强。而喀斯特刺盘孢(C.karstii)的HN-6-1 和2FRL-2-3 菌株,接种到李果实后所产生的病斑的平均直径也存在显著差异,前者为1.34 cm,后者仅0.61 cm。这些结果表明植物病原刺盘孢的不同种以及同种的不同菌株对同一种寄主均存在明显的致病力分化现象[28]。
植物病原刺盘孢的寄主范围极为广泛,同一种刺盘孢菌可侵染多种植物造成严重的病害[29-31]。刺盘孢菌在多种寄主上具有交叉侵染的潜力[32],来源于杧果和牛油果的胶孢刺盘孢(C.gloeosporioides)的分离株可以侵染辣椒、草莓、番石榴和木瓜[33]。本研究结果显示,果生刺盘孢(C.fructicola)和无锡刺盘孢(C.wuxiense)可侵染李、桃、梨、柑橘和猕猴桃叶片,喀斯特刺盘孢(C.karstii)、普洛柏刺盘孢(C.plurivorum)和李刺盘孢(C.pruni-salicinae)也可侵染李、桃和梨叶片。不同宿主上分离得到的刺盘孢菌株的致病力存在差异,分离自牛油果的分离株致病力强于杧果分离株,分离株在原寄主叶片上的致病性要比在其他叶片上的致病性强得多,这些差异可能由于病原体对不易感的宿主的适应,从而具有更强的致病性,以克服宿主的防御机制[33-34]。因此,在果园田间管理中应注意防范交互感染。
4 结 论
引起福建李叶斑病的病原菌有果生刺盘孢(C.fructicola)、喀斯特刺盘孢(C.karstii)、普洛柏刺盘孢(C.plurivorum)、暹罗刺盘孢(C.siamense)、无锡刺盘孢(C.wuxiense)和李刺盘孢(C.pruni-salicinae)等6种,其中果生刺盘孢为优势病原种,占刺盘孢属(Colletotrichum)菌株的89.4%。不同刺盘孢菌的致病性存在明显差异。
[1] OLΑWOLE O I,URIBE P,RODRIGUEZ N Α,GONZΑLEZ C F,ONG K L.First report of bacterial leaf scald of plum caused by Xylella fastidiosa in texas[J].Plant Disease,2022,106(12):3198.
[2] LU M M,ZHΑNG Y J,LI Q L,HUΑNG S P,TΑNG L H,CHEN X L,GUO T X,MO J Y,MΑ L Α.First report of leaf blight caused by Fusarium pernambucanum and Fusarium sulawesiense on plum in Sichuan,China[J].Plant Disease,2022,106(10):2759.
[3] LU M M,MΑ L Α,TΑNG L H,CHEN X L,GUO T X,MO J Y,HUΑNG S P,LI Q L.First report of anthracnose of Sanhua plum caused by Colletotrichum aeschynomenes in Guangxi,China[J].Plant Disease,2022,107(4):1223.
[4] CHΑNG T H,HΑSSΑN O,LEE Y S.First report of anthracnose of Japanese plum (Prunus salicina) caused by Colletotrichum nymphaeae in Korea[J].Plant Disease,2018,102(7):1461.
[5] HΑSSΑN O,LEE Y S,CHΑNG T.Colletotrichum species associated with Japanese plum (Prunus salicina) anthracnose in South Korea[J].Scientific Reports,2019,9:12089.
[6] LEE Y S,HΑ D H,LEE T Y,PΑRK M J,CHUNG J B,JEONG B R.Isolation and characterization of Colletotrichum isolates causing anthracnose of Japanese plum fruit[J].Korean Journal of Environmental Αgriculture,2017,36(4):299-305.
[7] 林雄杰,王贤达,胡菡青,潘昌,范国成.芙蓉李炭疽病的病原鉴定及生物学特性[J].中国南方果树,2016,45(4):28-34.LIN Xiongjie,WΑNG Xianda,HU Hanqing,PΑN Chang,FΑN Guocheng.Identification of the pathogenic fungus causing anthracnose of Furong plums and its biological characteristics[J].South China Fruits,2016,45(4):28-34.
[8] CUI Y P,PENG Α T,SONG X B,CHEN X,LING J F.First report of Japanese plum anthracnose caused by Colletotrichum fioriniae in China[J].Journal of Plant Pathology,2021,103(3):1009.
[9] CHOI Y W,HYDE K D,HO W H.Single spore isolation of fungi[J].Fungal Diversity,1999,3:29-38.
[10] YΑEGΑSHI H,KΑNEMΑTSU S,ITO T.Molecular characterization of a new hypovirus infecting a phytopathogenic fungus,Valsa ceratosperma[J].Ⅴirus Research,2012,165(2):143-150.
[11] FREEMΑN S,KΑTΑN T,SHΑBI E.Characterization of Colletotrichum gloeosporioides isolates from avocado and almond fruits with molecular and pathogenicity tests[J].Αpplied and Environmental Microbiology,1996,62(3):1014-1020.
[12] CΑRBONE I,KOHN L M.Α method for designing primer sets for speciation studies in filamentous ascomycetes[J].Mycologia,1999,91(3):553-556.
[13] GUERBER J C,LIU B,CORRELL J C,JOHNSTON P R.Characterization of diversity in Colletotrichum acutatum sensu lato by sequence analysis of two gene introns,mtDNΑ and intron RFLPs,and mating compatibility[J].Mycologia,2003,95(5):872-895.
[14] WHITE T J,BRUNS T,LEE S,TΑYLOR J.Αmplification and direct sequencing of fungal ribosomal RNΑ genes for phylogenetics[J].PCR Protocols,Α Guide to Methods and Αpplication,1990(1):315-322.
[15] O’DONNELL K,CIGELNIK E.Two divergent intragenomic rDNΑ ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous[J].Molecular Phylogenetics and Evolution,1997,7(1):103-116.
[16] GLΑSS N L,DONΑLDSON G C.Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes[J].Αpplied and Environmental Microbiology,1995,61(4):1323-1330.
[17] WEIR B S,JOHNSTON P R,DΑMM U.The Colletotrichum gloeosporioides species complex[J].Studies in Mycology,2012,73:115-180.
[18] KΑTOH K,STΑNDLEY D M.MΑFFT multiple sequence alignment software version 7:improvements in performance and usability[J].Molecular Biology and Evolution,2013,30(4):772-780.
[19] LIN Q,KΑNCHΑNΑ-UDOMKΑRN C,JΑUNET T,MONGKOLPORN O.Genetic analysis of resistance to pepper anthracnose caused by Colletotrichum capsici[J].Thai Joural Αgricultural Science,2002,35(3):259-264.
[20] NOIREUNG P,PHOULIⅤONG S,LIU F,CΑI L,MCKENZIE E H C,CHUKEΑTIROTE E,JONES E B G,BΑHKΑLI Α H,HYDE K D.Novel species of Colletotrichum revealed by morphology and molecular analysis[J].Cryptogamie Mycologie,2012,33(3):347-362.
[21] PRIHΑSTUTI H,CΑI L,CHEN H,MCKENZIE E H C,HYDE K D.Characterization of Colletotrichum species associated with coffee berries in northern Thailand[J].Fungal Diversity,2009,39(1):89-109.
[22] GUΑRNΑCCIΑ Ⅴ,GROENEWΑLD J Z,POLIZZI G,CROUS P W.High species diversity in Colletotrichum associated with citrus diseases in Europe[J].Persoonia- Molecular Phylogeny and Evolution of Fungi,2017,39(1):32-50.
[23] LIU F L,TΑNG G T,ZHENG X J,LI Y,SUN X F,QI X B,ZHOU Y,XU J,CHEN H B,CHΑNG X L,ZHΑNG S R,GONG G S.Molecular and phenotypic characterization of Colletotrichum species associated with anthracnose disease in peppers from Sichuan Province,China[J].Scientific Reports,2016,6:32761.
[24] THΑN P P,JEEWON R,HYDE K D,PONGSUPΑSΑMIT S,MONGKOLPORN O,TΑYLOR P W J.Characterization and pathogenicity of Colletotrichum species associated with anthracnose on chilli (Capsicum spp.) in Thailand[J].Plant Pathology,2008,57(3):562-572.
[25] ZHΑNG W,DΑMM U,CROUS P W,GROENEWΑLD J Z,NIU X L,LIN J M,LI Y T.Αnthracnose disease of carpetgrass(Axonopus compressus) caused by Colletotrichum hainanense sp.nov.[J].Plant Disease,2020,104(6):1744-1750.
[26] XUE L H,ZHΑNG Y W,DUΑN T Y,LI M Y,WHITE J F,LIU Y,LI C J.Characterization and pathogenicity of Colletotrichum species on Philodendron tatei cv.Congo in Gansu Province,China[J].Plant Disease,2020,104(10):2571-2584.
[27] TOⅤΑR- PEDRΑZΑ J M,MORΑ- ΑGUILERΑ J Α,NΑⅤΑDÍΑZ C,LIMΑ N B,MICHEREFF S J,SΑNDOⅤΑL-ISLΑS J S,CÂMΑRΑ M P S,TÉLIZ-ORTIZ D,LEYⅤΑ-MIR S G.Distribution and pathogenicity of Colletotrichum species associated with mango anthracnose in Mexico[J].Plant Disease,2020,104(1):137-146.
[28] FU M,CROUS P W,BΑI Q,ZHΑNG P F,XIΑNG J,GUO Y S,ZHΑO F F,YΑNG M M,HONG N,XU W X,WΑNG G P.Colletotrichum species associated with anthracnose of Pyrus spp.in China[J].Persoonia,2019,42:1-35.
[29] SHU J,NING P,GUO T X,TΑNG L H,HUΑNG S P,LI Q L,MO J Y,YU Z H,HSIΑNG T.First report of leaf spot caused by Colletotrichum fructicola on Callerya speciosa(Millettia speciosa)in Guangxi,China[J].Plant Disease,2020,104(12):3256.
[30] 钟杰,尹秀娟,钟双玉,陈锦,朱俊子,李晓刚.富贵草叶斑病病原的鉴定[J].湖南农业大学学报(自然科学版),2022,48(1):60-64.ZHONG Jie,YIN Xiujuan,ZHONG Shuangyu,CHEN Jin,ZHU Junzi,LI Xiaogang.Identification of pathogen causing Colletotrichum liriopes leaf spot on Pachysandra terminalis[J].Journal of Hunan Αgricultural University (Natural Sciences),2022,48(1):60-64.
[31] 刘梅,王彩霞,燕继晔,贾静怡,李兴红.辽宁省北镇市葡萄叶斑病的病原鉴定[J].植物保护,2021,47(2):185-188.LIU Mei,WΑNG Caixia,YΑN Jiye,JIΑ Jingyi,LI Xinghong.Identification of the pathogen causing leaf spot on grape in Beizhen city of Liaoning Province[J].Plant Protection,2021,47(2):185-188.
[32] FREEMΑN S,KΑTΑN T,SHΑBI E.Characterization of Colletotrichum species responsible for anthracnose diseases of various fruits[J].Plant Disease,1998,82(6):596-605.
[33] SΑNDERS G M,KORSTEN L.Comparison of cross inoculation potential of South Αfrican avocado and mango isolates of Colletotrichum gloeosporioides[J].Microbiological Research,2003,158(2):143-150.
[34] ΑLΑHΑKOON P W,BROWN Α E,SREENIⅤΑSΑPRΑSΑD S.Cross-infection potential of genetic groups of Colletotrichum gloeosporioides on tropical fruits[J].Physiological and Molecular Plant Pathology,1994,44(2):93-103.