中国柑橘以鲜食为主,无籽柑橘成为市场消费的主流和育种方向[1]。为提升产业竞争力,满足市场需求,培育柑橘无核品种一直是育种者的目标。三倍体为天然的无核材料,其在减数分裂时期,染色体发生紊乱,难以形成正常可育的配子,导致果实一般为无核[2]。由于染色体加倍、倍性增加,三倍体果实较大、抗逆性增强,对环境也具有更强的适应性。通过倍性杂交以培育三倍体是获得柑橘无核品种最为有效的途径之一,其中又以单胚性二倍体品种为母本与四倍体杂交方式最为普遍[3]。Soost 等[4]以单胚无酸柚与四倍体马叙葡萄柚杂交获得2个已广泛推广的无核品种Oroblanco 和Melgold。Aleza 等[5]配置以二倍体Fortune宽皮橘与四倍体Orlando柑橘品种等为亲本的77个杂交组合,获得4400多株三倍体再生植株,并从中筛选出一个综合性状优良的无核品种IVIA-600。近年来,华中农业大学柑橘团队也创制诸多以单胚性二倍体柑橘品种与四倍体体细胞杂种为亲本的杂交组合,获得一大批柑橘三倍体无核新种质[6]。
胚挽救在柑橘果树育种中是非常重要的技术,其核心是对由生理等因素导致种子不能成苗的合子胚人工接种于培养基上进行离体培育,并结合试管嫁接手段获得再生植株,极大地提升果树育种效率,加快育种进程[7]。彭珺[8]以2个二倍体柑橘品种与5个通过柑橘体细胞融合得到的四倍体品种进行倍性杂交,对幼胚进行离体培养,并结合流式细胞仪对再生植株进行倍性分析,获得三倍体141株。周锐[9]以不同柑橘材料为亲本,用相同的方法创制出147 株三倍体。此外,随着全基因组重测序技术逐渐成熟,对于特定品系的柑橘品种,开发该品种特异性且扩增良好的InDel(Insertion/Deletion)分子标记对杂交后代进行遗传鉴定,具有低成本、高效、遗传稳定性好、准确性高且结果可靠的优势[10]。王沦[11]以甜橙的基因组为参考基因组挖掘到268个该品种存在的高质量InDel标记。宋谢天等[12]利用测序数据开发出7对InDel标记能区分柑橘有性后代和无性后代。
笔者在本研究中以云南地方特色早熟品种东试早柚为母本,与2个同源四倍体(ZP、PT)和1个异源四倍体(NH)为父本,杂交授粉后利用胚挽救、流式细胞仪及InDel标记等技术手段获得具有丰富遗传背景的柑橘三倍体材料,为柑橘早熟无核新品种选育和相关基础研究奠定材料基础。
1 材料和方法
1.1 试验材料
用于创制柑橘三倍体无核种质的材料:云南西双版纳州热带作物科学研究所柚试验基地的早熟品种 东 试 早 柚[Citrus grandis(L.)Osbeck‘Dongshizao pummelo’],湖北武汉华中农业大学国家柑橘育种中心资源圃的纸皮[ZP,四倍体甜橙,C.sinensis (L.) Osbeck‘Paperrind orange’]、四倍体葡萄柚[PT,C.paradise Osbeck‘Grapefruit’]、NH[诺瓦橘柚+HB 柚,C.reticulata Blanco×C.paradisi Macf.+C.grandis(L.)Osbeck‘Hirado Buntan pummelo’,四倍体,体细胞融合杂种]。
1.2 杂交授粉后亲和性鉴定方法
花粉的收集和保存参照朱晨桥[13]的方法,杂交授粉及授粉后7 d的花柱染色使用苯胺蓝染色法,通过倒置荧光显微镜观察花粉管的生长状态来鉴定杂交亲和性[14]。
1.3 杂交后代的胚培育及再生植株的倍性检测
胚挽救参照强瑞瑞[15]的方法并适当修改。授粉后85 和100 d,采摘未成熟的果实暂置于4 ℃保存。无菌条件下,75%乙醇浸泡幼果15 min,立即置于乙醇灯上燃烧消毒灭菌,乙醇燃烧完全后用手术刀将果实剥开,取出种子。将种子尾部划一条缝并将其接种于萌发培养基(MT培养基+1 mg·L-1 GA3)中,置于培养室培养。培养1个月后,未萌发的种子仍于萌发培养基中继续培养;将已萌发的种子形成的胚状体置于生芽培养基(MT+0.5 mg·L-1 BA+0.5 mg·L-1 KT+0.1 mg·L-1 NAA+40 g蔗糖+8 g琼脂)中增殖生芽,待其长出2~3枚叶片后将茎切下,接种于生根培养基(1/2 MT+0.1 mg·L-1 IBA+0.5 mg·L-1 NAA+0.5 g·L-1活性炭+8 g琼脂)中诱导生根。
流式细胞仪(Cyflow space,Sysmex,Japan)倍性鉴定参照谢善鹏[16]的方法并适当修改。以二倍体沙田柚为对照,从待测样品上取0.5 cm2大小的新鲜叶片于干净的培养皿中,加约500 μL的细胞提取缓冲液(Nuclei extraction buffer,Cystain DNA 2 step),用刀片将其切碎,静置30~40 s 后加入约1.5 mL 的DNA 染色液(Staining buffer,Cystain DNA 2 step)进行染色,最后用30 μm 的微孔滤膜将样品过滤到2.5 mL 试管中,用流式细胞仪(Cyflow space,Sysmex,Japan)进行上样检测,FloMax 软件自动生成DNA含量分布曲线。
1.4 植物基因组DNA的提取与检测
采集东试早柚、ZP、PT、NH 及其各杂交子代成熟叶片并提取基因组DNA,提取方法采用改良CTAB法,具体步骤参照程运江[17]的博士学位论文,使用NanoDrop 1000 超微量分光光度计对DNA 进行质量检测,将质量合格的DNA初提液稀释至工作质量浓度(约200 ng·μL-1),保存于-20 ℃冰箱备用。
1.5 InDel分子标记的筛选
利用母本东试早柚的重测序数据以晚白柚的基因组为参考基因组进行序列比对,获取InDel变异位点,筛选含有50~200 bp 差异的位点。在InDel 变异位点上下游设计引物(表1),以东试早柚、ZP、PT、NH 的DNA 为模板进行PCR扩增,PCR扩增反应体系20 μL,其中包括10 μL Mix,1 μL DNA,InDel 上下游引物各0.5 μL,加入ddH2O 补足体积。PCR 扩增产物使用2.5%琼脂糖凝胶检测,选择清晰稳定的特异性带型。
表1 东试早柚遗传鉴定所用的InDel 引物信息
Table 1 InDel primers used in the genetic identification of Dongshizao pummelo
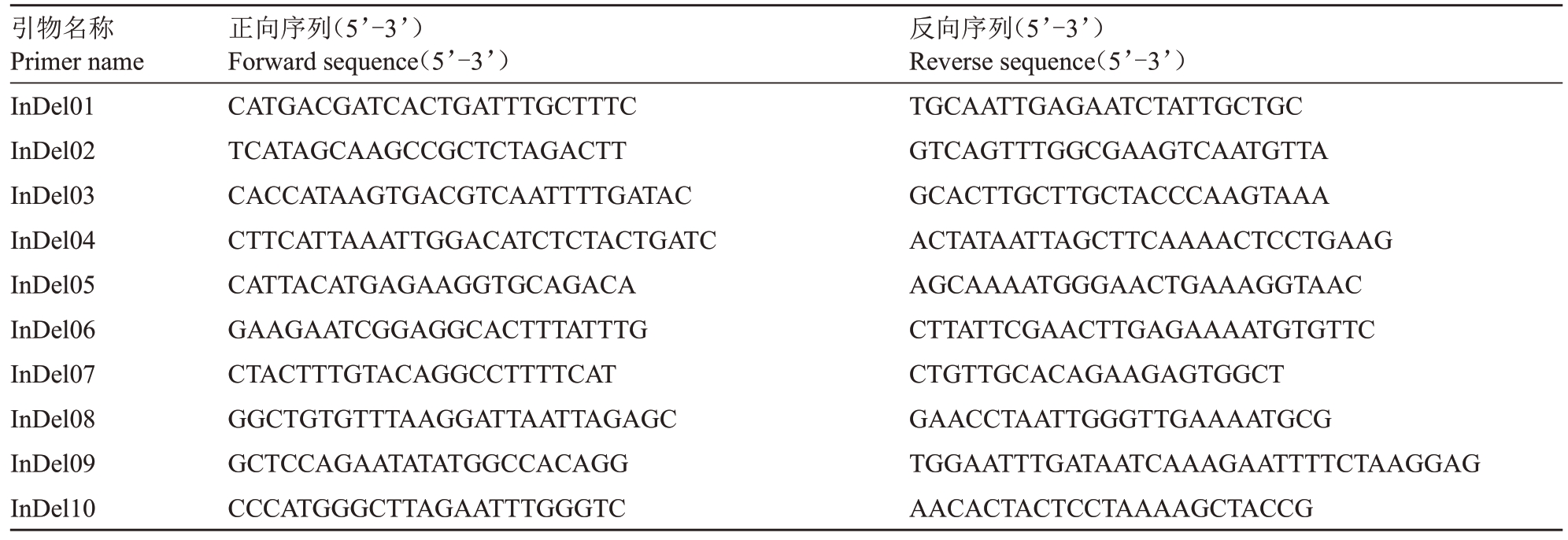
引物名称Primer name InDel01 InDel02 InDel03 InDel04 InDel05 InDel06 InDel07 InDel08 InDel09 InDel10正向序列(5’-3’)Forward sequence(5’-3’)CATGACGATCACTGATTTGCTTTC TCATAGCAAGCCGCTCTAGACTT CACCATAAGTGACGTCAATTTTGATAC CTTCATTAAATTGGACATCTCTACTGATC CATTACATGAGAAGGTGCAGACA GAAGAATCGGAGGCACTTTATTTG CTACTTTGTACAGGCCTTTTCAT GGCTGTGTTTAAGGATTAATTAGAGC GCTCCAGAATATATGGCCACAGG CCCATGGGCTTAGAATTTGGGTC反向序列(5’-3’)Reverse sequence(5’-3’)TGCAATTGAGAATCTATTGCTGC GTCAGTTTGGCGAAGTCAATGTTA GCACTTGCTTGCTACCCAAGTAAA ACTATAATTAGCTTCAAAACTCCTGAAG AGCAAAATGGGAACTGAAAGGTAAC CTTATTCGAACTTGAGAAAATGTGTTC CTGTTGCACAGAAGAGTGGCT GAACCTAATTGGGTTGAAAATGCG TGGAATTTGATAATCAAAGAATTTTCTAAGGAG AACACTACTCCTAAAAGCTACCG
2 结果与分析
2.1 东试早柚与四倍体柑橘杂交授粉后亲和性鉴定
以东试早柚作母本与2 个同源四倍体(ZP、PT)、1 个异源四倍体(NH)作父本进行倍性杂交。在东试早柚×ZP、东试早柚×PT、东试早柚×NH的杂交组合中,对授粉后的花柱进行苯胺蓝染色来观察花粉管的生长状态,发现大量花粉管能生长至花柱底部,均表现为亲和(图1)。
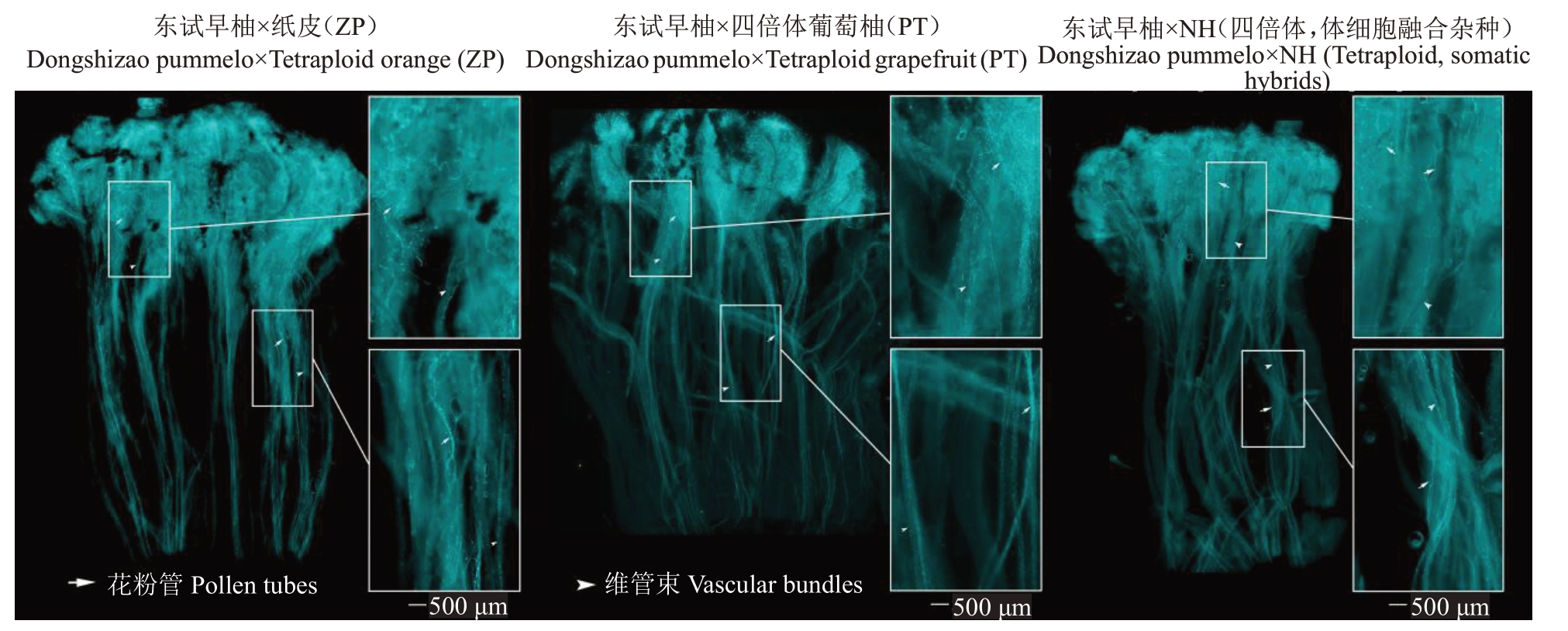
图1 杂交授粉后花柱的荧光成像
Fig.1 Fluorescence images of pollen tubes in pistils after different pollinations
2.2 胚挽救获得再生植株
由于子代胚乳中母本和父本基因组比例为2∶2(2x×4x),后期种子完全败育[2]。故而在种子未败育之前,对幼胚进行离体挽救培养(图2)。授粉后85 d,取东试早柚×NH杂交组合的果实种子进行胚挽救,接种771粒种子,获得再生植株15株。授粉后100 d,取东试早柚×PT、东试早柚×ZP 杂交组合的果实种子进行胚挽救,分别接种570、482粒种子,各获得再生植株96、117株(表2),组合间再生率存在差异,同源四倍体为父本的2个组合较高,在20%左右,异源四倍体为父本的组合较低,只有1.9%,相差近10倍。
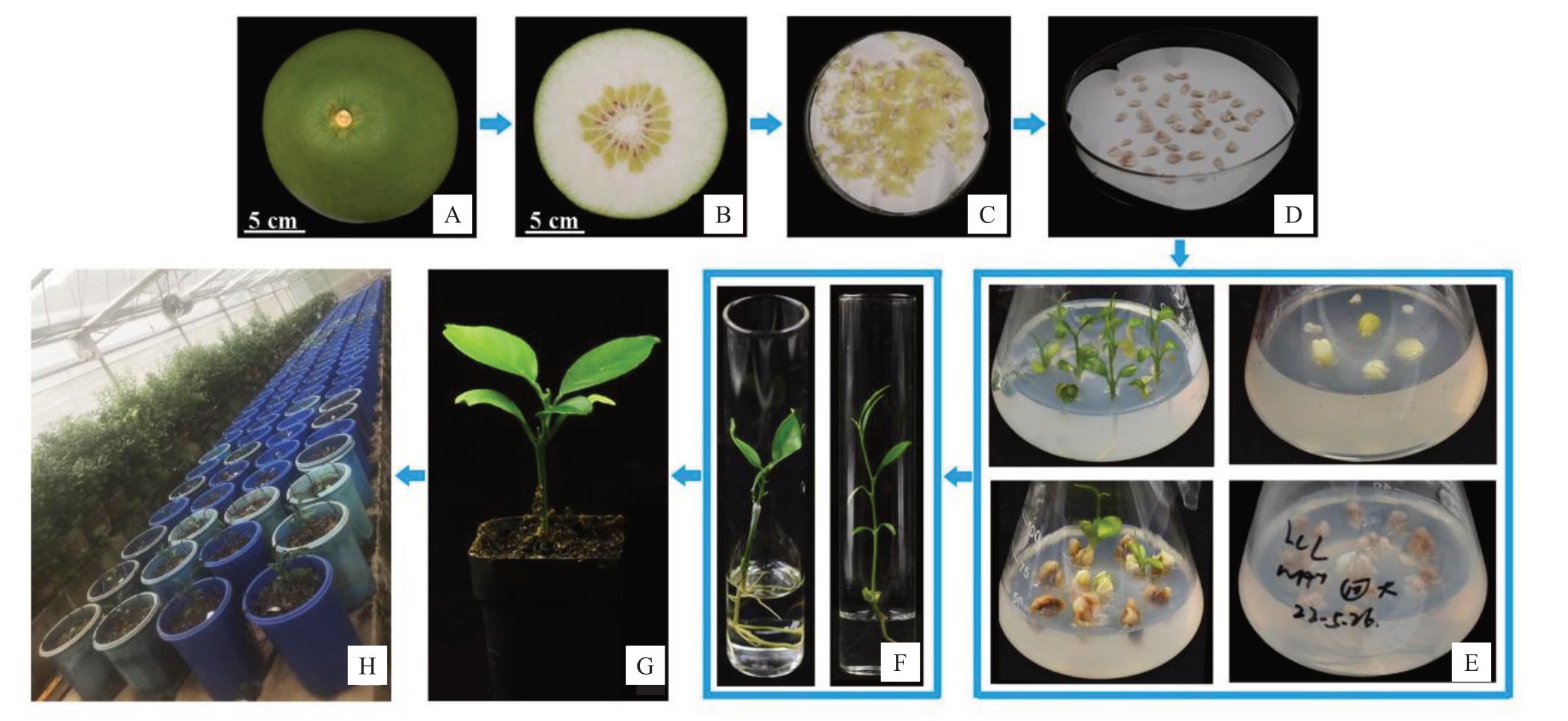
图2 胚挽救流程
Fig.2 Process of embryo rescue
A~B.果实;C~D.种子;E.种子播种于MT 培养基中;F.炼苗;G~H.移栽小苗。
A-B.Fruits;C-D.Seeds;E.The seeds sowing in MT medium;F.Seedlings hardening;G-H.Seedlings transferred to the pots.
表2 2x×4x 杂交组合授粉情况统计
Table 2 The results of embryo rescue and plant regeneration of 2x×4x crosses using cultivars
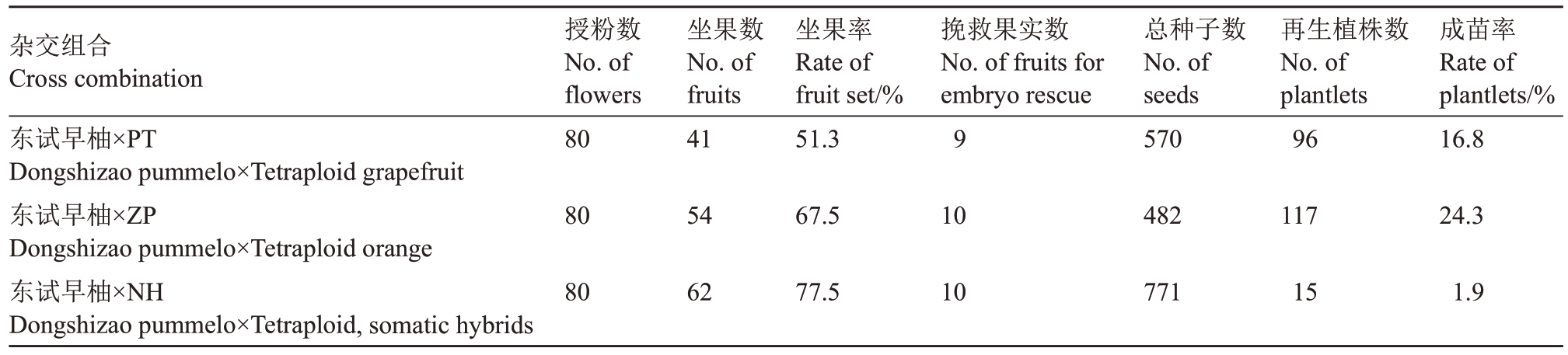
杂交组合Cross combination东试早柚×PT Dongshizao pummelo×Tetraploid grapefruit东试早柚×ZP Dongshizao pummelo×Tetraploid orange东试早柚×NH Dongshizao pummelo×Tetraploid,somatic hybrids授粉数No.of flowers 80坐果数No.of fruits 41坐果率Rate of fruit set/%51.3挽救果实数No.of fruits for embryo rescue 9总种子数No.of seeds 570再生植株数No.of plantlets 96成苗率Rate of plantlets/%16.8 80 54 67.5 10 482 117 24.3 80 62 77.5 10 771 15 1.9
2.3 再生植株倍性分析
利用流式细胞仪对东试早柚与3个四倍体柑橘杂交获得228 株子代中的168 株再生植株进行倍性检测(图3),在东试早柚×ZP杂交组合中,94株再生植株检测到三倍体幼苗60株,占再生子代的63.8%;东试早柚×PT杂交组合中,63株再生植株检测到三倍体幼苗60株和四倍体幼苗1株,分别占再生植株的95.2%和1.67%;东试早柚×NH 杂交组合中11 株再生植株检测到三倍体植株8 株,占再生植株的72.7%(表3)。
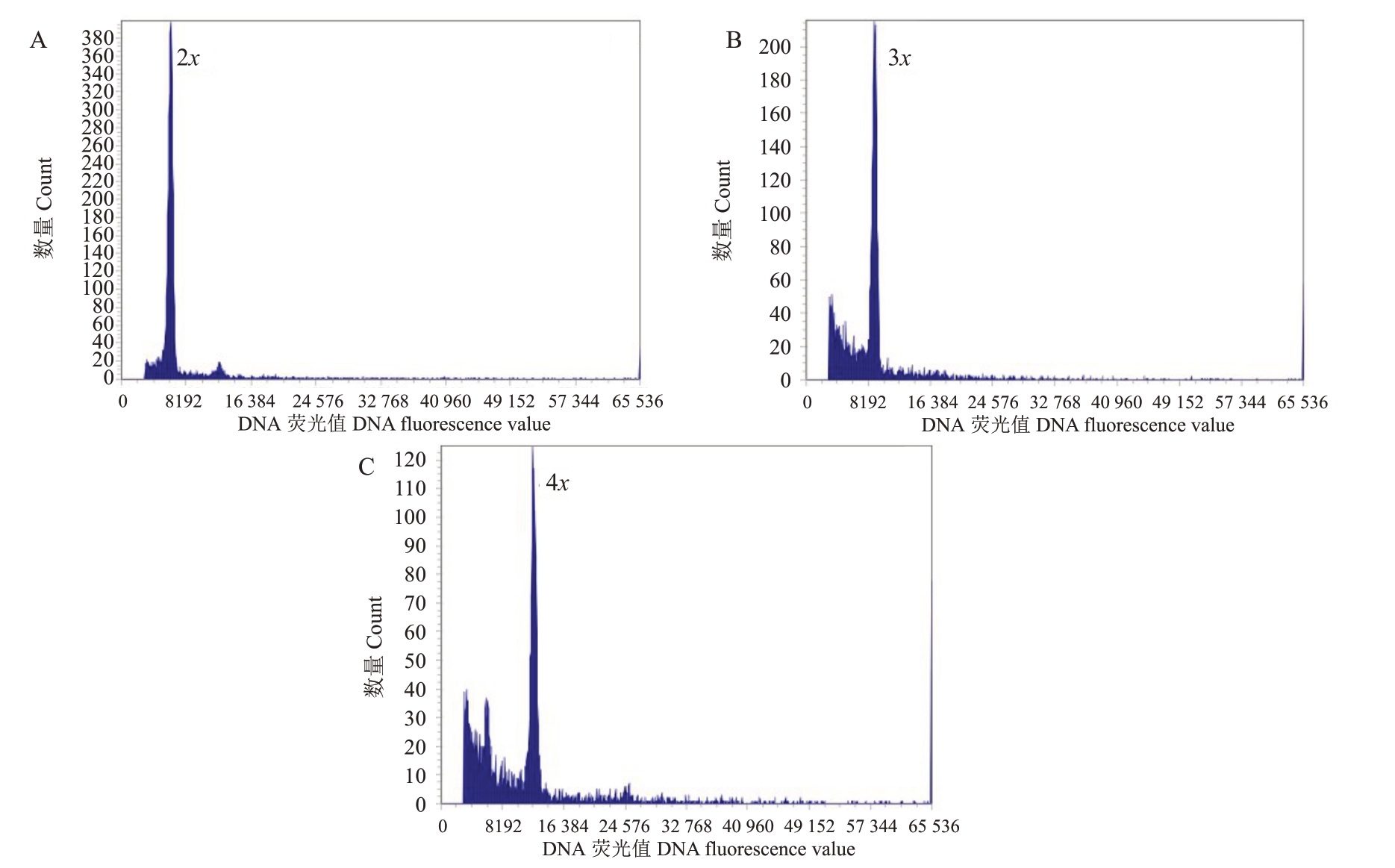
图3 流式细胞仪倍性鉴定
Fig.3 Ploidy level analysis by flow cytometry
A.二倍体沙田柚,2n=2x=18;B.东试早柚×四倍体柑橘的三倍体后代,2n=3x=27;C.东试早柚×四倍体柑橘的四倍体后代,2n=4x=36。
A.Diploid,Shatian pummelo,2n = 2x = 18;B.Triploids obtained from Dongshizao pummelo × tetraploid citrus,2n = 3x = 27;C.Tetraploid obtained from Dongshizao pummelo×tetraploid citrus,2n=4x=36.
表3 东试早柚×四倍体柑橘授粉杂交组合再生植株倍性鉴定结果
Table 3 The results of seedlings ploidy identification of 2x×4x crosses using cultivars

授粉组合Cross combination东试早柚×PT Dongshizao pummelo×Tetraploid grapefruit东试早柚×ZP Dongshizao pummelo×Tetraploid orange东试早柚×NH Dongshizao pummelo×Tetraploid,somatic hybrids检查的再生植株(系)No.of seedlings checked 63 94 11 3x(系)No.of triploids 60 60 8 4x(系)No.of tetraploids 1 0 0多倍体(系)No.of polyploids 61 60 8
2.4 再生植株的遗传来源分析
以晚白柚的基因组为参考基因组,利用东试早柚、葡萄柚及甜橙重测序数据开发10 对InDel 引物进行杂交子代筛选,其中InDel05 和InDel10 能区分东试早柚与ZP、PT,无法区分NH;InDel07 和In-Del08 虽能区分所有的亲本,但是其部分特异性条带不明显(图4)。因此,选择InDel05引物对东试早柚×PT 和东试早柚×ZP 杂交子代进行鉴定,InDel07引物对东试早柚×NH杂交子代进行鉴定(图5)。结果表明129株多倍体再生植株均扩增出具有双亲差异性显著的带型。
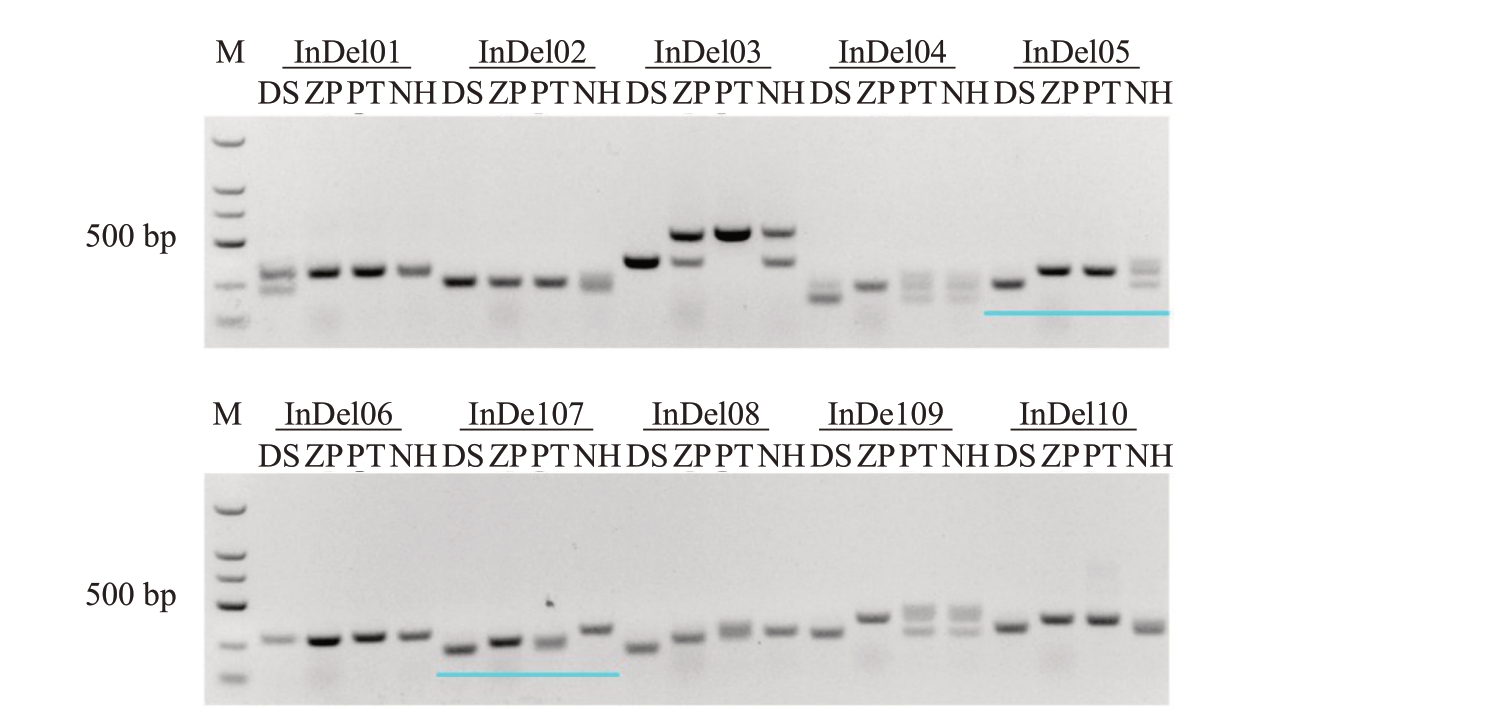
图4 不同柑橘种质InDel 引物鉴定结果
Fig.4 Results of InDel markers identification of different citrus germplasms
M.DL5000 DNA 分子标准;DS.东试早柚;ZP.纸皮,四倍体甜橙;PT.四倍体葡萄柚;NH.四倍体,体细胞融合杂种。
M.DL5000 DNA Marker;DS.Dongshizao pummelo;ZP.Tetraploid orange;PT.Tetraploid grapefruit;NH.Tetraploid,somatic hybrids.

图5 杂种F1代植株InDel 引物鉴定结果
Fig.5 Results of InDel markers identification in F1 generation plants of hybrid
DS.东试早柚,母本;ZP.纸皮,四倍体甜橙,父本;PT.四倍体葡萄柚,父本;NH.四倍体,体细胞融合杂种,父本;*.四倍体再生植株。
DS.Dongshizao pummelo, female parent; ZP.Tetraploid orange, male parent; PT.Tetraploid grapefruit, male parent; NH.Tetraploid, somatic hybrids,male parent;*.Tetraploid seedling.
3 讨 论
柑橘三倍体育种以获得果实无核为主要目标性状,同时能兼具不同成熟期、易剥皮、高糖低酸等优异性状。Fatta Del Bosco 等[18]以宽皮橘等为亲本通过倍性杂交,以期获得无核且易剥皮的新种质来取代Avana 和Tardivo di Ciaculli 多籽宽皮橘品种。西班牙则以无核且晚熟为目标性状,通过三倍体育种获得Safor[19]和Garbí[20]等具有无籽、中晚熟柑橘品种。染色体加倍也会导致三倍体植株表现出果皮增厚、果面粗糙、果实低糖高酸、枝刺增多且增长等诸多不利的性状。Grosser 等[21]以二倍体克里曼丁橘为亲本,倍性杂交获得的三倍体后代表现出枝刺长、枝刺增多等不利性状;但是以二倍体Sugar Belle 为亲本,得到的三倍体后代表现出果实早熟、枝刺短、枝刺减少等优良性状。因此,杂交后代性状的表现与亲本的选择密切相关。笔者在本研究中选择果实早熟、无籽、高糖低酸的云南地方特色品种东试早柚为母本,融合双亲优良性状的四倍体体细胞杂种NH、晚熟且丰产的同源四倍体ZP 和PT 为父本,通过三倍体育种以期获得果实早熟、丰产、品质更佳等诸多综合双亲优良性状的柑橘无核新种质。
在柑橘中,常以二倍体(♀)与四倍体(♂)、四倍体(♀)与二倍体(♂)进行倍性杂交,不正常的胚与胚乳的倍性比会致使杂交获得的合子胚提前败育[22]。胚挽救是对受各种不利因素影响而致使早期败育的合子胚离体培养,是大多数果树育种中普遍使用的一种方法[7]。解凯东等[23]创制以8 个二倍体柑橘品种、6个四倍体柑橘品种为亲本,通过倍性杂交获得再生植株2832株,并结合流式细胞仪对再生植株检测获得三倍体植株401 株等一大批倍性材料。笔者在本研究中以二倍体单胚性东试早柚为母本,2个同源四倍体和1个异源四倍体柑橘品种为父本,利用胚挽救手段获得228株再生植株,并使用流式细胞仪对168 株再生植株进行倍性检测,获得三倍体植株128株,还意外获得1株四倍体材料。
目前,在柑橘育种中特异性InDel 标记已成为鉴定杂交子代遗传来源的重要手段。汤雨晴等[24]利用金兰柚全基因组重测序数据开发出24对InDel标记,均能够有效区分金兰柚不同于其他的柚类品种。韩健等[25]基于基因组重测序数据开发出可有效检测沙田柚与枳杂交子代的InDel 标记,从1279株子代中鉴定获得698 株杂种苗。笔者在本研究中以晚白柚的基因组为参考基因组,开发10 对候选InDel标记用于区分东试早柚及ZP、PT、NH柑橘种质,筛选出2 对高质量InDel 标记对倍性检测获得的128 株三倍体和1株四倍体再生植株进行遗传鉴定,均扩增出亲本特异性条带。因此,结合倍性检测的结果,获得的流式细胞仪129 株多倍体植株均为双亲杂交子代,其中获得1 株四倍体是由东试早柚×PT 杂交而来,推测其可能是由母本产生未减数的雌配子与父本产生的二倍体雄配子杂交授粉受精而来的。
4 结 论
通过有性杂交,结合胚胎抢救、流式细胞仪及InDel 分子标记等技术在较短时间内创制出3 个组合的柑橘三倍体128株和四倍体杂种1株,为柑橘三倍体无核育种提供了材料。
[1]邓秀新.中国柑橘育种60 年回顾与展望[J].园艺学报,2022,49(10):2063-2074.DENG Xiuxin.A review and perspective for Citrus breeding in China during the last six decades[J].Acta Horticulturae Sinica,2022,49(10):2063-2074.
[2]宋健坤.柑橘三倍体种质资源的创造及遗传分析[D].武汉:华中农业大学,2006.SONG Jiankun.Creation and genetic analysis of triploid Citrus germplasm[D].Wuhan:Huazhong Agricultural University,2006.
[3]OLLITRAULT P,DAMBIER D,LURO F,FROELICHER Y.Ploidy manipulation for breeding seedless triploid Citrus[J].Plant Breeding Reviews,2008,30:323-352.
[4]SOOST R K,CAMERON J W.‘Melogold’,A triploid pummelo-grapefruit hybrid[J].HortScience,1985,20(6):1134-1135.
[5]ALEZA P,JUÁREZ J,CUENCA J,OLLITRAULT P,NAVARRO L.Extensive citrus triploid hybrid production by 2x × 4x sexual hybridizations and parent-effect on the length of the juvenile phase[J].Plant Cell Reports,2012,31(9):1723-1735.
[6]解凯东,王惠芹,王晓培,梁武军,谢宗周,伊华林,邓秀新,GROSSER J W,郭文武.单胚性二倍体为母本与异源四倍体杂交大规模创制柑橘三倍体[J].中国农业科学,2013,46(21):4550-4557.XIE Kaidong,WANG Huiqin,WANG Xiaopei,LIANG Wujun,XIE Zongzhou,YI Hualin,DENG Xiuxin,GROSSER J W,GUO Wenwu.Extensive Citrus triploid breeding by crossing monoembryonic diploid females with allotetraploid male parents[J].Scientia Agricultura Sinica,2013,46(21):4550-4557.
[7]梁青,陈学森,刘文,吴燕.胚抢救在果树育种上的研究及应用[J].园艺学报,2006,33(2):445-452.LIANG Qing,CHEN Xuesen,LIU Wen,WU Yan.Research and application of embryo rescue techniques in fruit tree breeding[J].Acta Horticulturae Sinica,2006,33(2):445-452.
[8]彭珺.以2 个多胚性宽皮柑橘为母本倍性杂交培育三倍体新种质[D].武汉:华中农业大学,2019.PENG Jun.Production of citrus triplouid plants by interploid crosses with two ployembryonic Mandarins as female parents[D].Wuhan:Huazhong Agricultural University,2019.
[9]周锐.柑橘特异资源四倍体发掘及倍性杂交创制三倍体[D].武汉:华中农业大学,2020.ZHOU Rui.Exploration of tetraploid seedlings and production of triploid plants via sexual ploidy hybridization in Citrus[D].Wuhan:Huazhong Agricultural University,2020.
[10]FANG Q Y,WANG L,YU H W,HUANG Y,JIANG X L,DENG X X,XU Q.Development of species-specific InDel markers in Citrus[J].Plant Molecular Biology Reporter,2018,36(4):653-662.
[11]王沦.柑橘驯化选择及体细胞变异的基因组基础[D].武汉:华中农业大学,2018.WANG Lun.Genomic basis of Citrus domestication and somatic mutation[D].Wuhan:Huazhong Agricultural University,2018.
[12]宋谢天,田啸宇,王楠,周银,谢源源,谢宗周,柴利军,叶俊丽,邓秀新.利用InDel 标记筛选多胚山金柑珠心苗后代[J].果树学报,2023,40(7):1312-1317.SONG Xietian,TIAN Xiaoyu,WANG Nan,ZHOU Yin,XIE Yuanyuan,XIE Zongzhou,CHAI Lijun,YE Junli,DENG Xiuxin.InDel marker-assisted selection of nucellar seedlings in polyembryonic Fortunella hindsii[J].Journal of Fruit Science,2023,40(7):1312-1317.
[13]朱晨桥.柑橘模式材料的开发与金柑属植物系统发育学研究[D].武汉:华中农业大学,2020.ZHU Chenqiao.Development of Citrus model material and phylogenetic study of genus Fortunella[D].Wuhan:Huazhong Agricultural University,2020.
[14]HU J B,XU Q,LIU C C,LIU B H,DENG C L,CHEN C W,WEI Z M,AHMAD M H,PENG K,WEN H,CHEN X L,CHEN P,LARKIN R M,YE J L,DENG X X,CHAI L J.Downregulated expression of S2-RNase attenuates self-incompatibility in‘Guiyou No.1’pummelo[J].Horticulture Research,2021,8:199.
[15]强瑞瑞.以宽皮柑橘为母本倍性杂交培育三倍体[D].武汉:华中农业大学,2016.QIANG Ruirui.Triploid citrus plants obtained from crossing the diploid Citrus reticulata Blanco with tetraploid somatic hybrids[D].Wuhan:Huazhong Agricultural University,2016.
[16]谢善鹏.柑橘11 个地方品种资源四倍体高效发掘及三倍体新种质创制[D].武汉:华中农业大学,2022.XIE Shanpeng.Efficient exploration of tetraploid seedlings from 11 local Citrus cultivars and production of triploid plants[D].Wuhan:Huazhong Agricultural University,2022.
[17]程运江.柑橘体细胞胞质遗传及叶绿体SSR 引物开发研究[D].武汉:华中农业大学,2004.CHENG Yunjiang.Somatic cell cytoplasmic inheritance and chloroplast simple sequence repeat (SSR) primer development in Citrus[D].Wuhan:Huazhong Agricultural University,2004.
[18]FATTA DEL BOSCO S,SIRAGUSA M,ABBATE L,LUCRETTI S,TUSA N.Production and characterization of new triploid seedless progenies for mandarin improvement[J].Scientia Horticulturae,2007,114(4):258-262.
[19]CUENCA J,ALEZA P,JUÁREZ J,PINA J A,NAVARRO L.‘Safor’mandarin:A new Citrus mid-late triploid hybrid[J].HortScience,2010,45(6):977-980.
[20]ALEZA P,CUENCA J,JUÁREZ J,PINA J A,NAVARRO L.‘Garbí’mandarin:A new late-maturing triploid hybrid[J].Hort-Science,2010,45(1):139-141.
[21]GROSSER J W,GMITTER F G.Protoplast fusion for production of tetraploids and triploids:Applications for scion and rootstock breeding in citrus[J].Plant Cell,Tissue and Organ Culture,2011,104(3):343-357.
[22]GUO W W,XIAO S X,DENG X X.Somatic cybrid production via protoplast fusion for citrus improvement[J].Scientia Horticulturae,2013,163:20-26.
[23]解凯东,王晓培,王惠芹,梁武军,谢宗周,郭大勇,伊华林,邓秀新,GROSSER J W,郭文武.以柑橘多胚性二倍体母本倍性杂交培育三倍体[J].园艺学报,2014,41(4):613-620.XIE Kaidong,WANG Xiaopei,WANG Huiqin,LIANG Wujun,XIE Zongzhou,GUO Dayong,YI Hualin,DENG Xiuxin,GROSSER J W,GUO Wenwu.High efficient and extensive production of triploid Citrus plants by crossing polyembryonic diploids with tetraploids[J].Acta Horticulturae Sinica,2014,41(4):613-620.
[24]汤雨晴,杨惠栋,闫承璞,王斯妤,王雨亭,胡钟东,朱方红.基于重测序的‘金兰柚’基因组InDel 标记的开发及应用[J].园艺学报,2023,50(1):15-26.TANG Yuqing,YANG Huidong,YAN Chengpu,WANG Siyu,WANG Yuting,HU Zhongdong,ZHU Fanghong.Development and application of Jinlan pummelo(Citrus maxima)InDel markers based on genome re-sequencing[J].Acta Horticulturae Sinica,2023,50(1):15-26.
[25]韩健,夏文文,杨贵兵,罗旭钊,蒋松良,李先信,邓子牛,马先锋.沙田柚×枳杂交群体创建与InDel 标记鉴定[J].果树学报,2023,40(2):223-229.HAN Jian,XIA Wenwen,YANG Guibing,LUO Xuzhao,JIANG Songliang,LI Xianxin,DENG Ziniu,MA Xianfeng.Establishment of Shatian pomelo × P.trifoliata hybrid population and In-Del marker identification[J].Journal of Fruit Science,2023,40(2):223-229.