多倍体是指含有3 套或者3 套以上完整染色体组的生物体,自然界中大部分植物都经历过多倍化事件,如小麦、棉花、油菜等重要的粮棉油作物,苹果、猕猴桃、香蕉等果树,果桑、油茶、柿等经济林树种都是典型的多倍体。多倍体植物通常会表现出生长迅速、细胞及部分器官巨大、新陈代谢旺盛、某些生化成分含量提高、育性降低、少籽或无籽、生活力强、对环境的适应性增强等特点[1]。多倍体育种已成为果树育种的重要方法之一,并取得了显著成效,如三倍体苹果乔纳金、四倍体葡萄巨峰、九倍体柿平核无等,在国内外的栽培种中占据着重要地位。
柿(Diospyros kaki Thunb.)绝大部分栽培品种为六倍体(2n=6x=90),极少数栽培品种为九倍体(2n=9x=135),目前未见有关柿种内单倍体的报道。柿可能为同源多倍体,或部分同源异源多倍体[2],但其起源和进化过程仍是未解之谜。柿的多倍体育种已取得重要进展。九倍体品种平核无是通过实生选育获得的,是日本第一主栽涩柿品种[3](图1),具有树势强,花芽多、落果少、丰产,适应性强等特点,在应对较差的立地条件和极端的环境变化方面表现突出;且其果实易脱涩,耐储存,脱涩后果肉无褐斑、肉质脆、汁多、味甜,深受广大消费者喜欢,在鲜食和加工方面具有显著优势。秋王是日本经人工培育获得的世界首例九倍体无核完全甜柿新品种,通过太秋未减数(2n)花粉与富有正常卵杂交获得,其口感似太秋,但着色更好且果面裂纹少,综合品质优于太秋,被评为日本最佳甜柿品种[4]。自然选育或人工创制培育的九倍体品种,凭其优良的综合性状表现,展现出极大的利用价值和发展潜力,同时表明多倍体育种是柿创新优异种质、选育突破性良种的重要途径。
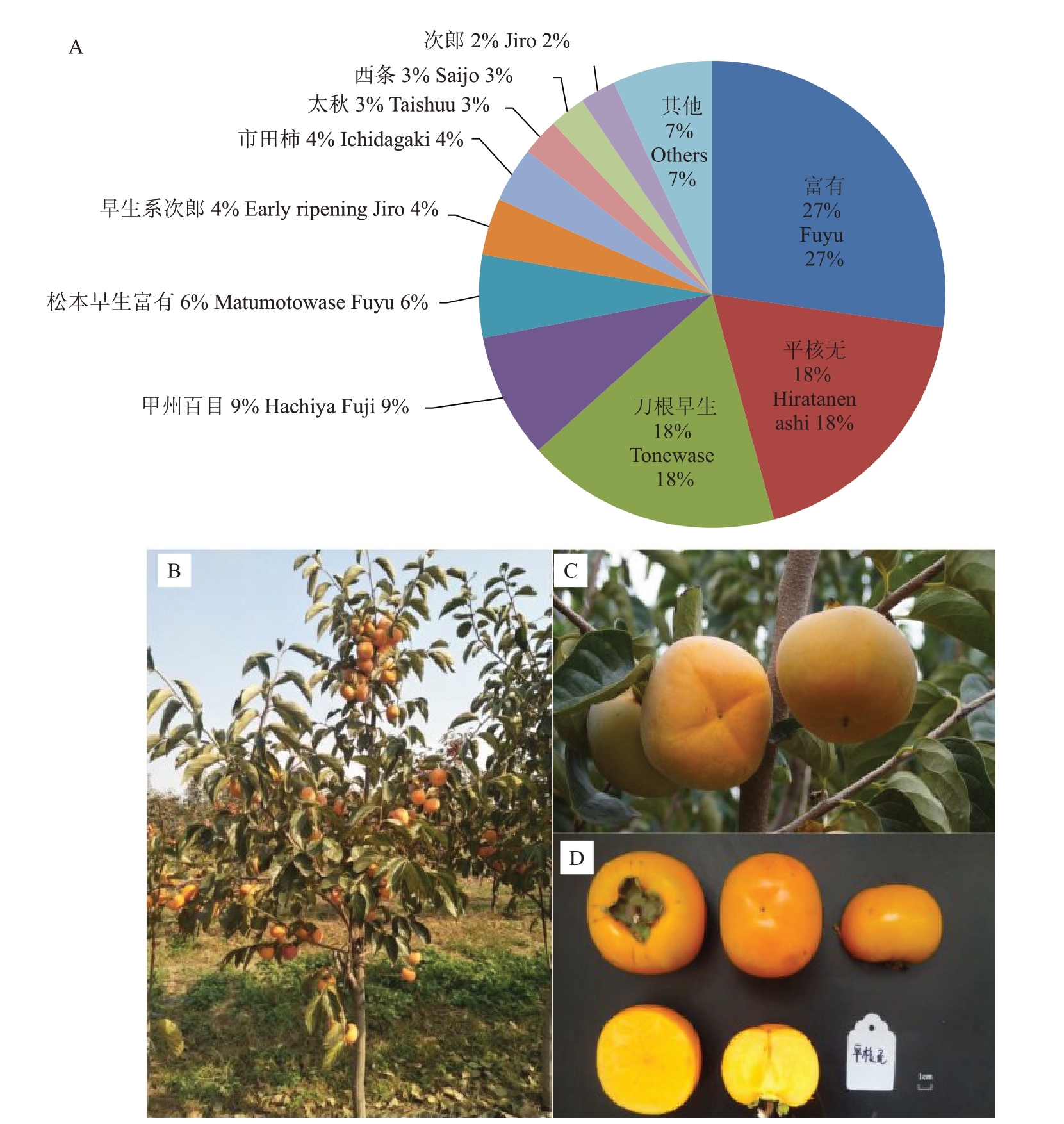
图1 九倍体品种平核无
Fig.1 The nonaploid D.kaki‘Hiratanenashi’
A.平核无在日本的栽培面积占比情况;B.平核无结果树;C.平核无结果枝;D.平核无果实。
A. The proportion of cultivated area of Hiratanenashi in Japan. B.A fruiting tree of Hiratanenashi. C.A bearing branch of Hiratanenashi. D. The fruit of Hiratanenashi.
笔者在本文中通过对国内外利用2n配子、体细胞染色体加倍、胚乳培养、原生质体融合等途径诱导柿多倍体的育种进展,以及形态学、染色体计数、流式细胞仪法等倍性鉴定方法的应用情况进行综述,总结近年来柿多倍体育种成果,以供柿育种研究人员参考。
1 柿倍性育种技术研究进展
普通栽培柿品种为六倍体,受高倍性限制,在此基础上开展柿多倍体育种,目前应用的技术主要包括利用2n配子诱导柿九倍体、理化诱导体细胞染色体加倍诱导多倍体、胚乳培养诱导柿多倍体,以及原生质体融合创制柿多倍体等。
1.1 利用2n配子创制柿九倍体
2n 配子在植物多倍化过程中发挥着至关重要的作用,利用自发或诱发获得的2n配子与正常配子杂交,可获得综合了杂种优势和倍性优势的种内三倍体,是植物育种工程中能获得期望性状最佳的倍性育种途径之一。柿九倍体实为种内三倍体,理论上是由未减数配子(2n)与正常配子(n)融合形成的,表现出植物三倍体的染色体行为和伪单性结实特性,生长发育正常,且其天然无核,该特性使其在鲜食,尤其是加工方面均具有很重要的应用价值。
平核无等自然九倍体的存在,表明柿种质可自然形成2n配子。前期研究表明,部分柿品种可自然形成2n 花粉,如禅寺丸(4.8%)、Yamato-gosho(6.3%)、Ama-yotsumizo(15.5%)等[5]。买旖旎等[6]对38份柿雄性种质天然2n花粉含量进行了评价,发现716 号和中柿4 号种质2n 花粉得率分别可达到7.17%和6.8%。但是,不同品种在不同年份产生2n花粉的比例不同,表明2n花粉的自然形成受到环境和基因型的双重影响。此外,有研究表明日本甜柿品种藤原御所可以自然形成高频率的2n 卵[7-8]。利用自然形成的2n 花粉或2n 卵进行杂交有望创制出九倍体新种质。Sugiura 等[5]利用禅寺丸2n 花粉与次郎杂交,结合幼胚挽救,获得九倍体新种质;Yamada等[9]和房剑锋[10]均利用禅寺丸与藤原御所杂交,获得九倍体和十二倍体新种质;Chijiwa 等[11]利用富有和太秋杂交,通过对发育不完全的种子进行胚培养,获得了九倍体新种质,经多年品种试验,审定了甜柿新品种福冈1号-秋王,该品种为目前品质最佳的甜柿品种;Sun 等[12]通过基因分型,证实秋王来源于太秋2n花粉与富有正常雌配子杂交。
自然形成的2n配子受环境和基因型影响,2n花粉比例相对较低且与正常花粉存在竞争关系,导致多倍体诱导效率较低,不能满足以创制九倍体甜柿新种质为目标的育种需求。因此,通过理化方法人工诱导更高比例的柿2n 花粉有望提高多倍体育种效率。用秋水仙碱等化学诱变剂处理,以及温度胁迫处理等均可诱导植物2n 配子的形成。谷晓峰等[13]利用秋水仙碱处理柿雄花芽诱导2n花粉,结果表明在雄花芽小孢子母细胞处于前期Ⅰ的双线期至终变期时,以0.3%~0.5%的秋水仙碱间隔6~7 h注射3 次,2n 花粉比例可达到40.6%。Yamada 等[14]利用低温4 ℃处理48 h 柿雄花芽,在处理后15~17 d 和17~18 d收集花粉,2n花粉比例显著提高。Mai等[15]在掌握柿树雄花芽小孢子母细胞减数分裂进程的基础上,对处于减数分裂前期的雄花芽施加42 ℃高温处理4 h,显著提高了2n 花粉比例(22%);随后改进措施,通过间隔多次处理,结合分批次收集花粉,显著提高2n 花粉比例达46%。目前研究成果主要是通过理化处理诱导出高比例柿2n花粉,但未有利用诱导获得的2n 花粉杂交创制出柿九倍体新种质的报道。此外,未见有利用理化处理的方法诱导柿2n雌配子的相关研究。
1.2 体细胞染色体加倍诱导柿多倍体
植物体细胞在受到外界环境刺激时可引起有丝分裂异常,进而造成体细胞染色体数目变异。有丝分裂异常可发生在合子或幼胚、分生组织及普通薄壁细胞中,合子或幼胚体细胞染色体加倍可形成完全多倍性的组织,而分生组织和薄壁细胞体细胞染色体加倍往往易形成嵌合体[16]。通过理化处理体细胞染色体加倍诱导柿多倍体已取得一定进展。Tamura 等[17]用0.1%秋水仙碱处理次郎的原生质体细胞6 d,处理后接种到培养基中诱导愈伤,最终获得十二倍体植株。谷晓峰等[18]以罗田甜柿试管苗离体叶片为材料,0.3%秋水仙碱处理4~6 d,诱导获得同质十二倍体。陈绪中等[19-20]以鄂柿1 号和秋焰甜柿离体叶片为外植体,0.5%秋水仙素处理4 d 后接种到培养基中,诱导获得了十二倍体再生植株;以罗田甜柿离体茎尖为外植体,0.08%秋水仙碱处理2 d后接种到培养基中,诱导获得了嵌合体植株。符昂等[21]以罗田甜柿组培苗为材料,25 μmol·L-1氟乐灵浸泡茎尖48 h 处理的存活率为53.3%,诱变率为25.0%;50 μmol·L-1的氟乐灵浸泡茎尖处理48 h 存活率为80.0%,诱变率为12.5%,获得的变异植株均为六倍体和十二倍体的嵌合体。此外,通过秋水仙碱处理刚萌发的柿种子,也可高效诱导出十二倍体新种质,诱导率可达10%(未发表数据)。通过体细胞染色体加倍获得十二倍体植株,再与六倍体柿进行杂交,是获得九倍体新种质的有效途径,因此,十二倍体植株也是重要的育种亲本材料。
1.3 胚乳培养诱导柿多倍体
被子植物的胚乳是双受精产物,属三倍体组织,理论上通过胚乳培养可直接获得种内三倍体植株。在胚乳培养时,供体植株的基因型、胚乳发育程度、胚因子、基本培养基类型、激素种类及用量、碳源、附加成分、培养条件等都会影响胚乳愈伤组织诱导率、分化率等;其中供体基因型是主要因素。前期科研人员在不同柿品种中进行了胚乳培养,取得一些进展。庄东红等[22]对黑柿、长良品种的胚乳进行培养,通过愈伤组织获得再生植株,经检测再生植株根尖染色体数目为2n=90(6x)或180(12x),但未获得九倍体植株。陈绪中等[23]对罗田甜柿自然授粉后70 d的胚乳进行了培养,采用不同的细胞分裂素和生长素组合,最终获得十二倍体植株。此外,蒲婷婷等对富有杂交种子的胚乳进行培养,通过优化愈伤诱导培养基和分化培养基,成功获得九倍体1株,十二倍体2株(未发表数据)。以上研究结果表明胚乳愈伤组织细胞在培养过程中发生了染色体数量的变化,易获得十二倍体或嵌合体,而获得理想的九倍体新种质较为困难。
1.4 原生质体融合创制柿多倍体
原生质体融合技术可获得多倍体植株,目前通过PEG 或电融合法已获得多种果树的体细胞杂种或胞质杂种[24]。六倍体柿与其他柿属植物存在很大的种间或倍性间杂交障碍,通过原生质体融合进行体细胞杂交,可以产生特殊倍性的种间杂种。Tamura 等[25-26]建立了日本柿的体细胞杂交技术,利用电融合技术将次郎和骏河2个六倍体品种的原生质体进行融合,并获得了种内十二倍体杂种(2n =12x=180);随后又将D. glandulosa(2n=2x=30)与六倍体次郎的原生质体进行融合,获得149个愈伤系,其中63 个分化获得植株,通过根尖染色体计数观察,证实获得的体细胞杂种均为八倍体(2n=8x=120)。谷晓峰等[27]以罗田甜柿休眠芽茎尖诱导的愈伤组织为材料,进行了原生质体分离、纯化、培养等条件优化,初步建立中国甜柿原生质体分离培养技术体系。通过柿与其近缘种进行原生质体融合,可将近缘种的一些重要性状引入到柿中,比如增强柿的抗病性、抗旱性、抗寒性等。
1.5 其他途径
芽变是柿种质创新的重要途径,日本已登记的新品种有66个,其中25个是芽变品种。九倍体柿品种平核无是一个重要的突变体材料,其芽变品种有早熟芽变、大果芽变、小果芽变等。Yakushiji等[28]发现一株平核无矮化的芽变植株,其茎长、节间长、叶片、花、果实等均小于平核无,且其可以形成正常的种子,经倍性鉴定后证实为八倍体(2n=8x=120),八倍体植株恢复形成正常种子的能力可能是其倍性由各奇倍性(9x)突变为偶倍性(8x)所致。
2 柿倍性鉴定技术研究进展
2.1 形态鉴定
形态鉴定可以依据植株茎粗细、叶形指数、生长快慢、花果和种子大小等指标来进行鉴定。陈绪中[19]在比较秋水仙素处理得到的十二倍体与六倍体对照中发现,多倍体甜柿植株与其他作物多倍体类似,茎干粗壮、节间较短、叶片较大,气孔大小与倍性具有相关性,但在多倍体间气孔密度上没有明显差异。房剑锋[10]在利用2n 卵培育出的六倍体和九倍体新种质的研究中观察到叶片气孔大小与倍性呈正相关,而与气孔密度呈负相关。张永芳等[29]观察柿属8种植物发现四倍体德阳柿花粉粒大小介于二倍体与六倍体之间。
2.2 染色体计数
通过细胞学方法进行染色体计数最直接、准确,相比于形态观察缩短了周期且更为准确。目前柿属植物染色体计数通常用压片法来进行。1980年,李懋学[30]以对二氯苯为预处理试剂报道了染色体数2n=90的柿材料。1990年,Zhuang等[31]检测平核无、刀根早生染色体数目为2n=135,且发育不成熟和退化的种胚植株中包含染色体数目106~114的非整倍体。1999 年,杨勇等[32]对中国绝大多数柿产区及日本原产柿品种进行染色体计数,在次郎种子中同样观察到染色体数目为83 或87 的非整倍体。2000年,唐仙英等[33]对20 个无核柿品种进行染色体计数,并通过种子发育分析认为中国柿品种的无核与染色体倍性没有相关性。
2.3 流式细胞术
运用流式细胞仪可测定植物基因组大小并进行倍性鉴定,其优点是快速、简单、高效,重复性好。早在1998年,Tamura等[26]就运用流式细胞仪检测了柿品种次郎及杂交后代等的核DNA含量,进一步鉴定次郎为六倍体、D.glandulosa为二倍体等,同时也发现了倍性为八倍体和十二倍体的嵌合体。陈绪中等[20]等运用流式细胞仪初步证实了秋水仙素诱导鄂柿1 号离体叶片获得的变异株为十二倍体。Yakushiji等[28]运用流式细胞术测得2005年日本发现主栽品种九倍体涩柿平核无芽变产生的矮化品种Hasshu 的DNA 含量介于六倍体次郎与九倍体平核无之间,结合染色体计数,证实其为八倍体。但是,在对柿新种质进行流式细胞仪检测时,发现同一种质不同叶片部位的样品在检测时的出峰位置不同,影响其倍性的判定。这可能是由于柿子叶片富含多糖、单宁、酚类物质等,造成细胞内黏性过高,黏性物质致使细胞质和细胞核紧密黏在一起,影响分离的细胞核纯度,并加速细胞核老化,阻碍流式细胞仪中鞘液的流动,以致于不能准确鉴定样品的倍性[34]。因此,需要进一步筛选适合柿的裂解液,并改进样品制备的处理方法。
对于柿倍性鉴定而言,其染色体多且小,用染色体计数方法需考虑合适的材料,如根尖和茎尖等有丝分裂较为活跃的组织,这些组织相对流式细胞术所需的叶片较难获得,且压片需要掌握一定的技巧,操作难度较大,费时费力。因此,可以通过流式细胞术对大批材料的倍性进行初步鉴定,再结合染色体计数法对重点候选材料进行精准倍性鉴定。
3 问题与展望
2n配子在有性多倍化、高频率传递杂合性和上位性等方面有其他育种方法无法比拟的优势。相对于细胞学特征难以观察的2n雌配子,2n花粉检测简单易行,在植物倍性育种方面应用更为广泛。但是2n 花粉在应用方面也存在一些问题:首先,为满足杂交育种对2n花粉量的需求,需要提高2n花粉的产生频率,即建立高效的2n花粉理化诱导技术;其次,2n 花粉萌发相对迟缓,导致其与正常花粉竞争力差,因此为提高倍性育种效率,需要建立有效的2n花粉分离技术,目前已报道的2n花粉分离方法主要有筛选法[35-36]、静电分离法[37]、速度沉降法[38]和流式细胞仪法[39]等,而柿的2n花粉分离仅有筛选法的报道,可以尝试其他更方便并能保证其活力的分离方法;再次柿2n 花粉与正常配子杂交后,因胚乳不平衡会出现种子胚乳败育现象,需要结合幼胚组织培养对其进行挽救,胚挽救获得的组培苗通过试管苗嫁接可以缩短育种周期,但目前报道的嫁接成活率最高为45%[40],需要进一步优化嫩枝嫁接技术。
2n雌配子不存在竞争问题,授粉后即能获得种子,利用价值更高。但因其细胞学特征难以观察,一般只能通过对其杂交后代倍性的检测来确定2n 雌配子的发生,严重限制了其在果树倍性育种中的应用。随着现代生物技术的发展,对2n配子发生机制的研究日益增多[41-42]。通过对2n配子形成机制的研究,解析相关基因和调控机制,不仅可以利用分子设计育种对现有材料进行遗传改良,使其自发形成高比例的2n 配子,也可以开发相关连锁分子标记,筛选2n配子高产植株,促进2n配子在育种中的高效利用[43]。因此,如何将分子育种技术与倍性育种相结合,加速柿遗传改良进度,是亟须进一步探讨和研究的问题,也是未来柿育种的发展方向。
体细胞染色体加倍诱导多倍体是果树上常见的无性多倍化途径,包括在体诱导和离体诱导两种方式,前者主要是以秋水仙碱等诱变剂处理顶芽、腋芽,后者是处理种子、组培离体叶片和茎尖等。在体诱导往往获得嵌合体现象严重,进一步纯化耗时耗力;而通过种子或愈伤途径诱导多倍体时,诱导加倍后的单细胞再生成植株,获得纯合体的概率较大[24];通过处理离体茎尖诱导多倍体时,诱变剂浸泡处理效果优于直接加入培养基中,但也易出现嵌合体[44]。柿组培体系已经建立,并通过诱导离体叶片或茎尖等成功获得一些十二倍体材料,但是诱导效率较低。以种子为材料,通过秋水仙碱处理刚萌发的种子,十二倍体诱导效率可达10%,种子播种后可直接成苗,省时省力,是一种高效的多倍体诱导途径,可以快速创制大批多倍体新种质,为优异新品种选育提供材料基础。
胚乳培养是获得种内三倍体的重要途径之一,但是胚乳组织在形成愈伤并进一步分化成植株的过程中,会发生染色体数目的变化,恢复成二倍性细胞或突变成四倍性细胞,导致种内三倍体的诱导效率降低。因此,以创制九倍体柿新种质为目的的胚乳培养,需要对其培养基配方及培养条件进一步优化,并对引起胚性愈伤组织染色体数目变化的调控因子进行研究。此外,原生质体融合创制多倍体已在多种果树上获得成果,其中以柑橘原生质体融合技术最为成熟;柿在此方面也开展了部分研究,但与其他果树相比仍然存在很大差距,需进一步加强这一方面的研究。
除多倍体育种外,果树单倍体育种方法也日益成熟,获得的单倍体或双单倍体新种质进一步用于基因组测序,可以推动多年生果树的分子生物学和遗传学研究[45]。单倍体育种的主要途径包括[46-47]:①种间或属间远缘杂交,花粉虽不能正常受精,但可以刺激卵细胞发育成胚;②物理照射或化学诱变,花粉失去活力,授粉后刺激卵细胞孤雌发育,产生单倍体;③未授粉的子房或胚珠培养;④花药和花粉培养;⑤利用基因编辑技术CRISPR 产生单倍体。目前虽未见有关柿单倍体育种的报道,但柿与油柿、柿与美洲柿等种间远缘杂交工作已经相继开展,且配套的幼胚挽救技术已经成熟,因此,多种单倍体诱导途径皆可尝试。柿作为传统木本粮食树种,在中国栽培历史悠久,但其起源与进化历程仍不清楚,并且其六倍体基因组复杂难解,对重要经济性状形成的分子机制解析缺乏完整的遗传信息,严重阻碍了柿分子育种的进程。因此,开展柿单倍体育种,对柿分子遗传学相关研究具有重要意义。
[1] 康向阳.林木三倍体育种研究进展及展望[J].中国科学:生命科学,2020,50(2):136-143.KANG Xiangyang. Research progress and prospect of triploid breeding of forest trees[J]. Scientia Sinica (Vitae),2020,50(2):136-143.
[2] AKAGI T,TAO R,TSUJIMOTO T,KONO A,YONEMORI K.Fine genotyping of a highly polymorphic ASTRINGENCYlinked locus reveals variable hexasomic inheritance in persimmon (Diospyros kaki Thunb.) cultivars[J]. Tree Genetics & Genomes,2012,8(1):195-204.
[3] SATO A,ONOUE N. Recent persimmon cultivar composition and breeding activities in Japan[C]//YONEMORI K,TAIRA S.Abstract Book,7th International Symposium on Persimmon in Nara,Japan.Nara,Japan:the Institute of Fruit Tree and Tea Science of NARO,2021:22.
[4] CHIJIWA H,ASAKUMA H,ISHIZAKA A. Development of seedless PCNA persimmon (Diospyros kaki Thunb.) cv. Fukuoka K1 Gou and the effect of gibberellin spray and/or disbudding on fruit set[J]. Horticultural Research (Japan),2013,12(3):263-267.
[5] SUGIURA A,OHKUMA T,CHOI Y A,TAO R,TAMURA M.Production of nonaploid (2n = 9x) Japanese persimmons (Diospyros kaki) by pollination with unreduced (2n = 6x) pollen and embryo rescue culture[J]. Journal of the American Society for Horticultural Science,2000,125(5):609-614.
[6] 买旖旎,李树战,索玉静,孙鹏,韩卫娟,刁松锋,王丽媛,李华威,傅建敏.柿不同种质中的天然2n 花粉得率的评价及诱导时期的判定[J].中国农业大学学报,2019,24(12):44-52.MAI Yini,LI Shuzhan,SUO Yujing,SUN Peng,HAN Weijuan,DIAO Songfeng,WANG Liyuan,LI Huawei,FU Jianmin. Identification of natural 2n pollens in different persimmon germplasms and ascertainment of their induction peroid[J].Journal of China Agricultural University,2019,24(12):44-52.
[7] TAO R,YAMADA A,ESUMI T,MOTOSUGI H,SUGIURA A.Ploidy variations observed in the progeny of hexaploid Japanese persimmon (Diospyros kaki)‘Fujiwaragosho’[J]. Horticultural Research(Japan),2003,2(3):157-160.
[8] YAMADA A,TAO R. High frequency sexual polyploidisation observed in hexaploid Japanese persimmon (Diospyros kaki)‘Fujiwaragosho’[J]. The Journal of Horticultural Science and Biotechnology,2006,81(3):402-408.
[9] YAMADA A,TAO R. Controlled pollination with sorted reduced and unreduced pollen grains reveals unreduced embryo sac formation in Diospyros kaki Thunb.‘Fujiwaragosho’[J].Journal of the Japanese Society for Horticultural Science,2007,76(2):133-138.
[10] 房剑锋.利用2n 卵创制无核甜柿新种质的研究[D].武汉:华中农业大学,2010.FANG Jianfeng.Studies on the production of nonaploid seedless pollination-constant nonastringent persimmon (PCNA) by pollination with 2n egg[D].Wuhan:Huazhong Agricultural University,2010.
[11] CHIJIWA H,KUWAHARA M,HIRAKAWA N,TETSUMURA T.Generation of nonaploid persimmons(Diospyros kaki Thunb.)by embryo culture of imperfect seeds derived from a cross between‘Fuyu’and‘Taishuu’[J].Journal of the Japanese Society for Horticultural Science,2008,77(4):358-363.
[12] SUN P,NISHIYAMA S,ASAKUMA H,VOORRIPS R E,FU J M,TAO R.Genomics-based discrimination of 2n gamete formation mechanisms in polyploids:A case study in nonaploid Diospyros kaki‘Akiou’[J]. G3-Genes Genomes Genetics,2021,11(8):jkab188.
[13] 谷晓峰,罗正荣.柿品种禅寺丸的花粉染色体加倍研究[J].中国农业科学,2003,36(4):426-428.GU Xiaofeng,LUO Zhengrong. Study on pollen chromosome doubling in Zenjimaru persimmon[J]. Scientia Agricultura Sinica,2003,36(4):426-428.
[14] YAMADA A,TAO R,SUGIURA A. Influence of low temperature before flowering on the occurrence of unreduced pollen in Japanese persimmon (Diospyros kaki Thunb.)[J]. HortScience,2005,40(1):24-28.
[15] MAI Y N,LI H W,SUO Y J,FU J M,SUN P,HAN W J,DIAO S F,LI F D. High temperature treatment generates unreduced pollen in persimmon(Diospyros kaki Thunb.)[J].Scientia Horticulturae,2019,258:108774.
[16] 杨继. 植物多倍体基因组的形成与进化[J]. 植物分类学报,2001,39(4):357-371.YANG Ji.The formation and evolution of polyploid genomes in plants[J].Acta Phytotaxonomica Sinica,2001,39(4):357-371.
[17] TAMURA M,TAO R,SUGIURA A. Production of dodecaploid plants of Japanese persimmon (Diospyros kaki L.) by colchicine treatment of protoplasts[J].Plant Cell Reports,1996,15(7):470-473.
[18] 谷晓峰,罗正荣.秋水仙素处理‘罗田甜柿’获得12 倍体再生植株[J].园艺学报,2003,30(3):325-327.GU Xiaofeng,LUO Zhengrong. Regeneration of dodecaploid plants from in vitro leave of‘Luotian Tianshi’persimmon treated with colchicine[J]. Acta Horticulturae Sinica,2003,30(3):325-327.
[19] 陈绪中.甜柿多倍体创制的初步研究[D].武汉:华中农业大学,2003.CHEN Xuzhong. Preliminary studies on polyploidy creation in non-astringent persimmon[D]. Wuhan:Huazhong Agricultural University,2003.
[20] 陈绪中,罗正荣.鄂柿1 号离体叶片秋水仙素诱导和再生植株倍性鉴定[J].果树学报,2005,22(5):554-556.CHEN Xuzhong,LUO Zhengrong. Ploidy verification of regenerated plants of Eshi 1 persimmon from in vitro leaves treated with colchicine[J]. Journal of Fruit Science,2005,22(5):554-556.
[21] 符昂,刘梦锜,刘一凤,张青林,徐莉清,罗正荣.‘罗田甜柿’多倍体诱导及其生物学特性研究[R].山东临朐:第九届全国柿生产和科研进展研讨会,2018.FU Ang,LIU Mengyi,LIU Yifeng,ZHANG Qinglin,XU Liqing,LUO Zhengrong. Studies on polyploid induction and biological characteristics of sweet persimmon Luotian[R]. Linqu,Shandong:The 9th National Symposium on Persimmon Production and Research Progress,2018.
[22] 庄东红,石田雅士.柿树胚乳培养及其再生植株染色体倍性变化的研究[J].汕头大学学报(自然科学版),1995,10(1):42-47.ZHUANG Donghong,MASASHI I. Changes in chromosomal ploidy in regenerated plant obtained from cultured endosperm of Diospyros kaki[J].Journal of Shantou University(Natural Science Edition),1995,10(1):42-47.
[23] 陈绪中,罗正荣.‘罗田甜柿’胚乳培养获得十二倍体再生植株[J].园艺学报,2004,31(5):589-592.CHEN Xuzhong,LUO Zhengrong.Dodecaploid plantlets regenerated from endosperm culture of‘Luotiantianshi’persimmon[J].Acta Horticulturae Sinica,2004,31(5):589-592.
[24] 石庆华,刘平,刘孟军.果树倍性育种研究进展[J].园艺学报,2012,39(9):1639-1654.SHI Qinghua,LIU Ping,LIU Mengjun. Advances in ploidy breeding of fruit trees[J].Acta Horticulturae Sinica,2012,39(9):1639-1654.
[25] TAMURA M,TAO R,SUGIURA A. Regeneration of somatic hybrids from electrofused protoplasts of Japanese persimmon(Diospyros kaki L.)[J].Plant Science,1995,108(1):101-107.
[26] TAMURA M,TAO R,SUGIURA A. Production of somatic hybrids between Diospyros glandulosa and D.kaki by protoplast fusion[J].Plant Cell,Tissue and Organ Culture,1998,54(2):85-91.
[27] 谷晓峰,罗正荣.罗田甜柿原生质体分离、培养及植株再生[J].园艺学报,2002,29(4):369-371.GU Xiaofeng,LUO Zhengrong. Studies on the isolation,culture of protoplast and plant regeration in Luotiantianshi persimmon[J].Acta Horticulturae Sinica,2002,29(4):369-371.
[28] YAKUSHIJI H,YAMASAKI A,KOBAYASHI S,KANEYOSHI J,AZUMA A,SUGIURA H,SATO A. Characteristics of a dwarf octoploid mutation arising from a nonaploid persimmon cultivar[J].The Horticulture Journal,2016,85(3):209-216.
[29] 张永芳,胡超琼,杨勇,朱仁胜,郭静,王仁梓.柿属8 种植物花粉形态观察[J].园艺学报,2016,43(6):1167-1174.ZHANG Yongfang,HU Chaoqiong,YANG Yong,ZHU Rensheng,GUO Jing,WANG Renzi. Pollen morphology observation of eight resources in Diospyros[J].Acta Horticulturae Sinica,2016,43(6):1167-1174.
[30] 李懋学. 对二氯苯在植物染色体预处理中的应用[J]. 遗传,1980,2(6):30-32.LI Maoxue. Application of p-diehlorohenzene in pretreatment for plant chromosomes[J].Hereditas,1980,2(6):30-32.
[31] ZHUANG D H,KITAJIMAA,ISHIDA M,SOBAJIMA Y.Chromosome numbers of Diospyros kaki cultivars[J]. Journal of the Japanese Society for Horticultural Science,1990,59(2):289-297.
[32] 杨勇,王仁梓,李高潮,王雯.柿属植物及柿品种染色体数目研究[J].西北农业学报,1999,8(3):64-66.YANG Yong,WANG Renzi,LI Gaochao,WANG Wen. Study on chromosome numbers of Diospyros and their varieties[J].Acta Agriculturae Boreali-Occidentalis Sinica,1999,8(3):64-66.
[33] 唐仙英,罗正荣.部分中国柿品种及其胚培养后代的染色体倍性研究[J].园艺学报,2000,27(4):235-239.TANG Xianying,LUO Zhengrong.Ploidy of adult trees and plantlet cultured from embryos of some seedless cultivars of Chinese persimmon[J].Acta Horticulturae Sinica,2000,27(4):235-239.
[34] 朱道圩,杨宵,郅玉宝,易明林,理莎莎,符真珠.用流式细胞仪鉴定中华猕猴桃的多倍体[J].植物生理学通讯,2007,43(5):905-908.ZHU Daoyu ,YANG Xiao,ZHI Yubao,YI Minglin,LI Shasha,FU Zhenzhu. Identification of polyploidy in Actinidia chinensis Planch. by flow cytometer[J]. Plant Physiology Communications,2007,43(5):905-908.
[35] 谷晓峰,唐仙英,罗正荣.柿品种‘禅寺丸’未减数花粉分离体系的建立[J].果树学报,2001,18(1):32-34.GU Xiaofeng,TANG Xianying,LUO Zhengrong. Study on the establishment of separation system for unreduced pollens in‘Zenjimaru’persimmon variety[J]. Journal of Fruit Science,2001,18(1):32-34.
[36] 张平冬,周晴,桑亚茹,吴剑,李智群,赵一帆.杨树2n 花粉的诱导和分离纯化方法:202011141926.4[P].2021-01-15.ZHANG Pingdong,ZHOU Qing,SANG Yaru,WU Jian,LI Zhiqun,ZHAO Yifan.Methods for induction,separation and purification of 2n pollen from Poplar:202011141926.4[P]. 2021-01-15.
[37] 商显宝.花粉分选机理探讨及花粉分选装置的改进[D].泰安:山东农业大学,2006.SHANG Xianbao. Research on the pollens’electrified and the improvement of the pollens’electrostatic separating device[D].Tai’an:Shandong Agricultural University,2006.
[38] SIMON C J,SANFORD J C.Separation of 2n potato pollen from a heterogeneous pollen mixture by velocity sedimentation[J].HortScience,1990,25(3):342-344.
[39] 傅嘉欣,刘成明,熊海,胡桂兵,甘吉昌,毛晓丹.从花粉中分选2n 花粉的方法:CN110542641A[P].2020-11-06.FU Jiaxin,LIU Chengming,XIONG Hai,HU Guibing,GAN Jichang,MAO Xiaodan. Method for sorting 2n pollen from pollen:CN110542641A[P].2020-11-06.
[40] 吴彩红.甜柿十二倍体试管苗嫁接与移栽研究[D].武汉:华中农业大学,2004.WU Caihong. Studies on grafting and transplanting in dodecaploid plantlet of nonastringent persimmon[D]. Wuhan:Huazhong Agricultural University,2004.
[41] ZHOU Q,CHENG X T,KONG B,ZHAO Y F,LI Z Q,SANG Y R,WU J,ZHANG P D.Heat shock-induced failure of meiosisⅠto meiosis Ⅱtransition leads to 2n pollen formation in a woody plant[J].Plant Physiology,2022,189(4):2110-2127.
[42] VAVRDOVÁ T,SAMAJ J,KOMIS G. Phosphorylation of plant microtubule-associated proteins during cell division[J].Frontiers in Plant Science,2019,10:238.
[43] 张正海,康向阳.植物2n 配子发生及其遗传标记研究进展[J].遗传,2006,28(1):105-109.ZHANG Zhenghai,KANG Xiangyang. Advances in researches on genetic markers of 2n gametes[J]. Hereditas,2006,28(1):105-109.
[44] 高宁宁,李晓慧,康利允,梁慎,常高正,李海伦,王慧颖,王琰,徐小利,赵卫星.单倍体甜瓜染色体加倍技术研究[J].中国瓜菜,2021,34(6):28-32.GAO Ningning,LI Xiaohui,KANG Liyun,LIANG Shen,CHANG Gaozheng,LI Hailun,WANG Huiying,WANG Yan,XU Xiaoli,ZHAO Weixing. Study on chromosome doubling technique of haploid muskmelon[J].China Cucurbits and Vegetables,2021,34(6):28-32.
[45] 王婵,刘建勋,王托和,张立荣,赵向田.单倍体育种技术在玉米种质创新上的应用与实践[J].现代农业科技,2019(10):32-33.WANG Chan,LIU Jianxun,WANG Tuohe,ZHANG Lirong,ZHAO Xiangtian. Application and practice of haploid breeding technology on maize germplasm innovation[J]. Modern Agricultural Science and Technology,2019(10):32-33.
[46] 陈海强,刘会云,王轲,张双喜,叶兴国.植物单倍体诱导技术发展与创新[J].遗传,2020,42(5):466-482.CHEN Haiqiang,LIU Huiyun,WANG Ke,ZHANG Shuangxi,YE Xingguo. Development and innovation of haploid induction technologies in plants[J].Hereditas,2020,42(5):466-482.
[47] 白继鹏,王洪洋,李灿辉.马铃薯双单倍体诱导及辅助育种研究进展[J].中国瓜菜,2021,34(4):1-7.BAI Jipeng,WANG Hongyang,LI Canhui. Research progress on potato dihaploid induction and assistant breeding[J]. China Cucurbits and Vegetables,2021,34(4):1-7.