库尔勒香梨(Pyrus sinkiangensis Yü)简称香梨,属于蔷薇科(Rosaceae)梨属(Pyrus)中白梨系统,是新疆梨和西洋梨的自然杂交后代,是新疆主栽梨品种[1]。但在香梨果实中存在着“公梨”(宿萼果)与“母梨”(脱萼果)之分,相比宿萼果,脱萼果则具有果形正、果面光洁、果核小、石细胞少和风味佳等诸多优点。由于遗传因素影响,香梨存在严重的萼片宿存现象,极大地影响了其品质,对销售量及经济效益造成了一定影响[2]。
香梨萼片脱落是由多种因素协调控制的复杂的生理过程,逆境条件、植物内源激素及相关酶均可以影响香梨萼片脱落[3]。新疆南疆地区极度缺水,易产生干旱胁迫造成器官衰老,这是引起器官非正常脱落的主要原因之一[4]。同时,梨树的物质运输、蒸腾和光合作用同样受土壤含水量的直接影响,适当的水分胁迫有利于提高果实的品质[5]。干旱胁迫时,萼筒产生的大量活性氧(reactive oxygen species,ROS)是一类具有强氧化能力、能持续进行反应的物质,主要包括O2-·(超氧阴离子)、H2O2(过氧化氢)和·OH(羟自由基)等[6]。超氧化物歧化酶(superoxide dismutase,SOD)、过氧化物酶(peroxidase,POD)和过氧化氢酶(catalase,CAT)等是萼筒抗氧化酶系统[7]。当活性氧的含量超过清除酶能力时,需清除多余的活性氧才能维持自身的平衡[8]。
在通常情况下,细胞中活性氧浓度很低,可以被SOD、CAT 和POD 清除,因而不会造成伤害。在细胞衰老时,ROS 平衡遭到破坏,ROS 浓度超过了伤害阈值,致使蛋白质、核酸和酶结构被氧化破坏,尤其是膜脂中的不饱和双链酸最容易受到攻击,继而发生过氧化作用。过氧化过程又会产生新的羟自由基,从而加重膜脂质的过氧化,破坏膜的完整性,最终导致细胞死亡[9]。当ROS 浓度适中的时候,其作为信号分子参与调控细胞程序性死亡(programmed cell death,PCD)等生理事件的发生[10]。
笔者在本试验中通过不同物候期灌水处理测定短果枝水势、ROS 含量及其相关清除酶活性,探究不同时期灌水对香梨果实萼片脱落的影响,并结合石蜡切片、DAPI 染色、TUNEL 检测、DNA laddering等方法阐明香梨萼片脱落的PCD特征。
1 材料和方法
1.1 试验材料
试验于2022 年3—4 月在新疆阿拉尔市塔里木大学进行。供试品种为以杜梨为砧木、长势一致的27 年生香梨树,株高度4.5 m,株行4 m×5 m,南北行向。灌溉方式为漫灌,2021年冬灌水时间为10月底,灌水定额为300 mm,土壤类型为壤砂土,果园常规管理。
1.2 试验设计
本试验以5 株树为1 小区,每小区1 个处理,随机排列,设置5次重复,以不灌水为对照。漫灌处理的梨树于每个处理绕树起一条保护墒,具体漫灌试验设计如表1。从3 月15 日开始每隔3 d 测定各个处理的土壤含水量,并于4 月10 日(大蕾期后10 d)和4 月20 日(大蕾期后20 d),分别采集2 个处理和对照的脱萼果和宿萼果萼筒及短果枝,取萼筒部位,一部分固定在乙醇-福尔马林-醋酸固定液(ethanolformalin-acetic acid solution,FAA)中用于DAPI 染色、DNA ladder 检测、TUNEL 标记和石蜡切片显微结构观察,另一部分经液氮处理后保存于-80 ℃冰箱,用于·OH 清除率、O2-·生成速率、H2O2含量和抗氧化酶(SOD、POD和CAT)活性测定。
表1 漫灌试验设计
Table 1 Test design of flood irrigation
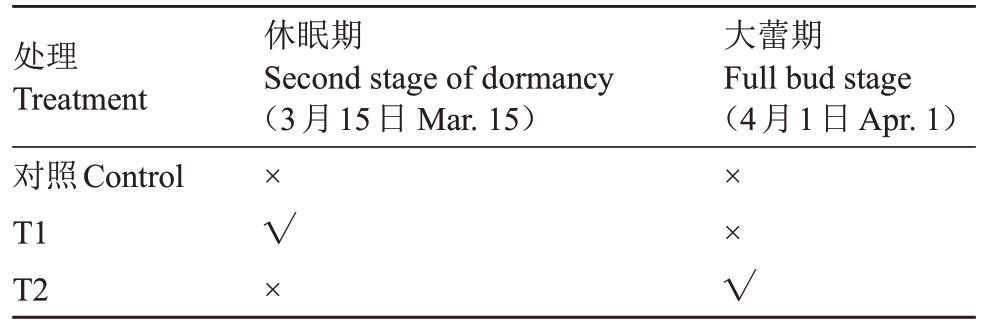
注:√为灌溉,×为不灌溉。
Note:√means irrigation,×means no irrigation.
处理Treatment对照Control T1 T2休眠期Second stage of dormancy(3月15日Mar.15)大蕾期Full bud stage(4月1日Apr.1)×√×××√
1.3 测定方法
1.3.1 脱萼率 花后30 d,调查自然条件下香梨的脱萼率[11]。
脱萼率/%=脱萼果/总果数×100。
1.3.2 土壤含水量的测定 利用烘干法[12]测定土壤含水量。
1.3.3 短果枝水势测定 短果枝水势采用小液流法测定[13]。
1.3.4 活性氧(·OH、O2-·和H2O2)代谢测定 ·OH清除率参照李贵荣等[14]的方法测定;O2-·生成速率参照石润霖等[15]的方法测定;H2O2含量参照Liu等[16]的方法测定。
1.3.5 抗氧化酶(SOD、POD 和CAT)活性测定SOD 活性采用氮蓝四唑(nitro blue tetrazolium,NBT)光还原法[17]测定;POD活性采用愈创木酚比色法[18]测定;CAT活性采用紫外吸收法测定[19]。
1.3.6 香梨萼片PCD过程的形态特征(1)显微结构观察。样本选取灌水处理的为宿萼,不灌水处理的为脱萼。样本从FAA 固定液中取出使用系列浓度的乙醇溶液与透明剂完成样本的脱水和透明,使用石蜡进行包埋,切片机切片,烘箱烘片,使用系列浓度的乙醇溶液与透明剂复水,使用1%番红染液和0.5%固绿染液双重染色,中性树胶封片,使用显微镜进行观察拍照[20]。
(2)DNA ladder 检测。利用5×TAE buffer 和琼脂糖制备2%琼脂糖凝胶,向胶槽中点入处理组与对照组样品DNA 及marker,150 V 电泳30 min 用于检测。电泳结果经溴化乙锭(ethidium bromide,EB)染色并照相。试验进行3次重复[21]。
(3)DAPI 染色和TUNEL 标记。石蜡包埋组织切片先脱蜡和水化加蛋白酶K通透,用1×PBS洗涤样品2~3次,滴加100 μL 1×Equilibration Buffer室温平衡10~30 min,依次加入34 μL ddH2O、10 μL 5×Equilibration Buffer、5 μL BrightGreen Labeling Mix、1 μL Recombinant TdT Enzyme,37 ℃标记60 min终止,然后用1×PBS 洗涤,再用1×PBS 新鲜配制的2 mL·L-1 DAPI 溶液在黑暗中对样品进行复染,染色时室温放置5 min。立即在荧光显微镜下分析样本,用标准的荧光过滤装置在460 nm 荧光下观察DAPI的蓝色荧光和在520 nm±20 nm的荧光下观察绿色荧光[22]。
2 结果与分析
2.1 花前和花期灌水对香梨萼片脱落的影响
由表2 可知,T1、T2 处理与对照脱萼率呈显著差异,T1、T2 的脱萼率较对照分别下降了21.41%、18.62%,而T1、T2间脱萼率并无显著差异。这说明不灌水处理更利于香梨萼片脱落。
表2 花前和花期灌水对香梨萼片脱落的影响
Table 2 Effect of pre-flowering and flowering irrigation on calyx shedding of fragrant pear
注:不同字母表示在0.05 水平差异显著。下同。
Note: Different letters indicate significant difference at the 0.05 level.The same below.
T2 37.95 b处理Treatment脱萼率Calyx rate/%对照Control 56.57 a T1 35.16 b
2.2 花前和花期灌水对土壤含水量的影响
从图1中可以看出,与对照相比较,T1含水量呈现先上升再下降的变化趋势,其中第一次上升是3月16 日,其原因是灌水后的第一天含水量变高,而第二次上升则是因为3月20—21日连续下雨;T2含水量的变化也是受降水影响在3 月20—21 日升高,而后开始下降,在4 月1 日因灌水而再次升高,而对照除受降雨影响基本呈现持续下降趋势外,且含水量均低于T1、T2灌水处理。

图1 花前和花期灌水对土壤含水量的影响
Fig.1 Effect of pre-flowering and flowering irrigation on soil water content
2.3 花前和花期灌水对香梨短果枝水势的影响
从图2 中可以看出,T1、T2 处理和对照在大蕾期后10 d和大蕾期后20 d短果枝水势的变化趋势基本一致。在大蕾期后10 d,T1、T2 处理和对照的香梨短果枝水势变化范围为-1.08~-0.54 MPa;在大蕾期后20 d,T1、T2 处理和对照的香梨短果枝水势变化范围为-1.29~-0.84 MPa。对比大蕾期后10 d 和大蕾期后20 d 发现,T1、T2 处理和对照的短果枝水势变化分别下降了-0.31、-0.33 和-0.20 MPa,其中对照(不灌水处理)短果枝水势下降幅度最小,T2下降幅度最大。
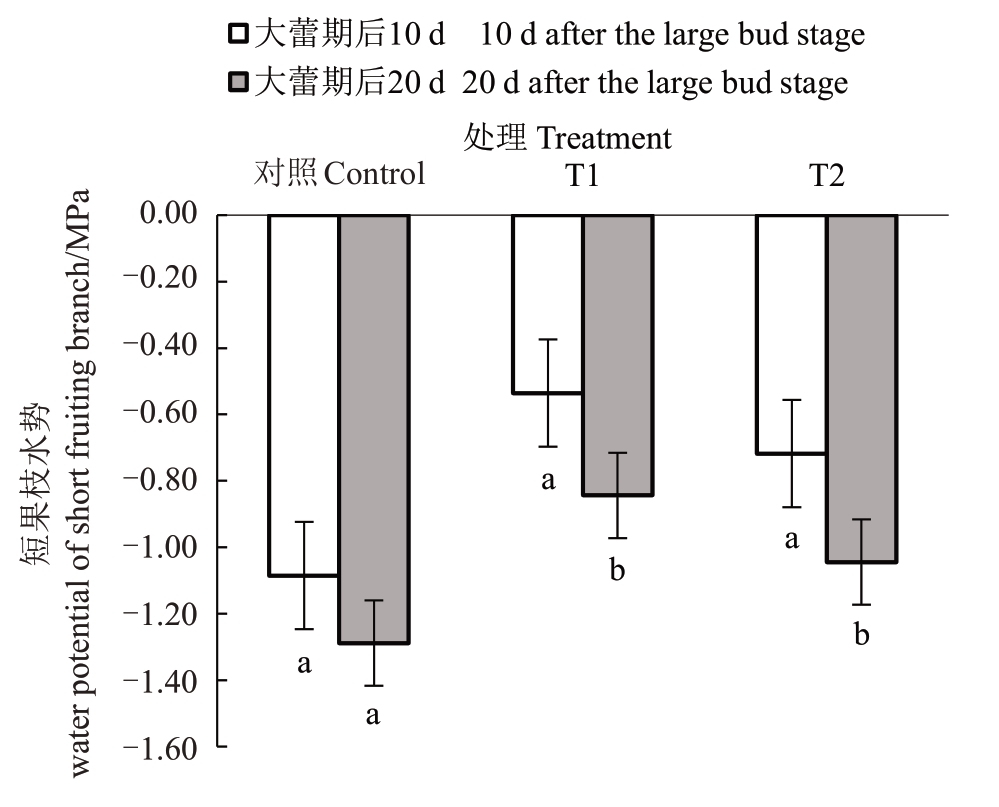
图2 花前和花期灌水对香梨短果枝水势的影响
Fig.2 Effect of pre-flowering and flowering irrigation on the water potential of fragrant pear short fruiting branches
2.4 大蕾期后10 d 和大蕾期后20 d 土壤含水量和短果枝水势与脱萼率的相关性分析
如图3 所示,大蕾期后10 d 土壤含水量与萼片脱萼率呈负相关,但并不显著(r<-0.811 4),大蕾期后20 d土壤含水量与脱萼率呈极显著(r>-0.917 2)负相关;大蕾期后10 d 短果枝水势与脱萼率呈显著(-0.811 4<r<-0.917 2)负相关,大蕾期后20 d短果枝水势与脱萼率呈极显著(r>-0.917 2)负相关。
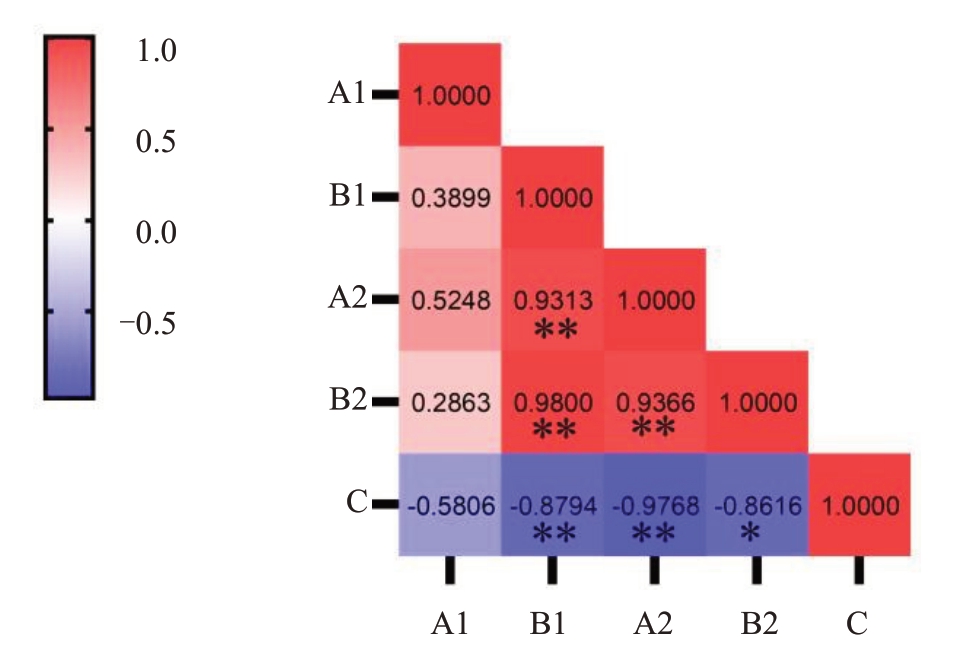
图3 大蕾期后10 d 和大蕾期后20 d 土壤含水量和短果枝水势与脱萼率的相关性分析
Fig.3 Correlation analysis of soil water content and water potential of short fruiting branches with calyx removal rate at 10 days after the large bud stage and 20 days after the large bud stage
相关系数临界值,a=0.05 时,r=0.811 4;a=0.01 时,r=0.917 2。A.土壤含水量;B.短果枝水势;C.脱萼率。数字1 代表大蕾期后10 d,数字2 代表大蕾期后20 d;*代表显著,**代表极显著。
Critical value of correlation coefficient, r=0.811 4 for a=0.05 a=0.01,r=0.917 2.A.Soil water content;B.Water potential of short fruiting branches;C.Calyx removal rate;number 1 represents 10 days after the large bud stage and number 2 represents 20 days after the large bud stage;*represents significant,**is significant.
2.5 花前和花期灌水对香梨萼筒活性氧代谢的影响
2.5.1 花前和花期灌水对香梨萼筒·OH 清除率的影响 由图4 可知,T1、T2 处理和对照大蕾期后10 d 脱萼比宿萼萼筒的·OH 清除率分别显著升高了79.01%、48.02%、71.54%;大蕾期后20 d 脱萼比宿萼萼筒的·OH 清除率分别显著升高了144.42%、98.74%、208.65%;大蕾期后10 d 与大蕾期后20 d宿萼萼筒的·OH 清除率随干旱胁迫的持续并无显著变化,相反大蕾期后10 d 与大蕾期后20 d 脱萼的·OH 清除率随干旱胁迫的持续变化显著;相对于大蕾期后10 d 脱萼,大蕾期后20 d 脱萼的·OH清除率分别显著增加了26.18%、28.54%、46.24%,其中对照增幅最大为46.24%,T1 增幅最小为26.18%。
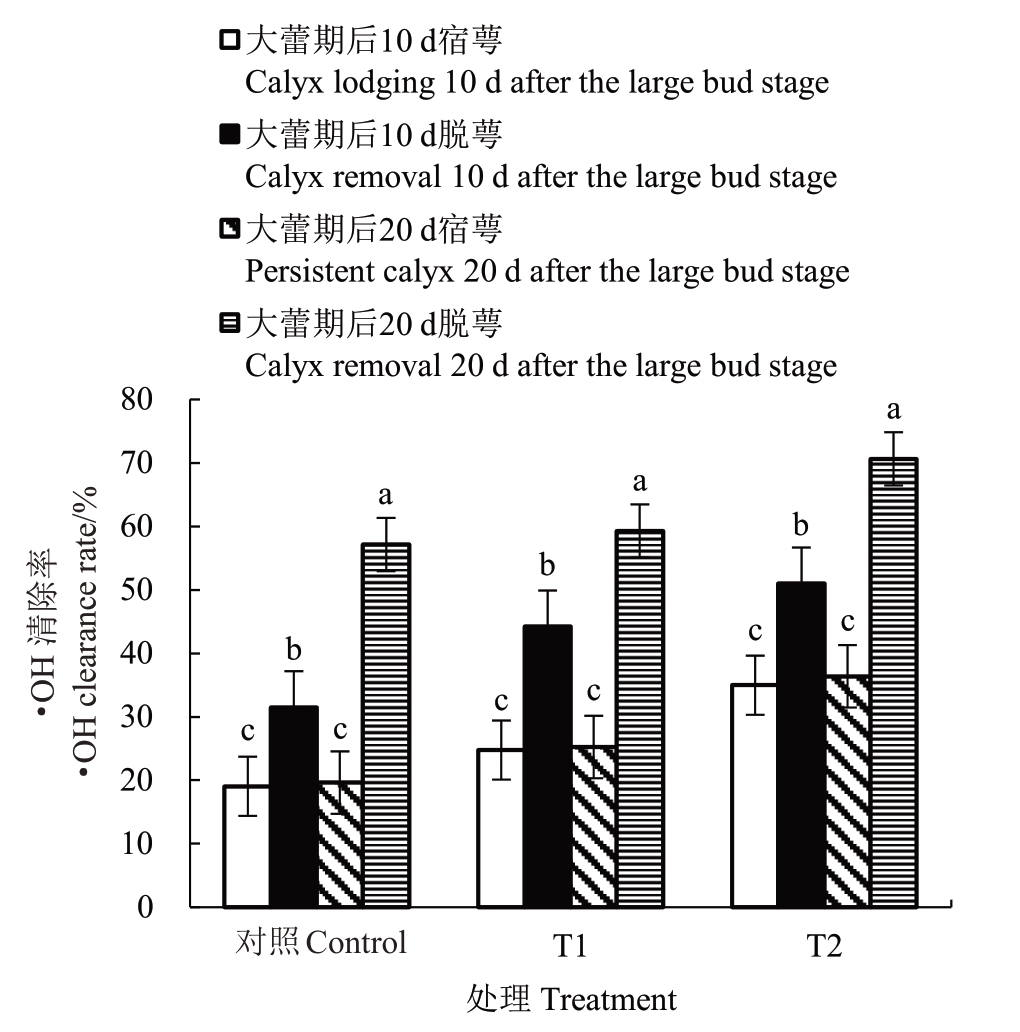
图4 花前和花期灌水对香梨萼筒·OH 清除率的影响
Fig.4 Effect of pre-flowering and flowering irrigation on the scavenging rate of fragrant pear calyx tube·OH
2.5.2 花前和花期灌水对香梨萼筒O2-·生成速率的影响 由图5 可知,大蕾期后10 d T1、T2 处理和对照脱萼比宿萼萼筒的O2-·生成速率分别显著升高了21.90%、15.53%、29.91%;大蕾期后20 d脱萼比宿萼萼筒的O2-·生成速率分别显著升高了44.05%、42.61%、118.47%;大蕾期后10 d 与大蕾期后20 d 宿萼的O2-·生成速率随干旱胁迫的持续并无显著变化,相反大蕾期后10 d 和大蕾期后20 d 脱萼的O2-·生成速率随干旱胁迫的持续变化显著;大蕾期后20 d脱萼的O2-·生成速率较大蕾期后10 d分别显著增加了15.08%、27.25%、36.12%,其中对照增幅最大为36.12%,T1增幅最小为15.08%。
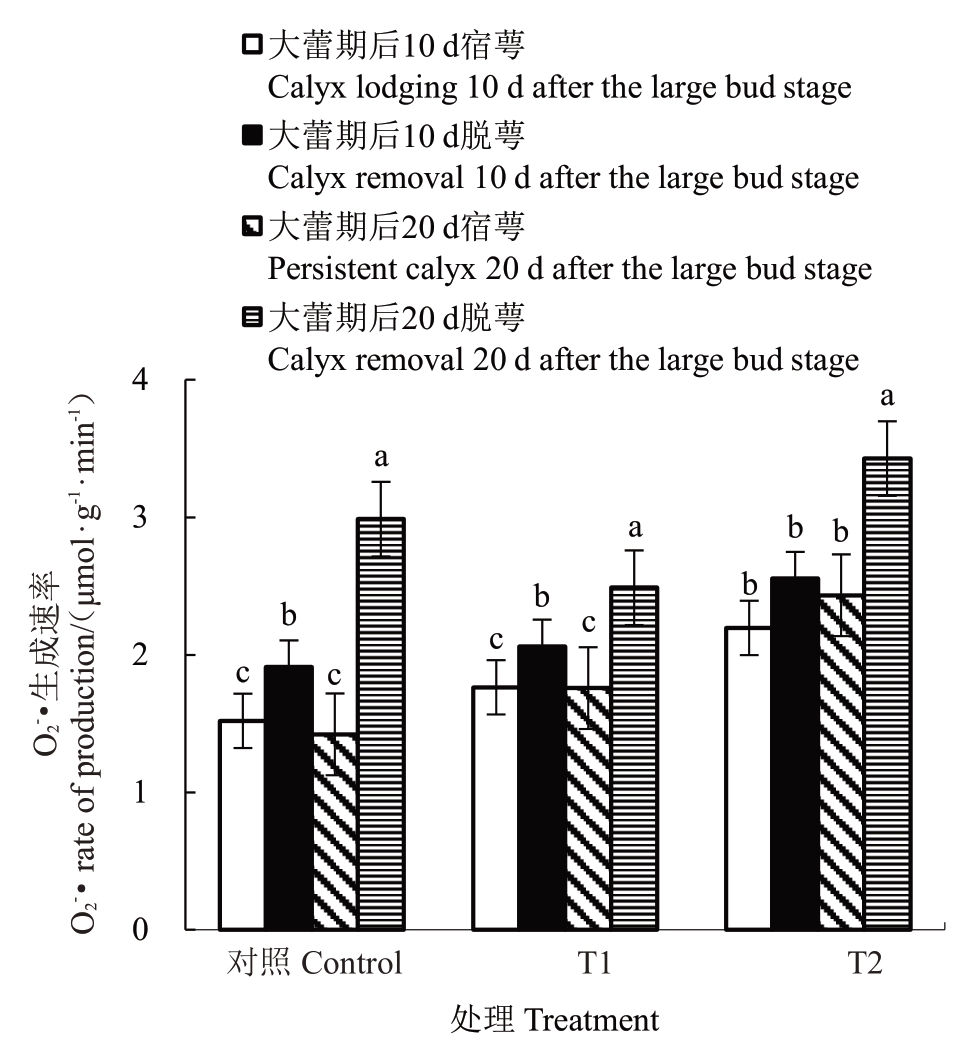
图5 花前和花期灌水对香梨萼筒O2-·生成速率的影响
Fig.5 Effect of pre-flowering and flowering irrigation on the O2-·production rate of fragrant pear calyx tube
2.5.3 花前和花期灌水对香梨萼筒H2O2含量的影响 由图6 可知,大蕾期后10 d T1、T2 处理和对照脱萼比宿萼萼筒的H2O2 含量分别显著升高了67.38%、122.36%、84.99%;大蕾期后20 d,脱萼比宿萼萼筒的H2O2 含量分别显著升高了147.58%、256.06%、290.49%;大蕾期后10 d 与大蕾期后20 d宿萼的H2O2含量随干旱胁迫的持续并无显著变化,反之大蕾期后10 d 和大蕾期后20 d 脱萼的H2O2含量随干旱胁迫的持续变化显著;大蕾期后20 d脱萼的H2O2 含量较大蕾期后10 d 分别显著增加了37.20%、37.80%、53.84%,其中对照增幅最大为53.84%,T1增幅最小为37.20%。T1、T2处理和对照宿萼萼筒H2O2含量无显著变化,而脱萼萼筒H2O2含量变化显著,其中对照的H2O2含量随干旱的持续变化最显著。
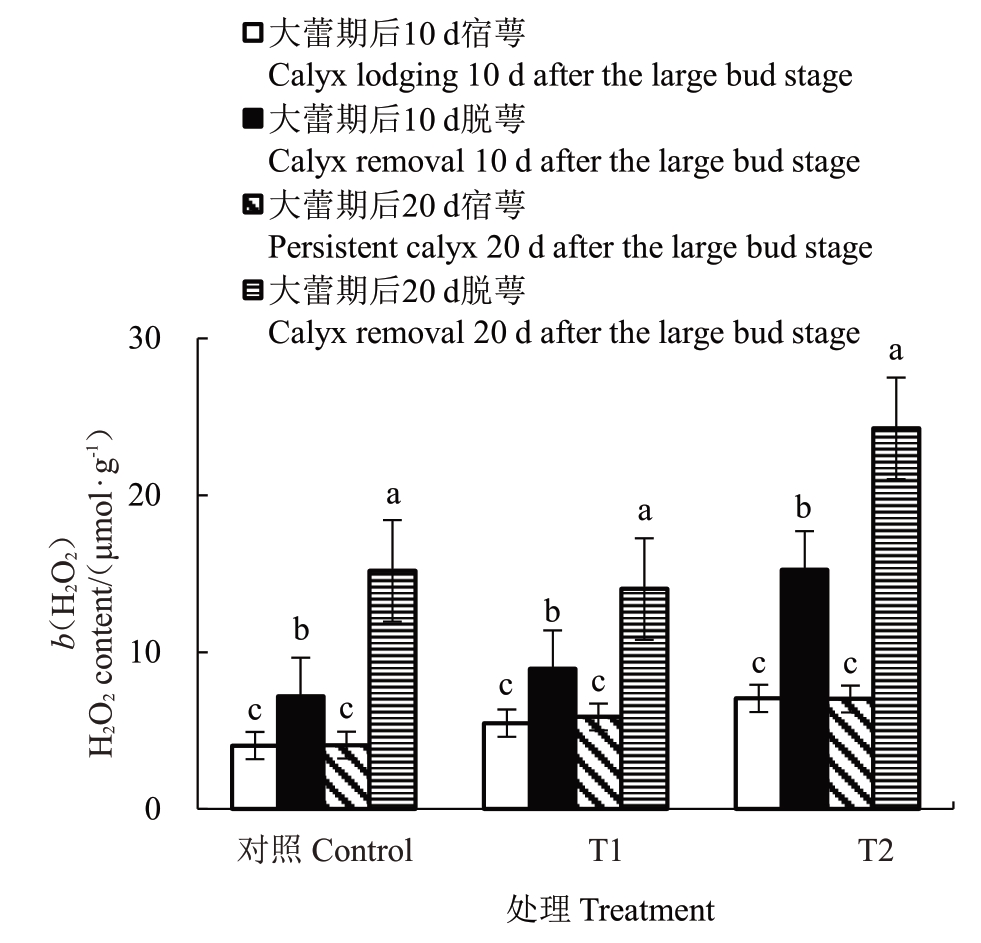
图6 花前和花期灌水对香梨萼筒H2O2含量的影响
Fig.6 Effect of pre-flowering and flowering irrigation on the H2O2 content of fragrant pear calyx tube
2.6 花前和花期灌水对活性氧清除酶活性的影响
2.6.1 花前和花期灌水对香梨萼筒SOD 活性的影响 由图7 可知,T1、T2 处理和对照大蕾期后10 d脱萼和宿萼均有显著差异,其中对照变化最显著,为17.10%;大蕾期后20 d 脱萼和宿萼均有显著差异,其中对照变化最显著,为45.57%。
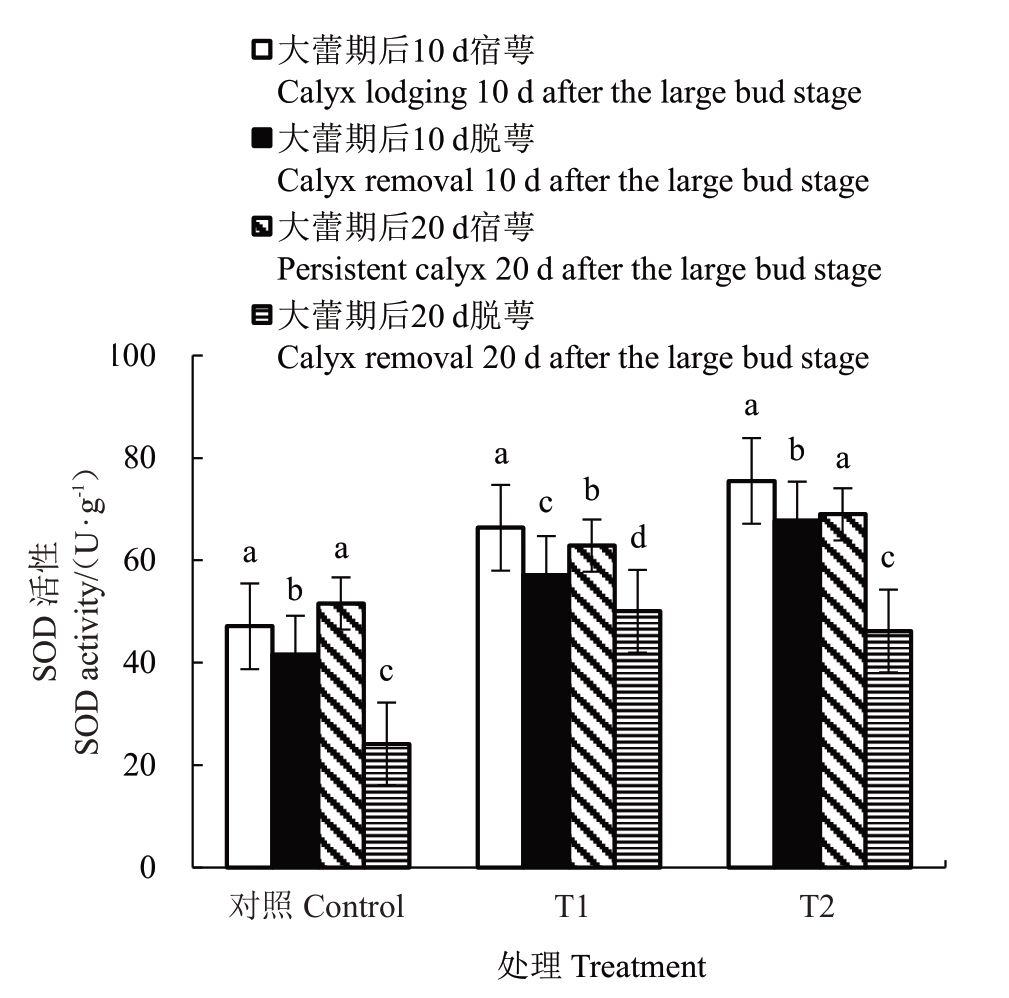
图7 花前和花期灌水对香梨萼筒SOD 活性的影响
Fig.7 Effect of pre-flowering and flowering irrigation on the SOD activity of fragrant pear calyx tube
2.6.2 花前和花期灌水对香梨萼筒POD 活性的影响 由图8 可知,T1、T2 处理和对照大蕾期后10 d脱萼和宿萼萼筒的POD活性均有显著差异,其中对照变化最显著,为38.26%;大蕾期后20 d 脱萼和宿萼萼筒的POD活性均有显著差异,其中对照变化最显著,为69.76%。
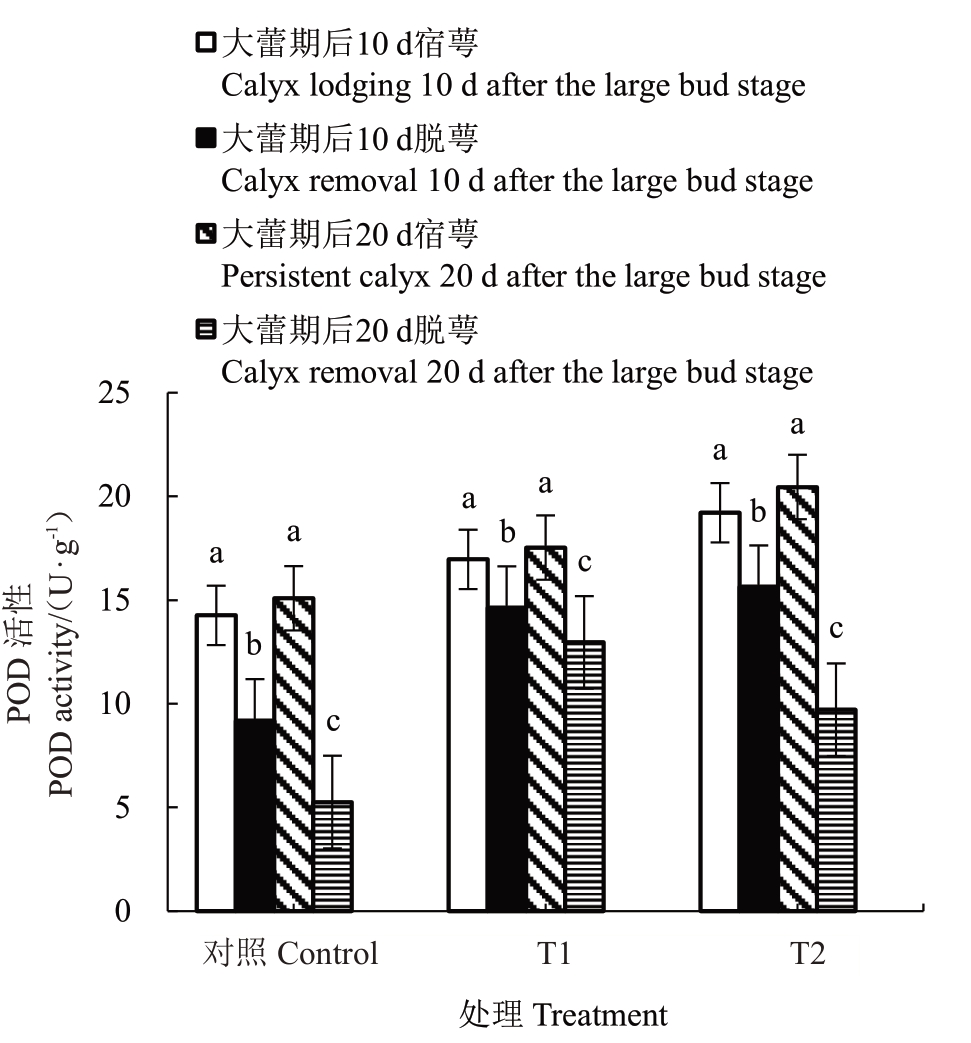
图8 花前和花期灌水对香梨萼筒POD 活性的影响
Fig.8 Effect of pre-flowering and flowering irrigation on the POD activity of fragrant pear calyx tube
2.6.3 花前和花期灌水对香梨萼筒CAT 活性的影响 由图9 可知,T1、T2 处理和对照大蕾期后10 d脱萼和宿萼萼筒的CAT活性均有显著差异,其中对照变化最显著,为35.55%;大蕾期后20 d 脱萼和宿萼萼筒的CAT活性均有显著差异,其中对照变化最显著,为62.47%。
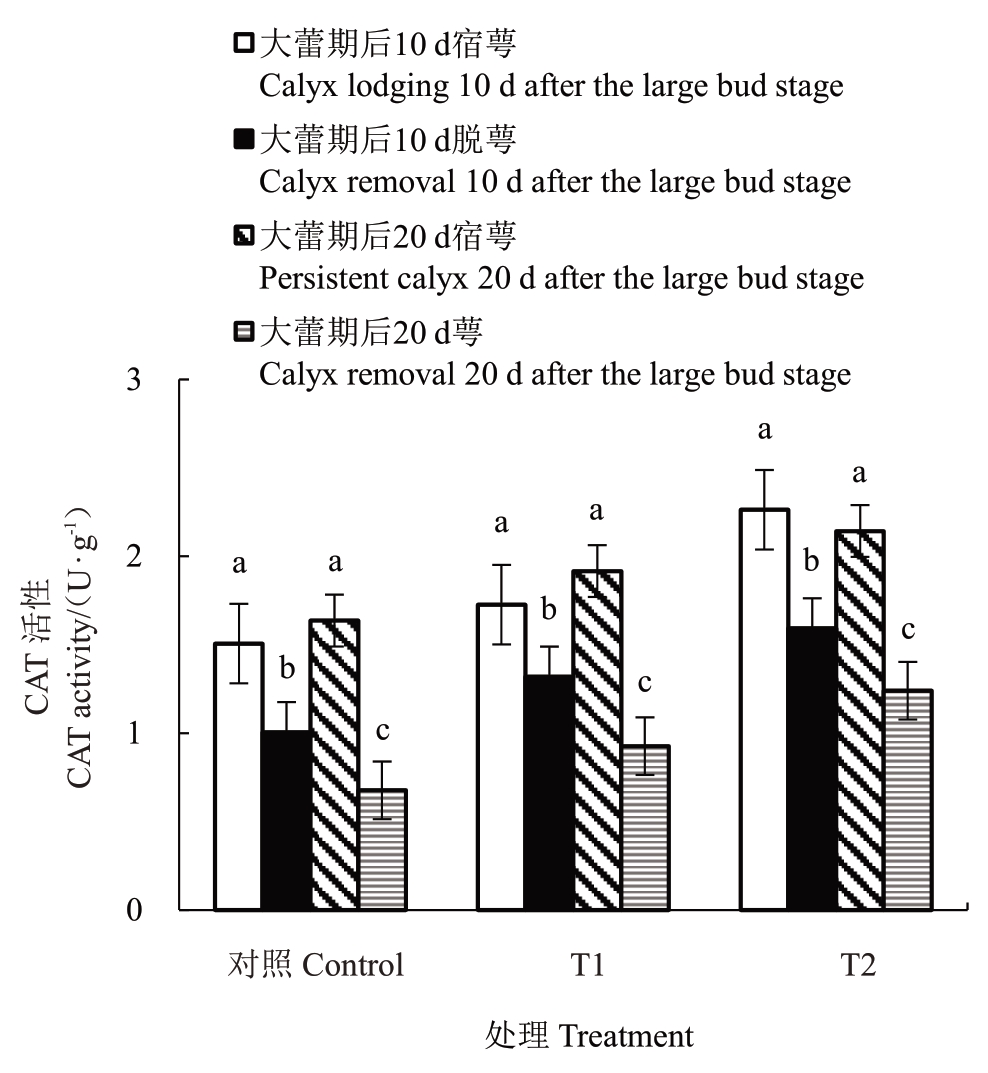
图9 花前和花期灌水对香梨萼筒CAT 活性的影响
Fig.9 Effect of pre-flowering and flowering irrigation on CAT activity in fragrant pear calyx cylinders
2.7 大蕾期后10 d 与大蕾期后20 d 萼筒活性氧含量和抗氧化酶活性与脱萼率的相关性分析
如图10所示,大蕾期后10 d脱萼的·OH清除率与脱萼率呈极显著(r>0.917 2)正相关,O2-·生成速率与脱萼率呈显著(0.811 4<r<0.917 2)正相关,H2O2含量与脱萼率呈极显著(r>0.917 2)正相关;大蕾期后20 d 脱萼的·OH 清除率与脱萼率呈显著(0.811 4<r<0.917 2)正相关,O2-·生成速率与脱萼率呈极显著(r>0.917 2)正相关,H2O2含量与脱萼率呈极显著(r>0.917 2)正相关。大蕾期后10 d 脱萼的SOD活性与脱萼率呈负相关,但不显著(r<-0.811 4),POD 活性与脱萼率呈极显著(r>-0.917 2)负相关,CAT 活性与脱萼率呈显著(-0.811 4<r<-0.917 2)负相关;大蕾期后20 d脱萼的SOD活性与脱萼率呈显著(r>-0.811 4)负相关,POD 活性与脱萼率呈极显著(r>-0.917 2)负相关,CAT 活性与脱萼率呈极显著(r>-0.917 2)负相关。
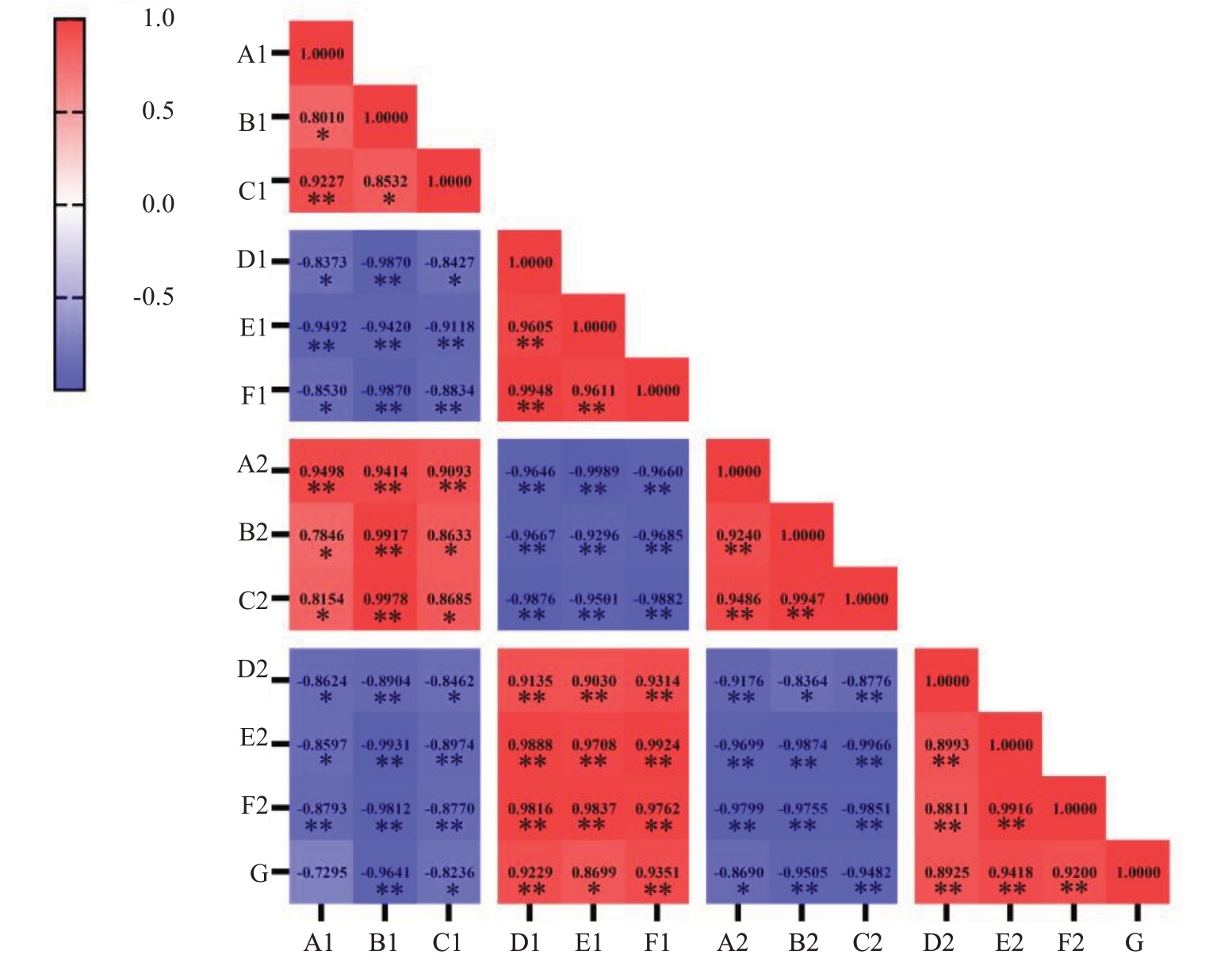
图10 大蕾期后10 d 与大蕾期后20 d 萼筒活性氧含量和抗氧化酶活性与脱萼率相关性分析
Fig.10 Correlation analysis of reactive oxygen content and antioxidant enzyme activity of calyx tube with desiccation rate at 10 days after large bud stage and 20 days after large bud stage
相关系数临界值,α=0.05 时,r=0.811 4;α=0.01 时,r=0.917 2。A.SOD;B.POD;C.CAT;D.·OH 清除率;E.O2-·生成速率;F.H2O2 含量;G.脱萼率。数字1 代表大蕾期后10 d,数字2 代表大蕾期后20 d;*代表显著,**代表极显著。
Critical value of correlation coefficient, r=0.811 4 for α=0.05; r=0.917 2 for α=0.01 A.SOD; B.POD; C.CAT; D.·OH scavenging rate; E.O2-·production rate; F.H2O2 content; G.Desiccation rate; number 1 represents 10 days after the large bud stage, number 2 represents 20 days after the large bud stage;*Represents significant,**Represents extremely significant.
2.8 香梨萼片PCD过程的形态特征
2.8.1 外观形态特征 图11 为香梨萼片离层发育的外观形态特征。图11-a~b为大蕾期后10 d形成的宿萼果,萼片离区随干旱的持续并无明显变化;图11-c~d)为大蕾期后20 d的脱萼果,萼片离区在花后10 d出现明显的黄色离层,并且随着干旱的持续,花后20 d萼片离层已经出现明显的脱落。
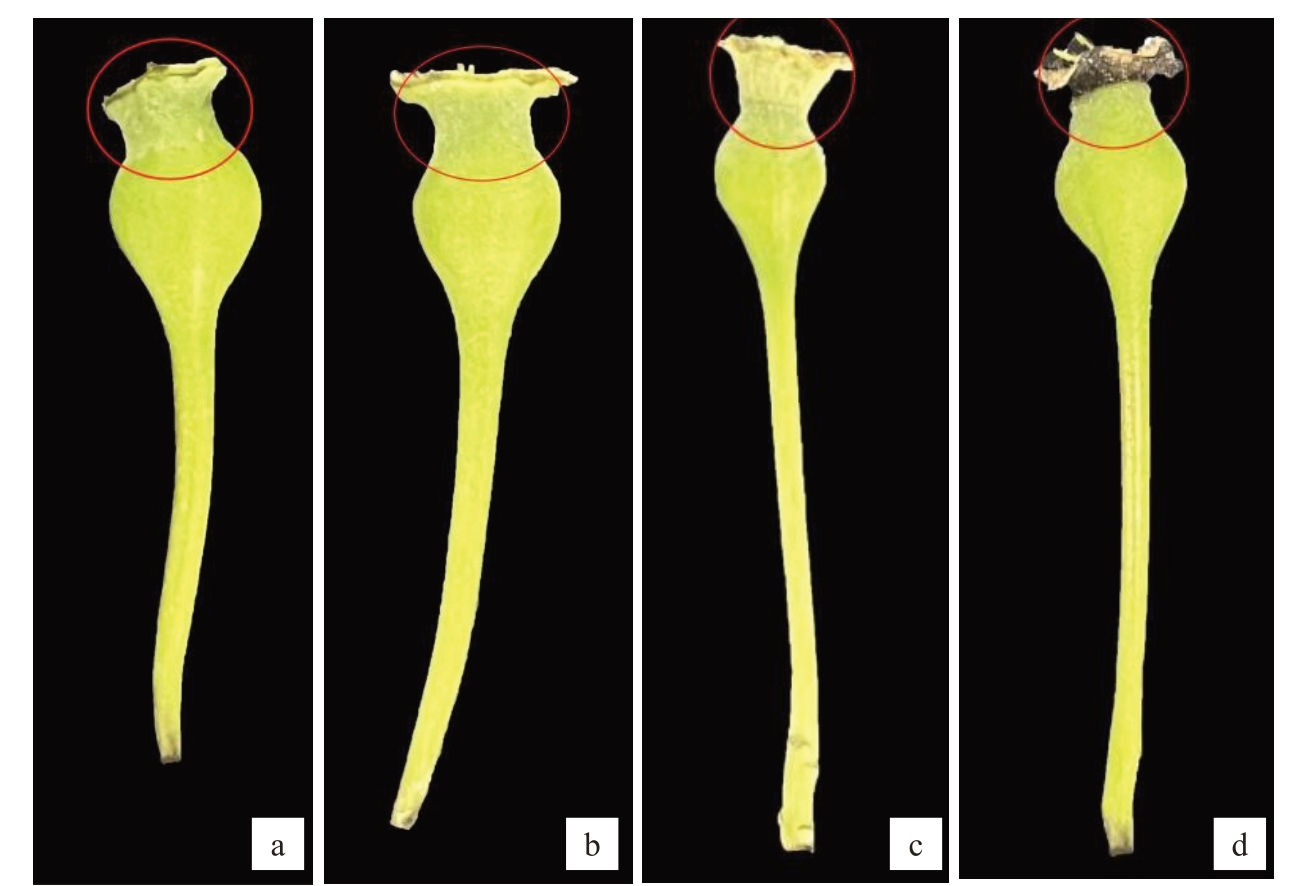
图11 脱萼果宿萼果外观形态
Fig.11 Morphology of the appearance of the desiccated fruit with persistent calyx
a.大蕾期后10 d 宿萼;b.大蕾期后20 d 宿萼;c.大蕾期后10 d 脱萼;d.大蕾期后20 d 脱萼下同。图12,图14 同。
a.Persistent calyx 10 days after large bud stage; b.Persistent calyx 20 days after large bud stage; c.Decalcification 10 days after large bud stage;d.Decalcification 20 days after large bud stage.Figure 12 and 14 are the same.
2.8.2 显微结构观察 大蕾期后10 d 和大蕾期后20 d 宿萼离区细胞并未发现有明显番红着色现象(图12-a~b);大蕾期后10 d 脱萼离区细胞番红着色比例为48%(图12-c),大蕾期后20 d 脱萼离区细胞番红着色比例高达85%(图12-d),说明脱萼离区随干旱胁迫的持续木质化程度加重,促进萼片脱落。

图12 脱萼果和宿萼果显微结构的观察
Fig.12 Observation of microstructure of desiccated and persistent calyx fruit
箭头所指位置为离层形成区域。
The location indicated by the arrow is the off-layer formation area.
2.8.3 DNA ladder 检测 从图13 可知,大蕾期后10 d和大蕾期后20 d宿萼对应的1、2泳道细胞的总DNA 表现为1 条完整的带,表明该时期未发生PCD。大蕾期后10 d 脱萼对应的3 泳道可以看到DNA 的断裂程度加重,呈现模糊的DNA 梯状条带(DNA Ladder),此时该离区细胞DNA 降解。大蕾期后20 d脱萼对应的4泳道DNA电泳图谱呈“涂片状”,梯状条带消失,可能是因为随着PCD 的深化,细胞内部DNA降解为更小的片段,并被细胞吸收利用,转化为细胞壁的一部分。DNA 电泳图谱显示,离区细胞从大蕾期后10 d 开始发生明显的PCD,出现明显的DNA 梯状条带。随着PCD 的深入发展,DNA电泳图谱逐渐增宽并且强度下降,表明小片段的DNA迅速增多,随着PCD不断深化,最后被吸收利用。该检测结果证明了萼片脱落发生时离区细胞的PCD死亡特征。
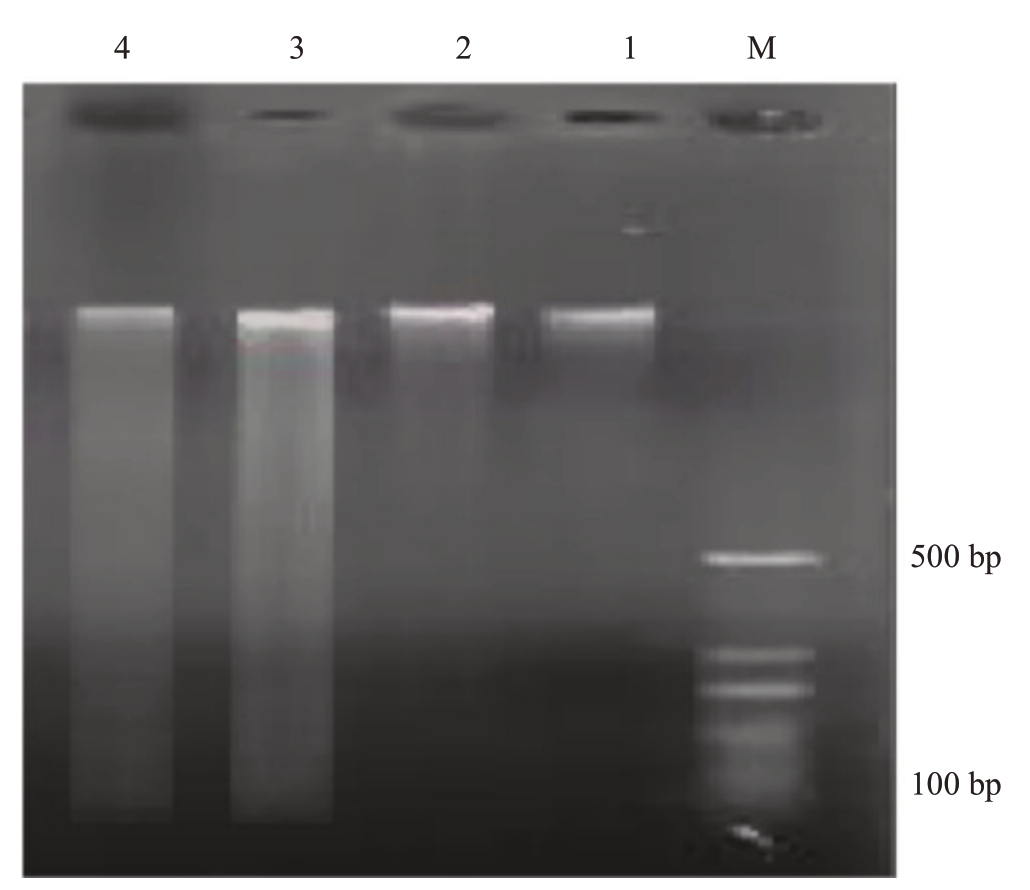
图13 DNA ladder 检测
Fig.13 DNA ladder detection
泳道M.100 bp 间隔的分子质量Marker;泳道1.大蕾期后10 d宿萼;泳道2.大蕾期后20 d 宿萼;泳道3.大蕾期后10 d 脱萼;泳道4.大蕾期后20 d 脱萼。
Lane M.molecular weight marker at 100 bp intervals; lane 1.calyx lodging 10 days after large bud stage;lane 2.calyx lodging 20 days after large bud stage; lane 3.calyx removal 10 days after large bud stage;lane 4.calyx removal 20 days after large bud stage.
2.8.4 TUNEL 标记和DAPI 染色 大蕾期后10 d和大蕾期后20 d 宿萼离区细胞TUNEL 标记和DAPI 染色观察并无变化,未发现死亡细胞(图14-a~b);大蕾期后10 d 脱萼,TUNEL 标记可以看到离层细胞有少数的细胞核呈现为阳性,并且是在已经出现离层的离区细胞上发生的(图14-c-1);大蕾期后20 d 脱萼,TUNEL 标记的细胞核随着干旱的持续,细胞核基本消失不见,说明此时离区细胞的细胞核已经被吸收利用(图14-d-1)。大蕾期后10 d,TUNEL 标记的离区阳性细胞核开始出现并逐渐增多,随着PCD 的加深,离区细胞的细胞核普遍表现为阳性,并迅速萎缩变小直至消失。并且随着PCD的进行大蕾期后10 d 脱萼离层出现明显断裂(图14-c-3),大蕾期后20 d 脱萼离层断裂更加明显(图14-d-3)。
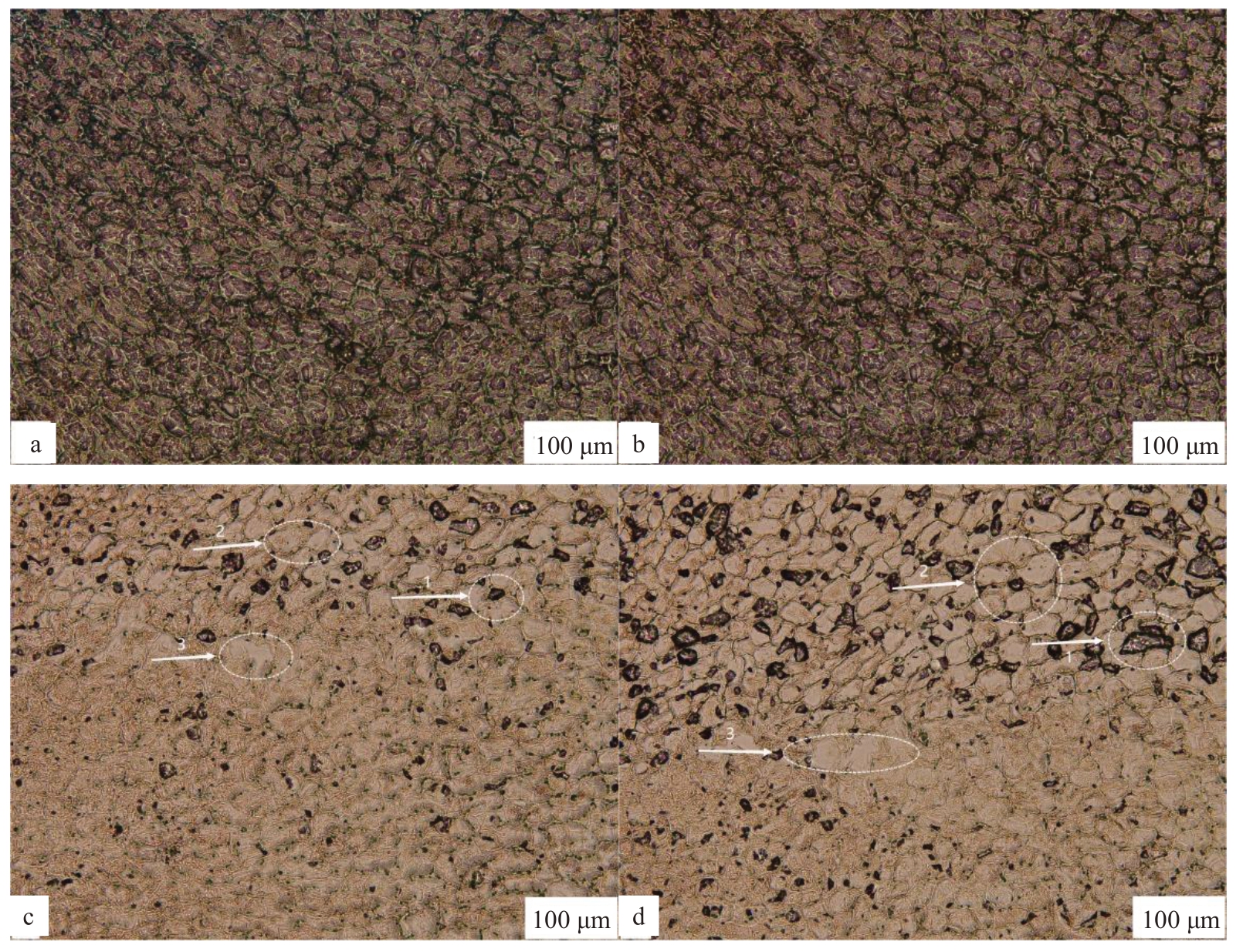
图14 TUNEL 标记和DAPI 染色
Fig.14 TUNEL markup and DAPI staining
图中1、2 代表细胞核的变化,3 代表离层断裂位置。
1,2 in the figure represent the changes of the nucleus,3 represents the location of the delamination break.
大蕾期后10 d脱萼(图14-c-2),DAPI染色出现明显的细胞核浓缩,并且呈月牙形的染色质向核膜靠拢,表明细胞已经开始凋亡;大蕾期后20 d 脱萼(图14-d-2),DAPI 染色离区细胞出现细胞破裂,细胞呈大小不一的碎片,并被细胞膜所包裹,此时的细胞已经基本全部凋亡。
3 讨 论
干旱胁迫造成器官衰老,引起器官的非正常脱落。任莹莹[23]研究发现,同一梨园内,浇水困难的干燥区的梨树脱萼果率高于正常浇水区的梨树,且靠近水渠一侧的树体宿萼果多,而其他方向平均较少,充足的水分会导致香梨宿萼果增多。这与本研究中的结果基本一致,即花前和花期灌水与对照(不灌水处理)相比,对照(不灌水处理)短果枝水势下降幅度最小,土壤含水量因持续的干旱胁迫处于较低水平,并且与香梨的脱萼率呈显著负相关,香梨的脱萼率高于花前和花期灌水处理。
本研究中大蕾期后10 d 脱萼的·OH 清除率、O2-·生成速率和H2O2的含量呈上升趋势,并且随着干旱胁迫时间的持续大蕾期后20 d 脱萼的·OH、O2-·和H2O2在不断累积,同时对比花前和花期灌水处理,对照(不灌水处理)脱萼的·OH、O2-·和H2O2随胁迫时间的持续变化最为明显。杨伟等[24]在对干旱胁迫下抗旱性老芒麦幼苗的研究中发现,随着干旱胁迫时间的持续和强度的增强,植物·OH 清除率、O2-·生成速率和H2O2的含量呈上升趋势。张木清等[25]研究发现,干旱胁迫会促进细胞内·OH、O2-·和H2O2的累积,这与本文研究结果一致。
马福林等[26]研究表明,受到干旱胁迫后,植物体内抗氧化酶活性并不都处于持续上升的过程;随着干旱胁迫的加剧,植物体内抗氧化酶活性的变化是动态的,即在受到胁迫初期呈现上升的趋势,随着胁迫的加剧又处于下降的趋势。本研究得到了相同的结果,即花前和花期灌水处理和对照脱萼萼筒的SOD、POD、CAT 活性在大蕾期后10 d 均呈上升趋势;随着胁迫的加剧,脱萼萼筒的SOD、POD、CAT活性在大蕾期后20 d 均呈下降趋势,并且对照相对于花前和花期灌水处理的ROS 清除酶活性下降最明显。
ROS是植物PCD的关键影响因子,与发育过程和环境应激反应有关,ROS含量较低可以提高植物的抗逆性,而较高则可能导致PCD,并且ROS 含量的变化会影响PCD进程,从而影响离区发育。在干旱胁迫下,一定含量的ROS 自由基对植物生长有利,但是过多的ROS 自由基会毒害植物,破坏植物的光合系统和细胞膜稳定性,从而使植物的生长受到抑制[27]。在本研究中,花前和花期灌水处理和对照在大蕾期后10 d 和大蕾期后20 d 宿萼萼筒的ROS含量随着干旱胁迫的加剧变化并不显著,与之相对应的抗氧化酶活性也处于平稳状态;同时大蕾期后20 d脱萼的ROS含量明显高于大蕾期后10 d,并且相对应的抗氧化酶活性也随干旱胁迫的加剧明显降低,脱萼率与活性氧呈极显著正相关,与抗氧化酶活性呈极显著负相关。这与谷岩等[28]结论相一致。说明随着干旱胁迫的加剧,萼筒的抗氧化酶不足以清除多余的ROS,从而影响PCD进程。
刘喜明等[29]认为巨尾桉脱落树皮离区细胞形成和分离中细胞属于主动的PCD,本试验通过石蜡切片、DAPI 染色、TUNEL 检测、DNA laddering得出了相似的结论。本研究结果表明,离区细胞已经具备了明显的PCD 特征,随着离区细胞的发展,PCD 程度不断深化。TUNEL检测和DNA梯度检测的结论均为阐明离区细胞死亡的PCD 特征提供了充足的证据。
4 结 论
综上所述,在不同物候期灌水对香梨萼片脱落的影响并不显著,而持续的干旱胁迫萼片脱落效果最为明显,并且随着干旱胁迫的加剧萼筒ROS含量升高导致抗氧化酶无法清除从而影响萼筒离区发育,影响PCD进程,促进香梨萼片脱落。
[1]刘艳,吴运建.库尔勒香梨研究进展[J].新疆农垦科技,2015,38(2):23-26.LIU Yan,WU Yunjian.Research progress of Korla fragrant pear[J].Xinjiang Farm Research of Science and Technology,2015,38(2):23-26.
[2]陈园园,罗嘉亮,李凯,宋宇琴,李六林.梨脱萼果与宿萼果品质比较[J].北京农学院学报,2018,33(1):15-21.CHEN Yuanyuan,LUO Jialiang,LI Kai,SONG Yuqin,LI Liulin.Comparative studies on quality and texture of leaving calyx and persistent calyx fruits in pear[J].Journal of Beijing University of Agriculture,2018,33(1):15-21.
[3]蔡庆生.植物生理学[M].北京:中国农业大学出版社,2011:275-277.CAI Qingsheng.Plant physiology[M].Beijing:China Agricultural University Press,2011:275-277.
[4]SIMONS R K.Tissue response of young developing apple fruits to freeze injury1[J].Journal of the American Society for Horticultural Science,1969,94(4):376-382.
[5]程平,赵明玉,李宏,张志刚,马文涛,刘帮,郝凤.干旱胁迫对苹果树生长、光合特性及果实品质的影响[J].云南大学学报(自然科学版),2022,44(2):405-414.CHENG Ping,ZHAO Mingyu,LI Hong,ZHANG Zhigang,MA Wentao,LIU Bang,HAO Feng.Effects of drought on growth,photosynthetic characteristics and fruit quality of apple trees[J].Journal of Yunnan University (Natural Sciences Edition),2022,44(2):405-414.
[6]胡彦,何虎翼,何龙飞.活性氧与植物细胞程序性死亡[J].文山师范高等专科学校学报,2005(2):125-128.HU Yan,HE Huyi,HE Longfei.Reactive oxygen species and programmed cell death in higher plants[J].Journal of Wenshan Teachers College,2005,18(2):125-128.
[7]薛鑫,张芊,吴金霞.植物体内活性氧的研究及其在植物抗逆方面的应用[J].生物技术通报,2013(10):6-11.XUE Xin,ZHANG Qian,WU Jinxia.Research of reactive oxygen species in plants and its application on stress tolerance[J].Biotechnology Bulletin,2013(10):6-11.
[8]杨淑慎,高俊凤.活性氧、自由基与植物的衰老[J].西北植物学报,2001,21(2):215-220.YANG Shushen,GAO Junfeng.Influence of active oxygen and free radicals on plant senescence[J].Acta Botanica Boreali-Occidentalia Sinica,2001,21(2):215-220.
[9]王爱国,邵从本,罗广华,郭俊彦,梁厚果.大豆下胚轴线粒体的衰老与膜脂的过氧化作用[J].植物生理学报,1988,14(3):269-273.WANG Aiguo,SHAO Congben,LUO Guanghua,GUO Junyan,LIANG Houguo.Senescence and peroxidation of membrane lipid in mitochondria of soybean hypocotyl[J].Physiology and Molecular Biology of Plants,1988,14(3):269-273.
[10]MITTLER R.Oxidative stress,antioxidants and stress tolerance[J].Trends in Plant Science,2002,7(9):405-410.
[11]张倩,李萧婷,吴翠云,张锐,包建平,陶书田.不同灌溉方式对库尔勒香梨果实品质的多变量分析[J].新疆农业科学,2022,59(3):578-587.ZHANG Qian,LI Xiaoting,WU Cuiyun,ZHANG Rui,BAO Jianping,TAO Shutian.Multivariate analysis of fruit quality of Korla pear irrigated by different methods[J].Xinjiang Agricultural Sciences,2022,59(3):578-587.
[12]马璠,张鹏辉,王健,郑腾辉,张庆玮.土壤样品含水量快速测定方法的比较研究[J].安徽农业科学,2014,42(20):6591-6593.MA Fan,ZHANG Penghui,WANG Jian,ZHENG Tenghui,ZHANG Qingwei.Comparison of rapid methods for soil water content[J].Journal of Anhui Agricultural Sciences,2014,42(20):6591-6593.
[13]朱秀云,马玉,梁梦.小液流法测定植物水势实验综述报告[J].科技视界,2020(10):178-180.ZHU Xiuyun,MA Yu,LIANG Meng.A review of the experiments for the determination of water potential in plant tissues by small liquid flow method[J].Science&Technology Vision,2020(10):178-180.
[14]李贵荣,杨胜圆.党参多糖的提取及其对活性氧自由基的清除作用[J].化学世界,2001,42(8):421-422.LI Guirong,YANG Shengyuan.Extraction of Codonopsis pilosula polysaccharide and its effects of anti-active oxygen free radicals[J].Chemical World,2001,42(8):421-4224.
[15]石润霖,尹亚辉,杜维,李瑞龙,王金辉,董亮,赵长新.酿酒酵母老化过程中超氧化物歧化酶活性和O2-·生成速率变化的研究[J].食品科技,2015,40(9):2-6.SHI Runlin,YIN Yahui,DU Wei,LI Ruilong,WANG Jinhui,DONG Liang,ZHAO Changxin.Investigation of changes in superoxide dismutase activity and O2-· production rates during the aging process of Saccharomyces cerevisiae cells[J].Food Science and Technology,2015,40(9):2-6.
[16]LIU Y,PAN Q H,YANG H R,LIU Y Y,HUANG W D.Relationship between H2O2 and jasmonic acid in pea leaf wounding response[J].Russian Journal of Plant Physiology,2008,55(6):765-775.
[17]衣海青,王建华.芦丁和抗坏血酸对NBT 光还原法测定植物组织SOD 活性的干扰[J].植物生理学通讯,1996,32(4):276-278.YI Haiqing,WANG Jianhua.Interference of rutin and ascorbic acid on determination of SOD activity in plant tissues by NBT photoreduction method[J].Plant Physiology Communications,1996,32(4):276-278.
[18]郑炳松.现代植物生理生化研究技术[M].北京:气象出版社,2006:91-92.ZHENG Bingsong.Research techniques in contemporary plant physiology and biochemistry[M].Beijing:China Meteorological Press,2006:91-92.
[19]孙群,胡景江.植物生理学研究技术[M].杨凌:西北农林科技大学出版社,2006:167-170.SUN Qun,HU Jingjiang.Research technology of plant physiology[M].Yangling:Northwest Agriculture and Forestry University Press,2006:167-170.
[20]周乃富,张俊佩,刘昊,查巍巍,裴东.木本植物非均质化组织石蜡切片制作方法[J].植物学报,2018,53(5):653-660.ZHOU Naifu,ZHANG Junpei,LIU Hao,ZHA Weiwei,PEI Dong.New protocols for paraffin sections of heterogeneous tissues of woody plants[J].Chinese Bulletin of Botany,2018,53(5):653-660.
[21]王蕊.葫芦巴碱诱导烟草Bright Yellow-2 细胞凋亡及其分子机制研究[D].哈尔滨:东北林业大学,2010.WANG Rui.Mechanism of apoptosis in bright yellow-2 tobacco suspension cell induced by trigonelline[D].Harbin:Northeast Forestry University,2010.
[22]张婧.板栗短雄花序发育期间细胞程序性死亡研究[D].北京:北京农学院,2007.ZHANG Jing.Studies on the programmed cell death during mutant short catkin development in chestnut[D].Beijing:Beijing University of Agriculture,2007.
[23]任莹莹.影响库尔勒香梨果实品质的相关因子及提高果实品质的措施研究[D].乌鲁木齐:新疆农业大学,2007.REN Yingying.Studies on influencing related factors of fruit quality and measures of improving fruit quality in Korla fragrant pear[D].Urumqi:Xinjiang Agricultural University,2007.
[24]杨伟,刘文辉,马祥,马晖玲.干旱胁迫对2 种不同抗旱性老芒麦幼苗ROS 积累及抗氧化系统的影响[J].草地学报,2020,28(3):684-693.YANG Wei,LIU Wenhui,MA Xiang,MA Huiling.Effects of ROS accumulation and antioxidant system in two different drought resistant Elymus sibiricus under drought stress[J].Acta Agrestia Sinica,2020,28(3):684-693.
[25]张木清,陈如凯,余松烈.水分胁迫下蔗叶活性氧代谢的数学分析[J].作物学报,1996,22(6):729-735.ZHANG Muqing,CHEN Rukai,YU Songlie.Mathematical analysis for the active oxygen metabolism in the drought-stressed leaves of sugarcane[J].Acta Agronomica Sinica,1996,22(6):729-735.
[26]马福林,马玉花.干旱胁迫对植物的影响及植物的响应机制[J].宁夏大学学报(自然科学版),2022,43(4):391-399.MA Fulin,MA Yuhua.Effect of drought stress on plants and their response mechanism[J].Journal of Ningxia University(Natural Science Edition),2022,43(4):391-399.
[27]王晓雪,李越,张斌,贾举庆,冯美臣,张美俊,杨武德.干旱胁迫及复水对燕麦根系生长及生理特性的影响[J].草地学报,2020,28(6):1588-1596.WANG Xiaoxue,LI Yue,ZHANG Bin,JIA Juqing,FENG Meichen,ZHANG Meijun,YANG Wude.Effects of drought stress and rehydration on root growth and physiological characteristics of oats[J].Acta Agrestia Sinica,2020,28(6):1588-1596.
[28]谷岩,梁煊赫,王振民,何文安,赵福林,吴春胜.不同抗旱性玉米苗期叶片活性氧代谢对水分胁迫的响应[J].安徽农业科学,2009,37(29):14089-14091.GU Yan,LIANG Xuanhe,WANG Zhenmin,HE Wen’an,ZHAO Fulin,WU Chunsheng.Response of activated oxygen metabolism to water stress in the leaves of different drought-resistant corns in seedling stage[J].Journal of Anhui Agricultural Sciences,2009,37(29):14089-14091
[29]刘喜明,陈瑞英,陈居静,于再君.巨尾桉韧皮部周皮形成过程的研究[J].福建林学院学报,2013,33(3):262-266.LIU Ximing,CHEN Ruiying,CHEN Jujing,YU Zaijun.Study on the periderm forming process in Eucalyptus grandis×E.urophylla phloem[J].Journal of Fujian College of Forestry,2013,33(3):262-266.