改革开放以来,我国果树产业发展取得巨大成就,年产值约1万亿元,从业人口约1亿,果树种植面积和产量居世界首位[1]。同时随着我国经济持续增长,居民消费水平不断升级,对高品质农产品的需求与日俱增。果实品质主要以理化和感官两个方面作为评价指标,前者主要包括营养物质的种类和含量,后者主要指风味物质、色素物质和果实肉质等[2]。其中果实风味作为评价果实品质的内在指标,也是决定其市场占有率、种植面积的重要因素,因此成为科学研究的重点。
涩味是果实的基本风味之一,通常存在于未成熟的果实当中,并随着果实成熟逐渐减弱。但是部分栽培品种[3-4]和野生资源在果实成熟之后涩味依然比较明显[5],阻碍了野生种质的利用和优良品种选育。果实涩味与单宁(tannins)、儿茶素(catechin,C)、表儿茶素(epicatechin,EC)、绿原酸(chlorogenic acid,CA)、新绿原酸(neochlorogenic acid)等多酚物质含量相关[6-8],且在不同果树上的遗传调控存在差异。笔者在本研究中从果实涩味物质分类、合成与积累,不同果树涩味物质研究方面取得的进展,果实脱涩机制与技术几个方面进行综述,并提出研究建议,以期为低涩味或无涩味的新品种选育提供思路。
1 果实涩味物质分类
涩味即收敛感,是触觉神经末梢被刺激之后在口腔表面产生的干燥、收紧、粗糙感[9]。研究表明,涩味化合物主要包括单宁、儿茶素、表儿茶素、绿原酸、新绿原酸等多酚物质,其中单宁对涩味的影响最大[6-8]。
单宁,是植物体内的多酚类次生代谢产物,它产生涩味的机制是其结构中的酚羟基能够与唾液蛋白发生缩合反应,使唾液蛋白发生沉淀,引起口腔的收敛和皱缩[10]。根据在醇溶液中的溶解性,单宁可被分为可溶性单宁(soluble tannin)和不溶性单宁(insoluble tannin),其中可溶性单宁可溶于甲醇,而不溶性单宁在无水甲醇中的溶解度极低[11]。另外根据化学结构,果实中的单宁可被分为水解单宁(hydrolysable tannins,HTs)和缩合单宁(condensed tannins,CTs)两大类[12]。HTs由酸及其衍生物与葡萄糖或者多元醇以酯键相连形成,并可以被酸或者酶水解;根据水解产生的酚酸种类,水解单宁又被分为没食子单宁和鞣花单宁[13]。研究表明鞣花单宁是石榴中主要的涩味物质[14]。CTs也被称为原花青素(proanthocyanidins,PAs),是由儿茶素和表儿茶素等黄烷-3-醇单元结构缩合而形成的聚合物[15],并能够在热酸作用下缩合成花色素[16]。据报道,果实涩味与原花青素以及其单体物质儿茶素、表儿茶素含量密切相关[6-8]。然而,果实涩味并不一定与单宁含量呈线性正相关,还受单宁结构、种类的影响[17]。
酚酸也是广泛存在于植物种子、果皮、蔬菜叶中的一类多酚物质,其结构中包含一个羧酸基团。植物体内的酚酸主要包括羟基苯甲酸和羟基肉桂酸,其中绿原酸是由咖啡酸和奎宁酸结合形成的可溶性羟基肉桂酸[18]。新绿原酸是绿原酸的同分异构体,且两者均对果实涩味有一定影响。研究表明,桃果实中绿原酸和新绿原酸与果实涩味的相关系数r分别为0.711和0.660[4]。苹果中也有类似研究,即果实成熟时,酚酸在涩味显著果实中的含量高于低涩味果实中的含量[6]。但是酚酸导致涩味的机制目前尚不清晰。
2 涩味物质合成与积累
多酚是导致果实涩味的主要物质,其在植物体内主要通过苯丙烷、类黄酮、酚酸三个途径合成。多酚合成的前体物质是苯丙氨酸,然后在苯丙氨酸裂解酶(PAL)、肉桂酸4-羟化酶(C4H)和4-香豆酰CoA 连接酶(4CL)3 种酶的催化下,依次生成肉桂酸、香豆酸和4-香豆酰CoA,这个过程称为苯丙烷类代谢途径。其中PAL 是该过程的关键酶和限速酶。之后,多酚代谢经4-香豆酰CoA转入酚酸和类黄酮两条途径(图1)。
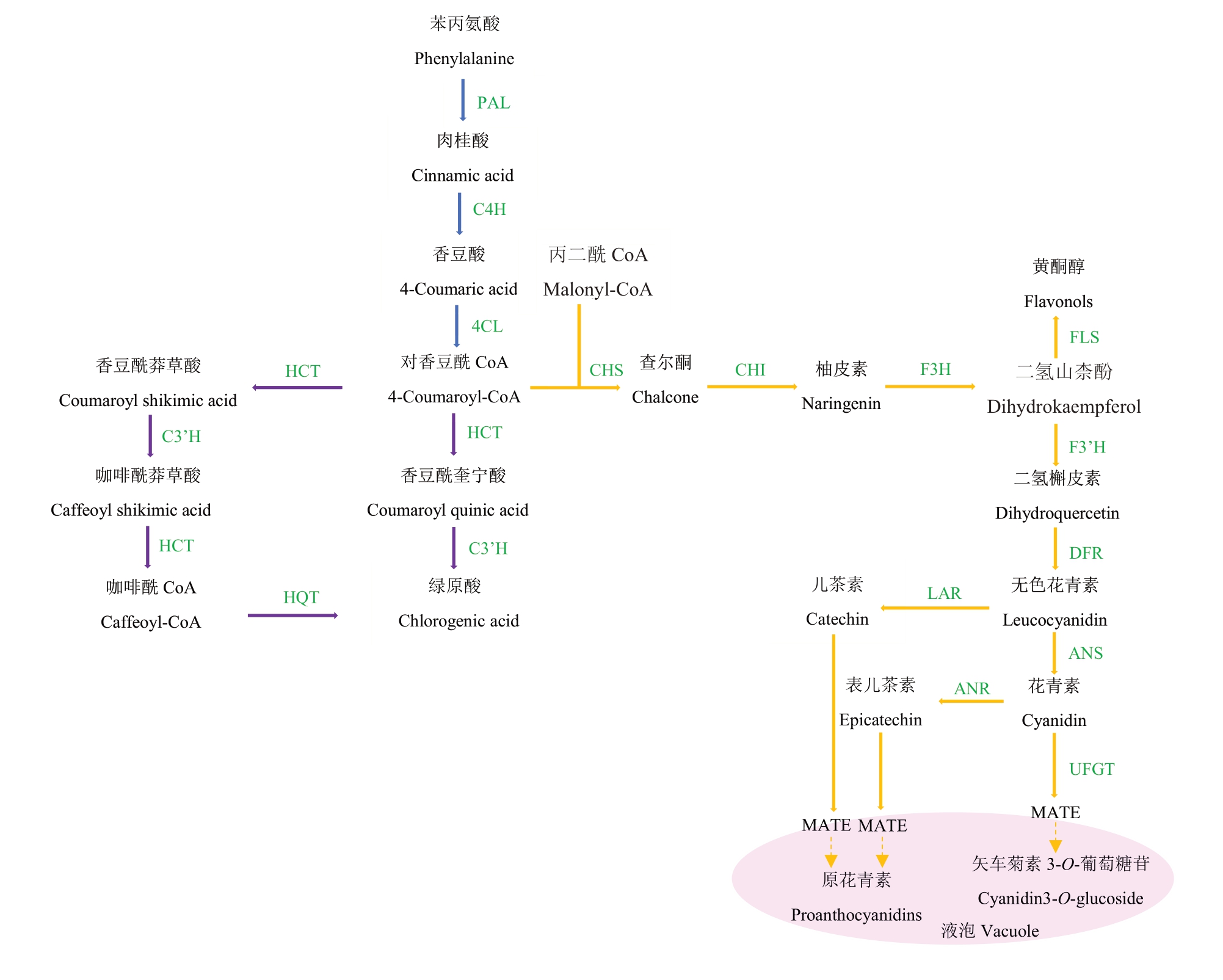
图1 植物涩味物质代谢途径[19]
Fig.1 Metabolic pathways of plant astringent substances[19]
蓝色箭头代表苯丙烷代谢途径,紫色箭头代表酚酸代谢途径,黄色箭头代表类黄酮代谢途径,绿色字代表代谢途径中的酶,粉色椭圆代表液泡。PAL.苯丙氨酸裂解酶;C4H.肉桂酸4-羟化酶;4CL.4-香豆酰CoA 连接酶;CHS.查尔酮合成酶;CHI.查尔酮异构酶;F3H.黄烷酮3-羟化酶;F3’H.黄烷酮3’-羟化酶;DFR.二氢黄酮醇4-还原酶;LAR.无色花青素还原酶;ANR.花青素还原酶;ANS.花青素合成酶;FLS.黄酮醇合酶;UFGT.类黄酮3-O-葡萄糖基转移酶;MATE.MATE-type 转运子;C3’H.香豆酸3’-羟化酶;HCT.羟基桂皮酰转移酶;HQT.奎宁酸羟基桂皮酰转移酶。
The blue arrow represents the phenylpropane metabolic pathway, the purple arrow represents the phenolic acid metabolic pathway, the yellow arrow represents the flavonoid metabolic pathway,the green word represents the enzyme in the metabolic process,and the pink oval represents the vacuole. PAL. Phenylalanine ammonia-lyase; C4H. Cinnamate-4-hydroxylase; 4CL. 4-coumarate-CoA ligase; CHS. Chalcone synthase; CHI. Chalcone isomerase; F3H. Flavanone 3-hydroxylase; F3’H. Flavanone 3’- hydroxylase; DFR. Dihydroflavonol 4-reductase; LAR. Leucocyanidin reductase;ANR. Anthocyanidin reductase; ANS. Anthocyanidin synthase; FLS. Flavonol synthase; UFGT. UDP glucose:flavonoid 3-O-glucosyltransferase;MATE. Multidrug and toxic compound extrusion transporters; C3’H. Cinnamate 3’- hydroxylase; HCT. Hydroxycinnamoyl transferase; HQT. Hydroxycinnamoyl-CoA quinate transferase.
根据KEGG 数据库中的代谢通路,4-香豆酰CoA 可以通过两条途径生成酚酸:一条是在羟基桂皮酰转移酶(HCT)、香豆酸3-羟化酶(C3’H)催化下依次生成香豆酰莽草酸、咖啡酰莽草酸和咖啡酰辅酶A,另一条途径是直接生成香豆酰奎宁酸,而咖啡酰辅酶A和香豆酰奎宁酸可分别被奎宁酸羟基桂皮酰转移酶(HQT)和C3’H催化生成绿原酸。HCT是酚酸合成途径中的关键酶[20]。杜仲NuHCT[21]和烟草NtHCT[22]能够影响绿原酸及黄酮类化合物合成。绿原酸合成途径中另一个关键酶是C3’H,属于CYP450 家族。通过体外酶活性研究,发现该基因编码的产物可催化咖啡酰莽草酸和咖啡酰奎宁酸的羟基化反应并催化绿原酸的合成[23]。
4-香豆酰CoA 和丙二酰CoA 在查尔酮合成酶(CHS)作用下生成查尔酮,使多酚代谢转入类黄酮生物合成途径。查尔酮异构酶(CHI)、黄烷酮3-羟化酶(F3H)等能够催化查尔酮生成二氢山柰酚等二氢黄酮醇类物质。二氢黄酮醇是花色苷、单宁和其他类黄酮化合物的共同前体产物,能经二氢黄酮醇4-还原酶(DFR)催化形成无色花青素。无色花青素在植物体内有两个分支:能在花青素合成酶(ANS)作用下生成有色花青素,然后有色花青素通过花色素还原酶(ANR)催化得到表儿茶素,也可以直接经无色花色素还原酶(LAR)催化形成儿茶素[24];最后表儿茶素和儿茶素聚合生成原花青素[25]。
原花青素的单体物质儿茶素和表儿茶素在细胞质中生成,但是原花青素只在液泡中积累,因此单体物质需要运输到液泡中进行聚合和储存。原花青素单体可以通过MATE[26-27]和GST[28]等转运蛋白运输,也可以通过囊泡运输[29]。此外原花青素单体在液泡中的聚合还受漆酶影响。DkLAC1促使可溶性单宁聚合为不溶性单宁[30];FaTT10则促使草莓中原花青素单体的聚合[31]。
3 不同果实中涩味物质代谢调控
3.1 柿
柿是我国的特色果树之一,在我国已有2000多年的栽培历史[32]。研究表明,柿果实中的涩味物质主要是原花青素,在新鲜涩柿果实中,其含量约占鲜果质量的2%[33]。柿品种众多,根据果实成熟时能否在树上自然脱涩以及涩味性状遗传特点,可将栽培品种分为完全甜柿(PCNA)和非完全甜柿(非PCNA)[34]。其中PCNA 果实在树上就能自然脱涩,而非PCNA 型果实在完全成熟之前仍然具有涩味,需要人工脱涩才能食用。
目前,F3’5’H、ANR 和LAR 已被报道与柿原花青素的生物合成相关,且PCNA 型果实中F3’5’H、ANR 表达量显著低于非PCNA 型[35-36]。通过对果实发育过程中ANR 和LAR 的表达量进行测定,发现DkANR 的表达量显著高于DkLAR[35],这与原花青素结构中表儿茶素含量较高的结果相一致[37]。在发育过程中,DkANR的表达量与原花青素含量存在正相关;当果实成熟时,ANR表达受到强烈抑制,且伴随原花青素含量的减少[37]。因此DkANR 可能是柿原花青素生物合成的关键基因。另外柿原花青素的合成与积累也受到转录因子的调节作用。通常来说,MYB 转录因子常与bHLH、WD40 蛋白形成MBW复合体共同调控多酚物质的生物合成,同时也可单独发挥调控作用。DkMYB2能够与bHLH转录因子结合,共同增强ANR 启动子活性,也可以单独激活ANR 启动子活性;而DkMYB4 则必须与bHLH 共同发挥作用[37-38]。DkMYB4能够与DkANS、DkF3’5’H、DkANR启动子区的MYBCORE顺式基序结合,但对DkLAR的表达没有影响[39]。同时DkMYB4的表达受到ABA响应因子DkbZIP5的调控。DkbZIP5能够识别DkMYB4 启动子区的ABA 响应元件ABRE,激活DkMYB4活性,正向调控原花青素的合成[40]。此外,柿中鉴定到的WD40 蛋白DkWDR1 能与DkMYB4结合并抑制DkMYB4的表达[37]。
同时负调控因子也参与柿果实中原花青素的生物合成。DkMYB14能够抑制类黄酮生物合成途径中相关基因的表达,直接抑制原花青素的合成[41]。miRNA858b 通过负调控靶基因DkMYB19、Dk-MYB20 的表达,抑制果实和叶片中原花青素的积累[42]。值得指出的是,部分调节因子通过分解涩味物质或者改变其结构,从而改善果实风味。Dk-MYB14可以激活乙醛生物合成途径的相关基因,促使乙醛的合成;而乙醛结构中的醛基能与可溶性单宁的酚羟基发生酚醛缩合反应,使可溶性单宁转为不溶性的单宁,使果实脱涩[41,43];DkLAC1 通过改变单宁的聚合度,最终使果实涩味减弱[30](图2)。
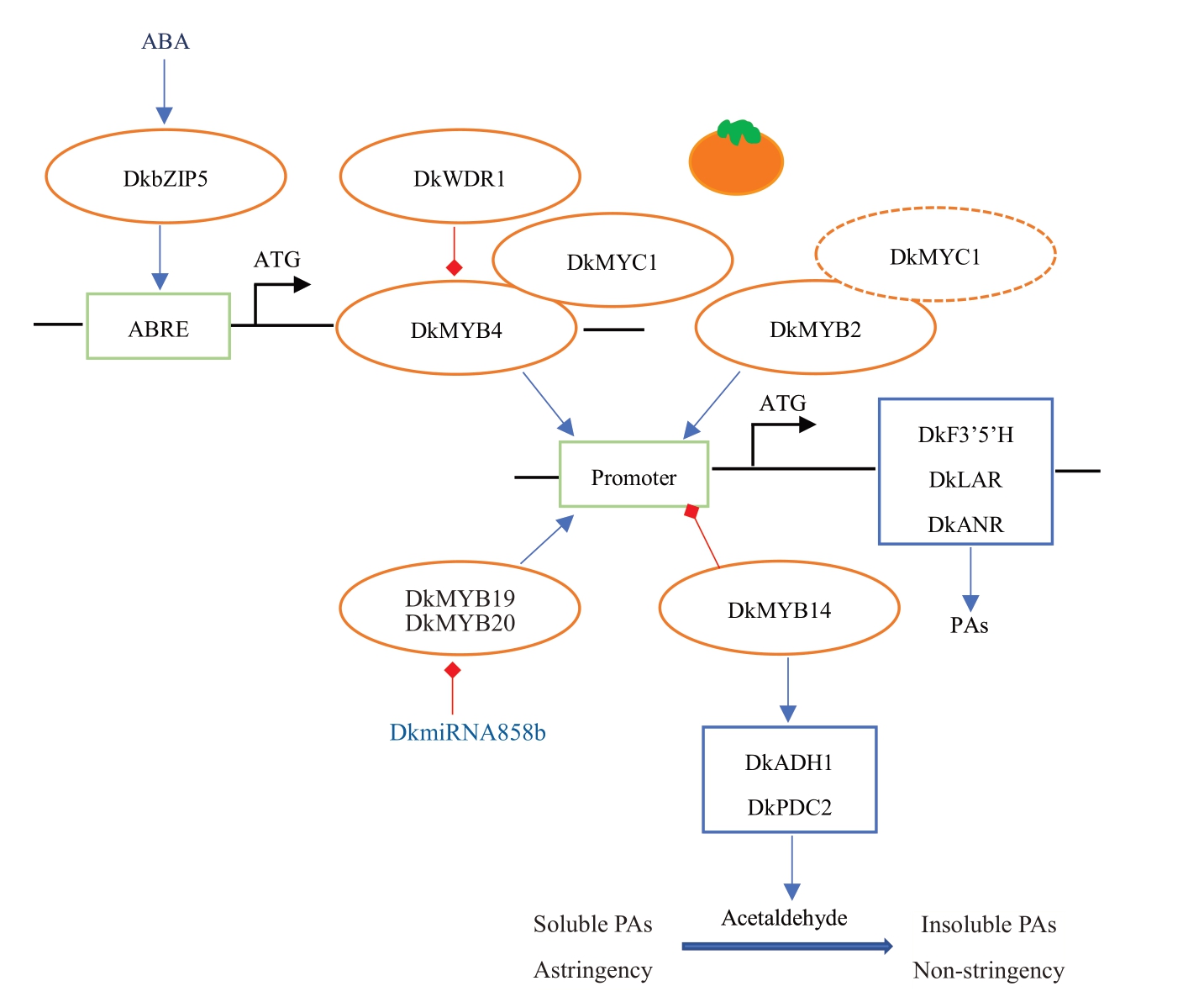
图2 柿果实涩味物质代谢调控模式[37-38,41]
Fig.2 Diagram of the metabolic regulation pattern of astringent substances in persimmon[37-38,41]
蓝色箭头代表正向调控,红色箭头代表负向调控,橘色椭圆代表转录因子,蓝色方框代表结构基因,绿色方框代表顺式作用元件。虚线表示DkMYB2 既可以与bHLH 转录因子形成复合体,也可以单独发挥调控作用。
The blue arrow represents positive regulation, the red arrow represents negative regulation, the orange oval represents the transcription factor, the blue box represents the structural gene,and the green box represents the cis-action element.The dotted line indicates that DkMYB2 can either form a complex with the bHLH transcription factor or play a regulatory role alone.
3.2 葡萄
单宁是葡萄酒中重要的苦味和涩味成分,赋予葡萄酒饱满度和骨架感,因此也被誉为葡萄酒的灵魂[44]。葡萄各组织部位都含有单宁,但不同组织在单宁含量、聚合度、结构方面存在差异,其中果皮单宁的主要组成单位是儿茶素、表儿茶素、表儿茶素没食子酸酯和表没食子儿茶素,聚合度为31~33[45];而种子中的单宁则富含表儿茶素,聚合度较低[24]。
在葡萄中,存在2 个与儿茶素合成高度相关的基因LAR1 和LAR2[46]。值得指出的是,MYB 因子VvMYBPA1 和VvMYBPA2 可以激活ANR 和LAR1的表达,但不能激活LAR2[47-48],而VvMYBPAR 则同时激活ANR、LAR1、LAR2、CHS、MATE 的表达,促进果实原花青素合成与运输[49]。VvMYB5a/5b 能够与bHLH 家族的转录因子AtEGL3 共同调控VvCHI 和VvLAR1 的启动子活性,在果实发育早期正向调控葡萄果皮、果肉和种子中原花青素的合成[50-51]。葡萄中鉴定到的bHLH 家族转录因子VvMYC1[52]、VvMYCA1[53]通过与MYB 转录因子相互作用,共同激活原花青素代谢通路上的结构基因。同时从葡萄中分离出的VvWDR1 常与MYB 和bHLH 形成转录复合体,调控花青素和原花青素合成[54]。另外,WRKY 家族的WKRY26 能够与VvMYB5a 互作,激活VvCHI 以及液泡酸化相关基因,故在原花青素合成和积累中起正向调控作用[55]。此外还鉴定到一系列负调控因子。VvMYBC2-L1/L3 在过表达的情况下会降低原花青素的含量[56]。miRNA TAS4 能够使VvMYBPA1、VvMYBPA2 的同源基因VvMYBA6 和VvMYBA7沉默,从而负调控花和果实中原花青素的合成[57](图3)。

图3 葡萄涩味物质代谢调控模式
Fig.3 Diagram of the metabolic regulation pattern of grape astringent substances in grape
蓝色箭头代表正向调控,红色箭头代表负向调控,橘色椭圆代表转录因子,蓝色方框代表结构基因,绿色方框代表顺式作用元件。
The blue arrow represents positive regulation, the red arrow represents negative regulation, the orange oval represents the transcription factor, the blue box represents the structural gene.
另外,环境因素也参与涩味物质的代谢过程。光能够诱导VvMYBF1 的表达,从而提高VvFLS1 的转录水平[58]。遮光处理不但会减少原花青素的生物合成,还会导致其结构中三羟基化亚基比例和平均聚合度降低[11],而果实涩味随单宁聚合度的增大而增强[59]。但是紫外线的强度并不影响原花青素的含量与结构组成[59]。水分亏缺会提高果皮中MYBPA1、MYBPA2 的表达丰度,增加原花青素的含量和聚合度[60]。此外用赤霉素(gibberellin,GA3)和塞苯隆(thidiazuron,TDZ)处理,也可使葡萄果皮和果肉中的可溶性单宁含量高于对照组[61]。
3.3 苹果
果实成熟时,绿原酸、儿茶素、表儿茶素和原花青素的含量在涩味显著苹果中分别是低涩味苹果的1.91 倍、2.91 倍、2.05 倍和1.99 倍[6]。故这些多酚物质很可能是苹果涩味的主要来源。通过mQTL连锁分析和GWAS全基因组关联分析,均在16号染色体上定位到与儿茶素、表儿茶素和原花青素相关的单一强关联信号,这说明三者物质之间可能存在共同的分子调控机制[62-64]。
苹果涩味物质的代谢在转录水平上受到调节。苹果中参与原花青素合成的MYB转录因子可以分成TT2型[65]和PA1型[66]。PA1型转录因子MdMYBPA1能通过调节类黄酮生物合成途径中不同结构基因的表达水平,分别促进原花青素和花青素的生物合成。在常温条件下,TT2型转录因子MdMYB9、Md-MYB11、MdMYB12 能够激活MdMYBPA1 启动子活性,后者通过与LAR 和ANR 启动子区的MBS 元件结合,促使果实中原花青素的合成;而在低温光照条件下,MdbHLH33 直接结合在MdMYBPA1启动子的低温响应(LTR)顺式元件上,两者共同增强UFGT和ANS 启动子活性,促进果实中花青素合成与积累[66]。NAC 家族转录因子MdNAC52 既可以与Md-MYB9 和MdMYB11 的启动子结合间接参与原花青素和花青素合成,又能与MdLAR、MdANR 启动子结合直接参与原花青素合成[67]。此外,MdHY5、Md-WRKY41、MdMYB12 三个转录因子能够形成调控模块,级联调控红肉苹果原花青素的生物合成,其中MdWRKY41 能够下调MdMYB12、MdLAR 和MdANR 的表达,抑制原花青素的积累;而光响应因子MdHY5 抑制MdWRKY41 转录[68]。另外,与在柿中的研究结果类似,miRNA858 能够抑制MdMYB9和MdMYBPA1的表达[69]。
苹果涩味物质的合成同时受到激素的调节作用。茉莉酸甲酯(MeJA)能够通过上调MdMYB9 和MdMYB11 的表达增加原花青素的积累[70]。油菜素类固醇BR 可诱导MdJa2 的产生,MdJa2 能与Md-BZR1形成复合物,通过抑制MdMYB9、MdMYB12启动子活性而抑制原花青素的合成[71](图4)。
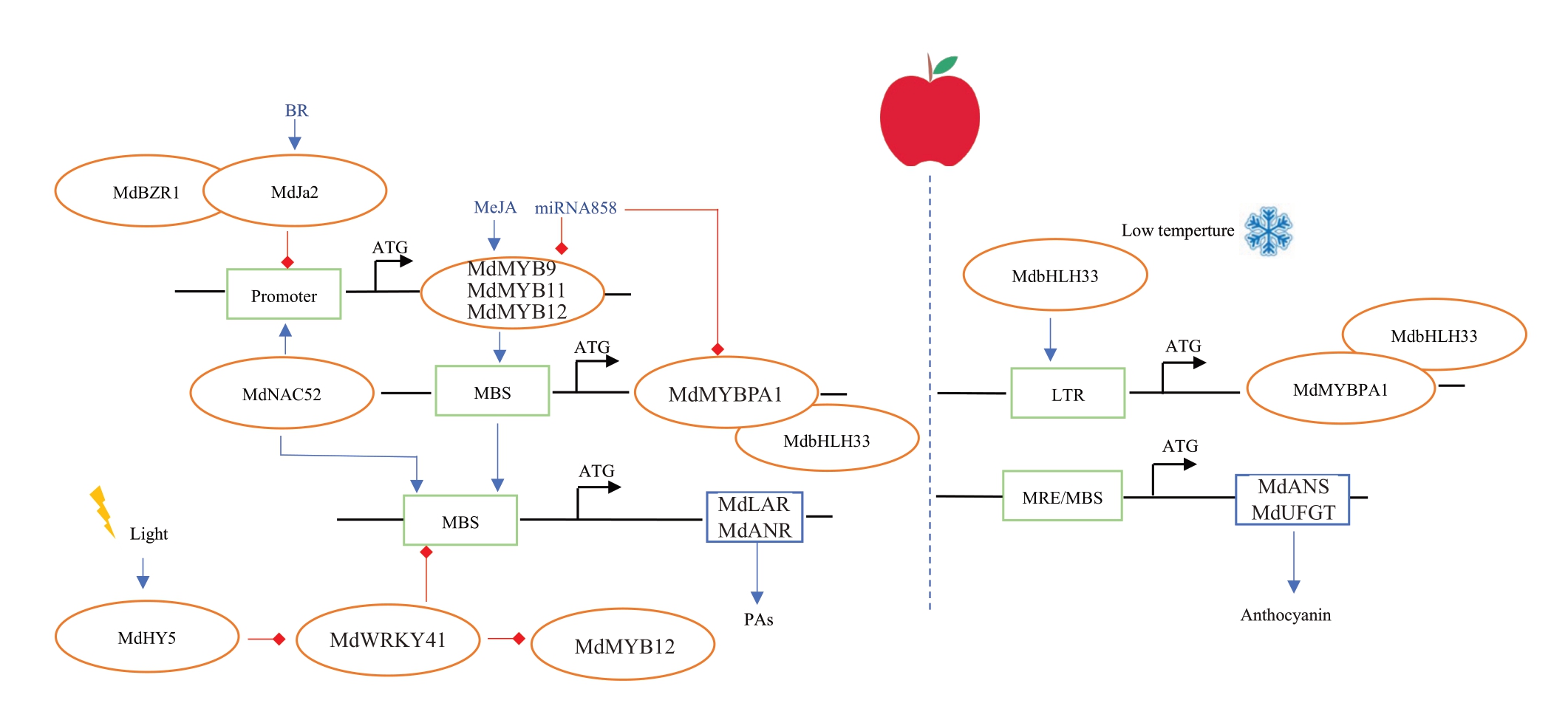
图4 苹果果实涩味物质代谢调控模式[66]
Fig.4 Diagram of the metabolic regulation pattern of astringent substances in apple[66]
蓝色箭头代表正向调控,红色箭头代表负向调控,橘色椭圆代表转录因子,蓝色方框代表结构基因,绿色方框代表顺式作用元件。
The blue arrow represents positive regulation, the red arrow represents negative regulation, the orange oval represents the transcription factor, the blue box represents the structural gene,and the green box represents the cis-action element.
3.4 桃
桃中涩味物质种类和含量在不同品种中存在显著差异。通过对187 份桃果实中的多酚含量和种类进行分析,发现儿茶素含量(w,后同)的变异范围为0.00~157.46 mg·kg-1,表儿茶素含量为0.00~5.80 mg·kg-1,原花青素B1 含量则为0.00~382.91 mg·kg-1,新绿原酸含量为0.79~74.51 mg·kg-1,绿原酸含量为5.27~107.93 mg·kg-1 [72]。水蜜桃中的涩味物质以表儿茶素、绿原酸为主;蟠桃中主要是表儿茶素和儿茶素,酚酸主要是绿原酸和新绿原酸;油桃品种中则分别是儿茶素和绿原酸[73]。另外桃中涩味物质的积累与果实颜色相关。其中白肉桃和黄肉桃中原花青素的积累量都随着果实成熟而降低,而DBF_基因型红肉桃则相反,其含量在果实发育过程中急剧升高,在成熟期达到最高[3]。同时研究也表明白肉桃和黄肉桃中LAR 的酶活力和基因表达量与原花青素的积累相关性较高,而ANR与红肉桃中原花青素的积累更为相关[3],这与白肉桃和黄肉桃中儿茶素含量高,而红肉桃中表儿茶素含量高的研究结果相一致[74]。
同时一系列转录因子参与涩味物质的代谢。在bHLH3 的激活作用下,PpMYBPA1 能够增强DFR、LAR 和ANR 的启动子活性,但对UFGT 启动子活性影响较低,因此在平衡原花青素和花色苷含量方面发挥重要作用[75]。PpMYB7也是调控原花青素合成的重要转录因子,与PpMYBPA1 相比,其可选择的bHLH 伴侣更为广泛,能同时被bHLH3 和bHLH33激活[76]。另外桃中的PpbZIP5也可以通过对ABA的响应激活PpMYBPA1、PpMYB7 的转录。同时PpMYB18通过与PpMYBPA1竞争bHLH结构,而成为原花青素生物合成的抑制因子[77]。此外,随着高通量测序技术的不断发展,与桃果实涩味相关的新基因也被不断挖掘。丁体玉[72]将儿茶素、表儿茶素、原花青素B1、新绿原酸和绿原酸定位到LG4 上的14 069 638和LG7上的462 241两个位点,区间内共有9 个候选基因。Cao 等[78]在桃基因组2 号、3 号、5号和7号染色体上鉴定到与儿茶素和表儿茶素含量相关的位点,还挖掘到候选基因Prupe.2G087000。目前,桃中与酚酸生物合成的相关基因鲜有报道。Zhou 等[79]表明桃叶片中绿原酸的含量有可能与HCT、C3’H这两个基因的表达量相关(图5)。
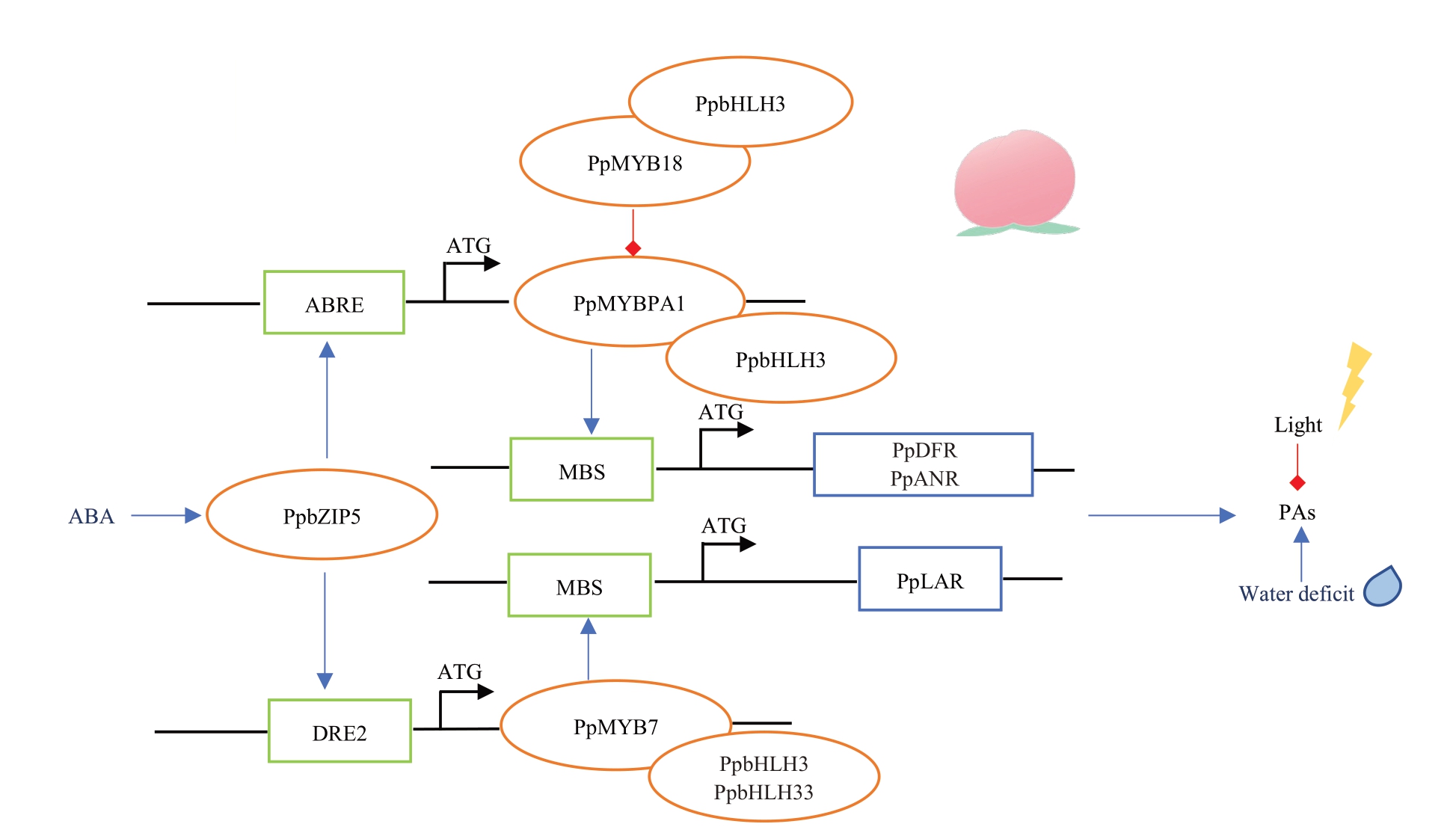
图5 桃果实涩味物质代谢调控模式
Fig.5 Diagram of the regulation pattern of astringent substance metabolism in peach
蓝色箭头代表正向调控,红色箭头代表负向调控,橘色椭圆代表转录因子,蓝色方框代表结构基因,绿色方框代表顺式作用元件。
The blue arrow represents positive regulation, the red arrow represents negative regulation, the orange oval represents the transcription factor, the blue box represents the structural gene,and the green box represents the cis-action element.
此外,桃果实涩味物质含量也受环境因素的影响。酚酸和黄烷醇对光较敏感,套袋显著抑制这两类物质的合成[80],在行间铺设反光膜则可以促进酚酸和单宁的积累[81]。水分亏缺可以明显提高油桃Caldesi 2000果皮中原花青素和酚酸的含量,而在Flordastar中则引起果皮原花青素和花青素含量增加[82]。
4 果实脱涩技术
涩味通常存在于未成熟的果实中,并随着果实成熟逐渐减弱,但是部分栽培品种和野生资源中涩味物质的含量在果实成熟之后依然较高。如DBF_基因型的红肉桃色泽艳丽,富含多酚物质,抗氧化能力强,深受消费者青睐,但是其果实中原花青素的含量在果实成熟时达到最高[3,72],对果实风味产生了不利影响。因而除了从分子调控方面探索涩味物质的代谢过程,还应当关注果实脱涩技术的研究,为提高果实品质和进行种质创新提供技术支撑。
果实脱涩的机制主要包括两个方面:一是在脱涩过程中产生的乙醛能与可溶性单宁发生酚醛缩合反应,通过将可溶性单宁转为不溶性单宁,降低果实涩味[43];二是在脱涩过程中,果肉中的果胶、原生质膜和细胞壁会与多糖发生凝胶反应,形成果胶和单宁复合体,使涩味消失[83]。目前在柿果实脱涩方面的研究较多,如冷水脱涩[84]、温水脱涩[85]、N2脱涩和CO2脱涩[86]等。但是冷水和温水脱涩易导致果实变软、褐化、风味变淡等,不适合大规模处理;而N2处理虽然能够使果实保持较好脆度,并不在果皮表面产生褐斑,但是该方法成本较高,只适合大规模处理[87]。目前CO2处理是广泛使用的柿果实脱涩方法,同时为防止果肉脱涩后褐变,常将1-甲基环丙烯(1-MCP)与CO2结合使用[88]。
5 展 望
近年来,随着我国果树产业的迅速发展和人民消费水平的提升,对果品市场的要求愈加严格。利用野生资源或者地方品种培育新型种质,是满足人民群众对绿色、优质、营养、多样化果品需求的有效途径,但在这一过程中易引入涩味性状。目前在果实涩味物质种类、生物合成途径、代谢调控、果实脱涩等方面取得了一系列成果,这为完善果实涩味形成机制奠定了基础,也为选育低涩味或无涩味的果树新品种提供了依据。但是果实涩味研究方面还存在不足,建议加强以下方面的研究:(1)建立精准果实涩味评价标准。《果树种质资源描述符》中把果实涩味分为无、微、中、多4 个等级[89],但在实际工作中,不同等级之间的界限还较为模糊。另外,涩味敏感度因人而异,主观判断也会使涩味评价结果出现偏差。因此,需要采用科学的感官评价方法[90],建立精准的果实涩味评价标准。(2)完善果实涩味的形成机制,探究不同果实中涩味物质的遗传规律。果实中的涩味物质种类较多,但是不同涩味物质导致的涩味强弱并不明确;儿茶素和表儿茶素聚合生成主要涩味物质—单宁的途径尚不清晰,且对绿原酸和新绿原酸的研究尚不充分。同时由于涩味物质的含量与种类在不同果树中存在差异,同源基因在不同物种中的调控途径存在特异性,所以充分利用多种试验手段,完善果实涩味形成机制,探究不同果实中涩味物质遗传规律,发掘关键基因,对满足不同的育种需求具有重要指导意义。(3)加强果实脱涩研究。目前脱涩技术的研究集中于柿果实上,但生产实践中发现其他果实中的部分种质也存在涩味明显的现象,却缺乏相应的研究。故有必要开展不同果树果实脱涩方法的探索,从而为提高果实品质和进行种质创新提供技术支撑。(4)寻求风味与抗氧化能力之间的平衡,进行种质创新。单宁、绿原酸和新绿原酸都具有强抗氧化性,在果树生长发育、抵抗生物胁迫[91]和非生物胁迫[92]以及促进人体健康方面发挥着积极的调节作用[93-94]。但是涩味物质含量过高又会影响果实风味,降低果实品质。因此有必要通过种质创新,选育具有强抗氧化能力的低涩味新品种。
[1] 刘凤之,王海波,胡成志.我国主要果树产业现状及“十四五”发展对策[J].中国果树,2021(1):1-5.LIU Fengzhi,WANG Haibo,HU Chengzhi. Current situation of main fruit tree industry in China and it’s development countermeasure during the“The 14th Five Year Plan”period[J]. China Fruits,2021(1):1-5.
[2] 赵智慧,周俊义.果树果实内在品质形成及评价方法研究进展[J].河北农业大学学报,2002,25(S1):111-114.ZHAO Zhihui,ZHOU Junyi. The review of development and evaluation of fruits internal quality[J]. Journal of Agricultural University of Hebei,2002,25(S1):111-114.
[3] 严娟,宋志忠,蔡志翔,沈志军,马瑞娟,俞明亮.3 种果肉颜色桃原花青素积累[J].江苏农业学报,2018,34(3):651-656.YAN Juan,SONG Zhizhong,CAI Zhixiang,SHEN Zhijun,MA Ruijuan,YU Mingliang. Proanthocyanidin accumulation in peach fruit with three types of flesh color[J]. Jiangsu Journal of Agricultural Sciences,2018,34(3):651-656.
[4] 徐子媛,严娟,蔡志翔,孙朦,宿子文,沈志军,马瑞娟,俞明亮.桃果实糖酸和酚类物质与口感风味的相关性[J].江苏农业学报,2022,38(1):190-199.XU Ziyuan,YAN Juan,CAI Zhixiang,SUN Meng,SU Ziwen,SHEN Zhijun,MA Ruijuan,YU Mingliang. Correlation between soluble sugar,organic acid and phenolic substances with tasted flavor in peach fruit[J]. Jiangsu Journal of Agricultural Sciences,2022,38(1):190-199.
[5] HUANG R,FANG W,XIE X Q,LIU Y T,XU C M. Identification of key astringent compounds in aronia berry juice[J]. Food Chemistry,2022,393:133431.
[6] 乜兰春,孙建设.苹果果实酚类物质含量与果实苦涩关系的研究[J].园艺学报,2005,32(5):778-782.NIE Lanchun,SUN Jianshe.Relationship between the content of phenolic compounds and the taste of astringency and bitterness in apple fruit[J].Acta Horticulturae Sinica,2005,32(5):778-782.
[7] 王坤范,陈秀芳.桃果实多酚氧化酶性质的研究[J].北京农业大学学报,1995,21(4):370-376.WANG Kunfan,CHEN Xiufang. The study of polyphenol oxidase property in peach[J].Acta Agriculturae Universitatis Pekinensis,1995,21(4):370-376.
[8] 赵文杰,薛冰,胡明华,敬思群.葡萄皮渣中单宁的提取纯化及含量测定[J].中国酿造,2010,29(8):152-156.ZHAO Wenjie,XUE Bing,HU Minghua,JING Siqun. Extraction and purification technology of tannins from grape residue and determination of tannins contents[J]. China Brewing,2010,29(8):152-156.
[9] SOARES S,BRANDÃO E,MATEUS N,DE FREITAS V. Sensorial properties of red wine polyphenols:Astringency and bitterness[J]. Critical Reviews in Food Science and Nutrition,2017,57(5):937-948.
[10] 狄莹,石碧. 植物单宁化学研究进展[J]. 化学通报,1999,62(3):2-6.DI Ying,SHI Bi.Advances in plant tannin chemistry[J].Chemistry,1999,62(3):2-6.
[11] 张宝善,陈锦屏,卢勇.水果的涩味研究[J].食品研究与开发,1998,19(1):31-34.ZHANG Baoshan,CHEN Jinping,LU Yong.Study of the astringency of the fruit[J].Food Research and Development,1998,19(1):31-34.
[12] BHAT T K,SINGH B,SHARMA O P.Microbial degradation of tannins:A current perspective[J]. Biodegradation,1998,9(5):343-357.
[13] 舒畅,赵韩栋,焦文晓,范新光,姜微波.植物单宁的生物活性研究进展[J].食品工业科技,2018,39(17):328-334.SHU Chang,ZHAO Handong,JIAO Wenxiao,FAN Xinguang,JIANG Weibo.Research progress on the bioactivity of plant origin tannins[J]. Science and Technology of Food Industry,2018,39(17):328-334.
[14] ELFALLEH W,TLILI N,NASRI N,YAHIA Y,HANNACHI H,CHAIRA N,YING M,FERCHICHI A. Antioxidant capacities of phenolic compounds and tocopherols from Tunisian pomegranate (Punica granatum) fruits[J]. Journal of Food Science,2011,76(5):707-713.
[15] 卜洪洋.原花青素提取纯化工艺及生产过程红外分析[D].北京:北京林业大学,2016.BU Hongyang. Extraction-purification process and IR analysis production process of procyanidins[D]. Beijing:Beijing Forestry University,2016.
[16] 罗晓文,刘敏,齐晓花,徐强,陈学好. 果实涩味分子研究进展[J].分子植物育种,2013,11(5):647-656.LUO Xiaowen,LIU Min,QI Xiaohua,XU Qiang,CHEN Xuehao. Molecular research progress in fruit astringent[J]. Molecular Plant Breeding,2013,11(5):647-656.
[17] 何强,姚开,石碧.植物单宁的营养学特性[J].林产化学与工业,2001,21(1):80-85.HE Qiang,YAO Kai,SHI Bi.Nutriological properties of vegetable tannins[J]. Chemistry & Industry of Forest Products,2001,21(1):80-85.
[18] KUMAR N,GOEL N. Phenolic acids:natural versatile molecules with promising therapeutic applications[J]. Biotechnology Reports,2019,24:e00370.
[19] BILLET K,MALINOWSKA M A,MUNSCH T,UNLUBAYIR M,DE BERNONVILLE T D,BESSEAU S,COURDAVAULT V,OUDIN A,PICHON O,CLASTRE M,GIGLIOLI-GUIVARC’H N,LANOUE A. Stilbenoid-enriched grape cane extracts for the biocontrol of grapevine diseases[M]//MÉRILLON J M,RAMAWAT K G. Plant defence:Biological control progress in biological control. Cham:Springer International Publishing,2020:215-239.
[20] 耿飒,徐存拴,李玉昌. 木质素的生物合成及其调控研究进展[J].西北植物学报,2003,23(1):171-181.GENG Sa,XU Cunshuan,LI Yuchang.Advance in biosynthesis of lignin and its regulation[J].Acta Botanica Boreali-Occidentalia Sinica,2003,23(1):171-181.
[21] 李铁柱,杜红岩,朱高浦.杜仲绿原酸生物合成途径相关基因的差异表达[J].经济林研究,2013,31(4):32-38.LI Tiezhu,DU Hongyan,ZHU Gaopu.Differential expression of related genes of chlorogenic acid biosynthetic in Eucommia ulmoides[J].Nonwood Forest Research,2013,31(4):32-38.
[22] 李洋,李明,岳玮,丁新华,储昭辉.烟草NtHCT 基因对次生代谢物质绿原酸和类黄酮合成的影响[J].中国烟草学报,2015,21(6):127-131.LI Yang,LI Ming,YUE Wei,DING Xinhua,CHU Zhaohui. Effect of NtHCT gene on synthesis of chlorogenic acid and flavonoid in tobacco[J].Acta Tabacaria Sinica,2015,21(6):127-131.
[23] 亓希武,徐道华,于盱,房海灵,李维林,梁呈元.金银花香豆酸-3-羟化酶基因LjC3H2 的克隆及表达分析[J].分子植物育种,2018,16(4):1092-1099.QI Xiwu,XU Daohua,YU Xu,FANG Hailing,LI Weilin,LIANG Chengyuan. Cloning and expression analysis of LjC3H2 from Lonicera japonica Thunb.[J]. Molecular Plant Breeding,2018,16(4):1092-1099.
[24] DIXON R A,LIU C G,JUN J H. Metabolic engineering of anthocyanins and condensed tannins in plants[J]. Current Opinion in Biotechnology,2013,24(2):329-335.
[25] FENG H J,LI Y J,WANG S F,ZHANG L L,LIU Y C,XUE F,SUN Y Q,WANG Y M,SUN J.Molecular analysis of proanthocyanidins related to pigmentation in brown cotton fibre(Gossypium hirsutum L.)[J]. Journal of Experimental Botany,2014,65(20):5759-5769.
[26] YANG S C,JIANG Y,XU L Q,SHIRATAKE K,LUO Z R,ZHANG Q L. Molecular cloning and functional characterization of DkMATE1 involved in proanthocyanidin precursor transport in persimmon(Diospyros kaki Thunb.)fruit[J].Plant Physiology and Biochemistry,2016,108:241-250.
[27] CHEN S Y,TANG Y M,HU Y Y,WANG Y,SUN B,WANG X R,TANG H R,CHEN Q.FaTT12-1,a multidrug and toxin extrusion (MATE) member involved in proanthocyanidin transport in strawberry fruits[J].Scientia Horticulturae,2018,231:158-165.
[28] PÉREZ-DÍAZ R,MADRID-ESPINOZA J,SALINAS-CORNEJO J,GONZÁLEZ-VILLANUEVA E,RUIZ-LARA S.Differential roles for VviGST1,VviGST3,and VviGST4 in proanthocyanidin and anthocyanin transport in Vitis vinifera[J]. Frontiers in Plant Science,2016,7:1166.
[29] ZHAO J,PANG Y Z,DIXON R A.The mysteries of proanthocyanidin transport and polymerization[J]. Plant Physiology,2010,153(2):437-443.
[30] HU Q N,LUO C,ZHANG Q L,LUO Z R.Isolation and characterization of a Laccase gene potentially involved in proanthocyanidin polymerization in oriental persimmon (Diospyros kaki Thunb.) fruit[J]. Molecular Biology Reports,2013,40(4):2809-2820.
[31] HOU G Y. FaTT10,a laccasel-like polyphenol oxidase involved in the accumulation of proanthocyanins monomerin strawberry fruit[J]. International Journal of Agriculture and Biology,2021,25(6):1222-1230.
[32] 王仁梓.关于罗田甜柿原产地问题的探讨[J].中国果树,1983(2):16-19.WANG Renzi.Discussion on the origin of Luotian sweet persimmon[J].China Fruits,1983(2):16-19.
[33] 费学谦,王劲风,周立红,龚榜初,吴开云.甘、涩柿果实主要化学成份的研究[J].林业科学研究,1994,7(1):106-110.FEI Xueqian,WANG Jinfeng,ZHOU Lihong,GONG Bangchu,WU Kaiyun. A study on chemical compositions of astringent and non-astringent type fruits of persimmons[J]. Forest Research,1994,7(1):106-110.
[34] YONEMORI K,MATSUSHIMA J,SUGIURA A. Differences in tannins of non-astringent and astringent type fruits of Japanese persimmon(Diospyros kaki Thunb.)[J].Journal of the Japanese Society for Horticultural Science,1983,52(2):135-144.
[35] AKAGI T,IKEGAMI A,SUZUKI Y,YOSHIDA J,YAMADA M,SATO A,YONEMORI K. Expression balances of structural genes in shikimate and flavonoid biosynthesis cause a difference in proanthocyanidin accumulation in persimmon (Diospyros kaki Thunb.)fruit[J].Planta,2009,230(5):899-915.
[36] NAKAGAWA T,NAKATSUKA A,YANO K,YASUGAHIRA S,NAKAMURA R,SUN N,ITAI A,SUZUKI T,ITAMURA H. Expressed sequence tags from persimmon at different developmental stages[J].Plant Cell Reports,2008,27(5):931-938.
[37] GIL-MUÑOZ F,SÁNCHEZ-NAVARRO J A,BESADA C,SALVADOR A,BADENES M L,DEL MAR NAVAL M,RÍOS G.MBW complexes impinge on anthocyanidin reductase gene regulation for proanthocyanidin biosynthesis in persimmon fruit[J].Scientific Reports,2020,10:3543.
[38] AKAGI T,IKEGAMI A,YONEMORI K. DkMyb2 wound-induced transcription factor of persimmon (Diospyros kaki Thunb.),contributes to proanthocyanidin regulation[J]. Planta,2010,232(5):1045-1059.
[39] AKAGI T,IKEGAMI A,TSUJIMOTO T,KOBAYASHI S,SATO A,KONO A,YONEMORI K.DkMyb4 is a MYB transcription factor involved in proanthocyanidin biosynthesis in persimmon fruit[J].Plant Physiology,2009,151(4):2028-2045.
[40] AKAGI T,KATAYAMA-IKEGAMI A,KOBAYASHI S,SATO A,KONO A,YONEMORI K. Seasonal abscisic acid signal and a basic leucine zipper transcription factor,DkbZIP5,regulate proanthocyanidin biosynthesis in persimmon fruit[J].Plant Physiology,2012,158(2):1089-1102.
[41] CHEN W X,ZHENG Q Y,LI J W,LIU Y,XU L Q,ZHANG Q L,LUO Z R. DkMYB14 is a bifunctional transcription factor that regulates the accumulation of proanthocyanidin in persimmon fruit[J].The Plant Journal,2021,106(6):1708-1727.
[42] YANG S C,ZHANG M,XU L Q,LUO Z R,ZHANG Q L.MiR858b inhibits proanthocyanidin accumulation by the repression of DkMYB19 and DkMYB20 in persimmon[J]. Frontiers in Plant Science,2020,11:576378.
[43] TAIRA S,SATOH I,WATANABE S. Relationship between differences in the ease of removal of astringency among fruits of Japanese persimmon(Diospyros kaki Thunb.)and their ability to accumulate ethanol and acetaldehyde[J]. Journal of the Japanese Society for Horticultural Science,1992,60(4):1003-1009.
[44] 朱虹,张平,李新榜.葡萄酒的灵魂之一:不可或缺的单宁[J].中外葡萄与葡萄酒,2008(5):47-51.ZHU Hong,ZHANG Ping,LI Xinbang.One of the soul of wine:The indispensable tannins[J].Sino-Overseas Grapevine&Wine,2008(5):47-51.
[45] SOUQUET J M,CHEYNIER V,BROSSAUD F,MOUTOUNET M. Polymeric proanthocyanidins from grape skins[J]. Phytochemistry,1996,43(2):509-512.
[46] WEI X F,JU Y L,MA T T,ZHANG J X,FANG Y L,SUN X Y.New perspectives on the biosynthesis,transportation,astringency perception and detection methods of grape proanthocyanidins[J].Critical Reviews in Food Science and Nutrition,2021,61(14):2372-2398.
[47] BOGS J,JAFFÉ F W,TAKOS A M,WALKER A R,ROBINSON S P. The grapevine transcription factor VvMYBPA1 regulates proanthocyanidin synthesis during fruit development[J].Plant Physiology,2007,143(3):1347-1361.
[48] TERRIER N,TORREGROSA L,AGEORGES A,VIALET S,VERRIÈS C,CHEYNIER V,ROMIEU C. Ectopic expression of VvMybPA2 promotes proanthocyanidin biosynthesis in grapevine and suggests additional targets in the pathway[J]. Plant Physiology,2009,149(2):1028-1041.
[49] KOYAMA K,NUMATA M,NAKAJIMA I,GOTO-YAMAMOTO N,MATSUMURA H,TANAKA N. Functional characterization of a new grapevine MYB transcription factor and regulation of proanthocyanidin biosynthesis in grapes[J].Journal of Experimental Botany,2014,65(15):4433-4449.
[50] DELUC L,BARRIEU F,MARCHIVE C,LAUVERGEAT V,DECENDIT A,RICHARD T,CARDE J P,MÉRILLON J M,HAMDI S. Characterization of a grapevine R2R3-MYB transcription factor that regulates the phenylpropanoid pathway[J].Plant Physiology,2006,140(2):499-511.
[51] DELUC L,BOGS J,WALKER A R,FERRIER T,DECENDIT A,MERILLON J M,ROBINSON S P,BARRIEU F. The transcription factor VvMYB5b contributes to the regulation of anthocyanin and proanthocyanidin biosynthesis in developing grape berries[J].Plant Physiology,2008,147(4):2041-2053.
[52] HICHRI I,HEPPEL S C,PILLET J,LÉON C,CZEMMEL S,DELROT S,LAUVERGEAT V,BOGS J.The basic helix-loophelix transcription factor MYC1 is involved in the regulation of the flavonoid biosynthesis pathway in grapevine[J]. Molecular Plant,2010,3(3):509-523.
[53] MATUS J T,POUPIN M J,CAÑÓN P,BORDEU E,ALCALDE J A,ARCE-JOHNSON P. Isolation of WDR and bHLH genes related to flavonoid synthesis in grapevine (Vitis vinifera L.)[J].Plant Molecular Biology,2010,72(6):607-620.
[54] GIL-MUÑOZ F,SÁNCHEZ-NAVARRO J A,BESADA C,SALVADOR A,BADENES M L,DEL MAR NAVAL M,RÍOS G.MBW complexes impinge on anthocyanidin reductase gene regulation for proanthocyanidin biosynthesis in persimmon fruit[J].Scientific Reports,2020,10:3543.
[55] AMATO A,CAVALLINI E,ZENONI S,FINEZZO L,BEGHELDO M,RUPERTI B,TORNIELLI G B. A grapevine TTG2-like WRKY transcription factor is involved in regulating vacuolar transport and flavonoid biosynthesis[J]. Frontiers in Plant Science,2017,7:1979.
[56] CAVALLINI E,MATUS J T,FINEZZO L,ZENONI S,LOYOLA R,GUZZO F,SCHLECHTER R,AGEORGES A,ARCE-JOHNSON P,TORNIELLI G B. The phenylpropanoid pathway is controlled at different branches by a set of R2R3-MYB C2 repressors in grapevine[J].Plant Physiology,2015,167(4):1448-1470.
[57] ROCK C D.Trans-acting small interfering RNA4:key to nutraceutical synthesis in grape development?[J].Trends in Plant Science,2013,18(11):601-610.
[58] CZEMMEL S,STRACKE R,WEISSHAAR B,CORDON N,HARRIS N N,WALKER A R,ROBINSON S P,BOGS J. The grapevine R2R3-MYB transcription factor VvMYBF1 regulates flavonol synthesis in developing grape berries[J].Plant Physiology,2009,151(3):1513-1530.
[59] KOYAMA K,IKEDA H,POUDEL P R,GOTO-YAMAMOTO N.Light quality affects flavonoid biosynthesis in young berries of Cabernet Sauvignon grape[J].Phytochemistry,2012,78:54-64.
[60] CÁCERES-MELLA A,TALAVERANO M I,VILLALOBOSGONZÁLEZ L,RIBALTA-PIZARRO C,PASTENES C. Controlled water deficit during ripening affects proanthocyanidin synthesis,concentration and composition in Cabernet Sauvignon grape skins[J]. Plant Physiology and Biochemistry,2017,117:34-41.
[61] 程大伟,陈锦永,顾红,黄海娜,靳路真,张威远,张洋,郭西智.GA3 与TDZ 组合对巨玫瑰葡萄果实理化指标和苦涩味物质含量的影响[J].南方农业学报,2018,49(5):922-929.CHENG Dawei,CHEN Jinyong,GU Hong,HUANG Haina,JIN Luzhen,ZHANG Weiyuan,ZHANG Yang,GUO Xizhi. Effects of GA3 and TDZ combination on bitter and astringent taste compounds of Vitis vinifera×V.labrusca[J].Journal of Southern Agriculture,2018,49(5):922-929.
[62] ALI KHAN S,CHIBON P Y,DE VOS R C H,SCHIPPER B A,WALRAVEN E,BEEKWILDER J,VAN DIJK T,FINKERS R,VISSER R G F,VAN DE WEG E W,BOVY A,CESTARO A,VELASCO R,JACOBSEN E,SCHOUTEN H J. Genetic analysis of metabolites in apple fruits indicates an mQTL hotspot for phenolic compounds on linkage group 16[J]. Journal of Experimental Botany,2012,63(8):2895-2908.
[63] CHAGNÉ D,KRIEGER C,RASSAM M,SULLIVAN M,FRASER J,ANDRÉ C,PINDO M,TROGGIO M,GARDINER S E,HENRY R A,ALLAN A C,MCGHIE T K,LAING W A. QTL and candidate gene mapping for polyphenolic composition in apple fruit[J].BMC Plant Biology,2012,12:12.
[64] MCCLURE K A,GONG Y,SONG J,VINQVIST-TYMCHUK M,PALMER L C,FAN L H,BURGHER-MACLELLAN K,ZHANG Z Q,CELTON J M,FORNEY C F,MIGICOVSKY Z,MYLES S. Genome-wide association studies in apple reveal loci of large effect controlling apple polyphenols[J]. Horticulture Research,2019,6:107.
[65] GESELL A,YOSHIDA K,TRAN L T,CONSTABEL C P.Characterization of an apple TT2-type R2R3 MYB transcription factor functionally similar to the poplar proanthocyanidin regulator PtMYB134[J].Planta,2014,240(3):497-511.
[66] WANG N,QU C Z,JIANG S H,CHEN Z J,XU H F,FANG H C,SU M Y,ZHANG J,WANG Y C,LIU W J,ZHANG Z Y,LU N L,CHEN X S. The proanthocyanidin-specific transcription factor MdMYBPA1 initiates anthocyanin synthesis under low-temperature conditions in red-fleshed apples[J]. The Plant Journal,2018,96(1):39-55.
[67] 孙庆国.MdNAC52 调控苹果花青苷和原花青素生物合成的机理[D].泰安:山东农业大学,2020.SUN Qingguo. Mechanism of NAC transcription factor Md-NAC52 regulating biosynthesis of apple anthocyanins and proanthocyanidins[D]. Tai’an:Shandong Agricultural University,2020.
[68] MAO Z L,JIANG H Y,WANG S,WANG Y C,YU L,ZOU Q,LIU W J,JIANG S H,WANG N,ZHANG Z Y,CHEN X S.The MdHY5- MdWRKY41- MdMYB transcription factor cascade regulates the anthocyanin and proanthocyanidin biosynthesis in red-fleshed apple[J].Plant Science,2021,306:110848.
[69] 李志强.Mdm-miR858 靶向MdMYB9 和MdMYBPA1 负调控红肉苹果中花青苷的生物合成[D].泰安:山东农业大学,2022.LI Zhiqiang.Mdm-miR858 targets MdMYB9 and MdMYBPA1 to negatively regulate anthocyanin biosynthesis in red-fleshed apples[D].Tai’an:Shandong Agricultural University,2022.
[70] AN X H,TIAN Y,CHEN K Q,LIU X J,LIU D D,XIE X B,CHENG C G,CONG P H,HAO Y J. MdMYB9 and MdMYB11 are involved in the regulation of the JA-induced biosynthesis of anthocyanin and proanthocyanidin in apples[J]. Plant and Cell Physiology,2015,56(4):650-662.
[71] SU M Y,WANG S,LIU W J,YANG M,ZHANG Z Y,WANG N,CHEN X S. MdJa2 participates in the brassinosteroid signaling pathway to regulate the synthesis of anthocyanin and proanthocyanidin in red-fleshed apple[J]. Frontiers in Plant Science,2022,13:830349.
[72] 丁体玉. 桃种质资源多酚类物质评价及其QTL 定位[D]. 北京:中国农业科学院,2017.DING Tiyu. The evaluation of polyphenol in peach and its QTL mapping[D]. Beijing:Chinese Academy of Agricultural Sciences,2017.
[73] 卢娟芳,刘盛雨,芦旺,席万鹏.不同类型桃果肉酚类物质及抗氧化活性分析[J].中国农业科学,2017,50(16):3205-3214.LU Juanfang,LIU Shengyu,LU Wang,XI Wanpeng. Phenolic profiles and antioxidant activity of fruit pulp from different types of peaches[J]. Scientia Agricultura Sinica,2017,50(16):3205-3214.
[74] 严娟,蔡志翔,沈志军,张斌斌,钱巍,俞明亮.桃3 种颜色果肉中10 种酚类物质的测定及比较[J].园艺学报,2014,41(2):319-328.YAN Juan,CAI Zhixiang,SHEN Zhijun,ZHANG Binbin,QIAN Wei,YU Mingliang. Determination and comparison of 10 phenolic compounds in peach with three types of flesh color[J].Acta Horticulturae Sinica,2014,41(2):319-328.
[75] RAVAGLIA D,ESPLEY R V,HENRY-KIRK R A,ANDREOTTI C,ZIOSI V,HELLENS R P,COSTA G,ALLAN A C.Transcriptional regulation of flavonoid biosynthesis in nectarine(Prunus persica)by a set of R2R3 MYB transcription factors[J].BMC Plant Biology,2013,13:68.
[76] ZHOU H,KUI L W,LIAO L,GU C,LU Z Q,ALLAN A C,HAN Y P. Peach MYB7 activates transcription of the proanthocyanidin pathway gene encoding leucoanthocyanidin reductase,but not anthocyanidin reductase[J]. Frontiers in Plant Science,2015,6:908.
[77] ZHOU H,KUI L W,WANG F R,ESPLEY R V,REN F,ZHAO J B,OGUTU C,HE H P,JIANG Q,ALLAN A C,HAN Y P.Activator-type R2R3-MYB genes induce a repressor-type R2R3-MYB gene to balance anthocyanin and proanthocyanidin accumulation[J].The New Phytologist,2019,221(4):1919-1934.
[78] CAO K,LI Y,DENG C H,GARDINER S E,ZHU G R,FANG W C,CHEN C W,WANG X W,WANG L R.Comparative population genomics identified genomic regions and candidate genes associated with fruit domestication traits in peach[J].Plant Biotechnology Journal,2019,17(10):1954-1970.
[79] ZHOU W,JIA M Y,ZHANG G C,SUN J,LI Q L,WANG X L,HUA J,LUO S H.Up-regulation of phenylpropanoid biosynthesis system in peach species by peach aphids produces anthocyanins that protect the aphids against UVB and UVC radiation[J].Tree Physiology,2021,41(3):428-443.
[80] 周君,陈宗玲,张琼,王红清.套袋对桃果实成熟过程中酚酸类和类黄酮类物质积累的影响[J]. 园艺学报,2009,36(12):1717-1724.ZHOU Jun,CHEN Zongling,ZHANG Qiong,WANG Hongqing.Effects of bagging on accumulation of phenolic acids and flavonoids in peach pericarp during fruit maturity[J]. Acta Horticulturae Sinica,2009,36(12):1717-1724.
[81] PLIAKONI E D,NANOS G D,GIL M I. Two-season study of the influence of regulated deficit irrigation and reflective mulch on individual and total phenolic compounds of nectarines at harvest and during storage[J]. Journal of Agricultural and Food Chemistry,2010,58(22):11783-11789.
[82] NAJLA S,VERCAMBRE G,GÉNARD M.Effects of water deficit and variations of fruit microclimate on peach fruit growth and quality[J].Plant Stress,2011,49(5):33-38.
[83] TAIRA S,ONO M,MATSUMOTO N. Reduction of persimmon astringency by complex formation between pectin and tannins[J].Postharvest Biology and Technology,1997,12(3):265-271.
[84] 熊宇婷,曾明,王璠,李国权,王彦波,吴美华,杜晓云.不同脱涩方法对‘赣方1 号’柿低温贮藏及果实品质的影响[J].中国南方果树,2014,43(6):90-93.XIONG Yuting,ZENG Ming,WANG Fan,LI Guoquan,WANG Yanbo,WU Meihua,DU Xiaoyun. Effects of different deastringation methods on low-temperature storage and fruit quality of‘Ganfang No. 1’persimmon[J]. South China Fruits,2014,43(6):90-93.
[85] 林菲. 柿子保鲜及脱涩技术研究[D]. 福州:福建农林大学,2013.LIN Fei. Studies on fresh-keeping and postharvest de-astringency handles of persimmon fruits[D]. Fuzhou:Fujian Agriculture and Forestry University,2013.
[86] ARNAL L,RIO M A. Removing astringency by carbon dioxide and nitrogen-enriched atmospheres in persimmon fruit cv. Rojo brillante[J].Journal of Food Science,2003,68(4):1516-1518.
[87] 张鹏,韩双双,李春媛,李江阔,薛友林.涩柿脱涩技术研究进展[J].包装工程,2019,40(11):9-16.ZHANG Peng,HAN Shuangshuang,LI Chunyuan,LI Jiangkuo,XUE Youlin. Research progress on de-astringent technology of persimmon[J].Packaging Engineering,2019,40(11):9-16.
[88] 程青,梁平卓,李莹,李宝.1-甲基环丙烯和CO2组合处理抑制柿果实脱涩软化的效应及其细胞壁成分的变化[J].中国农业大学学报,2015,20(4):92-99.CHENG Qing,LIANG Pingzhuo,LI Ying,LI Bao. Effects of 1-MCP on fruit softening and cell wall component variation of persimmon variety treated with CO2[J].Journal of China Agricultural University,2015,20(4):92-99.
[89] 蒲富慎.果树种质资源描述符:记载项目及评价标准[M].北京:农业出版社,1990.PU Fushen. Descriptors of fruit tree germplasm resources:Record items and evaluation criteria[M]. Bingjing:China Agriculture Press,1990.
[90] 由美千惠,秦智伟,辛明,周秀艳.黄瓜种质资源食味性感官品质评价[J].中国瓜菜,2021,34(12):101-106.YOU Meiqianhui,QIN Zhiwei,XIN Ming,ZHOU Xiuyan.Sensory evaluation of taste of cucumber germplasm resources[J].China Cucurbits and Vegetables,2021,34(12):101-106.
[91] MARTÍNEZ G,REGENTE M,JACOBI S,DEL RIO M,PINEDO M,DE LA CANAL L. Chlorogenic acid is a fungicide active against phytopathogenic fungi[J]. Pesticide Biochemistry and Physiology,2017,140:30-35.
[92] GOURLAY G,HAWKINS B J,ALBERT A,SCHNITZLER J P,PETER CONSTABEL C. Condensed tannins as antioxidants that protect poplar against oxidative stress from drought and UV-B[J].Plant,Cell&Environment,2022,45(2):362-377.
[93] HEO H J,LEE C Y. Epicatechin and catechin in cocoa inhibit amyloid beta protein induced apoptosis[J].Journal of Agricultural and Food Chemistry,2005,53(5):1445-1448.
[94] VIZZOTTO M,PORTER W,BYRNE D,CISNEROS-ZEVALLOS L. Polyphenols of selected peach and plum genotypes reduce cell viability and inhibit proliferation of breast cancer cells while not affecting normal cells[J]. Food Chemistry,2014,164:363-370.