油梨(Persea americana Mill.)又称鳄梨、酪梨、牛油果等,为樟科(Lauraceae)鳄梨属(Persea)速生常绿乔木果树,原产于中美洲、南美洲热带及亚热带地区。油梨的果肉脂肪含量高,糖分含量低,没有胆固醇,并含有大量的单不饱和脂肪酸、富含多种维生素和矿物质,因此被称为“完美的水果”[1-2]。1918年油梨被引进中国台湾,目前在海南、广东、广西、福建、云南、四川、浙江、贵州、湖南等地均有种植[3]。近年来,中国油梨种植面积和产量增幅较大,在2010年以后的10年里,中国油梨种植面积和产量分别以1.52%和1.31%的年均增长率稳步增长,2020年中国油梨栽培面积和生产量分别为1.8 万hm2和11.7万t(https://www.fao.org/faostat/zh/#data/QCL)。
随着种植面积的逐年扩大,病害的发生日趋严重,病害导致果实产量和品质下降,成为阻碍中国油梨产业快速发展的重要因素之一。目前,国内外已报道引起油梨叶片和果实的真菌病害有Colletotrichum fructicola[4]、C. siamense[5]、C. karstii[6]、C. kahawae subsp.ciggaro[7]、Glomerella acutata[8]等引起的炭疽病;Corynespora cassiicola[9]引起的叶斑病;Neofusicoccum luteum[10]、Lasiodiplodia theobromae、N.parvum[11]、N. australe[12]、Pestalotiopsis spp.、P. clavispora[13]、Diaporthe rudis[14]引 起 的 蒂 腐 病;Erysiphe sp.[15]、Podosphaera perseae-americanae[16]引 起 的 白粉病;Trichothecium roseum[12]引起的粉腐病;Phytophthora cactorum[17]、N. mangiferae[18]、N. parvumand、Botryosphaeria dothidea[19]引起的果腐病;N. parvum[20]、Pseudocercospora purpurea[21]引 起 的 黑 斑病。其中,炭疽病是引起油梨叶片和果实最广泛和最严重的病害之一。
2020年10月3日,笔者在海南省白沙县和儋州市的两个哈斯油梨种植基地进行炭疽病病害调查,并将具有典型病状的油梨叶片和果实带回实验室,对病害样本进行病原菌分离和纯化后,利用柯赫氏法则进行致病性测定,并结合形态学和分子生物学对病原菌的种类进行鉴定,以明确引起白沙县和儋州市油梨产区炭疽病的病原菌,以期为该病害的诊断以及田间防控措施的制定提供理论依据。
1 材料和方法
1.1 病株采集
表现有典型炭疽病的哈斯品种油梨叶片和果实采自海南省白沙黎族自治县大岭农场附近的某种植基地(109°6′14.076″E,19°26′37.464″N)和儋州市南辰农场(109°29′32.856″E,19°29′36.960″N),采集时间为2020年10月3日。
1.2 试验试剂
DNA 快速抽提试剂盒(OMEGA BIO-TEK)、DNA 片段回收试剂盒、Taq DNA 聚合酶、DL2000 Marker 和通用引物(表1)[22]。马铃薯葡萄糖培养基(PDA)于121 ℃高压灭菌20 min后备用。
表1 研究使用的引物
Table 1 Primers used in this study
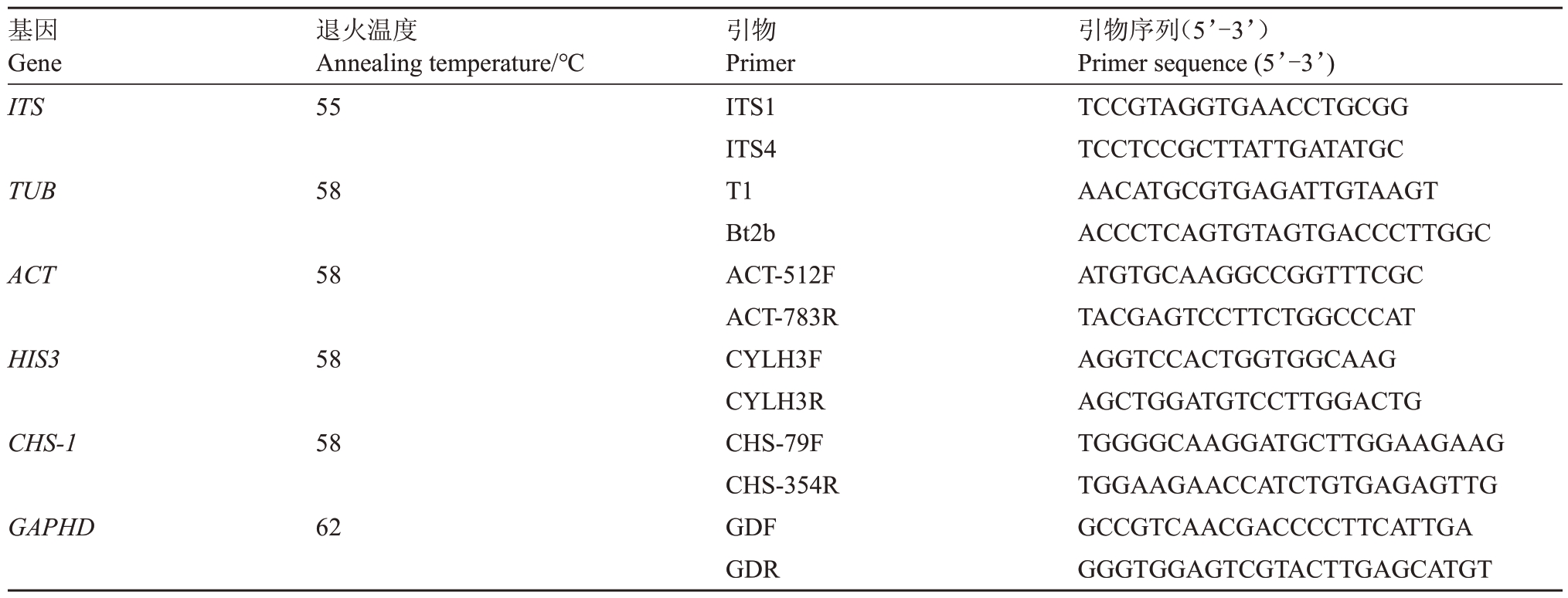
引物Primer ITS1 ITS4 T1 Bt2b ACT-512F ACT-783R CYLH3F CYLH3R CHS-79F CHS-354R GDF GDR基因Gene ITS退火温度Annealing temperature/℃55 TUB 58 ACT 58 HIS3 58 CHS-1 58 GAPHD 62引物序列(5’-3’)Primer sequence(5’-3’)TCCGTAGGTGAACCTGCGG TCCTCCGCTTATTGATATGC AACATGCGTGAGATTGTAAGT ACCCTCAGTGTAGTGACCCTTGGC ATGTGCAAGGCCGGTTTCGC TACGAGTCCTTCTGGCCCAT AGGTCCACTGGTGGCAAG AGCTGGATGTCCTTGGACTG TGGGGCAAGGATGCTTGGAAGAAG TGGAAGAACCATCTGTGAGAGTTG GCCGTCAACGACCCCTTCATTGA GGGTGGAGTCGTACTTGAGCATGT
1.3 菌株的分离及纯化
根据组织分离法,选取具有典型症状的发病叶片和果实,用自来水清洗干净,自然晾干,用无菌剪刀于叶片病健交界处剪取5 mm2组织块,用无菌手术刀取病健交界处5 mm2大小的果皮块。先用75%乙醇对叶片组织块和果皮块分别消毒20 s 和30 s,2% NaClO 消毒1 min 和3 min,再用无菌水分别清洗3次(每次30 s),置于PDA培养基上,每皿4个组织块或果皮块,3 次重复。28 ℃光照培养3 d,用无菌接种针挑取菌丝尖端进行纯化培养。待产孢后挑取单孢获得纯化培养菌株,将纯化的菌株转接于PDA斜面培养基,于28 ℃保存备用。
1.4 致病性测定
采用刺伤或无伤接种方式,供试菌株为HNBSL01~03、HNDZL02~07 和HNBSF03,接种材料为健康的5龄哈斯油梨树上的叶片和健康的离体哈斯油梨果实。分离菌株在PDA 培养基上28 ℃、连续光照培养5 d后,在菌落边缘打取直径为5 mm的菌饼,将HNBSL01~03和HNDZL02~07的菌饼贴在无菌针刺伤的叶片上,表面覆盖无菌吸水纸,保湿7 d,每个处理3 枚叶片,每枚叶片设置8 个接种点,3 次重复,以接种空白PDA培养基作为对照[9];待菌株产孢后配制1×106个·mL-1的孢子悬浮液,将20 μL 孢子悬浮液均匀喷洒在无伤叶片上,表面覆盖无菌吸水纸,保湿7 d,每个处理3枚叶片,3次重复,以接种20 μL 无菌水作为对照[23];将从菌株HNBSF03 打取的菌饼贴在无菌针刺伤的果实上,表面覆盖无菌吸水纸,保湿7 d,每个处理3 个果实,每个果实设置2个接种点,3次重复,以接种空白PDA培养基作为对照[5]。待出现相同症状后,参考1.3从病斑处重新取样分离并纯化。
1.5 病原菌形态特征的观察
将纯化后的病原菌转接到PDA 培养基上,25 ℃12 h光暗交替培养。7 d后观察并记录菌落的形态、颜色、产孢情况和菌丝生长情况。待菌落产孢后,挑取培养物在显微镜下观察分生孢子,记录其形态特征并测量分生孢子的大小。采用玻片萌发法诱导附着胞,配制1×104个·mL-1浓度的孢子悬浮液,吸取少量悬浮液滴在无菌载玻片上,于28 ℃下保湿培养,24 h后观察并记录附着胞的形态。
1.6 分子生物学鉴定
用无菌药匙刮取纯化后菌株的气生菌丝100 mg,用真菌基因组DNA 快速抽提试剂盒(OMEGA BIO-TEK)提取菌株的基因组DNA。分别对病原菌的核糖体内转录间隔区(internal transcribed spacer,ITS)、肌动蛋白基因(actin,ACT)、β-微管蛋白基因(β-tubulin,TUB2)、几丁质合成酶A 基因(chitin synthetase A,CHS-1)、3-磷酸甘油醛脱氢酶基因(glyceraldehydes-3-phosphate dehydrogenase,GAPDH)和组蛋白基因(histone3,HIS3)进行PCR 扩增(表1)[22]。PCR反应体系体积均为25 μL,包括DNA模板1.0 µL,2×Es Taq MasterMix (Dye) 12.5 µL,上下引物各1.0 µL,ddH2O 9.5 µL。94 ℃变性2 min,各引物按表1 中相应的退火温度退火30 s,72 ℃延伸30 s,共35 个循环,最后72 ℃延伸2 min。用1%琼脂糖凝胶电泳检测扩增产物,检测到目的片段后委托生工生物工程(上海)股份有限公司完成测序。将获得的基因序列提交至GenBank 数据库,并获得序列号。通过BLAST 比对搜索从GenBank 数据库中下载其他炭疽菌属菌株序列。使用SequenceMatrix 软件按照ITS-ACT-TUB2-CHS-1-GAPHD-HIS3 顺序进行序列拼接,使用MEGA 7.0选择最大似然法(maximum likelihood method,ML)和T92+G+I 核苷酸替代模型构建系统发育树,以自展法(bootstrap)重复1000 次检测系统树中节点的置信度[24]。
2 结果与分析
2.1 田间症状描述
2020年10月,在海南省白沙黎族自治县大岭农场 附 近 的 某 种 植 基 地(109°6′14.076″ E,19°26′37.464″N)发现油梨叶片炭疽病病株。发病主要从叶片的叶缘或叶尖出现点状或连接成片的褪绿病斑;发病中期,叶缘或叶尖病斑逐渐由淡黄色发展为褐色,病斑边缘深褐色且伴有黄色晕圈;后期叶尖或叶缘部分呈大面积灰褐色坏死病斑,病健交界处颜色加深且有黄色晕圈(图1-A~B)。在儋州市南辰农场(109°29′32.856″ E,19°29′36.960″ N)发现油梨叶片炭疽病病株。油梨叶片炭疽病病株从叶片上出现近圆形或不规则形的褪绿小病斑,病斑中心呈黄色,外缘为淡黄色;发病中期,病斑中央呈深棕色,外缘颜色较浅为棕色,且具淡黄色晕圈;后期病部中央出现大面积棕色坏死病斑,且有大量散生或轮生的小黑点出现,并有明显同心轮纹,外缘为深棕色(图1-C~D)。成熟果实发病初期,果实表面出现浅棕色圆形或近圆形的小病斑;随着病斑不断扩大,病斑中心略有凹陷,中央颜色为黑色,边缘深棕色;后期果实出现大面积的坏死病斑,且病斑的凹陷内产生大量黏稠状橘红色分生孢子堆,有白色霉层覆盖,最终导致整个果实腐烂(图1-E~F)。

图1 油梨炭疽病在叶片和果实上的症状
Fig.1 Symptoms of anthracnose of avocado on leaves and fruit
A.叶片上的症状;B.叶尖和叶缘发病的症状;C.叶片中央发病的症状;D.叶片中央发病的症状(示病斑呈轮纹状);E.成熟果实上的症状(示白色的霉层和橘红色的孢子堆);F.白色的霉层和橘红色的孢子堆(放大)。
A. Symptoms on the leaves; B. Symptoms on leaf tips and margins; C. Symptoms on central leaves; D. Symptoms on central leaves (Whorled spots);E.Symptoms on mature fruit(White mold layer and orange conidiomata);F.White mold layer and orange conidiomata(Amplification).
2.2 菌株的分离与致病性测定
从采自白沙市具典型病斑的14 份病叶中分离出3 株真菌菌株(HNBSL01~03),自儋州市采集的10 份病叶中分离获得4 株菌株(HNDZL02~07),从白沙市采集的具典型炭疽病病斑的5 份病果中分离出菌株HNBSF03。采用刺伤和无伤接种的方法将这些菌株分别接种到活体健康的油梨叶片和健康果实上。刺伤接种HNBSL01 菌株的菌饼2 d 后,接种叶片开始发病,接种点出现黄棕色病斑,边缘有淡黄色晕圈,并逐渐向外圈扩展;10 d 后,病斑变为深褐色,并且病斑上有黏稠状橘红色分生孢子堆产生,与田间症状相同(图2-A~B)。对照组均未发病(图2-C);无伤接种HNBSL01 菌株的孢子悬浮液2 d 后,接种叶片开始发病,叶片边缘出现褐色病斑,并逐渐向外扩展;10 d 后,病斑变为深褐色且边缘变为黑色,与田间症状相同(图2-D~E)。对照组均未发病(图2-F)。从发病部位再次分离纯化,获得了与HNBSL01 形态相同的菌株。而接种HNBSL02 和HNBSL03 菌株的叶片均未发病。经柯赫氏法则检验,菌株HNBSL01 为油梨叶片炭疽病的致病菌。刺伤接种HNDZL02 菌饼第3天,接种部位出现浅褐色小病斑,并有黄色晕圈,随后病斑逐渐扩大,10 d 后,病斑呈深褐色并产生黏稠状橙色分生孢子堆,边缘具淡黄色晕圈,与田间症状相同(图2-D~E)。对照组均未见任何症状(图2-F)。从病斑处进行再分离纯化,获得与HNDZL02形态相同的菌株,而接种HNDZL03~07菌株叶片均未发病,说明HNDZL02为油梨叶片炭疽病的病原菌。刺伤接种HNBSF03 菌株2 d 后,接种果实出现浅棕色病斑并逐渐向四周扩展,7 d后,病斑中央略凹陷且有大量黏稠状粉红色分生孢子堆产生,病部呈深棕色,边缘棕色,与田间症状相一致(图2-G~H)。对照组果实均未发病(图2-I)。对发病组织进行再分离并纯化后,得到与HNBSF03 形态相同的菌株,根据柯赫氏法则,HNBSF03为油梨果实炭疽病的病原菌。

图2 油梨叶片和果实炭疽病的致病性测定
Fig.2 The pathogenicity of anthracnose on leaves and fruits of avocado
A.接种菌株HNBSL01 第10 天的叶片症状;B.病斑产生分生孢子堆;D、E.接种HNBSL01 孢子悬浮液第10 天的叶片症状;G.接种菌株HNDZL02 第10 天的叶片症状;H. 病斑产生分生孢子堆;J. 接种菌株HNBSF03 第7 天的果实症状;K.病斑产生分生孢子堆;C、F、I、L.PDA空白对照。
A. Leaf symptoms on day 10 of inoculated strain HNBSL01; B. Conidiomata on leaves; D、E. No wounding inoculated conidial suspension of strain HNBSL01 on day 10. G. Leaf symptoms on day 10 of inoculated strain HNDZL02; H. Conidiomata on leaves; J. Fruit symptoms on day 7 of inoculated strain HNBSF03;K.Conidiomata on fruit;C,F,I,L.Control.
2.3 菌株培养性状
将HNBSL01、HNDZL02 和HNBSF03 菌 株 在PDA培养基上于25 ℃12 h光暗交替环境下培养7 d,结果显示,HNBSL01菌株菌落白色,边缘整齐,菌落直径为68~70 mm(¯x=69 mm),菌丝浓密,气生菌丝发达,菌落正面的颜色为白色,背面的颜色为象牙白色,菌株未产生分生孢子堆(图3-A1),30 d 后菌丝层表面产生大量橘黄色黏稠状分生孢子堆,散生或成簇聚集(图3-A2);HNDZL02菌株菌落白色,边缘整齐,菌落直径为70~72 mm(¯x=71 mm),菌丝浓密,气生菌丝发达,菌落正面靠近菌饼部分的颜色为黛绿色、外缘为白色,背面中心39~42 mm的颜色为鸦青色、外缘为象牙白色,菌株未产生分生孢子堆(图3-B1),30 d后菌丝层表面产生大量姜黄色黏稠状分生孢子堆,散生或成簇聚集(图3-B2);HNDZF03菌株菌落白色,边缘整齐,菌落直径为77~79 mm(¯x=78 mm),菌丝浓密,气生菌丝发达,菌落正面菌饼周围略带墨灰色的颜色、其余部分为白色,背面靠近菌饼24~26 mm处的颜色为竹青色、外缘为象牙白色,菌株未产生分生孢子堆(图3-C1),30 d 后菌丝层表面产生大量橘红色黏稠状分生孢子堆,散生或成簇聚集(图3-C2)。
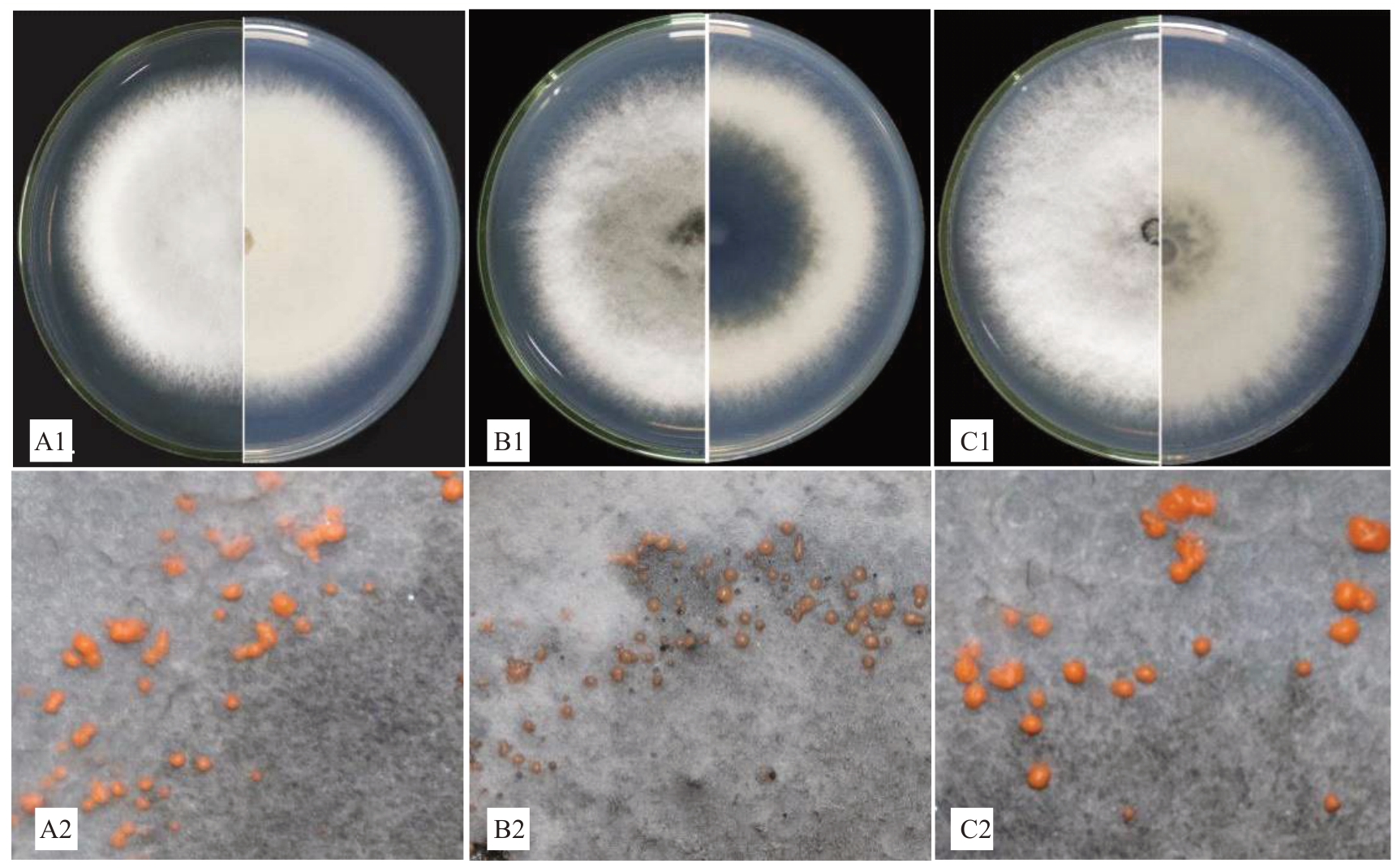
图3 油梨炭疽病菌的菌落
Fig.3 Colonies of pathogen on avocado anthracnose
A1.HNBSL01 的菌落(7 d,左:正面;右:背面);A2.HNBSL01 的孢子堆(30 d);B1.HNDZL02 的菌落(7 d,左:正面;右:背面);B2.HNDZL02 的孢子堆(30 d);C1.HNBSF03 的菌落(7 d,左:正面;右:背面);C2.HNDZL03 的孢子堆(30 d)。
A1. colonies of HNBSL01 (7 d, Left: Front; Right: Back); A2. Conidiomata of HNBSL01 (30 d); B1. colonies of HNDZL02 (7 d, Left: Front;Right:Back);B2.Conidiomata of HNDZL02(30 d);C1.colony of HNBSF03(7 d,Left:Front;Right:Back);C2.Conidiomata of HNDZL03(30 d).
2.4 菌株形态特征
菌株HNBSL01 的分生孢子无色,单胞,直或稍弯曲,圆柱状,两端钝圆或一端钝圆另一端渐尖,含油球,壁薄且表面光滑,大小为14.11~16.97(15.61)μm×3.74~4.89(4.32)μm(n=100),长宽比为3.47~3.77(图4-A1~A2);附着胞浅褐色至褐色,棍棒状、椭圆形、近球形或球形,全缘,壁厚,大小为7.73~10.24(9.03)μm×4.37~6.16(5.15)μm(n=100)(图4-A3~A4);菌株HNDZL02 的分生孢子无色,单胞,直,短圆柱状,两端钝圆,不含油球,薄壁,表面光滑,大小为13.84~16.96(15.67)μm×5.38~6.52(5.97)μm(n=100),长宽比为2.57~2.60(图4-B1~B2);附着胞浅棕色至棕色,椭圆形或不规则形,全缘或具钝锯齿状裂片,壁厚,大小为9.95~13.76(11.88)μm×4.82~6.22(5.53)μm(n=100)(图4-B3~B4);菌株HNBSF03 的分生孢子无色,单胞,直或略弯,棍棒状,顶端渐尖,基部钝圆,不含油球,薄壁,光滑,大小为13.77~17.65(15.49)μm×4.15~5.47(4.66)μm(n=100),长宽比为3.23~3.32(图4-C1~C2);附着胞褐色至深褐色,卵圆形至圆形,全缘,壁厚,大小为6.65~9.78(8.38)μm×4.95~6.91(6.25)μm(n=100)(图4-C3~C4)。根据上述形态学特征,菌株HNBSL01 和HNDZL02 与胶孢炭疽菌(Colletotrichum gloeosporioides complexes)复合种相似[25],HNBSF03 与C. coelogynes 和 长 直孢炭疽菌(C. gigasporum complexes)复合种相似[26-27]。

图4 病原菌(菌株HNBSL01、HNDZL02 和HNBSF03)的形态特征
Fig.4 Morphological characteristics of the pathogens(strains HNBSL01,HNDZL02 and HNBSF03)
A1、A2.HNBSL01 分生孢子;A3、A4.HNBSL01 附着胞;B1、B2.HNDZL02 分生孢子;B3、B4.HNDZL02 附着胞;C1、C2.HNDZL03 分生孢子;C3、C4.HNDZL03 附着胞;标尺=10 μm。
A1,A2.Conidia of HNBSL01;A3,A4.Appressoria of HNBSL01;B1,B2.Conidia of HNDZL02;B3,B4.Appressoria of HNDZL02;C1,C2.Conidia of HNDZL03;C3,C4.Appressoria of HNDZL03;Bars=10 μm.
2.5 病原菌分子生物学鉴定
提取病原菌基因组DNA并对ITS、ACT、TUB2、CHS-1、GAPHD和HIS3序列片段进行PCR扩增,菌株HNBSL01 得到片段大小分别为548、252、728、280、252 和383 bp;HNDZL02 得到的片段大小分别为550、251、734、278、253 和388 bp;HNBSF03 获得的片段大小分别为534、249、745、276、270和384 bp。将获得的基因片段在GenBank中进行BLAST分析,并下载与3 个菌株的6 个基因片段相似度达95%以上的序列,以Monilochaetes infuscans CBS 869.96 作为外群,对6 个基因序列联合构建系统发育树(表2)。结果表明:菌株HNBSL01 与C. siamense 聚为一个进化分支,自举支持率为81%;菌株HNDZL02与C. fructicola 聚为一个进化分支,自举支持率为100%;菌株HNBSF03 与C.gigasporum 聚为一个进化分支,自举支持率为100%(图5)。因此,根据形态学鉴定和系统发育分析,将引起白沙市油梨叶片炭疽病的病原菌鉴定为C.siamense;引起儋州市油梨叶片炭疽病的病原菌鉴定为C.fructicola;引起白沙市油梨果实炭疽病的病原菌鉴定为C. gigasporum。
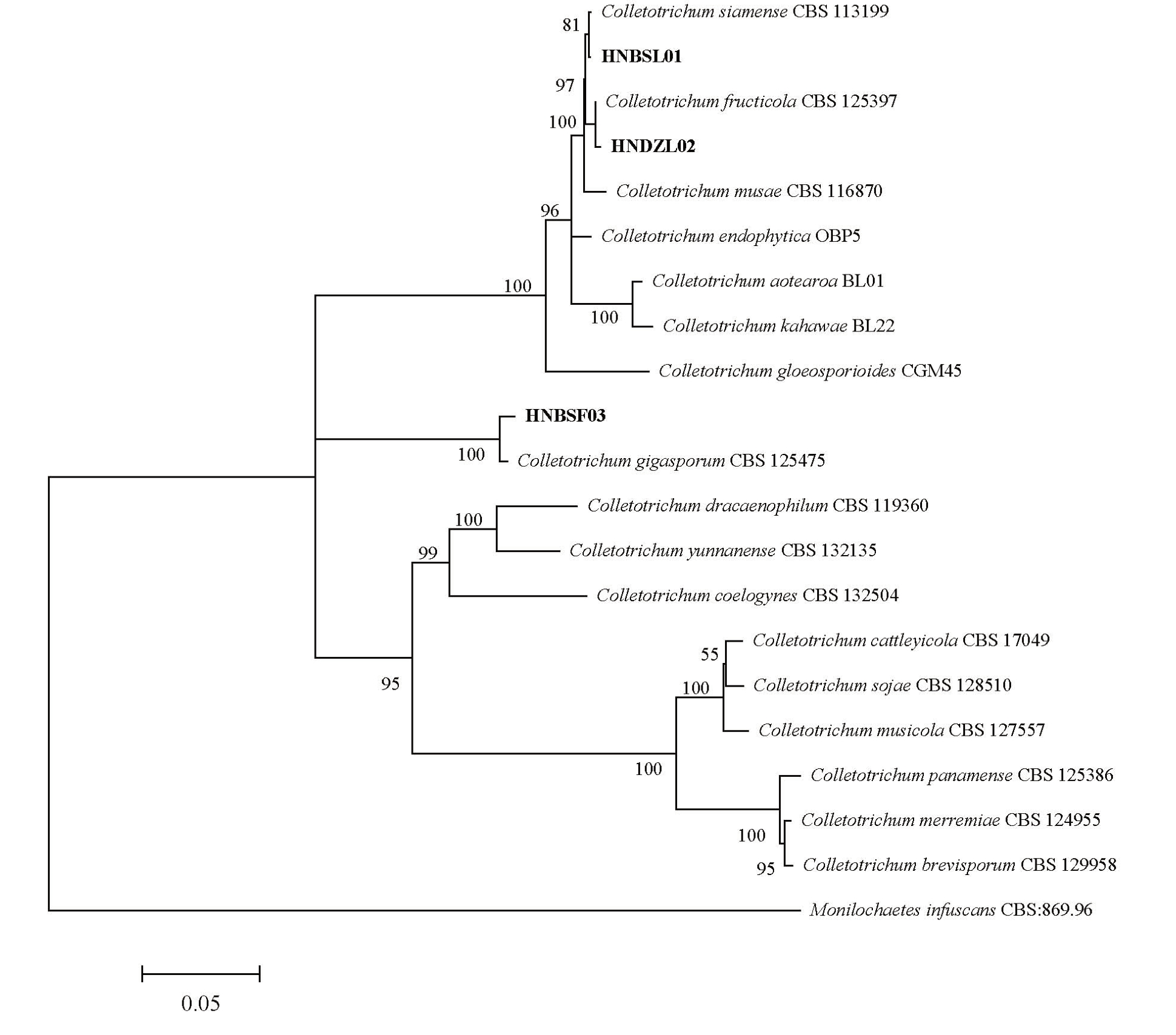
图5 基于ITS-ACT-TUB2-CHS-1-GAPHD-HIS3 序列以最大似然法对分离菌株进行多基因序列系统发育树的构建
Fig.5 Maximum likelihood for multigene sequence phylogenetic tree of isolated strains based on ITS,ACT,TUB2,CHS-1,GAPHD and HIS3 sequences
表2 本研究系统发育分析中的菌株信息
Table 2 Information on strains in the phylogenetic analysis of this study

注:加粗表示本研究获得的菌株。下同。
Note:The isolates obtained in this study are expressed in bold.The same below.
种Speices C.siamense菌株标号Culture No.CBS 113199基因库登录号GenBank registration number ITS KC297066 ACT KC296930 TUB KC297090 CHS-1 KC296985 GAPHD KC297008 HIS KC297044 C.fructicola CBS 125397寄主Host Protea cynaroides Unknown MH863502 JX009581 JX010409 JX009874 JX010032 KY856315 C.endophytica OBP5 KJ947310 KJ947187 KJ947210 KJ947233 KJ947279 KJ947256 C.gigasporum CBS 125475 Piper nigrum Unknown MH863697 KF687789 KF687874 KF687766 KF687836 KF687852 C.coelogynes CBS 132504 Unknown MG600713 MG600920 MG600980 MG600836 MG600776 MG600882 C.dracaenophilum CBS 119360 Unknown MG600711 MG600918 MG600978 MG600834 MG600774 MG600880 C.yunnanense CBS 132135 Unknown MH865960 JX519239 JX519248 JX519231 JX546706 JX546755 C.cattleyicola CBS 17049 Unknown MG600758 MG600963 MG601025 MG600866 MG600819 MG600905 C.sojae CBS 128510 Unknown MG600751 MG600956 MG601018 MG600862 MG600812 MG600901 C.musicola CBS 127557 Unknown MG600737 MG600943 MG601004 MG600854 MG600799 MG600896 C.merremiae CBS 124955 Unknown MG600765 MG600969 MG601032 MG600872 MG600825 MG600910 C.brevisporum CBS 129958 Unknown MG600763 MG600967 MG601030 MG600870 MG600823 MG600909 C.panamense CBS 125386 Unknown MG600766 MG600970 MG601033 MG600873 MG600826 MG600911 Monilochaetes infuscans C.musae CBS 869.96 Unknown JQ005780 JQ005843 JQ005864 JQ005801 JX546612 JQ005822 CBS 116870 Musa sp.JQ005777 JQ005840 JX010413 JQ005798 JX010050 JQ005819 C.aotearoa BL01 MN273059 MN273187 MN273219 MN273123 MN273091 MN273155 C.gloeosporioides CGM45 JX669445 JX827425 JX827449 JX827431 JX827437 JX827443 C.kahawae BL22国家Country津巴布韦Zimbabwe巴拿马Panama印度India越南Viet Nam德国Germany德国Germany中国China德国Germany德国Germany德国Germany德国Germany德国Germany德国Germany荷兰Netherlands美国USA中国China马来西亚Malaysia中国China MN273080 MN273208 MN273240 MN273144 MN273112 MN273176 C.siamense C.fructicola C.gigasporum HNBSL01 HNDZL02 HNBSF03 Areca catechu Glycine max L.Areca catechu Avocado Avocado Avocado中国China中国China中国China MW406820 MW406823 MW406832 MW426508 MW426511 MW426520 MW526372 MW526375 MW526384 OP504013 OP504016 OP504025 MW683343 MW683346 MW683355 MW556568 MW556571 MW556580
3 讨 论
炭疽菌属(Colletotrichum),属半知菌类Fungi Imperfecti、腔孢纲Coelomycetes、黑盘孢目Melanconiales、黑盘孢科Melanconiaceae[28]。该属真菌分布范围广,寄主繁多,特别是热带水果容易受炭疽菌侵染,目前已在多种重要经济作物上报道,如咖啡、番石榴、苹果、火龙果、杧果和草莓等。炭疽菌属病原菌包括600多种,其中,C.gloeosporioides和C.boninense 复合种为油梨炭疽病常见的病原菌[6-7]。炭疽菌通常危害油梨的叶、花和果实,造成叶斑、叶枯、花腐和果腐症状,影响油梨的生长,严重时会导致落叶、落花和烂果,甚至造成植株死亡,降低油梨的产量和质量。同时,炭疽病还会危害采后的油梨果实,造成贮藏果实腐烂,给农户带来严重的经济损失。
笔者在本研究中通过对海南省白沙县和儋州市的油梨主要种植基地中采集具有典型炭疽病症状的叶片和果实进行病原菌的分离与纯化,通过形态学观察、多基因联合建树分析以及致病性测定,将叶片炭疽病病原鉴定为C.siamense和C.fructicola,果实炭疽病病原为C.gigasporum,通过显微镜观察分生孢子和附着胞的形态和大小,发现C.siamense 与Li等[5]在中国报道的与Honger等[29]在加纳报道的引起油梨果实炭疽病的C. siamense 在形态和大小上相似。C.fructicola与Li等[4]在中国报道的引起油梨果实炭疽病的病原菌形态大小相似,但笔者在本研究中的附着胞较Fuentes-Aragón 等[30]报道的引起墨西哥油梨果实炭疽病的C.fructicola的附着胞长,可能是分离菌株的地区、分离部位和品种不同导致的差异。C. gigasporum 与Hunupolagama 等[31]报道的引起斯里兰卡油梨果实炭疽病的田间症状和病原菌形态相同,但分生孢子和附着胞的大小有较大差异,笔者在本研究中的分生孢子大小为13.77~17.65(15.49)μm×4.15~5.47(4.66)μm(n=100)和附着胞大小为6.65~9.78(8.38)μm×4.95~6.91(6.25)μm(n=100),而Hunupolagama 等[31]研 究 的 分 生 孢 子 为18.00~30.00(22.50)μm×7.00~10.00(8.00)μm,附着胞大小为18.75 μm×8.00 μm,这种差异可能是分离菌株的国家不同以及菌种随时间变化造成的。
本研究结果表明,在海南省白沙县和儋州市主要的油梨种植区,与油梨相关的Colletotrichum物种具多样性,这可能与环境条件的多样性有关,如温度和降雨量,还可能与样品采集时间、地区和分离部位有关。此外,引起白沙县的油梨果实炭疽病和叶片炭疽病的病原菌不同,可能原因是果实上的病原菌来源不同,果实上的病原菌可能来自叶片和枝干,而不同病原菌的侵染能力不同,导致可成功侵染果实和叶片的病原菌存在差异。这项研究增进了笔者对海南油梨炭疽病相关的Colletotrichum 物种多样性的了解。笔者需要进一步地研究来确定各个油梨炭疽病菌菌株在生物学和致病性上的差异,以及造成这种差异的分子机制。关于油梨炭疽菌的病原学和流行病学的研究很少,有待进一步研究。
4 结 论
通过组织分离与纯化、致病性测定、病原菌形态特征及多基因联合建树分析,首次明确了引起海南省白沙黎族自治县大岭农场附近的某种植基地油梨叶片炭疽病的病原菌为C.siamense、油梨果实炭疽病的病原菌为C.gigasporum;引起儋州市南辰农场油梨叶片炭疽病的病原菌为C.fructicola。
[1] LIN X B,ZHANG H,HU L N,ZHAO G Y,SVANBERG S,SVANBERG K. Ripening of avocado fruits studied by spectroscopic techniques[J]. Journal of Biophotonics,2020,13(8):e202000076.
[2] COMERFORD K B,AYOOB K T,MURRAY R D,ATKINSON S A. The role of avocados in complementary and transitional feeding[J].Nutrients,2016,8(5):316.
[3] 张良,张德生,刘康德.海南省油梨产业发展的环境分析与对策[J].中国农业资源与区划,2015,36(4):78-84.ZHANG Liang,ZHANG Desheng,LIU Kangde. Environment analysis and policy for development of avocado industry in hainan[J]. Chinese Journal of Agricultural Resources and Reginoal Planning,2015,36(4):78-84.
[4] LI S,LIU Z,ZHANG W. First report of anthracnose disease on avocado (Persea americana) caused by Colletotrichum fructicola in China[J].Plant Disease,2022,106(9):2529
[5] LI M,FENG W L,YANG J Y,GAO Z Y,ZHANG Z K,ZHANG W,WANG S M,WANG W B,GONG D Q,HU M J.First report of anthracnose caused by Colletotrichum siamense on avocado fruits in China[J]. Crop Protection,2022,155:105922.
[6] UYSAL A,KURT Ş. First report of fruit and leaf anthracnose caused by Colletotrichum karstii on avocado in Turkey[J]. Crop Protection,2020,133:105145.
[7] KWON J H,CHOI O,LEE Y,KIM S H,KANG B S,KIM J W.Anthracnose on postharvest avocado caused by Colletotrichum kahawae subsp. ciggaro in South Korea[J]. Canadian Journal of Plant Pathology,2020,42(4):508-513.
[8] AVILA-QUEZADA G,SILVA-ROJAS H V. First report of the anamorph of Glomerella acutata causing anthracnose on avocado fruits in Mexico[J].Plant Disease,2007,91(9):1200.
[9] QIU F,XU G,XIE C P,LI X,ZHENG F Q,MIAO W G. First report of Corynespora cassiicola causing leaf spot on avocado(Persea americana) in China[J]. Plant Disease,2020,104(6):1857.
[10] TAPIA L,LARACH A,RIQUELME N,GUAJARDO J,BESOAIN X. First report of Neofusicoccum luteum causing stemend rot disease on avocado fruits in Chile[J]. Plant Disease,2020,104(7):2027.
[11] WANJIKU E K,WACEKE J W,WANJALA B W,MBAKA J N.Identification and pathogenicity of fungal pathogens associated with stem end rots of avocado fruits in Kenya[J].International Journal of Microbiology,2020,2020:4063697.
[12] SHARMA G,MAYMON M,FREEMAN S. First detailed report of Trichothecium roseum causing post-harvest pink rot of avocado in Israel[J].Plant Disease,2016,100(4):856.
[13] VALENCIA A L,TORRES R,LATORRE B A. First report of Pestalotiopsis clavispora and Pestalotiopsis spp. causing postharvest stem end rot of avocado in Chile[J].Plant Disease,2011,95(4):492.
[14] TORRES C,CAMPS R,AGUIRRE R,BESOAIN X. First report of Diaporthe rudis in Chile causing stem-end rot on‘Hass’avocado fruit imported from California[J]. Plant Disease,2016,100(9):1951.
[15] SUAREZ S N,SAUCEDO J R,SANAHUJA G,LOPEZ P.First report of a putative new Erysiphe sp. causing powdery mildew on avocado in Florida[J]. Plant Disease,2018,102(2):448.
[16] SIAHAAN S A S,HIDAYAT I,KRAMADIBRATA K,MEEBOON J,TAKAMATSU S. Podosphaera perseae-americanae,a new powdery mildew species on Persea americana (avocado)from Indonesia[J].Mycoscience,2016,57(6):417-421.
[17] LÓPEZ-HERRERA C J,PÉREZ-JIMÉNEZ R M,ZEA-BONILLA T.First report of Phytophthora cactorum causing fruit rot on avocado in Spain[J].Plant Disease,2005,89(12):1363.
[18] NI H F,LIOU R F,HUNG T H,CHEN R S,YANG H R. First report of a fruit rot disease of avocado caused by Neofusicoccum mangiferae[J].Plant Disease,2009,93(7):760.
[19] FIRMINO A C,FISCHER I H,JOSÉ JÚNIOR T H,ROSA D D,FURTADO E L.Identificação de espécies de Fusicoccum causadoras de podridão em frutos de abacate[J]. Summa Phytopathologica,2016,42(1):100-102.
[20] MOLINA-GAYOSSO E,SILVA-ROJAS H V,GARCÍA-MORALES S,AVILA-QUEZADA G. First report of black spots on avocado fruit caused by Neofusicoccum parvum in Mexico[J].Plant Disease,2012,96(2):287
[21] RODRÍGUEZ-POLANCO E,REINA-NOREÑA J A,TAMAYOMOLANO P J,RODRÍGUEZ-POLANCO L A,VARÓN-DEVIA E H.Validation of black spot[(Pseudocercospora purpurea(Cooke) Deighton] management strategies in avocado crops in northern Tolima (Colombia)[J]. Revista Colombiana De Ciencias Hortícolas,2020,14(2):178-191.
[22] 马瑞,徐刚,郑樊,郑妃庆,谢昌平.海南省温郁金炭疽病的病原鉴定[J].植物保护,2018,44(4):81-86.MA Rui,XU Gang,ZHENG Fan,ZHENG Feiqing,XIE Changping.Identification of the pathogen causing anthracnose on Curcuma wenyujin in Hainan,China[J]. Plant Protection,2018,44(4):81-86.
[23] YANG B,JIN X H,FENG Q C,XIAO K L,ZHANG H Z,TANG T T,SHAN L Y,GUO W. Colletotrichum species causing leaf spot diseases of Liriope cymbidiomorpha(Ined.)in China[J].Australasian Plant Pathology,2020,49(2):137-139.
[24] KUMAR S,STECHER G,TAMURA K. MEGA7:molecular evolutionary genetics analysis version 7.0 for bigger datasets[J].Molecular Biology and Evolution,2016,33(7):1870-1874.
[25] WEIR B S,JOHNSTON P R,DAMM U. The Colletotrichum gloeosporioides species complex[J].Studies in Mycology,2012,73(1):115-180.
[26] DAMM U,SATO T,ALIZADEH A,GROENEWALD J Z,CROUS P W. The Colletotrichum dracaenophilum,C. magnum and C.orchidearum species complexes[J].Studies in Mycology,2019,92:1-46.
[27] LIU F,CAI L,CROUS P W,DAMM U.The Colletotrichum gigasporum species complex[J].Persoonia,2014,33(1):83-97.
[28] 邢来君,李明春,魏东盛.普通真菌学[M].2 版.北京:高等教育出版社,2010:389.XING Laijun,LI Mingchun,WEI Dongsheng. General mycology[M].2nd ed.Beijing:Higher Education Press,2010:389.
[29] HONGER J O,OFFEI S K,ODURO K A,ODAMTTEN G T,NYAKU S T. Identification and molecular characterisation of Colletotrichum species from avocado,citrus and pawpaw in Ghana[J]. South African Journal of Plant and Soil,2016,33(3):177-185.
[30] FUENTES-ARAGÓN D,JUÁREZ-VÁZQUEZ S B,VARGASHERNÁNDEZ M,SILVA-ROJAS H V.Colletotrichum fructicola,a member of Colletotrichum gloeosporioides sensu lato,is the causal agent of anthracnose and soft rot in avocado fruits cv.‘Hass’[J].Mycobiology,2018,46(2):92-100.
[31] HUNUPOLAGAMA D M,WIJESUNDERA R L C,CHANDRASEKHARAN N V,WIJESUNDERA W S S,KATHRIARACHCHI H S,FERNANDO T H P S. Characterization of Colletotrichum isolates causing avocado anthracnose and first report of C. gigasporum infecting avocado in Sri Lanka[J]. Plant Pathology&Quarantine,2015,5(2):132-143.