西瓜(Citrullus lanatus)属葫芦科1 年生蔓生植物,原产非洲,是我国重要的经济作物,在我国的栽培面积和产量均居世界第一[1]。西瓜有7个种,且属内栽培类型繁多[2],具有一定栽培规模的有黏籽西瓜、籽用西瓜和普通(栽培)西瓜。黏籽西瓜是西非国家的重要经济作物[3],果肉白色、质地硬且味苦不可食,以食用种子为主。籽用西瓜是我国西北旱区的主要经济作物[4],籽瓜籽具有较高的营养价值和经济价值,瓜瓤有利尿、润肺、益肝和健脾等功效[5]。普通西瓜即鲜食甜西瓜,果实甘甜可口,富含多种营养物质。西瓜果实中含有多种游离氨基酸[6],它们在西瓜的生长发育中发挥重要作用[7-8];部分游离氨基酸自身为呈味氨基酸或参与风味物质的形成,且在人体中发挥重要作用,极大提高了西瓜的营养和风味价值[9]。对不同类型西瓜果实在动态发育过程中的游离氨基酸进行研究,既可以全面了解其含量和积累模式的差异,也可以针对特定游离氨基酸进行遗传改良以选育出高目标游离氨基酸含量的西瓜新品种,同时还能在代谢组协助种质分类中呈现潜在价值。代谢组学主要研究分子质量小于1 ku的代谢物[10]。目前,代谢组学检测技术已经在水稻[11]、黄瓜[12]、番茄[13-15]和辣椒[16]等多种作物的研究中发挥重要作用,为研究植株生长、代谢物合成、品种驯化改良和遗传机制揭示等提供了新的手段。游离氨基酸代谢物参与植物生长发育、细胞生长和分化、能量代谢、次生代谢、转录和翻译、调控及抗性等形成过程。通过代谢组学检测技术鉴定出多种游离氨基酸,有助于了解各游离氨基酸之间的差异,同时有助于发掘植物中游离氨基酸的营养价值[9]。对植物果实游离氨基酸代谢物的研究也有助于种质分类。袁平丽等[6,17]研究表明代谢组差异和表型差异均可以作为区分西瓜品种的重要依据,有助于充分了解和利用西瓜种质资源;刘圆等[18]表明不同甜瓜品种特有的香气物质及特征性酯类种类和含量均不同,这些差异构成了各品种甜瓜特有的典型性香气。西瓜果实中含有丰富的游离氨基酸,但目前相关研究主要集中在含量较其他水果或蔬菜更高的瓜氨酸和精氨酸方面[19-20]。李蒙蒙等[21]研究了嫁接对西瓜果实中瓜氨酸含量的影响;Davis等[22]通过测定多个西瓜品种中瓜氨酸含量,选择出高瓜氨酸含量的品种;Assefa 等[23]研究了瓜氨酸、精氨酸含量与可溶性固形物含量及瓤色的相关性。目前,对西瓜果实中其他游离氨基酸的研究报道较少。虽然对西瓜果实中含量较高的游离氨基酸的研究已经存在一定的基础,但不同类型西瓜果实中多种游离氨基酸的组分和含量及发育过程中的积累模式并未被完全揭示和详细报道。笔者在本研究中采用果实表型差异明显的黏籽西瓜、籽用西瓜和普通西瓜3个类型的西瓜为试材,系统测定和分析了3个类型西瓜果实中多种游离氨基酸的组分及含量和积累模式的差异,明确3个类型西瓜果实中差异积累的游离氨基酸组分和含量及积累模式的差异,并结合前人研究将游离氨基酸含量和积累模式特征与品种类型联系在一起进行分析。通过对PI532726、宁夏红籽瓜和冰糖脆3个不同类型的西瓜果实在生长发育过程中游离氨基酸的含量构成及积累模式差异情况的探究,为西瓜营养、西瓜风味、抗逆评价和西瓜种质分类提供数据支撑和参考,并为西瓜遗传育种和西瓜中游离氨基酸的遗传机制研究提供参考。
1 材料和方法
1.1 试验材料
供试材料种子由中国农业科学院郑州果树研究所国家西甜瓜中期库和多倍体西瓜遗传育种课题组提供。根据种质特点选取PI532726(黏籽品种)、宁夏红籽瓜(籽瓜品种)及冰糖脆(栽培品种)3个种质类型的西瓜品种作为试验材料(表1)。
表1 试验材料基础信息
Table 1 Basic information of experimental materials

?
供试材料于2019 年春季种植于中国农业科学院新乡综合试验基地大棚中,种子统一破壳处理,清水浸泡2.5 h,置于培养箱中32 ℃条件下催芽,芽长0.5 cm 时播种于营养钵中,苗期统一管理。实生苗播种后35 d移栽至大棚,随机区组设计,双蔓整枝,行间距1.5 m,株间距0.5 m,田间管理一致。花期人工自交授粉,在第二雌花节位留瓜,并悬挂吊牌标记授粉日期。根据授粉日期,在果实分别发育至10、18、26、34 d 时根据品种发育特征,每个品种选取长势良好、均匀一致的6个西瓜,纵切后对每2个西瓜果实的果肉进行5点混合取样作为1个生物学重复,共3 个生物学重复。对样品进行液氮速冻,随后使用冷冻干燥机进行冷冻干燥。
1.2 游离氨基酸含量的检测
1.2.1 标准溶液与样品溶液的配制 氨基酸标准品:L-丙氨酸(Ala)、L-精氨酸(Arg)、L-瓜氨酸(Cit)、L-鸟氨酸(Orn)、L-天冬酰胺(Asn)、L-天冬氨酸(Asp)、L-胱氨酸(Cys)、γ-氨基丁酸(GABA)、L-谷氨酰胺(Gln)、L-谷氨酸(Glu)、L-甘氨酸(Gly)、L-组氨酸(His)、L-异亮氨酸(ILe)、L-亮氨酸(Leu)、L-赖氨酸(Lys)、L-甲硫氨酸(Met)、L-苯丙氨酸(Phe)、L-脯氨酸(Pro)、L-羟脯氨酸(HYP)、L-丝氨酸(Ser)、L-苏氨酸(Thr)、L-酪氨酸(Tyr)、L-缬氨酸(Val)、肌氨酸(Sar)和L-茶氨酸(The),纯度均≥98%,购自郑州盛百欣生物科技有限公司。将各标准品分别配制成5000、2500、1250、625、312.5 mmol·L-1 5 个浓度梯度,于-20 ℃保存待测。
取1.00 g 冷冻干燥的果肉样品研磨成细粉状,使用100 mL 的沸水冲泡,随后放入95 ℃水浴锅水浴10 min,期间搅拌混匀5 次,水浴结束趁热使用0.22 μm 水相滤膜进行抽滤,冷却至室温后定容至125 mL,置于-20 ℃保存待测。
1.2.2 高效液相色谱条件 采用岛津公司的高效液相色谱仪对游离氨基酸进行测定,色谱仪型号:LC-20AD;色谱条件,荧光检测器A Ex:250 nm,Em:395 nm;色谱柱:C18,5 μm,4.6 mm×250 mm。流动相A(95 mL 800 mmol·L-1磷酸二氢钾溶液+ 5 mL 800 mmol·L-1磷酸氢二钠溶液+10 mL 1 mol·L-1盐酸三乙胺溶液+55 mL 乙腈+835 mL 水);流动相B(100 mL 800 mmol·L-1磷酸二氢钾溶液+500 mL乙腈+400 mL 超纯水);流速1 mL·min-1;二元梯度高压:泵A+泵B 总浓度为100%,洗脱梯度为泵A 浓度:0~25 min(100%),25~40 min(97%),40~55 min(81%),55~68 min(74%),68~88 min(0%)。
1.2.3 游离氨基酸含量的测定 取10 μL样品溶液于玻璃内衬管中,并加入20 μL衍生试剂和70 μL衍生用缓冲试剂,放入进样瓶涡旋混合10 s,转移至55 ℃烘箱中加热10 min,取出样品瓶放置于高效液相色谱样品瓶架上,开启检测按钮,重复测定3次。
1.3 数据处理
以3次生物学重复测定结果的平均值作为最终含量值。采用Excel 对试验数据进行平均数、标准差和相对标准偏差分析处理和作图。使用TBtools软件进行热图绘制,利用IBM SPSS Statistics 26 软件的因子模型对数据进行主成分分析,使用Origin 2018 64 Bit绘制主成分分析图。
2 结果与分析
2.1 3个类型西瓜果实发育过程中游离氨基酸含量分析
参考4个发育时期中游离氨基酸的最高含量,发现瓜氨酸、甘氨酸和谷氨酰胺在3 个类型西瓜果实中含量均较高,且存在显著差异;瓜氨酸在PI532726中为0.11 g·kg-1,在宁夏红籽瓜中为0.12 g·kg-1,而在冰糖脆中为0.39 g·kg-1。精氨酸(0.1~0.2 g·kg-1)仅在冰糖脆中含量较高(0.12 g·kg-1),而在PI532726和宁夏红籽瓜中分别为0.018 和0.068 g·kg-1。γ-氨基丁酸、谷氨酸、缬氨酸、异亮氨酸、天冬酰胺和苯丙氨酸在3 个不同类型西瓜果实中含量均在0.01~0.06 g·kg-1之间,茶氨酸、羟脯氨酸、酪氨酸、肌氨酸、丙氨酸、亮氨酸、鸟氨酸和半胱氨酸在3个类型西瓜果实中含量均低于0.01 g·kg-1。其余几种游离氨基酸在3个不同类型西瓜中含量差异显著,脯氨酸和甲硫氨酸在冰糖脆中含量分别为0.028和0.013 g·kg-1,而在PI532726和宁夏红籽瓜中含量均低于0.01 g·kg-1。丝氨酸在宁夏红籽瓜和冰糖脆中含量为0.011 和0.015 g·kg-1,而在PI532726中含量均低于0.01 g·kg-1(表2,图1)。
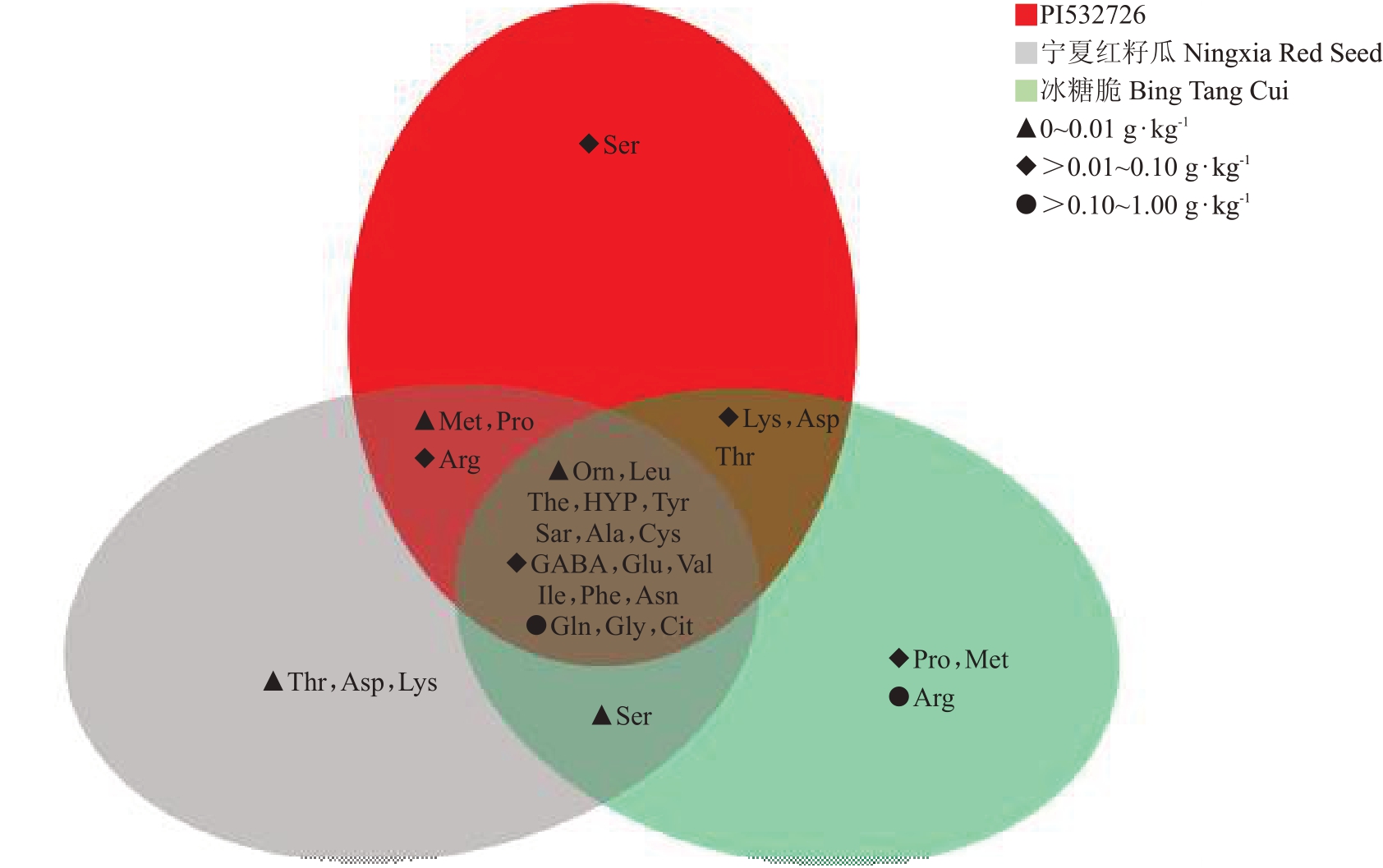
图1 3 个西瓜品种果实中不同游离氨基酸含量分析
Fig.1 Analysis of different free amino acids content in fruits of three watermelon varieties
表2 3 个不同类型品种4 个发育时期果肉游离氨基酸含量
Table 2 Contents of free amino acids in pulp of three watermelon varieties at four developmental stages (g·kg-1)

?
对3个类型品种西瓜4个发育时期果实中的24个指标标准化后进行主成分分析,提取特征值>1的2 个主成分。其中,PI532726 特征值分别为14.503、5.511,宁夏红籽瓜特征值分别为19.006、3.368,冰糖脆特征值分别为10.73、9.79,累计方差贡献率均大于83%,具有较强信息代表性,达到分析要求。PCA 载荷图(loading plot)可用来寻找差异变量,距离原点越远表示对样本贡献率越大。从载荷图(图2)可以看出,对PI532726 贡献率较大的游离氨基酸是甘氨酸和谷氨酰胺,对宁夏红籽瓜贡献率较大的游离氨基酸是甘氨酸、瓜氨酸和谷氨酰胺,对冰糖脆贡献率较大的游离氨基酸是甘氨酸、瓜氨酸、精氨酸和谷氨酰胺。聚类热图(图3)分析能够清晰地看出3个品种西瓜果实中的甘氨酸、瓜氨酸、精氨酸和谷氨酰胺存在显著差异的积累量与积累模式,而游离氨基酸进化树也能清晰地呈现出3个类型品种之间的差异,其中,PI532726和宁夏红籽瓜中游离氨基酸的代谢特征更相似。综上可以看出,在3 个类型的西瓜中存在含量显著差异的游离氨基酸,可能在不同种质类型的西瓜品种中作为区分种质的标志性差异代谢物存在。
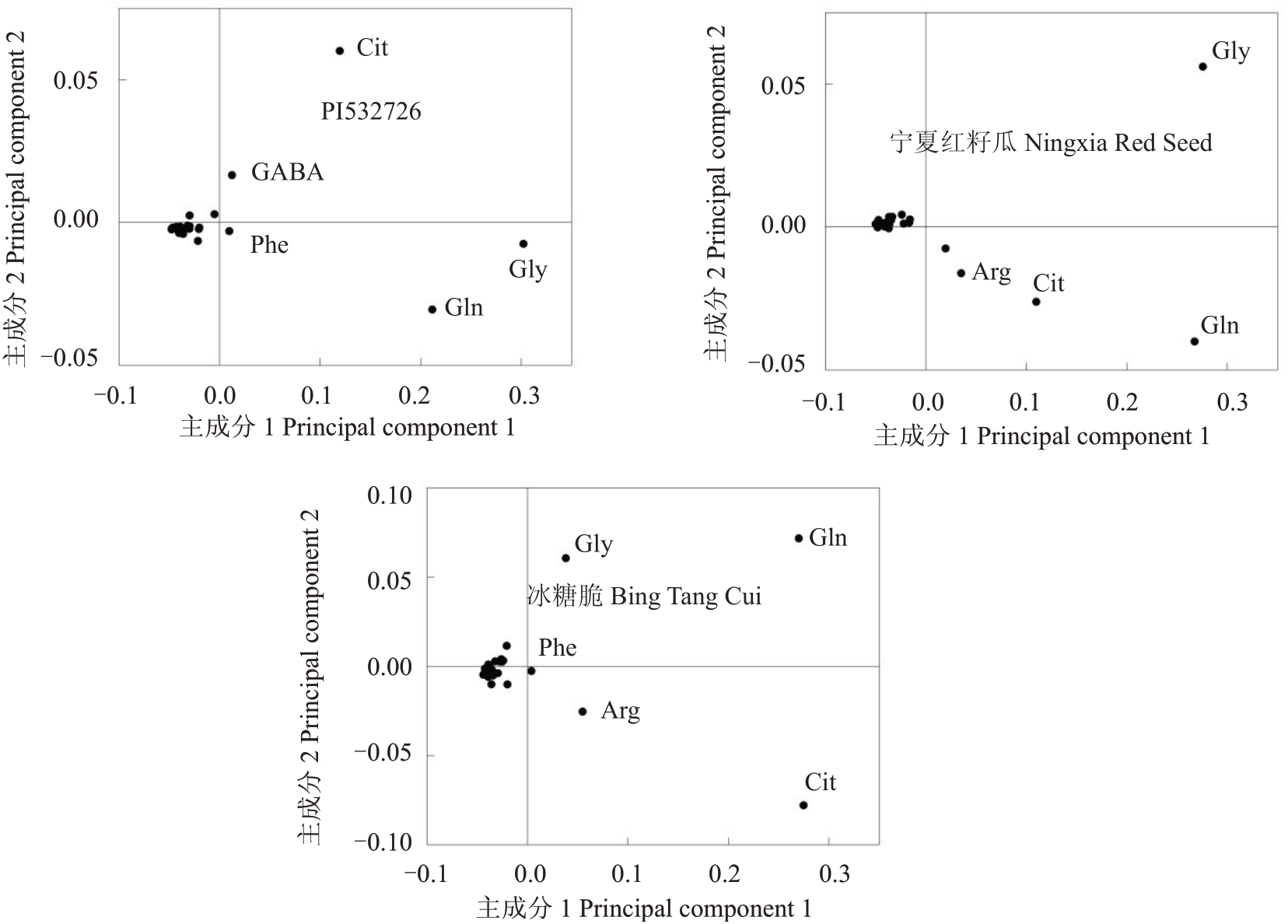
图2 3 个类型西瓜果实不同时期游离氨基酸的PCA 载荷图
Fig.2 PCA loadings of free amino acids in three watermelon varieties at different stages
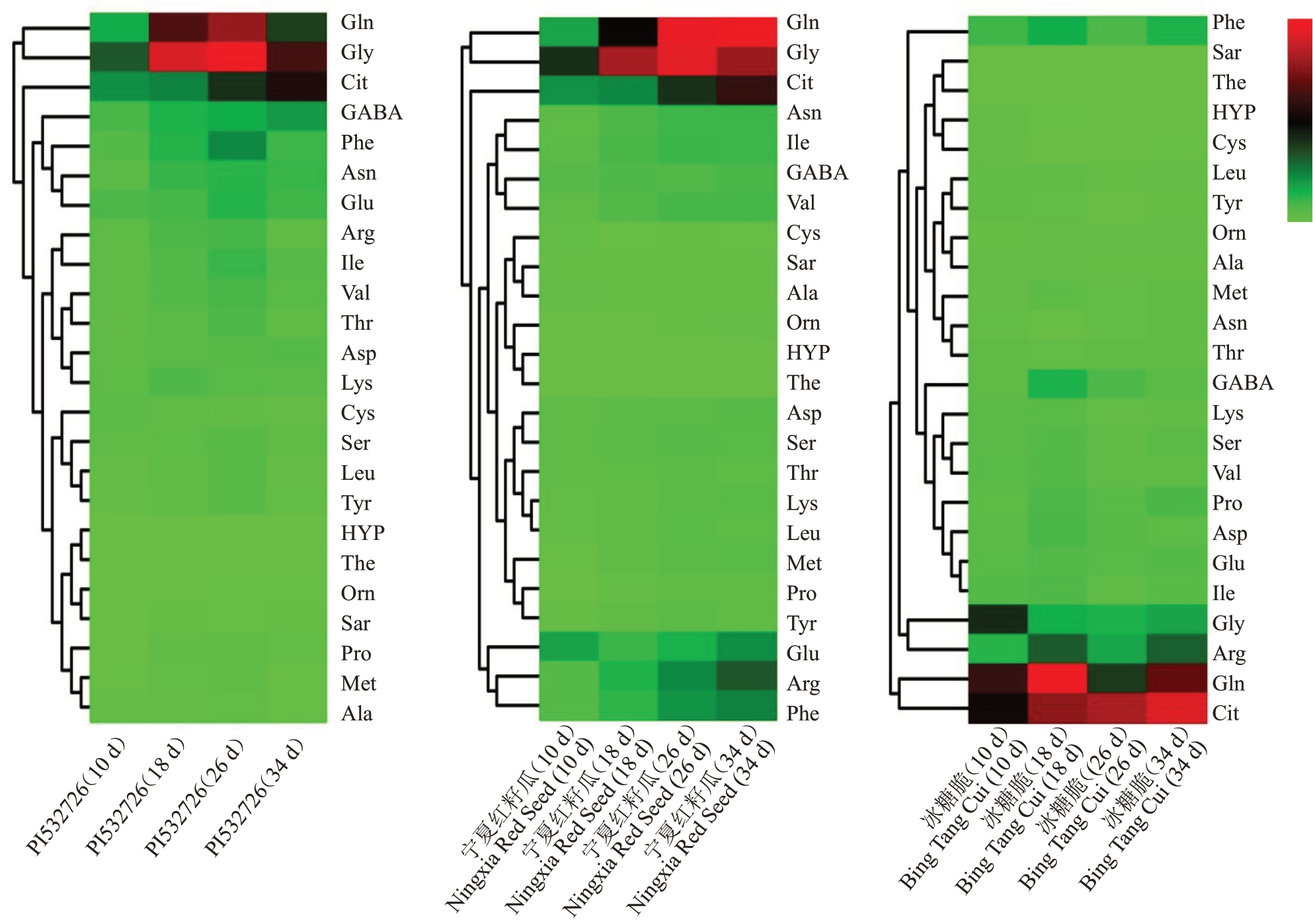
图3 3 个类型品种西瓜果实游离氨基酸数据聚类分析
Fig.3 Cluster analysis of free amino acid data in three watermelon varieties
将检测到的24 种游离氨基酸作为总游离氨基酸。PI532726发育过程中甘氨酸(26%~34%)、谷氨酰胺(12%~24%)、瓜氨酸(8%~23%)和γ-氨基丁酸(4%~9%)含量占比较大。宁夏红籽瓜中甘氨酸(20%~35%)、谷氨酰胺(12%~28%)、瓜氨酸(9%~15%)、谷氨酸(4%~12%)和精氨酸(4%~9%)含量占比较大。冰糖脆中瓜氨酸(23%~45%)、谷氨酰胺(21%~35%)、甘氨酸(4%~19%)和精氨酸(5%~11%)含量占比较大。不同发育时期中,3 个类型西瓜中多种游离氨基酸比例情况存在显著差异(图4),表明3 个类型西瓜果实中存在含量比例始终较大的游离氨基酸,且存在显著差异。这些游离氨基酸在果实发育中的积累情况可能与西瓜的品种和种质类型有关。

图4 3 个品种西瓜果实中重要游离氨基酸含量比例
Fig.4 Composition of important free amino acids in watermelon fruits of three varieties
仅对含量比例≥4%的游离氨基酸进行标注。
Only free amino acids whose proportion is greater than or equal to 4%are labeled.
2.2 3个类型西瓜果实中游离氨基酸含量分析
将检测到的24 种游离氨基酸作为总游离氨基酸。PI532726中总游离氨基酸含量的变化呈抛物线型(上升-下降),宁夏红籽瓜中呈上升型,而冰糖脆西瓜中呈“N”型(上升-下降-上升)。冰糖脆的4个发育时期的总游离氨基酸含量均高于PI532726 和宁夏红籽瓜。PI532726和宁夏红籽瓜的总游离氨基酸积累曲线重合度较高(图5)。
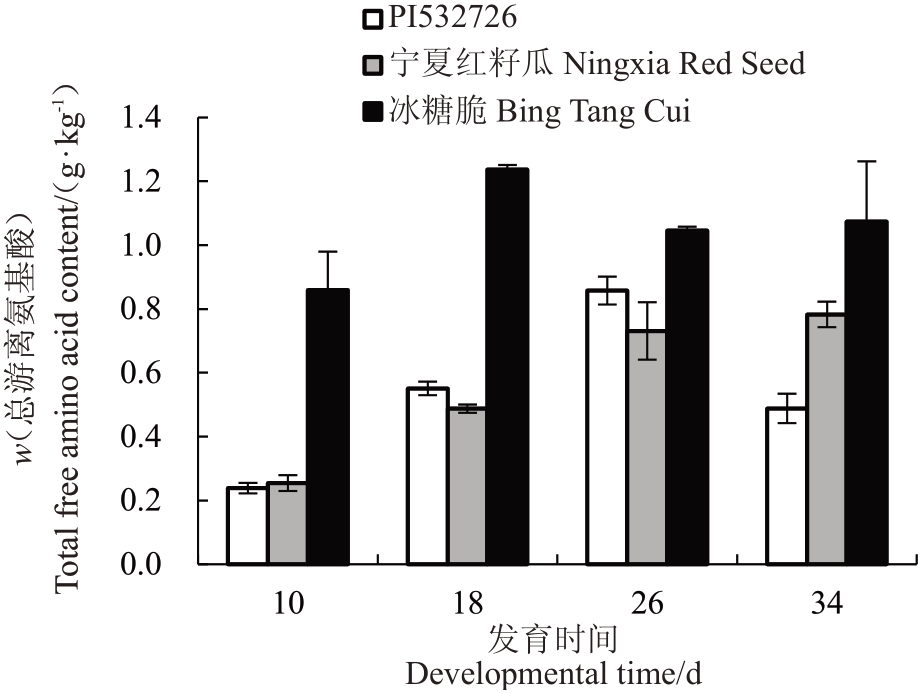
图5 3 个品种西瓜果实4 个不同发育时期游离氨基酸总量对比
Fig.5 Comparison of the total amount of free amino acids in fruits of three watermelon varieties at four different developmental stages
分别对各游离氨基酸积累模式进行分析。PI532726中瓜氨酸、γ-氨基丁酸、天冬氨酸和鸟氨酸含量的积累模式呈上升型,半胱氨酸含量呈下降型,谷氨酰胺、甘氨酸、精氨酸、谷氨酸、苯丙氨酸、天冬氨酸和异亮氨酸含量的积累模式呈抛物线型。宁夏红籽瓜中丙氨酸和半胱氨酸含量的积累模式呈下降型,谷氨酸含量呈“V”型(下降-上升),甘氨酸、亮氨酸、丝氨酸和酪氨酸含量呈抛物线型,瓜氨酸,谷氨酰胺、精氨酸、苯丙氨酸、γ-氨基丁酸、天冬氨酸、天冬酰胺、赖氨酸、缬氨酸、甲硫氨酸、鸟氨酸和茶氨酸含量呈上升型。冰糖脆中甘氨酸和丙氨酸含量呈下降型,瓜氨酸和鸟氨酸含量呈上升型,天冬氨酸和γ-氨基丁酸含量呈抛物线型,谷氨酰胺、精氨酸、苯丙氨酸、谷氨酸、亮氨酸、异亮氨酸、赖氨酸、脯氨酸、丝氨酸、缬氨酸和甲硫氨酸含量呈“N”型(上升-下降-上升),苏氨酸含量呈“倒N”型(下降-上升-下降)。PI532726与宁夏红籽瓜中多种游离氨基酸含量变化曲线重合度较高,如甘氨酸、亮氨酸、天冬酰胺、苏氨酸、异亮氨酸、丝氨酸、γ-氨基丁酸和酪氨酸等,而PI532726与冰糖脆中无变化曲线重合度较高的游离氨基酸,宁夏红籽瓜和冰糖脆中仅半胱氨酸含量的变化曲线重合度较高(图6)。进一步对所有样本的游离氨基酸数据进行PCA 分析,发现主成分1 将PI532726 和宁夏红籽瓜聚类在一起,将冰糖脆单独聚为一类(图7),表明PI532726 和宁夏红籽瓜果实中的游离氨基酸代谢网络和代谢形式可能更相似。
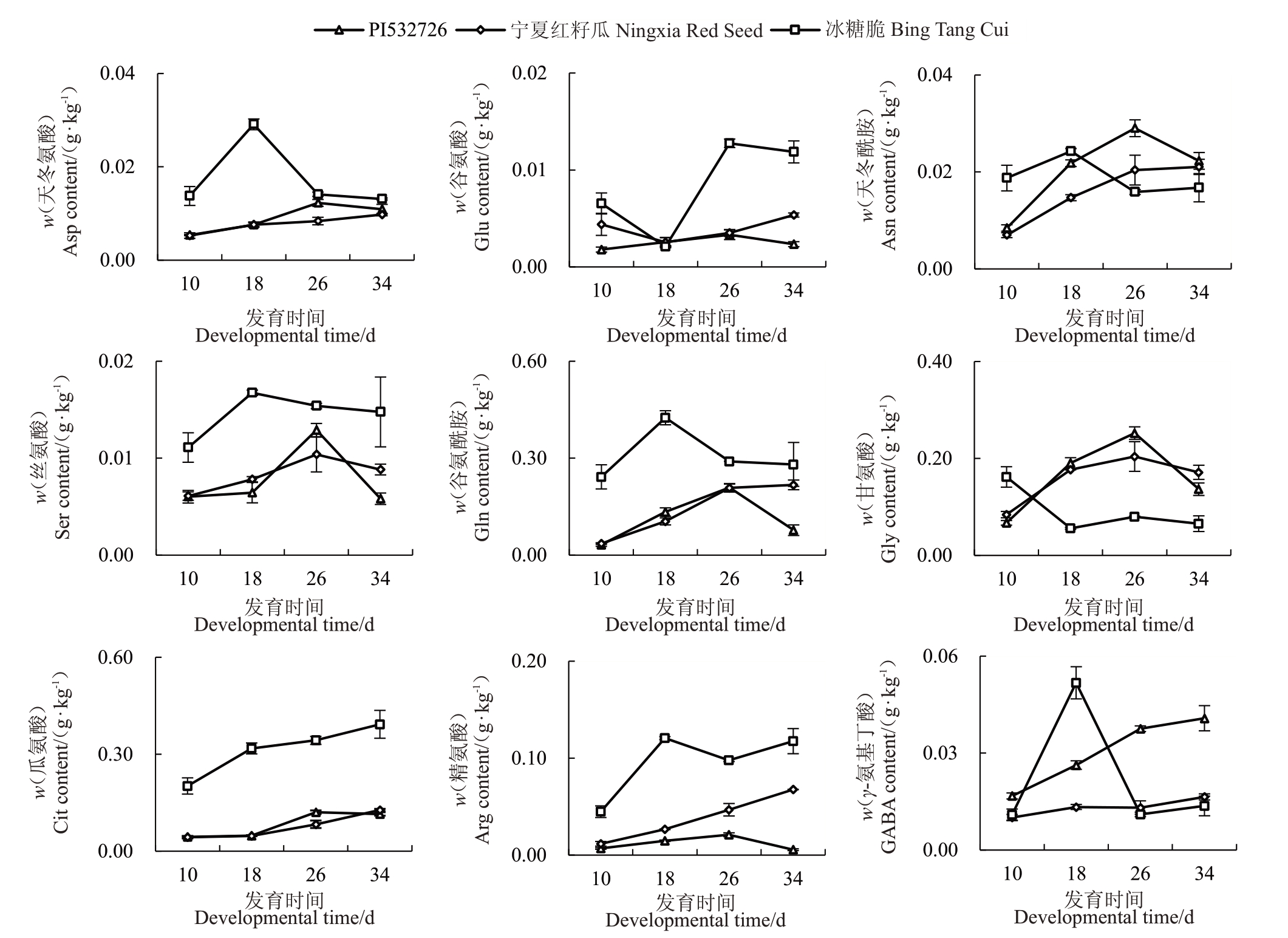
图6 3 个类型西瓜果实中各游离氨基酸不同时期的积累
Fig.6 Changes in free amino acids in fruits of three watermelon varieties at different periods

图6 (续) Fig.6 (Continued)
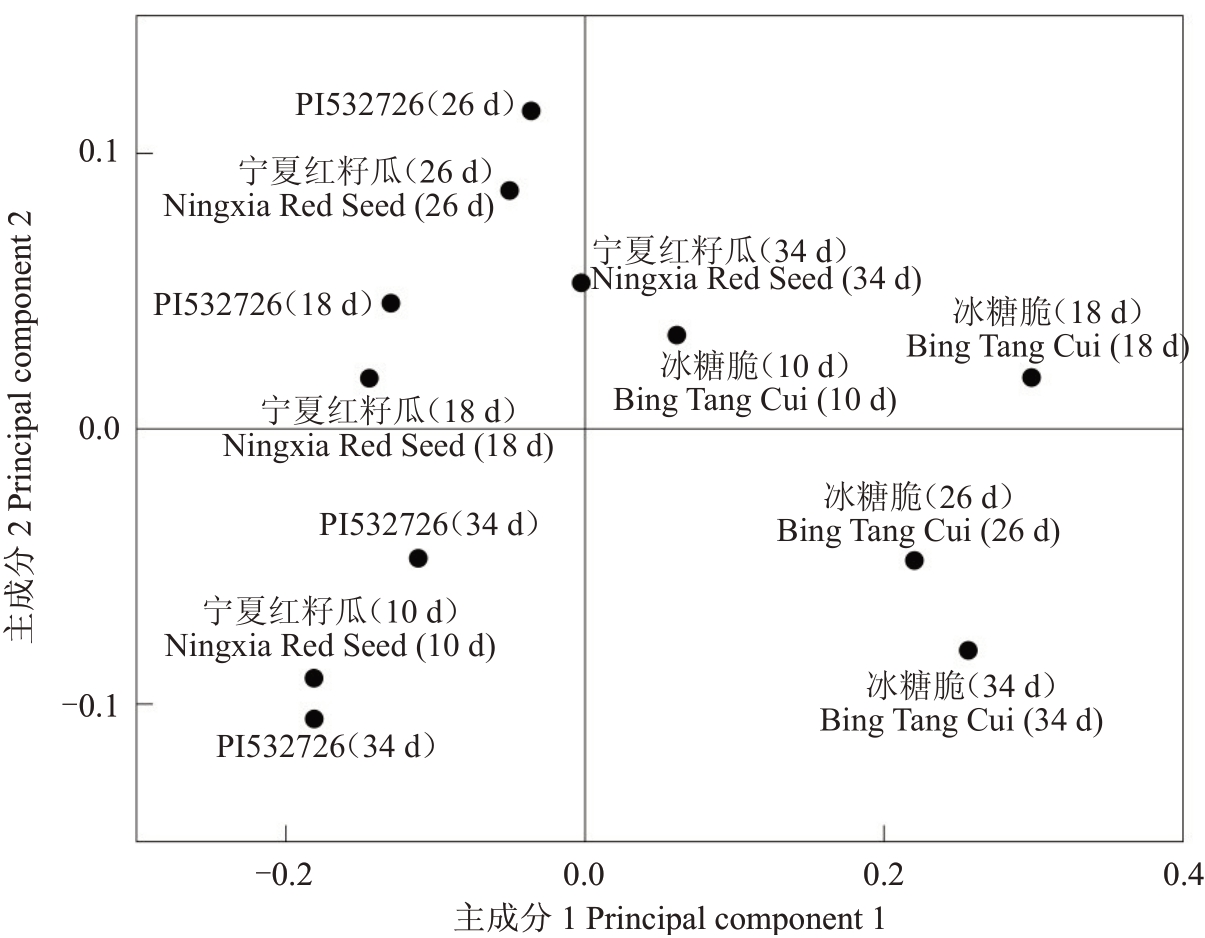
图7 3 个类型品种西瓜果实游离氨基酸不同时期PCA 得分图
Fig.7 PCA scores of free amino acids in three watermelon varieties at different periods
2.3 植物中的游离氨基酸代谢网络
3个类型西瓜品种果实中存在着特异的游离氨基酸,进一步将检测到的游离氨基酸代谢物放置于代谢途径中(图8)。通过代谢路径能够更清晰地看出,瓜氨酸、精氨酸和鸟氨酸直接参与了植物中的鸟氨酸循环,γ-氨基丁酸、酪氨酸和天冬酰胺等参与三羧酸循环,甘氨酸和谷氨酰胺则与多种游离氨基酸建立联系。其中,三羧酸循环是三大营养素(糖类、脂类、氨基酸)的最终代谢通路,又是糖类、脂类、氨基酸代谢联系的枢纽;鸟氨酸循环中间产物对植物的生长发育、生物与非生物胁迫起着重要的作用,也与植物氮素利用密切相关。将一些重要目标游离氨基酸放置于复杂的氨基酸代谢途径中,与其他游离氨基酸的代谢联系在一起,有助于了解不同品种西瓜中的游离氨基酸积累特性,对西瓜的选育和分类有着重要的指导意义,可为品种划分及定向育种提供参考。
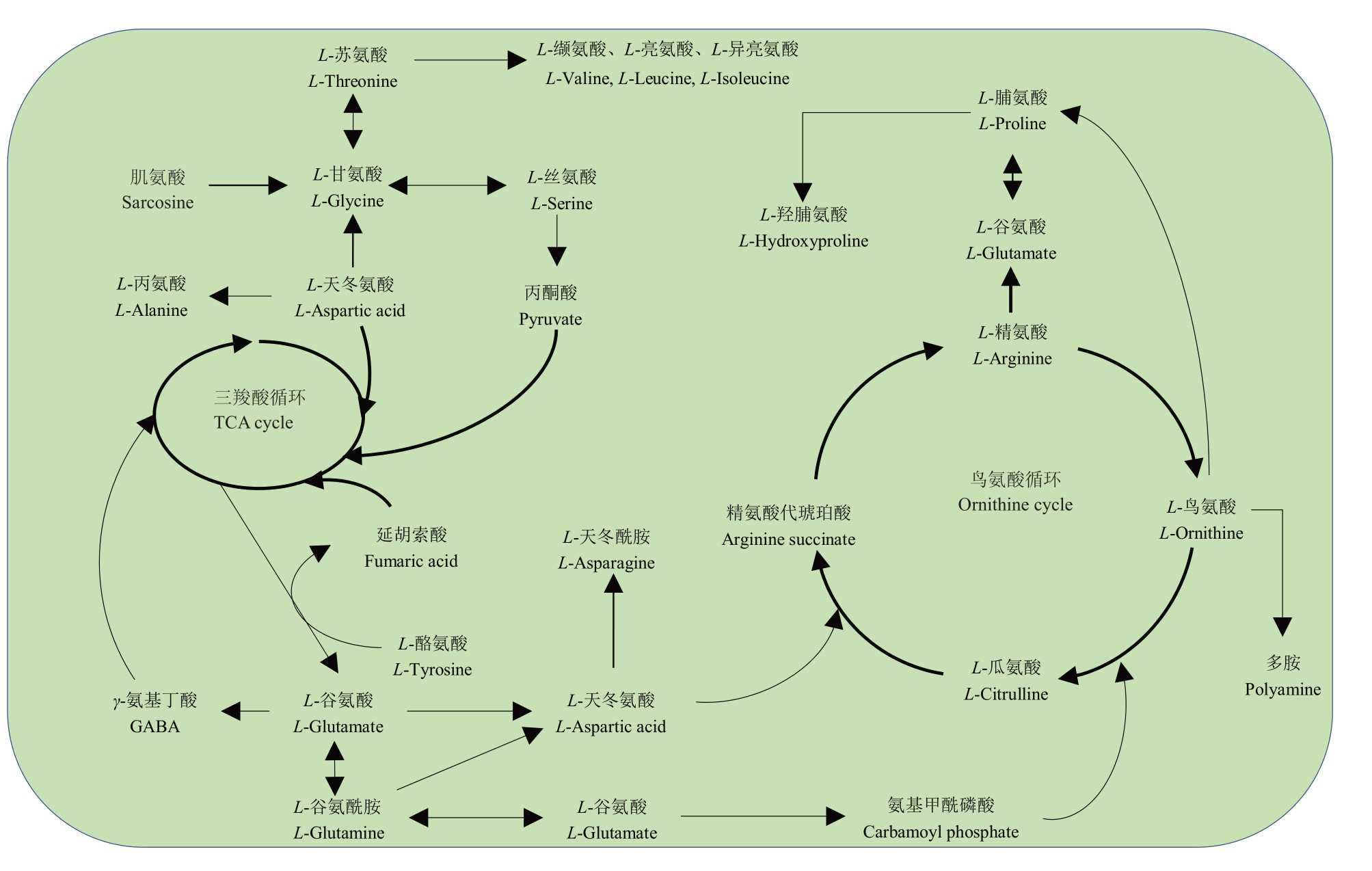
图8 西瓜果肉中重要游离氨基酸可能的生物代谢路径
Fig.8 Possible biological metabolism pathways of important free amino acids in the watermelon pulp
3 讨 论
游离氨基酸作为植物体内重要的组分之一,是植物体完成生命活动的必要保证,广泛参与植物发育及各种代谢途径。甘氨酸是植物体内重要氮源,同时对干旱、寒冷和化感胁迫均有缓解甚至抵消作用[7];谷氨酰胺作为植物体中重要氮源,可以分解为谷氨酸、天冬氨酸、丙酮酸、乳酸、丙氨酸和柠檬酸等,参与多种生化反应;瓜氨酸在植物的抗逆过程中起到重要的作用;精氨酸在植物中是重要的氮素储藏营养物,也是重要的信使分子多胺(polyamine,PA)和一氧化氮(NO)等合成的前体物质,参与植物生长发育、抗逆等在内的几乎所有生理生化过程[8];游离脯氨酸是细胞质中重要的渗透剂和防脱水剂,对植物体内的酶和膜结构有保护作用,能增强植株的抗旱性[24],目前多数研究都以测定叶片中游离脯氨酸的含量来研究植物的抗旱性[25];植物体内的赖氨酸和亮氨酸能够激发转氨酶和脱氢酶的活性[26];组氨酸与植物抵抗重金属胁迫有关[27];天冬酰胺具有螯合Cd、Pb和Zn的能力,能够降低这些重金属的毒性[28];半胱氨酸是甲硫氨酸、谷胱甘肽和植物螯合肽的合成前体物质,有助于植物抗氧化胁迫、抗重金属毒害、抵抗外界病原菌侵入和植物免疫[29-30]。游离氨基酸也可以抑制植物生长,如体外低微摩尔级的谷氨酸对拟南芥根系负向调控,形成主根短而分枝侧根多的根部形态[31];高浓度芳香族氨基酸(如苯丙氨酸、色氨酸、酪氨酸)会抑制拟南芥种子萌发和幼苗生长[32]。综上所述,游离氨基酸在植物体内的积累及代谢网络等是今后研究的重点。笔者在本研究中针对西瓜中游离氨基酸进行研究分析,对了解西瓜氨基酸的生理机制意义重大,研究中发现谷氨酰胺、瓜氨酸、甘氨酸和精氨酸含量很高,这可能与西瓜物种特异的代谢调节通路有密切的关系,对植物的生化与遗传机制研究有很大的参考价值。
游离氨基酸不仅在植物生长发育中发挥重要作用,同时也可以作为重要的营养和风味物质。甘氨酸在动物体中具有抗炎、免疫调节和细胞保护等作用[33];谷氨酰胺是人体中含量最丰富的循环性氨基酸,是免疫细胞基因表达的重要组成部分[34];瓜氨酸对治疗男性疾病有着很好的效果[18];精氨酸在人体多个器官系统中发挥重要作用[35]。游离氨基酸还能通过多种代谢途径形成有机酸、糖类、香气物质等风味营养物质[36-37]。草莓香气产生与缬氨酸有关[38];甜瓜[39-41]、草莓[42-43]、香蕉[44-45]、苹果[46]等果实中异亮氨酸、亮氨酸、缬氨酸和丙氨酸等与果实中重要风味物质酯类物质的形成密切相关;番茄中部分挥发性物质形成的前体是甘氨酸、缬氨酸、亮氨酸、异亮氨酸和苯丙氨酸等氨基酸[47];番木瓜的芳香组分中的酯类化合物由脂肪酸和氨基酸转化而来[48]。笔者在本研究中测得了多种游离氨基酸的含量,其中瓜氨酸、甘氨酸、谷氨酰胺和精氨酸含量高且都有很高的营养价值,也有很大的研究价值。本研究结果为西瓜中营养物种和风味物质的研究提供了参考。
不同种质类型西瓜的育种目标差异会造成果实表型的差异,代谢物也发生着变化,育种过程中可能很多代谢物被正向选择或被负向选择。研究表明,代谢组学可以作为表型和基因组的桥梁,放大表型或者基因组差异,可以帮助科研人员找到不同种质之间表型和基因差异之外的差异性状,探寻不同种质间的区别[49]。部分代谢物的变化甚至能够直接或者间接影响生物部分性状[50-51]。游离氨基酸代谢途径复杂且参与多种生化途径。本研究基于不同发育时期多种游离氨基酸的数据,显示籽用西瓜和普通西瓜在发育过程中游离氨基酸代谢物方面的明显差异,这为西瓜种质分类的研究提供了参考。在笔者研究的基础上,相关研究可以扩大代谢物的测定量,与基于植株表型和DNA 分子标记的植物分类鉴定相互补充,并结合基因组、转录组等数据分析西瓜不同栽培类群的关系,辅助西瓜分类;同时也可以参考本研究数据进一步在自然群体中对目标游离氨基酸进行检测,开展基于代谢组的全基因组关联分析(metabolome genome-wide association study,mGWAS),挖掘调控目标游离氨基酸积累的关键候选基因。通过代谢组学mGWAS分析和遗传图谱定位等方式对游离氨基酸的遗传机制进行进一步的研究,可以逐步解析西瓜果实游离氨基酸的生化和遗传机制,从而为代谢组学辅助西瓜育种奠定基础。
4 结 论
3个类型的西瓜果实中含有高含量的瓜氨酸、甘氨酸和谷氨酰胺(0.1~0.5 g·kg-1),且含量差异较大,精氨酸仅在冰糖脆中含量高(0.12 g·kg-1);PI532726和宁夏红籽瓜中游离氨基酸含量和积累模式相似,与冰糖脆差异显著;对多种游离氨基酸含量和积累模式结合分析可能在西瓜种质分类中发挥作用。
[1] 刘文革,何楠,赵胜杰,路绪强.我国西瓜品种选育研究进展[J].中国瓜菜,2016,29(1):1-7.LIU Wenge,HE Nan,ZHAO Shengjie,LU Xuqiang. Advances in watermelon breeding in China[J]. China Cucurbits and Vegetables,2016,29(1):1-7.
[2] ZHANG H Y,FAN J G,GUO S G,REN Y,GONG G Y,ZHANG J E,WENG Y Q,DAVIS A,XU Y. Genetic diversity,population structure,and formation of a core collection of 1197 Citrullus accessions[J].HortScience,2016,51(1):23-29.
[3] ACHIGAN-DAKO E G,AVOHOU E S,LINSOUSSI C,AHANCHEDE A,VODOUHE R S,BLATTNER F R. Phenetic characterization of Citrullus spp. (Cucurbitaceae) and differentiation of egusi-type (C. mucosospermus)[J]. Genetic Resources and Crop Evolution,2015,62(8):1159-1179.
[4] 陈菁菁,许勇,张建农,陈年来.我国籽用西瓜生产与研究进展[J].中国蔬菜,2015(12):12-18.CHEN Jingjing,XU Yong,ZHANG Jiannong,CHEN Nianlai.Production and research of seed using watermelon in China[J].China Vegetables,2015(12):12-18.
[5] 林淑敏,刘谨,刘彤,陶东,王诚忠.甘肃鲜食籽瓜产业开发前景[J].农业科技与信息,2011(21):9-11.LIN Shumin,LIU Jin,LIU Tong,TAO Dong,WANG Chengzhong. Development prospect of fresh edible seed melon industry in Gansu Province[J]. Agricultural Science-Technology and Information,2011(21):9-11.
[6] 袁平丽,何楠,赵胜杰,路绪强,朱红菊,刁卫楠,龚成胜,UMER M J,刘文革.籽瓜、黏籽和普通西瓜的果实代谢组比较[J].中国农业科学,2021,54(19):4179-4195.YUAN Pingli,HE Nan,ZHAO Shengjie,LU Xuqiang,ZHU Hongju,DIAO Weinan,GONG Chengsheng,UMER M J,LIU Wenge. Metabolomics comparative study on fruits of edible seed watermelon,egusi and common watermelon[J]. Scientia Agricultura Sinica,2021,54(19):4179-4195.
[7] 仇奕之,李宜珅,杨鹏军,张旭强,杨宁.甘氨酸对不同胁迫条件下高山离子芥试管苗的保护作用[J].兰州大学学报(自然科学版),2018,54(2):200-207.QIU Yizhi,LI Yishen,YANG Pengjun,ZHANG Xuqiang,YANG Ning. Protective effects of glycine on Chorispora bungeana plantlets under different stresses[J]. Journal of Lanzhou University(Natural Sciences),2018,54(2):200-207.
[8] 杨洪强,高华君.植物精氨酸及其代谢产物的生理功能[J].植物生理与分子生物学学报,2007,33(1):1-8.YANG Hongqiang,GAO Huajun.Physiological function of arginine and its metabolites in plants[J].Journal of Plant Physiology and Molecular Biology,2007,33(1):1-8.
[9] 万学闪,刘文革,阎志红,赵胜杰,何楠,刘鹏,代军委.西瓜果实发育过程中番茄红素、瓜氨酸和Vc 等功能物质含量的变化[J].中国农业科学,2011,44(13):2738-2747.WAN Xueshan,LIU Wenge,YAN Zhihong,ZHAO Shengjie,HE Nan,LIU Peng,DAI Junwei. Changes of the contents of functional substances including lycopene,citrulline and ascorbic acid during watermelon fruits development[J]. Scientia Agricultura Sinica,2011,44(13):2738-2747.
[10] 刘贤青,罗杰. 植物代谢组学技术研究进展[J]. 科技导报,2015,33(16):33-38.LIU Xianqing,LUO Jie.Advances of technologies and research in plant metabolomics[J]. Science &Technology Review,2015,33(16):33-38.
[11] CHEN W,GONG L,GUO Z L,WANG W S,ZHANG H Y,LIU X Q,YU S B,XIONG L Z,LUO J. A novel integrated method for large-scale detection,identification,and quantification of widely targeted metabolites:Application in the study of rice metabolomics[J].Molecular Plant,2013,6(6):1769-1780.
[12] WEI G,TIAN P,ZHANG F X,QIN H,MIAO H,CHEN Q W,HU Z Y,CAO L,WANG M J,GU X F,HUANG S W,CHEN M S,WANG G D. Integrative analyses of nontargeted volatile profiling and transcriptome data provide molecular insight into VOC diversity in cucumber plants (Cucumis sativus)[J]. Plant Physiology,2016,172(1):603-618.
[13] TIEMAN D,ZHU G T,RESENDE M F R,LIN T,NGUYEN C,BIES D,RAMBLA J L,BELTRAN K S O,TAYLOR M,ZHANG B,IKEDA H,LIU Z Y,FISHER J,ZEMACH I,MONFORTE A,ZAMIR D,GRANELL A,KIRST M,HUANG S W,KLEE H. A chemical genetic roadmap to improved tomato flavor[J].Science,2017,355(6323):391-394.
[14] OSORIO S,CARNEIRO R T,LYTOVCHENKO A,MCQUINN R,SØRENSEN I,VALLARINO J G,GIOVANNONI J J,FERNIE A R,ROSE J K C. Genetic and metabolic effects of ripening mutations and vine detachment on tomato fruit quality[J].Plant Biotechnology Journal,2020,18(1):106-118.
[15] WANG A M,LI R S,REN L,GAO X L,ZHANG Y G,MA Z M,MA D F,LUO Y H. A comparative metabolomics study of flavonoids in sweet potato with different flesh colors [Ipomoea batatas(L.)Lam.][J].Food Chemistry,2018,260:124-134.
[16] 张正海,曹亚从,于海龙,王立浩,张宝玺.辣椒果实主要品质性状遗传和代谢物组成研究进展[J].园艺学报,2019,46(9):1825-1841.ZHANG Zhenghai,CAO Yacong,YU Hailong,WANG Lihao,ZHANG Baoxi. Genetic control and metabolite composition of fruit quality in Capsicum[J].Acta Horticulturae Sinica,2019,46(9):1825-1841.
[17] 袁平丽,龚成胜,何楠,刁卫楠,UMER M J,朱红菊,杨东东,ANEES M,路绪强,KASEB M O,赵胜杰,刘文革.黏籽西瓜、籽瓜和普通西瓜的植物学性状比较分析[J].中国瓜菜,2021,34(9):15-25.YUAN Pingli,GONG Chengsheng,HE Nan,DIAO Weinan,UMER M J,ZHU Hongju,YANG Dongdong,ANEES M,LU Xuqiang,KASEB M O,ZHAO Shengjie,LIU Wenge.Comparative study on agronomic characters of egusi,edible-seed and common watermelon[J]. China Cucurbits and Vegetables,2021,34(9):15-25.
[18] 刘圆,齐红岩,王宝驹,郭亮,苏欣.不同品种甜瓜果实成熟过程中香气物质动态分析[J].华北农学报,2008,23(2):49-54.LIU Yuan,QI Hongyan,WANG Baoju,GUO Liang,SU Xin.Dynamic analysis of aromatic compounds in different cultivars of melon during the fruit ripeness[J].Acta Agriculturae Boreali-Sinica,2008,23(2):49-54.
[19] FISH W. The expression of citrulline and other members of the arginine metabolic family in developing watermelon fruit[J]. International Journal of Agriculture Innovations and Research,2014,2(5):665-672.
[20] ALI Aslam,王伟伟,何楠,路绪强,赵胜杰,朱红菊,刘文革.不同葫芦科作物中瓜氨酸含量的比较[J].中国瓜菜,2020,33(6):6-11.ASLAM A,WANG Weiwei,HE Nan,LU Xuqiang,ZHAO Shengjie,ZHU Hongju,LIU Wenge. Comparison of citrulline contents in different Cucurbitaceae crops[J]. China Cucurbits and Vegetables,2020,33(6):6-11.
[21] 李蒙蒙,路绪强,赵胜杰,何楠,刘文革.嫁接对西瓜果实瓜氨酸含量及合成途径关键酶基因表达的影响[J]. 果树学报,2019,36(7):857-865.LI Mengmeng,LU Xuqiang,ZHAO Shengjie,HE Nan,LIU Wenge. Effects of grafting on citrulline content and expression of key enzyme genes in watermelon fruits[J]. Journal of Fruit Science,2019,36(7):857-865.
[22] DAVIS A R,WEBBER C L,FISH W W,WEHNER T C,KING S,PERKINS-VEAZIE P.L-citrulline levels in watermelon cultigens tested in two environments[J]. HortScience,2011,46(12):1572-1575.
[23] ASSEFA A D,HUR O S,RO N Y,LEE J E,HWANG A J,KIM B S,RHEE J H,YI J Y,KIM J H,LEE H S,SUNG J S,KIM M K,NOH J J. Fruit morphology,citrulline,and arginine levels in diverse watermelon (Citrullus lanatus) germplasm collections[J].Plants,2020,9(9):1054.
[24] 王邦锡,黄久常,王辉,王辉.不同植物在水分胁迫条件下脯氨酸的累积与抗旱性的关系[J].植物生理学报,1989,15(1):46-51.WANG Bangxi,HUANG Jiuchang,WANG Hui,WANG Hui.The correlation of proline accumulation and drought resistance in various plants under water stress condition[J].Physiology and Molecular Biology of Plants,1989,15(1):46-51.
[25] 张国盛.干旱、半干旱地区乔灌木树种耐旱性及林地水分动态研究进展[J].中国沙漠,2000,20(4):363-368.ZHANG Guosheng. Research progress on trees and shrub drought-resistance and woodland water activity in arid and semirid region[J].Journal of Desert Research,2000,20(4):363-368.
[26] 郑易之,范文静,刘科,刘昀,刘国宝.大豆ASR 蛋白富含组氨酸结构域在结合金属离子中的作用[J].华南师范大学学报(自然科学版),2015,47(5):91-98.ZHENG Yizhi,FAN Wenjing,LIU Ke,LIU Yun,LIU Guobao.The role of soybean ASR protein histidine-rich domain on binding metal ions[J]. Journal of South China Normal University(Natural Science Edition),2015,47(5):91-98.
[27] KRÄMER U,COTTER-HOWELLS J D,CHARNOCK J M,BAKER A J M,SMITH J A C.Free histidine as a metal chelator in plants that accumulate nickel[J]. Nature,1996,379(6566):635-638.
[28] DOMÍNGUEZ-SOLÍS J R,LÓPEZ-MARTÍN M C,AGER F J,YNSA M D,ROMERO L C,GOTOR C. Increased cysteine availability is essential for cadmium tolerance and accumulation in Arabidopsis thaliana[J]. Plant Biotechnology Journal,2004,2(6):469-476.
[29] NING H X,ZHANG C H,YAO Y,YU D Y. Overexpression of a soybean O- acetylserine (thiol) lyase- encoding gene GmOASTL4 in tobacco increases cysteine levels and enhances tolerance to cadmium stress[J]. Biotechnology Letters,2010,32(4):557-564.
[30] ÁLVAREZ C,ÁNGELES BERMÚDEZ M,ROMERO L C,GOTOR C,GARCÍA I.Cysteine homeostasis plays an essential role in plant immunity[J].New Phytologist,2012,193(1):165-177.
[31] WALCH-LIU P,LIU L H,REMANS T,TESTER M,FORDE B G. Evidence that l-glutamate can act as an exogenous signal to modulate root growth and branching in Arabidopsis thaliana[J].Plant and Cell Physiology,2006,47(8):1045-1057.
[32] VOLL L M,ALLAIRE E E,FIENE G,WEBER A P M.The Arabidopsis phenylalanine insensitive growth mutant exhibits a deregulated amino acid metabolism[J].Plant Physiology,2004,136(2):3058-3069.
[33] MCCARTY M F,O'KEEFE J H,DINICOLANTONIO J J. Dietary glycine is rate-limiting for glutathione synthesis and may have broad potential for health protection[J]. The Ochsner Journal,2018,18(1):81-87.
[34] PIMENTEL R F W,FERNANDES S L. Effects of parenteral glutamine in critically ill surgical patients:A systematic review and meta-analysis[J]. Nutrición Hospitalaria,2020,34(3):616-621.
[35] 朱重阳,李鑫,温泉,张军.L-精氨酸对人肝细胞中内源凝血因子Ⅷ表达的激活作用[J].中国生物制品学杂志,2012,25(10):1303-1306.ZHU Chongyang,LI Xin,WEN Quan,ZHANG Jun. L-arginine activates expression of endogenous coagulation factor Ⅷin human normal liver cells[J].Chinese Journal of Biologicals,2012,25(10):1303-1306.
[36] 王莹,史振声,王志斌,李凤海.植物对氨基酸的吸收利用及氨基酸在农业中的应用[J].中国土壤与肥料,2008(1):6-11.WANG Ying,SHI Zhensheng,WANG Zhibin,LI Fenghai. Absorption and utilization of amino acids by plant and application of amino acids on agiculture[J]. Soil and Fertilizer Sciences in China,2008(1):6-11.
[37] LEWINSOHN E,SITRIT Y,BAR E,AZULAY Y,IBDAH M,MEIR A,YOSEF E,ZAMIR D,TADMOR Y. Not just colors:carotenoid degradation as a link between pigmentation and aroma in tomato and watermelon fruit[J]. Trends in Food Science&Technology,2005,16(9):407-415.
[38] PÉREZ A G,SANZ C,OLÍAS R,RÍOS J J,OLÍAS J M.Evolution of strawberry alcohol acyltransferase activity during fruit development and storage[J]. Journal of Agricultural and Food Chemistry,1996,44(10):3286-3290.
[39] 齐红岩,关小川,李岩,李金燃,邱丽妍.嫁接对薄皮甜瓜果皮和果肉中主要酯类、游离氨基酸及酯类合成相关酶活性的影响[J].中国农业科学,2010,43(9):1895-1903.QI Hongyan,GUAN Xiaochuan,LI Yan,LI Jinran,QIU Liyan.Effects of grafting on main esters,free amino acids content and related enzyme activities in oriental sweet melon peel and flesh tissues[J].Scientia Agricultura Sinica,2010,43(9):1895-1903.
[40] 唐贵敏,于喜艳,赵登超,王利平.不同品种厚皮甜瓜果实成熟过程中挥发性物质成分分析[J].中国蔬菜,2007(4):7-11.TANG Guimin,YU Xiyan,ZHAO Dengchao,WANG Liping.GC-MS analysis of volative components in different cultivars of muskmelon during fruit development[J].China Vegetables,2007(4):7-11.
[41] 王宝驹,齐红岩,刘圆,王佳辉.薄皮甜瓜果实不同部位中的挥发性酯类物质与氨基酸的关系[J].植物生理学通讯,2008,44(2):215-220.WANG Baoju,QI Hongyan,LIU Yuan,WANG Jiahui.The relationship between volatile esters and free amino acids in different parts of ripe melon (Cucumis melo L.)[J]. Plant Physiology Communications,2008,44(2):215-220.
[42] 乜兰春,孙建设,黄瑞虹.果实香气形成及其影响因素[J].植物学通报,2004,39(5):631-637.NIE Lanchun,SUN Jianshe,HUANG Ruihong. The biosynthesis and affecting factors of aroma in some fruits[J].Chinese Bulletin of Botany,2004,39(5):631-637.
[43] PÉREZ A G,OLÍAS R,LUACES P,SANZ C. Biosynthesis of strawberry aroma compounds through amino acid metabolism[J].Journal of Agricultural and Food Chemistry,2002,50(14):4037-4042.
[44] WYLLIE S G,FELLMAN J K. Formation of volatile branched chain esters in bananas(Musa sapientum L.)[J].Journal of Agricultural and Food Chemistry,2000,48(8):3493-3496.
[45] TRESSL R,DRAWERT F. Biogenesis of banana volatiles[J].Journal of Agricultural and Food Chemistry,1973,21(4):560-565.
[46] MATICH A,ROWAN D.Pathway analysis of branched-chain ester biosynthesis in apple using deuterium labeling and enantioselective gas chromatography-mass spectrometry[J].Journal of Agricultural and Food Chemistry,2007,55(7):2727-2735.
[47] BUTTERY R G. Quantitative and sensory aspects of flavour of tomato and other vegetables and fruits[M]//ACREE T E,TERANISHI R. Flavor science:Sensible principles and techniques.New York:ACS Professional Reference Book,1993:259-286.
[48] BALBONTÍN C,GAETE-EASTMAN C,VERGARA M,HERRERA R,MOYA-LEON M A. Treatment with 1-MCP and the role of ethylene in aroma development of mountain papaya fruit[J]. Postharvest Biology and Technology,2007,43(1):67-77.
[49] LUO J. Metabolite-based genome-wide association studies in plants[J].Current Opinion in Plant Biology,2015,24:31-38.
[50] BERNILLON S,BIAIS B,DEBORDE C,MAUCOURT M,CABASSON C,GIBON Y,HANSEN T H,HUSTED S,DE VOS R C H,MUMM R,JONKER H,WARD J L,MILLER S J,BAKER J M,BURGER J,TADMOR Y,BEALE M H,SCHJOERRING J K,SCHAFFER A A,ROLIN D,HALL R D,MOING A. Metabolomic and elemental profiling of melon fruit quality as affected by genotype and environment[J]. Metabolomics,2013,9(1):57-77.
[51] VAN TREUREN R,VAN EEKELEN H D L M,WEHRENS R,DE VOS R C H. Metabolite variation in the lettuce gene pool:Towards healthier crop varieties and food[J]. Metabolomics,2018,14(11):146.