阳光玫瑰(Shine Muscat)葡萄属于二倍体欧美杂种(Vitis vinifera×V.labrusca),果实具有外观美、香味浓、糖度高等优点,种植面积发展迅速。近年来,中国北方地区阳光玫瑰生理性黄化现象普遍发生,其症状主要表现为上部叶肉细胞失绿,而叶脉正常,严重时整株叶片黄化,甚至造成树体死亡[1]。目前叶片黄化已成为限制北方地区阳光玫瑰葡萄优质生产的重要因素之一。
叶片黄化的原因有多种,环境因素、土壤条件、栽培管理、嫁接砧木、树体营养以及遗传背景等因素都可能导致叶片发生黄化。为了解决葡萄叶片黄化问题,前人对该现象发生机制做过一些探索,目前关注更多的是土壤环境因素引起有效铁含量降低以及冻害等因素造成根系发育不良等因素引起的叶片黄化[2-3]。露地百合叶片黄化是植株缺乏氮、镁、铁、锌元素所致[4],而核桃叶片黄化主要原因是缺乏氮、铁元素,且发生叶片黄化的植株产量下降50%~70%[5]。
葡萄叶片黄化现象多发生在中国北方地区,发生时间集中在花序生长期和开花期,中、轻度黄化植株在5月下旬到6月初开始逐渐复绿。随着阳光玫瑰种植面积不断扩大,叶片黄化现象对产业造成的影响日益凸显。目前,有关葡萄叶片黄化的研究多停留在土壤、叶片营养元素分析,并以此阐明黄化原因,对于更深层次的生理生化复杂过程的研究甚少。此外对黄化现象的矫正措施仍以施用硫酸亚铁为主,但由于碱性土壤中Fe2+很快会被氧化固定,效果并不理想。本研究在土壤和叶片营养元素分析的基础上,对阳光玫瑰黄化植株生理特征进行更深一步研究分析,并设计出3种矫正方案,对比其矫正效果,旨在为探究叶片黄化的原因提供理论依据。
1 材料和方法
1.1 试验材料
为了研究阳光玫瑰葡萄叶片黄化发生的规律,对郑州周边24 个种植阳光玫瑰的葡萄园区进行调查,这些园区包括黄化园和正常园,试验材料为各园中不同黄化程度植株和正常植株;大田试验地点位于河南省荥阳市蔡寨村葡萄园,地处北纬34°36′、东经113°07′,属温暖带大陆性季风气候,土壤平均pH值8.04,试验材料为该园中叶片严重黄化以及正常的4 年生自根树,树形为单干双臂形,架高185 cm,株距1 m,行距2.5 m,南北行向,露地栽培,园内所有阳光玫瑰植株统一进行无核处理和田间栽培管理,该园区阳光玫瑰2022 年5 月初出现不同程度叶片黄化现象。盆栽试验以1年生阳光玫瑰自根苗为试验材料,试验于中国农业科学院郑州果树研究所智能温室进行。
1.2 试验设计与方法
1.2.1 试验设计 (1)调查阳光玫瑰叶片黄化情况。对不同黄化程度的植株进行分级。黄化叶片占全株25%左右为轻度黄化;黄化叶片占全株50%左右为中度黄化;黄化叶片占75%以上为重度黄化。
在各园区采集不同黄化程度植株以及正常植株根际土壤各500 g,一共采集到48份土壤样品,送实验室烘干后测定pH值、EC值、有机质含量以及全铁和有效铁含量。
(2)黄化矫正试验设计。对不同黄化程度植株和叶片拍照,采集不同黄化程度植株根际土壤样本各500 g,根系样本10 g,采集不同黄化程度叶片样本各15个。
对黄化植株设3 组处理(T1、T2、T3),如表1 所示,设置黄化植株对照(黄CK,清水处理)、正常植株对照(绿CK,清水处理)2个对照组,每组5个重复。
表1 矫正处理方案
Table 1 Corrective treatment plan

?
叶面喷施用喷壶均匀地喷施全株叶片正反两面,灌根在树冠投影边缘近树干三分之一处挖30 cm左右深、直径约35 cm的坑,将药剂充分溶解后灌根后覆土。观察到黄化叶片复绿后对各处理组进行拍照,采集各处理组及对照组根际土壤样本各500 g,叶片样本各随机取15 个。本试验叶片样本试验和采集部位均选取从基部向上第8枚成龄叶。
处理后用SPAD 叶绿素仪测其叶绿素相对含量,每隔3 d 测1 次,每组叶片3 次重复,取平均数。处理15 d 后采集土壤和叶片送实验室处理,对土壤样本测定pH 值及速效氮、速效磷、速效钾、全铁、有效铁、有效锌、有效铜、硝态氮、铵态氮的含量,叶片样本测定全氮、全磷、全钾、全铁、全铜、全锌、有效铁的含量以及高铁还原酶(FCR)活性,根系测定全铁含量和根系活力,元素测定每组3次重复,根系活力和酶活性每组5次重复。
(3)盆栽试验。2022 年3 月下旬将生长势基本一致的1 年生自根苗种植于装有培养基质(V 草炭土∶V 蛭石∶V 珍珠岩=3∶1∶1)的塑料花盆中,花盆上口直径、下口直径和高度分别为26、22、18 cm,每盆1 株,常规管理。小苗长至12片叶完全展开时开始摘心,并抹去多余副梢。7 月14 日开始用NaHCO3 溶液和NaOH溶液处理。
分别用90 mmol·L-1 NaHCO3溶液和pH值为9.0的NaOH 溶液定量灌溉处理,对照组用等量清水灌溉。每次灌溉溶液(清水)每盆2 L,每隔3 d灌溉1 次,每个处理5次重复。观察叶片生长状态,处理50 d 后,取根部向上第9~10 枚叶片和根系,测定有效铁和全铁含量,每个指标测3 次重复。测定叶片FCR活性,每组5次重复。
1.2.2 测定方法 土壤pH 值参照NY/T 1121.2—2006测定;土壤EC值用电导法测定;土壤有机质含量参照NY/T 1121.6—2006 测定;土壤碱解氮含量采用扩散法测定[6];土壤速效磷含量采用碳酸氢钠浸提钼锑抗比色法测定[6];土壤速效钾含量采用乙酸铵浸提火焰光度计法测定[6];土壤有效铁、锌、铜含量参照国家环境标准HJ 804—2016 二乙烯三胺五乙酸浸提-电感耦合等离子体发射光谱法测定;土壤全铁含量采用硝酸消解、ICP-ms测定[7];土壤硝态氮含量采用氯化钾浸提双波长比色法测定[8];土壤铵态氮含量采用靛酚蓝比色法测定[6];叶片全氮含量用元素分析仪法测定[9];叶片全磷和全钾含量参照NY/T 2017—2011 测定;叶片有效铁含量采用比色法测定;叶片全铁、全锌、全铜以及根系全铁含量参照GB 5009.268—2016测定。
1.3 数据处理
用Excel 2016数据分析软件对数据进行统计和作图,用SPASSPRO(众言科技)在线软件进行数据分析。
2 结果与分析
2.1 阳光玫瑰黄化情况的调查
不同黄化程度植株见图1,调查结果发现叶片黄化发生时间集中在花序生长期和开花期,发生初期都是幼叶的叶脉间和新梢先开始黄化,逐渐发展至下部叶片。黄化植株较正常植株新生根很少,轻度黄化植株在5月下旬到6月初开始逐渐复绿,重度黄化植株叶片干枯或植株死亡。

图1 不同黄化程度的叶片
Fig.1 Leaves with different degrees of chlorosis
A.轻度黄化;B.中度黄化;C.重度黄化。
A.Light chlorosis;B.Moderate chlorosis;C.Severe chlorosis.
通过调查不同园区土壤样品的pH值、EC值、有机质含量以及全铁和有效铁含量,对叶片黄化现象发生的原因进行初步分析,结果(表2)表明48 份土壤样品pH 值均大于7.0,pH 值大于7.5 的样品占66%,pH值大于8的样品占25.41%。即使重度黄化的园区土壤pH 值也可能8.0 以上。EC 值除个别园区大于1000 μS·cm-1以外,93.75%的园区都处于正常范围(150~900 μS·cm-1)。参照李宝鑫等[10]发布的土壤元素含量标准,样品中有效铁含量为缺乏(5-10 mg·kg-1)的仅占20.83%,有效铁含量为中等水平(10~30 mg·kg-1)的占79.17%,因此大部分园区土壤中有效铁并不缺乏。植株黄化程度和土壤指标相关性分析结果表明,黄化程度与土壤pH 值、EC 值、有机质含量以及全铁和有效铁含量相关性均不显著。而土壤pH值与EC值之间呈显著负相关(表3)。有机质含量与土壤有效铁含量之间呈显著正相关,与土壤全铁含量呈极显著正相关。
表2 不同园区土壤调查
Table 2 Soil survey of different parks

注:黄化程度0、1、2 和3 分别表示正常、轻度、中度和重度。
Note:The degree of chlorosis 0,1,2 and 3 indicates normal,mild,moderate and severe,respectively.
?
表3 植株黄化程度与土壤pH 值、EC 值、有机质含量以及铁元素含量相关性分析
Table 3 Correlation analysis between chlorosis level of plants and soil pH,EC value,organic matter content as well as Fe content

注:**、*分别代表0.01、0.05 的显著水平。下同。
Note:**,*represent significance levels of 0.01,0.05,respectively.The same below.
?
2.2 大田黄化与正常植株的对比分析
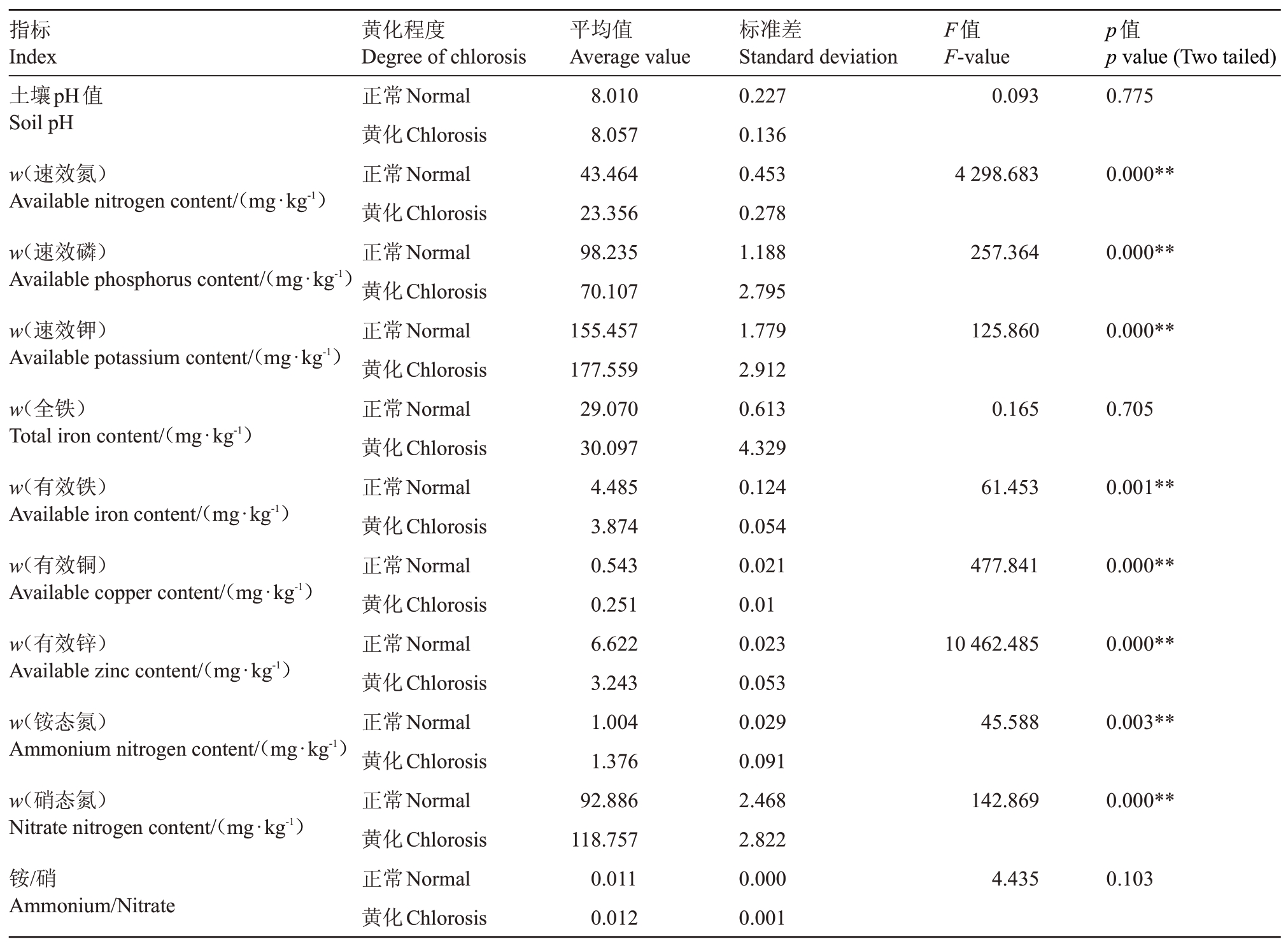
2.2.1 黄化植株和正常植株根际土壤pH 值和矿质元素含量对比分析 如表4 所示,通过对比分析根际土壤pH值和矿质元素含量,表明黄化植株和正常植株土壤pH 值均大于8.0,偏碱性,两者差异不显著,土壤中氮存在形式均以硝态氮为主,即铵态氮含量显著低于硝态氮,两者铵硝比差异不显著。黄化植株土壤中速效氮、速效磷、有效铁、有效铜、有效锌含量均低于正常植株,且差异均达到极显著水平(p<0.01)。黄化植株根际土壤速效氮、速效磷含量分别比正常植株低46.26%和28.63%,黄化植株有效铁和有效铜含量比正常植株低53.78%和51.03%。黄化植株土壤中速效钾含量比正常植株高14.22%,差异达到极显著水平(p<0.01)。二者土壤全铁含量差异不显著。
表4 黄化植株和正常植株根际土壤pH 值和矿质元素含量对比
Table 4 Comparison of pH and mineral elements contents in rhizosphere soils of etiolated and normal plants
?
2.2.2 不同黄化程度植株叶片矿质元素与根系铁含量对比分析 如表5、表6 所示,通过对比不同黄化程度叶片矿质元素与根系铁含量,不同程度黄化叶片中氮、磷、钾、铜、锌含量均显著高于正常植株,有效铁含量显著低于正常植株,差异随黄化程度的加重逐渐增大。虽然不同程度黄化叶片全铁含量均显著低于正常叶片,但随黄化程度的加重,黄化叶片中全铁含量逐渐增加,即重度黄化叶片全铁含量显著高于轻度黄化叶片。黄化植株根系中铁元素含量是叶片中的4~5 倍,比正常植株高16.40%,差异显著(p<0.05)。因此,黄化植株中铁元素并不缺乏。
表5 不同黄化程度植株叶片矿质元素含量对比
Table 5 Comparison of mineral elements contents of leaves from plants with different chlorosis degrees

注:不同小写字母表示在0.05 水平差异显著。下同。
Note:Different lowercase letters indicate significant differences at the 0.05 level.The same below.
?
表6 黄化植株与正常植株根系中铁元素对比
Table 6 Comparison of iron elements in roots of yellow and normal plants

?
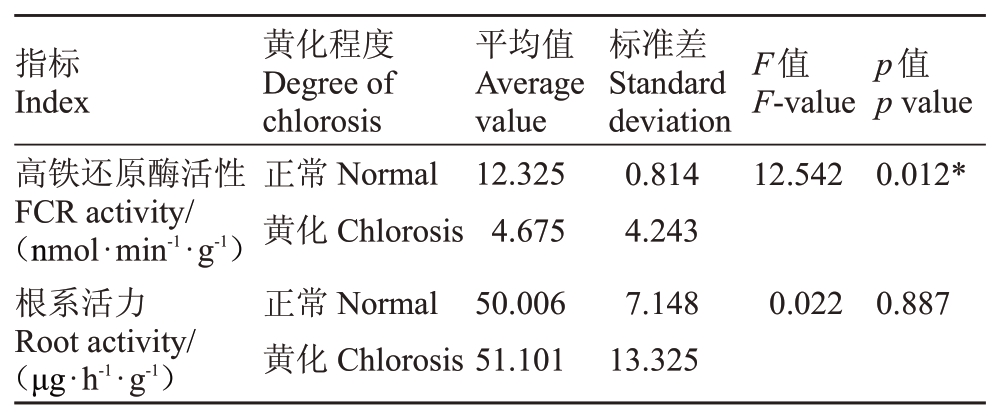
2.2.3 叶片高铁还原酶(FCR)活性和根系活力差异分析 如表7 所示,通过分析黄化叶片和正常叶片FCR 活性和根系活力,表明黄化植株的根系活力与正常植株差异不显著,黄化植株根系活力甚至高于正常植株,黄化叶片中FCR 活性低于正常叶片,仅是正常植株的37.93%,差异显著(p<0.01),因此Fe3+的还原在黄化叶片中受到了抑制。
表7 叶片高铁还原酶活性和根系活力差异分析
Table 7 Difference analysis of ferric reductase(FCR)activity in leaves and root activity
?
2.3 盆栽试验
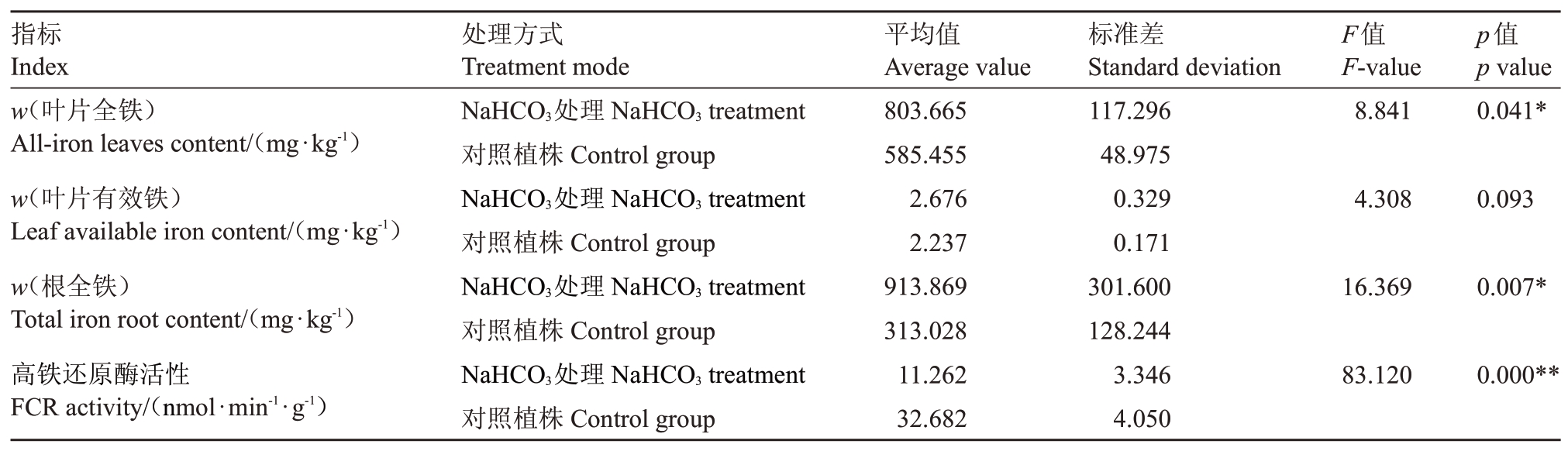
如图2 所示,处理50 d 后,用NaHCO3处理的阳光玫瑰植株叶片出现明显黄化症状,而用pH值为9的NaOH溶液处理的植株叶片未出现黄化。通过分析NaHCO3处理植株与对照植株之间叶片和根系中铁元素含量以及叶片FCR 活性的差异,结果(表8)表明NaHCO3处理植株叶片和根全铁含量均显著高于对照植株。叶片有效铁含量在两者之间无显著差异。NaHCO3处理植株叶片FCR 活性低于对照植株,差异极显著。

图2 不同处理方式对比
Fig.2 Comparison of different processing methods
A.90 mmol·L-1 NaHCO3 溶液处理;B.pH 为9 的NaOH 溶液处理。
A.90 mmol·L-1 NaHCO3 solution treatment;B.NaOH solution treatment with pH 9.
表8 处理组与对照组差异分析
Table 8 Analysis of differences between treatment group and control group
?
2.4 大田黄化植株矫正试验
2.4.1 不同处理对黄化的矫正效果 用3种不同的处理对阳光玫瑰黄化植株进行处理,处理6 d后观察到黄化叶片明显复绿。不同处理的效果存在差异,如图3~图6 所示,T1 处理植株上部叶片大部分复绿,中下部叶片恢复较差,恢复过程中叶片上形成与药剂喷施位置一致的绿斑。T2 处理同样上部叶片大部分复绿,中下部叶片恢复较差,T2 处理总体复绿情况比T1 处理差。T3 处理上部叶片全部复绿,中部叶片大部分复绿,复绿情况好于处理T1 和T2。黄化对照组如图7 所示。如图8 所示,各处理组矫正处理后叶绿素相对含量差距明显缩小,其中T3处理的叶绿素相对含量甚至高于绿色对照。

图3 处理T1 前(A)后(B)对比
Fig.3 Treatment before(A)and after(B)treatment T1
A.处理前;B.处理6 d 后(红框标记处为同一株的相同部位)。下同。
A.Before processing;B.6 days after treatment(the same part of the same strain was marked with red box).The same below.

图4 处理T1 处理叶片后产生的斑点
Fig.4 Spot generated by treatment T1 leaves

图5 处理T2 前(A)后(B)对比
Fig.5 Comparison before(A)and after(B)treatment T2

图6 处理T3 前(A)后(B)对比
Fig.6 Comparison before(A)and after(B)treatment T3

图7 黄化对照处理前(A)后(B)对比
Fig.7 Comparison of chlorosis control group before(A)and after(B)treatment

图8 处理后各组叶片叶绿素相对含量的变化
Fig.8 Change of relative content of chlorophyll in leaves of each group after treatment
2.4.2 不同处理对叶片矿质元素含量的影响 图9~图11为不同处理叶片元素含量,结果表明各组叶片中氮元素含量T2 处理>黄化对照>T3 处理>T1处理>正常对照,但各组间差异并不显著。叶片中磷元素含量黄化对照>T2处理>T1处理>T3处理>正常对照,其中T2 处理显著高于正常对照,黄化对照显著高于T3 处理和正常对照。叶片中钾元素含量黄化对照>T2处理>T3处理>T1处理>正常对照,其中T2处理显著高于T1处理,黄化对照显著高于处理T1、T2、T3 和正常对照,T2 处理显著高于T3 处理和正常对照,T3 处理显著高于正常对照。叶片中铜元素含量黄化对照>T2 处理>T3 处理>T1 处理>正常对照,但各组间差异并不显著。叶片中锌元素含量黄化对照>T3 处理>T2 处理>T1 处理>正常对照,其中T1 处理显著低于T3 处理和黄化对照,T2处理显著低于T3处理和黄化对照,T3处理显著高于正常对照,正常对照显著低于黄化对照。叶片中铁元素含量T2 处理>黄化对照>T3处理>T1 处理>正常对照,但各组差异并不显著。叶片中有效铁含量T1处理>T2处理>T3处理>正常对照>黄化对照,其中T1 处理显著高于处理T2、T3、黄化对照和正常对照,T2 处理显著高于黄化对照和正常对照,T3 处理显著高于黄化对照和正常对照。以上差异均在p<0.05水平。
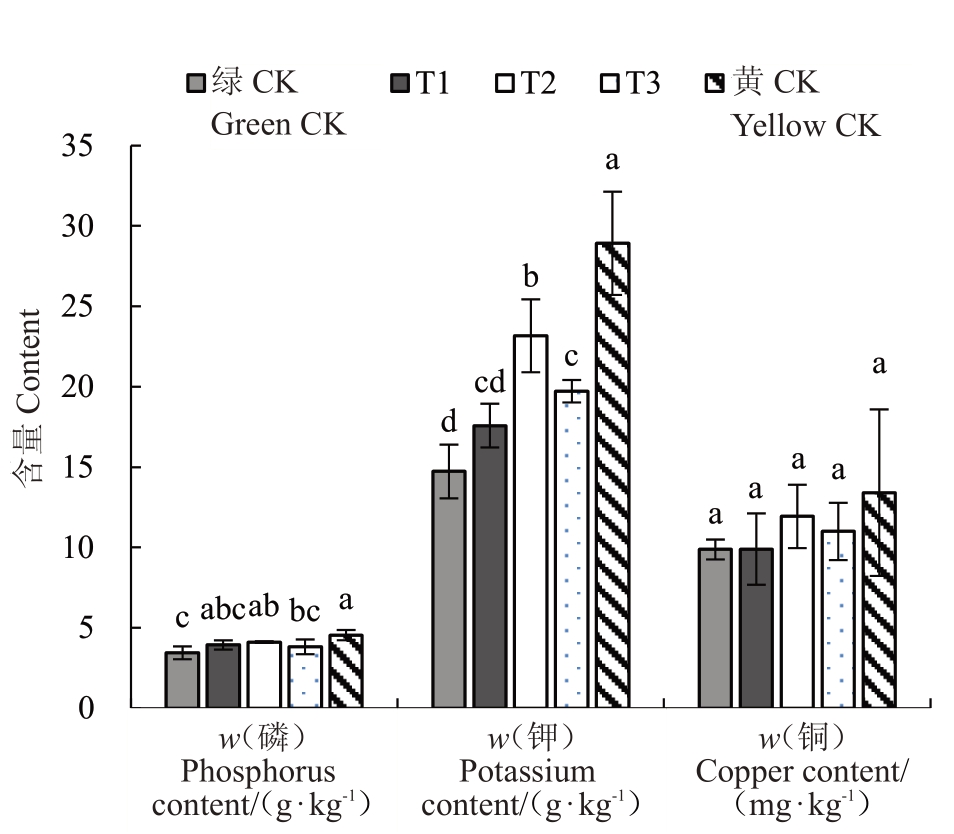
图9 不同处理对叶片磷、钾、铜含量的影响
Fig.9 Effect of different treatments on Phosphorus,Potassium and Copper contents of leaves

图10 不同处理对叶片铁含量的影响
Fig.10 Effect of different treatments on leaf iron content
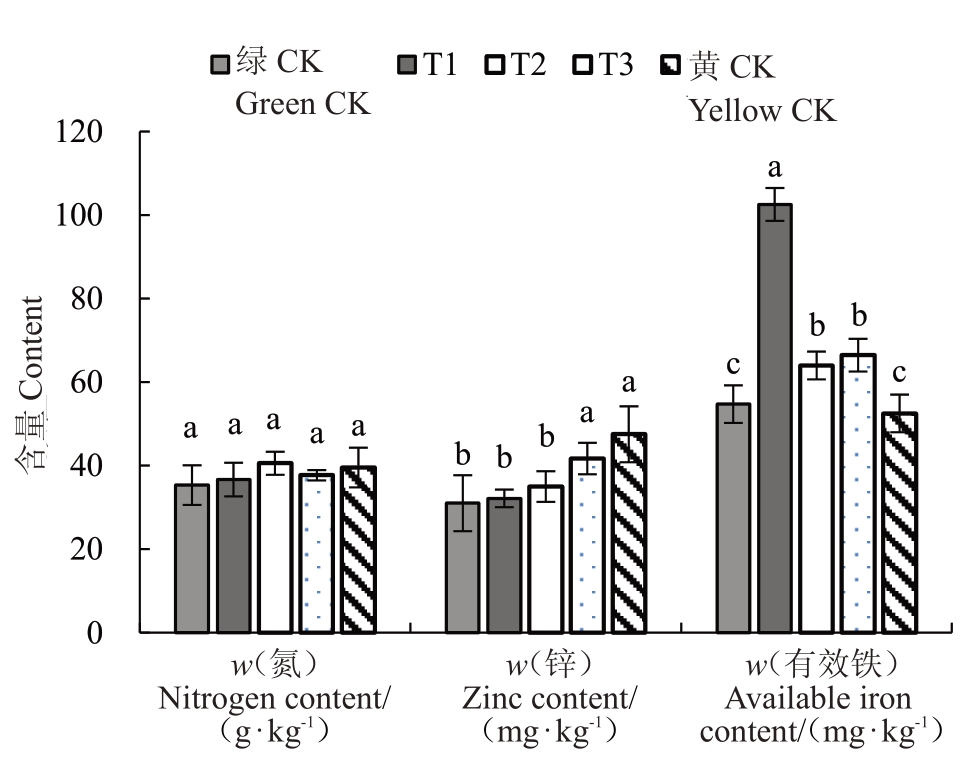
图11 不同处理对叶片氮、锌、有效铁含量的影响
Fig.11 Effect of different treatments on nitrogen,zinc and available iron contents of leaves
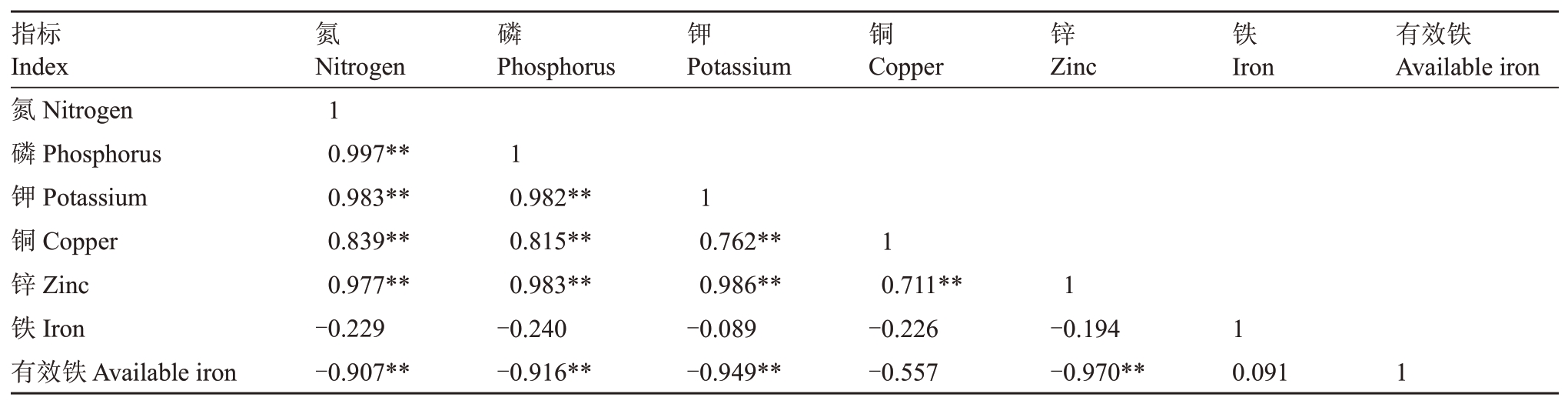
2.4.3 叶片矿质元素含量相关性分析 根据处理后各组叶片矿质元素含量的变化,对其进行相关性分析。结果(表9)表明,叶片中氮含量与磷、钾、铜、锌含量呈极显著正相关,与有效铁含量呈极显著负相关。叶片磷含量与钾、铜、锌含量呈极显著正相关,与有效铁含量呈极显著负相关。叶片中钾含量与铜、锌含量呈极显著正相关,与有效铁含量呈极显著负相关。叶片中铜含量与锌含量呈极显著正相关。叶片锌含量与有效铁含量呈极显著负相关。
表9 叶片矿质元素含量相关性分析
Table 9 Correlation analysis of mineral elements content of leaves
?
3 讨 论
叶片黄化通常与植株体内营养元素失衡关系密切,一般认为土壤高pH 值引起土壤有效铁降低,植株没有得到充足的有效铁供应是发生叶片黄化的主要原因[11-13]。但在本研究调查中发现虽然所有土壤样本均为碱性,但大部分阳光玫瑰葡萄黄化园土壤中有效铁并不缺乏,黄化程度与土壤pH 值、有机质含量以及土壤铁元素含量的相关性并不显著。因此,土壤高pH值不是导致叶片黄化的关键因素。
植株对铁元素的吸收和转运关键在于植株对Fe3+的还原机制。铁的吸收起始于根皮层中的标准还原酶对Fe3+的还原,这种酶活性受pH 值影响,如果质外体的pH 较高,铁的还原会受到抑制,铁元素不能顺利吸收到根细胞中,从而导致铁在根质外体中积累[14-15]。铁被吸收到根细胞后进入木质部前会被氧化为Fe3+,并与有机酸络合成螯合物,通过木质部转移到植物上部[16]。铁的第二次还原在叶中,叶肉细胞对铁的吸收取决于质膜上的FCR对Fe3+的还原,其活性依然受植物体内环境pH值的影响[17]。以上两个还原过程在受到抑制时均能引起叶片的缺铁黄化。本研究大田试验结果表明,即使在黄化植株的根系中也富含大量的铁,含量为叶片中的几倍,显著高于正常植株,叶片中FCR活性显著低于正常植株,黄化叶片的全铁含量随黄化程度加重呈上升趋势。上述结果与前人研究得出的结论一致[18-21]。这说明黄化植株内并不缺铁,黄化植株中铁积累与铁还原和转运过程在叶片和根系中受到抑制有关,而观察到正常叶片全铁含量显著高于不同程度黄化叶片可能是由于试验材料中黄化植株根部存在的抑制作用较为强烈,使通过质外体运输到叶片的铁元素远少于正常植株,最终即使由于叶片中的抑制作用使铁元素在叶片质外体积累,其总铁含量也显著低于正常植株。
有学者研究发现HCO3-和NO3-是引起植物质外体pH 值上升的主要原因[22-24]。在土壤高pH 值的条件下,一方面植物根系呼吸持续产生的CO2在土壤黏重或田间含水量高的情况下更容易产生并积累HCO3-;另一方面土壤中NH4+在碱性条件下容易发生去质子反应,生成NH3并挥发到空气中,因此土壤中氮元素通常以NO3-为主要存在形式[25]。本研究结果与之一致。HCO3-和NO3-分别通过中和质膜H+泵排出的H+和被植物细胞吸收后参与H+的共转运和OH-反转运使根质外体pH 增高,根系吸收的HCO3-和NO3-也可以通过木质部运输转移到叶片中[26-27]。有试验已证明HCO3-和NO3-也可以使叶片质外体pH 上升[22]。因此,HCO3-和NO3-与植物对铁元素的吸收和转运受阻密切相关。本试验中以盆栽试验的方法,通过用NaHCO3溶液处理阳光玫瑰植株,诱导其叶片发生黄化,元素含量和FCR活性测定结果表明在HCO3-诱导的黄化植株中也能观察到铁元素在叶片和根系中积累,FCR 活性受到抑制,这与大田试验结果基本一致。因此上述结论可以进一步证明大田阳光玫瑰植株叶片发生黄化与HCO3-有关。
对于叶片缺铁引起的叶片黄化,采取土壤增施铁肥或者叶面喷施硫酸亚铁等含铁药剂是常规的挽救措施。但一般直接往石灰性土壤中加入Fe2+并不会产生效果,因为Fe2+马上会被氧化固定,但添加有机物可增强铁有效性,所以通常使用螯合铁来矫正黄化[28]。不同的螯合剂与铁生成的螯合物稳定性不同,效果也不同:乙二胺四乙酸铁(Fe-EDTA)稳定性在pH 值超过6 时降低,而乙二胺二邻苯基乙酸铁(Fe-EDDHA)即使土壤pH 值高于9 依然保持稳定性[29-30]。施用螯合铁这种做法成本较高,每年都要重复,因为铁会迅速固定在土壤中或从根区淋溶出来[31]。一些学者发现在土壤中施加NH4+会抑制根系对NO3-的吸收,同时使质外体的pH 值下降,改善植物铁营养[22,32]。此外,通过根施或叶片喷施酸溶液也可以绕过HCO3-和NO3-对铁吸收和转运的抑制,也可以增强叶片对铁元素的固定作用[33]。Tagliavini等[34]通过喷施柠檬酸使黄化的猕猴桃叶片复绿。Sahu等[35]通过喷施硫酸等酸性物质成功缓解豌豆的缺铁黄化。也有一些学者通过喷施吲哚乙酸(IAA)以及丝孢霉素等酸性药剂刺激质膜中质子泵,以此来治疗植物缺铁黄化[25,31]。本试验中针对HCO3-和NO3-制定3种矫正方案,T1处理使用螯合铁与柠檬酸组合;T2处理单纯喷施柠檬酸;T3处理使用硫酸铵溶液根施。为了避免NH4+在碱性土壤条件下快速挥发,试验时在溶液中添加了1%的柠檬酸。结果表明,3种方案处理后植株都有不同程度复绿,以根施硫酸铵效果最优。说明对于该园出现的叶片黄化现象,解除对根系铁元素吸收和转运的抑制作用矫正效果要优于解除叶片对铁元素的固定。矫正试验的结果进一步验证了大田试验和盆栽试验中得出的结论。
元素相关性分析结果表明,叶片中有效铁含量与氮、磷、钾、锌含量均呈极显著负相关。各处理叶片不同程度复绿后,叶片中氮、磷、钾、锌、铜元素含量相较于黄化对照均有下降,但复绿情况较差的处理T2 叶片中总铁含量依然高于复绿情况较好的处理T3。说明各处理组虽不同程度复绿,但铁元素在叶片中转运受到的抑制作用依然存在。
葡萄作为策略Ⅰ植物,当存在缺铁胁迫时,植株会通过增加H+的分泌(根际的酸化),从而增强还原酶活性和溶解根际中的铁来促进铁的吸收,因此葡萄抗缺铁黄化能力与根际酸化能力具有很强的相关性,易缺铁黄化品种的根际酸化能力低于抗缺铁黄化品种[36]。葡萄不同种的抗缺铁的能力也不同,一般认为欧洲葡萄(V.vinifera L.)和冬葡萄(V.berlandieri Planche)抗缺铁黄化能力较强,而美洲种(V.labrusca L.)、河岸葡萄(V.riparia Michaux.)和沙地葡萄(V. rupestris Sheele)抗缺铁黄化能力较差[37]。本研究的试验材料阳光玫瑰属于欧美杂种(V.vinifera×V.labrusca),含有较多美洲种的血缘,因此在生产中更容易出现缺铁黄化。
Köseoğlu[38]和吴利园[39]在桃树黄化叶片中发现氮、磷、钾以及铁的含量均高于正常叶片。刘春燕[40]和卢春生[41]在葡萄中缺铁黄化叶片中也分别发现磷、钾和氮、钾的含量高于正常叶。以上结论与本研究结果一致。对于黄化叶片经常伴随出现的高磷含量,Mengel 等[25]通过盆栽试验的方法向葡萄生长土壤中施入大量磷,但未发现植株黄化,因此黄化叶中高水平磷含量是导致黄化病发生的结果而不是原因。本研究中发现黄化叶片中锌、铜含量高于正常叶片。Wallace等[42]认为铜、锌等金属在植物中会与铁竞争配体,从而降低植物对铁的利用。本研究通过对叶片矿质元素相关性分析,结果表明叶片有效铁含量与氮、磷、钾、锌含量均呈极显著负相关,铜、锌含量均与氮、磷、钾含量呈极显著正相关。植株中各元素含量此消彼长,因此在日常栽培管理中理应重视树体营养的均衡补充。
4 结 论
由于土壤高pH值诱导,HCO3-和NO3-在土壤中大量积累,使根和叶中铁还原酶活性降低,植株对铁元素吸收和转运受到抑制,积累在根系和叶片质外体的铁元素不能顺利被还原转运到根细胞和叶肉细胞,最终引起阳光玫瑰叶片缺铁黄化。黄化矫正方案以根施铵肥和酸性物质、解除根系铁还原受到的抑制作用复绿效果最佳。
[1] 刘文科,杜连凤,刘东臣.果树铁营养诊断及缺铁失绿症的施肥矫治研究现状[J].中国农学通报,2002,18(4):67-70.LIU Wenke,DU Lianfeng,LIU Dongchen.A review on diagnosis methods for iron nutrition status of fruit trees and fertilization correction for Fe-deficiency chlorosis[J]. Chinese Agricultural Science Bulletin,2002,18(4):67-70.
[2] 刘春燕,周龙,罗洁,吴晨光,刘永强,方海龙.吐鲁番葡萄黄化园土壤养分与地上部的相关性[J].新疆农业科学,2017,54(10):1920-1929.LIU Chunyan,ZHOU Long,LUO Jie,WU Chenguang,LIU Yongqiang,FANG Hailong.Study on the correlation between soil nutrients and above-ground parts in grape yellow orchard of Turpan[J].XinjiangAgricultural Sciences,2017,54(10):1920-1929.
[3] 张坤,郝燕,王玉安. 兰州春季葡萄叶片黄化的气温因素初探[J].甘肃农业科技,2019(10):70-73.ZHANG Kun,HAOYan,WANGYu’an.Preliminary study on temperature factors of grape leaf yellowing in spring in Lanzhou[J].Gansu Agricultural Science and Technology,2019(10):70-73.
[4] 李华鹏,杜方.露地百合叶片黄化与营养元素的关系[J].山西农业科学,2019,47(10):1734-1741.LI Huapeng,DU Fang. Study on the relationship between leaf yellowing and nutrient elements in open field lily[J]. Journal of Shanxi Agricultural Sciences,2019,47(10):1734-1741.
[5] 刘金生,陈旭亮,韦才学,包福兴,孙布尔,陈虹.乌什县核桃叶片黄化与土壤矿质营养的关系[J]. 天津农业科学,2019,25(6):68-71.LIU Jinsheng,CHEN Xuliang,WEI Caixue,BAO Fuxing,SUN Buer,CHEN Hong. Relationships of walnut leaf yellowing and soil mineral nutrients in Wushi County[J]. Tianjin Agricultural Sciences,2019,25(6):68-71.
[6] 鲍士旦. 土壤农化分析[M]. 3 版. 北京:中国农业出版社,2000:54-108.BAO Shidan. Soil and agricultural chemistry analysis[M]. 3rd ed.Beijing:China Agriculture Press,2000:54-108.
[7] 杨丽,李和平. 测试条件对土壤中33 种元素同时测定的影响[J].河南预防医学杂志,2018,29(11):824-827.YANG Li,LI Heping. Effect of test conditions on determination of 33 elements in the soil by inductively coupled plasma mass spectromentry[J]. Henan Journal of Preventive Medicine,2018,29(11):824-827.
[8] 宋歌,孙波,教剑英.测定土壤硝态氮的紫外分光光度法与其他方法的比较[J].土壤学报,2007,44(2):288-293.SONG Ge,SUN Bo,JIAO Jianying. Comparison between ultraviolet spectrophotometry and other methods in determination of soil nitrate-N[J].Acta Pedologica Sinica,2007,44(2):288-293.
[9] 陈雅涵,谢宗强,薛丽萍.碳氮元素分析仪测试土壤与植物样品的流程优化[J].现代化工,2016,36(4):185-187.CHEN Yahan,XIE Zongqiang,XUE Liping. Process optimization for measurements of soil and plant samples with carbon/nitrogen element analyzer[J].Modern Chemical Industry,2016,36(4):185-187.
[10] 李宝鑫,杨俐苹,卢艳丽,师校欣,杜国强.我国葡萄主产区的土壤养分丰缺状况[J]. 中国农业科学,2020,53(17):3553-3566.LI Baoxin,YANG Liping,LU Yanli,SHI Xiaoxin,DU Guoqiang. Status of soil fertility in main grape producing areas of China[J].Scientia Agricultura Sinica,2020,53(17):3553-3566.
[11] 晁无疾,周敏,张铁强,付京晶,丁双陆.葡萄缺铁性黄化病调查与矫治试验[J].中外葡萄与葡萄酒,2000(2):25-27.CHAO Wuji,ZHOU Min,ZHANG Tieqiang,FU Jingjing,DING Shuanglu. Investigation and treatment test of iron deficiency chlorosis of grape[J].Sino-Overseas Grapevine&Wine,2000(2):25-27.
[12] 李登科,李撵科,韩亚维.猕猴桃黄化致病因素及综合防治[J].西北园艺(果树),2022(2):30-31.LI Dengke,LI Nianke,HAN Yawei. Etiopathogenesis and integrated prevention and treatment of kiwifruit yellowing[J].Northwest Horticulture,2022(2):30-31.
[13] 何天明,刘泽军,覃伟铭,田永平,张绍铃.土壤因子对库尔勒香梨缺铁失绿症发生的影响[J].西北农业学报,2013,22(1):97-103.HE Tianming,LIU Zejun,QIN Weiming,TIAN Yongping,ZHANG Shaoling. Effects of soil factors on iron-deficit chlorosis of Kuerle fragrant pear (Pyrus bretschneideri Rehd.)[J].Acta Agriculturae Boreali-Occidentalis Sinica,2013,22(1):97-103.
[14] WANG T G,PEVERLY J H.Investigation of ferric iron reduction on the root surfaces of common reeds using an EDTA-BPDS method[J].Journal of Plant Nutrition,1999,22(6):1021-1032.
[15] SUSIN S,ABADIAA,GONZALEZ-REYES J A,LUCENA J J,ABADIA J.The pH requirement for in vivo activity of the irondeficiency-induced“Turbo”ferric chelate reductase (a comparison of the iron-deficiency-induced iron reductase activities of intact plants and isolated plasma membrane fractions in sugar beet)[J].Plant Physiology,1996,110(1):111-123.
[16] BROWN J C,JOLLEY V D. Plant metabolic responses to irondeficiency stress[J].BioScience,1989,39(8):546-551.
[17] KOSEGARTEN H U,HOFFMANN B,MENGEL K. Apoplastic pH and Fe3 + reduction in intact sunflower leaves[J]. Plant Physiology,1999,121(4):1069-1079.
[18] MENGEL K. Iron availability in plant tissues-iron chlorosis on calcareous soils[J].Plant and Soil,1994,165(2):275-283.
[19] JACOBSON L. Iron in the leaves and chloroplasts of some plants in relation to their chlorophyll content[J]. Plant Physiology,1945,20(2):233-245.
[20] MENGEL K,SCHERER H W,Malissiovas N.Chlorosis with respect to soil chemistry and the nutrition of vines [grapes][J].Mitteilungen Rebe und Wein, Obstbau und Fruechtenverwertung(Austria),1979,28(4):151-156.
[21] MENGEL K,MALISSIOVAS N. Bicarbonat als auslösender faktor der eisenchlorose bei der weinrebe (Vitis vinifera)[J]. Vitis,1981,20(3):235-243.
[22] MENGEL K,PLANKER R,HOFFMANN B. Relationship between leaf apoplast pH and iron chlorosis of sunflower (Helianthus annuus L.)[J].Journal of Plant Nutrition,1994,17(6):1053-1065.
[23] MENGEL K,GEURTZEN G.Iron chlorosis on calcareous soils.Alkaline nutritional condition as the cause for the chlorosis[J].Journal of Plant Nutrition,1986,9(3/4/5/6/7):161-173.
[24] 邹春琴,张福锁.叶片质外体pH 降低是铵态氮改善植物铁营养的重要机制[J].科学通报,2003,48(16):1791-1795.ZOU Chunqin,ZHANG Fusuo.The decrease of pH of leaf apoplast is an important mechanism for ammonium nitrogen to improve iron nutrition in plants[J].Chinese Science Bulletin,2003,48(16):1791-1795.
[25] MENGEL K,BREININGER M T,BÜBL W. Bicarbonate,the most important factor inducing iron chlorosis in vine grapes on calcareous soil[J].Plant and Soil,1984,81(3):333-344.
[26] TOULON V,SENTENAC H,THIBAUD J B,DAVIDIAN J C,MOULINEAU C,GRIGNON C. Role of apoplast acidification by the H+pump[J].Planta,1992,186(2):212-218.
[27] MILLER G W.Carbon dioxide-bicarbonate absorption,accumulation,effects on various plant metabolic reactions,and possible relations to lime-induced chlorosis[J].Soil Science,1960,89(5):241-245.
[28] PAPASTYLIANOU I. Timing and rate of iron chelate application to correct chlorosis of peanut[J].Journal of Plant Nutrition,1993,16(7):1193-1203.
[29] HERNANDEZ-APAOLAZA L,GÁRATE A,LUCENA J J.Efficacy of commercial Fe(III)-EDDHA and Fe(III)-EDDHMA chelates to supply iron to sunflower and corn seedlings[J]. Journal of Plant Nutrition,1995,18(6):1209-1223.
[30] ALVAA K.Solubility and iron release characteristics of iron chelates and sludge products[J].Journal of Plant Nutrition,1992,15(10):1939-1954.
[31] TAGLIAVINI M,ABADÍA J,ROMBOLÀ A D,ABADÍA A,TSIPOURIDIS C,MARANGONI B. Agronomic means for the control of iron deficiency chlorosis in deciduous fruit trees[J].Journal of Plant Nutrition,2000,23(11/12):2007-2022.
[32] BRETELER A,SMIT A L.Effect of ammonium nutrition on uptake and metabolism of nitrate in wheat[J]. Netherlands Journal of Agricultural Science,1974,22(2):73-81.
[33] MENGEL K. Iron availability in plant tissues-iron chlorosis on calcareous soils[J].Plant and Soil,1994,165(2):275-283.
[34] TAGLIAVINI M,SCUDELLARI D,MARANGONI B,TOSELLI M.Acid-spray regreening of kiwifruit leaves affected by limeinduced iron chlorosis[M]//ABADÍA J. Nutrition in Soils and Plants.Dordrecht:Springer,1995:191-195.
[35] SAHU M P,SHARMA D D,JAIN G L,SINGH H G.Effects of growth substances,sequestrene 138-Fe and sulphuric acid on iron chlorosis of garden peas (Pisum sativum L.)[J]. Journal of Horticultural Science,1987,62(3):391-394.
[36] BRANCADORO L,RABOTTI G,SCIENZA A,ZOCCHI G.Mechanisms of Fe-efficiency in roots of Vitis spp.in response to iron deficiency stress[J].Plant and Soil,1995,171(2):229-234.
[37] 贺普超.葡萄学[M].北京:中国农业出版社,1999:1-2.HE Puchao.Grapeviology[M].Beijing:China Agriculture Press,1999:1-2.
[38] KÖSEOĞLU A T. Effect of iron chlorosis on mineral composition of peach leaves[J]. Journal of Plant Nutrition,1995,18(4):765-776.
[39] 吴利园.陕西三原、泾阳叶片黄化桃园叶片及土壤养分含量分析[D].杨凌:西北农林科技大学,2018.WU Liyuan. Analysis of nutrient contents in leaves and soil of yellows peach orchard in Sanyuan and Jingyang,Shaanxi[D].Yangling:Northwest A&F University,2018.
[40] 刘春燕.吐鲁番市葡萄黄化病致病因子研究[D].乌鲁木齐:新疆农业大学,2018.LIU Chunyan. Study on pathogenic factors of grape chlorosis in Turpan city[D].Urumqi:Xinjiang Agricultural University,2018.
[41] 卢春生. 葡萄缺铁失绿发生原因与防治[J]. 新疆农业科学,1998,35(1):32-34.LU Chunsheng. Causes and prevention of iron deficiency and chlorosis of grapes [J]. Xinjiang Agricultural Sciences,1998,35(1):32-34.
[42] WALLACE A,WALLACE G A,CHA J W. Some modifications in trace metal toxicities and deficiencies in plants resulting from interactions with other elements and chelating agents:The special case of iron[J]. Journal of Plant Nutrition,1992,15(10):1589-1598.