嗜果刀孢菌(Wilsonomyces carpophilus)是引起新疆伊犁地区天山野果林野杏(Prunus armeniaca L.)真菌性穿孔病的病原菌[1]。由嗜果刀孢菌引起的穿孔病是危害核果类果树的重要病害,而野杏(P.armeniaca L.)是天山野果林原始植物区系组成物种之一,对维持稳定的野果林生态系统起着重要作用[2]。因此,由嗜果刀孢菌引起的野杏穿孔病的防治对保护野杏种质资源及野果林生态系统的恢复有着重要意义。目前,嗜果刀孢菌的防治主要以化学防治为主。Azmy 等[3]在2007—2008 年在埃及Nobariya地区的杏树上喷施克菌星(Punch)、烯唑醇(Sumi-8)、腈菌唑(Sythane-24)、戊菌唑(Topas-100)、多菌灵(Cam-zen)、肟菌酯(Flint)、吡唑醚菌酯(Pyraclostrobin)和氢氧化铜(Copper acrobat)8 种杀菌剂进行杏树嗜果刀孢菌的防治,发现腈菌唑、多菌灵、烯唑醇、克菌星和戊菌唑5 种杀菌剂在1 mg·L-1时的防效能够完全抑制杏树穿孔病的发生。此外,克菌丹(captan)、己唑醇(hexaconazole)、苯醚甲环唑(difenoconazole)等[4]都可用来防治穿孔病。钱超等[5]和王召元等[6]使用代森锌、代森锰锌、苯醚甲环唑和甲基硫菌灵进行桃穿孔病田间防治,发现代森锌和代森锰锌800倍液对桃穿孔病均有不错的防治效果。赵俊芳等[7]通过对比克菌康、戊唑醇和叶枯唑3 种杀菌剂对杏李嗜果刀孢菌的田间药效,发现戊唑醇2000倍液对杏李穿孔病的防效较好,防治效果达83.74%。近年来,伊犁地区天山野果林野杏穿孔病的大面积发生导致了野杏资源的减少,而有关嗜果刀孢菌化学药剂防治的研究年限较早,且相关防治均在平原地区栽培杏园内开展,同时伊犁地区天山野果林尚未见防治研究报道。
生物防治能有效避免环境污染和病原物产生抗药性等问题,因此越来越受到国内外专家学者的重视。Azmy 等[3]发现哈茨木霉(Trichoderma harzianum)和绿色木霉(T.viride)能有效防止桃、杏和李穿孔病的发生,但枯草芽孢杆菌(Bacillus subtilis)的防效较低。Karlidag 等[8]利用芽孢杆菌OSU-142 悬浮液(109 CFU·mL-1)有效防止了杏穿孔病的发生。目前,国外生物防治均使用成品菌株制剂,国内尚未见嗜果刀孢菌生物防治的相关报道。因此,笔者在本研究中以嗜果刀孢菌YA21 为供试菌株,通过生长速率法和孢子萌发法筛选防治杀菌剂,并采用平板对峙法筛选拮抗细菌,为嗜果刀孢菌的化学和生物防治提供理论依据。
1 材料和方法
1.1 供试材料
供试培养基:马铃薯琼脂糖培养基(potato dextrose agar,PDA)配方为马铃薯200 g、葡萄糖20 g、琼脂糖20 g、蒸馏水1 L;LB液体培养基配方为胰蛋白胨10 g、酵母粉5 g、NaCl 10 g、蒸馏水1 L;LB 固体培养基是在LB 液体培养基的基础上添加琼脂粉18 g。所有培养基均在121 ℃灭菌30 min后使用。
含药培养基的制备:将不同杀菌剂按推荐倍数经预试验调整后分别稀释至相应的倍数备用。将配置好的PDA 培养基每瓶99 mL 分装在250 mL 锥形瓶中,待灭菌完成后冷却至60 ℃加入配置好的杀菌剂,比例为V 药剂∶V 培养基=1∶9,以不加杀菌剂的PDA平板作为对照,振荡摇匀后倒入直径90 mm 的培养皿中备用[9]。
供试菌株:嗜果刀孢菌YA21(GenBank:OQ547194)于野杏芽中分离获得;所有供试菌株均保存于新疆农业大学林学与风景园林学院森林保护学实验室。
各供试杀菌剂试验稀释倍数根据商品药剂推荐稀释倍数及预试验结果调整为最终试验所用稀释倍数。杀菌剂见表1。
表1 供试药剂及试验稀释倍数
Table 1 Test agents and test concentrations

杀菌剂名称Pesticide 50%多菌灵50%carbendazim 80%代森锰锌80%mancozeb 75%百菌清75%chlorothalonil 40%福·福锌15%thiram+25%ziram 722 g·L-1 霜霉威盐酸盐722 g·L-1 propamocarb hydrochloride 27%戊唑·噻霉酮2%benzisothiazolinone+25%tebuconazole 36%喹啉·戊唑醇24%oxine-copper+12%tebuconazole 20%吡唑·嘧菌酯20%pyraclostrobin剂型Pesticide formulation可湿性粉剂WP Wettable powder可湿性粉剂WP Wettable powder可湿性粉剂WP Wettable powder可湿性粉剂WP Wettable powder水剂AS Aqueous solutions水乳剂EW Emulsion in water稀释倍数Dilution multiple 2000,3000,4000,5000,6000 2000,3000,4000,5000,6000 400,600,800,1000,1200 1000,2000,3000,4000,5000 600,1800,2400,3000,3600 2000,4000,8000,16 000,32 000悬浮剂SC Suspension concentrate微囊悬浮剂CS Microcapsule suspension生产厂家Manufacturer四川润尔科技有限公司Sichuan Runer Technology Co.,Ltd河北伊诺生化有限公司Hebei Yinuo Biochemical Co.,Ltd江西中迅农化有限公司Jiangxi Zhongxun Agrochemical Co.,Ltd河北冠龙农化有限公司Hebei Guanlong Agrochemical Co.,Ltd拜耳股份公司Bayer陕西大华特科技实业有限公司Shaanxi dahuate Technology Industry Co.,Ltd顺毅股份有限公司Shunyi Co.,Ltd江西中迅农化有限公司Jiangxi Zhongxun Agrochemical Co.,Ltd 4000,6000,8000,10 000,12 000 2000,4000,6000,8000,10 000
1.2 不同杀菌剂对嗜果刀孢菌菌丝生长的抑制作用
用直径5 mm灭菌打孔器打取菌饼,将菌饼菌丝一面向下放置于不同杀菌剂不同稀释倍数和对照PDA 平板中央,每个处理3 次重复,置于25 ℃恒温培养箱培养,每天观察其生长状况,采用十字交叉法测量菌落直径,根据式(1)计算菌丝生长抑制率,采用菌丝生长速率法[10]对杀菌剂毒力进行评估。以药剂稀释倍数的自然对数值为自变量(x),以抑菌率的概率为因变量(y),利用最小二乘法建立毒力回归方程(y=ax+b),计算出各供试杀菌剂的有效中浓度(median effective concentration,EC50),进行不同杀菌剂毒力大小的评估[11]。

1.3 不同杀菌剂对嗜果刀孢菌分生孢子萌发的抑制作用
纯培养菌落在显微镜下观察到有分生孢子产生后,向产孢平板内加入无菌水,用接种环在菌落表面轻轻刮取使分生孢子脱落,将分生孢子悬浮液用双层无菌纱布过滤,过滤后的溶液使用离心机1000 r·min-1离心5 min,弃上清液,再加入无菌水配置成107 个·mL-1 的孢子悬浮液。将配置好的孢子悬浮液与药液混匀,比例为V 药液∶V 孢子悬浮液=1∶1,25 ℃恒温保湿培养,根据预试验结果14 h后进行观察。采用孢子萌发法比较嗜果刀孢菌分生孢子对8种杀菌剂毒力的敏感性。孢子萌发标准为当芽管长度超过孢子最大直径长度的一半时即视为萌发[4]。以加入清水的孢子悬浮液作为对照,对照萌发率超过90%时,统计8 种杀菌剂不同稀释倍数下的孢子萌发情况,根据式(2)和式(3),每个稀释倍数检查200个孢子,计算孢子萌发率和孢子萌发抑制率。孢子萌发对各杀菌剂敏感性毒力回归方程建立同1.2。

利用Microsoft Excel 2019和GraphPad Prism 8.0软件进行数据整理、统计与分析,求出各杀菌剂对嗜果刀孢菌菌丝和孢子的毒力回归方程、EC50及相关系数。
1.4 拮抗菌株的分离与纯化
选取具有典型野杏穿孔病症的叶片,用无菌水冲洗干净,无菌滤纸滤干水分。取病健交界部位切成5 mm的小块,用3%次氯酸钠溶液浸泡30 s,用无菌镊子放置于LB 固体培养基表面,每皿放置5 块,28 ℃恒温培养箱中黑暗培养7 d。将采集的土壤样品通过稀释涂布法分离,取10 g土样倒入150 mL的锥形瓶中,加无菌水至100 mL,充分振荡,即制成10-3、10-5、和10-7的悬浮液,用移液枪吸取200 μL 的上清液置于含有0.05 g·L-1盐酸四环素的PDA 培养基上,用无菌玻璃棒涂抹均匀,设置3 次重复,放于28 ℃恒温培养箱培养,待有明显菌落出现后立即进行纯化。待病原菌落长出后,将其周围生长的单菌落划线转接至新的LB 培养基中,转接2~3 次直至获得纯化的单菌落,再将纯化的单菌落转接到LB液体培养基中28 ℃、150 r·min-1振荡培养24 h,-20 ℃冰箱中保藏备用。
1.5 拮抗菌株对嗜果刀孢菌的抑制效果
采用平板对峙法[12]测定拮抗菌株对嗜果刀孢菌的抑制效果。用直径5 mm的打孔器在活化培养5 d的菌落边缘取菌饼置于PDA 平板中央。挑取单菌落细菌菌株在菌饼两侧划线接种菌株,以只接种菌饼的平板为对照,每个处理3次重复,放入恒温培养箱25 ℃培养,待对照菌落生长至满皿或停止生长后,测量菌落直径,依据式(4)计算抑制率。

1.6 拮抗菌株的鉴定
1.6.1 拮抗菌株的培养特征观察及生理生化特性测定 挑取纯化后的单菌落划线转接至新的LB固体培养基表面,28 ℃黑暗培养3 d后观察,记录单菌落的大小、颜色、透明度和表面质地等形态学特征[13],同时进行革兰氏染色,测定拮抗菌株的生理生化特性。
1.6.2 拮抗菌株分子生物学鉴定 用Ezup 柱式细菌基因组DNA 抽提试剂盒提取拮抗菌株的DNA,采用细菌通用引物进行PCR扩增,引物序列为:27F(5’-AGAGTTTGATCCTGGCTCAG-3’)和1492R(5’-TACCTTGTTACGACTT-3’),引物由生工生物工程(上海)股份有限公司合成。PCR扩增反应总体系25µL:2×Taq PCR Master Mix 12.5µL,上下游引物各0.5 μL,模板DNA 1 µL、ddH2O 补充至25 μL。PCR扩增条件为:95 ℃预变性5 min;94 ℃变性30 s,57 ℃退火30 s,72 ℃延伸90 s,30个循环;72 ℃延伸10 min。PCR 产物经1%琼脂糖凝胶电泳(150 V,20 min)检测后,凝胶成像系统分析结果。切割PCR 产物电泳条带所需DNA 目的条带,使用San-Prep柱式DNA胶回收试剂盒回收DNA片段。
将回收成功的PCR产物送至生工生物工程(上海)股份有限公司进行测序,在NCBI 网站(https://www.ncbi.nlm.nih.gov/)进 行BLAST 同 源 序 列 比对[14],选取同源性较高的已知菌株序列,利用MEGA 6.0软件进行序列分析,采用Neighbor-jioning法构建系统发育树,确定菌株的分类地位。
1.7 拮抗菌株对不同病原菌的抑菌效果测定
方法同1.4。供试菌株见表2。
表2 供试菌株及来源
Table 2 The species and sources of strains in this study

序号Number 1 2 3 4 5 6 7 8 9病原菌Pathogenic bacteria Phaeosphaeria avenaria菌株编号Strain No.GL314-1序号Number 10病原菌Pathogenic bacteria Nectria dematiosa菌株编号Strain No.3142-2 Didymella maydis HC228-1 11 Cytospora ulmi 3336-2 Ascochyta nigripycnidia TET305 12 Nectria nigrescens 3456-2 Dothiorella omnivora 3442 13 Nothophoma quercina HC230-1-1 Aureobasidium pullulansYA92 14 Phoma herbarum GL323-3 Didymella glomerata YA99 15 Didymella aliena XB170 Monilinia laxa XHG 3m2 16 Nigrospora gorlenkoana HC261-1 Cytospora donetzica 3410-1 17 Chaetomium elatum HC273-1 Cytospora pruinopsis 3336-1寄主Host金丝桃叶绣线菊Spiraea hypericifolia金丝桃叶绣线菊S.hypericifolia金丝桃叶绣线菊S.hypericifolia野杏P.armeniaca野杏P.armeniaca野杏P.armeniaca野杏P.armeniaca榆树Ulmus pumila榆树U.pumila 18 Dothiorella sarmentorum 3111-1-1寄主Host榆树U.pumila榆树U.pumila榆树U.pumila榆树U.pumila刚毛忍冬Lonicera hispida腺齿蔷薇Rosa albertii山楂Crataegus pinnatifida苦豆子Sophora alopecuroides蔷薇Rosa sp.
2 结果与分析
2.1 不同杀菌剂对嗜果刀孢菌菌丝生长的毒力测定结果
8种杀菌剂对嗜果刀孢菌的菌落生长均表现出抑制作用,但不同的杀菌剂抑菌效果有着明显的差异。由表3 可知,50%多菌灵各稀释倍数梯度药液对菌丝生长均有强抑制作用,嗜果刀孢菌菌丝均未生长,在抑菌试验结束后将含50%多菌灵培养基上的菌饼回接至正常培养基,菌丝恢复生长;722 g·L-1霜霉威盐酸盐和36%喹啉·戊唑醇对嗜果刀孢菌菌丝的生长抑制作用较强,EC50 分别为0.322 5 和0.329 8 mg·L-1;27%戊唑·噻霉酮、20%吡唑·嘧菌酯和80%代森锰锌的抑制作用次之,EC50 分别为10.66、37.18 和41.81 mg·L-1;40%福·福锌的抑制作用较弱,EC50为114.5 mg·L-1;75%百菌清的抑制效果最差,EC50为918.8 mg·L-1。
表3 供试药剂对嗜果刀孢菌菌丝生长的抑制效果
Table 3 Test results of indoor toxicity of the tested drug to W.carpophilus
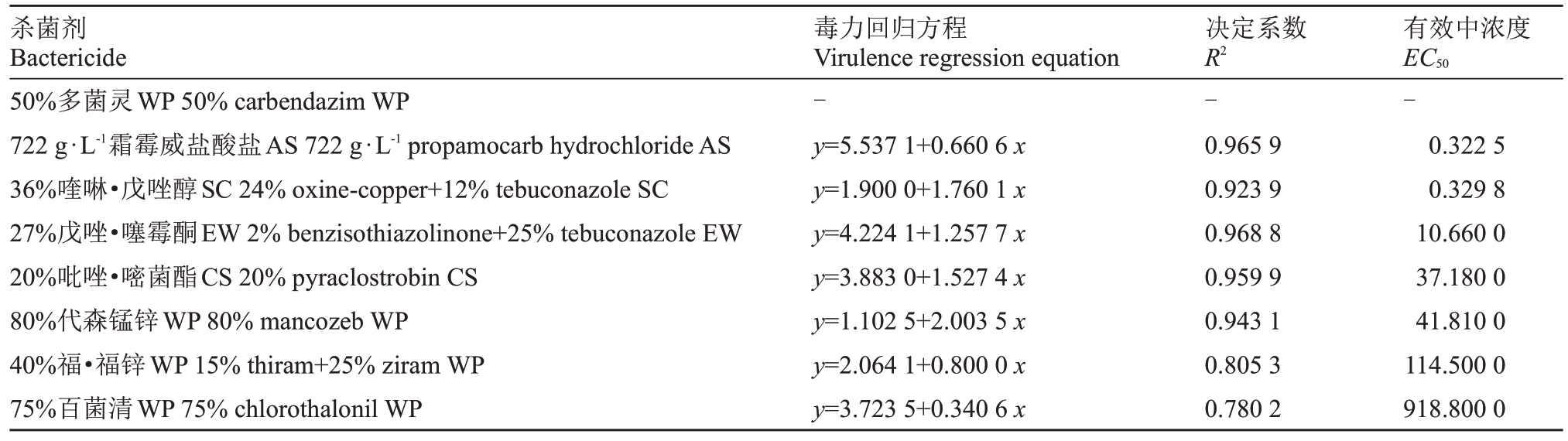
杀菌剂Bactericide 50%多菌灵WP 50%carbendazim WP 722 g·L-1霜霉威盐酸盐AS 722 g·L-1 propamocarb hydrochloride AS 36%喹啉·戊唑醇SC 24%oxine-copper+12%tebuconazole SC 27%戊唑·噻霉酮EW 2%benzisothiazolinone+25%tebuconazole EW 20%吡唑·嘧菌酯CS 20%pyraclostrobin CS 80%代森锰锌WP 80%mancozeb WP 40%福·福锌WP 15%thiram+25%ziram WP 75%百菌清WP 75%chlorothalonil WP毒力回归方程Virulence regression equation-y=5.537 1+0.660 6 x y=1.900 0+1.760 1 x y=4.224 1+1.257 7 x y=3.883 0+1.527 4 x y=1.102 5+2.003 5 x y=2.064 1+0.800 0 x y=3.723 5+0.340 6 x决定系数R2-0.965 9 0.923 9 0.968 8 0.959 9 0.943 1 0.805 3 0.780 2有效中浓度EC50-0.322 5 0.329 8 10.660 0 37.180 0 41.810 0 114.500 0 918.800 0
2.2 不同杀菌剂对嗜果刀孢菌分生孢子萌发的毒力测定结果
嗜果刀孢菌分生孢子在25 ℃保湿培养14 h后,对照的萌发率为97%。8种杀菌剂对嗜果刀孢菌分生孢子的萌发都有一定的抑制作用。8种杀菌剂中27%戊唑·噻霉酮和722 g·L-1霜霉威盐酸盐的抑制效果最好,EC50分别为0.060 5 和0.164 0 mg·L-1;其次是80%代森锰锌,EC50为2.352 mg·L-1;20%吡唑·嘧菌酯和36%喹啉·戊唑醇的抑制效果较弱,EC50 分别为34.41 和58.50 mg·L-1;40%福·福锌和50%多菌灵的抑制效果相对前5种杀菌剂较差,EC50分别为162.6 和223.2 mg·L-1;75%百菌清的抑制效果最差,EC50为1103 mg·L-1(表4)。
表4 供试药剂对分生孢子萌发的抑制结果
Table 4 Toxicity determination of the test reagent to the conidia in the laboratory
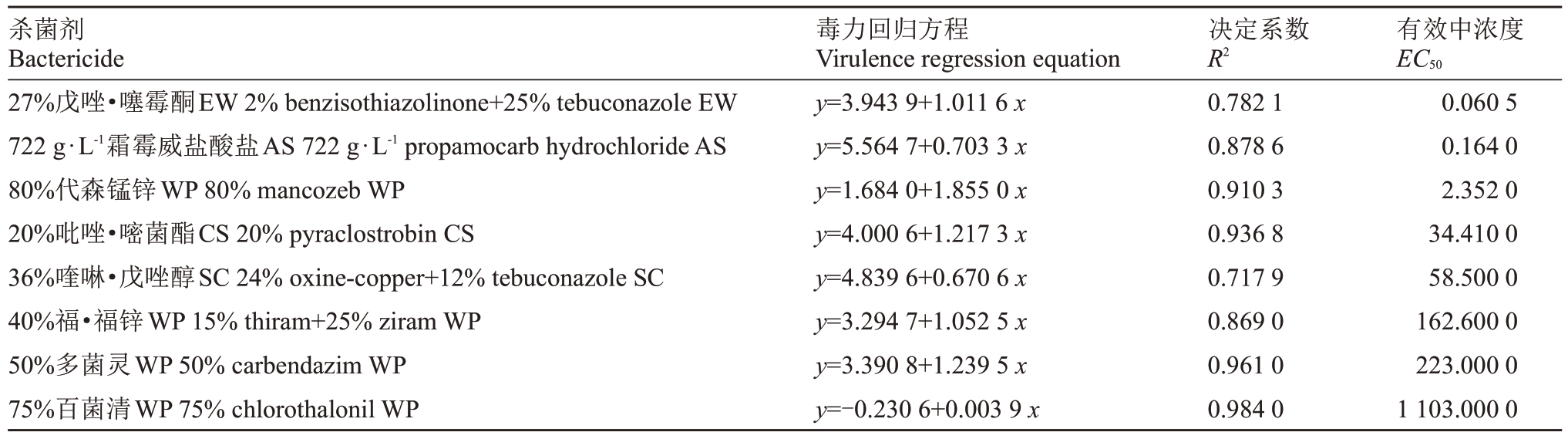
杀菌剂Bactericide 27%戊唑·噻霉酮EW 2%benzisothiazolinone+25%tebuconazole EW 722 g·L-1霜霉威盐酸盐AS 722 g·L-1 propamocarb hydrochloride AS 80%代森锰锌WP 80%mancozeb WP 20%吡唑·嘧菌酯CS 20%pyraclostrobin CS 36%喹啉·戊唑醇SC 24%oxine-copper+12%tebuconazole SC 40%福·福锌WP 15%thiram+25%ziram WP 50%多菌灵WP 50%carbendazim WP 75%百菌清WP 75%chlorothalonil WP毒力回归方程Virulence regression equation y=3.943 9+1.011 6 x y=5.564 7+0.703 3 x y=1.684 0+1.855 0 x y=4.000 6+1.217 3 x y=4.839 6+0.670 6 x y=3.294 7+1.052 5 x y=3.390 8+1.239 5 x y=-0.230 6+0.003 9 x决定系数R2 0.782 1 0.878 6 0.910 3 0.936 8 0.717 9 0.869 0 0.961 0 0.984 0有效中浓度EC50 0.060 5 0.164 0 2.352 0 34.410 0 58.500 0 162.600 0 223.000 0 1 103.000 0
2.3 拮抗菌株XHG-1-3m2的种类鉴定
2.3.1 拮抗菌株XHG-1-3m2 的培养特征与生理生化特性 菌株XHG-1-3m2 在LB 固体培养基表面28 ℃黑暗培养3 d后,培养特征如图1所示。该菌为杆状、单菌落圆形、边缘不整齐,前期乳白色,后期颜色逐渐加深、不透明、微隆起,表面不光滑,鉴定为革兰氏染色阳性。
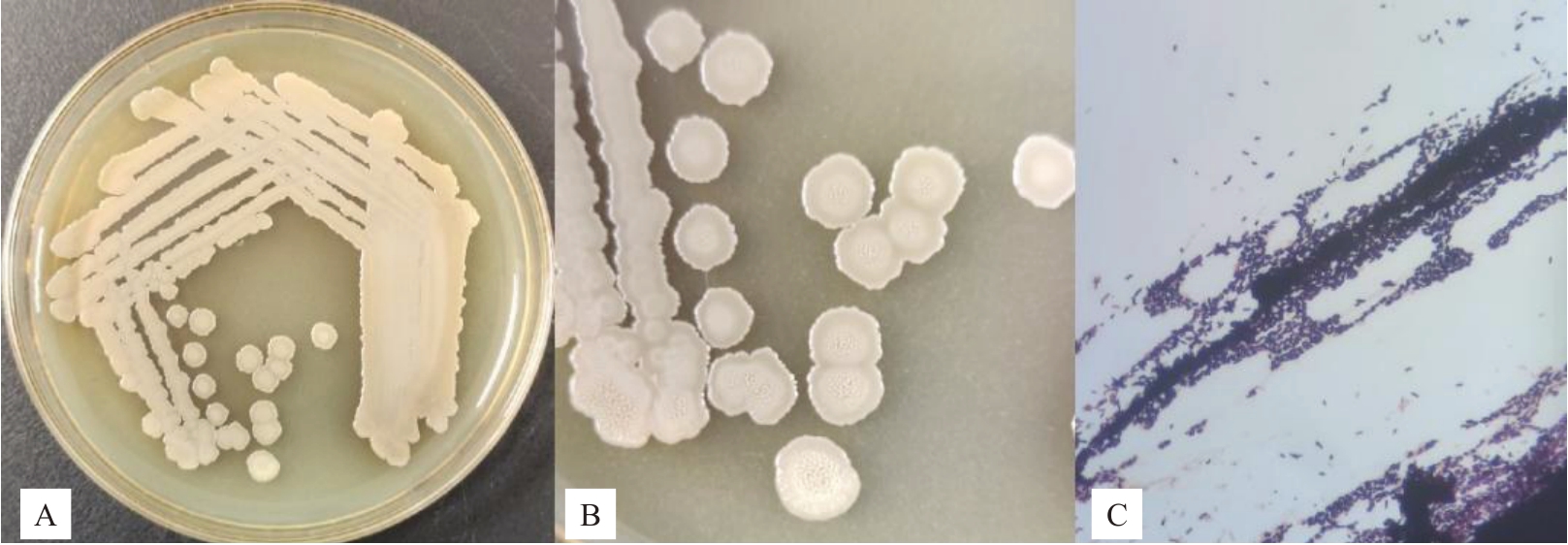
图1 菌株XHG-1-3m2 的形态及革兰氏染色图
Fig.1 Morphology and gram staining of strain XHG-1-3m2
A、B 为菌株XHG-1-3m2 菌落形态;C 为革兰氏染色。
A,B are the colony morphology of strain XHG-1-3m2;C is gram stain.
2.3.2 拮抗菌株XHG-1-3m2 的生理生化特性测定 菌株XHG-1-3m2 乙酰甲基甲醇试验和硝酸还原反应均为阳性(表5),好氧,能使明胶液化,可以水解淀粉等,符合萎缩芽孢杆菌的生理生化特征。
表5 菌株XHG-1-3m2 的生理生化特征测定结果
Table 5 Determination results of physiological and biochemical characteristics of XHG-1-3m2

注:“+”为阳性,“-”为阴性。
Note:“+”is positive,“-”is negative.
生理生化特性Physiological and biochemical characteristics乙酰甲基甲醇试验V-P柠檬酸盐Ixazomib丙酸盐Metacetonic acid D-木糖D-Xylose L-阿拉伯糖L-Arabinopyranose D-甘露醇D-Glucitol测定结果Measurement results测定结果Measurement results+ - - - - -生理生化特性Physiological and biochemical characteristics明胶液化Gelatin liquefaction 7%氯化钠生长7%sodium chloride growth pH 5.7生长pH 5.7 growth硝酸盐还原Nitrate reduction淀粉水解Starch hydrolysis厌氧生长Anaerobic growth+ + + + + -
2.3.3 拮抗菌株XHG-1-3m2 的分子生物学鉴定菌株XHG-1-3m2 的16S rDNA 扩增序列片段长度为1487 bp,GenBank 登录号为OQ438428。菌株XHG-1-3m2 在NCBI 数据库BLAST 结果与Bacil-lus atrophaeus 序列相似度达到100%,采用邻接法构建系统发育树(图2),菌株XHG-1-3m2与B.atrophaeus 聚为同一支,综合培养特征学及分子生物学分析确定菌株XHG-1-3m2 为萎缩芽孢杆菌(B.atrophaeus)。
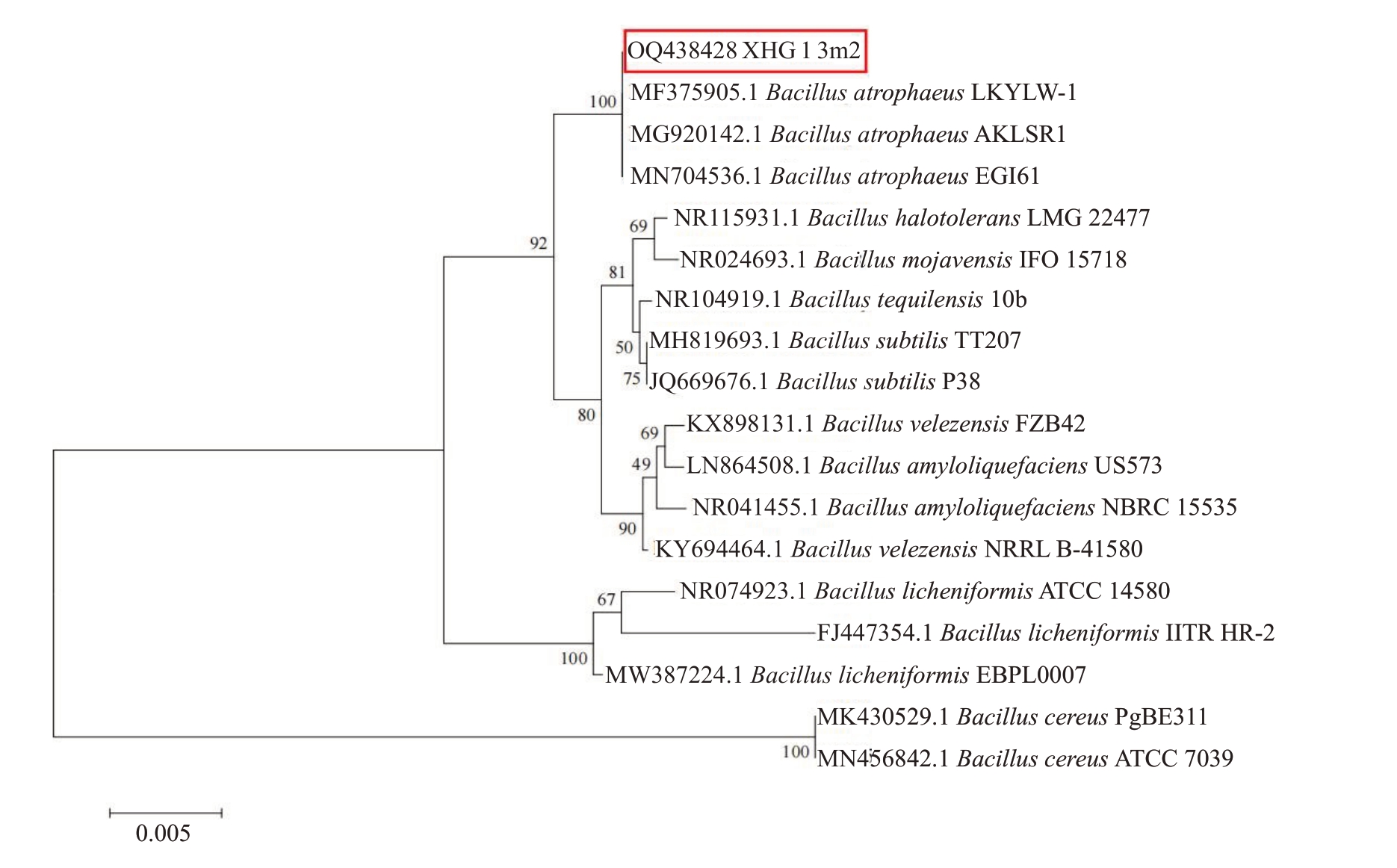
图2 基于16S rDNA 基因序列构建的菌株XHG-1-3m2 的系统发育树
Fig.2 Phylogenetic tree of strain XHG-1-3m2 constructed based on 16S rDNA gene sequences
2.4 拮抗菌株XHG-1-3m2对嗜果刀孢菌菌丝生长的影响
采用平板对峙法测定拮抗菌株XHG-1-3m2 对嗜果刀孢菌的抑制效果达88.88%(图3)。嗜果刀孢菌在受到菌株XHG-1-3m2 的抑制后菌落前沿颜色加深;挑取菌丝在显微镜下观察,发现该菌菌丝畸形,节间缩短,末端相比正常菌丝膨大,粗细不均匀或产生多个分支或细胞质外渗的现象,影响了菌丝的正常生长。

图3 菌株XHG-1-3m2 对嗜果刀孢菌菌丝形态的影响
Fig.3 Effect of strain XHG-1-3m2 on the mycelial morphology of W.carpophilus
A.对照嗜果刀孢菌落;B.受抑制嗜果刀孢菌落;C.正常菌丝;D.受抑制菌丝。
A.The control colony of W.carpophilus;B.The inhibited colony;C.Normal hypha;D.The inhibited hypha.
2.5 拮抗菌株XHG-1-3m2对不同病原菌的抑菌测定结果
通过采用平板对峙法将菌株XHG-1-3m2 划线接种培养,发现菌株XHG-1-3m2对17种病原菌都有抑制效果(表6,图4),其中对M.laxa(R1~R2)有明显的抑制效果,抑制率为89.41%,对病菌D.aliena(M1~M2)、A.pullulans(G1~G2)、A.nigripycnidia(Q1~Q2)、C.ulmi(N1~N2)、P.herbarum(L1~L2)、C.pruinopsis(D1~D2)、D.maydis(P1~P2)的抑制率为60%~70%;对D.glomerata(J1~J2)、N.dematiosa(K1~K2)、N.quercina(O1~O2)、C.elatum(H1~H2)、N.nigrescens(F1~F2)、P.avenaria(A1~A2)、C.donetzica(C1~C2)、D.omnivora(B1~B2)、D.sarmentorum(I1~I2)的抑制率为50%~60%;对病菌N.gorlenkoana(E1~E2)无明显的抑制效果。

图4 菌株XHG-1-3m2 的抑菌广谱性结果
Fig.4 Results of broad-spectrum bacteriostasis of strain XHG-1-3m2
字母-1 为对照;字母-2 为处理。
The letter-1 is control;Letter-2 is processing.
表6 菌株XHG-1-3m2 对不同病原菌的抑菌结果
Table 6 Bacteriostatic results of strain XHG-1-3m2 against different pathogenic bacteria
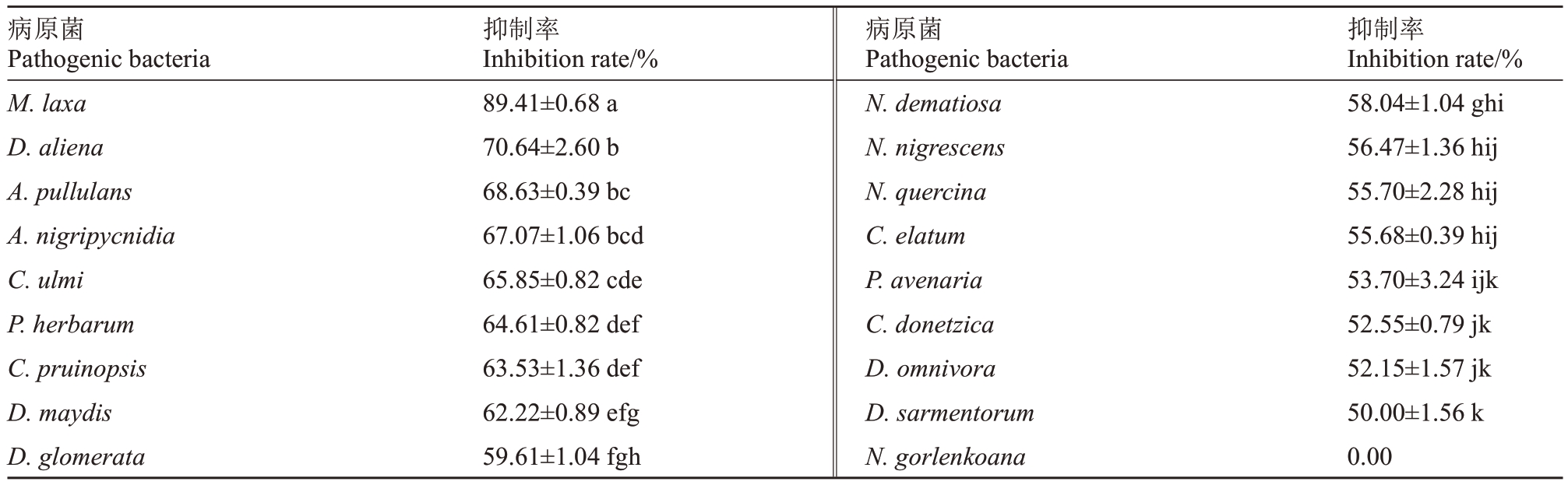
注:表中数值为(平均值±标准误差),同列不同小写字母表示处理间在p<0.05 水平差异显著。
Note:The values in the table represent(the mean±standard error),and different lowercase letters in the same column indicate significant differences among treatments at p<0.05.
抑制率Inhibition rate/%58.04±1.04 ghi 56.47±1.36 hij 55.70±2.28 hij 55.68±0.39 hij 53.70±3.24 ijk 52.55±0.79 jk 52.15±1.57 jk 50.00±1.56 k 0.00病原菌Pathogenic bacteria M.laxa D.aliena A.pullulans A.nigripycnidia C.ulmi P.herbarum C.pruinopsis D.maydis D.glomerata抑制率Inhibition rate/%89.41±0.68 a 70.64±2.60 b 68.63±0.39 bc 67.07±1.06 bcd 65.85±0.82 cde 64.61±0.82 def 63.53±1.36 def 62.22±0.89 efg 59.61±1.04 fgh病原菌Pathogenic bacteria N.dematiosa N.nigrescens N.quercina C.elatum P.avenaria C.donetzica D.omnivora D.sarmentorum N.gorlenkoana
3 讨 论
嗜果刀孢菌先后在2019 年和2020 年被程元等[15]和叶双华[16]证实在伊犁哈萨克族自治州野果林主要分布的县域大量存在,并明确了真菌性穿孔病在野果林中的危害。目前,嗜果刀孢菌防治均在平原地区开展,缺乏山区防治的研究报道,且关于新疆地区由嗜果刀孢菌引起的真菌性穿孔病的防治研究也尚未见相关报道。笔者在本研究中采用菌丝生长速率法和孢子萌发抑制法研究了多菌灵、霜霉威、喹啉·戊唑醇、戊唑·噻霉酮、吡唑·醚菌酯、代森锰锌、福·福锌和百菌清8 种杀菌剂室内对嗜果刀孢菌菌丝生长及孢子萌发的毒力,结果表明,供试的8种杀菌剂均具有一定的抑菌作用,其中多菌灵对菌丝生长抑制作用最强,在试验中将杀菌剂稀释倍数由500~2500 倍液调整至2000~6000 倍液,所接菌饼菌丝均未生长,在培养25 d后将菌饼回接至正常PDA培养基后菌丝恢复生长,表明该杀菌剂对嗜果刀孢菌菌丝生长具有强烈的抑制作用。Azmya 等[3]2007—2008年在杏树上喷施多菌灵防治杏穿孔病,结果显示50%多菌灵500倍液喷施后能完全抑制穿孔病的发生,1000 和2000 倍液喷施后发病率为30%~40%。此外,在春季树体发芽前喷施4~5°Bé石硫合剂、在生长季喷施代森锌等对穿孔病的防治也能起到不错的效果[5-6]。本研究中722 g·L-1霜霉威盐酸盐具有内吸、保护作用且兼具低毒、高效等特性,对菌丝生长和孢子萌发均具有较强的抑制作用。36%喹啉·戊唑醇对菌丝抑制效果较强,27%戊唑·噻霉酮对孢子抑制效果较强,这2 种复配杀菌剂的室内抑菌效果较好,其有效成分多为三唑类杀菌剂,具有促进植物生长的作用,被广泛应用于多种真菌病害防治。因此,在防治真菌性穿孔病的过程中,将这3种杀菌剂轮换使用,可以在提高防效的同时延缓病原菌抗药性的产生。
笔者在本研究中首次从田间发病的野杏植株上分离到能够对嗜果刀孢菌具有抑制效果的拮抗细菌萎缩芽孢杆菌B.atrophaeus,且该菌对其他多种病原真菌均具有不错的抑制效果。芽孢杆菌属是一类好氧或兼性厌氧的革兰氏阳性菌,该属细菌的重要特性是能够产生芽孢抵抗各种不利条件和环境[17]。化学药剂防治是目前控制植物病害大面积发生的主要手段,但化学药剂的长期使用对自然环境的破坏难以恢复且存在病原菌出现耐药性等潜在问题。生防菌的研究和应用逐渐被研究者重视起来,芽孢杆菌属的枯草芽孢杆菌B.subtilis、解淀粉芽孢杆菌B.amyloliquefaciens、萎缩芽孢杆菌B.atrophaeus和贝莱斯芽孢杆菌B.velezensis都是应用广泛的拮抗细菌。
朱杰等[12]利用平板稀释法从枸杞根际土壤中筛选鉴定出的菌株QH-588为B.atrophaeus,该菌株不仅能够明显抑制樱桃叶斑病菌的菌丝生长,还能够抑制樱桃叶斑病菌的孢子萌发。与该结果不同,本研究中嗜果刀孢菌在接种菌株XHG-1-3m2 后于后续的试验观察中发现病原菌未能产孢,这表明B.atrophaeus 对嗜果刀孢菌的防效可能会更好。刘欣等[18]从土样中分离鉴定出对苹果树腐烂病具有良好拮抗效果的菌株DM3-18 为B.atrophaeus。王璐[19]在研究中发现B.atrophaeus CP 297对黄曲霉也有明显的抑制效果。付莉媛[20]从草莓根际土壤等样品中分离筛选出4株芽孢杆菌对草莓根腐病菌具有良好的拮抗作用,经鉴定为B.velezensis和B.subtilis。敖静等[21]筛选出对黄瓜枯萎病具有较强抑制作用的B.amyloliquefaciens菌株XN-3。郝芳敏等[22]从甜瓜根部分离得到对甜瓜多种真菌病害病原菌均有抑制作用的多黏类芽孢杆菌NBmelon-1,该菌对甜瓜幼苗的生长还具有促进作用。李娜等[23]从黄瓜根萎病根际土分离得到在黄瓜苗期对尖孢镰刀菌(Fuasrium oxypsorum)有较好防治效果的弗雷德里克斯堡假单胞菌33-1(Pseudomonas frederiksbergensisi)。
萎缩芽孢杆菌对多种病原真菌的抑制效果都比较明显,具有抑菌广谱性。此外,4种生防细菌对病原菌抑制机制相似,都是通过影响病原菌菌丝正常生长导致畸形和抑制产孢或孢子萌发的方式达到抑菌效果。芽孢杆菌的抑菌机制多样,能够产生抗菌物质如抗生素、细菌素、酶类或其他抗菌蛋白等,通过不同的途径抑制甚至杀死病原菌,也能够通过产生促进植株生长的物质如合成植物激素、固氮等方式增强植物的生长能力,提高抗病性,从而达到防治或减少病害发生的效果[19,24]。笔者在本研究中筛选出了嗜果刀孢菌的拮抗菌株XHG-1-3m2,对抑菌机制进行了初步探索,但对于其抑菌物质的种类、含量及具体抑菌机制尚不明确,有待进一步探索和研究。
4 结 论
笔者在本研究中所选用的8种杀菌剂对嗜果刀孢菌的菌丝生长和孢子萌发都有一定的抑制作用。50%多菌灵对嗜果刀孢菌菌丝生长抑制作用较强;27%戊唑·噻霉酮对嗜果刀孢菌分生孢子萌发抑制效果较强。笔者在本研究中首次从野杏发病叶片上分离筛选出对嗜果刀孢菌具有良好抑制效果的拮抗菌株XHG-1-3m2,综合培养特征和16s rDNA 序列分析确定菌株XHG-1-3m2为萎缩芽孢杆菌(B.atrophaeus)。
[1]YE S H,JIA H Y,CAI G F,TIAN C M,MA R.Morphology,DNA phylogeny,and pathogenicity of Wilsonomyces carpophilus isolate causing shot-hole disease of Prunus divaricata and Prunus armeniaca in wild- fruit forest of western Tianshan Mountains,China[J].Forests,2020,11(3):319.
[2]林培钧.天山伊犁野果林在人类生态和果树起源上的地位[J].农业考古,1993(1):133-137.LIN Peijun.The position of wild fruit forest in Yili,Tianshan Mountain in human ecology and the origin of fruit trees[J].Agricultural Archaeology,1993(1):133-137.
[3]AZMY A,KORRA A.Management of shot-hole disease of stone fruit trees caused by Stigmina carpophila[J].Journal of Plant Protection and Pathology,2010,1(12):973-989.
[4]NABI A S,SHAH M U D,PADDER B A,DAR M S,AHMAD M.Morpho-cultural,pathological and molecular variability in Thyrostroma carpophilum causing shot hole of stone fruits in India[J].European Journal of Plant Pathology,2018,151(3):613-627.
[5]钱超,季红健,陈加红.桃穿孔病的田间防治试验[J].山西果树,2017(6):3-4.QIAN Chao,JI Hongjian,CHEN Jiahong.Field control test of peach perforation disease[J].Shanxi Fruits,2017(6):3-4.
[6]王召元,李永红,常瑞丰,陈湖,韩继成,刘国俭.桃穿孔病的发生规律与综合防治措施[J].河北果树,2018(6):29-30.WANG Zhaoyuan,LI Yonghong,CHANG Ruifeng,CHEN Hu,HAN Jicheng,LIU Guojian.Occurrence regularity and comprehensive control measures of peach perforation[J].Hebei Fruits,2018(6):29-30.
[7]赵俊芳,常聚普,乔趁峰,吉洪坤,杨玉巧,景向方.几种药剂防治杏李穿孔病田间药效对比试验[J].北方园艺,2010(18):178-179.ZHAO Junfang,CHANG Jupu,QIAO Chenfeng,JI Hongkun,YANG Yuqiao,JING Xiangfang.Comparative experiment on field efficacy of several chemicals to control apricot and plum perforation[J].Northern Horticulture,2010(18):178-179.
[8]KARLIDAG H,ESITKEN A,ERCISLI S,DONMEZ M F.The use of PGPR (plant growth promoting rhizobacteria) in organic apricot production[J].Acta Horticulturae,2010,862:309-312.
[9]姜彩鸽,杨小伟,张怡,王国珍,王广录,方治永.12 种杀菌剂对葡萄灰霉病菌的室内毒力测定[J].宁夏农林科技,2017,58(8):33-35.JIANG Caige,YANG Xiaowei,ZHANG Yi,WANG Guozhen,WANG Guanglu,FANG Zhiyong.Indoor toxicity of 12 fungicides against grape Botrytis cinerea Pers.[J].Ningxia Journal of Agriculture and Forestry Science and Technology,2017,58(8):33-35.
[10]陈宏州,杨敬辉,肖婷,吴祥,吉沐祥,庄义庆.12 种杀菌剂对葡萄灰霉病菌的毒力测定[J].江苏农业科学,2015,43(1):124-127.CHEN Hongzhou,YANG Jinghui,XIAO Ting,WU Xiang,JI Muxiang,ZHUANG Yiqing.Toxicity determination of 12 fungicides to Botrytis cinerea[J].Jiangsu Agricultural Sciences,2015,43(1):124-127.
[11]孙蕾.吉林省葫芦科作物炭疽病的病原鉴定及药剂筛选[D].长春:吉林农业大学,2019.SUN Lei.Pathogen identification and screening of the fungicides of Cucurbitaceae crop anthracnose caused by Colletotrichum in Jinlin Province[D].Changchun:Jilin Agricultural University,2019.
[12]朱杰,程亮,张纲,陈红雨,付丽颖,姚强,郭青云.樱桃叶斑病生防菌株萎缩芽孢杆菌菌株QH-588 的筛选鉴定[J].南方农业学报,2021,52(11):3022-3033.ZHU Jie,CHENG Liang,ZHANG Gang,CHEN Hongyu,FU Liying,YAO Qiang,GUO Qingyun.Screening and identification of Bacillus atrophaeus QH-588 for biological control of cherry leaf spot[J].Journal of Southern Agriculture,2021,52(11):3022-3033.
[13]王敏,赵颖,蔡桂芳,王雪萍,张志东,马荣.野核桃枝枯病菌拮抗细菌的筛选及鉴定[J].新疆农业大学学报,2019,42(4):261-266.WANG Min,ZHAO Ying,CAI Guifang,WANG Xueping,ZHANG Zhidong,MA Rong.Screening and identification of the antagonistic bacteria against wild walnut branch rot[J].Journal of Xinjiang Agricultural University,2019,42(4):261-266.
[14]周俊辉,游春平.香蕉枯萎病菌4 号小种拮抗细菌的筛选与鉴定[J].果树学报,2011,28(2):278-283.ZHOU Junhui,YOU Chunping.Screening and identification of antagonistic bacteria against Fusarium oxysporum f.sp. cubense race 4[J].Journal of Fruit Science,2011,28(2):278-283.
[15]程元,淮稳霞,姚艳霞,林若竹,刘忠军,赵文霞.新疆巩留县杏果实斑点病病原菌鉴定[J].林业科学研究,2019,32(2):117-122.CHENG Yuan,HUAI Wenxia,YAO Yanxia,LIN Ruozhu,LIU Zhongjun,ZHAO Wenxia.The pathogen identification of apricot fruit spots disease in Gongliu County,Xinjiang[J].Forest Research,2019,32(2):117-122.
[16]叶双华.伊犁野果林中Juglanconis 和Wilsonomyces 属引起的病害病原菌种类研究[D].乌鲁木齐:新疆农业大学,2020.YE Shuanghua.Identification of Juglanconis and Wilsonomyces pathogens species in Ili wild-fruit forest[D].Urumqi:Xinjiang Agricultural University,2020.
[17]刘秀花,梁峰,刘茵,翟兴礼.河南省土壤中芽孢杆菌属资源调查[J].河南农业科学,2006,35(8):67-71.LIU Xiuhua,LIANG Feng,LIU Yin,ZHAI Xingli.Genus Bacillus resources of soils in Henan Province[J].Journal of Henan Agricultural Sciences,2006,35(8):67-71.
[18]刘欣,马强,宿静瑶,付崇毅,杜美娥,李正男,孙平平.苹果树腐烂病拮抗菌株的分离、筛选和鉴定[J].中国果树,2022(5):50-56.LIU Xin,MA Qiang,SU Jingyao,FU Chongyi,DU Meie,LI Zhengnan,SUN Pingping.Isolation,screening and identification of antagonistic strains against apple valsa canker[J].China Fruits,2022(5):50-56.
[19]王璐.萎缩芽孢杆菌CP 297 和解淀粉芽孢杆菌CP 2014 对毛桃致腐霉菌的抑菌机理及保鲜应用[D].太谷:山西农业大学,2019.WANG Lu.Bacteriostatic mechanism and preservation application of Bacillus atrophaeus CP 297 and Bacillus amyloliquefaciens CP 2014 against pathogenic mold isolated from peach[D].Taigu:Shanxi Agricultural University,2019.
[20]付莉媛.北京地区草莓根腐病致病菌的分离鉴定及拮抗菌筛选[D].秦皇岛:河北科技师范学院,2021.FU Liyuan.Isolation and identification of the pathogen of strawberry root-rot disease in Beijing and screening of its antagonistic bacteria[D].Qinhuangdao:Hebei Normal University of Science&Technology,2021.
[21]敖静,李杨,刘晓辉,高晓梅,孙玉禄.一株黄瓜枯萎病拮抗细菌的筛选鉴定及抑菌作用初探[J].云南农业大学学报(自然科学),2022,37(3):429-434.AO Jing,LI Yang,LIU Xiaohui,GAO Xiaomei,SUN Yulu.Screening and identification of antagonistic bacteria against Fusarium wilt of cucumber and preliminary study on its antifungal effect[J].Journal of Yunnan Agricultural University(Natural Science),2022,37(3):429-434.
[22]郝芳敏,臧全宇,马二磊,丁伟红,王毓洪,黄芸萍.甜瓜多种真菌病害拮抗细菌NBmelon-1 的鉴定及其促生和生防效果[J].中国瓜菜,2021,34(7):14-19.HAO Fangmin,ZANG Quanyu,MA Erlei,DING Weihong,WANG Yuhong,HUANG Yunping.Identification,biocontrol and growth promoting effects of antagonistic bacteria NBmelon-1 of various fungal diseases in melon[J].China Cucurbits and Vegetables,2021,34(7):14-19.
[23]李娜,李晶,付麟雲,刘锦霞,丁品,武建荣.1 株优良生防细菌的筛选及其对甘肃温室黄瓜枯萎病的防治效果[J].中国瓜菜,2022,35(1):86-90.LI Na,LI Jing,FU Linyun,LIU Jinxia,DING Pin,WU Jianrong.Screening and identification of an excellent biocontrol bacteria against cucumber Fusarium wilt in heliogreenhouse of Gansu and its biological effect[J].China Cucurbits and Vegetables,2022,35(1):86-90.
[24]豆雅楠.苹果树腐烂病病原菌的分离鉴定及其拮抗芽孢杆菌的筛选鉴定[D].兰州:西北师范大学,2018.DOU Ya’nan.Isolation and identification of pathogens of apple Valsa canker and screening of antagonistic Bacillus to the pathogens[D].Lanzhou:Northwest Normal University,2018.