柑橘溃疡病是一种由柑橘黄单胞杆菌(Xanthomonas citri subsp.citri,Xcc)引起的世界性检疫病害,可侵染柑橘叶片、枝梢和果实,对柑橘产业危害严重[1]。柑橘不同组织受溃疡病菌侵染后,在表层形成典型的火山口状病斑,严重时导致落叶落果[2]。而pthA4 被鉴定为在Xcc 侵染过程中发挥主要作用的致病效应子,当pthA4从Xcc中被敲除后无法引起火山口状病斑的形成[3]。植物的器官脱落是生命周期不同阶段的一个关键过程,是植物界广泛存在的一种生理现象,应确保植物适时脱落不需要的器官,如叶、花、果实等[4]。它也是一个重要的农艺性状,对一些作物的产量、质量和采后贮藏有重大影响[5-6]。
植物通过脱落器官来适应外界环境,器官的正常脱落与植物的生长发育息息相关,这对植物自身有利,如衰老、果实或种子成熟、受精等[7-8]。病原菌入侵或非生物胁迫等环境因素也可能引发器官脱落[4, 9-10]。许多研究已经证明,植物叶片受到病原菌侵染时过早脱落,从而导致产量下降、经济效益降低。例如,苹果高度感染灰霉病菌或冠状马松氏菌会导致早期叶片脱落[11-12],柑橘分枝杆菌引起的油斑病导致叶片脱落[13],樱桃感染环状尾孢菌也会出现严重的早期叶片脱落现象[14]。在蓝莓中,叶斑病会导致植株在夏秋季叶片脱落过早发生,从而降低次年的产量[15]。叶斑病通过减少光合作用的叶面积以及降低叶的光合作用能力来降低产量[16-17]。上述研究均对由病害引起的叶片过早脱落对产量的影响进行了讨论。柑橘类果树出现这种不正常脱落的危害极大,会使植株损失大量有机养分,从而导致枝梢枯萎、树势衰弱以及落花落果等,给柑橘产业带来了巨大的经济损失。
器官脱落发生在离区(abscission zone,AZ),是一个细胞形态上不同的区域,通常位于植物器官基部的固定位置,由数层较小、密集排列、细胞质紧密的细胞组成[4, 18]。离区通常在发育的早期就已经形成,常呈现为带状,处于静止状态。离区的形成是脱落过程的第一步,但离区形成不一定启动脱落,只有通过进一步的分化成熟才具备脱落的能力。在正常情况下,离区会将器官与植物母体牢固结合,当感受到发育信号、环境变化或胁迫时,离区细胞会响应这些信号,从而触发脱落[4]。一旦触发脱落,离区细胞膨大,各种细胞壁水解酶降解中间层(果胶层),从而使细胞分离,发生脱落。脱落后,脱落的“疤痕”上会形成一层新的保护性表皮层,以保护残留在植物上的器官免受病原菌感染和水分流失[4,7,19]。
植物器官的脱落是由发育信号和多种环境刺激诱导的,涉及多个调控网络,是一个高度协调、受到精确调控的事件,涉及细胞组织结构改变、代谢变化和基因表达变化等多种过程[20-21]。以往有关器官脱落的研究主要集中在花器官、花梗和种子脱落的分子机制上[20, 22-23],对叶和叶柄脱落的研究较少,有关柑橘溃疡病引起柑橘落叶的机制尚不清楚,其他关于病害引起叶片脱落机制的研究也较少。因此笔者在本研究中以易感柑橘溃疡病的冰糖橙叶片为材料,通过采用不同的活体接种方式和活体接种不同浓度的病原菌,观察溃疡病菌引起叶片脱落的时间,筛选促进冰糖橙叶片脱落的溃疡病菌接种方式以及接种浓度。通过树脂切片观察叶片脱落过程中离区组织结构的动态变化,探索溃疡病菌诱导冰糖橙叶片脱落的特征和规律,为进一步深入研究柑橘溃疡病引起的柑橘落叶落果现象提供研究思路。
1 材料和方法
1.1 植物材料
笔者在本研究中所用植物材料为2年生枳砧冰糖橙无病毒容器苗,所有植物材料均放置于湖南农业大学国家柑橘改良中心长沙分中心植物培养室中培养,培养室条件为温度(30±1)℃、湿度80%~90%、光周期为12 h 光照-12 h 黑暗。植物材料接种病原菌后放置于湖南农业大学国家柑橘改良中心长沙分中心植物病理温室中培养,培养室条件为温度(30±1)℃、湿度80%~90%、光周期为12 h 光照-12 h 黑暗。
1.2 细菌菌株与活体接种
笔者在本研究中使用的细菌菌株为柑橘溃疡病菌DL-509菌株(以下简称Xcc)、Xcc 049A(主要致病效应子pthA4缺失的Xcc菌株)以及水稻白叶枯病菌(X.oryzae pv. oryzae,Xoo);Xcc 由国家柑橘改良中心长沙分中心分离和保存,Xcc 049A 和Xoo 分别由上海交通大学陈功友教授团队和湖南农业大学植物保护学院戴良英教授团队惠赠。
将冻存于-80 ℃的Xcc、Xcc 049A、Xoo 菌株于固体LB 培养基上活化,28 ℃恒温培养48 h。随后挑取单菌落至5 mL 液体LB 培养基中,在28 ℃、220 r·min-1条件下培养18 h,取1 mL 菌液至40 mL液体LB 培养基中在28 ℃、220 r·min-1条件下培养12 h,4 ℃、7500 r·min-1离心5 min,去上清液后加入10 mmol·L-1 MgCl2溶液重悬,使重悬菌液OD600为0.60±0.02,此时菌液浓度为109 CFU·mL-1,随后进行梯度浓度稀释,依次获得浓度为103~109 CFU·mL-1的Xcc、Xcc 049A 以及108 CFU·mL-1的Xoo 菌液,103、104 CFU·mL-1的菌液用于菌落计数,106~109 CFU·mL-1菌液用于注射和浸泡接种冰糖橙叶片,各15 个重复。选用冰糖橙植株新梢上达到最大叶面积但未完全转为深绿的叶片进行活体注射和浸泡接种病原菌。每天拍照记录接种不同病原菌的冰糖橙叶片出现的症状及变化,观察叶片脱落的发生部位及时间,并统计落叶率。
注射接种:使用1 mL无菌注射器将稀释好的菌液缓慢注射进叶片背部(翼叶除外)至均匀充满叶片,注射接种完成后使用无菌脱脂棉擦干叶片表面残留的菌液。
浸泡接种:向稀释好的重悬菌液中加入1/5000体积的表面活性剂Sil-wet L-77,振荡混匀,将冰糖橙叶片(翼叶除外)置于菌液中浸泡5 s,随后取出叶片自然风干。
1.3 树脂切片
冰糖橙叶片注射接种108 CFU·mL-1的Xcc重悬液,2、4 dpi(days post inoculation)对叶片离区取样固定(图1)。使用飞鹰牌剃须刀片快速切下叶片离区组织,立即放入FAA 固定液中,使用JOANLAB VP-30L 无油隔膜真空泵抽真空15 min,缓慢放气,更换新的FAA 固定液后置于4 ℃条件下固定1~2 d用于后续试验。参照周乃富等[23]报道的方法进行离区样品软化,全程注意密封避免试剂挥发。在室温条件下,使用梯度体积分数的乙醇进行脱水,70%乙醇处理1 h,85%乙醇处理1 h,95%乙醇处理1.5 h,无水乙醇处理1 h,更换新的无水乙醇处理1 h。脱水完成后使用树脂(KULZER Technovit 7100)进行包埋,待树脂固化后置于65 ℃恒温处理24 h,随后使用超薄切片机(Leica EM UC6)进行超薄组织切片,采用组织纵向切片,切片示意图如图1-A~B 所示。切片厚度设置为5 μm,切片置于0.1%甲苯胺蓝染液中展片,在65 ℃恒温条件下烘片10 min,烘片完成后使用Zeiss Axio Imagine 2.0 显微镜观察切片。
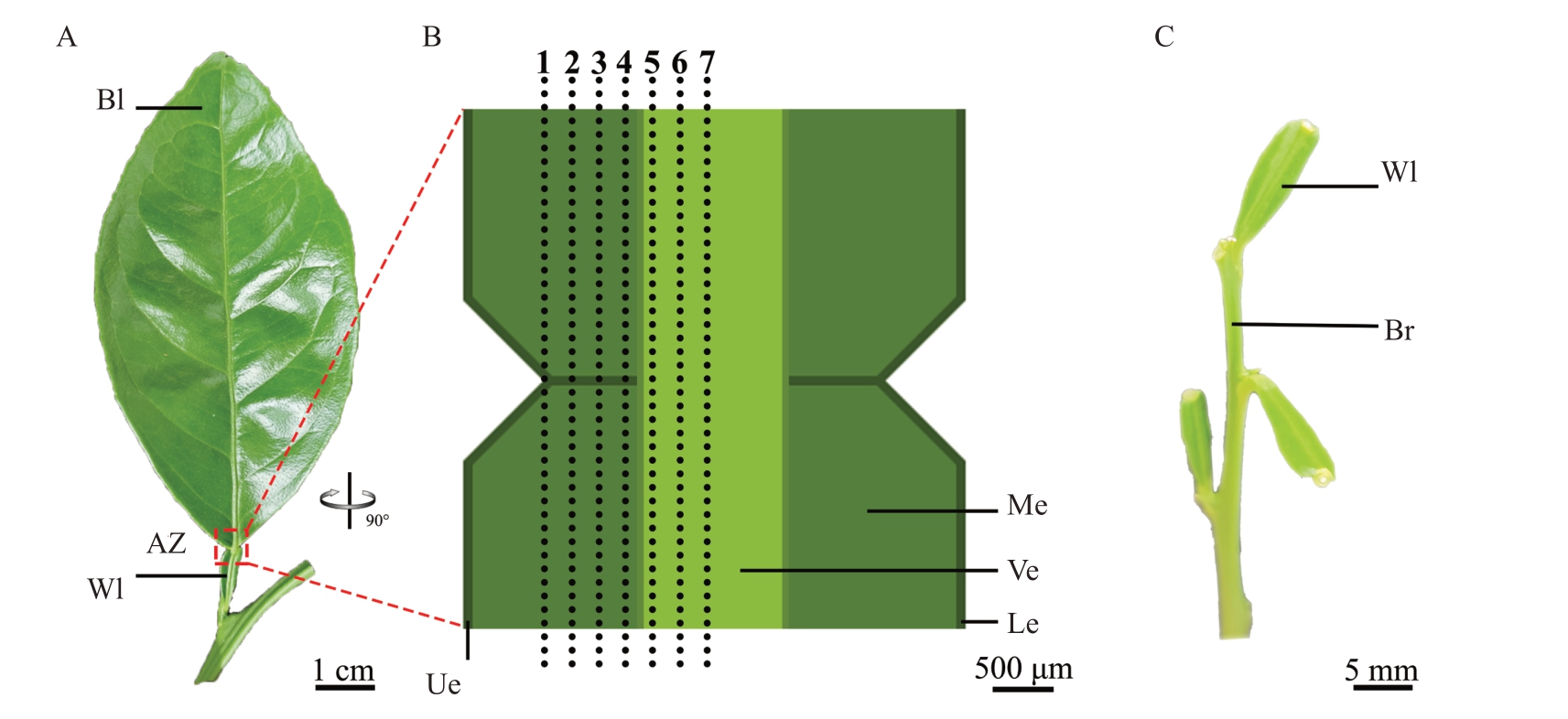
图1 冰糖橙叶片脱落及离区切片示意图
Fig.1 Schematic diagram of leaf abscission and slices of abscission zone in Bingtang sweet orange
A.冰糖橙叶片;B.离区AZ 的切片示意图,黑色虚线展示离区AZ 的纵切片位置,1~7 依次为由外向内的纵切片(#1~#7),#1 为最外层切片,#7为中间位置切片;C.冰糖橙叶片接种病原菌脱落后的翼叶和枝条;AZ.离区;Bl.本叶;Wl.翼叶;Ue.上表皮;Le.下表皮;Me.叶肉;Ve.叶脉;Br.枝条。
A.Leaf of Bingtang sweet orange;B.The schematic diagram of the slices in the AZ,with a black dashed line showing the position of the longitudinal slices in the AZ.Sections 1 to 7 are the outward inward longitudinal slices(#1 to#7),with#1 being the outermost slice and#7 being the middle slice; C.The wing leaf and branch of Bingtang sweet orange after shedding induced by inoculating with pathogenic bacteria;AZ.Abscission zone;Bl.Blade;Wl.Wing leaf;Ue.Upper epidermis;Le.Lower epidermis;Me.Mesophyll;Ve.Vein;Br.Branch.
1.4 离区细胞计数及单个细胞平均面积分析
冰糖橙叶片注射接种108 CFU·mL-1的Xcc,在2、4 dpi 叶片离区的纵向组织切片中,取由外向内的切片#4(图1-B 切片示意图#4)进行单位面积(28 476.56 μm2)离区细胞计数及单个细胞平均面积分析。细胞计数使用Image J软件,显著性分析使用Excel 2016 软件,绘图使用Chiplot Online(https://www.chiplot.online/)。
2 结果与分析
2.1 不同浓度Xcc诱导冰糖橙叶片脱落差异分析
为了筛选适宜的促进冰糖橙叶片脱落的Xcc接种浓度,使用106~108 CFU·mL-1的Xcc 注射接种冰糖橙叶片,置于植物病理温室中观察叶片感病症状的特征并统计分析落叶率。结果显示,注射接种108 CFU·mL-1 Xcc 后,3 dpi 本叶背面出现水渍状突起病斑,4 dpi本叶开始脱落,落叶率为31%,6 dpi本叶全部脱落,水渍状病斑完全覆盖叶片背面,未形成溃疡病典型的火山口状突起病斑(图2-A、D),离区AZ为本叶脱落部位,翼叶表面未观察到明显症状且未发生脱落(图1-A、C);注射接种107 CFU·mL-1 Xcc后,3 dpi 本叶背面的水渍状突起病斑较注射接种108 CFU·mL-1 Xcc减少,6 dpi叶片开始脱落,落叶率为26%,8 dpi叶片全部脱落(图2-A~B,D),本叶脱落部位与注射接种108 CFU·mL-1 Xcc相同,翼叶表面未观察到明显症状且未发生脱落(图1-A、C);注射接种106 CFU·mL-1 Xcc后,4 dpi本叶背面出现水渍状突起病斑,病斑较注射接种108 CFU·mL-1和107 CFU·mL-1 Xcc 减少,9 dpi 叶片开始脱落,落叶率为24%(图2-A~D),本叶脱落部位与注射接种108 CFU·mL-1 Xcc相同,翼叶表面未观察到明显症状且未发生脱落(图1-A、C)。冰糖橙叶片注射接种108CFU·mL-1的Xcc导致叶片背面水渍状病斑增多,促进叶片脱落,即随着Xcc浓度增加,叶片脱落的时间缩短。

图2 不同浓度Xcc 诱导冰糖橙叶片脱落的差异分析
Fig.2 Differential analysis of leaf abscission induced by different concentrations of Xcc in Bingtang sweet orange
A.注射接种108 CFU·mL-1 Xcc 的冰糖橙叶片症状;B.注射接种107 CFU·mL-1 Xcc 的冰糖橙叶片症状;C.注射接种106 CFU·mL-1 Xcc 的冰糖橙叶片症状;D.不同浓度Xcc 诱导冰糖橙落叶的落叶率。dpi.接种后天数。下同。
A.Symptoms of leaves by injecting of 108 CFU·mL-1 Xcc in Bingtang sweet orange;B.Symptoms of leaves by injecting of 107 CFU·mL-1 Xcc in Bingtang sweet orange; C.Symptoms of leaves by injecting of 106 CFU·mL-1 Xcc in Bingtang sweet orange; D.Leaf abscission rate of Bingtang sweet orange induced by different concentrations of Xcc.dpi.Days post inoculation.The same below.
2.2 不同病原菌诱导冰糖橙叶片脱落差异分析
野生型Xcc 的致病效应子pthA4 是诱导柑橘叶片形成典型溃疡病症状的主要调节因子[25],但pthA4在冰糖橙叶片受Xcc 诱导落叶中的功能尚不明确。Xcc 049A 为pthA4 缺失的Xcc 菌株,Xoo 与Xcc 同属于黄单胞杆菌属,是引起水稻白叶枯病的水稻致病变种,主要危害水稻,但其不危害柑橘,在柑橘叶片和果实上均不表现症状。注射接种108 CFU·mL-1 Xcc显著促进冰糖橙叶片脱落,因此使用108 CFU·mL-1的Xcc、Xcc 049A、Xoo菌液侵染冰糖橙叶片,置于植物病理温室中观察叶片出现症状的特征并统计分析落叶率。结果显示,注射接种Xcc后,3 dpi本叶背面出现水渍状突起病斑,4 dpi 本叶开始脱落,落叶率为31%,6 dpi 本叶全部脱落,水渍状病斑完全覆盖本叶背面(图3-A,D),离区AZ 为本叶脱落部位,翼叶表面未观察到明显症状且未发生脱落(图1-A,C);注射接种Xcc 049A 后,3 dpi 本叶背面叶脉呈水渍状,叶片未出现水渍状突起病斑,6 dpi本叶脱落,落叶率为36%,时间较注射接种相同浓度的Xcc 延迟2 d(图3-A~B,D),本叶脱落与注射接种Xcc的叶片相同,翼叶未观察到明显症状且未发生脱落(图1-A,C);而注射接种Xoo 后,叶片表面未观察到明显症状且未发生脱落(图3-C~D),表明注射接种Xcc引起的叶片脱落中,致病效应子pthA4 显著促进冰糖橙叶片脱落,可能还有其他因素影响Xcc 诱导叶片脱落的发生。
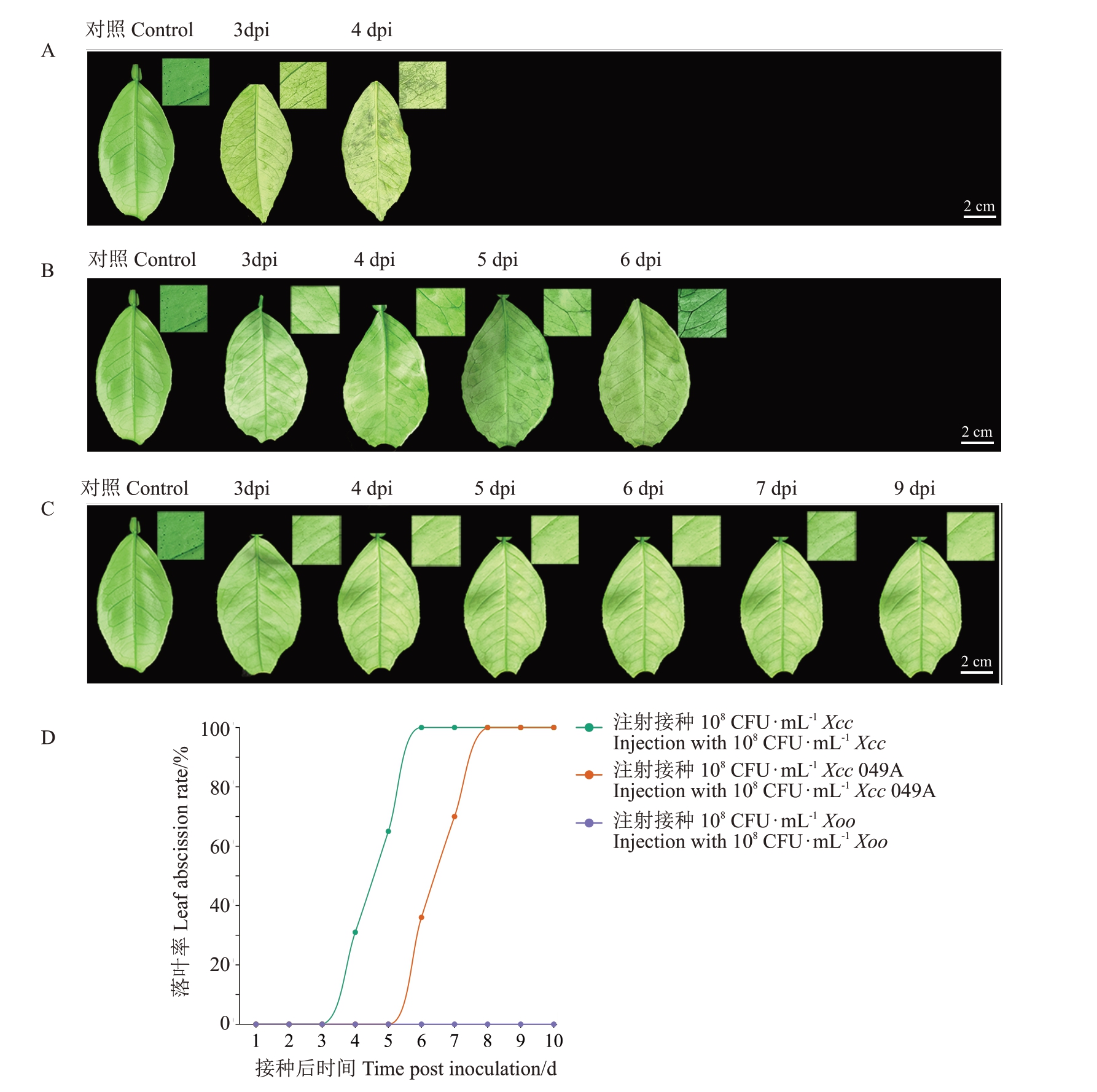
图3 不同病原菌诱导冰糖橙叶片脱落的差异分析
Fig.3 Differential analysis of leaf abscission induced by different pathogens in Bingtang sweet orange
A.注射接种108 CFU·mL-1 Xcc 的冰糖橙叶片症状;B.注射接种108 CFU·mL-1 Xcc 049A 的冰糖橙叶片症状;C.注射接种108 CFU·mL-1 Xoo 的冰糖橙叶片症状;D.不同病原菌诱导冰糖橙落叶的落叶率。
A.Symptoms of leaves by injecting of 108 CFU·mL-1 Xcc in Bingtang sweet orange; B.Symptoms of leaves by injecting of 108 CFU·mL-1 Xcc 049A in Bingtang sweet orange;C.Symptoms of leaves by injecting of 108 CFU·mL-1 Xoo in Bingtang sweet orange;D.Leaf abscission rate induced by different pathogens in leaves of Bingtang sweet orange.
2.3 不同接种方式诱导冰糖橙叶片脱落差异分析
不同的溃疡病菌接种量引起的冰糖橙叶片感病症状和叶片脱落时间不同,病原菌浓度越高,病斑越多,显著促进叶片脱落。为了探明不同的Xcc 接种方式是否影响冰糖橙叶片出现的症感病状以及冰糖橙叶片受Xcc 诱导的落叶率,使用108 CFU·mL-1的Xcc分别采用注射和浸泡两种不同方式活体接种冰糖橙叶片,置于植物病理温室中观察叶片出现症状的特征并统计分析落叶率。结果显示,注射接种108 CFU·mL-1 Xcc 后,3 dpi 本叶背面出现水渍状突起病斑,4 dpi 本叶开始脱落,落叶率为31%,6 dpi本叶全部脱落,水渍状病斑完全覆盖本叶背面(图4-A,D),离区AZ 为本叶脱落部位,翼叶表面未观察到明显症状且未发生脱落(图1-A,C);浸泡接种108 CFU·mL-1 Xcc 后,8 dpi 本叶背面出现愈伤状突起病斑,14 dpi病斑完全覆盖整个叶片背面,叶片卷曲,15 dpi未观察到本叶或翼叶脱落(图4-B,D);为了探索浸泡接种Xcc 的方式能否诱导冰糖橙叶片脱落,将Xcc 菌液浓度增高至109 CFU·mL-1 Xcc 后,3 dpi 本叶背面出现愈伤状突起病斑,8 dpi 病斑完全覆盖整个本叶背面,叶片卷曲,15 dpi 仍未观察到本叶或翼叶脱落(图4-B,D)。以上结果表明,不同的病原菌接种方式导致叶片发病症状产生差异,注射接种较浸泡接种显著促进冰糖橙叶片脱落。因此使用注射接种108 CFU·mL-1 Xcc促进冰糖橙叶片脱落。
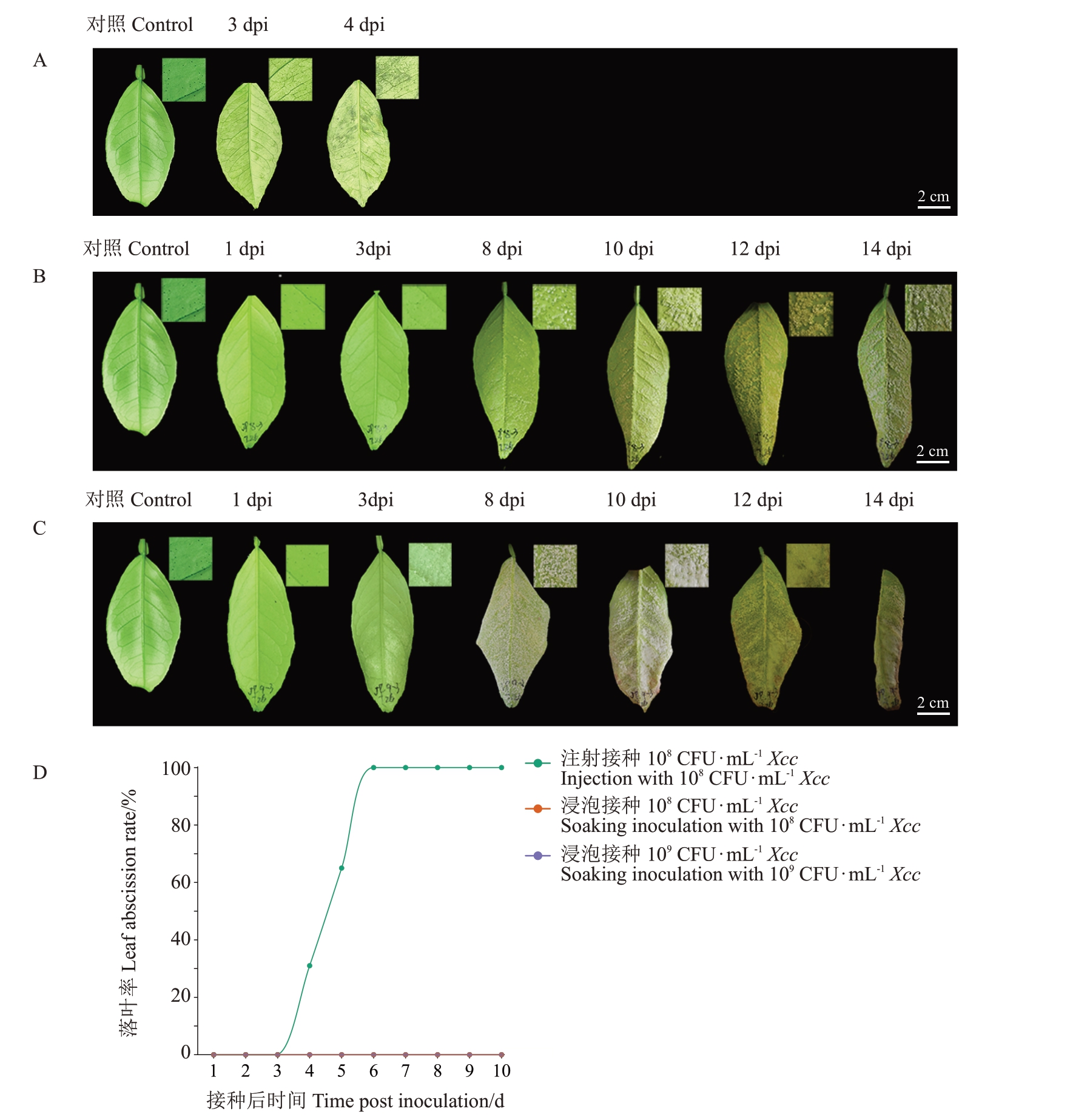
图4 Xcc 不同接种方式导致的冰糖橙叶片脱落差异分析
Fig.4 Differential analysis of leaf abscission induced by different inoculation methods of Xcc in Bingtang sweet orange
A.注射接种108 CFU·mL-1 Xcc 的冰糖橙叶片症状;B.浸泡接种108 CFU·mL-1 Xcc 的冰糖橙叶片症状;C.浸泡接种109CFU·mL-1 Xcc 的冰糖橙叶片症状;D.Xcc 不同接种方式下冰糖橙叶片的落叶率。
A.Symptoms of leaves by injecting of 108 CFU·mL-1 Xcc in Bingtang sweet orange;B.Symptoms of leaves by soaking inoculation of 108 CFU·mL-1 Xcc in Bingtang sweet orange;C.Symptoms of leaves by soaking inoculation of 109 CFU·mL-1 Xcc in Bingtang sweet orange;D.Leaf abscission rate of Bingtang sweet orang under different inoculation methods of Xcc.
2.4 Xcc诱导冰糖橙叶片脱落过程中离区组织结构变化
在器官脱落过程中,离区响应脱落信号分化形成离层和保护层,细胞分离最终导致器官脱落[3]。为了观察Xcc 诱导冰糖橙叶片脱落过程中离区组织结构的动态变化过程,在冰糖橙叶片注射接种108 CFU·mL-1 Xcc后,0、2、4 dpi对叶片离区AZ取样固定,经树脂切片后在显微镜下观察离区组织结构的变化特征。结果显示,在正常冰糖橙叶片离区AZ的纵向切片中,切片#1中观察到本叶与翼叶的表皮细胞之间贴近但未发生连接,切片#2~#7 中观察到离区已经形成,为细胞质紧密、排列密集的小细胞群(图5);2 dpi 离区AZ 组织结构较对照无明显变化(图6~图8);在4 dpi离区AZ中,切片#1中观察到离区组织结构与对照相似,切片#2~#4 中观察到离区细胞分离,离层和保护层形成,切片#5~#7中观察到离区细胞分层,排列整齐,细胞未分离(图6~图8),即离区AZ在离层和保护层衔接处从外周向中轴逐渐分离形成两层,分化形成成熟的离层和保护层,最终导致本叶脱落。以上结果表明,Xcc 诱导冰糖橙叶片离区AZ中细胞分离,导致本叶脱落。

图5 冰糖橙叶片离区的切片观察
Fig.5 Observation on the abscission zone of leaves in Bingtang sweet orange by slicing
正常冰糖橙叶片离区AZ 的纵向切片图;#1~#7 为图1 中所展示的AZ 由外向内的纵切片图,a~g 为切片#1~#7 中红色虚线框内离区的放大图。
Longitudinal slice of AZ in the abscission zone of normal leaves in Bingtang sweet orange, #1-#7 are the vertical slices of AZ shown in Figure 1 from the outside to the inside,and a-g are enlarged views of the red dashed boxes in slices#1-#7.

图6 Xcc 诱导冰糖橙叶片脱落离区的切片观察(切片#1)
Fig.6 Observation of Xcc-induced abscission zone in leaves of Bingtang sweet orange by slicing(slices#1)
冰糖橙叶片注射接种108 CFU·mL-1 Xcc 后0、2、4 dpi 离区AZ 的组织纵向切片中切片#1 的比较;a~c 为红色虚线框内离区的放大图。
Comparison of section#1 in the longitudinal sections of AZ tissues at 0,2,and 4 dpi after injection of 108 CFU·mL-1 Xcc into the leaves of Bingtang sweet orange;a-c are enlarged views of the offset area within the red dashed box.

图7 Xcc 诱导冰糖橙叶片脱落离区的切片观察(切片#2、#4)
Fig.7 Observation of Xcc-induced abscission zone in leaves of Bingtang sweet orange by slicing(slices#2,#4)
冰糖橙叶片注射接种108 CFU·mL-1 Xcc 后0、2、4 dpi 离区AZ 的纵向组织切片中切片#2、#4 的比较;A 为切片#2 的比较,B 为切片#4 的比较;a~f 为红色虚线框内离区的放大图。
Comparison of section #2, #4 in the longitudinal sections of AZ tissues at 0, 2, and 4 dpi after injection of 108 CFU·mL-1 Xcc into the leaves of Bingtang sweet orange;A is the comparison of slice # 2, B is the comparison of slice # 4; a-f are enlarged views of the offset area within the red dashed box.

图8 Xcc 诱导冰糖橙叶片脱落离区的切片观察(切片#5、#7)
Fig.8 Observation of Xcc-induced abscission zone in leaves of Bingtang sweet orange by slicing(slices#5,#7)
冰糖橙叶片注射接种108 CFU·mL-1 Xcc 后0、2、4 dpi 离区AZ 的纵向组织切片中切片#5、#7 的比较,A 为切片#5 的比较,B 为切片#7 的比较;a~f 为红色虚线框内离区的放大图。
Comparison of section #5, #7 in the longitudinal sections of AZ tissues at 0, 2, and 4 dpi after injection of 108 CFU·mL-1 Xcc into the leaves of Bingtang sweet orange;A is the comparison of slice#5,B is the comparison of slice#7;a-f are enlarged views of the offset area within the red dashed box.
2.5 离区细胞计数及单个细胞平均面积分析
随着器官脱落的发生,离区细胞会发生膨大,这是脱落的主要驱动力[26-28]。为了探明Xcc 诱导冰糖橙叶片脱落过程中离区细胞是否膨大,在冰糖橙叶片注射接种108 CFU·mL-1 Xcc 后,对叶片离区0、2、4 dpi的组织纵切片的切片#4中的离区细胞进行单位面积(28 476.56 μm2)细胞计数以及单个细胞平均面积分析。分析结果显示,注射接种108 CFU·mL-1 Xcc后,2、4 dpi 离区细胞数量较对照减少,单个细胞平均面积较对照增大(图9)。表明Xcc 诱导冰糖橙叶片离区细胞膨大,导致本叶脱落。
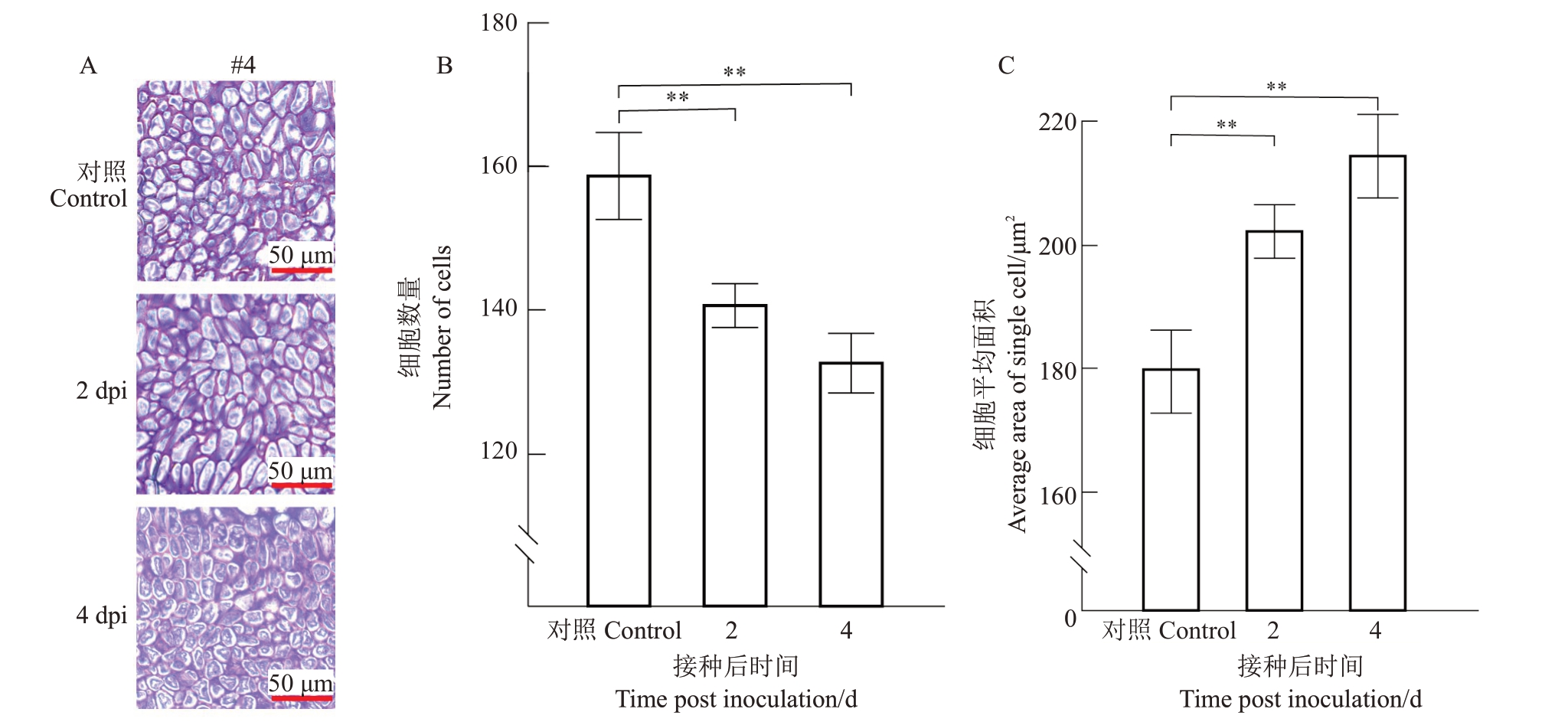
图9 冰糖橙叶片接种Xcc 后单位面积离区细胞数量和单个细胞平均面积分析
Fig.9 Analysis of the number of cells per unit area and average area of individual cells in the abscission zone of the leaves in Bingtang sweet orange after inoculation with Xcc
A.注射接种108 CFU·mL-1 Xcc 后0、2、4 dpi 离区AZ 树脂切片的切片#4 中,取单位面积离区进行细胞计数和单个细胞平均面积分析;B.细胞数量;C.单个细胞平均面积。**表示在p<0.01 水平差异极显著(T 检验)。
A.After injection with 108 CFU·mL-1 Xcc,in section#4 of resin sections in AZ at 0,2,and 4 dpi,taking unit area to count number of cells and calculate the average area of individual cell;B.Number of cells;C.Average area of individual cells.**represents extremely significant different at p<0.01 using T-test.
3 讨 论
植物的器官脱落是生命周期不同阶段的一个关键过程,是植物界广泛存在的一种生理现象,确保植物适时脱落不需要的器官如叶、花、果实等[4]。柑橘溃疡病可侵染柑橘叶片、枝梢和果实,严重时导致落叶落果,对柑橘产业危害严重。病原菌侵染导致的叶片脱落通常发生在感染后期,而在落叶中存活的病原菌则可能引起下一轮的侵染。Patharkar等[10]的研究表明,拟南芥茎生叶受假单胞菌侵染后叶片脱落,这个过程与宿主的防御信号有关。Teper等[29]的研究表明,金橘接种Xcc后,免疫反应增强,例如PR基因的上调、水杨酸的积累、过敏反应、细胞死亡和早期叶片脱落,有助于金橘抵抗Xcc。所以叶片脱落可能是植物应对病原体侵染的一种主动防御机制,在其他免疫反应不能抑制病原体定殖时,通过脱落受感染的叶片,减少植物体内的病原菌,防止病原菌传播到健康部位[10,29]。结果显示,Xcc能诱导冰糖橙叶片脱落,注射接种108 CFU·mL-1 Xcc 显著促进叶片脱落,随着Xcc 浓度的降低,Xcc 诱导冰糖橙叶片脱落的程度降低。笔者在本试验中使用不同的细菌菌株以及不同接种方式接种病原菌至叶片内,结果表明,注射接种Xcc诱导了冰糖橙叶片的脱落,且加大Xcc 接种浓度显著促进了柑橘叶片脱落,相较于叶片自然感病后落叶和其他的病原菌接种方式,可明显缩短试验周期,便于研究柑橘溃疡病引起的叶片脱落发生机制。笔者在本研究中仅观察到Xcc侵染冰糖橙叶片后出现早期叶片脱落,尚未探明叶片脱落过程中免疫反应是否增强,如PR 基因的上调、水杨酸的积累、过敏反应以及细胞死亡是否被诱导等,探明Xcc 诱导的柑橘叶片脱落过程中的免疫调控网络具有重要意义。冰糖橙受Xcc侵染导致叶片脱落可能也是叶片响应病原菌的入侵,启动自身的防御机制,从而保护未受病原菌侵染的其他组织和器官。如果叶片脱落是植物主动的防御反应,那么防御增强突变体可能会比野生型植株更易发生叶片脱落[30]。据报道,金橘对Xcc 具有抗性,其对Xcc的免疫反应是由其主要致病效应子pthA4 介导的,Xcc 中pthA4 的突变不会降低金橘叶片中病原菌的生长量,但消除了溃疡病症状、细胞死亡和叶片脱落等免疫反应[29]。结合笔者课题组前期鉴定的柑橘属独特抗柑橘溃疡病种质枸橼C-05 的叶片在接种105 CFU·mL-1 Xcc 后部分叶片在6~8 dpi 脱落,与接种107 CFU·mL-1 Xcc 的冰糖橙叶片脱落时间接近,表明Xcc 诱导的叶片脱落可能与柑橘对Xcc 的防御机制相关,后续需进一步验证在同样的病原菌侵染条件下,柑橘抗病种质是否会更容易发生叶片脱落。除pthA4 外,金橘叶片接种效应子xps 突变的Xcc,表现出免疫反应和叶片脱落的延迟,但病原菌的生长量少于野生型Xcc,所以免疫反应的减少可能是由于xps 的缺失,或xps 突变的Xcc 生长量过少,未达到病原体识别需要的细菌种群阈值[29]。本研究结果表明,与接种108 CFU·mL-1的Xcc相比,冰糖橙叶片接种同浓度的Xcc 049A后,脱落时间显著延迟,说明pthA4的存在能显著促进叶片脱落,其他效应子可能也参与诱导柑橘叶片脱落[31-35]。但笔者尚未对接种Xcc和Xcc 049A后的冰糖橙叶片中病原菌的生长量进行检测,只探讨了pthA4 在诱导冰糖橙叶片脱落过程中的作用,尚未研究其他致病效应子在诱导叶片脱落中的功能。探明溃疡病菌致病效应子在诱导叶片脱落中的作用,可作为未来研究方向。此外,拟南芥叶片上细菌侵染位置决定了叶片脱落的程度,当叶片局部被侵染时,只有部分细胞发生分离[10]。在本研究中,加大病原菌浓度,显著促进Xcc诱导的冰糖橙本叶脱落,但对于Xcc侵染叶片的位置以及局部性是否会影响叶片的脱落和离区的分化尚不清楚,未来可以深入研究。Xcc 含量检测结果表明,沙田柚叶片本叶中注射接种Xcc 后在翼叶中也检测到少量Xcc。本研究表明,Xcc诱导的冰糖橙叶片脱落仅限于本叶脱落,而翼叶不发生脱落,可能是由于Xcc 注射接种都是在冰糖橙本叶,本叶中Xcc含量显著多于翼叶。笔者仅在15 d的试验观察期间记录叶片脱落发生的部位以及统计落叶率,15 dpi 往后翼叶是否发生脱落尚未可知。或者本叶的脱落以及翼叶不发生脱落是由冰糖橙植株本身响应Xcc而启动的防御机制,与Xcc侵染的部位无明显关联,但此方面的内容尚不清楚,未来可进一步研究。有关柑橘溃疡病诱导柑橘果实脱落的机制尚不清楚,但本研究为解释病原菌引起的落叶落果现象提供了新的思路,未来有关柑橘果实脱落的研究应作为重要的方向。
与动物细胞不同,大多数植物细胞都被细胞壁的中间层紧紧地粘在一起,严格阻碍邻近细胞的细微位置变化。离区是器官脱离植物母体的位置,离区细胞在未发育成熟时并不具备脱落的功能,只有在感受到脱落信号后,通过进一步分化,形成离层和保护层,从而启动脱落[4,36]。离区常伴随着器官发育成熟而形成,但只有在感受到特定的脱落信号后,才会启动脱落进程[37]。笔者在本研究中通过对冰糖橙叶片受Xcc诱导脱落过程中离区组织的树脂切片完整展示了离区组织结构的动态变化过程。结果显示,正常的冰糖橙叶片中离区已经存在,为相对较小、细胞质紧密的细胞群,处于静止状态,不具备成熟离区的功能;在Xcc 侵染后,离区AZ 完成了离层与保护层的分化,离区细胞在离层和保护层衔接处从外周向中轴逐渐分离,最终导致叶片脱落。Wilmowicz等[38-39]的研究表明,在脱落的过程中EPIP肽诱导特定的细胞修饰,如细胞分裂反应的高细胞活性、细胞质中的合成颗粒和大量胞间连丝的出现。笔者在本研究中仅使用树脂切片展示了Xcc诱导的冰糖橙叶片脱落过程中离区组织结构的动态变化,未能观察到离区细胞的超微结构,未来可通过透射电子显微镜深入观察离区细胞超微结构的变化。所有的细胞分离事件都可能需要力的作用,就像种子干燥时种子荚壁上的张力一样,脱落的力量可能来自离区细胞的扩张,或者在有果实的情况下可能来自重力[40]。早在20世纪,科学家们就认为离区细胞扩大产生的机械剪切力是脱落的主要驱动力[26]。随着脱落的发生,离区细胞会发生膨大[27-28]。笔者在本研究中发现在Xcc 诱导的冰糖橙叶片脱落过程中,离区细胞数量减少,单个细胞平均面积增大,说明离区细胞在叶片脱落前膨大,这可能为叶片脱落提供了驱动力。Du 等[41]通过分析蒺藜苜蓿叶片脱落过程中叶柄的断裂强度和抖动脱落试验证明了脱落突变体叶片脱落的能力。本研究中,尚未明确Xcc 诱导的冰糖橙脱落过程中离区的断裂强度,未来可进一步研究。在Xcc诱导冰糖橙叶片脱落过程中,叶片脱落仅发生于离区AZ,叶柄处不发生脱落。受Xcc 诱导发生脱落的离区AZ 和叶柄处潜在的离区之间,组织结构的变化是否存在差异,细胞是否膨大等都尚未可知。探明不同离区之间的差异变化,对于更好地理解冰糖橙叶片受Xcc 诱导脱落具有重要意义。Lee 等[42]的研究表明,在病原菌入侵后,拟南芥叶片中木质素积累,形成一种类似凯氏带的物理屏障,空间上限制了病原菌的传播,从而终止其生长。在拟南芥花器官脱落过程中,离区细胞分化形成两种类型的细胞,残留的器官表面形成表皮细胞,起保护作用,随花器官脱落而脱落的离区细胞则产生一种木质素构成的蜂窝状结构,起到机械“支撑”的作用,在空间上将细胞壁的破裂限制于此处。所以木质素的积累可以在空间上限制病原菌的传播[7]。在Xcc诱导的冰糖橙叶片脱落过程中,离区细胞是否也会分化形成两种类型的细胞并积累木质素,一方面确保脱落的精准发生,另一方面防止病原菌的传播,因此叶片脱落仅发生于离区AZ中,但这方面的内容还不清楚,未来可作为重要的研究方向。
在温带气候中,最显著的细胞分离过程是秋天树木的叶片脱落,为过冬做准备[43]。光周期和较冷的温度会在秋季引发叶片脱落[44]。叶片衰老中第一个可见的表型变化是叶片颜色的变化,这是由于叶绿素的优先分解并伴随着叶绿体的分解[44]。在柑橘亚科中,除每年秋季落叶的枳为落叶果树,其余均为常绿果树,笔者在本研究中使用的植物材料冰糖橙叶片生长周期一般为2~3 a(年)。在Xcc 诱导的枳和冰糖橙叶片脱落过程中,叶片在脱落前会逐渐变黄,但在Xcc 诱导柑橘叶片脱落过程中叶色的变化与枳自然落叶叶色变化之间是否存在差异,不同诱因诱导的柑橘叶片脱落的部位是否存在差异,以及离区的组织结构变化是否相同,未来需深入研究。
4 结 论
Xcc的注射接种方式较其他接种方式能够有效诱导冰糖橙叶片脱落,且Xcc接种浓度越高,叶片脱落越快;其次,含有致病效应子pthA4 的Xcc 会加速叶片脱落,叶片离区的组织切片动态观察表明离区细胞会逐步膨大并由外向内分离,最后叶片脱落。
[1]胡天其.柑桔溃疡病的发生与防治的研究综述[J].世界农业,1988(7):30-32.HU Tianqi.Review on the occurrence and control of Citrus canker disease[J].World Agriculture,1988(7):30-32.
[2]STOVER E,DRIGGERS R,RICHARDSON M L,HALL D G,DUAN Y P,LEE R F.Incidence and severity of Asiatic Citrus canker on diverse Citrus and Citrus-related germplasm in a Florida field planting[J].HortScience,2014,49(1):4-9.
[3]HU Y,ZHANG J L,JIA H G,SOSSO D,LI T,FROMMER W B,YANG B,WHITE F F,WANG N,JONES J B.Lateral organ boundaries 1 is a disease susceptibility gene for citrus bacterial canker disease[J].Proceedings of the National Academy of Sciences of the United States of America,2014,111(4):E521-E529.
[4]PATHARKAR O R,WALKER J C.Advances in abscission signaling[J].Journal of Experimental Botany,2018,69(4):733-740.
[5]ZHAO W,BALDWIN E A,BAI J H,PLOTTO A,IREY M.Comparative analysis of the transcriptomes of the calyx abscission zone of sweet orange insights into the huanglongbing-associated fruit abscission[J].Horticulture Research,2019,6:71.
[6]OLSSON V,BUTENKO M A.Abscission in plants[J].Current Biology,2018,28(8):R338-R339.
[7]LEE Y,YOON T H,LEE J,JEON S Y,LEE J H,LEE M K,CHEN H Z,YUN J,OH S Y,WEN X H,CHO H K,MANG H,KWAK J M.A lignin molecular brace controls precision processing of cell walls critical for surface integrity in Arabidopsis[J].Cell,2018,173(6):1468-1480.
[8]CHO S K,LARUE C T,CHEVALIER D,WANG H C,JINN T L,ZHANG S Q,WALKER J C.Regulation of floral organ abscission in Arabidopsis thaliana[J].Proceedings of the National Academy of Sciences of the United States of America,2008,105(40):15629-15634.
[9]PATHARKAR O R,WALKER J C.Core mechanisms regulating developmentally timed and environmentally triggered abscission[J].Plant Physiology,2016,172(1):510-520.
[10]PATHARKAR O R,GASSMANN W,WALKER J C.Leaf shedding as an anti-bacterial defense in Arabidopsis cauline leaves[J].PLoS Genetics,2017,13(12):e1007132.
[11]ROSENBERGER D A,ENGLE C A,MEYER F W.Effects of management practices and fungicides on sooty blotch and flyspeck diseases and productivity of‘Liberty’apples[J].Plant Disease,1996,80(7):798-803.
[12]SHARMA I M,BHARDWAJ S S.Efficacy and economics of different fungicide spray schedules in controlling premature defoliation disease in apple[J].Plant Disease Research Ludhiana,2003,18(1):21-24.
[13]HIDALGO H,SUTTON T B,ARAUZ F.Epidemiology and control of Citrus greasy spot on Valencia orange in the humid tropics of Costa Rica[J].Plant Disease,1997,81(9):1015-1022.
[14]SZTEJNBERG A.Etiology and control of cherry leaf spot disease in Israel caused by Cercospora circumscissa[J].Plant Disease,1986,70(4):349.
[15]CLINE W O.Blueberry bud set and yield following the use of fungicides for leaf spot control in North Carolina[J].Acta Horticulturae,2002,574:71-74.
[16]JESUS JUNIOR W C,VALE F X R,COELHO R R,PAUL P A,HAU B,FILHO A B,ZAMBOLIM L,BERGER R D.Relationships between angular leaf spot,healthy leaf area,effective leaf area and yield of Phaseolus vulgaris[J].European Journal of Plant Pathology,2003,109(6):625-632.
[17]LOPES D B,BERGER R D.The effects of rust and anthracnose on the photosynthetic competence of diseased bean leaves[J].Phytopathology,2001,91(2):212-220.
[18]ADDICOTT F T,LYNCH R S.Physiology of abscission[J].Annual Review of Plant Physiology,1955,6:211-238.
[19]GAO Y R,LIU C,LI X D,XU H Q,LIANG Y,MA N,FEI Z J,GAO J P,JIANG C Z,MA C.Transcriptome profiling of petal abscission zone and functional analysis of an Aux/IAA family gene RhIAA16 involved in petal shedding in rose[J].Frontiers in Plant Science,2016,7:1375.
[20]MENG X Z,ZHOU J G,TANG J,LI B,DE OLIVEIRA M V V,CHAI J J,HE P,SHAN L B.Ligand-induced receptor-like kinase complex regulates floral organ abscission in Arabidopsis[J].Cell Reports,2016,14(6):1330-1338.
[21]ESTORNELL L H,AGUSTÍ J,MERELO P,TALÓN M,TADEO F R.Elucidating mechanisms underlying organ abscission[J].Plant Science,2013,199/200:48-60.
[22]LI R Z,SHI C L,WANG X Y,MENG Y,CHENG L N,JIANG C Z,QI M F,XU T,LI T L.Inflorescence abscission protein Sl-IDL6 promotes low light intensity-induced tomato flower abscission[J].Plant Physiology,2021,186(2):1288-1301.
[23]REICHARDT S,PIEPHO H P,STINTZI A,SCHALLER A.Peptide signaling for drought-induced tomato flower drop[J].Science,2020,367(6485):1482-1485.
[24]周乃富,张俊佩,刘昊,查巍巍,裴东.木本植物非均质化组织石蜡切片制作方法[J].植物学报,2018,53(5):653-660.ZHOU Naifu,ZHANG Junpei,LIU Hao,ZHA Weiwei,PEI Dong.New protocols for paraffin sections of heterogeneous tissues of woody plants[J].Chinese Bulletin of Botany,2018,53(5):653-660.
[25]JIA H G,ORBOVIC V,JONES J B,WANG N.Modification of the PthA4 effector binding elements in Type I CsLOB1 promoter using Cas9/sgRNA to produce transgenic Duncan grapefruit alleviating XccΔpthA4:dCsLOB1.3 infection[J].Plant Biotechnology Journal,2016,14(5):1291-1301.
[26]SEXTON R,ROBERTS J A.Cell biology of abscission[J].Annual Review of Plant Physiology,1982,33:133-162.
[27]KIM J.Four shades of detachment:Regulation of floral organ abscission[J].Plant Signaling&Behavior,2014,9(11):e976154.
[28]盛云燕,杨丽敏,戴冬洋,张佳欣,王岭,王迪,才羿,田丽美.甜瓜果柄离区细胞学观察及成熟脱落基因AL3 的初步定位[J].园艺学报,2022,49(2):341-351.SHENG Yunyan,YANG Limin,DAI Dongyang,ZHANG Jiaxin,WANG Ling,WANG Di,CAI Yi,TIAN Limei.Cytological observation of fruit peduncle abscission zone and preliminary mapping of mature fruit abscission AL3 gene in melon[J].Acta Horticulturae Sinica,2022,49(2):341-351.
[29]TEPER D,XU J,LI J Y,WANG N.The immunity of Meiwa kumquat against Xanthomonas citri is associated with a known susceptibility gene induced by a transcription activator-like effector[J].PLoS Pathogens,2020,16(9):e1008886.
[30]PATHARKAR O R.Quantification of cauline leaf abscission in response to plant pathogens[M]//GASSMANN W.Plant Innate Immunity.New York:Humana,2019:127-139.
[31]TEPER D,XU J,PANDEY S S,WANG N.PthAW1,a transcription activator-like effector of Xanthomonas citri subsp.citri,promotes host-specific immune responses[J].Molecular Plant Microbe Interactions,2021,34(9):1033-1047.
[32]WEI C D,DING T,CHANG C Q,YU C P,LI X W,LIU Q G.Global regulator PhoP is necessary for motility,biofilm formation,exoenzyme production,and virulence of Xanthomonas citri subsp.citri on Citrus plants[J].Genes,2019,10(5):340.
[33]徐鑫焱,李艳娇,户勋,邹华松.柑橘溃疡病菌胞外蛋白水解酶对其致病力的作用[J].福建农林大学学报(自然科学版),2020,49(1):1-9.XU Xinyan,LI Yanjiao,HU Xun,ZOU Huasong.The role of extracellular protease on the virulence of Xanthomonas citri subsp.citri[J].Journal of Fujian Agriculture and Forestry University(Natural Science Edition),2020,49(1):1-9.
[34]TEPER D,ZHANG Y N,WANG N.TfmR,a novel TetR-family transcriptional regulator,modulates the virulence of Xanthomonas citri in response to fatty acids[J].Molecular Plant Pathology,2019,20(5):701-715.
[35]ROESCHLIN R A,UVIEDO F,GARCÍA L,MOLINA M C,FAVARO M A,CHIESA M A,TASSELLI S,FRANCO-ZORRILLA J M,FORMENT J,GADEA J,MARANO M R.PthA4AT,a 7.5-repeats transcription activator-like (TAL) effector from Xanthomonas citri ssp.citri,triggers Citrus canker resistance[J].Molecular Plant Pathology,2019,20(10):1394-1407.
[36]SERRA O,GELDNER N.The making of suberin[J].New Phytologist,2022,235(3):848-866.
[37]NAKANO T,FUJISAWA M,SHIMA Y,ITO Y.Expression profiling of tomato pre-abscission pedicels provides insights into abscission zone properties including competence to respond to abscission signals[J].BMC Plant Biology,2013,13:40.
[38]WILMOWICZ E,KUĆKO A,TRANBARGER T J,OSTROWSKI M,NIEDOJADŁO J,KARWASZEWSKI J,KAPUŚCIŃSKA D,PANEK K.EPIP as an abscission promoting agent in the phytohormonal pathway[J].Plant Physiology and Biochemistry,2022,178(1):137-145.
[39]WILMOWICZ E,KUĆKO A,OSTROWSKI M,PANEK K.INFLORESCENCE DEFICIENT IN ABSCISSION-like is an abscission-associated and phytohormone-regulated gene in flower separation of Lupinus luteus[J].Plant Growth Regulation,2018,85(1):91-100.
[40]PATHARKAR O R,WALKER J C.Connections between abscission,dehiscence,pathogen defense,drought tolerance,and senescence[J].Plant Science,2019,284:25-29.
[41]DU J,LU S Y,CHAI M F,ZHOU C E,SUN L,TANG Y H,NAKASHIMA J,KOLAPE J,WEN Z Z,BEHZADIRAD M,ZHONG T X,SUN J,ZHANG Y W,WANG Z Y.Functional characterization of PETIOLULE-LIKE PULVINUS (PLP) gene in abscission zone development in Medicago truncatula and its application to genetic improvement of alfalfa[J].Plant Biotechnology Journal,2021,19(2):351-364.
[42]LEE M H,JEON H S,KIM S H,CHUNG J H,ROPPOLO D,LEE H J,CHO H J,TOBIMATSU Y,RALPH J,PARK O K.Lignin-based barrier restricts pathogens to the infection site and confers resistance in plants[J].The EMBO Journal,2019,38(23):e101948.
[43]JIN X,ZIMMERMANN J,POLLE A,FISCHER U.Auxin is a long-range signal that acts independently of ethylene signaling on leaf abscission in Populus[J].Frontiers in Plant Science,2015,6:634.
[44]WOO H R,KIM H J,LIM P O,NAM H G.Leaf senescence:Systems and dynamics aspects[J].Annual Review of Plant Biology,2019,70:347-376.