葡萄作为四大水果之一,因其营养丰富,适应性强,普遍受到消费者和生产者的青睐,在世界范围内被广泛栽培。然而,葡萄生产过程中经常遭受霜霉病、白腐病等真菌病害的危害,严重影响葡萄的产量和品质。葡萄霜霉病是全世界范围内严重危害葡萄的真菌病害之一[1],该病原菌主要以卵孢子形态在病残组织内越冬,5 月份通过气流或雨滴溅散传播,典型的症状为叶片背面出现白色霜状霉层,即病菌的孢子囊和孢子梗,同时侵染嫩梢、卷须、叶柄和幼果等部位,严重危害葡萄的营养器官和生殖器官[2-3]。
木质素是一种多酚聚合物,被木纤维、其他维管束细胞和厚壁细胞包围[4-5],是植物中重要的次生物质,不仅可以增强植物细胞和组织的强度,有利于植物组织中的水分运输,同时也能提高植物抵抗病虫害的能力,其总量是仅次于纤维素的第二大有机物[6-7]。木质素的生物合成是苯丙氨酸或酪氨酸在一系列酶的催化下逐渐转化为木质素单体,继而形成木质素的过程[8-9],该过程由3个途径组成:苯丙烷途径、木质素合成的特定途径以及木质素单体向木质素的糖基化转运和聚合的途径。咖啡酸O-甲基转移酶(COMT)是苯丙烷代谢途径中重要的甲基化酶[10-11],COMT有多种功能,如催化咖啡酸的甲基化,5-羟基苯基醛生成阿魏酸、芥末醛等,还能催化S-腺苷L-蛋氨酸(SAM或AdoMet)的甲基基团形成阿魏酸和S-腺苷L-同型半胱氨酸(SAH或AdoHcy)来调节木质素的合成,且其N 端在没有金属离子的环境下就能进行同源二聚化,除参与木质素合成外还在类黄酮和芥子酸酯等物质中发挥催化作用[12-13]。
前人研究表明,COMT基因家族包含多个成员,如毛杨中有25个[14],拟南芥和甘蓝型油菜中分别有14个[15]和42个[16]。在其他物种中也有相关的研究,如烟草[17]、燕麦[18]、松树[19]、水稻[20]、大麦[21]和蓝莓果实[22]。植物病原菌侵染和植株果实发育包含木质素的积累过程,而COMT 基因已被证明在木质素积累过程中起着关键作用。Petitot 等[23]发现非洲水稻COMT3 在根结线虫侵染过程中表达量明显升高;Fornalé 等[24]抑制玉米COMT 基因的表达后发现其总木质素含量和S单位/G单位比降低;Wang等[25]过表达COMT-3D 基因使得转基因小麦的耐病性与木质素含量得到了提高。因此,COMT 基因对植物抵抗生物和非生物胁迫尤为重要。但在葡萄中尚未对COMT基因家族的特征和功能进行全面研究。笔者在本研究中参考乃国洁等[26]的生物信息学方法,鉴定了葡萄COMT基因家族,分析其蛋白质理化性质、染色体定位、保守结构域和基序分析,同时研究不同抗性葡萄品种COMT 基因接种霜霉病的表达模式,旨在挖掘葡萄霜霉病响应的关键COMT 基因,有利于进一步明确该基因家族在葡萄抗病反应过程中的作用,为葡萄抗病品种的选育奠定基础。
1 材料和方法
1.1 葡萄COMT基因的鉴定及理化性质分析
葡萄基因组数据库(v2.1)来自phytozome 13(https://Phytozome-next.igi.doe.gov),首先在拟南芥数据库(https://www.arabidopsis.org)搜索O-甲基转移酶基因,基于Pfam 数据库的隐马尔可夫模型(PF00891),利用phytozome 13非冗余蛋白质数据库中BLASTP 工具搜索比对,获取葡萄COMT 家族候选基因,删除重复和冗余序列,确定VvCOMT 基因家族。利用在线ExPASy(http://web.expasy.org/)和Plant-PLocserve(http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/)工具预测VvCOMT蛋白的理化性质,包括蛋白长度、分子质量、等电点等,并预测亚细胞定位,利用线上分析软件ProtParam(http://web.expasy.org/protparam)进行蛋白质二级结构预测。
1.2 系统发育树的构建及染色体定位
运用MEGA 11 软件中的Clustal W 程序将拟南芥、水稻、玉米、大豆和番茄等物种蛋白序列进行多序列比对,并通过邻接法(neighbour-joining,NJ)和最大似然法(maximum likelihood,ML)构建系统发育树,Bootstrap 检验设定1000 次重复,以评价系统发育树的统计可靠性。利用iTOL(http://iTOL.embl.de)在线软件对进化树进行美化。
为了解VvCOMTs基因在基因组内的分布,通过JGI 数据库获得基因组注释文件中提供的位置信息,利用TBtools软件[27]将葡萄COMT基因定位到相应的染色体上。
1.3 VvCOMT家族基序与启动子顺式作用分析
为更好地理解和调控VvCOMTs的基因功能,利用 在 线 程 序MEME(v4.3)(http://meme.nbcr.net/meme/)分析VvCOMTs 序列特征蛋白保守模块(motif),查找的motif数量设置为20,运行参数为默认。
从欧洲葡萄数据库中查找VvCOMTs 起始密码子ATG 上游2 kb 的序列,提交使用在线程序Plant-CARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/)预测VvCOMT 启动子区域的顺式作用元件,将获得的顺式作用元件提交至TBtools软件中进行启动子可视化。
1.4 VvCOMT 基因家族成员在葡萄霜霉病中的表达分析
为了解VvCOMTs在抗霜霉病侵染中的作用,采集病叶制作霜霉病病原孢子(浓度为1×105个·mL-1),材料选择抗病品种摩尔多瓦和感病品种夏黑接种制备的霜霉病菌,接种后0、6、48、96、120 h分别采样,液氮速冻于-80 ℃保存。样品RNA 提取采用试剂盒法(OMEGA,美国),利用UEIris RT mix with DNase(All-in-One)合成cDNA,使用实时荧光定量PCR(qRT-PCR)技术分析基因表达水平,荧光定量反应体系10 μL:TB Green®Premix Ex Taq™(TaKaRa,大连)5 μL,模板0.5 μL,上、下游引物1 μL,ddH2O 3.5 μL。荧光定量反应程序为:50 ℃2 min,95 ℃2 min,然 后95 ℃15 s,60 ℃30 s,39 次 循 环。Thresh值按PCR仪默认为30,分别记录每个反应荧光信号由本底进入指数增长阶段的拐点所对应的循环数(threshold cycle,Ct),然后用2-△△CT法[28]以未接种霜霉病菌的叶片为对照,对不同时间点VvCOMTs基因的相对表达量进行分析。所有样本使用3次生物重复进行分析。使用葡萄Actin作为内参引物,本研究中使用的所有引物均列于表1。
表1 用于qRT-PCR 反应的引物序列
Table 1 The primer sequences used for qRT-PCR amplification
基因名称Gene name VvCOMT1 VvCOMT2 VvCOMT3 VvCOMT4 VvCOMT5 VvCOMT6 VvCOMT7 VvCOMT8 VvCOMT9 VvCOMT10 VvCOMT11 VvCOMT12 VvCOMT13 VvCOMT14 VvCOMT15 VvCOMT16 VvCOMT17 VvCOMT18 VvCOMT19 VvCOMT20 VvCOMT21 VvCOMT22 VvCOMT23 VvCOMT24 VvCOMT25 VvCOMT26 VvACT上游引物Forward primer(5’-3’)CCCCTTTTGCACTTGATCG CCGCCTTCTCACCACTTACG TTGAAAGCAGCCTTGGAGC TCCCAACTTGTCAGCCTTCG CTCATGCGTTTGCTGGTGC AGCTTGGCACCGATTGTTC GTGACGATGGGTGCAAGAAG TCATGCGTGTTCTTGTTCAATC AAAATGATGACCCCACTCCG TGGGCACAAGTGAAAACGG AGCAGCGTATAAAGATGGGTTC CCCCGACATCATTCACAACC GGCTTTGGCAGTTGGTGAG TCAGTGCGTGTACCGTCTCAT TCAGTGCGTGTACCGTCTCAT TTCCAAAATGAAGACCCCACC AGAAAGTGCGGACGAAGAGG TCCTTGCCTCCATCGTCTTA TGATCCCACTCCGTTCCAC GACCCCACCCCATTTCATAC GAAGCAAGAGGCGGCAGAG GGGGATAGGGGAGAGGATGT GCAAGCGTGCTGATTACCAA CTGCGTGCAAACAACCCG ACAGTCTCAGCCAACATTCCTT CTCGGTCAACATTCCTTTCG AACCCCAAGGCCAACAGAGAAAA下游引物Reverse primer(5’-3’)CCACTGTGACCTTGTCCTGAAT CGCTGTCATCAACTCCTCCTG AGGGTTGTGGGTGGGGAT TTCCCTCTGAACCAGTCTCCTA GATAAGTTGGAAGTTGGGTTGTCT TGGTTCCCATAGTTCCAGAGG GTAGCATTCAGTGATGGAGG TGCGTAAGCACATACCCCTC AATACGCCCTTGCCCTCC GATTGGGCGGGCGTGA TCGAAGTTAATGCCCTTGATGT CCCTCTTCGTCCGCACTTT TTGTGGATGATGTCTGGGATG CCCCTCTTCTTCCTTACCTTGT CACATACCCCTCTTCTTCCTCA CCGACCTCAACCAACCACC CGAGCAGTAGCAAGAAGGGTG TTCTTGCTCATTGTGGTCGG AAGCAGCACGCTGATGACTAA CAACCCTTCAAACACCTCCTT CGGGTTGTTTGTGGCTAAGTG TTCCCACACCCACCCATCGA TCCCCTCCTACAACCTCCAA CTTGAGCCGCCCACCATC ACTCCTTCTGGGTCCTTTCTTT ACTCCTTCTGGGTCCTTTCTTT CACCATCACCAGAATCCAGCACA
1.5 统计分析
采用SPSS Stantistics v.26.0 软件对数据进行方差分析(ANOVA)。采用最小显著性差异(p<0.05)进行显著性分析。
2 结果与分析
2.1 葡萄COMT 基因的鉴定和蛋白质理化性质分析
通过生物信息学分析获得26条VvCOMT基因,按照染色体位置分别命名为VvCOMT1~26。基因结构和保守结构域分析表明,26个葡萄COMT都具有一个名为Methyltransf_2 结构域的C 端催化结构域(PF00891),包括SAM/SAH 结合袋和底物结合位点[29],SAM/SAH 结合袋高度保守,而底物结合位点对不同组中的蛋白质具有特异性[30](图1)。利用ExPASy 在线工具进行蛋白理化性质分析(表2),84.6%的葡萄COMT氨基酸长度超过300 aa,且氨基酸数分布在189~395个之间,其中VvCOMT24(VIT_215s0048g02460)序列最长,有395 个氨基酸,VvCOMT3(VIT_208s0032g01130)序列最短,氨基酸数只有189。蛋白质相对分子质量在21 179.45(VvCOMT3)~43 521.88 Ku(VvCOMT24);等电点分布在5.18(VvCOMT22)~6.23(VvCOMT3)之间,且等电点都小于7;蛋白质不稳定系数在27.21(VvCOMT23)~43.30(VvCOMT5),其中6 条不稳定系数大于40 属于不稳定蛋白;亲水指数在-0.158(VvCOMT16)~0.079(VvCOMT7)之间均为两性蛋白;亚细胞定位结果显示26个COMT蛋白定位于细胞质和细胞外。

图1 VvCOMT1,VvCOMT5 和VvCOMT19 蛋白序列与TaCOMT-3a 蛋白的多重序列比对
Fig.1 Multiple sequence alignment of VvCOMT1,VvCOMT5 and VvCOMT19 with TaCOMT-3a genes
绿色.SAM 结合;蓝色.底物结合;橙色.催化残基。
Green.SAM binding;Blue.Substrate binding;Orange.Catalytic residues.
表2 葡萄COMT 基因家族的理化性质
Table 2 Physicochemical properties of COMT gene in grape
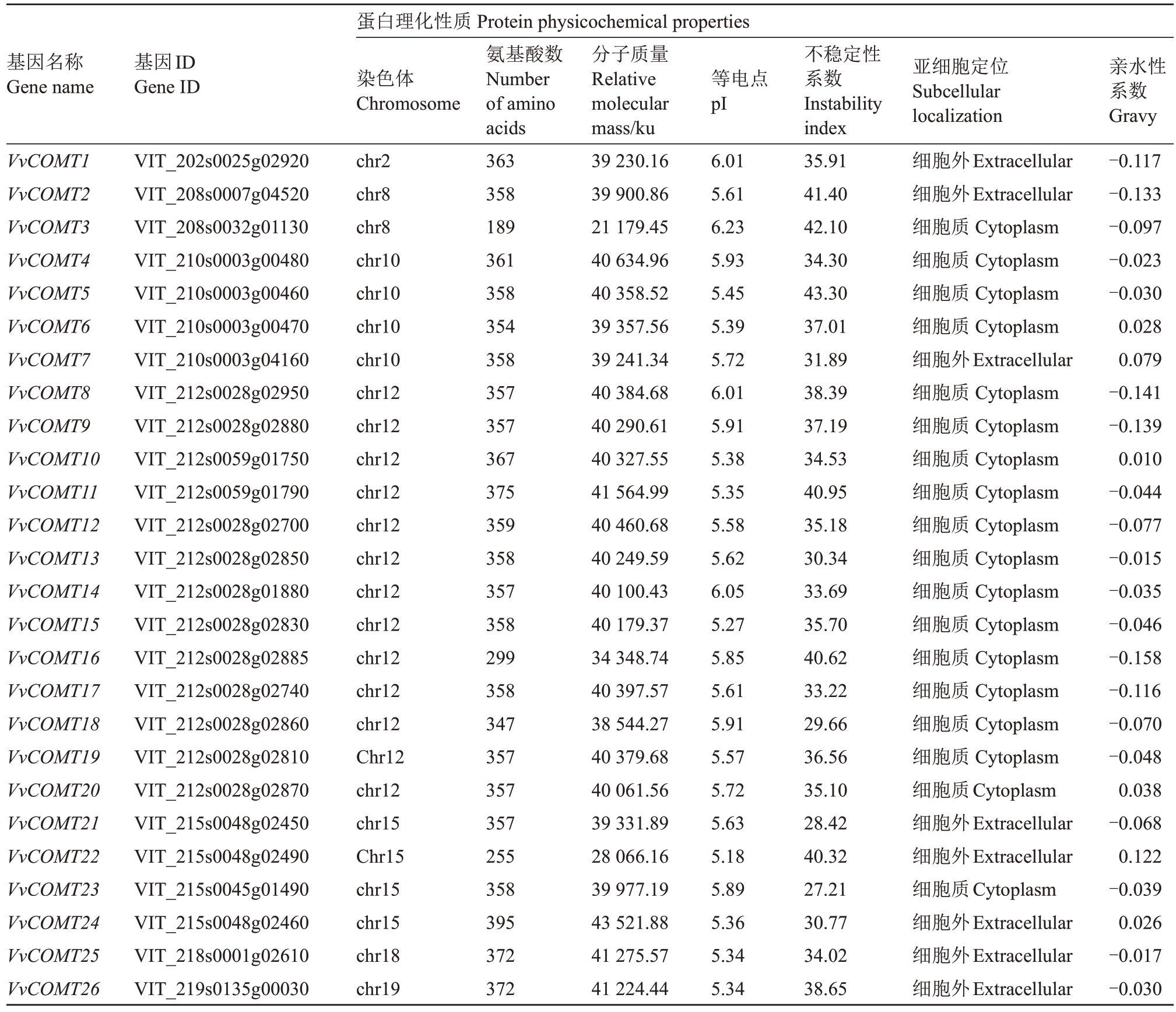
蛋白理化性质Protein physicochemical properties基因名称Gene name基因ID Gene ID 染色体Chromosome等电点pI VvCOMT1 VvCOMT2 VvCOMT3 VvCOMT4 VvCOMT5 VvCOMT6 VvCOMT7 VvCOMT8 VvCOMT9 VvCOMT10 VvCOMT11 VvCOMT12 VvCOMT13 VvCOMT14 VvCOMT15 VvCOMT16 VvCOMT17 VvCOMT18 VvCOMT19 VvCOMT20 VvCOMT21 VvCOMT22 VvCOMT23 VvCOMT24 VvCOMT25 VvCOMT26 VIT_202s0025g02920 VIT_208s0007g04520 VIT_208s0032g01130 VIT_210s0003g00480 VIT_210s0003g00460 VIT_210s0003g00470 VIT_210s0003g04160 VIT_212s0028g02950 VIT_212s0028g02880 VIT_212s0059g01750 VIT_212s0059g01790 VIT_212s0028g02700 VIT_212s0028g02850 VIT_212s0028g01880 VIT_212s0028g02830 VIT_212s0028g02885 VIT_212s0028g02740 VIT_212s0028g02860 VIT_212s0028g02810 VIT_212s0028g02870 VIT_215s0048g02450 VIT_215s0048g02490 VIT_215s0045g01490 VIT_215s0048g02460 VIT_218s0001g02610 VIT_219s0135g00030 chr2 chr8 chr8 chr10 chr10 chr10 chr10 chr12 chr12 chr12 chr12 chr12 chr12 chr12 chr12 chr12 chr12 chr12 Chr12 chr12 chr15 Chr15 chr15 chr15 chr18 chr19氨基酸数Number of amino acids 363 358 189 361 358 354 358 357 357 367 375 359 358 357 358 299 358 347 357 357 357 255 358 395 372 372分子质量Relative molecular mass/ku 39 230.16 39 900.86 21 179.45 40 634.96 40 358.52 39 357.56 39 241.34 40 384.68 40 290.61 40 327.55 41 564.99 40 460.68 40 249.59 40 100.43 40 179.37 34 348.74 40 397.57 38 544.27 40 379.68 40 061.56 39 331.89 28 066.16 39 977.19 43 521.88 41 275.57 41 224.44 6.01 5.61 6.23 5.93 5.45 5.39 5.72 6.01 5.91 5.38 5.35 5.58 5.62 6.05 5.27 5.85 5.61 5.91 5.57 5.72 5.63 5.18 5.89 5.36 5.34 5.34不稳定性系数Instability index 35.91 41.40 42.10 34.30 43.30 37.01 31.89 38.39 37.19 34.53 40.95 35.18 30.34 33.69 35.70 40.62 33.22 29.66 36.56 35.10 28.42 40.32 27.21 30.77 34.02 38.65亚细胞定位Subcellular localization细胞外Extracellular细胞外Extracellular细胞质Cytoplasm细胞质Cytoplasm细胞质Cytoplasm细胞质Cytoplasm细胞外Extracellular细胞质Cytoplasm细胞质Cytoplasm细胞质Cytoplasm细胞质Cytoplasm细胞质Cytoplasm细胞质Cytoplasm细胞质Cytoplasm细胞质Cytoplasm细胞质Cytoplasm细胞质Cytoplasm细胞质Cytoplasm细胞质Cytoplasm细胞质Cytoplasm细胞外Extracellular细胞外Extracellular细胞质Cytoplasm细胞外Extracellular细胞外Extracellular细胞外Extracellular亲水性系数Gravy-0.117-0.133-0.097-0.023-0.030 0.028 0.079-0.141-0.139 0.010-0.044-0.077-0.015-0.035-0.046-0.158-0.116-0.070-0.048 0.038-0.068 0.122-0.039 0.026-0.017-0.030
2.2 系统发育树的构建和蛋白质二级结构分析
为更好地了解葡萄与其他植物COMT的相似性和差异性,利用26 条葡萄COMT 蛋白与8 条拟南芥、14 条玉米、28 条水稻、16 条大豆和12 条番茄共104条蛋白序列构建了系统发育树(图2)。VvCOMT的系统发育分析显示,26个VvCOMT蛋白序列可分为两组:GroupⅠ包含17个VvCOMT蛋白,其余9个VvCOMT蛋白属于Group Ⅱ。葡萄COMT基因家族成员呈现集中分布在2 个类群之中,在进化过程中较保守,具有高度的相似性,其中与拟南芥和水稻亲缘关系较近,与玉米的亲缘关系最远,表明葡萄与玉米之间的COMT基因差异显著。

图2 采用最大似然法构建的葡萄与其他植物OMT 基因家族成员的系统发育树
Fig.2 Phylogenetic tree of grapes and members of the OMT gene family of other plants constructed using maximum likelihood
Vv.葡萄;Os.水稻;Zm.玉米;Md.苹果。
Vv.Grapes;Os.Rice;Zm.Corn;Md.Apple.
通过ProtParam 在线分析工具预测葡萄COMT基因家族成员的二级结构(表3),葡萄COMT 基因家族均含有α-螺旋、β-转角、无规则卷曲和延伸链4种构型,其中α-螺旋和无规则卷曲两种构型的总占比为70%,而β-转角与延伸链两种构型则只占总比的30%。
表3 VvCOMT 家族蛋白二级结构
Table 3 VvCOMT family protein secondary structure
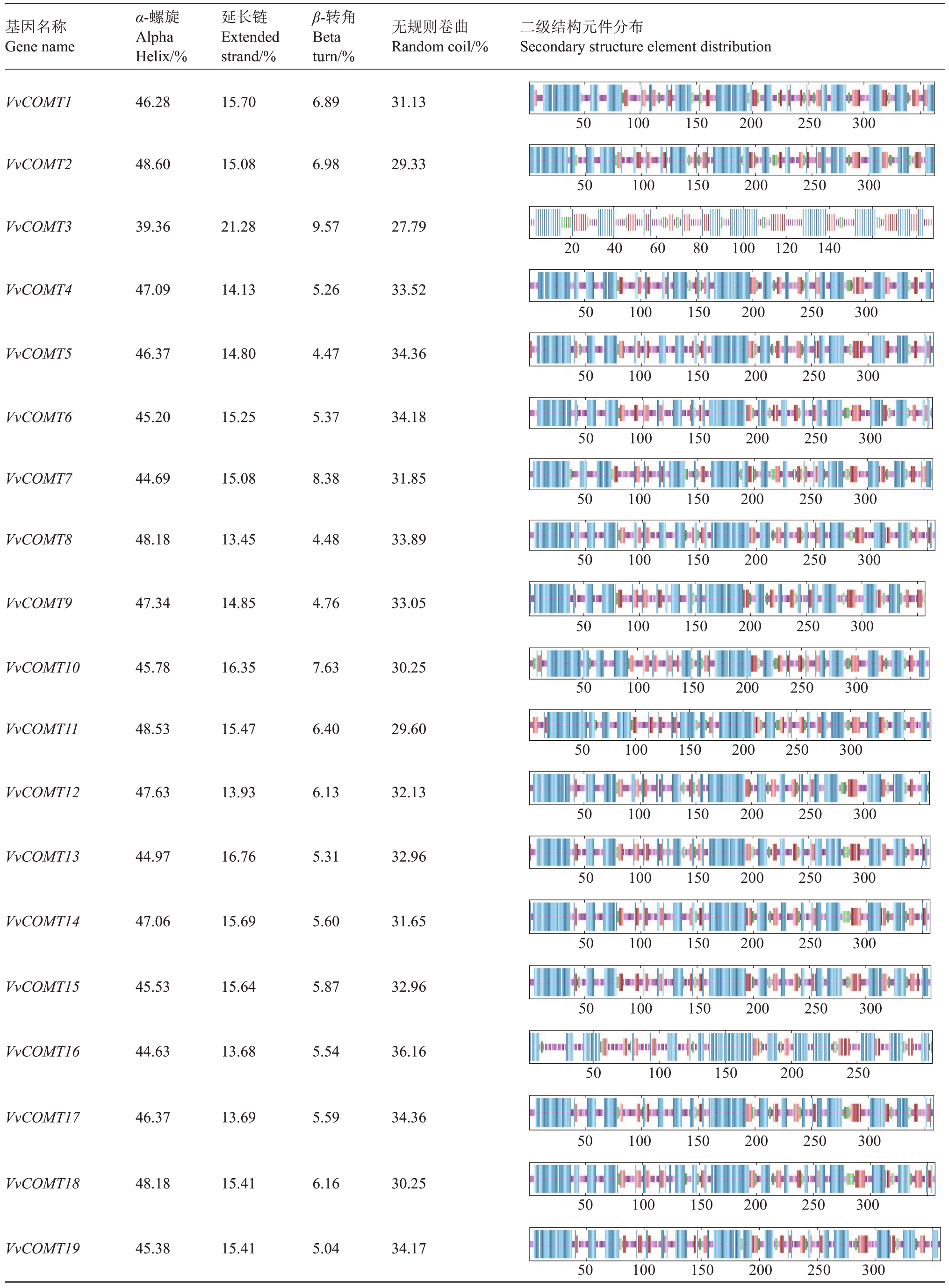
基因名称Gene name α-螺旋Alpha Helix/%延长链Extended strand/%β-转角Beta turn/%无规则卷曲Random coil/%二级结构元件分布Secondary structure element distribution VvCOMT1 VvCOMT2 VvCOMT3 VvCOMT4 VvCOMT5 VvCOMT6 VvCOMT7 VvCOMT8 VvCOMT9 VvCOMT10 VvCOMT11 VvCOMT12 VvCOMT13 VvCOMT14 VvCOMT15 VvCOMT16 VvCOMT17 VvCOMT18 VvCOMT19 46.28 48.60 39.36 47.09 46.37 45.20 44.69 48.18 47.34 45.78 48.53 47.63 44.97 47.06 45.53 44.63 46.37 48.18 45.38 15.70 15.08 21.28 14.13 14.80 15.25 15.08 13.45 14.85 16.35 15.47 13.93 16.76 15.69 15.64 13.68 13.69 15.41 15.41 6.89 6.98 9.57 5.26 4.47 5.37 8.38 4.48 4.76 7.63 6.40 6.13 5.31 5.60 5.87 5.54 5.59 6.16 5.04 31.13 29.33 27.79 33.52 34.36 34.18 31.85 33.89 33.05 30.25 29.60 32.13 32.96 31.65 32.96 36.16 34.36 30.25 34.17images/BZ_31_1332_631_2240_746.pngimages/BZ_31_1332_764_2239_879.pngimages/BZ_31_1332_897_2236_1013.pngimages/BZ_31_1332_1031_2238_1149.pngimages/BZ_31_1332_1166_2238_1287.pngimages/BZ_31_1332_1305_2235_1419.pngimages/BZ_31_1332_1437_2236_1551.pngimages/BZ_31_1332_1569_2241_1680.pngimages/BZ_31_1332_1697_2219_1824.pngimages/BZ_31_1332_1841_2228_1955.pngimages/BZ_31_1332_1973_2232_2087.pngimages/BZ_31_1332_2105_2229_2226.pngimages/BZ_31_1332_2244_2230_2362.pngimages/BZ_31_1332_2379_2229_2503.pngimages/BZ_31_1332_2521_2232_2642.pngimages/BZ_31_1332_2660_2235_2780.pngimages/BZ_31_1332_2797_2238_2924.pngimages/BZ_31_1332_2942_2241_3063.png50 100 150 200 250 300
表3 (续) Table 3 (Continued)
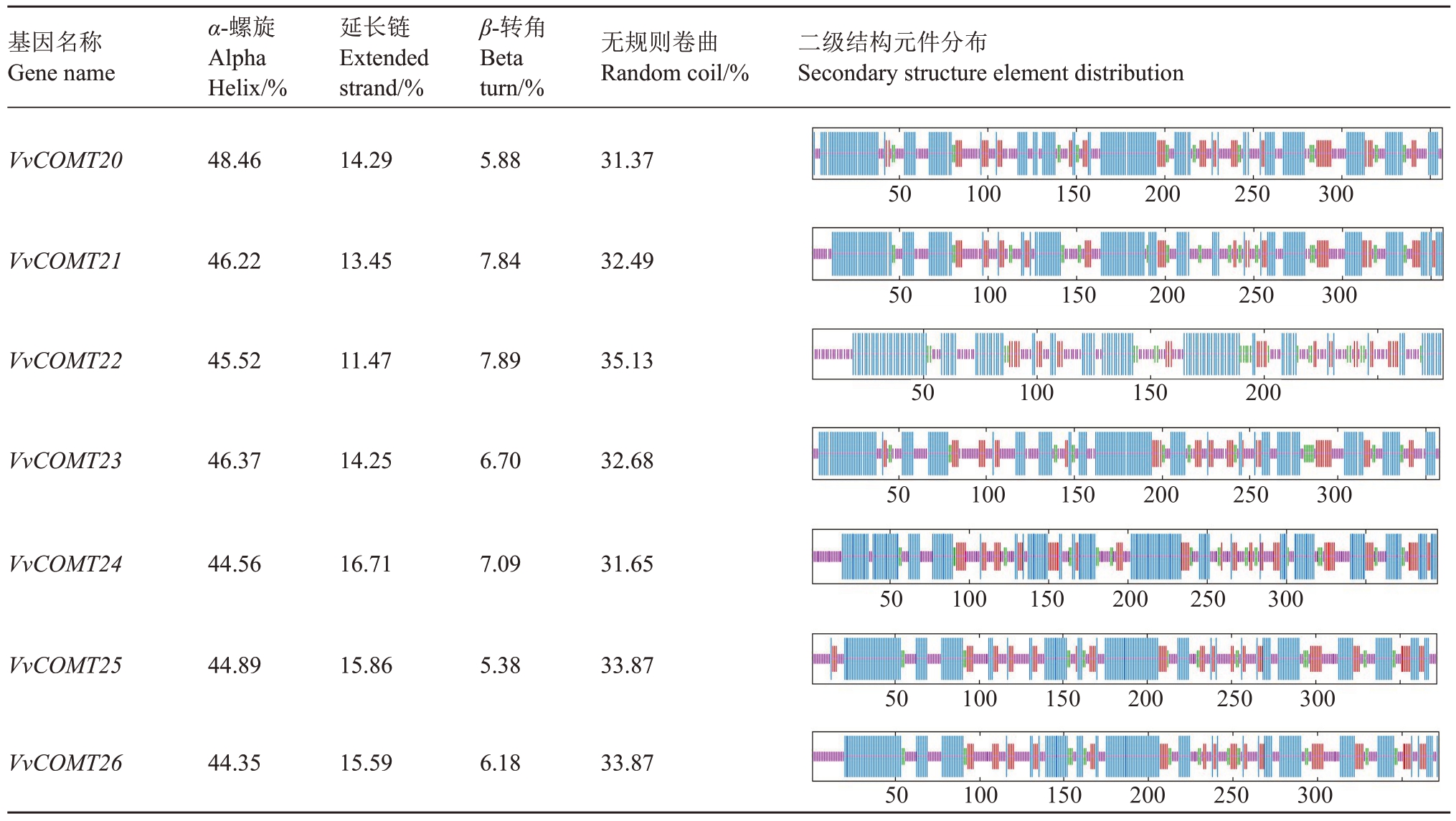
基因名称Gene name α-螺旋Alpha Helix/%延长链Extended strand/%β-转角Beta turn/%无规则卷曲Random coil/%二级结构元件分布Secondary structure element distribution VvCOMT20 48.46 14.29 5.88 31.37 50 100 150 200 250 300 VvCOMT21 46.22 13.45 7.84 32.49 50 100 150 200 250 300 VvCOMT22 45.52 11.47 7.89 35.13 50 100 150 200 VvCOMT23 46.37 14.25 6.70 32.68 50 100 150 200 250 300 VvCOMT24 44.56 16.71 7.09 31.65 50 100 150 200 250 300 VvCOMT25 44.89 15.86 5.38 33.87 50 100 150 200 250 300 VvCOMT26 44.35 15.59 6.18 33.87 50 100 150 200 250 300
2.3 染色体定位
利用TBtools软件进行染色体定位分析,结果显示,26条基因在7条染色体骨架上呈无规则分布,且不同染色体骨架上的基因分布密度不同(图3),其中第12 号染色体上基因分布最多,含有13 条VvCOMT 基因,第2、18 和19 号染色体上成员最少,各含有1条VvCOMT基因。
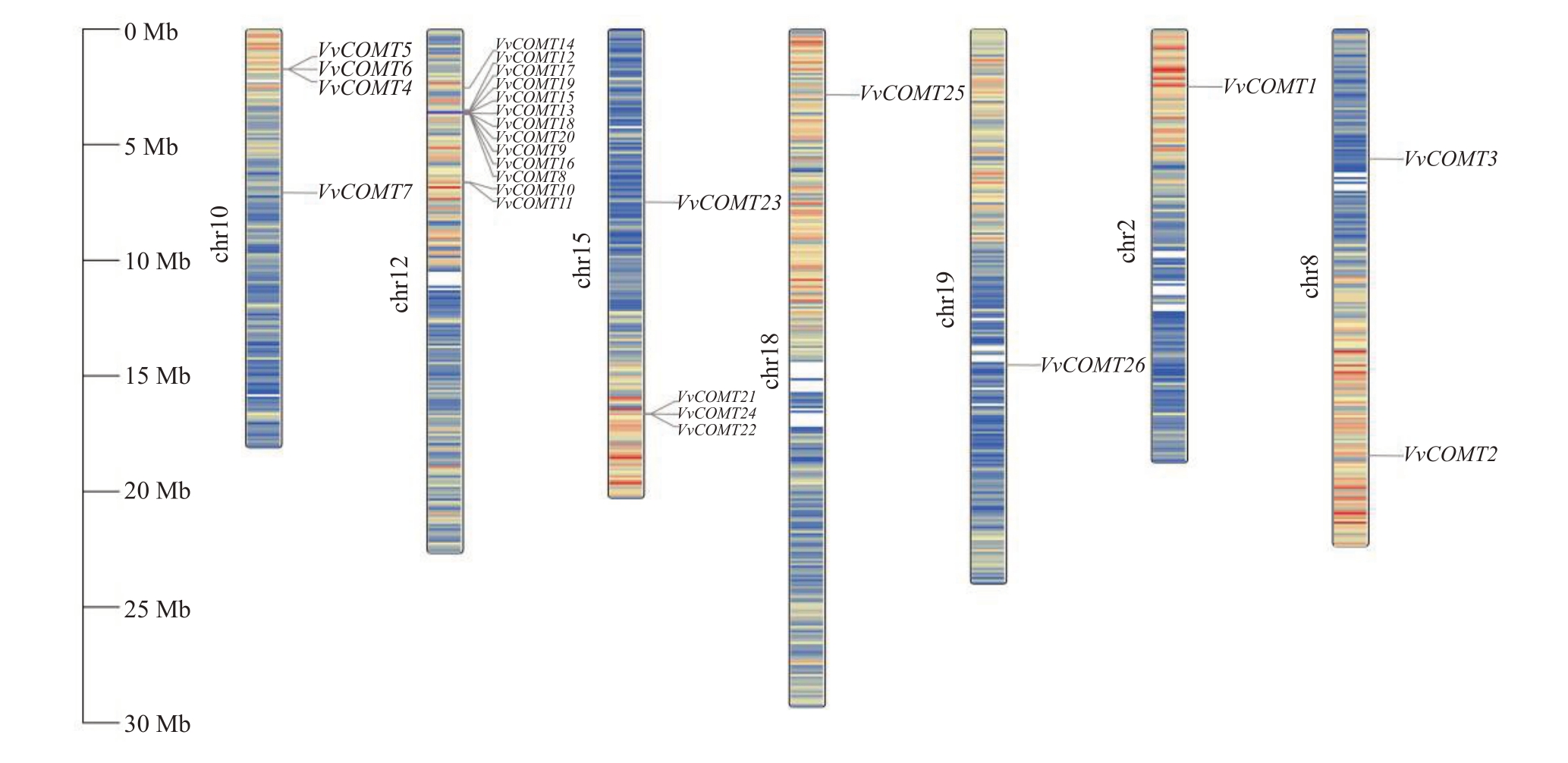
图3 VvCOMT 基因的染色体定位
Fig.3 Chromosomal localization of VvCOMT gene
2.4 基因结构与基序分析
利用MEME在线工具分析,发现葡萄COMT基因存在19 个较为保守的motif(图4),每条VvCOMT基因分布4~14 个motif,其中motif 6 存在于所有的COMT 基因,表明motif 6 具有很强的保守性。同时发现同一类群的COMT 基因包含的motif 相同,如Group Ⅰ中亲缘关系较近的VvCOMT 都含有motif 5、7、8、17 和19,Group Ⅱ与之不同,大多数COMT基因都含有motif 13、14 和16,VvCOMT4 中只含有motif 2、3、6、10 基序,不同的分支所包含基序的不同可能是VvCOMT 进化过程中发生功能分化的原因之一。

图4 基于进化关系的VvCOMT 家族成员的保守基序和外显子-内含子结构
Fig.4 The conserved motifs and exon-intron structures of VvCOMT family members based on the evolutionary relationship
根据VvCOMT系统发育关系,26个基因被分为3个亚组(图4),其中Ⅰ组与Ⅱ组与COMT基因外显子-内含子结构相似,都含有2 个外显子和1 个内含子,长度在302~775 bp之间,而Ⅲ组则包含3~4个外显子,且在同一进化枝中的外显子数量接近,说明系统发育树的可靠性。此外,26个COMT基因中形成9个旁系同源对,其中VvCOMT4/5/6,VvCOMT8/9及VvCOMT10/11步长值高达100。
2.5 启动子顺式作用元件分析
通过对葡萄COMT 基因家族成员上游2000 bp启动子区的顺式作用元件进行分析,笔者发现葡萄26 条COMT 基因中共存在243 个顺式作用元件,其中192 个激素相关元件(其中乙烯相关元件109 个,脱落酸相关元件39 个,水杨酸相关元件21 个,茉莉酸相关元件18 个,赤霉素相关元件5 个),真菌诱导相关元件20 个,防御和应激反应相关元件31 个(图5)。说明葡萄COMT基因可能参与激素响应和逆境胁迫响应过程。

图5 葡萄COMT 基因家族启动子顺式作用元件
Fig.5 Cis-elements of grape COMT gene family promoters
ABRE.脱落酸响应元件;TC-rich.防御和应激反应过程中涉及的相关响应元件;ERE.乙烯响应元件;TCA-element.水杨酸响应元件;W box.真菌诱导响应元件;GARE-motif.赤霉素响应元件;LTR.低温响应元件;TGACG-motif.茉莉酸响应元件;MBS.干旱响应元件。
ABRE.Abscisic acid response element;TC-rich.Relevant response elements involved in the process of defense and stress response; ERE.Ethylene response element;TCA-element.Salicylic acid response element;W box.Fungal induction response element;GARE-motif.Gibberellin response element;LTR.Low temperature response element;TGACG-motif.Jasmonic acid response element;MBS.Drought response element.
2.6 葡萄COMT 基因家族成员接种霜霉病的表达分析
为探究葡萄COMT 基因对霜霉病的响应,利用荧光定量PCR技术,分析抗病品种摩尔多瓦和感病品种夏黑在接种霜霉病后COMT 基因家族成员的表达量。由图6和图7可以看出,在抗病品种摩尔多瓦中,25 个VvCOMT 在霜霉病胁迫下均显著上调,其中42%的COMT 基因在接种后的48 h 即显著上调;而在感病品种夏黑中,VvCOMT1、2、、10、15、26和VvCOMT27 在接种后病原菌后出现显著下调,63%的COMT基因在接种后的24 h出现显著上调,其中VvCOMT2在2个品种中均无显著上调,表明其不响应霜霉病菌的侵染。此外,COMT基因在抗病品种的表达量显著高于感病品种,GroupⅠ中VvCOMT5/6/8/9和19在抗病品种中分别比感病品种高出13、15、21、120和7倍;Group Ⅱ中VvCOMT1和VvCOMT7在抗病品种中分别比感病品种高出102 和3580 倍;Ⅲ家族成员VvCOMT25 与VvCOMT26 比较特殊,均显著下调。综合COMT 基因在抗感品种中的表达,VvCOMT1、5、6、7、8、9 和VvCOMT19 上调最为显著,其最有可能在葡萄抵抗霜霉病菌胁迫过程中发挥着更重要的作用。
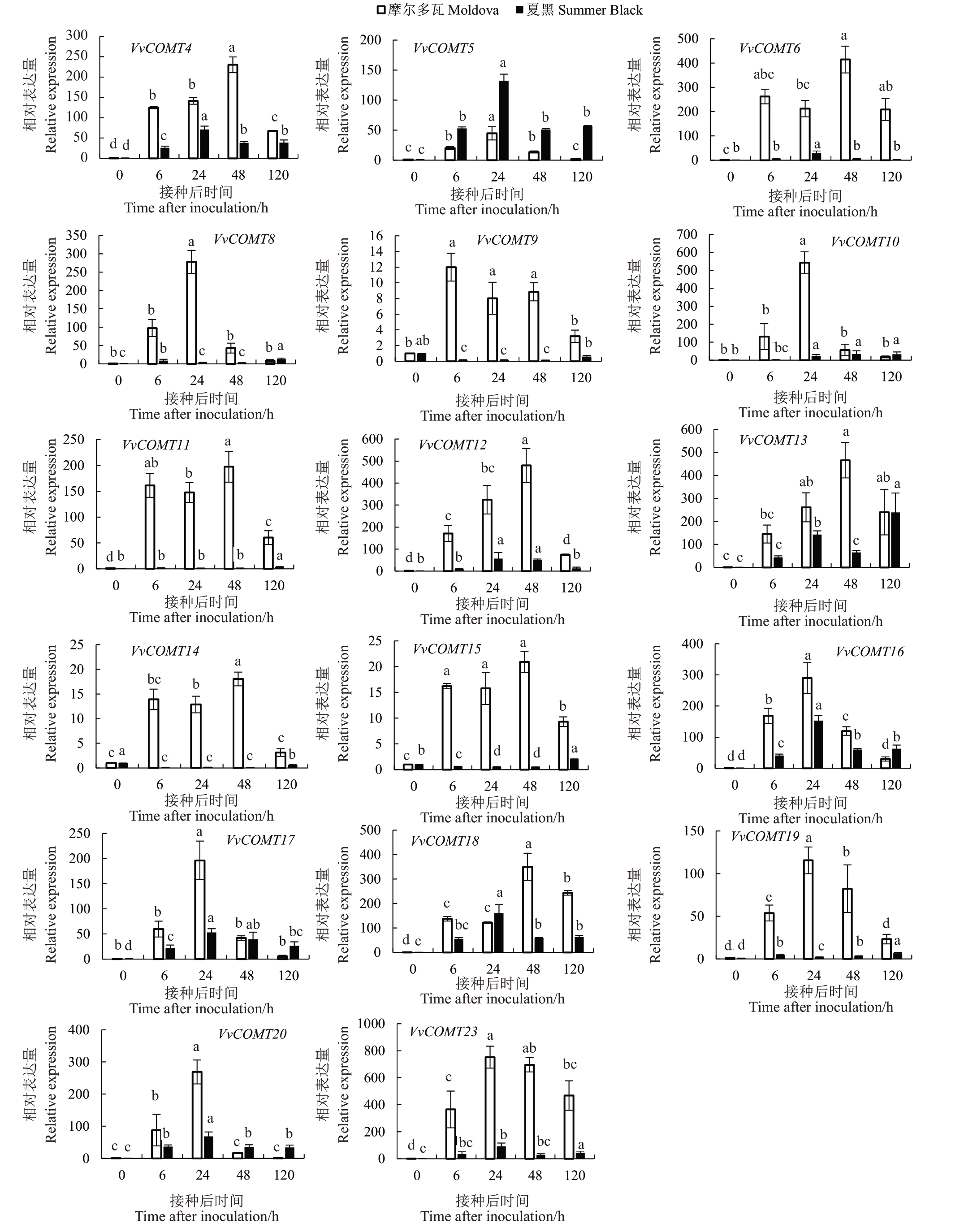
图6 VvCOMT 基因家族中GroupⅠ成员在不同品种接种霜霉病后的表达水平
Fig.6 Group in the VvCOMT gene GroupⅠexpression level of members after inoculation of different with downy mildew
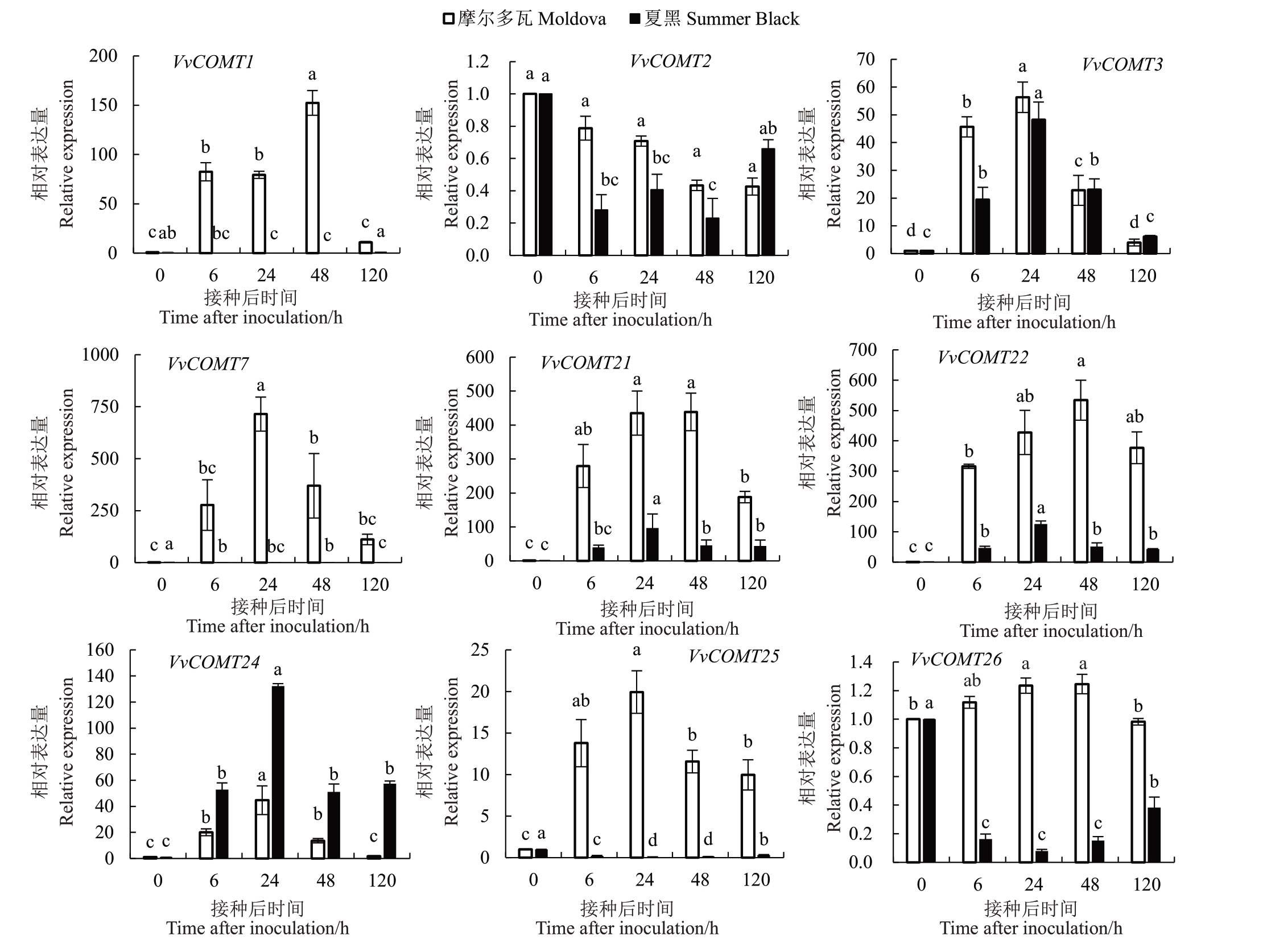
图7 VvCOMT 基因家族中Group Ⅱ成员在不同品种接种霜霉病后的表达水平
Fig.7 Group in the VvCOMT gene Group Ⅱexpression level of members after inoculation of different varieties with downy mildew
3 讨 论
植物O-甲基转移酶(OMTs)构成一大类酶,其中Ⅰ型OMT 形成功能发散的基团,并使多种底物(如类黄酮,生物碱和二苯乙烯)甲基化,主要以COMT 为代表[31]。在植物木质素生物合成、抵御病原菌侵染和抗逆胁迫中发挥重要作用。本研究基于phytozome 13 数据库,利用拟南芥COMT 基因对葡萄COMT基因家族进行了鉴定与分析,确定了26个VvCOMT 基因,所有基因均包含咖啡酸辅酶AO-甲基转移酶结构域,系统进化分析结果与基因结构及保守域分析结果一致,具有相同数量的内含子和保守基序的基因家族成员优先聚为一类,这与先前的研究结果一致[32],此外,各个亚类基因家族成员的蛋白质理化性质存在差异,本研究得到的26个COMT 基因二级结构同拟南芥COMT 基因家族的蛋白质组成和结果相似,其中α-螺旋和无规则卷曲比例最高,这与玉米COMT 基因二级结构相同[33]。启动子是RNA 聚合酶识别、结合和开始转录的一段DNA 序列,它含有RNA 聚合酶特异性结合和转录起始所需的保守序列,关系到基因的时空表达,是基因的开关,分析其启动子顺式作用元件有助于推测基因的潜在功能。VvCOMT 基因的启动子中含有许多响应激素调控的作用元件(TGAelemet 生长素响应元件,TCA-elemet 响应水杨酸响应元件,ABRE 脱落酸响应元件,TGACG-motif 茉莉酸甲酯响应元件),防御和应激反应作用元件提示基因在应对胁迫方面有重大作用,如VvCOMT1、5、13、14、19、20 和VvCOMT26 都含有防御和应激反应相关元件(TC-rich)。启动子分析结果表明,COMT 基因家族作用于葡萄的抗病虫、抗逆等方面,这与其他物种中COMT 基因家族的研究结果相一致[34-35]。
据报道,COMT 与COMT-like 基因在植物次生物质合成和抗逆胁迫反应中起着关键性作用[36-37]。单木质素生物合成是细胞壁贴合(cell wall apposition)过程中的关键,也是植物抵御病原菌的首要防线之一[38]。这一理论在其他物种中也得以验证,小麦中COMT-3D 基因过表达可以提高其对纹枯病的抗性[25],增加木质素的积累;棉花中n-乙酰转移酶1(GhSNAT1)和咖啡酸O-甲基转移酶(GhCOMT)沉默导致褪黑素的生物合成减少,从而影响木质素和棉酚的合成,降低了对棉花黄萎病的抗性[39];COMT的表达量下调降低了6个月龄的杨树中木质素的含量水平[40];水稻中咖啡酸O-甲基转移酶的过表达通过5-甲氧基色胺途径同样也增加了褪黑素的产生,以此提高植物抗性[41]。同样的,在VvCOMT 基因家族在受到葡萄霜霉病侵染时,抗病品种COMT 基因家族除VvCOMT2外均在接种霜霉病后48 h出现显著上调,而在感病品种中只有63%的COMT 基因在接种后的24 h内出现短时上调,且抗病力不同的品种,其表达量和表达模式也不同,摩尔多瓦多在6~48 h之间显著表达,而夏黑则是24 h后表达量提高,且抗病品种中96%的基因表达量都高于感病品种,说明在抗病品种抵御霜霉病时COMT 基因发挥重要作用,这与笔者先前预期的结果一致。植物生长发育过程中不会像动物拥有体细胞适应性免疫系统能够主动避开病原微生物和病虫害,只能依靠植物合成的化学成分和本身的一些结构作为屏障[42-43]。而在葡萄中,不同品种的抗霜霉病程度不一样,其COMT 基因家族的表达量也不一样,结果中显示VvCOMT1、5、6、7、8、9 和VvCOMT19 基因在抗病品种摩尔多瓦中表达量显著高于感病品种夏黑,同时发现VvCOMT1、5、19 启动子区域中包含抗防御和应激反应响应元件,可以证明这些基因在葡萄抗霜霉病侵染中发挥重要作用,后期可对这几个基因进行过表达处理,获得阳性转基因苗,以期挖掘出VvCOMT 基因家族更多的潜在功能,为葡萄抗病品种的培育做出贡献。
4 结 论
笔者在本研究中鉴定了葡萄26 个COMT 基因家族成员,在接种霜霉病后均有表达,尤其在抗病品种中表达较显著,推测COMT 基因在葡萄抗霜霉病侵染过程中发挥重要的作用,可为下一步研究其在抗病过程中的分子功能和在基因中的调控奠定基础。
[1]王璐璐,孙云开,徐旋,周洁,王清明,祝学珍.葡萄霜霉病的发病规律和防治研究进展[J].园艺与种苗,2022,42(9):31-33.WANG Lulu,SUN Yunkai,XU Xuan,ZHOU Jie,WANG Qingming,ZHU Xuezhen.Research progress on pathogenesis and control of grape downy mildew [J].Horticulture & Seed,2022,42(9):31-33.
[2]李卓,周婷婷,杨超,李映程,孙琦,任毓忠,赵宝龙,张莉,李国英.葡萄霜霉病菌侵染抗病和感病品种过程的组织学观察[J].园艺学报,2017,44(5):861-870.LI Zhuo,ZHOU Tingting,YANG Chao,LI Yingcheng,SUN Qi,REN Yuzhong,ZHAO Baolong,ZHANG Li,LI Guoying.Histological studies on the infection processs of the grape downy mildew between susceptible cultivar and resistant cultivar[J].Acta Horticulturae Sinica,2017,44(5):861-870.
[3]杜兴兰.葡萄霜霉病和白粉病生物防治的研究[D].保定:河北农业大学,2008.DU Xinglan.Biological control of grape downy mildew and powdery mildew[D].Baoding:Hebei Agricultural University,2008.
[4]GUO W F,JIN L,MIAO Y H,HE X,HU Q,GUO K,ZHU L F,ZHANG X L.An ethylene response-related factor,GbERF1-like,from Gossypium barbadense improves resistance to Verticillium dahliae via activating lignin synthesis[J].Plant Molecular Biology,2016,91(3):305-318.
[5]BOERJAN W,RALPH J,BAUCHER M.Lignin biosynthesis[J].Annual Review of Plant Biology,2003,54(1):519-546.
[6]VANHOLME R,DE MEESTER B,RALPH J,BOERJAN W.Lignin biosynthesis and its integration into metabolism[J].Current Opinion in Biotechnology,2019,56:230-239.
[7]LEWIS N G,YAMAMOTO E.Lignin:Occurrence,biogenesis and biodegradation[J].Annual Review of Plant Physiology and Plant Molecular Biology,1990,41:455-496.
[8]ROJE S. S-Adenosyl-L-methionine:Beyond the universal methyl group donor[J].Phytochemistry,2006,67(15):1686-1698.
[9]LAM K C,IBRAHIM R K,BEHDAD B,DAYANANDAN S.Structure,function,and evolution of plant O-methyltransferases[J].Genome,2007,50(11):1001-1013.
[10]LU S W,ZHUGE Y X,HAO T Y,LIU Z J,ZHANG M W,FANG J G.Systematic analysis reveals O-methyltransferase gene family members involved in flavonoid biosynthesis in grape[J].Plant Physiology and Biochemistry,2022,173:33-45.
[11]JOSHI C P,CHIANG V L.Conserved sequence motifs in plant S-adenosyl-L-methionine-dependent methyltransferases[J].Plant Molecular Biology,1998,37(4):663-674.
[12]BERIM A,GANG D R.Methoxylated flavones:Occurrence,importance,biosynthesis[J].Phytochemistry Reviews,2016,15(3):363-390.
[13]BENNETT M R,SHEPHERD S A,CRONIN V A,MICKLEFIELD J.Recent advances in methyltransferase biocatalysis[J].Current Opinion in Chemical Biology,2017,37:97-106.
[14]SHI R,SUN Y H,LI Q Z,HEBER S,SEDEROFF R,CHIANG V L.Towards a systems approach for lignin biosynthesis in Populus trichocarpa:Transcript abundance and specificity of the monolignol biosynthetic genes[J].Plant and Cell Physiology,2010,51(1):144-163.
[15]MOINUDDIN S G A,JOURDES M,LASKAR D D,KI C,CARDENAS C L,KIM K W,ZHANG D Z,DAVIN L B,LEWIS N G.Insights into lignin primary structure and deconstruction from Arabidopsis thaliana COMT (caffeic acid O-methyl transferase)mutant Atomt1[J].Organic&Biomolecular Chemistry,2010,8(17):3928-3946.
[16]LI W,LU J X,LU K,YUAN J L,HUANG J H,DU H,LI J N.Cloning and phylogenetic analysis of Brassica napus L.caffeic acid O-methyltransferase 1 gene family and its expression pattern under drought stress[J].PLoS One,2016,11(11):e0165975.
[17]PELLEGRINI L,GEOFFROY P,FRITIG B,LEGRAND M.Molecular cloning and expression of a new class of ortho-diphenol-O-methyltransferases induced in tobacco (Nicotiana tabacum L.) leaves by infection or elicitor treatment[J].Plant Physiology,1993,103(2):509-517.
[18]HEATH R,HUXLEY H,STONE B,SPANGENBERG G.cDNA cloning and differential expression of three caffeic acid Omethyltransferase homologues from perennial ryegrass (Lolium perenne)[J].Journal of Plant Physiology,1998,153(5/6):649-657.
[19]CHUNG N,ZHANG X D,KREAMER A,LOCCO L,KUAN P F,BARTZ S,LINSLEY P S,FERRER M,STRULOVICI B.Median absolute deviation to improve hit selection for genomescale RNAi screens[J].Journal of Biomolecular Screening,2008,13(2):149-158.
[20]CHOI G H,LEE H Y,BACK K.Chloroplast overexpression of rice caffeic acid O-methyltransferase increases melatonin production in chloroplasts via the 5-methoxytryptamine pathway in transgenic rice plants[J].Journal of Pineal Research,2017,63(1):e12412.
[21]DALY P,MCCLELLAN C,MALUK M,OAKEY H,LAPIERRE C,WAUGH R,STEPHENS J,MARSHALL D,BARAKATE A,TSUJI Y,GOEMINNE G,VANHOLME R,BOERJAN W,RALPH J,HALPIN C.RNAi-suppression of barley caffeic acid O-methyltransferase modifies lignin despite redundancy in the gene family[J].Plant Biotechnology Journal,2019,17(3):594-607.
[22]LIU Y S,WANG Y Z,PEI J B,LI Y D,SUN H Y.Genome-wide identification and characterization of COMT gene family during the development of blueberry fruit[J].BMC Plant Biology,2021,21(1):5.
[23]PETITOT A S,KYNDT T,HAIDAR R,DEREEPER A,COLLIN M,DE ALMEIDA ENGLER J,GHEYSEN G,FERNANDEZ D.Transcriptomic and histological responses of African rice (Oryza glaberrima) to Meloidogyne graminicola provide new insights into root-knot nematode resistance in monocots[J].Annals of Botany,2017,119(5):885-899.
[24]FORNALÉ S,SONBOL F M,MAES T,CAPELLADES M,PUIGDOMÈNECH P,RIGAU J,CAPARRÓS-RUIZ D.Downregulation of the maize and Arabidopsis thaliana caffeic acid Omethyl-transferase genes by two new maize R2R3-MYB transcription factors[J].Plant Molecular Biology,2006,62(6):809-823.
[25]WANG M X,ZHU X L,WANG K,LU C G,LUO M Y,SHAN T L,ZHANG Z Y.A wheat caffeic acid 3-O-methyltransferase TaCOMT-3D positively contributes to both resistance to sharp eyespot disease and stem mechanical strength[J].Scientific Reports,2018,8:6543.
[26]乃国洁,卢世雄,马维峰,李艳梅,陈佰鸿,毛娟.葡萄EIN3/EIL 转录因子家族成员鉴定及表达特征分析[J].果树学报,2021,38(6):856-870.NAI Guojie,LU Shixiong,MA Weifeng,LI Yanmei,CHEN Baihong,MAO Juan.Genome-wide identification and expression characteristic analysis of EIN3/EIL transcription factor family in grape[J].Journal of Fruit Science,2021,38(6):856-870.
[27]CHEN C J,CHEN H,ZHANG Y,THOMAS H R,FRANK M H,HE Y H,XIA R.TBtools:An integrative toolkit developed for interactive analyses of big biological data[J].Molecular Plant,2020,13(8):1194-1202.
[28]LIVAK K J,SCHMITTGEN T D.Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method[J].Methods,2001,25(4):402-408.
[29]RAMMESMAYER G,PICHORNER H,ADAMS P,JENSEN R G,BOHNERT H J.Characterization of IMT1,myo-inositol Omethyltransferase,from Mesembryanthemum crystallinum[J].Archives of Biochemistry and Biophysics,1995,322(1):183-188.
[30]DO C T,POLLET B,THÉVENIN J,SIBOUT R,DENOUE D,BARRIÈRE Y,LAPIERRE C,JOUANIN L.Both caffeoyl Coenzyme A 3-O-methyltransferase 1 and caffeic acid O-methyltransferase 1 are involved in redundant functions for lignin,flavonoids and sinapoyl malate biosynthesis in Arabidopsis[J].Planta,2007,226(5):1117-1129.
[31]IBRAHIM R K,BRUNEAU A,BANTIGNIES B.Plant O-methyltransferases:Molecular analysis,common signature and classification[J].Plant Molecular Biology,1998,36(1):1-10.
[32]包颖亮.构树咖啡酸-O-甲基转移酶基因的克隆及生物信息学分析[D].呼和浩特:内蒙古农业大学,2018.BAOYingliang.Cloning and bioinformatics analysis of caffeic acid O-methyl-transferase gene from Broussonetia papyrifera[D].Hohhot:Inner Mongolia Agricultural University,2018.
[33]徐碧莹,张敏艳,黄伟鹏,沙哈尼,吴委林,郑大浩.玉米O-甲基转移酶(ZmOMT)基因家族的生物信息学分析[J/OL].分子植物育种,2021:1-12.[2021-11-05].https://kns.cnki.net/kcms/detail/46.1068.s.20211104.1914.021.html.XU Biying,ZHANG Minyan,HUANG Weipeng,SHA Hani,WU Weilin,ZHENG Dahao.Bioinformatics analysis of maize O-methyltransferase (ZmOMT) gene family[J/OL].Molecular Plant Breeding,2021:1-12.[2021-11-05].https://kns.cnki.net/kcms/detail/46.1068.s.20211104.1914.021.html.
[34]WU C C,ZUO D Y,XIAO S P,WANG Q L,CHENG H L,LV L M,ZHANG Y P,LI P B,SONG G L.Genome-wide identification and characterization of GhCOMT gene family during fiber development and Verticillium wilt resistance in cotton[J].Plants,2021,10(12):2756.
[35]陈安琪.川芎咖啡酸-O-甲基转移酶(LcCOMT)的功能鉴定与应用研究[D].成都:西南交通大学,2021.CHEN Anqi.Functional identification and application of Ligusticumchuanxiongcaffcieacid-O-methyltransferase(LcCOMT)[D].Chengdu:Southwest Jiaotong University,2021.
[36]LEE J E,VOGT T,HAUSE B,LÖBLER M.Methyl jasmonate induces an O-methyltransferase in Barley[J].Plant and Cell Physiology,1997,38(7):851-862.
[37]TOQUIN V,GRAUSEM B,GEOFFROY P,LEGRAND M.Structure of the tobacco caffeic acid O- methyltransferase(COMT) II gene:Identification of promoter sequences involved in gene inducibility by various stimuli[J].Plant Molecular Biology,2003,52(3):495-509.
[38]COLLINGE D B.Cell wall appositions:The first line of defence[J].Journal of Experimental Botany,2009,60(2):351-352.
[39]LI C,HE Q L,ZHANG F,YU J W,LI C,ZHAO T L,ZHANG Y,XIE Q W,SU B R,MEI L,ZHU S J,CHEN J H.Melatonin enhances cotton immunity to Verticillium wilt via manipulating lignin and gossypol biosynthesis[J].The Plant Journal,2019,100(4):784-800.
[40]BARAKAT A,CHOI A,YASSIN N B M,PARK J S,SUN Z C,CARLSON J E.Comparative genomics and evolutionary analyses of the O-methyltransferase gene family in Populus[J].Gene,2011,479(1/2):37-46.
[41]BYEON Y,CHOI G H,LEE H Y,BACK K.Melatonin biosynthesis requires N-acetylserotonin methyltransferase activity of caffeic acid O-methyltransferase in rice[J].Journal of Experimental Botany,2015,66(21):6917-6925.
[42]HO-YUE-KUANG S,ALVARADO C,ANTELME S,BOUCHET B,CÉZARD L,LE BRIS P,LEGÉE F,MAIA-GRONDARD A,YOSHINAGA A,SAULNIER L,GUILLON F,SIBOUT R,LAPIERRE C,CHATEIGNER- BOUTIN A L.Mutation in Brachypodium caffeic acid O-methyltransferase 6 alters stem and grain lignins and improves straw saccharification without deteriorating grain quality[J].Journal of Experimental Botany,2016,67(1):227-237.
[43]李伟,熊谨,陈晓阳.木质素代谢的生理意义及其遗传控制研究进展[J].西北植物学报,2003,23(4):675-681.LI Wei,XIONG Jin,CHEN Xiaoyang.Advances in the research of physiological significances and genetic regulation of lignin metabolism[J].Acta Botanica Boreali-Occidentalia Sinica,2003,23(4):675-681.