枇杷(Eriobotrya japonica Lindl.)为蔷薇科枇杷属植物中的普通枇杷栽培种[1],属于亚热带常绿果树,果实于春末夏初成熟,在广州3月上旬至4月初成熟。早钟6号枇杷是中国第一个杂交育成的推广面积最大的枇杷品种[2],经1999年引入广东省栽培,因果实品质优良、早结丰产性好,目前已成为广东省枇杷主栽品种之一[3]。然而在果实成熟期,如遇高温天气,早钟6号枇杷果实易出现果皮皱缩现象,严重影响了果实品质和经济效益。郑少泉[4]的研究表明,枇杷果实成熟后期遇高温干旱强日照,果面温度升高,水分供应不足,容易造成萎蔫,往往在2~3 d的高温晴天后发生大量果皮皱缩现象。王荔等[5]研究了早钟6号枇杷果实皱皮的发生规律,发现皱皮现象集中发生在果实完熟前夕、果柄离层形成时。研究表明,采前柑橘果实皱缩受环境因子、品种、树体激素水平等的影响,喷施生长素能够显著降低皱缩果率[6-8]。然而采前果实皱缩相关的分子机制研究较少,早钟6号枇杷成熟期果实皱缩的相关研究鲜有报道,而对于抗皱缩品种的筛选,以及易皱缩品种与抗皱缩品种间的相关基因表达差异尚未有研究。
笔者课题组前期统计了枇杷资源圃中与早钟6号枇杷果实成熟期相近的13 份枇杷种质资源的果实皱缩率,其中早钟6 号枇杷果实皱缩率达19.8%,有2份种质(思贺大果枇杷和蜜糖枇杷)果实皱缩率为0,即没有出现皱缩现象。这2份种质中蜜糖枇杷的果实较小,因此笔者在本研究中选择果实较大的种质思贺大果枇杷作为抗皱缩品种,与易皱缩品种早钟6号进行比较分析,对早钟6号枇杷不同皱缩程度的果实和思贺大果枇杷进行了转录组测序分析,以期初步筛选与枇杷果实皱缩相关的基因、不同品种抗皱缩能力的差异基因以及相关的代谢通路,为进一步解析枇杷皱缩相关的分子机制,培育抗皱缩枇杷品种奠定理论基础。
1 材料和方法
1.1 材料
试验材料为早钟6 号和思贺大果枇杷果实,果实样品采自广东省农业科学院果树研究所枇杷资源圃,采摘时间2021 年3 月30 日,果实成熟度8~9 成熟。早钟6 号枇杷分3 个不同的样品:ZZS1 代表早钟6 号正常果实,ZZS2 代表早钟6 号轻度皱缩果,ZZS3 代表早钟6 号皱缩果。思贺大果枇杷果实未出现皱缩现象,采取其正常果,标记为DG,果实照片见图1。
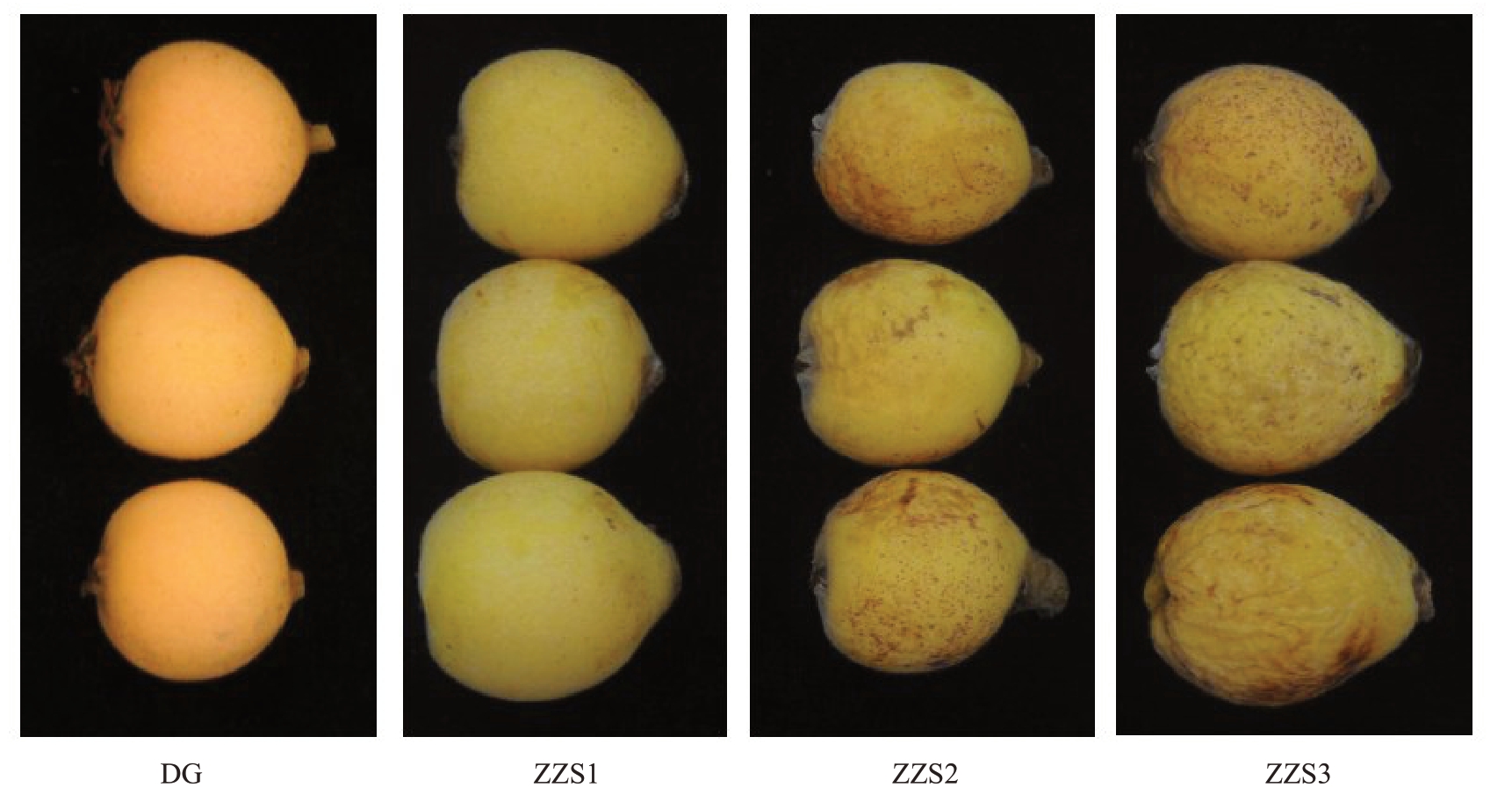
图1 早钟6 号枇杷和思贺大果枇杷果实样品
Fig.1 Zaozhong 6 and Sihedaguo loquat fruit samples
1.2 生理指标测定方法
1.2.1 丙二醛含量 称取1.0 g枇杷果实样品,加入5.0 mL 100 g·L-1 TCA 溶液,研磨匀浆后,离心收集上清液。取2.0 mL上清液加入2.0 mL 0.67%TBA,混合后在沸水浴中煮沸20 min,取出冷却后离心收集上清液,测定OD450、OD532、OD600,根据公式计算丙二醛含量。
1.2.2 脯氨酸含量 称取2.0 g枇杷果实样品,加入5 mL 30 g·L-1磺基水杨酸溶液研磨匀浆后转入试管中,沸水浴10 min,冷却后离心15 min,上清液即为脯氨酸提取液。吸取2.0 mL 提取液于具塞玻璃试管中,加入2.0 mL 冰醋酸及3.0 mL 酸性茚三酮试剂,沸水浴30 min,取出冷却后加入4 mL甲苯,摇荡30 s,静置分层,取上层液测定OD520,根据标准曲线计算其含量。
1.2.3 超氧化物歧化酶(SOD)活性 酶液提取:取0.1 g 枇杷果实样品,按体积比1∶10 加入预冷的提取液(50 mmol·L-1 pH 7.8 的磷酸缓冲液)和少量的石英砂,充分研磨后,于4 ℃下12 000 r·min-1离心15 min,所得上清液即可用于测定SOD活性。
SOD 活性测定:依次加入0.3 µmol·L-1核黄素0.15 mL、13 mmol·L-1甲硫氨酸2.5 mL、63µmol·L-1氯化硝基氮蓝四唑0.25 mL、酶液0.05 mL,混匀后,照光10~15 min 后,于560 nm 测定吸光值。定义在测定条件下,抑制NBT光氧化还原50%所需的酶量为1个酶活性单位(U)。
1.2.4 过氧化物酶(POD)活性 酶液提取:称取5.0 g 枇杷果实样品,加入5.0 mL 提取缓冲液(含1 mmol·L-1 PEG、4%PVPP和1%Triton X-100),在冰浴条件下研磨成匀浆,于4 ℃,12 000×g离心30 min,收集上清液即为酶提取液。
POD活性测定:取0.5 mL酶提取液,加入3.0 mL 25 mmol·L-1 愈创木酚溶液和200 μL 0.5 mol·L-1 H2O2溶液迅速混合启动反应,在反应15 s 时开始测定OD470,每隔1 min 记录1 次,连续测定30 min,根据公式计算POD活性。
1.3 转录组测序与分析
转录组测序委托北京百迈客生物科技有限公司完成。提取枇杷ZZS1、ZZS2、ZZS3和DG果实样品RNA后,构建cDNA文库,文库构建完成后,使用QPCR方法对文库的有效浓度进行准确定量,以保证文库质量。库检合格后,用Illumina 平台进行测序。将下机数据进行过滤得到Clean Data,用HISAT2软件与参考基因组进行序列比对,比对分析完成后利用String Tie 对比对上的reads 进行组装和定量;用DESeq2 进行样品组间的差异表达分析,用clusterProfiler软件(3.10.1)对差异表达基因进行GO富集分析及KEGG通路富集分析。
1.4 数据分析
试验数据通过统计软件SPSS21.0进行分析,用Duncan新复极差法进行方差分析,检验差异显著性。
2 结果与分析
为了阐明早钟6号枇杷果实成熟期果实皱缩的生理及分子机制,测定了丙二醛、脯氨酸含量和超氧化物歧化酶(SOD)、过氧化物酶(POD)活性,并且对早钟6号枇杷正常果、轻度皱缩果、皱缩果以及资源圃中选出的无皱缩现象的思贺大果枇杷4个样品进行了转录组测序,通过差异基因表达分析,以明确与早钟6 号枇杷果实皱缩相关的基因,以及品种之间应对高温胁迫的关键差异基因。
2.1 枇杷果实在皱缩过程中的生理生化指标变化
如图2所示,早钟6号枇杷皱缩果(ZZS2、ZZS3)与正常果(ZZS1)相比,丙二醛和脯氨酸含量、SOD和POD活性均显著增加;丙二醛含量(b)由正常果的(34.46±4.1)μmol·g-1增加到(41.86±0.77)μmol·g-1;皱缩果(ZZS3)的脯氨酸含量比正常果增加81.9%,SOD 活性增加了41.6%,POD 活性增加了94.5%。说明在枇杷果实皱缩过程中,细胞膜脂过氧化程度加深、抗氧化酶活性增强。
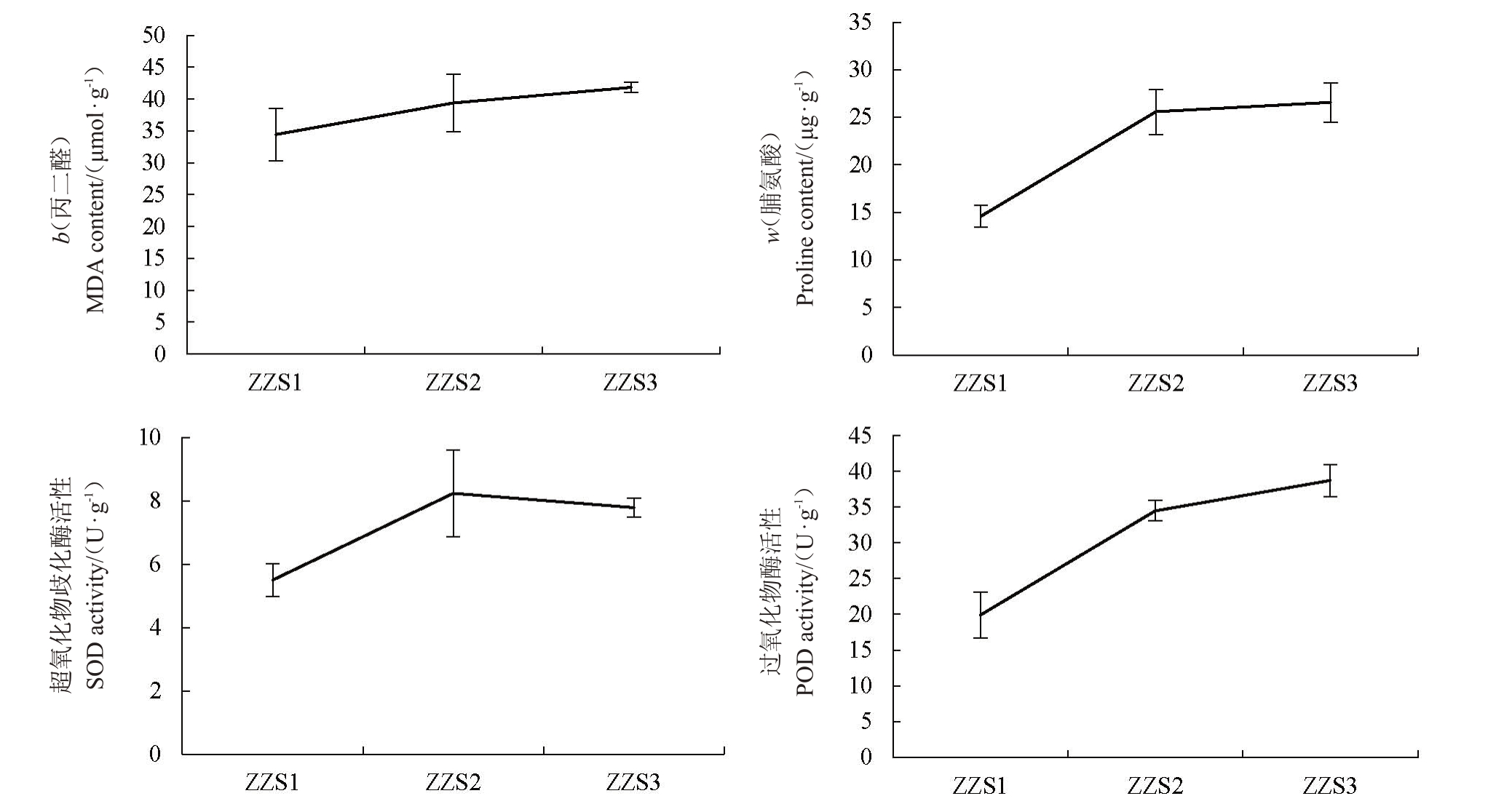
图2 早钟6 号枇杷在皱缩过程中生理生化指标变化情况
Fig.2 Changes of physiological and biochemical indexes of Zaozhong 6 loquat fruit during shrinkage process
2.2 枇杷果实转录组测序分析
对4 组样品(ZZS1、ZZS2、ZZS3、DG)进行转录组测序分析,共得到76.26 Gb Clean Data,各样品Q30碱基百分比均大于90%;GC含量在46.7~47.9之间(表1)。进行了ZZS1 vs ZZS2、ZZS1 vs ZZS3、ZZS2 vs ZZS3、DG vs ZZS1 四组比较的差异表达基因分析,如表2 和图3 所示,早钟6 号枇杷皱缩果(ZZS3)与正常果(ZZS1)相比,有差异表达基因4245个,其中上调表达2265个,下调表达1980个。早钟6号枇杷(ZZS1)与思贺大果枇杷(DG)相比较,有差异基因1647个,其中上调表达1028个,下调表达619个。
表1 枇杷果实样品转录组测序数据
Table 1 The transcriptome sequencing data of loquat fruit
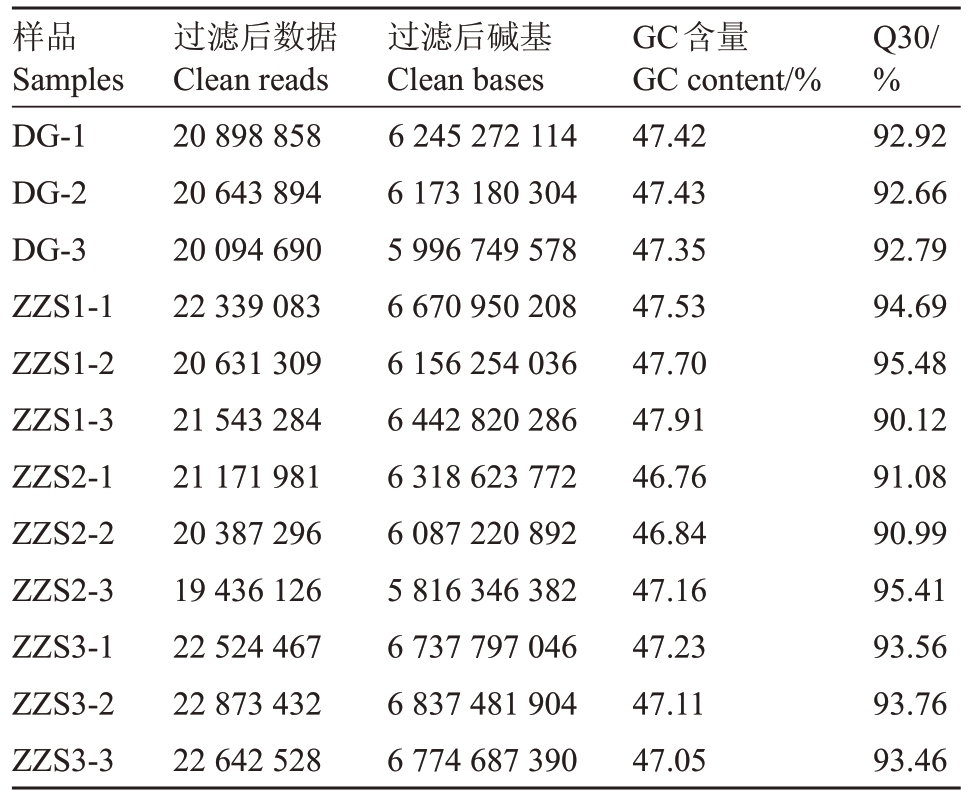
样品Samples DG-1 DG-2 DG-3 ZZS1-1 ZZS1-2 ZZS1-3 ZZS2-1 ZZS2-2 ZZS2-3 ZZS3-1 ZZS3-2 ZZS3-3过滤后数据Clean reads 20 898 858 20 643 894 20 094 690 22 339 083 20 631 309 21 543 284 21 171 981 20 387 296 19 436 126 22 524 467 22 873 432 22 642 528过滤后碱基Clean bases 6 245 272 114 6 173 180 304 5 996 749 578 6 670 950 208 6 156 254 036 6 442 820 286 6 318 623 772 6 087 220 892 5 816 346 382 6 737 797 046 6 837 481 904 6 774 687 390 GC含量GC content/%47.42 47.43 47.35 47.53 47.70 47.91 46.76 46.84 47.16 47.23 47.11 47.05 Q30/%92.92 92.66 92.79 94.69 95.48 90.12 91.08 90.99 95.41 93.56 93.76 93.46
表2 枇杷果实样品组间差异表达基因分析
Table 2 Analysis of different expressed genes between samples of loquat fruit
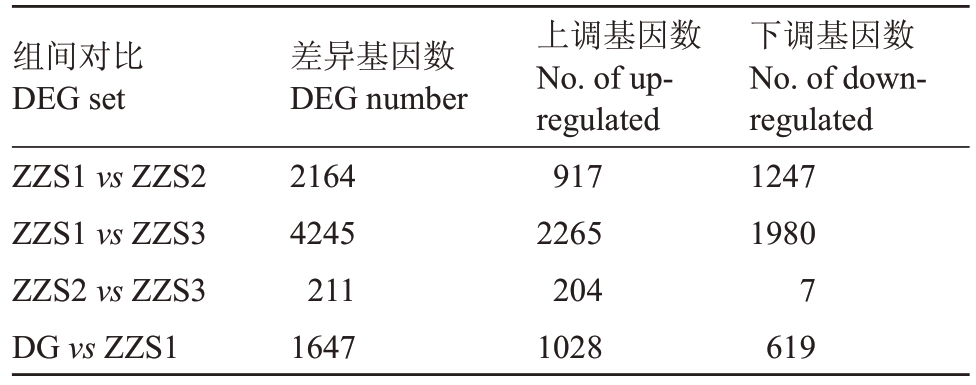
组间对比DEG set ZZS1 vs ZZS2 ZZS1 vs ZZS3 ZZS2 vs ZZS3 DG vs ZZS1差异基因数DEG number 2164 4245 211 1647上调基因数No.of upregulated 917 2265 204 1028下调基因数No.of downregulated 1247 1980 7 619

图3 枇杷果实样品组间差异表达基因火山图
Fig.3 Volcano diagrams of differentially expressed genes between loquat fruit sample groups
2.3 早钟6号枇杷不同程度皱缩果与正常果之间的差异表达基因分析
为了筛选影响早钟6 号枇杷皱缩的关键基因,对ZZS1 vs ZZS2,ZZS1 vs ZZS3 和ZZS2 vs ZZS3 三组比较进行了维恩图及差异表达基因分析(图4-A),找到3 组共有的差异表达基因27 个,其中20 个在3 组比较分析中均上调,4 个均下调,3 个在ZZS1 vs ZZS2 和ZZS1 vs ZZS3 比较中下调,在ZZS2 vs ZZS3中上调。根据COG功能分类(图4-B),这些基因参与了五大类功能,包括核苷酸运输与代谢(F,7.69%)、碳水化合物运输与代谢(G,15.38%)、脂质运输与代谢(I,7.69%)、次生代谢物合成、运输与代谢(Q,38.46%)以及一般功能类(R,30.77%)。根据GO分类,这些基因参与了信号转导、氧化还原、碳水化合物代谢、应对光刺激等生物学过程,参与细胞膜、内质网膜等组成成分,具有氧化还原酶活性、水解酶活性、纤维素酶活性、蛋白激酶活性等分子功能。

图4 不同程度皱缩果差异基因比较维恩图(A)及3 组共有差异基因COG 注释(B)
Fig.4 Vern diagram of three comparison pairs of different degrees of shrinkage fruit(A)and COG annotation of common differential genes in three compairation groups(B)
G3.ZZS1 vs ZZS2;G4.ZZS1 vs ZZS3;G5.ZZS2 vs ZZS3。中括号中的数字代表注释到特定功能的基因个数和注释到特定功能的基因所占的比例。下同。
Numbers in brackets represent the number of genes annotated to a specific function, and the proportion of genes annotated to the specific function.The same below.
在这27个共有的差异表达基因中,有20个在3组比较中表达量均上调,4个在3组比较中表达量均下调,这些基因可能与枇杷果实皱缩高度相关。20个上调基因包括:1 个丝氨酸/苏氨酸-蛋白激酶,根据KEGG注释,参与油菜素内脂信号转导途径;6个C1H46蛋白,根据KEGG注释,其中1个参与泛素介导的蛋白水解途径,1个参与萜类生物合成途径,其余4个未被注释到任何代谢途径(GO注释为氧化还原活性);2个基因参与淀粉和蔗糖代谢途径;1个基因参与苯唑嗪类生物合成;其余10个未被注释到任何代谢途径(包括1个水解酶类基因,1个NAC转录因子基因,1个F-box蛋白AFR-like基因,1个ras-related蛋白基因,2个DVH24蛋白基因,1个脱水素基因,另外3 个基因GO 注释为细胞膜组分)。4 个下调基因分别是1 个GDSL 酯酶/脂肪酶基因,参与脂肪代谢途径;1 个富含亮氨酸重复受体蛋白激酶基因,未被注释到任何代谢途径;1个内切葡聚糖酶基因,参与淀粉与蔗糖代谢途径;1个生长素响应蛋白基因,参与植物激素信号转导途径。
这24个在3组比较中表达量一致的基因表达聚类热图(图5)显示,其中有9个基因在重复间一致性较好,包括6 个上调表达的基因[EVM0002174(BRsignaling kinase,参与油菜素内脂信号转导途径)、EVM0008555(NAC 转录因子基因)、EVM0012379(Zinc-binding dehydrogenase,参与氧化还原过程)、EVM0017570(Aldo/keto reductase family,参与氧化还原过程)、EVM0027280(Ras family)、EVM0039679(Dehydrin,响应水分胁迫)]和3 个下调表达的基因[EVM0006727(GDSL esterase/lipase 7,参与脂肪代谢)、EVM0036225(SAUR,参与生长素信号转导途径)、EVM0031215(endoglucanase,参与淀粉和蔗糖代谢途径)]。

图5 在3 组比较中均上调和均下调的共有差异表达基因聚类热图
Fig.5 Cluster heat maps of common differentially expressed genes up-regulated or down-regulated in three comparisons
结果表明,在枇杷果实皱缩过程中,生长素和油菜素内酯这两类激素可能参与调控此过程,另外碳水化合物、萜类、脂类等代谢物在枇杷果实皱缩过程中也会发生变化;在分子水平上,脱水素基因表达量变化最明显,ZZS1 vs ZZS2和ZZS1 vs ZZS3两组比较的log2FC 值均>5,在皱缩果中表达量最高;并且发现NAC转录因子在3组比较中均上调表达,说明其可能参与调控枇杷果实皱缩相关的基因表达。
2.4 早钟6号枇杷正常果与思贺大果枇杷之间的差异表达基因分析
根据前期对资源圃中与早钟6 号枇杷果实同期成熟13 份资源皱缩情况的统计,从中筛选出成熟后不会出现皱缩现象的思贺大果与早钟6 号枇杷果实进行转录组测序分析,以期筛选出关键差异基因。如表2 所示,早钟6 号枇杷和思贺大果枇杷之间的差异基因有1647 个,其中上调表达1028 个,下调表达619个。这些基因是2 个品种之间所有的差异表达基因,其中一部分可能与果实响应高温并无关系,为了进一步筛选与枇杷果实高温耐受相关的基因,对DG vs ZZS1、ZZS1 vs ZZS2 和ZZS1 vs ZZS3 三组比较进行了维恩图分析(图6-A),找到3组共有的差异表达基因481个,根据COG功能分类(图6-B),主要参与碳水化合物运输和代谢、脂质运输和代谢、细胞壁/膜生物合成、次生代谢物合成、运输和分解、信号转导和防御机制等。
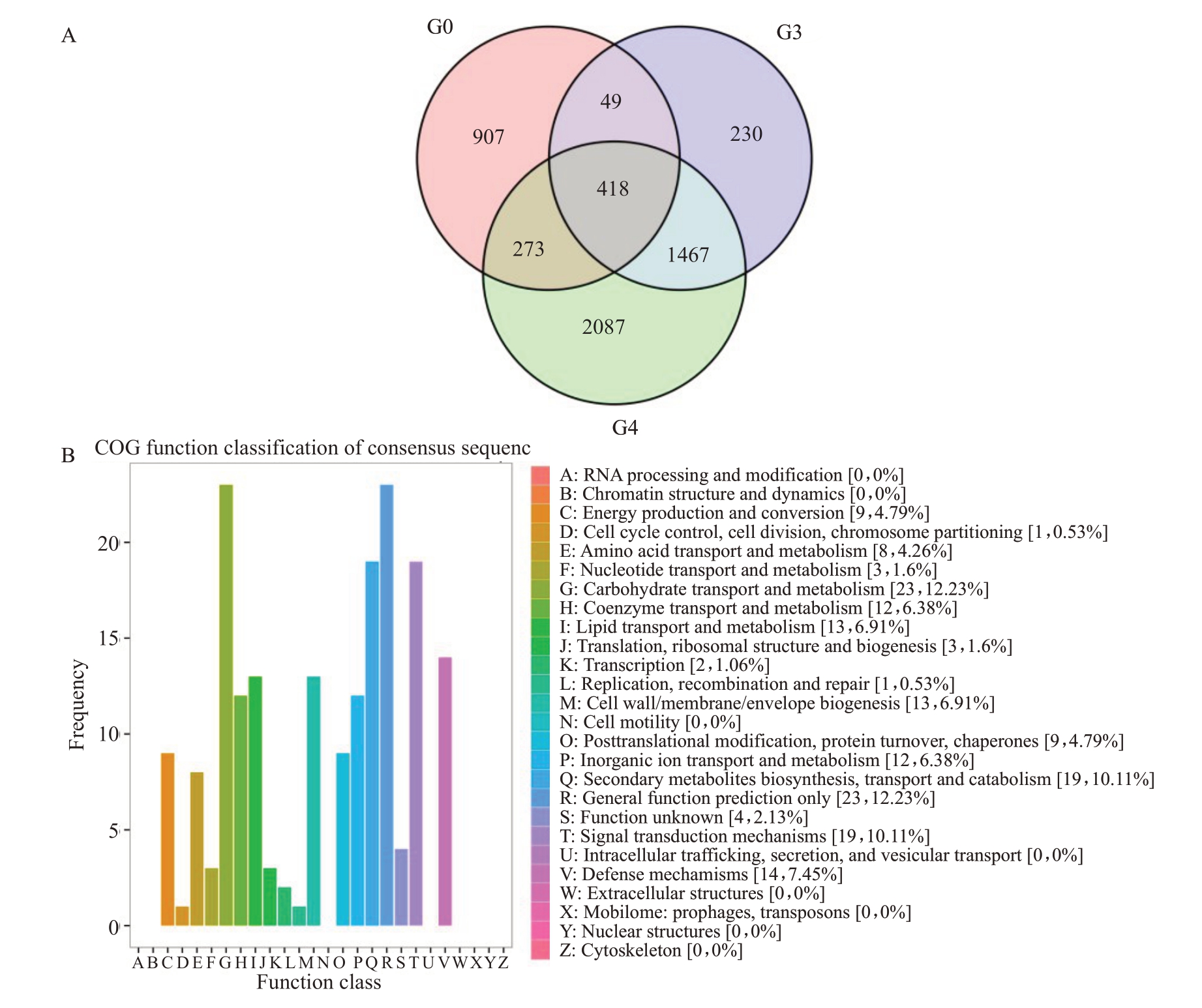
图6 三组差异基因比较维恩图(A)及共有差异基因COG 注释(B)
Fig.6 Vern diagram of three comparison pairs(A)and COG annotation of common differential genes in the three groups(B)
G0.DG vs ZZS1;G3.ZZS1 vs ZZS2;G4.ZZS1 vs ZZS3.
根据KEGG 分类,这些基因主要参与淀粉和蔗糖代谢途径、植物激素信号转导途径、苯丙烷生物合成途径(主要参与木质素合成)、糖酵解途径等。如图7所示,参与苯丙烷生物合成途径的12个基因中,有9 个与木质素的合成相关,分别是POD(2 个:EVM0030656 和EVM0044868)、COMT(3 个:EVM0000718,EVM0004717 和 EVM0043031)、CAD(EVM0015104)、CCR(EVM0022913)、4CL(EVM0033838)和F5H(EVM0043011),这9 个基因在DG vs ZZS1比较中均上调表达,说明木质素可能与不同品种应对高温胁迫能力相关;而在ZZS1 vs ZZS2和ZZS1 vs ZZS3两组比较中均下调表达,说明这些基因的表达量随着早钟6号枇杷果实皱缩程度的加深而下降。

图7 参与苯丙烷生物合成途径和植物激素信号转导途径的差异基因聚类热图
Fig.7 Heat maps of differential gene cluster involved in phenylpropanoid biosynthesis and plant hormone signal transduction pathways
参与植物激素信号转导途径的13个基因中,有6个参与生长素信号转导,包括2个GH3(EVM0012524和EVM0032063)、4个SAUR(EVM0015711、EVM00-31846、EVM0038736、EVM0039643)。另外,在3 组共有的差异表达基因中,发现有2 个基因(EVM0023097 和EVM0032997)参与油菜素内脂生物合成途径,这2 个基因在DG vs ZZS1、ZZS1 vs ZZS2 和ZZS1 vs ZZS3 三组比较中表达量均上调,NR注释为cytochrome P450 90A1-like(CYP90A1)。
3 讨 论
3.1 早钟6号枇杷果实皱缩过程中抗逆指标变化明显
早钟6 号枇杷果实随着皱缩程度加深,丙二醛含量、脯氨酸含量、SOD活性、POD活性等抗逆指标升高。研究表明,丙二醛含量是反映细胞膜损伤程度的指标,高温胁迫下,膜脂过氧化程度加深,细胞膜透性丧失,产生大量丙二醛等过氧化产物[9-10];同时由于高温逆境造成超氧阴离子自由基增加,导致体内活性氧积累,诱发SOD、POD 等抗氧化酶类活性升高[11-12]。脯氨酸在逆境下,可以平衡细胞代谢、维持细胞结构的完整性,多种果树遇温度胁迫,脯氨酸含量会大量增加[13-15]。在广州地区,早钟6号枇杷成熟期会遇到35~42 ℃的高温,因此笔者推测,早钟6 号枇杷果实成熟期果皮皱缩,响应逆境指标变化明显,可能是成熟期高温胁迫所致。
3.2 生长素和油菜素内脂可能参与枇杷果实皱缩过程
根据早钟6号枇杷不同皱缩程度果实的转录组分析,以及早钟6 号枇杷与思贺大果枇杷的转录组比较分析,发现有6 个差异基因(4 个SAUR 和2 个GH3)参与生长素信号转导,其中有2个在皱缩果中表达量上调,4个下调,说明生长素可能参与调节枇杷果实高温皱缩过程。Li等[6]的研究表明,Zn+NAA处理提高了砂糖橘果实生长素水平,减少了皱缩果率和落果率。高温胁迫能够诱导生长素的合成以及生长素信号响应基因SAUR 的表达[16-17]。番茄SLSAUR3、16、71、36 等基因在高温胁迫条件下表达量显著下降[18]。王红飞等[19]对黄瓜SAUR 基因家族的研 究 表 明,CsaSAUR1、CsaSAUR13、CsaSAUR15、CsaSAUR49、CsaSAUR50 等5 个基因受高温诱导表达量增加。柑橘SAUR基因家族中CclSAUR39的表达受低温胁迫的诱导,其对温度胁迫敏感[20]。Chen等[21]对枇杷果实受高温胁迫不同时间后的转录组测序分析,发现有58个差异表达基因参与生长素信号转导途径,其中有多个SAUR和GH3基因,说明生长素信号转导途径在枇杷应对高温胁迫过程中起到关键作用。
另外,笔者在本研究中发现2 个差异基因参与油菜素内脂生物合成途径,在早钟6 号枇杷皱缩果中的表达量高于其正常果,在抗皱缩果品种思贺大果枇杷中的表达量低于早钟6 号枇杷,说明枇杷果实中油菜素内脂可能参与调节果皮应对高温出现皱缩的程度。研究表明,油菜素内脂参与植物应对干旱、高温、冻害等非生物胁迫,提高抗氧化防御系统的能力,同时通过提高渗透调节物质脯氨酸的含量缓解逆境对植物的伤害[22-27];高温能够通过诱导PIF4和HY5转录因子调控油菜素内脂的合成,进而促进植株生长[28-29]。笔者在本研究中发现,油菜素内脂合成关键酶基因CYP90A1 在DG vs ZZS1、ZZS1 vs ZZS2 和ZZS1 vs ZZS3 三组比较中表达量均上调,说明其可能参与响应高温胁迫信号,进而影响枇杷果实中油菜素内脂的水平,以应对温度胁迫。
3.3 木质素合成代谢途径与枇杷果实皱缩相关
对抗皱缩种质思贺大果枇杷和易皱缩早钟6号枇杷及其不同程度皱缩果的转录组分析,发现差异表达基因中有9 个参与木质素合成(POD 2 个,COMT 3个和CAD、CCR、4CL、F5H各1个),与思贺大果枇杷相比,这些基因在早钟6 号枇杷中表达量上调,说明木质素含量及单体组分可能与不同品种抵抗高温胁迫的能力有关。李雪等[30-31]对枇杷果实木质化相关基因的功能分析发现,EjPAL 和Ej4CL基因的表达水平与木质素含量呈正相关,瞬时过量表达Ej4CL1 能够诱导烟草叶片木质素合成。而木质素合成下游基因COMT、CAD的差异表达则会影响木质素单体组分的含量[32-33],EjCAD1 的表达水平与果实木质化程度呈正相关[34]。枇杷果肉木质化研究较多的是在果实采后贮藏衰老的过程中果实出现质地生硬粗糙少汁的现象,可能与贮藏过程中发生冷害相关[35-37]。Shan 等[34]的研究发现,低温条件下,易发生木质化的枇杷品种洛阳青果实中的POD 表达水平显著高于不发生木质化的品种白沙。低温处理能够诱导枇杷果实木质化,并伴随着CAD活性的升高及EjCAD3 和EjCAD5 基因表达量的增加[38]。本研究中木质素合成相关基因在受高温影响的不同程度皱缩果实中出现差异表达,说明枇杷果实木质素的合成不仅与低温冷害有关,同样受到高温胁迫的影响。
3.4 NAC 转录因子可能参与调控枇杷果实皱缩相关基因的表达
对早钟6号枇杷果实不同皱缩程度的转录组比较(ZZS1 vs ZZS2、ZZS1 vs ZZS3、ZZS2 vs ZZS3)分析发现,NAC转录因子基因在3组中表达量均上调,说明其可能参与调控枇杷果实皱缩相关的基因表达。研究表明NAC 转录因子参与果实成熟衰老过程[39],如番茄突变体nor 的果实不能正常成熟,其原因是NAC结构域的基因不能正常表达[40];citNAC与奉节脐橙晚熟突变体的果实成熟及组织衰老密切相关[41];草莓NAC 转录因子调控细胞凋亡及衰老,导致采后草莓果实衰老[42]。本研究中,早钟6 号枇杷果实皱缩现象主要发生在成熟后期,其皱缩过程与果实成熟衰老过程一致,随着皱缩程度加重,NAC转录因子基因表达量增加,说明其可能参与调控这一过程。研究表明,NAC转录因子调控枇杷果实木质化,转录因子EjNAC 对木质素合成基因Ej4CL1具有转录激活效应[43],EjNAC3能与EjCAD-like基因启动子结合并激活其表达[44]。本研究中,木质素合成基因在枇杷不同程度皱缩果实中出现差异表达,说明NAC 转录因子有可能在枇杷果实皱缩过程中参与调控木质素合成相关基因的表达。
4 结 论
对早钟6号枇杷不同程度皱缩果实的转录组测序分析,发现可能参与调控这一过程的基因主要有24 个,包括丝氨酸/苏氨酸-蛋白激酶、C1H46 蛋白、F-box 蛋白AFR-like 基因、GDSL 酯酶基因、内切葡聚糖酶基因、NAC 转录因子基因等,参与油菜素内脂信号转导途径、泛素介导的蛋白水解途径、萜类生物合成、脂肪代谢、淀粉与蔗糖代谢以及植物激素信号转导途径。对思贺大果枇杷与早钟6号枇杷不同程度皱缩果实的转录组比较分析发现,9 个与木质素合成相关的基因在DG vs ZZS1 比较中均上调表达,说明木质素可能与不同品种应对高温胁迫能力相关;在3 组共有的差异表达基因中发现,6 个基因参与生长素信号转导,2 个基因参与油菜素内脂生物合成途径,说明生长素和油菜素内脂这两种激素可能参与调控枇杷果实应对高温胁迫的过程。
[1] 林顺权. 枇杷属野生种种质资源的研究与创新利用进展[J].园艺学报,2017,44(9):1704-1716.LIN Shunquan. A review on research of the wild species in genus Eriobotrya germplasm and their innovative utilization[J].Acta Horticulturae Sinica,2017,44(9):1704-1716.
[2] 林顺权. 新中国果树科学研究70 年:枇杷[J]. 果树学报,2019,36(10):1421-1428.LIN Shunquan.Fruit scientific research in New China in the past 70 years:Loquat[J]. Journal of Fruit Science,2019,36(10):1421-1428.
[3] 邱继水,曾帆,谢方启,周碧容,黄炳雄,陈锦瑞.早钟6 号枇杷在广东的引种观察[J].中国南方果树,2006,35(3):31-33.QIU Jishui,ZENG Fan,XIE Fangqi,ZHOU Birong,HUANG Bingxiong,CHEN Jinrui. Observation on introduction of Zaozhong 6 loquat in Guangdong Province[J]. South China Fruits,2006,35(3):31-33.
[4] 郑少泉.枇杷品种与优质高效栽培技术原色图说[M].北京:中国农业出版社,2005.ZHENG Shaoquan. Primary color illustration of loquat varieties and high quality and high efficiency cultivation techniques[M].Beijing:China Agriculture Press,2005.
[5] 王荔,张雪,黄旭明,王惠聪.枇杷皱皮果发生规律及其防治方法[J].浙江农业学报,2019,31(12):2019-2024.WANG Li,ZHANG Xue,HUANG Xuming,WANG Huicong.Study on occurrence of loquat fruit creasing and its control method[J].Acta Agriculturae Zhejiangensis,2019,31(12):2019-2024.
[6] LI J,LIANG C H,LIU X Y,HUAI B,CHEN J Z,YAO Q,QIN Y,LIU Z,LUO X Y. Effect of Zn and NAA co-treatment on the occurrence of creasing fruit and the peel development of‘Shatangju’mandarin[J]. Scientia Horticulturae,2016,201:230-237.
[7] GREENBERG J,KAPLAN I,FAINZACK M,EGOZI Y,GILADI B. Effects of auxins sprays on yield,fruit size,fruit splitting and the incidence of creasing of‘Nova’mandarin[J].Acta Horticulturae,2006(727):249-254.
[8] GREENBERG J,HOLTZMAN S,FAINZACK M,EGOZI Y,GILADI B,OREN Y,KAPLAN I. Effects of NAA and GA3 sprays on fruit size and the incidence of creasing of‘Washington’navel orange[J].Acta Horticulturae,2010,884:273-279.
[9] 余叔文,汤章城.植物生理与分子生物学[M].2 版.北京:科学出版社,1998.YU Shuwen,TANG Zhangcheng. Plant physiology and molecular biology[M].2nd ed.Beijing:Science Press,1998.
[10] 邓朝军,许奇志,蒋际谋,魏秀清,章希娟,郑少泉.高温胁迫对枇杷果皮热伤害的抗氧化特性影响[J].热带亚热带植物学报,2012,20(5):439-444.DENG Chaojun,XU Qizhi,JIANG Jimou,WEI Xiuqing,ZHANG Xijuan,ZHENG Shaoquan. Changes in antioxidant properties induced by heat injury in loquat peel under high temperature stress[J]. Journal of Tropical and Subtropical Botany,2012,20(5):439-444.
[11] 孙山.苹果绿色果皮光合生理特性及果皮灼伤机制的研究[D].泰安:山东农业大学,2009.SUN Shan. Study on photosynthetic characteristics and mechanism of sunburn in green peel of apple fruit[D]. Taian:Shandong Agricultural University,2009.
[12] 王静璞.高温、强光胁迫对苹果果实抗氧化能力的影响[D].保定:河北农业大学,2010.WANG Jingpu. Effect of high-temperature and excessive-light stresses on antioxidant capacity in apple fruits[D]. Baoding:Hebei Agricultural University,2010.
[13] 朱虹,祖元刚,王文杰,阎永庆.逆境胁迫条件下脯氨酸对植物生长的影响[J].东北林业大学学报,2009,37(4):86-89.ZHU Hong,ZU Yuangang,WANG Wenjie,YAN Yongqing.Effect of proline on plant growth under different stress conditions[J].Journal of Northeast Forestry University,2009,37(4):86-89.
[14] 李靖,孙淑霞,谢红江,陈栋,涂美艳,何俊涛,罗旭,鲍荣粉,江国良.枇杷花果冻害与若干生理生化指标的关系[J].果树学报,2011,28(3):453-457.LI Jing,SUN Shuxia,XIE Hongjiang,CHEN Dong,TU Meiyan,HE Juntao,LUO Xu,BAO Rongfen,JIANG Guoliang.The relationship between freezing injury and physiological indexes of loquat (Eriobotrya japonica) flowers and fruits[J]. Journal of Fruit Science,2011,28(3):453-457.
[15] 常晓晓,陆育生,林志雄,潘建平,邱继水,唐振强.枇杷幼果雨雪冻害的理化响应[J].中国农学通报,2017,33(31):68-73.CHANG Xiaoxiao,LU Yusheng,LIN Zhixiong,PAN Jianping,QIU Jishui,TANG Zhenqiang. The physiochemical response of young loquat fruit to freeze injury[J]. Chinese Agricultural Science Bulletin,2017,33(31):68-73.
[16] FRANKLIN K A,LEE S H,PATEL D,KUMAR S V,SPARTZ A K,GU C,YE S Q,YU P,BREEN G,COHEN J D,WIGGE P A,GRAY W M. Phytochrome-interacting factor 4 (PIF4) regulates auxin biosynthesis at high temperature[J]. Proceedings of the National Academy of Sciences of the United States of America,2011,108(50):20231-20235.
[17] REN H,GRAY W M. SAUR proteins as effectors of hormonal and environmental signals in plant growth[J]. Molecular Plant,2015,8(8):1153-1164.
[18] WU J,LIU S Y,HE Y J,GUAN X Y,ZHU X F,CHENG L,WANG J,LU G. Genome-wide analysis of SAUR gene family in Solanaceae species[J].Gene,2012,509(1):38-50.
[19] 王红飞,尚庆茂.黄瓜SAUR 基因家族的鉴定与表达分析[J].园艺学报,2019,46(6):1093-1111.WANG Hongfei,SHANG Qingmao.Genome-wide identification and expression analysis of the SAUR gene family in Cucumis sativus[J].Acta Horticulturae Sinica,2019,46(6):1093-1111.
[20] 王福生,余洪,胡洲,管德龙,张盼,朱世平,赵晓春. 柑橘属SAUR 基因家族的全基因组鉴定及表达分析[J]. 园艺学报,2020,47(1):23-40.WANG Fusheng,YU Hong,HU Zhou,GUAN Delong,ZHANG Pan,ZHU Shiping,ZHAO Xiaochun. Genome-wide analysis of SAUR gene family in Citrus[J].Acta Horticulturae Sinica,2020,47(1):23-40.
[21] CHEN Y P,DENG C J,XU Q Z,CHEN X P,JIANG F,ZHANG Y L,HU W S,ZHENG S Q,SU W B,JIANG J M.Integrated analysis of the metabolome,transcriptome and miRNome reveals crucial roles of auxin and heat shock proteins in the heat stress response of loquat fruit[J].Scientia Horticulturae,2022,294:110764.
[22] 朱广廉.油菜素甾醇类植物激素的研究进展[J].植物生理学通讯,1992,28(5):317-322.ZHU Guanglian. Advance in the study on brassinosteroids[J].Plant Physiology Communications,1992,28(5):317-322.
[23] 李凯荣,吴发启,王键. 天然油菜素内酯对黄土丘陵区苹果生长发育和产量的影响[J]. 水土保持学报,2003,17(3):174-177.LI Kairong,WU Faqi,WANG Jian. Effects of natural brassinolide on growth,development and yield of apple trees[J]. Journal of Soil Water Conservation,2003,17(3):174-177.
[24] KAGALE S,DIVI U K,KROCHKO J E,KELLER W A,KRISHNA P. Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses[J].Planta,2007,225(2):353-364.
[25] 兰彩耘.超量表达AtDWF4 基因对芥菜生长发育及抗寒性的影响[D].重庆:西南大学,2016.LAN Caiyun. Effect of AtDWF4 gene overexpression on growth,development and cold resistance in Brassica juncea[D].Chongqing:Southwest University,2016.
[26] 李嘉佳,李相怡,王磊,王世平.葡萄果实内油菜素内酯生物合成调控及生理效应[J/OL].分子植物育种,2021:1-10.(2021-06-15).https://kns.cnki.net/kcms/detail/46.1068. S.20210615.1225.004.html.LI Jiajia,LI Xiangyi,WANG Lei,WANG Shiping.The regulation of brassinolide biosynthesis and physiological effects in grape berries[J/OL].Molecular Plant Breeding,2021:1-10.(2021-06-15).https://kns.cnki.net/kcms/detail/46.1068.S.20210615.1225.004.html.
[27] 郑婷,程建徽,魏灵珠,向江,吴江.油菜素内酯及其在园艺植物中的研究进展[J/OL]. 分子植物育种,2022:1-9. (2022-01-13).https://kns.cnki.net/kcms/detail/46.1068.s.20220113.1105.004.html.ZHENG Ting,CHENG Jianhui,WEI Lingzhu,XIANG Jiang,WU Jiang. Progress of brassinosteroids and reasearch advancements on horticultural plants[J/OL]. Molecular Plant Breeding,2022:1- 9. (2022- 01- 13). https://kns.cnki.net/kcms/detail/46.1068.s.20220113.1105.004.html.
[28] MARTÍNEZ C,ESPINOSA-RUÍZ A,DE LUCAS M,BERNARDO-GARCÍA S,FRANCO-ZORRILLA J M,PRAT S.PIF4-induced BR synthesis is critical to diurnal and thermomorphogenic growth[J].The EMBO Journal,2018,37(23):e99552.
[29] LEE S,WANG W L,HUQ E. Spatial regulation of thermomorphogenesis by HY5 and PIF4 in Arabidopsis[J].Nature Communications,2021,12(1):3656.
[30] 李雪.枇杷果实木质化相关基因的克隆与功能分析[D].杭州:浙江大学,2016.LI Xue. Cloning and functional analysis of genes involved in flesh lignification of loquat fruit[D]. Hangzhou:Zhejiang University,2016.
[31] LI X,ZANG C,GE H,ZHANG J,GRIERSON D,YIN X R,CHEN K S.Involvement of PAL,C4H,and 4CL in chilling injury-induced flesh lignification of loquat fruit[J]. HortScience,2017,52(1):127-131.
[32] ABBOTT J C,BARAKATE A,PINÇON G,LEGRAND M,LAPIERRE C,MILA I,SCHUCH W,HALPIN C. Simultaneous suppression of multiple genes by single transgenes. Downregulation of three unrelated lignin biosynthetic genes in tobacco[J].Plant Physiology,2002,128(3):844-853.
[33] SIBOUT R,EUDES A,MOUILLE G,POLLET B,LAPIERRE C,JOUANIN L,SÉGUIN A. Cinnamyl alcohol dehydrogenase-C and-D are the primary genes involved in lignin biosynthesis in the floral stem of Arabidopsis[J].The Plant Cell,2005,17(7):2059-2076.
[34] SHAN L L,LI X,WANG P,CAI C,ZHANG B,SUN C D,ZHANG W S,XU C J,FERGUSON I,CHEN K S. Characterization of cDNAs associated with lignification and their expression profiles in loquat fruit with different lignin accumulation[J].Planta,2008,227(6):1243-1254.
[35] 郑永华,李三玉,席玙芳.枇杷冷藏过程中果肉木质化与细胞壁物质变化的关系[J].植物生理学报,2000,26(4):306-310.ZHENG Yonghua,LI Sanyu,XI Yufang. Changes of cell wall substances in relation to flesh woodiness in cold stored loquat fruits[J].Acta Photophysiologica Sinica,2000,26(4):306-310.
[36] 吴光斌,陈发河,张其标,杨姣.热激处理对冷藏枇杷果实冷害的生理作用[J].植物资源与环境学报,2004,13(2):1-5.WU Guangbin,CHEN Fahe,ZHANG Qibiao,YANG Jiao. Effects of heat shock treatment on chilling injury and physiological responses of Eriobotrya japonica fruit during cold storage[J].Journal of Plant Resources and Environment,2004,13(2):1-5.
[37] 蔡冲.枇杷果实采后木质化与品质调控[D].杭州:浙江大学,2006.CAI Chong. Regulation of lignification and quality changes in postharvest loquat fruit[D]. Hangzhou:Zhejiang University,2006.
[38] 徐蒙.枇杷果实冷害木质化的EjHAT1 和EjbHLH1 转录调控机制[D].杭州:浙江大学,2019.XU Meng. Transcriptional regulation of loquat fruit chilling induced lignification by EjHAT1 and EjbHLH1[D]. Hangzhou:Zhejiang University,2019.
[39] 张琪静,董文轩.NAC 转录因子在果实发育成熟过程中的作用研究进展[J].园艺学报,2018,45(10):2052-2062.ZHANG Qijing,DONG Wenxuan.Mechanisms of NAC transcription factor functions during fruit development and ripening[J].Acta Horticulturae Sinica,2018,45(10):2052-2062.
[40] MARTEL C,VREBALOV J,TAFELMEYER P,GIOVANNONI J J.The tomato mads-box transcription factor ripening inhibitor interacts with promoters involved in numerous ripening processes in a colorless nonripening-dependent manner[J]. Plant Physiology,2011,157(3):1568-1579.
[41] LIU Y Z,BAIG M N R,FAN R,YE J L,CAO Y C,DENG X X.Identification and expression pattern of a novel NAM,ATAF,and CUC-like gene from Citrus sinensis Osbeck[J].Plant Molecular Biology Reporter,2009,27(3):292-297.
[42] XU X B,YIN L L,YING Q C,SONG H M,XUE D W,LAI T F,XU M J,SHEN B,WANG H Z,SHI X Q. High-throughput sequencing and degradome analysis identify miRNAs and their targets involved in fruit senescence of Fragaria ananassa[J].PLoS One,2013,8(8):e70959.
[43] XU Q,WANG W Q,ZENG J K,ZHANG J,GRIERSON D,LI X,YIN X R,CHEN K S.A NAC transcription factor,EjNAC1,affects lignification of loquat fruit by regulating lignin[J]. Postharvest Biology and Technology,2015,102:25-31.
[44] GE H,ZHANG J,ZHANG Y J,LI X,YIN X R,GRIERSON D,CHEN K S. EjNAC3 transcriptionally regulates chilling- induced lignification of loquat fruit via physical interaction with an atypical CAD-like gene[J]. Journal of Experimental Botany,2017,68(18):5129-5136.